Abstract
In seventeen patients with rapid cycling bipolar disorder, time-series analyses detected synchronies between mood cycles and three lunar cycles that modulate the amplitude of the moon’s semi-diurnal gravimetric tides: the 14.8-day spring-neap cycle, the 13.7-day declination cycle, and the 206-day cycle of perigee-syzygies (“supermoons”). The analyses also revealed shifts among 1:2, 1:3, 2:3 and other modes of coupling of mood cycles to the two bi-weekly lunar cycles. These shifts appear to be responses to the conflicting demands of the mood cycles’ being entrained simultaneously to two different bi-weekly lunar cycles with slightly different periods. Measurements of circadian rhythms in body temperature suggest a biological mechanism through which transits of one of the moon’s semi-diurnal gravimetric tides might have driven the patients’ bipolar cycles, by periodically entraining the circadian pacemaker to its 24.84-hour rhythm and altering the pacemaker’s phase-relationship to sleep in a manner that is known to cause switches from depression to mania.
INTRODUCTION
The belief that the full moon can excite and disturb human behavior is deeply rooted in culture, language and clinical lore, but it tends to be regarded as a myth by the scientific community because it is not supported by epidemiological research.1,2 The failure to detect such an effect in the modern era, in which light pollution has nearly extinguished lunar-phase variation in brightness of the night sky, does not necessarily mean that this effect was absent in previous centuries when nights were darker, however.3,4 In their natural habitats, other species of primates generally do become more active at night under a full moon, and there are numerous examples of lunar cycles in other types of animals.5,6
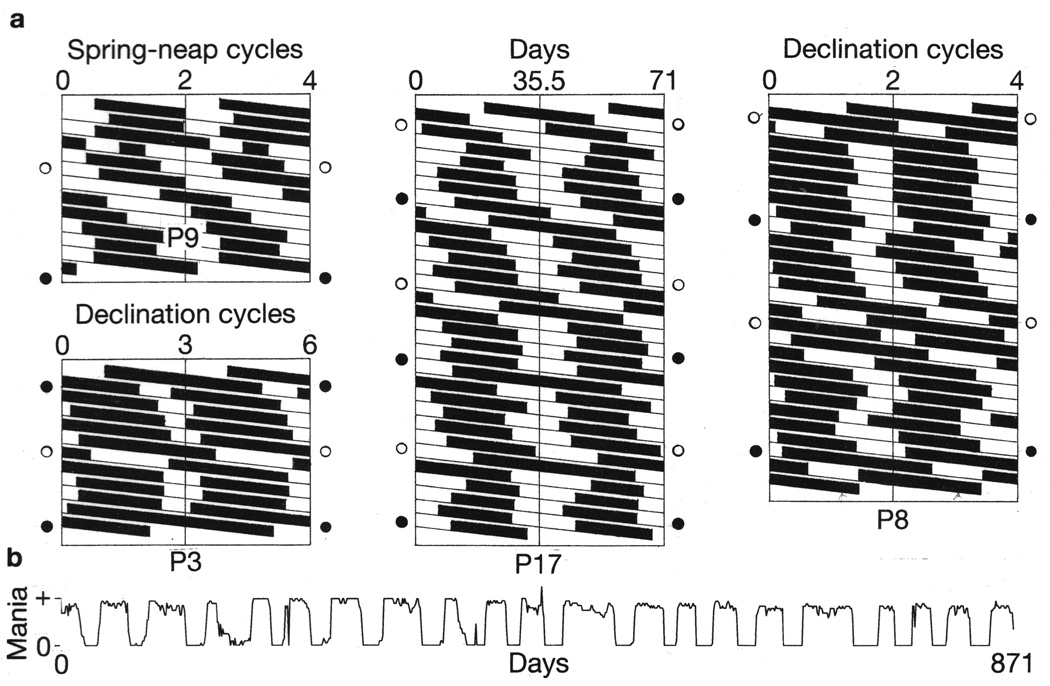
In the present study, the author has taken an approach that differs from that of epidemiological studies, by looking for evidence of lunar periodicities in extensive longitudinal records of course of illness in individual patients with rapid cycling bipolar disorder. This illness is characterized by a feature that helps to delineate its course over time—a dramatic and readily identifiable “switch process,” in which individuals shift abruptly from depression to mania, and vice versa, in the course of a single day (Figure 4b).7,8
The author undertook the study after examining the record of a patient (Patient 17) whose mood cycles exhibited a 35.5-d periodicity whose precision and stability over several years suggested that it might have arisen from a non-biological, clock-like source in the external environment (Figure 4). The fact that 35.5 days is the mean duration of double and triple multiples of two bi-weekly lunar tidal cycles, that 1/35.4 days is the mean frequency of every second and every third oscillation of one of those cycles, and that a recurring pattern in the mood cycles coincided with the 206-day lunar perigee-syzygy cycle suggested that the source of the 35.5-day periodicity might be the moon.
To investigate this possibility, the author conducted a retrospective study of sixteen additional rapid cycling bipolar patients. The study addressed the question of whether periodicities corresponding to bi-weekly tidal cycles and perigee-syzygy cycles were also present in these individuals’ courses of illness. It is worth noting that these types of lunar cycles do occur in other animals’ behavior, and that lunar gravimetric cycles might not be affected by the light pollution that has obscured the moon’s luminance cycle.6
Lunar tidal cycles
The spring-neap cycle exerts its maximum effect on the amplitude of the moon’s gravimetric tides every 14.8 days, when the sun, moon and earth are aligned at the full moon and at the new moon. The declination cycle exerts its maximum effect on the tides, in one of the two daily tides, every 13.7 days, when the declination of the moon’s orbit relative to the plane of the earth’s equator causes the moon to reach the northern or southern extreme of its orbit. This cycle is responsible for the diurnal inequality of the tides at many terrestrial locations.
The 206-day perigee-syzygy cycle exerts its maximum effect on the tides every 206 days, when the sun, moon and earth are aligned at the full moon or the new moon, and the moon most nearly approaches the perigee of its elliptical orbit—an event commonly referred to as a “supermoon.” There is an inequality in the height of the tides that occur with the full-moon and the new-moon phases of the spring-neap cycle. Every 206 days, this inequality shifts 360°, so that the higher tide coincides with its full-moon phase for seven months and then with its new-moon phase for seven months.
PATIENTS AND METHODS
Patients
Patients whose data are the focus of this report had forms of bipolar illness with the following characteristics: 1) a diagnosis of rapid-cycling bipolar disorder, according to criteria that prevailed at the time that they were studied;9–11 2) a circular course of illness in which sustained episodes of mania of several weeks’ duration regularly alternated with sustained episodes of depression of similar duration; 3) a prominent “switch process” in which patients transitioned abruptly from one mood state to the other over the course of a day or so;7,8 and, 4) a frequency of six or more complete (mania + depression) cycles, that is, twelve or more affective episodes per year.
The patient group consisted of six men (Patients 1, 4, 6 and 8–10) and eleven women (Patients 2, 3, 5, 7 and 11–17) aged 21–69. Three patients were post-menopausal (Patients 3, 11 and 17). Patients lived in ambient light in urban environments in their homes or in hospital rooms with windows. All patients had given written informed consent for their participation in studies that included longitudinal documentation of their course of illness, and that were approved by the Institutional Review Board of the Intramural Research Program of the National Institute of Mental Health, except Patient 8, who was seen in the author’s private practice and gave written informed consent for information concerning the course of his illness to be published, and Patients 1 and 10, whose mood cycles were documented by R. Gjessing in the 1920’s and 30’s in the pre-psychopharmacologcal era. The latter two patients were included in the analysis in order to explore the generalizability of methods and findings.9–12
Supplementary Table 1 provides further information about patient characteristics.
Assessments
Clinical course was documented with calendar records of dates of switches back and forth between depression and mania that had been maintained by patients themselves (Patients 3, 4, 6, 8 and 11), daily self-ratings of mood (Patients 7, 9, 13 14 and 17) and by specially trained research nurses’ behavioral daily ratings of depression and mania (Patients 1, 2, 5, 10, 12, 15 and 16).7,9,10,13 Research nurses also recorded the dates of Patients 12 and 16’s menses during the periods when their mood cycles were monitored.
Chi-square periodogram analyses were performed with each time series.14,15 Periods comprising whole numbers of days were sampled between 10 and 50 days (Patients 1–5, 7–10 and 12–17) or 20 and 100 days (Patients 6 and 11), and statistical significance for Qp values was set at .05 (one-tailed). The Qp value, which estimates the fraction of significant results that are null, is a multiple testing correction to p-values that is commonly used in genomics and proteomics. It provides more statistical power than a Bonferroni correction, but is still conservative. If the chi-square periodogram analysis looks for a specific periodicity, as opposed to any periodicity, corrections for multiple comparisons may not be necessary.16
Data Display
Raster plots of bipolar cycles were constructed by dividing longitudinal records of each time-series into segments equal to multiples of the bi-weekly lunar tidal cycles (N = 16) or 35.5 days (N = 3), the period that was prominent in the index patient’s mood cycles, and that is the mean of double and triple multiples of the two types of bi-weekly lunar tidal cycles. The segments were then displayed sequentially beneath one another, and the resulting array was double-plotted to the right, to reveal the inherent continuity of the data and to facilitate visual inspection of courses of the bipolar cycles across the plotting space.
Portions or all of twelve patients’ data were published previously without reference to lunar cycles.8–12,13,17–20 Supplementary Table S2 provides specific citations for each of these patients.
RESULTS
The seventeen patients were monitored for a combined total of 37.5 years, or 928 spring-neap cycles.
Onsets of the patients’ manic episodes were synchronous with every second (N = 8), third (N = 11), fourth (N = 2) and/or sixth (N = 1) bi-weekly lunar tidal cycle (Figures 1 and 2). In most patients, onsets of mania were predominately synchronous with multiples of one of the two types of bi-weekly tidal cycles, the declination cycle (N = 9) (Figure 1) or the spring-neap cycle (N = 8) (Figure 2). However, in seven patients (Patients 3, 4, 6, 8, 10, 15 and 16) onsets of mania were synchronous with each type of bi-weekly cycle at different times during the course of illness (Figures 1, 2 and Supplementary Figures S1, S2 and S4).
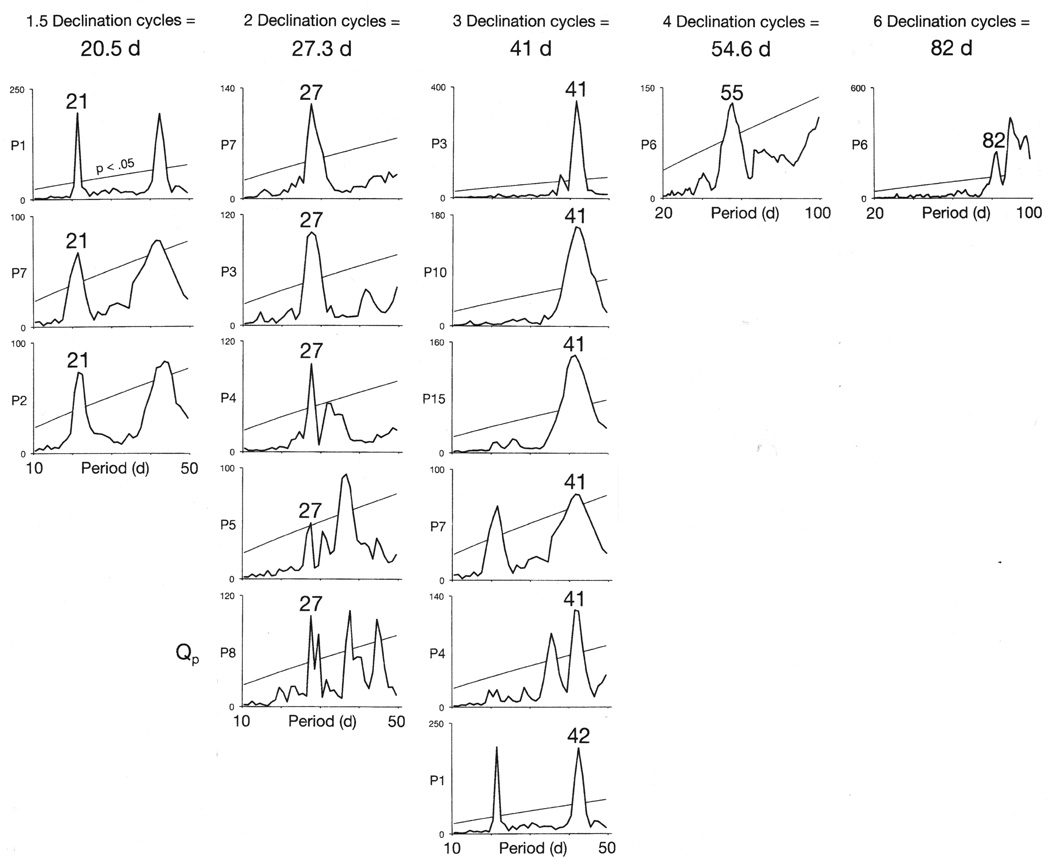
Figure 1.
Periodicities in ten rapid cycling bipolar patients’ mood cycles were synchronous with every second, third, fourth or sixth oscillation of the moon’s 13.7-d declination tidal cycle. Figure shows results of chi-square periodogram analyses of longitudinal observations of the patients’ courses of illness. Statistically significant periodicities that correspond to each of the multiples of the declination cycle are labeled. A number of patients exhibited more than one type of synchrony during their courses of illness.
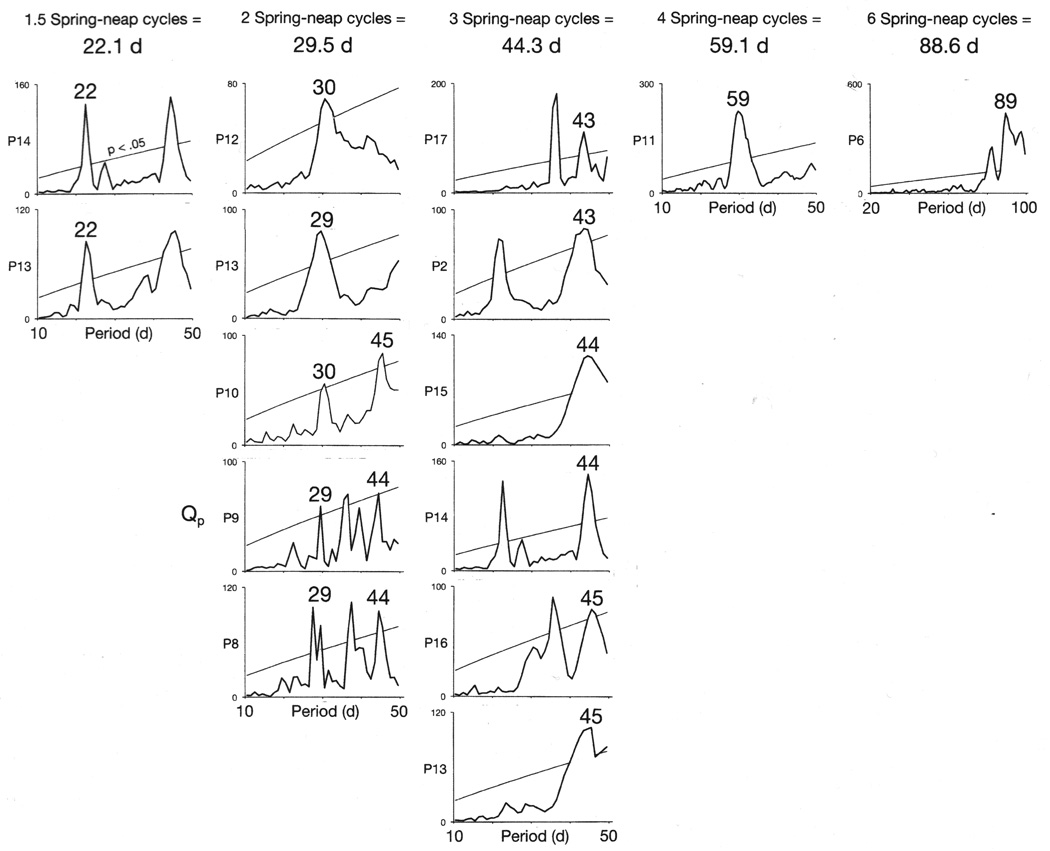
Figure 2.
Periodicities in twelve rapid cycling bipolar patients’ mood cycles were synchronous with every second, third, fourth or sixth oscillation of the moon’s 14.8-d spring-neap tidal cycle. Statistically significant periodicities in the chi-square periodograms that correspond to each of the multiples of the spring-neap cycle are labeled. A number of patients exhibited more than one type of synchrony during their courses of illness.
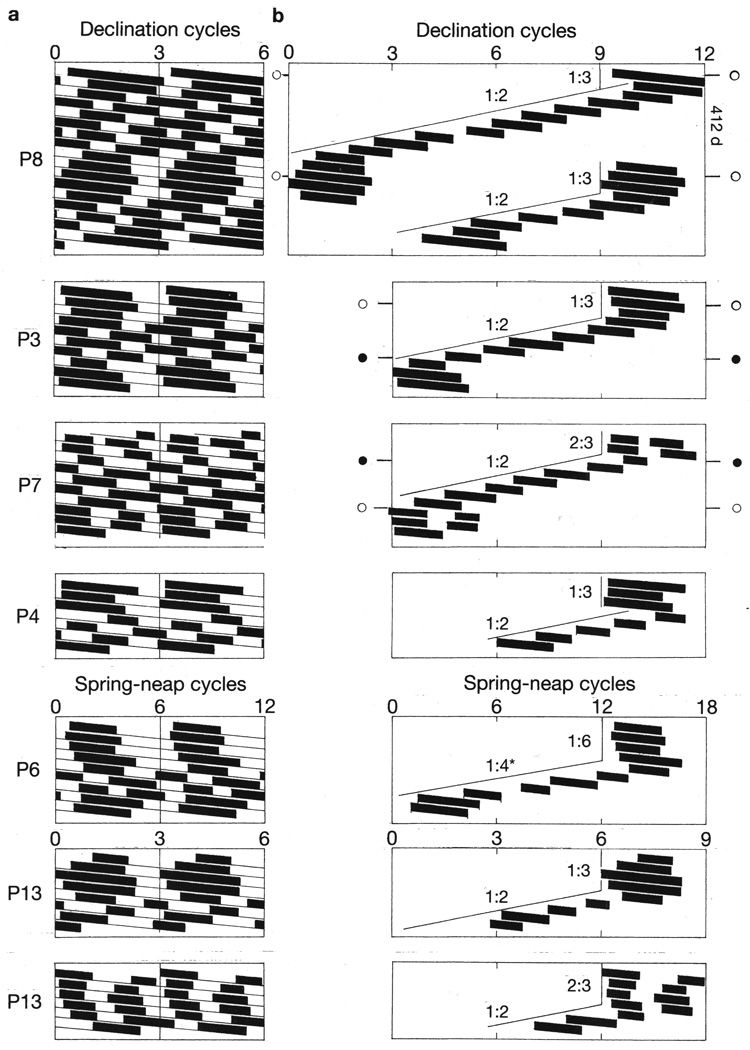
In thirteen patients (Patients 3, 4, 6–10 and 12–17), onsets of mania shifted their synchrony from a particular multiple of a bi-weekly lunar tidal cycle to a different multiple (Figures 1, 2, 3, 4, 5, 6, and 7 and Supplementary Figures S2 and S10). When onsets of mania shifted back to their initial mode of synchrony with a bi-weekly cycle, they re-established their original phase-relationship to that cycle (Figures 4, 7 and Supplementary Figures S2 and S4).
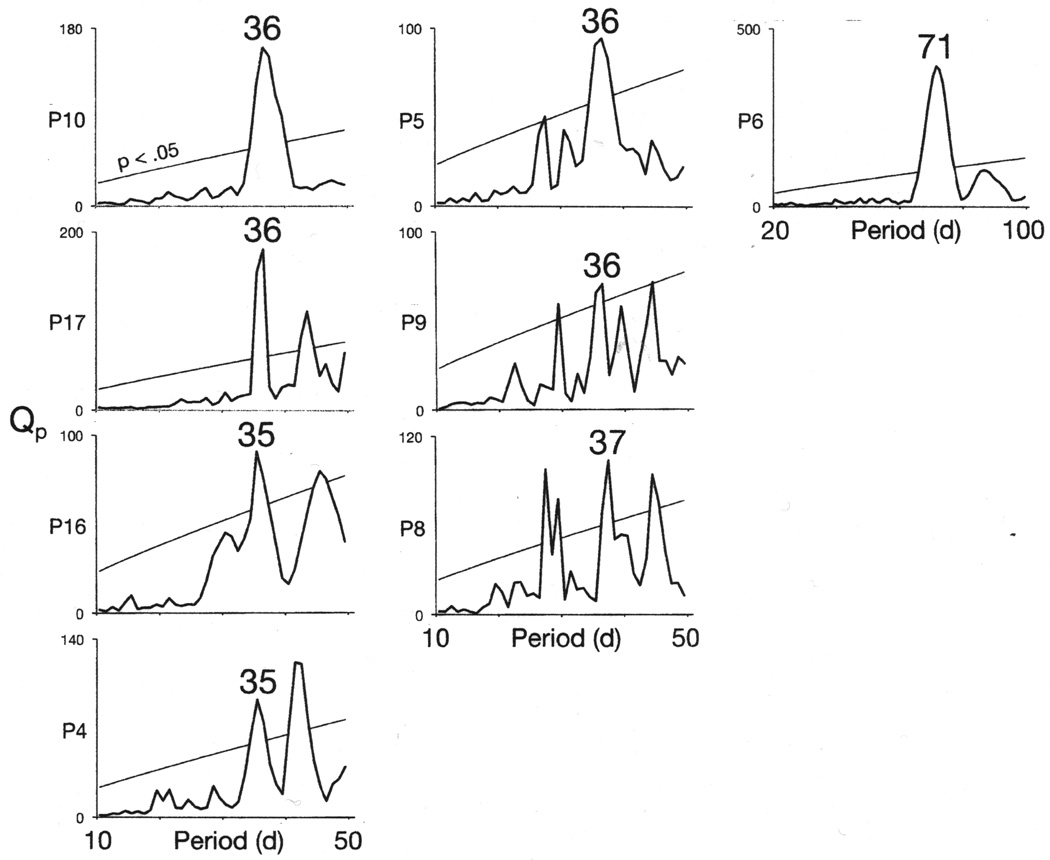
Figure 3.
Nearly half of seventeen rapid cycling bipolar patients’ mood cycles exhibited a circa 35.5-d periodicity. Statistically significant periodicities in the chi-sqaure periodograms that correspond to this interval are labeled.
Figure 4.
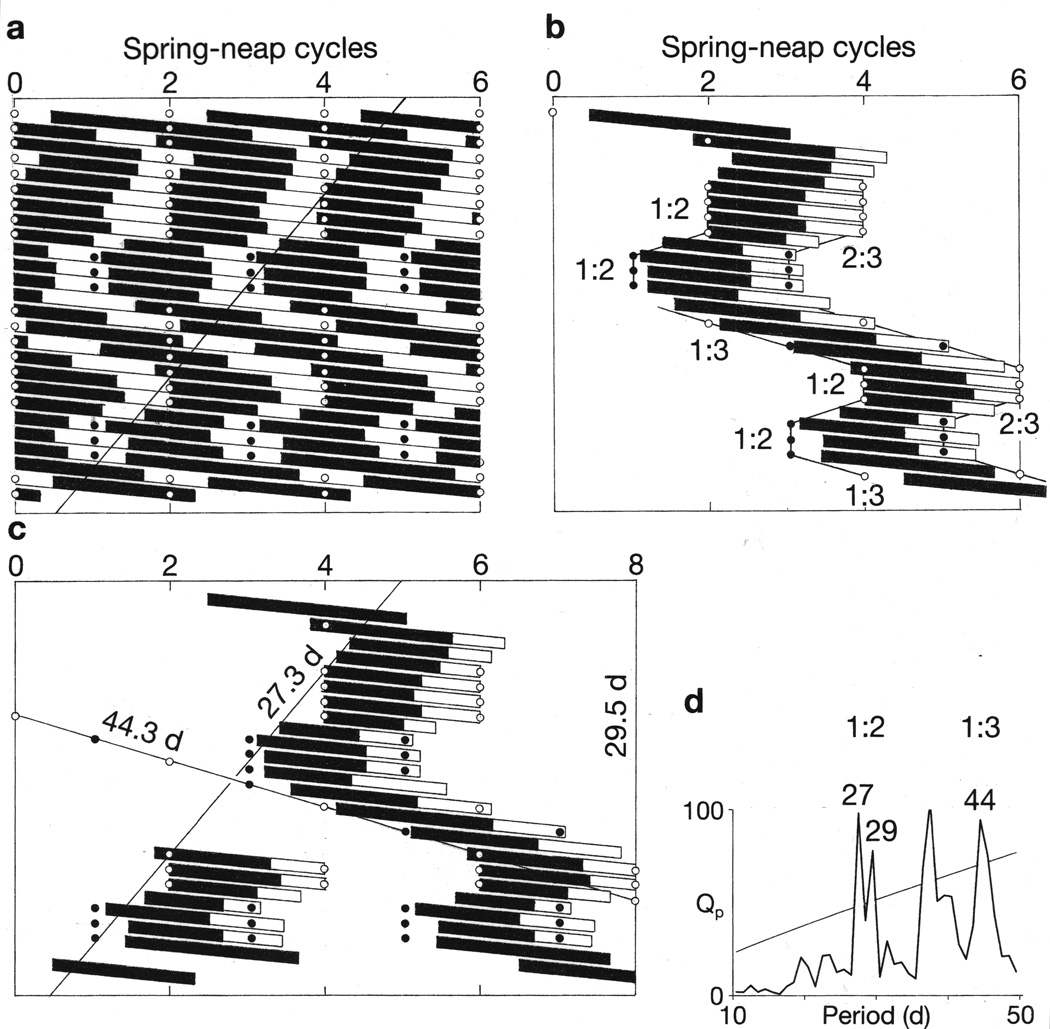
Bipolar mood cycles exhibited a synchrony with every second or every third bi-weekly lunar cycle that was periodically interrupted by 360° or 720° phase-displacements that coincided with lunar perigee-syzygies (supermoons). Raster double-plots (a) show manic episodes as black bars and depressive episodes as white bars. Open circles and closed circles adjacent to raster plots show times of 206-day recurrences of perigee-syzygies in their full-moon and new-moon phases, respectively. Patient identities are shown beneath raster plots. Vertical alignment of manic episodes indicates that they had become synchronous with every second or every third bi-weekly lunar cycle, as confirmed by chi-square periodogram analyses (Figures 1 and 2). Periodically, the course of manic episodes deviated diagonally to the right, leading to 360° or 720° phase-displacements of the bipolar cycles. These displacements regularly coincided with recurrences of perigee-syzygies. Each phase-displacement was followed by re-establishment of the bipolar cycles’ previous phase-relationship to the lunar cycles. For comparison with the raster plots, a linear plot of the time-series of Patient 17’s daily self-ratings of mania is shown (b). For further analyses of the relationships of Patients 8 and 17’s mood cycles to lunar cycles, see Figure 7 and Supplementary Figures S5 and S6.
Figure 5.
Eleven instances of shifts between 1:2 and 1:3 (or 2:3) modes of synchrony of mood cycles with bi-weekly lunar cycles. Double raster plots (a), as described for Figure 4. The courses of successive manic episodes across the plotting space (b) are shown together with numbers indicating their apparent mode of synchrony with bi-weekly lunar cycles. The numbers 1:2, 1:3 and 2:3 indicate, respectively, that every mood cycle had become synchronous with every second or third lunar cycle, or that every second mood cycle had become synchronous with every third lunar cycle. Uniquely, Patient 6’s mood cycles shifted from 1:6 resonance with spring-neap cycles to 1:4 resonance with declination cycles, as indicated by an asterisk (*).
Figure 6.
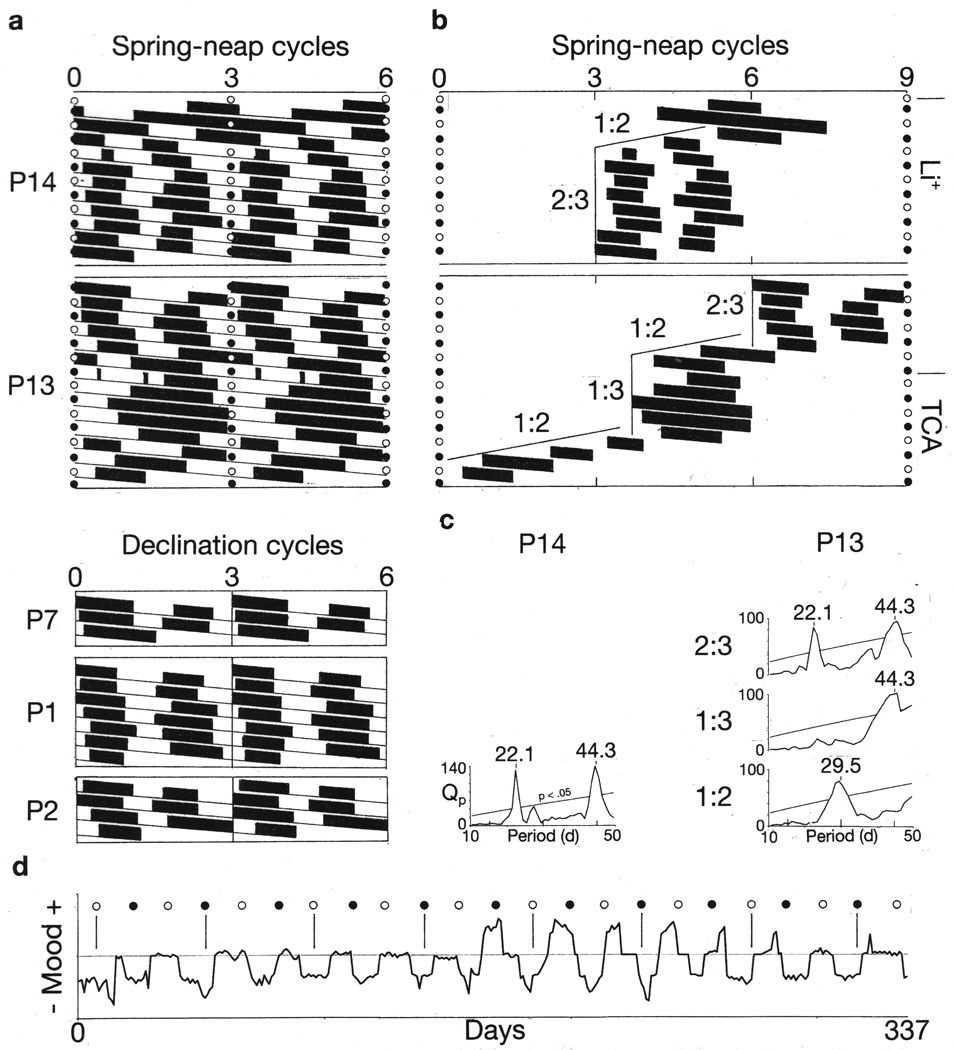
Five instances of synchrony of every second mood cycle with every third bi-weekly lunar cycle. Raster double plots of bipolar cycles (a) and courses of manic episodes across the plotting space (b), are shown as described for Figures 4 and 5. The occurrence of 2:3 synchrony of mood cycles with lunar cycles in Patients 13 and 14 is confirmed by chi-square periodogram analyses (c). As a reference, the duration of 1.5, 2 and 3 spring-neap cycles (22.1, 29.5 and 44.3 days, respectively) are shown above the periodograms. At different times, Patient 13’s mood cycles exhibited all three modes of synchrony with the lunar cycle (b, c). Patient 14’s periodogram and the first of Patient 12’s three periodograms show the first harmonic at circa 22.1 days, which is the fundamental period of the mood cycle, together with the second harmonic at circa 44.3 days, which is the period of recurrence of every third bi-weekly lunar cycle. Synchrony of every second mood cycle with every third lunar spring-neap cycle is evident in a linear plot of the time-series of Patient 14’s daily self-ratings of mood, in which times of full and new moons are indicated by open and closed circles, respectively (d).
In six patients (Patients 3, 4, 6–8 and 13), major shifts from shorter to longer cycle lengths appeared to coincide with 206-day recurrences of perigee-syzygies in the moon’s orbit (Figures 4 and 5).
In seven patients (Patients 3–6, 8–10, 16 and 17), mood cycles exhibited periodicities that were similar to the 35.5-day periodicity of the index patient’s mood cycles (Figure 3 and Supplementary Figures S1, S2 and S10).
In various patients, at various times, mood cycles exhibited five different modes of resonance with the bi-weekly lunar cycles:
A 1:2 mode in eight patients (Patients 3–5, 7–10 and 12), in which every mood cycle was synchronous with every second bi-weekly lunar cycle (Figures 1, 2, 4, 5, 7 and Supplementary Figures S10 and S11).
A 1:3 mode in seven patients (Patients 3, 4, 8–10, 15 and 16), in which every mood cycle was synchronous with every third bi-weekly lunar cycle (Figures 1, 2, 4–7 and Supplementary Figure S4).
A 2:3 mode in five patients (Patients 1, 2, 7, 13 and 14), in which every second mood cycle was synchronous with every third bi-weekly lunar cycle (Figures 1, 2 and 6).
A 1:4 mode in two patients (Patients 6 and 11) in which every mood cycle was synchronous with every fourth bi-weekly lunar cycle (Figures 1 and 2), and
A 1:6 mode in one patient (Patient 6), in which every mood cycle was synchronous with every sixth bi-weekly lunar cycle (Figures 1 and 2).
The findings in Patient 12 and 16’s menstrual cycles are analogous to those in their mood cycles. In both patients, menstrual cycles exhibited both 1:2 and 1:3 modes of synchrony with the spring-neap cycle, and onsets of menses tended to coincide with peaks of that cycle. Patient 16’s menses were synchronous with the full moon phase of every second spring-neap cycle in the first portion of her record and with the new moon phase in the second portion. Patient 12’s menses were synchronous with the new moon phase of every second spring-neap cycle (Supplementary Figure S12).
See figure legends for details.
DISCUSSION
The synchronies between lunar orbital cycles and bipolar mood cycles raise the possibility that the lunar cycles entrained or even generated the mood cycles. Although the present study did not address causality, several observations suggest that the hypothesis of a causal relationship between lunar cycles and mood cycles is plausible:
Diverse patients exhibited similar periodicities in their mood cycles, as if they arose from a common external source (Figures 1–3, 5 and 6).
These periodicities were synchronous with multiples of bi-weekly lunar tidal cycles.
Changes in the mood cycles’ frequency were not gradual, but rather were quantal or modular, as though the mood cycles were constrained to remain synchronous with integer multiples of the bi-weekly lunar cycles, with resonances of 1:2, 1:3 and 2:3 in most cases (Figures 5 and 6). Interestingly, such resonances are ubiquitous among the gravitational interactions between orbiting bodies in the solar system. For example, for every two orbits of Pluto, Neptune completes three—a 2:3 resonance. For every orbit of Jupiter’s moon Europa, its moon Io completes two—a 1:2 resonance. For every orbit of the trans-Neptune object, 2003 LG7, Neptune completes three—a 1:3 resonance.
After such frequency changes, when onsets of mania shifted back to their initial mode of synchrony with lunar tidal cycles, they re-established the same phase-relationships to those cycles that they had previously exhibited, as would occur if they were entrained to, or were generated by those cycles (Figure 4 and Supplementary Figure S4).
Major shifts in frequency from shorter mood cycles to longer mood cycles repeatedly coincided with 206-day recurrences of perigee-syzygies in six cases (Figures 4, 5 and Supplementary Figure S4).
With regard to causality, the case of index Patient 17 seems particularly compelling. Frequency shifts from shorter to longer mood cycles consistently coincided with 206-day recurrences of perigee-syzygies through five iterations (Figure 4). After such shifts, a stereotypic sequence of alternating advances and delays in the onsets of mania recurred repeatedly (Supplementary Figures S5a and S6). Furthermore, in each iteration, the sequences of alternating advances and delays could be mapped onto alternating recurrences of every second and every third spring-neap cycle and onto combinations of multiples of declination cycles and spring-neap cycles (Supplementary Figures S5b and S6). Finally, transits of full-moon and new-moon peaks of every second spring-neap cycle exhibited temporal relationships with onsets of mania in one sequence of mood cycles that were repeated exactly in the sequence of mood cycles that followed, one-half lunar year later (Supplementary Figure S5c). The co-occurrence of so many coincidences spanning so many iterations seems highly improbable.
Patient 8’s data suggest a causal explanation for the prevalence in the patient-group of shifts among 1:2, 1:3, 2:3 and other modes of resonance of mood cycles with lunar cycles. In Patient 8, the shifts appear to have facilitated the mood cycles’ being simultaneously entrained to both the spring-neap cycle and the declination cycle, in spite of the two lunar cycles’ having slightly different periods (Figure 7 and Supplementary Figure S6). Simultaneous entrainment to both the spring-neap cycle and the declincation cycle is not surprising, because the two cycles are inextricably linked. Both emanate from a single body’s orbit around the earth, and their oscillations are expressed additively in a single gravimetric variable.
Figure 7.
Bipolar mood cycles entrained simultaneously to both the declination cycle and the spring-neap cycle. Raster triple-plot of Patient 8’s mood cycles constructed with plotting intervals equal to two 14.8-d spring-neap cycles (a). Open and closed circles indicate selected times of occurrence of full-moon and new-moon peaks of the 14.8-day spring-neap cycle. Diagonal line indicates the transits across the plotting space of 27.3-day recurrences of every second peak of the 13.7-day declination cycle. Sequences in which mood cycles are vertically aligned indicate that they had become synchronous with every second spring-neap cycle. A sequence of successive individual manic-depressive cycles (b) extracted from the raster-plot (a) shows the path of their course across the plotting space. As they progress, the mood cycles shift among different modes of coupling with the spring-neap cycle. The sequence 1:2-2:3-1:2-1:3, which occurs over the course of one lunar year, is repeated exactly over the course of the subsequent lunar year. With these shifts, the mood cycles maintain relatively consistent phase-relationships with each of the bi-weekly lunar cycles, as shown by lines and circles (c). The 27.3-day line indicates times of recurrence of peaks of every second declination cycle. The 44.3-day line indicates times of recurrence of peaks of every third spring-neap cycle. The 29.5-day sequences of circles indicate times of recurrence of peaks of every second spring-neap cycle. The presence of all three periodicities in the course of the mood cycles is confirmed by chi-square periodogram analysis (d).
If lunar orbital cycles controlled the mood cycles in these patients, how might they have done so? The fact that the period of the declination cycle differs slightly from the period of the spring-neap cycle, whose every second peak coincides with the full moon, would seem to indicate that the declination cycle acts through a medium other than moonlight, such as, for example, gravitational forces, or more likely, perhaps, gravitational forces’ effects on other media, such as the earth’s magnetic field or its atmosphere.21–24
Longitudinal recordings of body temperature in Patients 5, 9, 12, and 17 and in other patients suggest a possible biological mechanism through which lunar gravimetric cycles might control mood cycles (Supplementary Figures S13–15).18 The recordings show that that the timing of circadian rhythms in temperature consistently shifted progressively later during periods that led up to switches from depression to mania, and that these changes coincided with every second or every third transit of a lunar semi-diurnal tide (Supplementary Figure S13). These observations raise the possibility that transits of the tide intermittently entrained a component of the human circadian (or circalunar) timing system to its 24.84-hour rhythm during phases of the bi-weekly cycles when the tide’s amplitude was highest and its strength as a forcing cycle would be greatest. This type of shift in the timing of circadian rhythms relative to the timing of sleep has been shown to cause switches from depression to mania and might therefore be a mechanism through which a semi-diurnal tide could drive the mood cycles (Supplementary Figure S16).13,25–29
The complexity and variety of the associations reported here between mood cycles and lunar orbital cycles might be non-physiological artifacts of modern lighting technology, which has greatly diminished the impact of the moon’s luminance cycle.30 In a natural lighting environment, the 29.5-day cycle of waxing and waning moonlight might once have reinforced entrainment of bio-behavioral cycles to 29.5-day recurrences of every second spring-neap gravimetric cycle and suppressed their entrainment to non-synchronous 27.3-day recurrences of every second declination gravimetric cycle, as well as to every third, fourth or sixth oscillation of either the spring-neap or the declination gravimetric cycles. The brightness of the full moon might also have reinforced the mood cycles’ entrainment to the full-moon phase of every second spring-neap gravimetric cycle and suppressed their entrainment to its new-moon phase. In these circumstances, the bio-behavioral cycles might once have exhibited the simple, one-to-one relationship with recurrences of the full-moon that has been sought, but not found, in modern epidemiological studies.3
Implicit in the foregoing discussion is a hypothesis that the putative biological system that responds to lunar cycles has two separate, but mutually reinforcing entrainment mechanisms—one that responds to the moon’s luminance cycles and one that responds to the moon’s gravimetric tidal cycles. If humans possess such a system, its function remains to be elucidated. In other animals, such systems most often serve to facilitate reproduction and avoidance of predation.6 Whatever its function in humans, light-pollution has made the operation of this system dysfunctional, in the scenario proposed above.
In the eight menstruating women, it is unclear whether lunar influences, if present, act directly on the mood cycles or—as Patient 12’s data might suggest—indirectly through the menstrual cycle (Supplementary Figure S11). The occurrence of hypersexuality in mania and loss of libido in depression does indicate that mood cycles and cycles in the reproductive neuroendocrine system are linked.
Antidepressant medications sometimes induce, or accelerate the frequency of, rapid mood cycling.12 These types of responses seem to have occurred in nine patients (Patients 3, 4, 6, 8, 9, 11, 14, 15 and 17), inasmuch as withdrawal of these agents was associated with slowing of cycling or onset of unremitting depression (see, for example, Supplemental Figure S7).10,13,19 If the relationship of mood cycles to lunar cycles is causal, this observation raises the possibility that antidepressant medications somehow sensitized the individuals to the influence of lunar cycles. This effect might result from the demonstrated capacity of some antidepressants to slow the intrinsic rhythm of the circadian pacemaker, thereby making it susceptible to capture by the slower-than-24-hour rhythm of the lunar tidal day.31,32
If bi-weekly lunar cycles drive rapid mood cycles by entraining the circadian pacemaker to the 24.8-hour lunar tidal day, how might one interfere with their influence? One approach would be to refrain from prescribing antidepressant medications while continuing maintenance treatment with lithium carbonate.12 Another approach would be to strengthen the pacemaker’s entrainment to the 24.0-hour solar day. This could be accomplished by exposing patients to longer periods of darkness at night, which have been shown to increase the amplitude of the pacemaker’s phase-responses to light and strengthen its coupling to the light-dark cycle.33 Consistent with this prediction, Patient 9 achieved a complete remission when he stayed in total darkness from 6 PM to 8 AM every night.10 Recently reported modifications of this procedure might make it more practical and convenient.34
In summary, rapid bipolar mood cycles exhibited synchronies with three different lunar cycles that modulate the amplitude of the moon’s semi-diurnal tides. Future research would be necessary to determine whether the findings can be replicated, and whether they can be extended more broadly to other biological phenomena. In this regard, recent reports of lunar month-variation of total sleep time in healthy individuals seem consistent with the finding of lunar month variation in onsets of mania, which are associated with marked changes in total sleep time (Supplementary Figure S16).35–41
The force that emanates from the moon whose variation is subject to all three types of lunar cycles is gravity. If the associations between lunar cycles and mood cycles are causal in nature, they would indicate that: (1) cycles in the Moon’s gravimetric tides govern the course of a type of bipolar illness; (2) humans possess a biological system that can detect and respond to lunar gravimetric cycles directly, or indirectly through an intervening variable; and (3) operations of this system are part of the mechanism that underlies the illness. The findings would also raise the possibility that modern light pollution has substantially altered the operations of this system. More broadly, the findings would open a new perspective on humans’ place in their cosmic neighborhood.
Supplementary Material
Acknowledgments
The author thanks the patients for their contribution of time and effort, Frederick K. Goodwin for collaboration and institutional support, Ellen Leibenluft for collaboration, data acquisition and administrative and clinical support, and Peter Barlow and Phillip Gold for suggestions and advice. The research was supported by the Intramural Research Program of the National Institute of Mental Health, Bethesda, Maryland, USA.
The views expressed in this report do not necessarily reflect the views of the NIMH, NIH or the US Federal Government.
Footnotes
CONFLICT OF INTEREST
The author has no competing financial interests in relation to the work described.
Supplementary information accompanies the paper on the Molecular Psychiatry website (http://www.nature.com/mp).
REFERENCES
- 1.Owens M, McGowan IW. Madness and the moon: The lunar cycle and psychopathology. German J Psychiatry. 2006;9:123–127. [Google Scholar]
- 2.Foster RG, Roenneberg T. Human responses to the geophysical daily, annual and lunar cycles. Curr Biol. 2008;18:R784–R794. doi: 10.1016/j.cub.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Raison CL, Klein HM, Steckler M. The moon and madness reconsidered. J Affect Disord. 1999;53:99–106. doi: 10.1016/s0165-0327(99)00016-6. [DOI] [PubMed] [Google Scholar]
- 4.Kronfeld-Schor N, Dominoni D, de la Iglesia H, Levy O, Herzog ED, Dayan T, Helfrich-Forster C. Chronobiology by moonlight. Proc R Soc B. 2013;280:20123088. doi: 10.1098/rspb.2012.3088. http://dx.doi.org/10.1098/rspb.2012.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nash LT. Moonlight and behavior in nocturnal and cathemeral primates, especially Lepilemur leucopus: Illuminating possible anti-predator efforts. In: Gursky SL, Nekaris KAI, editors. Developments in Primatology: Progress and Prospects. Berlin: Springer; 2007. pp. 173–187. [Google Scholar]
- 6.Naylor E. Moonstruck: How Lunar Cycles Affect Life. Oxford University Press; 2015. [Google Scholar]
- 7.Bunney WE, Jr, Murphy DL, Goodwin FK, Borge GF. The “switch process” in manic-depressive illness. Arch Gen Psychiatry. 1972;27:295–302. doi: 10.1001/archpsyc.1972.01750270005001. [DOI] [PubMed] [Google Scholar]
- 8.Wehr TA. Phase and biorhythm studies of affective illness. Ann Int Med. 1977;87:321–324. [Google Scholar]
- 9.Wehr TA, Wirz-Justice A, Goodwin FK, Breitmeier J, Craig C. 48-hour sleep-wake cycles in manic-depressive illness: naturalistic observations and sleep deprivation experiments. Arch Gen Psychiatry. 1982;39:559–565. doi: 10.1001/archpsyc.1982.04290050037008. [DOI] [PubMed] [Google Scholar]
- 10.Wehr TA, Turner EH, Shimada JM, Lowe CH, Barker C, Leibenluft E. Treatment of a rapidly cycling bipolar patient by using extended bed rest and darkness to stabilize the timing and duration of sleep. Biol Psychiatry. 1998;43:822–828. doi: 10.1016/s0006-3223(97)00542-8. [DOI] [PubMed] [Google Scholar]
- 11.Gjessing R. Somatology of Periodic Catatonia. Oxford: Pergamon Press; 1976. pp. 12–42. 1976. [Google Scholar]
- 12.Wehr TA, Goodwin FK. Rapid cycling in manic-depressives induced by tricyclic antidepressants. Arch Gen Psychiatry. 1979;36:555–559. doi: 10.1001/archpsyc.1979.01780050065007. [DOI] [PubMed] [Google Scholar]
- 13.Wehr TA, Wirz-Justice A, Duncan WC, Jr, Gillin JC, Goodwin FK. Phase-advance of the circadian sleep-wake cycle as an antidepressant. Science. 1979;206:710–713. doi: 10.1126/science.227056. [DOI] [PubMed] [Google Scholar]
- 14.Sokolove PG, Bushell WW. Chi square periodogram – its utility for analysis of circadian rhythms. J Theor Biol. 1978;72:131–160. doi: 10.1016/0022-5193(78)90022-x. [DOI] [PubMed] [Google Scholar]
- 15.Schmid B, Helfrich-Förster C, Yoshii T. A new ImageJ plug-in"ActogramJ” for chronobiological analyses. J Biol Rhythms. 2011;26:464–467. doi: 10.1177/0748730411414264. [DOI] [PubMed] [Google Scholar]
- 16.Zielinski T, Moore AM, Troup E, Halliday KJ, Millar AJ. Strengths and limitations of period estimation methods for circadian data. PLoS ONE. 2014;9(5):e96462. doi: 10.1371/journal.pone.0096462. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wehr TA, Goodwin FK. Tricyclics modulate frequency of mood cycles. Chronobiologia. 1979;6:377–385. [PubMed] [Google Scholar]
- 18.Wehr TA, Goodwin FK. Biological rhythms and manic-depressive illness. In: Wehr TA, Goodwin FK, editors. Biological Rhythms and Psychiatry. Pacific Grove, California: Boxwood Press; 1983. pp. 29–184. [Google Scholar]
- 19.Wehr TA. Seasonal affective disorders: A historical overview. In: Rosenthal NE, Blehar MC, editors. Seasonal Affective Disorders and Phototherapy. New York: The Guilford Press; 1989. pp. 11–32. [Google Scholar]
- 20.Wehr TA, Sack DA, Rosenthal NE, Cowdry RW. Rapid cycling affective disorder: Contributing factors and treatment responses in 51 patients. Am J Psychiatry. 1988;145:179–184. doi: 10.1176/ajp.145.2.179. [DOI] [PubMed] [Google Scholar]
- 21.Pertsev N, Dalin P. Lunar semimonthly signal in cloudiness: Lunar-phase or lunar-declination effect? J Atmospheric and Solar-Terrestrial Physics. 2010;72:713–717. [Google Scholar]
- 22.Grunskaya LV, Morozov VN, Zakirov AA, Rubai DV, Rubai RV. Solar and lunar tides in the geomagnetic field. Russian Physics Journal. 2011;54:133–146. [Google Scholar]
- 23.Gerasimov AV, Kostyuchenko VP, Solovieva AS, Olovnikov AM. Pineal gland as an endocrine gravitational lunasensor: Manifestation of moon-phase dependent morphological changes in mice. Biochemistry (Moscow) 2014;79:1069–1074. doi: 10.1134/S0006297914100083. [DOI] [PubMed] [Google Scholar]
- 24.Fisahn J, Klingele E, Barlow P. Lunar gravity affects leaf movement of Arabidopsis thaliana in the International Space Station. Planta. 2015;241:1509–1518. doi: 10.1007/s00425-015-2280-x. [DOI] [PubMed] [Google Scholar]
- 25.Schilgen B, Tölle R. Partial sleep deprivation as therapy for depression. Arch Gen Psychiatry. 1980;37:267–271. doi: 10.1001/archpsyc.1980.01780160037003. [DOI] [PubMed] [Google Scholar]
- 26.Berger M, Vollmann J, Hohagen F, Konig A, Lohner H, Voderholzer U, Riemann D. Sleep deprivation combined with consecutive sleep phase advance as a fast-acting therapy in depression. Amer J Psychiatry. 1997;154:870–872. doi: 10.1176/ajp.154.6.870. [DOI] [PubMed] [Google Scholar]
- 27.Benedetti F, Barbini B, Campori E, Fulgosi MC, Pontiggia A, Colombo C. Sleep phase advance and lithium to sustain the antidepressant effect of total sleep deprivation in bipolar depression. J Psychiat Res. 2001;35:323–329. doi: 10.1016/s0022-3956(01)00034-6. [DOI] [PubMed] [Google Scholar]
- 28.Wu JC, Bunney WE., Jr Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry. 2009;66:298–301. doi: 10.1016/j.biopsych.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 29.Echizenya M, Suda H, Takeshima M, Inomata Y, Shimizu T. Total sleep deprivation followed by sleep phase advance and bright light therapy in drug-resistant mood disorders. J Affect Disord. 2012;144:28–33. doi: 10.1016/j.jad.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 30.Gaston KJ, Duffy JP, Gaston S, Bennie J, Davies TW. Human alteration of natural light cycles: causes and consequences. Oecologia. 2014;176:917–931. doi: 10.1007/s00442-014-3088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wirz-Justice A, Campbell IC. Antidepressant drugs can slow or dissociate circadian rhythms. Experientia. 1982;38:1301–1309. doi: 10.1007/BF01954918. [DOI] [PubMed] [Google Scholar]
- 32.Duncan WC, Tamarkin L, Sokolove P, Wehr TA. Chronic clorgyline treatment of the Syrian Hamster: An analysis of circadian effects. J Biol Rhythms. 1988;3:305–322. doi: 10.1177/074873048800300401. [DOI] [PubMed] [Google Scholar]
- 33.Burgess JH, Eastman CI. Short nights reduce light-induced circadian phase delays in humans. Sleep. 2006;29:25–30. doi: 10.1093/sleep/29.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henriksen TEG, Skrede S, Fasmer OB, H Schoeyen H, Leskauskaite I, Bjorke-Berkeheussen J, et al. Blue-blocking glasses as additive treatment for mania: a single-blinded placebo-controlled trial. Bipolar Disord. 2016;18:221–232. doi: 10.1111/bdi.12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Röösli M, Jüni P, Braun-Fahrländer C, Brinkhof M, Low N, Egger M. Sleepless night, the moon is bright: longitudinal study of lunar phase and sleep. J Sleep Res. 2006;15:149–153. doi: 10.1111/j.1365-2869.2006.00520.x. [DOI] [PubMed] [Google Scholar]
- 36.Cajochen C, Altanay-Ekici S, Münch M, Frey S, Knoblauch V, Wirz-Justice A. Evidence that the lunar cycle influences human sleep. Curr Biol. 2013;23:1485–1488. doi: 10.1016/j.cub.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 37.Smith M, Croy I, Waye KP. Human sleep and cortical reactivity are influenced by lunar phase. Curr Biol. 2014;24:R551–R552. doi: 10.1016/j.cub.2014.05.018. [DOI] [PubMed] [Google Scholar]
- 38.Turányi CZ, Rónai KZ, Zoller R, Véber O, Czira ME, Újszászi Á, et al. Association between lunar phase and sleep characteristics. Sleep Med. 2014;15:1411–1416. doi: 10.1016/j.sleep.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 39.della Monica C, Atzori G, Dijk D-J. Effects of lunar phase on sleep in men and women in Surrey. J Sleep Res. 2015;24:687–694. doi: 10.1111/jsr.12312. [DOI] [PubMed] [Google Scholar]
- 40.Bauer M, Glenn T, Whybrow PC, Grof P, Rasgon N, Alda M, Marsh W, et al. Changes in self-reported sleep duration predict mood changes in bipolar disorder. Psychol Med. 2008;38:1069–1071. doi: 10.1017/S0033291708003280. [DOI] [PubMed] [Google Scholar]
- 41.Leibenluft E, Albert PS, Rosenthal NE, Wehr TA. Longitudinal relationships between sleep and mood in rapid cycling bipolar patients. Psychiatry Res. 1996;63:161–168. doi: 10.1016/0165-1781(96)02854-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.