Abstract
Central norepinephrine signaling influences a wide range of behavioral and physiological processes, and the ventral bed nucleus of the stria terminalis (vBNST) receives some of the densest norepinephrine innervation in the brain. Previous work describes norepinephrine neurons as projecting primarily unilaterally; however, recent evidence for cross-hemispheric catecholamine signaling challenges this idea. Here, we use fast-scan cyclic voltammetry and retrograde tracing to characterize cross-hemispheric norepinephrine signaling in the vBNST. We delivered stimulations to noradrenergic pathways originating in the A1/A2 and locus coeruleus and found hemispherically equivalent norepinephrine release in the vBNST regardless of stimulated hemisphere. Unilateral retrograde tracing revealed that medullary, but not locus coeruleus norepinephrine neurons send cross-hemispheric projections to the vBNST. Further characterization with pharmacological lesions revealed that stimulations of the locus coeruleus and its axon bundles likely elicit vBNST norepinephrine release through indirect activation. These experiments are the first to demonstrate contralateral norepinephrine release and establish that medullary, but not coerulean neurons are responsible for norepinephrine release in the vBNST.
Keywords: Norepinephrine, fast-scan cyclic voltammetry, cross-hemispheric, locus coeruleus, nucleus of the solitary tract, ventral bed nucleus of the stria terminalis
Graphical Abstract
Central norepinephrine signaling mediates a variety of processes including learning and memory, drug reward and withdrawal, and the behavioral and physiological responses to stress.1–5 Dysregulation of noradrenergic signaling is implicated in disorders ranging from drug addiction6 to Alzheimer’s disease,7 and the ventral bed nucleus of the stria terminalis (vBNST) is a site of some of the densest noradrenergic innervation in the brain.8 Limbic, forebrain, and brainstem inputs converge in the BNST to relay information about stressors and generate an appropriate physiological response through regulation of the hypothalamic-pituitary adrenal (HPA) axis.1 The vBNST receives noradrenergic input primarily from medullary neurons (A1/ A2) coursing through the ventral noradrenergic bundle (VNB) and, to a lesser extent, from the neurons of the locus coeruleus (LC) through the dorsal noradrenergic bundle (DNB).1,9–12 Norepinephrine is released in the vBNST during presentation of an aversive tastant, omission of an expected reward, and delivery of a noxious stimulus.13–15 Furthermore, the vBNST is an important structure in mediating the aversive components of drug-withdrawal,4,16,17 and norepinephrine signaling in the vBNST undergoes robust plasticity following stress or drug-withdrawal dependent on HPA axis function.18,19 Norepinephrine signaling in the vBNST can integrate information about aversive and stressful stimuli to generate an appropriate physiological response, thus how it is regulated is an important topic of investigation.
Early anatomical studies described catecholamine neurons as projecting solely to one hemisphere in rodents.20,21 However, more modern tracing studies challenge the exclusively unilateral nature of catecholaminergic projections, and provide evidence for crossing projections originating in cell groups ranging from the ventral tegmental area (VTA)22,23 to the LC.24,25 We recently asked to what extent cross-hemispheric projections contribute to striatal dopamine release in rats.26 Despite the reportedly small number of contralateral dopamine projections,22,23 we found that stimulating these projections resulted in physiologically relevant striatal dopamine release, which may confound the interpretation of unilateral manipulations.26 Since some norepinephrine neurons also exhibit crossing projections in rats,24,25,27,28 and primates,29,30 we hypothesized that, like dopamine, bilateral norepinephrine projections would influence measured norepinephrine release. In this work, we used fast-scan cyclic voltammetry in anesthetized rats to measure cross-hemispheric norepinephrine release in the vBNST. We found noradrenergic axon pathways can release norepinephrine in the contralateral vBNST, and we identified contralaterally projecting norepinephrine neurons using retrograde tracing. As a consequence of these investigations, we serendipitously discovered that LC and DNB stimulations produce norepinephrine overflow indirectly. This finding is contrary to our previous report suggesting coerulean projections are directly responsible for measured release in the vBNST.31 In agreement with anatomical evidence,1,9 we report that the neurons responsible for vBNST norepinephrine release are medullary in origin, and they exhibit cross-hemispheric functionality.
RESULTS AND DISCUSSION
Stimulation of Noradrenergic Axons Elicits Release in the Contralateral vBNST
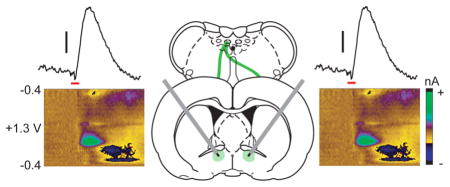
Early anatomical studies describe catecholamine neurons as projecting primarily unilaterally in rodents;20,21 however, more modern tracing studies have revealed some catecholamine neurons project contralateral to their origin.22–25 We recently showed crossing dopaminergic projections support dopamine release in the contralateral striatum and may contribute to interhemispheric signaling.26 To determine if norepinephrine neurons might also exhibit cross-hemispheric functionality and influence release in the vBNST, we used fast-scan cyclic voltammetry at dual carbon-fiber electrodes32 to measure norepinephrine release in anesthetized rats. We first lowered two carbon-fiber electrodes bilaterally into the vBNST, and a stimulating electrode unilaterally into the VNB (schematic in Figure 1a). Unilateral VNB stimulations produced norepinephrine release at both electrodes (examples in Figure 1b), supporting our hypothesis that norepinephrine projections exhibit cross-hemispheric functionality.
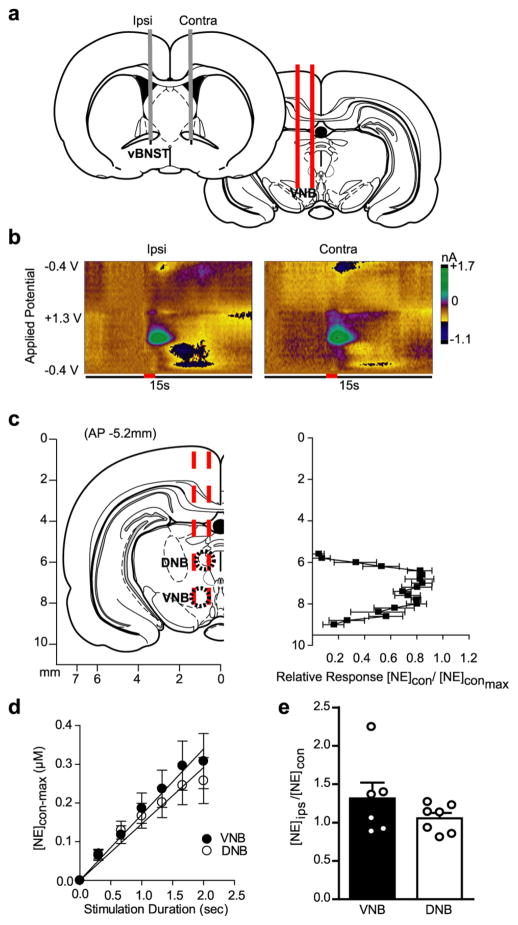
Figure 1.
Stimulation of noradrenergic axon bundles produces hemispherically equivalent norepinephrine release in the ventral bed nucleus of the stria terminalis (vBNST). (a) Schematic of dual carbon-fiber electrodes in the vBNST (gray) with unilateral stimulating electrode (red) in the ventral noradrenergic bundle (VNB). (b) Representative color plots demonstrating norepinephrine release to a 1s electrical stimulation (red bar) to the VNB recording ipsilateral (Ipsi) and contralateral (Contra) to the stimulation. Applied potential is plotted on the abiscca, recording time on the ordinate, and current is encoded in false color. (c) Effect of stimulation electrode placement on contralateral norepinephrine release. Data are plotted as norepinephrine release [NE]con over maximal norepinephrine release [NE]con-max as elicted by contralateral stimulations and are presented as average ± SEM (d) Effect of stimulation duration on norepinephrine release evoked by contralateral dorsal noradrenergic bundle (DNB) and VNB stimulations. Average ± SEM. (e) Within-animal comparison of norepinephrine release in the vBNST as elicited by ipsilateral and contralateral DNB and VNB stimulations. Average ± SEM with individual experiments overlaid.
To map the location of contralateral projections, we next held the carbon-fiber electrode at a fixed depth in the vBNST (−7.2 mm DV), and lowered the stimulating electrode ventrally through the contralateral noradrenergic bundles. We measured contralaterally evoked norepinephrine release over a large dorsal-ventral range, peaking at locations corresponding to the DNB (−6.4 mm DV) and VNB (−8.0 mm DV, Figure 1c), in agreement with previous ipsilateral characterization.31 Norepinephrine release evoked by contralateral DNB and VNB stimulation was linear with increasing stimulation duration (DNB slope = 0.146 ± 0.007 r2 = 0.97; VNB slope = 0.170 ± 0.005, r2 = 0.98; Figure 1d), similar to our previous report with ipsilateral stimulations.18 On average, maximal norepinephrine release in the vBNST was of comparable magnitude following stimulations of the contralateral DNB or VNB (DNB: 0.214 ± 0.066 μM; VNB: 0.192 ± 0.038 μM, n = 10, respectively), and ipsilateral DNB and VNB (DNB: 0.228 ± 0.063 μM; VNB: 0.191 ± 0.029 μM, n = 10, respectively). In a subset of animals, we performed within-animal comparisons of ipsilateral vs contralateral release by lowering the stimulating electrode ventrally through the ipsilateral, then contralateral hemisphere. Strikingly, the ratio of ipsilateral to contralateral release was equal between hemispheres following either DNB or VNB stimulations (Ipsi/Contra, VNB: 1.3 ± 0.20; DNB: 1.0 ± 0.08, n = 6 animals, P > 0.05, Figure 1e). In this way, cross-hemispheric norepinephrine in the vBNST closely resembled dopamine in the dorsomedial striatum, in that it exhibited equivalent release regardless of stimulated hemisphere.26
DSP-4 Treatment Does Not Attenuate vBNST Norepinephrine Release
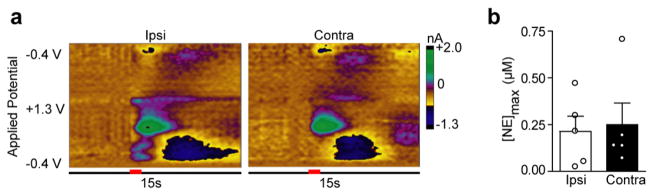
The vBNST is thought to receive a small input from the LC through the DNB.1,9 Since DNB stimulations produced hemispherically equivalent release amplitudes, we next placed our stimulating electrode in the contralateral LC to ascertain its contribution to vBNST norepinephrine release. Stimulations of the LC elicited robust norepinephrine regardless of stimulated hemisphere (Ipsi, 0.163 ± 0.082 μM; Contra, 0.250 ± 0.115 μM, n = 5, P > 0.05, Figure 2), and of similar magnitude to previously reported LC-evoked norepinephrine in the vBNST.31 However, coerulean inputs to the vBNST are sparse;9 thus, we next asked if off-target effects could contribute to robust norepinephrine release described here and in the previous study.31 We employed the selective neurotoxin DSP-4 to lesion norepinephrine terminals from the LC.33 Tissue content analysis revealed the treatment was effective at eliminating a significant degree of LC innervation, as concentrations were markedly reduced in the anteroventral thalamus (AV), a brain region receiving exclusively coerulean input (Table 1). Control values for the vBNST and the AV are similar to those previously reported from our lab18,31 and others.8,34 DSP-4 treatment significantly reduced norepinephrine and dopamine in the AV (unpaired t test, norepinephrine: t(9) = 3.579, P = 0.006; dopamine: t(9) = 2.586, P = 0.029), but did not exhibit an effect on the catecholamine content of the vBNST (norepinephrine: t(9) = 0.959, P = 0.363; dopamine: t(9) = 0.371, P = 0.719), in agreement with sparse coerulean innervation of the latter.9
Figure 2.
Locus coeruleus stimulations produce equivalent norepinephrine release in the ventral bed nucleus of the stria terminalis independent of stimulated hemisphere. (a) Representative color plots demonstrating norepinephrine release in the vBNST following a 1 s electrical stimulation (red bar) of the ipsilateral (Ipsi) and contralateral (Contra) locus coeruleus (LC). (b) Maximal norepinephrine release (NEmax) in the vBNST following Ipsi and Contra LC stimulations in all subjects.
Table 1.
Catecholamine Tissue Content in Target Regions for Untreated and DSP-4-Treated Animalsa
| NE (μg/g tissue)
|
DA (μg/g tissue)
|
|||
|---|---|---|---|---|
| untreated | DSP-4 | untreated | DSP-4 | |
| vBNST | 2.98 ± 0.80 | 2.14 ± 0.58 | 0.82 ± 0.25 | 0.84 ± 0.17 |
| AV | 1.82 ± 0.50 | 0.18 ± 0.10** | 1.62 ± 0.52 | 0.34 ± 0.20* |
Values are shown as mean ± SEM.
P < 0.05,
P < 0.01, compared to untreated values.
Abbreviations: NE, norepinephrine; DA, dopamine.
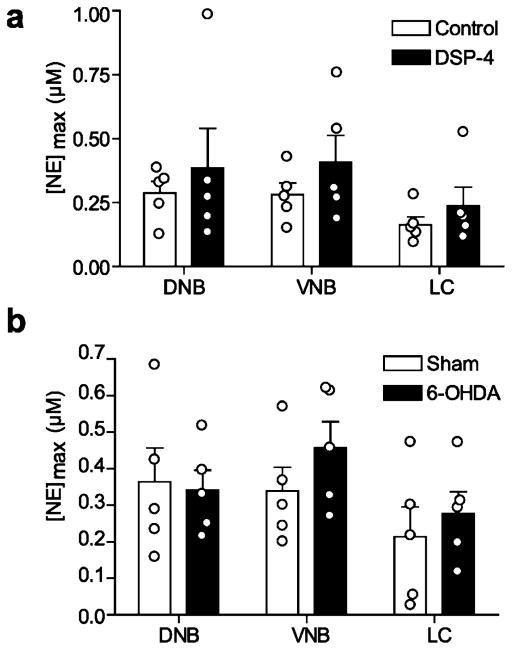
As expected from the tissue content findings, DSP-4 treatment significantly attenuated DNB-evoked norepinephrine release in the AV (control vs DSP-4, 0.216 ± 0.035 vs 0.0950 ± 0.0376 μM, n = 5, respectively, unpaired t test: t(8) = 2.363, p < 0.05, Figure S1). DSP-4 treatment did not affect vBNST release with ipsilateral VNB stimulations (control vs DSP-4, 0.281 ± 0.046 vs 0.408 ± 0 0.104 μM, n = 5 rats, respectively, Figure 3a) in agreement with our previous work.19 Surprisingly, DSP-4 treatment did not reduce vBNST norepinephrine evoked by ipsilateral LC or DNB stimulations (control vs DSP-4, DNB: 0.288 ± 0.046 vs 0.386 ± 0.154; LC 0.163 ± 0.032 vs 0.237 ± 0.073 μM, n = 5 rats, respectively, P > 0.05, Figure 3a), suggesting LC inputs to the vBNST were spared by DSP-4, or that release arises though an indirect effect.
Figure 3.
Chemical lesions of the LC do not impact vBNST norepinephrine release. (a) Maximal norepinephrine concentrations elicited by ipsilateral DNB, VNB, and LC stimulations in control (white) and DSP-4 treated rats (black). (b) Maximal norepinephrine concentrations elicted by ipsilateral DNB, VNB, and LC stimulations in sham (white), and 6-OHDA lesioned rats (black). Average ± SEM with individual experiments overlaid.
Physical, but Not 6-OHDA LC Lesions Attenuate vBNST Norepinephrine Release
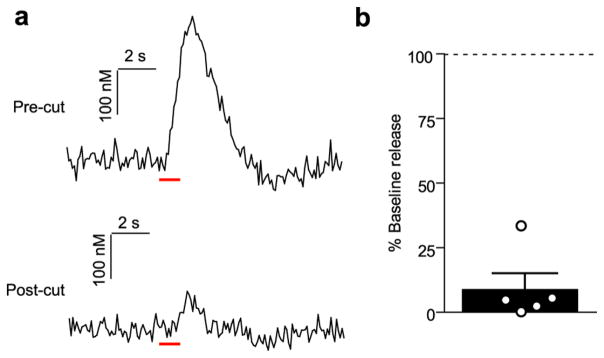
Since DSP-4 does not lesion the LC with 100% efficacy,35 and its actions on the LC noradrenergic system have been called into question,36 we next used bilateral 6-hydroxydopamine (6-OHDA) lesions targeted to the LC to corroborate the DSP-4 findings. Two weeks after 6-OHDA treatment, we anesthetized animals and recorded norepinephrine evoked from ipsilateral electrical stimulations. Similar to DSP-4 treatment, 6-OHDA lesions of the LC had no measurable effect on norepinephrine release in the vBNST (Sham vs 6-OHDA; DNB: 0.364 ± 0.092 vs 0.342 ± 0.054 μM; VNB: 0.339 ± 0.065 vs 0.457 ± 0.072 μM; LC 0.213 ± 0.082 vs 0.278 ± 0.060 μM, n = 5 rats, respectively, P > 0.05, Figure 3b). In our previous report of LC-evoked norepinephrine in the vBNST, release was suppressed after delivery of lidocaine to the stimulation site.31 Given that selective chemical ablations did not reduce norepinephrine release, we turned to a physical disconnection approach by performing a knife-cut at the level of the LC. Cutting the ipsilateral LC markedly reduced LC-evoked norepinephrine release in the vBNST (9.0 ± 6.1%, n = 5 rats, Figure 4), similar to lidocaine.31
Figure 4.
Knife-cut of the LC reduces LC-evoked vBNST norepinephrine. (a) Representative traces of norepinephrine release elicited by LC stimulations before, and after knife cut. Red bar denotes stimulation duration. (b) Norepinephrine release as a percentage of precut baseline. Average ± SEM with individual experiments overlaid.
A2, but Not LC Neurons Project Bilaterally to the vBNST
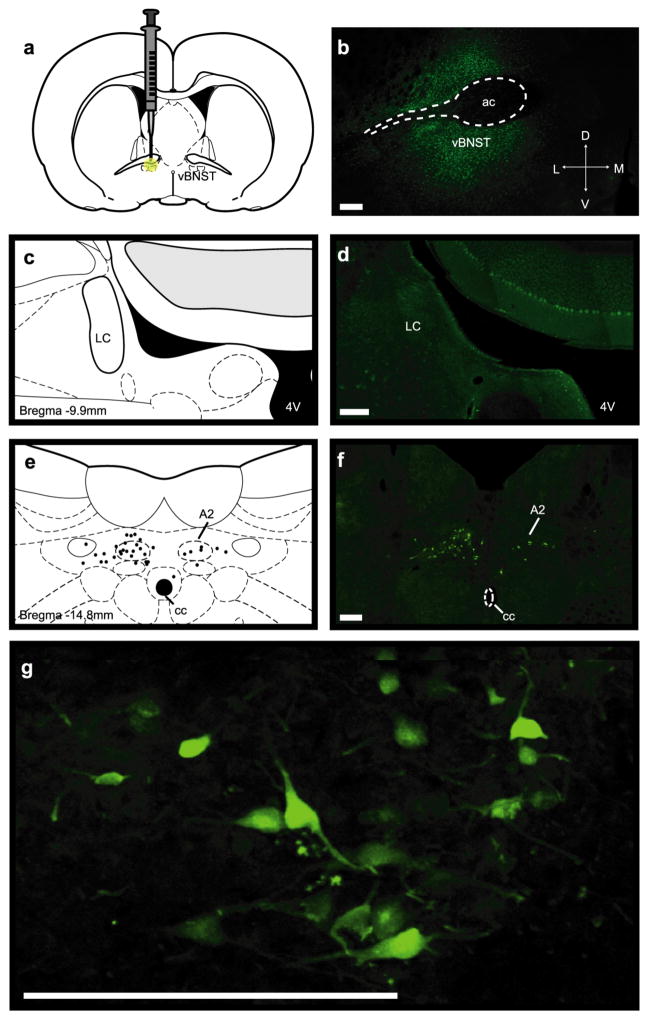
Since a physical, but not selective pharmacological lesion of the LC reduced norepinephrine overflow, we asked if the LC sends projections to the vBNST that might be spared by chemical treatments. We unilaterally injected fluorogold into the vBNST, and looked for retrograde labeling in the LC and the nucleus of the solitary tract (A2) (Schematic in Figure 5a, Representative injection site Figure 5b). Even after signal amplification with an antibody against fluorogold, we did not find any retrogradely labeled cells in the LC (Figure 5c,d). Instead, we found bilateral fluorogold labeling in the A2 (Figure 5e–g). A1 norepinephrine neurons also innervate the vBNST,1,7,9–11 which may provide an additional source of cross-hemispheric projections. However, further work is needed to assess if the A1 sends bilateral projections to the vBNST, and if they contribute to contralateral norepinephrine release. Nevertheless, we found strong bilateral retrograde labeling in the A2. The number of labeled cells ipsilateral to the tracer infusion was greater than those in the contralateral hemisphere (27 ± 1.7 vs 5.3 ± 1.2 cells, n = 3 rats), which was surprising since we measured similar concentrations of norepinephrine with ipsilateral and contralateral VNB stimulations.
Figure 5.
Unilateral fluorogold tracing in the vBNST. (a,b) Schematic and representative infusion site of fluorogold into the vBNST. (c,d) Apparent lack of fluorogold-positive cells in the ipsilateral LC and atlas section corresponding to the photomicrograph. (e,f) Bilateral fluorogold-positive cells in the A2 and camera lucida drawing of labeled cells in the atlas section corresponding to the photomicrograph. (g) Higher magnification image of fluorogold-positive cells in the ipsilateral A2. Scale bars are 200 μm. 4 V, fourth ventricle; ac, anterior commissure; cc, central canal.
Hemispherically equivalent release could arise from a number of mechanisms. First, norepinephrine concentrations released from contralateral projections were linear with respect to stimulation duration, in a similar manner as ipsilaterally evoked norepinephrine.18 This might indicate norepinephrine release is “capped” from ipsilateral projections by regulation mechanisms. In our previous characterization of cross-hemispheric dopamine, we found the ratio of ipsilateral to contralateral dopamine release was at least partially dependent on D2 autoreceptor control.26 Thus, autoreceptors may play a role in balancing norepinephrine concentrations between hemispheres as they do for dopamine. Alternatively, norepinephrine neurons may co-release glutamate, which was recently demonstrated to occur from some dopamine terminals.37 Co-release of other neurotransmitters may depolarize norepinephrine terminals in the vBNST and partially explain the apparent hemispheric equivalence. Indeed, bath application of excitatory amino acids can stimulate norepinephrine release in brain slices.38,39 It is also worth considering that stimulations of the VNB also target the VTA,32 which receives inputs from both the A2 group40 and the BNST.41 Activation of these projections may also contribute to electrically evoked norepinephrine release and hemispheric equivalence. Although the mechanism underlying hemispherically equivalent norepinephrine release is unknown, these data reveal that norepinephrine in the vBNST is similar to dopamine release in the dorsomedial striatum,26 in that it is of similar magnitude with ipsilateral or contralateral stimulations.
DNB Stimulations Produce vBNST Norepinephrine Indirectly
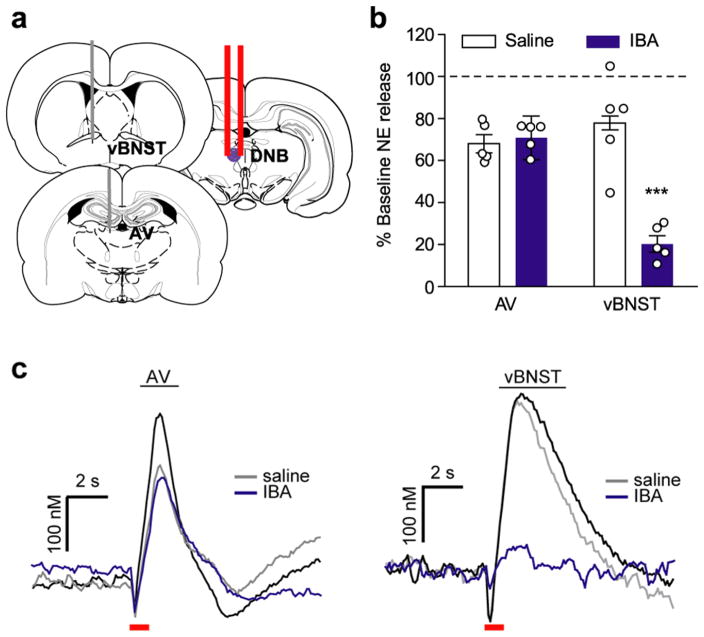
The LC sends most of its forebrain projections through the DNB.21 Since the LC was not labeled with fluorogold, we hypothesized DNB stimulations produced norepinephrine release through an indirect mechanism. To test the possibility that another midbrain structure was mediating norepinephrine release, we delivered ibotenic acid (IBA) to the DNB (schematic in Figure 6a). IBA is a glutamate analog that, through excitotoxicity, selectively inactivates cell bodies while leaving fibers of passage intact.42 Therefore, its neurotoxicity is not expected to impact the axons of the DNB; however, it would inactivate other proximal neurons. In agreement with this, norepinephrine release was not significantly altered in the AV (Saline vs IBA: 68.0 ± 4.3% vs 70.8 ± 10.4%, n = 5 rats, p > 0.05, Figure 6b,c), indicating in this region, release is mediated by direct activation of the DNB. However, IBA infusions in the DNB significantly reduced norepinephrine release in the vBNST (saline 77.9 ± 3.3% vs IBA 20.3 ± 3.8%, n = 5 rats, two-way repeated measures ANOVA: drug × region interaction F(2,16) = 16.52, effect of region F(2,16) = 21.47, effect of drug F(2,16) = 46.67, Bonferroni post hoc, p < 0.001, Figure 6b,c), suggesting DNB evoked release in the vBNST arises through an indirect mechanism.
Figure 6.
Ibotenic acid infusions in the DNB attenuate norepinephrine release in the vBNST, but not anteroventral thalamus (AV). (a) Schematic of recording locations and infusion of ibotenic acid (IBA) in the DNB. (b) Effect of saline (white) and IBA (purple) infusions on norepinephrine release in the vBNST and AV as a percent of baseline release (hashed line).***P < 0.01, two-way repeated measures ANOVA with Bonferroni post hoc. (c) Representative evoked norepinephrine in the AV and vBNST after saline (gray) and IBA (purple) infusions. Red bar denotes electrical stimulation.
Possible Mechanisms for Coerulean Evoked Norepinephrine in the vBNST
Based on the data obtained after selective chemical inactivation, we propose that norepinephrine release in the vBNST evoked from DNB and LC stimulations arises through an indirect mechanism. First, vBNST norepinephrine release was attenuated following IBA infusions in the DNB. Since IBA does not affect fibers of passage,42 the results from this experiment suggest that other midbrain nuclei are mediating release following DNB stimulation. At the coordinates used in this study, the DNB courses by several structures including the periaqueductal gray (PAG). Fluorogold infusions into the ventrolateral portion of the PAG bilaterally labels neurons in the A2 region.43 Thus, PAG activation may antidromically activate the A2 group to elicit vBNST release, or this release could arise from another unidentified mechanism. Second, selective chemical inactivation with DSP-4 and 6-OHDA strongly suggest that noradrenergic neurons of the LC are not responsible for release in the vBNST. However, both lidocaine and knife-cut would prevent the propogation of action potentials traveling down axons near the LC. It is therefore possible that projections from medullary noradrenergic neurons close to the LC are responsible for this release. Indeed, an anterograde tracing study revealed A2 neurons innervate regions proximal to the LC.44
Alternatively, the A2 projects to the nucleus paragiganto-cellularis (PGi),45 which in turn, sends projections to the LC.46 Therefore, LC stimulation may antidromically activate the PGi and subsequently the A2 to produce norepinephrine release in the vBNST. Furthermore, cross-talk between coerulean and medullary cell groups is supported by work demonstrating both norepinephrine cell groups contribute to opiate withdrawal syndrome,4,47 and coerulean lesions produce adaptations in norepinephrine signaling originating from A1/A2 neurons.19 Regardless of mechanism, the selective chemical lesions reveal that, although the DNB and LC can produce release in both ipsilateral and contralateral vBNST, it likely does so indirectly. Interestingly, the nonspecific activation we propose here may be responsible for the disparity in reports describing electrical self-stimulations of the LC or its pathways.48–51 Indeed, in animals trained to self-stimulate the LC, neither 6-OHDA lesions, nor electrolytic lesions of the DNB attenuate self-stimulation behavior,52,53 providing further support that stimulations of the LC/DNB activate other pathways.
There are two obvious approaches to address the specific neuronal populations contributing to vBNST norepinephrine release. The first would be to inactivate medullary norepinephrine neurons to confirm they are the sole population responsible for release in the vBNST. We have made repeated attempts to chemically ablate the A2 group, however these manipulations in the brainstem typically result in ceased respiratory activity and subsequent death of the subject. The second approach would rely on a more selective stimulation method, such as optogentics. Although optogenetic approaches have been widely successful for dopamine measurements,54 thus far we have been unable to measure norepinephrine release from optogenetic stimulations in intact rats. For the time being, electrical stimulations must suffice for studying vBNST norepinephrine release in anesthetized animals. It is in this context that we place our findings regarding LC and DNB evoked norepinephrine. Although this release likely arises through an indirect mechanism, we can not provide a definitive source. However, it is clear that care must be taken when interpreting data obtained with electrical stimulations of norepinephrine neurons and their projections.
Cross-Hemispheric Projections in the Context of Unilateral Manipulations
Neurodegenerative conditions such as Parkinson’s disease are often modeled with unilateral lesions of catecholaminergic neurons.55 However, the cross-hemispheric nature of catecholamine projections may confound the interpretation of data obtained following unilateral manipulations in rats. Indeed, stimulating intact, contralateral dopamine projections can release dopamine into an otherwise depleted hemisphere.26 Furthermore, in a recent report, researchers used a unilateral knife-cut of the DNB to rule out contributions of LC norepinephrine to catecholamine release measured in the medial prefrontal cortex.56 However, LC neurons project bilaterally to some regions24,25 and these contralateral projections may release physiologically relevant norepinephrine concentrations. It is clear from these findings that care must be taken when performing unilateral disconnection studies, since the unilateral nature of the monoamine projections originally described by Ungerstedt20,21 is now being called into question. Finally, exploiting the cross-hemispheric nature of catecholamine projections may prove useful in therapies such as deep-brain stimulation for restoring catecholamine concentrations in the brain.
CONCLUSIONS
In summary, we found norepinephrine release was elicited in the vBNST contralateral to the stimulation location. Stimulations of the DNB, VNB, and LC evoked norepinephrine of equal magnitude in both hemispheres. Norepinephrine evoked from LC stimulations occurred via nonspecific activation, as only physical, but not selective pharmacological lesions of the LC attenuated release. DNB stimulations also elicited norepinephrine in a nonspecific way, since inactivation of cells proximal to the DNB reduced evoked vBNST norepinephrine. Furthermore, fluorogold tracing revealed medullary, but not LC neurons, send bilateral projections to the vBNST. Taken together, these data show that, although norepinephrine is released in both hemispheres with unilateral activation, only medullary norepinephrine neurons are directly responsible for cross-hemispheric release in the vBNST. This previously undescribed property of norepinephrine neurons should be taken into account when performing unilateral manipulations.
METHODS
Animal Care
All experiments were performed in accordance with the Institutional Animal Care and Use Committee guidelines of the University of North Carolina at Chapel Hill (UNC). Sprague–Dawley rats (males, 300–400 g; Charles River, Wilmington, MA) were given food and water ad libitum and pair-housed in UNC animal facilities on a 12:12 h light:dark cycle.
Voltammetric Norepinephrine Measurements
Norepinephrine release was measured in anesthetized rats as described previously19 using HDCV.57 An average in vitro calibration factor of 6 nA/ μM was used to convert norepinephrine current to concentration following principal component analysis.58 For bilateral norepinephrine measurements, rats were anesthetized with urethane (1.5 g/kg) and placed in a stereotaxic frame (Kopf. Tujunga, CA). Holes were drilled for the vBNST (AP 0 mm, ML ± 1.2 mm), the DNB/VNB (AP −5.2 mm, ML +1.2 mm), and the LC (AP −9.8 mm, ML +1.3 mm), referenced from bregma and based on the atlas of Paxinos and Watson.59 A Ag/ AgCl reference electrode was placed in the left hemisphere and secured with a jeweler’s screw. A carbon fiber microelectrode (~100 μm active length) was lowered into the right vBNST (−7 to −7.5 mm DV) and a bipolar stimulating electrode (Plastics One, Roanoke, VA) was lowered ipsilateral to the carbon-fiber electrode in the LC (~ −7.0 mm DV), DNB (~ −6.5 mm DV), or VNB (~ −8.0 mm DV) until maximal norepinephrine release was attained. Both stimulating and carbon-fiber electrodes were subsequently secured with dental cement. A second carbon fiber microelectrode was lowered into the left, contralateral vBNST (−7 to 7.5 mm DV) until maximal norepinephrine was achieved. A total of 10 rats were used for these studies.
For mapping experiments and stimulation duration studies, first a carbon-fiber electrode was lowered into the right vBNST until maximal release with ipsilateral VNB stimulations was attained. Next, the stimulating electrode was raised to the DNB to determine maximum ipsilateral release. Then, the stimulating electrode was lowered through the contralateral hemisphere in 200 μm increments to map the effect of contralateral stimulating electrode placement in the vBNST. Sixty Hz stimulations of varying duration (20–120 pulses) were delivered at depths corresponding to maximal release from contralateral DNB and VNB stimulations and plotted vs stimulation duration. We compared maximal norepinephrine evoked by ipsilateral and contralateral DNB and VNB stimulations at the same recording electrode location. A total of 6 rats were used for these experiments. In another group of rats (n = 5), we compared maximal norepinephrine release in the vBNST following ipsilateral and contralateral LC stimulations.
DSP-4 Treatment
Adolescent rats (150–200 g) were administered DSP-4 (N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine) in two doses (0.5 mL, 50 mg/kg, i.p.) provided 3 days apart.19 Voltammetric (n = 5 DSP-4, n = 5 control) and tissue content (n = 6 DSP-4, n = 5 control) experiments were conducted 10–15 days after the last dose.
Tissue Content Analysis
A separate group of rats was anesthetized with urethane (1.5 g/kg), and their brains were rapidly removed and placed in ice-cold artificial cerebral spinal fluid (aCSF). Coronal sections (300 μm thick) containing the BNST or AV were collected with a VF-200 Compresstome (Precisionary Instruments Greenville, NC) in ice cold aCSF. The aCSF contained (in mM) 126 NaCl, 25 NaHCO3, 2.45 KCl, 12 NaH2PO4, 1.2 MgCl2, 2.4 CaCl2, 20 HEPES, and 11 glucose, and was adjusted to pH 7.4 and saturated with 95% O2 /5% CO2. Tissue containing the vBNST or AV was excised bilaterally with a 1 mm punch, and collected into preweighed tubes. The samples were mixed with 200 μL of 0.1 N HClO4 containing 1 μM hydroquinone, the internal standard, and subsequently homogenized using a sonic dismembrator (Fisher Scientific, Model 60, Pittsburgh, PA). The homogenate was spun down at 6000 rpm for 10 min, and the supernatant was removed and filtered using a 0.2 μm syringe filter. High performance liquid chromatography was performed as described previously.18,31 Briefly, 20 μL injections were made onto a reversed-phase column (5 μm, 4.6 × 5 mm, Waters Atlantis, Milford, MA). The mobile phase consisted of 0.1 M citric acid, 1 mM sodium hexylsulfate, 0.1 mM EDTA (pH = 3), and 10% methanol organic modifier at a flow rate of 1.0 mL/min. Norepinephrine and dopamine were detected with a thin layer radial electrochemical cell (BASi, West Lafayette, IN) at a potential of +800 mV vs Ag/AgCl. Data were collected at 60 Hz using a LabVIEW stripchart recorder program (Jorgenson Lab, UNC) and custom-built electronics. Concentration was determined by a ratio of analyte peak area to internal standard peak area, and normalized to wet tissue weight.
6-Hydroxydopamine Lesions
Rats underwent stereotaxic surgery under isoflurane anesthesia (4% induction, 1.5% maintenance), and an incision was made in the scalp to drill bilateral holes targeting the LC (AP −9.8 mm, ML ±1.4). An infusion cannula (Plastics One) was lowered to a depth of 7.0 mm from brain surface, and 1 μL of 10 mM 6-hydroxydopamine hydrobromide (6-OHDA)/ 0.01% w/v ascorbic acid (Sigma-Aldrich) in sterile saline (0.9%), or saline (sham-lesioned) was infused into each hemisphere with an infusion needle (33 ga, 10 mm, Plastics One) over 5 min. The scalp was closed with Vet Bond (3M, St Paul, MN) and rats were allowed to recover for 2 weeks before being anesthetized with urethane for voltammetric norepinephrine measurements.
Knife-Cut Experiments
Rat were anesthetized with urethane (1.5 g/kg) and affixed in a stereotaxic frame. Holes were drilled for the vBNST, VNB, and LC as described above. Once maximal release was attained with ipsilateral VNB stimulations the stimulating electrode was moved to the ipsilateral LC and adjusted for maximal release. The stimulating electrode was subsequently removed, and a surgical blade was lowered 0.2 mm past the depth of maximal LC release. The stimulating electrode was repositioned in the LC, and norepinephrine release was measured after subsequent LC stimulations. A total of 5 animals were used for these experiments.
FluoroGold Tracing
Rats underwent stereotaxic surgery under isoflurane anesthesia (4% induction, 1.5% maintenance). A small incision was made in the scalp, a hole was drilled in the skull to unilaterally target the BNST (AP 0.0 mm, ML 1.2 mm), and a 2 μL Hamilton syringe was lowered to a depth of 7.2 mm from brain surface. 200 nL of FluoroGold (4% w/v in 0.9% saline, Fluorochrome, Denver, CO) was infused slowly over 5 min using a microinjection unit (model 500, Kopf, Tujunga, CA). The syringe was left in place for an additional 5 min to minimize spread up the tract. The scalp was closed with vet bond (3M), and rats were allowed to recover for 2 weeks. Rats were then anesthetized with urethane (1.5 g/kg) and transcardially perfused with 0.1 M phosphate buffered saline (PBS, pH 7.4), followed by 4% paraformaldehyde dissolved in PBS. Brains were subsequently removed and postfixed for >24 h in 4% paraformaldehyde. The fixed tissue was cryoprotected for >24 h in 30% sucrose before 30 μm sections were collected in PBS using a freezing microtome (Leica, Germany).
Free-floating sections were incubated in 1%NaBH4/0.1 M PBS for 15 min to quench endogenous fluorescence, and then rinsed in PBS 3× 15 min. Sections were blocked in 10% Normal Goat Serum (NGS)/ 0.3% Triton x-100 for 2 h at room temperature. After blocking, sections were incubated in 1:1000 rabbit-anti-FluoroGold (Fluorochrome) in 3% NGS 0.1%Triton x-100 overnight at 4 °C. Sections were washed in PBS before being incubated for 2 h in 1:500 goat anti-rabbit IgG FITC conjugate (Millipore) in 2% bovine serum albumin/0.1 M PBS at room temperature. Sections were rinsed 3× in PBS, then mounted, dried, and coverslipped with fluoromount (Sigma-Aldrich) for imaging on an Olympus FV1000 confocal microscope.
Ibotenic Acid Infusion
Electrical stimulation of the DNB was repeated every 3 min over a 1 h period to establish a baseline for norepinephrine release. Thereafter the stimulating electrode was removed, and the tip of a 2 μL Hamilton syringe containing sterile saline was positioned 500 μm dorsal to the original stimulation depth. The saline was infused manually with a microinjection unit (Model 500, Kopf, Tujunga, CA) over a 20 min period, and the syringe was removed for reinsertion of the stimulating electrode. Stimulations commenced for another 1 h period before the infusion procedure was repeated with 2 μL ibotenic acid (130 mM in 2% Chicago Sky Blue prepared in sterile saline, Abcam, Cambridge, MA). The last 15 min of data collected for baseline, post-saline and post-IBA were used in analysis
Statistics
All statistical tests were performed in Graph Pad Prism. Two-tailed t tests were used to assess differences in ipsilateral vs contralateral release. Two-way analysis of variance (ANOVA) with Bonferroni post hoc was used to assess differences in release following DSP-4 or 6-OHDA treatment. Two-way repeated-measures ANOVA with Bonferroni post hoc was used to assess differences in norepinephrine release following IBA lesions.
Supplementary Material
Acknowledgments
This work was funded by NIH (DA 10900 to R.M.W.). The authors acknowledge Dr. Vladimir Ghukasyan at the University of North Carolina Confocal and Multiphoton Imaging Core for technical assistance (P30NS045892).
ABBREVIATIONS
- A2
nucleus of the solitary tract
- DNB
dorsal noradrenergic bundle
- DSP-4
N-(2-chloroethyl)-N-ethyl-2-bromobenzyl-amine
- IBA
ibotenic acid
- LC
locus coeruleus
- vBNST
ventral bed nucleus of the stria terminalis
- VNB
ventral noradrenergic bundle
Footnotes
Author Contributions
M.E.F. and E.S.B. performed and analyzed voltammetry experiments. J.A.J. assisted with DSP-4 experiments. M.E.F. performed HPLC analysis of catecholamine content and fluorogold tracing. M.E.F., E.S.B., and R.M.W. wrote the manuscript.
The authors declare no competing financial interest.
- Figure demonstrating norepinephrine release in the AV after DSP-4 treatment (PDF)
References
- 1.Forray MI, Gysling K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in the regulation of the hypothalamic-pituitary-adrenal axis. Brain Res Rev. 2004;47:145–160. doi: 10.1016/j.brainresrev.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Cecchi M, Khoshbouei H, Javors M, Morilak DA. Modulatory effects of norepinephrine in the lateral bed nucleus of the stria terminalis on behavioral and neuroendocrine responses to acute stress. Neuroscience. 2002;112:13–21. doi: 10.1016/s0306-4522(02)00062-3. [DOI] [PubMed] [Google Scholar]
- 3.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 4.Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- 5.Olson VG, Heusner CL, Bland RJ, During MJ, Weinshenker D, Palmiter RD. Role of noradrenergic signaling by the nucleus tractus solitarius in mediating opiate reward. Science. 2006;311:1017–1020. doi: 10.1126/science.1119311. [DOI] [PubMed] [Google Scholar]
- 6.Koob GF. Brain stress systems in the amygdala and addiction. Brain Res. 2009;1293:61–75. doi: 10.1016/j.brainres.2009.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinshenker D. Functional consequences of locus coeruleus degeneration in Alzheimer’s disease. Curr Alzheimer Res. 2008;5:342–345. doi: 10.2174/156720508784533286. [DOI] [PubMed] [Google Scholar]
- 8.Kilts CD, Anderson CM. The simultaneous quantification of dopamine, norepinephrine and epinephrine in micropunched rat brain nuclei by on-line trace enrichment HPLC with electrochemical detection: Distribution of catecholamines in the limbic system. Neurochem Int. 1986;9:437–445. doi: 10.1016/0197-0186(86)90086-0. [DOI] [PubMed] [Google Scholar]
- 9.Robertson SD, Plummer NW, de Marchena J, Jensen P. Developmental origins of central norepinephrine neuron diversity. Nat Neurosci. 2013;16:1016–1023. doi: 10.1038/nn.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banihashemi L, Rinaman L. Noradrenergic inputs to the bed nucleus of the stria terminalis and paraventricular nucleus of the hypothalamus underlie hypothalamic-pituitary-adrenal axis but not hypophagic or conditioned avoidance responses to systemic yohimbine. J Neurosci. 2006;26:11442–11453. doi: 10.1523/JNEUROSCI.3561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forray MI, Gysling K, Andres ME, Bustos G, Araneda S. Medullary noradrenergic neurons projecting to the bed nucleus of the stria terminalis express mRNA for the NMDA-NR1 receptor. Brain Res Bull. 2000;52:163–169. doi: 10.1016/s0361-9230(00)00229-x. [DOI] [PubMed] [Google Scholar]
- 12.Shin JW, Geerling JC, Loewy AD. Inputs to the ventrolateral bed nucleus of the stria terminalis. J Comp Neurol. 2008;511:628–657. doi: 10.1002/cne.21870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park J, Wheeler RA, Fontillas K, Keithley RB, Carelli RM, Wightman RM. Catecholamines in the bed nucleus of the stria terminalis reciprocally respond to reward and aversion. Biol Psychiatry. 2012;71:327–334. doi: 10.1016/j.biopsych.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park J, Bucher ES, Budygin EA, Wightman RM. Norepinephrine and dopamine transmission in 2 limbic regions differentially respond to acute noxious stimulation. Pain. 2015;156:318–327. doi: 10.1097/01.j.pain.0000460312.79195.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park J, Bucher ES, Fontillas K, Owesson-White C, Ariansen JL, Carelli RM, Wightman RM. Opposing catecholamine changes in the bed nucleus of the stria terminalis during intracranial self-stimulation and its extinction. Biol Psychiatry. 2013;74:69–76. doi: 10.1016/j.biopsych.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aston-Jones G, Delfs JM, Druhan J, Zhu YAN. The Bed Nucleus of the Stria Terminalis: A Target Site for Noradrenergic Actions in Opiate Withdrawal. Ann N Y Acad Sci. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- 17.Fox ME, Rodeberg NT, Wightman RM. Reciprocal Catecholamine Changes during Opiate Exposure and Withdrawal. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McElligott ZA, Fox ME, Walsh PL, Urban DJ, Ferrel MS, Roth BL, Wightman RM. Noradrenergic Synaptic Function in the Bed Nucleus of the Stria Terminalis Varies in Animal Models of Anxiety and Addiction. Neuropsychopharmacology. 2013;38:1665–1673. doi: 10.1038/npp.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox ME, Studebaker RI, Swofford NJ, Wightman RM. Stress and Drug Dependence Differentially Modulate Norepinephrine Signaling in Animals with Varied HPA Axis Function. Neuropsychopharmacology. 2015;40:1752–1761. doi: 10.1038/npp.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andén NE, Dahlstrom A, Fuxe K, Larsson K, Olson L, Ungerstedt U. Ascending Monoamine Neurons to the Telencephalon and Diencephalon. Acta Physiol Scand. 1966;67:313–326. [Google Scholar]
- 21.Ungerstedt U. Stereotaxic mapping of the monoamine pathways in the rat brain. Acta Physiol Scand. 1971;82:1–48. doi: 10.1111/j.1365-201x.1971.tb10998.x. [DOI] [PubMed] [Google Scholar]
- 22.Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J Comp Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- 23.Jaeger CB, Joh TH, Reis DJ. The effect of forebrain lesions in the neonatal rat: survival of midbrain dopaminergic neurons and the crossed nigrostriatal projection. J Comp Neurol. 1983;218:74–90. doi: 10.1002/cne.902180105. [DOI] [PubMed] [Google Scholar]
- 24.Simpson KL, Altman DW, Wang L, Kirifides ML, Lin RC, Waterhouse BD. Lateralization and functional organization of the locus coeruleus projection to the trigeminal somatosensory pathway in rat. J Comp Neurol. 1997;385:135–147. [PubMed] [Google Scholar]
- 25.Lee SB, Beak SK, Park SH, Waterhouse BD, Lee HS. Collateral projection from the locus coeruleus to whisker-related sensory and motor brain regions of the rat. J Comp Neurol. 2009;514:387–402. doi: 10.1002/cne.22012. [DOI] [PubMed] [Google Scholar]
- 26.Fox ME, Mikhailova MA, Bass CE, Takmakov P, Gainetdinov RR, Budygin EA, Wightman RM. Cross-hemispheric dopamine projections have functional significance. Proc Natl Acad Sci U S A. 2016;113:6985–6990. doi: 10.1073/pnas.1603629113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi RM, Palkovits M, Jacobowitz DM, Kopin IJ. Biochemical mapping of the noradrenergic projection from the locus coeruleus. A model for studies of brain neuronal pathways. Neurology. 1975;25:223–233. doi: 10.1212/wnl.25.3.223. [DOI] [PubMed] [Google Scholar]
- 28.Ader JP, Room P, Postema F, Korf J. Bilaterally diverging axon collaterals and contralateral projections from rat locus coeruleus neurons, demonstrated by fluorescent retrograde double labeling and norepinephrine metabolism. J Neural Transm. 1980;49:207–208. doi: 10.1007/BF01252126. [DOI] [PubMed] [Google Scholar]
- 29.Porrino LJ, Goldman-Rakic PS. Brainstem innervation of prefrontal and anterior cingulate cortex in the rhesus monkey revealed by retrograde transport of HRP. J Comp Neurol. 1982;205:63–76. doi: 10.1002/cne.902050107. [DOI] [PubMed] [Google Scholar]
- 30.Felten DL, Sladek JR., Jr Monoamine distribution in primate brain V. Monoaminergic nuclei: Anatomy, pathways and local organization. Brain Res Bull. 1983;10:171–284. doi: 10.1016/0361-9230(83)90045-x. [DOI] [PubMed] [Google Scholar]
- 31.Park J, Kile BM, Wightman RM. In vivo voltammetric monitoring of norepinephrine release in the rat ventral bed nucleus of the stria terminalis and anteroventral thalamic nucleus. Eur J Neurosci. 2009;30:2121–2133. doi: 10.1111/j.1460-9568.2009.07005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park J, Takmakov P, Wightman RM. In vivo comparison of norepinephrine and dopamine release in rat brain by simultaneous measurements with fast-scan cyclic voltammetry. J Neurochem. 2011;119:932–944. doi: 10.1111/j.1471-4159.2011.07494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fritschy JM, Grzanna R. Immunohistochemical analysis of the neurotoxic effects of DSP-4 identifies two populations of noradrenergic axon terminals. Neuroscience. 1989;30:181–197. doi: 10.1016/0306-4522(89)90364-3. [DOI] [PubMed] [Google Scholar]
- 34.Oke A, Solnick J, Adams RN. Catecholamine distribution patterns in rat thalamus. Brain Res. 1983;269:180–183. doi: 10.1016/0006-8993(83)90979-4. [DOI] [PubMed] [Google Scholar]
- 35.Bortel A. Nature of DSP-4-Induced Neurotoxicity. In: Kostrzewa MR, editor. Handbook of Neurotoxicity. Springer; New York: 2014. pp. 219–236. [Google Scholar]
- 36.Szot P, Miguelez C, White SS, Franklin A, Sikkema C, Wilkinson CW, Ugedo L, Raskind MA. A Comprehensive Analysis of the Effect of DSP4 on the Locus Coeruleus Noradrenergic System in the Rat. Neuroscience. 2010;166:279–291. doi: 10.1016/j.neuroscience.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang S, Qi J, Li X, Wang HL, Britt JP, Hoffman AF, Bonci A, Lupica CR, Morales M. Dopaminergic and glutamatergic microdomains in a subset of rodent mesoaccumbens axons. Nat Neurosci. 2015;18:386–392. doi: 10.1038/nn.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones SM, Snell LD, Johnson KM. Phencyclidine selectively inhibits N-methyl-D-aspartate-induced hippocampal [3H]norepinephrine release. J Pharmacol Exp Ther. 1987;240:492–497. [PubMed] [Google Scholar]
- 39.Vezzani A, Wu HQ, Samanin R. [3H]-norepinephrine release from hippocampal slices is an in vitro biochemical tool for investigating the pharmacological properties of excitatory amino acid receptors. J Neurochem. 1987;49:1438–1442. doi: 10.1111/j.1471-4159.1987.tb01011.x. [DOI] [PubMed] [Google Scholar]
- 40.Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- 41.Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jarrard LE. On the use of ibotenic acid to lesion selectively different components of the hippocampal formation. J Neurosci Methods. 1989;29:251–259. doi: 10.1016/0165-0270(89)90149-0. [DOI] [PubMed] [Google Scholar]
- 43.Chang Z, Okamoto K, Bereiter DA. Differential ascending projections of temporomandibular joint-responsive brainstem neurons to periaqueductal gray and posterior thalamus of male and female rats. Neuroscience. 2012;203:230–243. doi: 10.1016/j.neuroscience.2011.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Geerling JC, Loewy AD. Aldosterone-sensitive neurons in the nucleus of the solitary tract: efferent projections. J Comp Neurol. 2006;497:223–250. doi: 10.1002/cne.20993. [DOI] [PubMed] [Google Scholar]
- 45.Reyes BAS, Van Bockstaele EJ. Divergent projections of catecholaminergic neurons in the nucleus of the solitary tract to limbic forebrain and medullary autonomic brain regions. Brain Res. 2006;1117:69–79. doi: 10.1016/j.brainres.2006.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ennis M, Aston-Jones G. Activation of locus coeruleus from nucleus paragigantocellularis: A new excitatory amino acid pathway in brain. J Neurosci. 1988;8:3644–3657. doi: 10.1523/JNEUROSCI.08-10-03644.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maldonado R. Participation of noradrenergic pathways in the expression of opiate withdrawal: biochemical and pharmacological evidence. Neurosci Biobehav Rev. 1997;21:91–104. doi: 10.1016/0149-7634(95)00061-5. [DOI] [PubMed] [Google Scholar]
- 48.Crow TJ, Spear PJ, Arbuthnott GW. Intracranial self-stimulation with electrodes in the region of the locus coeruleus. Brain Res. 1972;36:275–287. doi: 10.1016/0006-8993(72)90735-4. [DOI] [PubMed] [Google Scholar]
- 49.Ritter S, Stein L. Self-stimulation of noradrenergic cell group (A6) in locus coeruleus of rats. J Comp Physiol Psychol. 1973;85:443–452. doi: 10.1037/h0035289. [DOI] [PubMed] [Google Scholar]
- 50.Amaral DG, Routtenberg A. Locus coeruleus and intracranial self-stimulation: a cautionary note. Behav Biol. 1975;13:331–338. doi: 10.1016/s0091-6773(75)91374-7. [DOI] [PubMed] [Google Scholar]
- 51.Simon H, Le Moal M, Cardo B. Self-stimulation in the dorsal pontine tegmentum in the rat. Behav Biol. 1975;13:339–347. doi: 10.1016/s0091-6773(75)91388-7. [DOI] [PubMed] [Google Scholar]
- 52.Corbett D, Skelton RW, Wise RA. Dorsal noradrenergic bundle lesions fail to disrupt self-stimulation from the region of locus coeruleus. Brain Res. 1977;133:37–44. doi: 10.1016/0006-8993(77)90047-6. [DOI] [PubMed] [Google Scholar]
- 53.Clavier RM, Routtenberg A. Brain stem self-stimulation attenuated by lesions of medial forebrain bundle but not by lesions of locus coeruleus or the caudal ventral norepinephrine bundle. Brain Res. 1976;101:251–271. doi: 10.1016/0006-8993(76)90267-5. [DOI] [PubMed] [Google Scholar]
- 54.Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, Stuber GD, Tye KM, Janak PH, Deisseroth K. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zigmond MJ, Abercrombie ED, Berger TW, Grace AA, Stricker EM. Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci. 1990;13:290–296. doi: 10.1016/0166-2236(90)90112-n. [DOI] [PubMed] [Google Scholar]
- 56.Shnitko TA, Robinson DL. Anatomical and pharmacological characterization of catecholamine transients in the medial prefrontal cortex evoked by ventral tegmental area stimulation. Synapse. 2014;68:131–143. doi: 10.1002/syn.21723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bucher ES, Brooks K, Verber MD, Keithley RB, Owesson-White C, Carroll S, Takmakov P, McKinney CJ, Wightman RM. Flexible software platform for fast-scan cyclic voltammetry data acquisition and analysis. Anal Chem. 2013;85:10344–10353. doi: 10.1021/ac402263x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodeberg NT, Johnson JA, Cameron CM, Saddoris MP, Carelli RM, Wightman RM. Construction of Training Sets for Valid Calibration of in Vivo Cyclic Voltammetric Data by Principal Component Analysis. Anal Chem. 2015;87:11484–11491. doi: 10.1021/acs.analchem.5b03222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier Academic Press; Amsterdam, Boston: 2005. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.