Abstract
Numerous genetic and functional studies implicate variants of Neuregulin-1 and its neuronal receptor ErbB4 in schizophrenia and many of its endophenotypes. While the neurophysiological and behavioral phenotypes of NRG1 mutant mice have been investigated extensively, practically nothing is known about the function of NRG2, the closest NRG1 homologue. We found that NRG2 expression in the adult rodent brain does not overlap with NRG1 and is more extensive than originally reported, including expression in the striatum and medial prefrontal cortex (mPFC), and therefore generated NRG2 knockout mice (KO) to study its function. NRG2 KOs have higher extracellular dopamine levels in the dorsal striatum but lower levels in the mPFC; a pattern with similarities to dopamine dysbalance in schizophrenia. Like ErbB4 KO mice, NRG2 KOs performed abnormally in a battery of behavioral tasks relevant to psychiatric disorders. NRG2 KOs exhibit hyperactivity in a novelty-induced open field, deficits in prepulse inhibition, hypersensitivity to amphetamine, antisocial behaviors, reduced anxiety-like behavior on the elevated plus-maze and deficits on the T-maze alteration reward test - a task dependent on hippocampal and mPFC function. Acute administration of clozapine rapidly increased extracellular dopamine levels in the mPFC and improved alternation T-maze performance. Similar to mice treated chronically with NMDAR antagonists, we demonstrate that NMDA receptor synaptic currents in NRG2 KOs are augmented at hippocampal glutamatergic synapses and are more sensitive to ifenprodil, indicating an increased contribution of GluN2B-containing NMDA receptors. Our findings reveal a novel role for NRG2 in the modulation of behaviors with relevance to psychiatric disorders.
Keywords: ErbB4, clozapine, ADHD, animal models, cognitive, GluN2B, NR2B, ketamine
INTRODUCTION
The Neuregulins (NRG 1-4) and their cognate neuronal receptor ErbB4 are among the numerous genetic variants associated with a risk for schizophrenia1–4 and many of its endophenotypes5. Moreover, studies using human induced pluripotent stem cells (iPSCs) from affected subjects6, as well as work in mice with targeted mutations in NRG17–9 and ErbB410, provide strong experimental evidence for “biological plausibility” of this pathway in psychiatric disorders11. Most psychiatric diseases, such attention-deficit hyperactivity disorder (ADHD) and schizophrenia, manifest during development and exhibit a high degree of heritability. While current pharmacological interventions for ADHD and schizophrenia target the dopamine system, multiple neurotransmitter systems including dopamine, glutamate, GABA and acetylcholine have been implicated in their pathophysiology.
The markedly different regional, temporal and cellular expression patterns of NRG isoforms in the brain suggest distinct and non-overlapping functions. NRG1 transcripts are abundantly expressed in the embryonic and fetal brain but become restricted during postnatal development12, whereas NRG2 transcript levels dramatically increase shortly after birth suggesting important functions in the postnatal and adult brain13. In the hippocampus and neocortex, ErbB4 is confined to GABAergic interneurons14, and in the ventral tegmental area (VTA) and substantia nigra compacta (SNc) ErbB4 is expressed in dopaminergic neurons15, 16 where it regulates activity from glutamatergic inputs17. NRG1 treatment acutely and dramatically increases extracellular dopamine levels in behaving rats18, and regulates glutamatergic synaptic plasticity in acute hippocampal slices 19 and the power of gamma oscillations20, 21- a type of local neuronal network activity associated with cognitive deficits in persons with psychiatric disorders22. Most, if not all, NRG effects are abolished in ErbB4 knockout (KO) mice10. Consistent with these findings, ErbB4 KOs exhibit altered excitation/inhibition (E/I) balance and numerous deficits in behavioral tasks modeling schizophrenia and its endophenotypes, including prepulse inhibition (somatosensory gating), rewarded T-maze alternation (requires working memory) and social interactions7–10.
Despite its prominent expression in the postnatal and adult brain, little is known about NRG2 function and its potential involvement in neurodegenerative and neuropsychiatric disorders. Children with de novo overlapping 5q31 microdeletions, encompassing three genes that include NRG2, share clinical features that include white matter abnormalities and severe developmental and intellectual delays23, 24. While the region encompassing the NRG2 locus (5q23-33) has been associated with risk for schizophrenia25–28 and genetically linked with ADHD in genome-wide scans of multigenerational extended families29, at the present time, genetic studies implicating this locus in psychiatric disorders is less compelling than for NRG1 signaling.
Functionally, like NRG1, a peptide harboring the NRG2 EGF-like domain acutely promotes the internalization of GABAARs30 and NMDARs31 in hippocampal and cortical ErbB4-expressing GABAergic interneurons, respectively. NMDAR- and NRG2/ErbB4-dependent signaling pathways form a negative feedback loop in GABAergic interneurons in which NMDAR activity is required for NRG2 ectodomain shedding and subsequent activation of ErbB4 which in turn down-regulates GluN2B-containing NMDARs31. The fact that NRG2 modulates glutamatergic transmission in GABAergic neurons and selectively affects GluN2B-containing NMDARs, a major proposed target of ketamine and the co-agonist glycine that lies at the heart of the hypoglutamatergic theory of schizophrenia32–34, raises the intriguing possibility that NRG2 could regulate brain development and neuronal circuits implicated in psychiatric disorders.
Here we have generated KO mice to investigate a role for NRG2 in several psychiatrically relevant behavioral paradigms, dopamine balance and glutamatergic transmission. This is the first study to analyze NRG2 functions in vivo, and to identify its importance for regulating dopaminergic and glutamatergic transmission and numerous behaviors associated with psychiatric diseases. As discussed, these findings warrant a renewed effort to investigate the possible association of NRG2 variants with psychiatric disorders.
MATERIALS AND METHODS
(see Supplementary Information for details)
Generation of NRG2 KOs
NRG2 KO mice were generated by recombineering (Fig. 1E) and the targeted allele confirmed by Southern blot and PCR. Mice were kept with food and water ad libitum and 12/12h light cycle. Procedures were performed in accordance with NIH Animal Welfare guidelines. As NRG2 heterozygote mice exhibited no phenotype in a number of tests (see Results), comparisons were between NRG2 KO and their corresponding non-mutant littermates called hereafter controls.
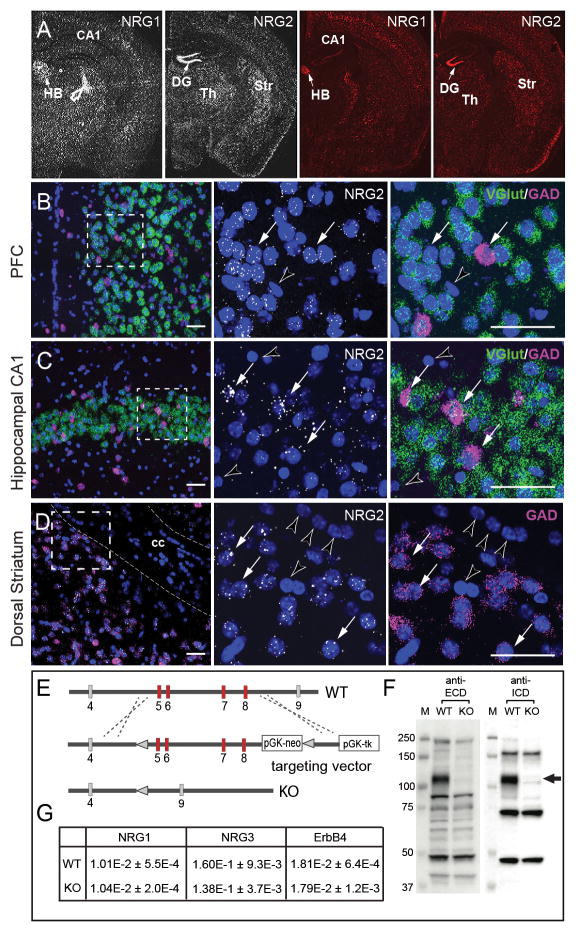
Figure 1. Expression patterns of NRG1 and NRG2 differ in adult brain.
A) ISH analyses for NRG1 and NRG2 were performed using either antisense 33P-labeled cRNA probes (left) or RNAScope oligonucleotides probe sets (right). Representative low-magnification dark-field and fluorescence images of adult mouse coronal sections show differential regional expression of both NRGs in the habenula (HB), hippocampal dentate gyrus (DG) and CA1, thalamus (Th), and striatum (Str). Arrows indicate areas of highest NRG1 (HB) and NRG2 (DG) expression; open arrow indicates an emulsion artifact. B-D) The distribution of NRG2 transcripts in glutamatergic and GABAergic neurons in the (B) prefrontal cortex (PFC), (C) hippocampus and (D) dorsal striatum were analyzed by triple ISH using RNAScope probes for NRG2 (white), vGlut (green) and GAD (magenta); nuclei were labeled with DAPI (blue). The middle and right panels correspond to the boxed area taken at higher magnification. Arrows indicate NRG2-expressing neurons, arrowheads mark neurons and presumably glial cells in the corpus callosum (cc) devoid of hybridization signal. Scale bar: 50 μm. E) Diagram of the Nrg2 region spanning exons 4 to 9 targeted by homologous recombination; targeted exons 5–8 shown in red (see Supplementary Information). F) Western blots of cerebellar lysates from NRG2 KO and WT mice probed with antibodies against the extracellular (ECD) and intracellular (ICD) domains of NRG2. Arrow indicates the position of the pro-NRG2 band absent in NRG2 KO brain tissue. G) Semi-quantitative RT-PCR analysis reveals no compensatory changes in ErbB4, NRG1 and NRG3 mRNA levels in whole brain RNA preparations from adult NRG2 KO compared to wild-type littermate mice. Data are relative to β-actin and shown as means ± S.E.M. (n=4 per group; p>0.05 for all targets; Student’s t-test).
RNA in situ hybridization
ISH experiments were performed on adult mouse brain sections (10–12 μm) using 33P-labeled cRNA probes, as described13, or by triple RNAscope fluorescence using NRG1 or NRG2 probes paired with V-Glut-1 and GAD-1 probes (Cat #s: 418181, 418191, 416631-C2, 400951-C3), according to the manufacturer’s protocol (Advanced Cell Diagnostics, USA).
Battery of behavior tests
Novelty-induced locomotor activity in the open field, prepulse inhibition of startle, elevated plus maze, and resident intruder tests were performed in adult littermates (14–18 week old), as described previously10. Amphetamine-induced (0–3 mg/kg) locomotor activity and responses of NRG2 KO mice to clozapine were tested by initially habituating mice for 30 min to the open field arena, administering the drug (i.p) and returning the mice to the same arena for measuring locomotor activity for 60 min. The rewarded T-maze consisted of a start arm and two identical goal arms with food wells at their ends. After training the animal to alternate between the arms, each mouse was given a free-choice run of 10 trials. Alternation rates were calculated as the percentage of correct entries of the total numbers of choice trials.
Electrophysiological Slice Recordings
Hippocampal slices (300 μm; 4–6 weeks) from NRG2 KO and littermate control mice were used to measure evoked EPSCs and LTP in whole-cell voltage-clamp mode as previously reported10, 18. To determine the NMDA/AMPA ratio, neurons were stimulated at 50% of the maximal intensity in the presence of 100 μM picrotoxin to measure NMDAR currents at Vh= +40 mV (50 ms post stimulation artifact) and AMPAR currents at Vh= −70 mv. AMPA and NMDA I/V curves were derived by recording synaptic currents in the presence of 50 μM APV or 10 μM CNQX, respectively, using a Multiclamp 700A amplifier (Molecular Devices, Sunnyvale, CA). Command voltage and current stimulus were controlled by pClamp 10.1 via a digital/analogue interface (Digidata 1322A, Molecular Devices). The acquisition rate was 4 kHz and digitized at 20 kHz. Recorded data were analyzed with Sigmaplot software (Systat Software, Inc, Chicago, IL).
Dopamine measurements
Extracellular dorsostriatal and mPFC dopamine was measured by microdialysis, followed by HPLC and electrochemical detection as described18. Microdialysis (2 mm probe, 18 kDa cutoff) was performed 1 week after guide cannulae implantation with continuous perfusion at 1 μl/min for 1 h. Samples were collected every 15 min into 5 μl 0.1 M HCl + 1 mM EDTA and stored at −80°C until analysis using an isocratic HPLC system with electrochemical detection. Quantification of extracellular and total dopamine was confirmed in independent samples by liquid chromatography, followed by mass spectrometry (Brains On-Line, San Francisco, CA).
RESULTS
Distinct expression patterns of NRG1 and NRG2 in the adult rodent brain
Earlier studies reported that NRG2 transcripts in the adult rodent brain are predominantly restricted to cerebellar, olfactory bulb and hippocampal granule neurons13, 35, 36. Here we used more sensitive and independent in situ hybridization (ISH) techniques, utilizing 33P-labeled cRNA and fluorescent RNAScope probes (see Methods) to analyze NRG2 expression. As shown in Fig. 1A, both radioactive (left) and fluorescence-based (right) approaches labeled the same regions for either the NRG1 or the NRG2 probes. As reported previously13, 35, 36, NRG2 ISH signals are strongest in hippocampal dentate gyrus granule cells; however, we also detected weaker signals in the neocortex, striatum and hippocampal CA1-CA3 neurons. By contrast, NRG1 expression is highest in cholinergic motor nuclei (not shown) and habenular nuclei (Fig. 1A), consistent with a prior report12, and its overall regional expression pattern differs markedly from NRG2. Neuronal subtype-specific analyses in the mPFC (Fig. 1B) and hippocampus (Fig. 1C) using triple ISH labeling with RNAscope probes revealed that NRG2 is expressed in both Gad1-positive GABAergic neurons and vGlut-1-positive glutamatergic neurons, albeit at lower levels, as well as in most striatal medium spiny neurons (Fig. 1D). RNAScope signals were not detected in mouse striatal sections hybridized with the DapB negative control probe, and NRG1 and NRG2 probes yielded distinct patterns of positive and negative cells that were entirely devoid of signal (Supplemental Figure S1). The virtual absence of background labeling in RNAScope is consistent with prior studies 37. These findings indicate that NRG2 is more widely expressed than originally reported and that its regional and temporal expression patterns differ from NRG1, suggesting distinct physiological functions for both ErbB receptor ligands.
Generation of NRG2 KO mice
The paucity of information about in vivo NRG2 functions prompted us to generated NRG2 KO mice by homologous recombination of exons 5–8 encoding the EGF-like (α and β isoforms) and transmembrane domains (Fig. 1E, see SI Methods). Western blot analyses using antibodies raised against either the extracellular or the intracellular domains confirmed the lack of detectable NRG2 protein in brain extracts from NRG2 KO mice (Fig. 1F). Furthermore, semi-quantitative RT-PCR revealed no significant compensatory effects of NRG2 deficiency on mRNA expression for NRG1, NRG3 and ErbB4 (Fig. 1G). Mutant mice were viable but exhibited transient anatomical delays during early postnatal development that became insignificant after 5 weeks of age (Fig. S2), similar to a previously generated NRG2 KO line that only targeted exon 4 of the EGF-like domain38. In our initial postnatal (birth to P21) basic behavioral assessment, NRG2 KO and WT littermates performed equally for negative geotaxis and cliff aversion, but showed a 1-day delay in eye opening, surface and air righting reflexes, auditory startle and negative geotaxis, all of which normalized by the second postnatal week of postnatal development. No differences in size or neonatal measures were observed between NRG2 heterozygote and WT littermates at any time during development (Fig. S2A). Moreover, no differences between gender and NRG2 heterozygote and WT littermate were observed in locomotor activity (Fig. S2B,C) nor in the elevated plus maze in adult mice (Fig. S2D,E); therefore, all subsequent comparisons were between NRG2 KOs and their control WT littermates.
NRG2 null mice have higher striatal dopamine levels and exhibit higher locomotor activity
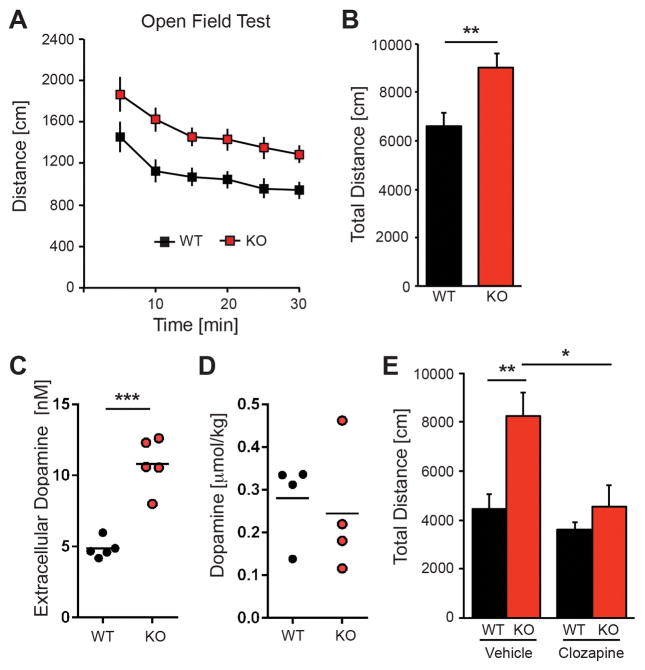
The expression of NRG2 in medium spiny neurons and the mPFC prompted us to test for spontaneous, novelty-induced activity in the open field (Fig. 2A,B). Both mutants and controls habituated to the novel environment, but NRG2 KO mice were more active during the entire observation period (F1,82=23.93, p<0.0001; two-way ANOVA) and covered a significantly longer total distance (9019±608 vs. 6602±560 cm; t26=2.87, p<0.01). Because the marked increase of locomotor activity suggested altered dopamine neurotransmission, we used microdialysis to measure extracellular dopamine (Fig. 2C) and its metabolites (Table S1) in the dorsal striatum of freely moving mice (see Supplementary Methods). Indeed, we found that dopamine levels were approximately 2-fold higher in NRG2 KOs relative to controls (10.82±0.82 vs. 4.86±0.30 nM, t8=6.81, p<0.0001). Consistent with the notion that extracellular dopamine levels in the dorsal striatum are regulated predominantly by DAT, HVA levels did not differ between genotypes (Table S1) whereas DOPAC levels were approximately 1.8-fold higher in the NRG2 KOs relative to controls (9.3 ± 1.0 vs. 5.2 ± 0.2 μM, p<0.01). Of note, total striatal dopamine content was not different between mutant and control mice (Fig. 2D), suggestive of an effect of NRG2 deficiency on modulation of dopamine release or uptake (see Discussion).
Figure 2. Hyperactivity of NRG2 KO mice is associated with elevated striatal dopamine and ameliorated by clozapine.
A) Novelty-induced locomotor activity in the open field during the 30-min observation period was consistently higher in NRG2 KO mice compared to their WT littermate controls. Data binned at 5 min intervals (n=15 for each group). B) Cumulative distance plot for data shown in (A). C,D) Extracellular dopamine levels (C) and total dopamine content (D) in NRG2 KO mice and WT littermate controls. Measurements for extracellular dopamine were performed using microdialysis in freely moving mice (n=4–5/genotype; unpaired two-tailed Student’s t-test). E) Following habituation, clozapine treatment (1 mg/kg, i.p.) restored normal locomotor activity in NRG2 KO mice, but was without effect in WT littermates (n=5–7/genotype). Results represent the mean ± SEM, *p<0.05, **p<0.01 and ***p<0.0001.
Positive symptoms in schizophrenia are associated with increases in striatal dopamine release (see39). The atypical antipsychotic clozapine, when administered acutely but not chronically 40, can ameliorate dopamine dysbalance and behaviors in animal models40, 41. Indeed, clozapine (1 mg/kg; i.p.) restored normal locomotor activity of NRG2 mutants when injected 25 min prior to the open field test (clozapine: 4565±884 vs. vehicle: 8255±964 cm; t7=3.36; p<0.05; Fig. 2E), such that the total distance traveled was similar to vehicle- or clozapine-treated WT controls (vehicle: 4444±670 cm or clozapine: 3595±369 cm; t9=0.097; p>0.05). Importantly, the lack of a clozapine effect on WT controls suggests that restoration of normal locomotor activity in hyperactive NRG2 KO mice is not due to generalized sedation (Fig. 2E).
Reduced levels of mPFC dopamine and impaired working memory in NRG2 null mice
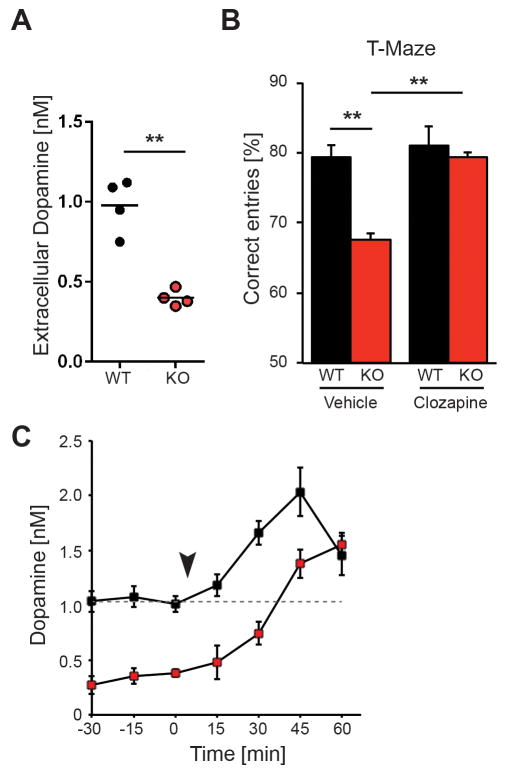
Functional imaging studies have repeatedly shown that mesostriatal and mesocortical/extrastriatal dopaminergic activity are inversely affected in schizophrenia39,42. Because striatal extracellular dopamine levels are elevated in NRG2 KOs (Fig. 2C), we measured baseline dopamine levels in the mPFC and determined performance in a working memory task (Fig. 3). Strikingly, we found that extracellular dopamine concentrations are significantly reduced in the mPFC of NRG2 KOs relative to littermate controls (0.40±0.02 vs. 0.98±0.08 nM; t8=8.513, p<0.0001; Fig. 3A). HVA levels were also reduced (Table S1), consistent with decreased dopamine availability for turnover by catechol-O-methyltransferase.
Figure 3. NRG2 null mice exhibit working memory deficits and reduced dopamine levels that are restored by clozapine.
A) Reduced extracellular dopamine levels in the mPFC of NRG2 KO mice (n=4/genotype; unpaired two-tailed Student’s t test). B) Poor performance by NRG2 KO mice in a T-maze reward alternation task as compared to WT littermates (left) can be restored by clozapine (1mg/kg, i.p.) administration (right; n=7/genotype) (two-way ANOVA with Sidak’s posthoc test). C) Extracellular dopamine levels in the mPFC of NRG2 KO mice (n=5) and WT littermates (n=5) before and after injection (arrowhead) of clozapine (two-way ANOVA for repeated measures with Sidak’s test for multiple comparison for post hoc analysis). Dotted line demarks basal dopamine concentration in controls prior to injection. Results represent the mean ± SEM, **p<0.01.
Numerous primate and rodent studies indicate that working memory performance relies on optimal dopamine levels in the DLPFC43, with low levels suggested to reduce signal-to-noise ratios during cognitive tasks44. We therefore tested the performance of NRG2 KOs in the T-maze rewarded alternation task to assess working memory. As shown in Fig. 3B (left), the percent of correct entries was lower for NRG2 KOs than for their WT littermates (68±2.9% vs. 79±1.9%, p<0.01, Sidak’s posthoc test). Because acute clozapine administration can increase mPFC dopamine levels in rodents40, we investigated if clozapine (1 mg/kg, i.p.) could improve performance of NRG2 KOs when administered 30–45 min before the task. As shown in Fig. 3B (right), clozapine indeed improved task performance in drug-treated relative to vehicle-treated NRG2 KOs (79±0.82% vs. 68±2.9%; p<0.01). Importantly, rates of correct entries were not significantly different between clozapine-treated NRG2 KOs (79±0.82%) and vehicle- (79±1.9%) or clozapine-treated (81±1%) WT littermates (significance for clozapine treatment: F1,24=9.352, p<0.01, two-way ANOVA). To determine if cortical dopamine levels correlated with these behavioral findings, we measured extracellular concentrations in the mPFC of NRG2 KO and control littermates before and following clozapine treatment (Fig. 3C). Interestingly, we found that clozapine steadily increased extracellular dopamine concentration up to 45 min post-injection in both controls (from 1.01±0.07 to 2.03±0.22 nM; t6=4.04, p<0.005) and NRG2 KOs (from 0.38±0.03 to 1.38±0.13 nM; t6=7.46, p<0.0005), the latter leveling off 45 min post-injection (F1,6= 28.92, p=0.0017). Notably, dopamine concentrations in NRG2 KOs between 30 and 45 min post-injection (when the T-maze task was performed) were near baseline levels in control mice. In summary, NRG2 KOs are hyperdopaminergic in the striatum and hypodopaminergic in the mPFC, and respond to acute clozapine treatment by elevating mPFC dopamine levels that correlate with improved working memory performance.
NRG2 KO mice exhibit multiple behavioral deficits associated with psychiatric disorders
Based on the observed dopamine dysbalance, as well as the genetic and functional association of the NRG-ErbB4 signaling pathway with schizophrenia1–4 and its endophenotypes5, we assessed NRG2 KOs in rodent behavioral tasks with relevance to psychiatric disorders and reported to be altered in ErbB4 KO mice, including tests for amphetamine sensitivity, sensorimotor gating, social interactions and anxiety-like behaviors45.
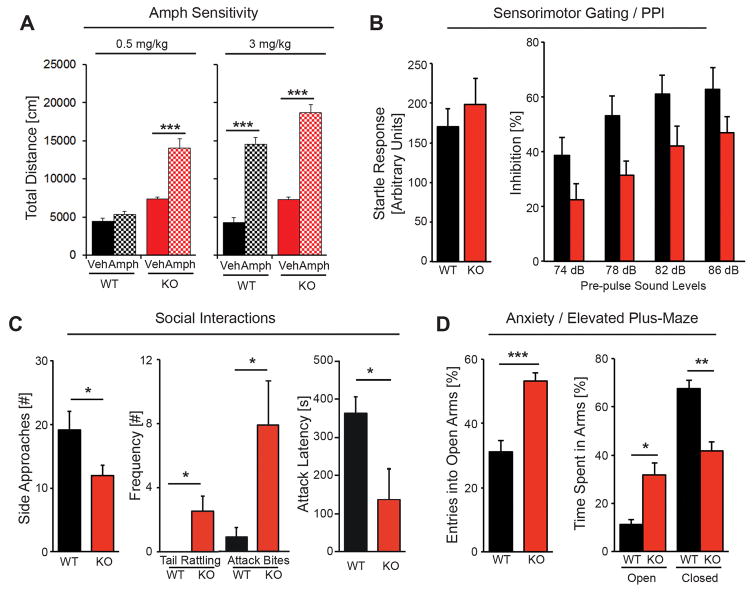
Individuals with schizophrenia manifest an augmented release of striatal dopamine (see39) and a subgroup exhibit higher sensitivity to psychosis induced by stimulants such as amphetamine (Amph) 46–48, whereas a large percentage of ADHD subjects exhibit a reduction in activity at higher psychostimulant doses 49. To investigate if NRG2 KO mice are hypersensitive to psychostimulants or instead reduce their activity at higher Amph levels, we first performed a dose-response experiment (0.0 to 3.0 mg/kg; i.p.) to determine the threshold for Amph-mediated stimulation of locomotor activity in WT mice habituated in an open field chamber (Supplemental Fig. S3). Based on this analysis, we then chose to test the effects vehicle, 0.5 (subthreshold) or 3.0 mg/kg (suprathreshold) Amph on locomotor activity of NRG2 KO mice and their WT littermates after they were habituated to the open area. As shown in Fig. 4A (left), total distance traveled approximately doubled following injection of 0.5 mg/kg Amph compared to vehicle-injected NRG2 KO mice (14146 ± 1090 vs. 7363 cm ± 268 p<0.001) whereas the same dose had no effect on WT littermates (F3,36=51.03, p<0.0001; One-way ANOVA). On the other hand, 3.0 mg/kg Amph increased the total distance traveled in both NRG2 KO and WT littermates by ~2.5-fold relative to their respective vehicle-injected controls (Fig. 4A, right), indicating that both cohorts can respond to Amph but only NRG2 KO mice exhibit hypersensitivity to this psychostimulant.
Figure 4. NRG2 mutant mice exhibit numerous behavioral deficits associated with endophenotypes in psychiatric disorders.
A) Both control (black) and NRG2 KO (red) habituated mice display increased motor activity in response to 3.0 mg/kg (i.p., right panels) of Amph (checkered), relative to vehicle-injected mice (solid color), whereas only NRG2 KOs exhibited hyperactivity following a subthreshold dose 0.5 mg/kg of Amph (left panels; n=10/genotype; One-way ANOVA). B) Startle responses (left) are similar in adult NRG2 KO and littermate control mice. PPI, measured at 74, 78, 82, and 86 dB prepulse stimulus intensities (right), is reduced in NRG2 KO mice (n= 15–19/genotype; repeated two-way ANOVA). C) NRG2 KO mice display abnormal social interactions in the resident-intruder task characterized by a reduced number of side approaches (left), and increased aggressiveness manifesting as increased frequency of tail rattling (middle) and attack bites, and decreased latency to attack (right; n=12–13/genotype; Student’s t-test). D) NRG2 KO mice exhibit reduced anxiety-like behavior in the elevated-plus maze, entering more often (right) and spending more time (left) in the open arms than control littermates. Entries into the open arms are shown as a percentage of all entries, and time spent in the open arms is plotted as a percentage of the total observation time; times spent in the middle are not shown (n=6–8/genotype; Student’s t-test). Results represent the mean ± SEM; *p<0.05, **p<0.005 and ***p<0.0005.
Prepulse inhibition (PPI) of the acoustic startle response, which is conserved across species, is a robust sensorimotor gating reflex that is regulated by dopamine and impaired in schizophrenia and potentially other psychiatric disorders50, 51. Prompted by our observation of elevated striatal dopamine levels in NRG2 KOs, we measured the acoustic startle response and PPI in mutant and control mice. No genotype effect on acoustic startle was observed in response to a 120 dB sound (Fig. 4B, left). Inhibition of the startle response increased with increasing prepulse acoustic intensities in both NRG2 KOs and controls, but is lower in NRG2 KOs (F1,32= 5.53, p<0.05). PPI was significantly lower across all sound intensity levels in NRG2 KO mice compared to WT littermate controls (Fig 4B, right; F3,96=12.50, p<0.0001); no interaction between genotype and prepulse intensity was found.
We examined NRG2 KOs for social interaction-like behaviors, testing for aggression by scoring the frequency of approaches, tail rattling, biting and latency to attack using a resident intruder test (Fig. 4C). The frequency of side approaches by NRG2 KO mice toward an intruder was significantly reduced compared to WT controls (12±1.5 vs 19±2.9; t23=2.06, p<0.05), whereas olfactory investigation time was not different (134.3±22.7s vs 128.5±28.2s). NRG2 KOs also displayed significantly higher frequency of tail rattling (2.5±0.9 vs 0±0; t23=2.49 p<0.05) and attack bites (7.9 ± 2.7 vs 0.9 ± 0.6; t23=2.35 p<0.05), and a reduced latency to attack (151±77 vs 371±47 sec; t23=2.42, p<0.05). These data indicate NRG2 KO mice are aggressive and socially impaired.
Based on our earlier behavioral studies indicating that ErbB4 KO mice exhibit reduced anxiety-like behaviors10, we analyzed the preference of NRG2 KO mice for the open vs. closed arms of an elevated plus-shaped maze (Fig. 4D) as a measure commonly attributed to reduced anxiety-like or risk-taking behavior52. We found that NRG2 KOs enter into the open arms more often than controls (left; 53±3 vs. 31±4%; t12=4.78, p<0.0005). In addition, NRG2 KOs spent more time in the open arms relative to controls (32±5 vs. 11±5%; t12=3.25, p<0.005) while controls spent significantly more time exploring the closed arms (68±4 vs. 42±4%; t12=4.54, p<0.0005); we observed no freezing behavior during exploration of the open arms by either genotype. Taken together with our earlier study10, these results suggest that NRG2 signaling through ErbB4 receptors in the adult brain may account, at least in part, for anxiety-like related behaviors or risk-taking behaviors.
Analysis of NRG2 KO effects on neurotransmission and plasticity at glutamatergic synapses
NRG/ErbB4 signaling regulates glutamatergic transmission and plasticity at hippocampal Schaeffer collateral-to-CA1 (SC-CA1) synapses on pyramidal neurons that lack ErbB414 through an indirect mechanism that requires ErbB4 in GABAergic interneurons 10, 53 and dopamine signaling18. Of note, we recently showed that NRG2 promotes the association of ErbB4 with GluN2B-containing NMDARs and downregulates NMDAR EPSCs in acute mPFC slices 31. Moreover, because chronic NMDAR antagonist treatment in rodents paradoxically augments synaptic NMDAR currents54, resulting in dopamine imbalance32, and because dopamine modulates NMDAR kinetics and levels55 (see Discussion), we investigated if dopamine dysregulation in NRG2 KO mice affects glutamatergic transmission and plasticity.
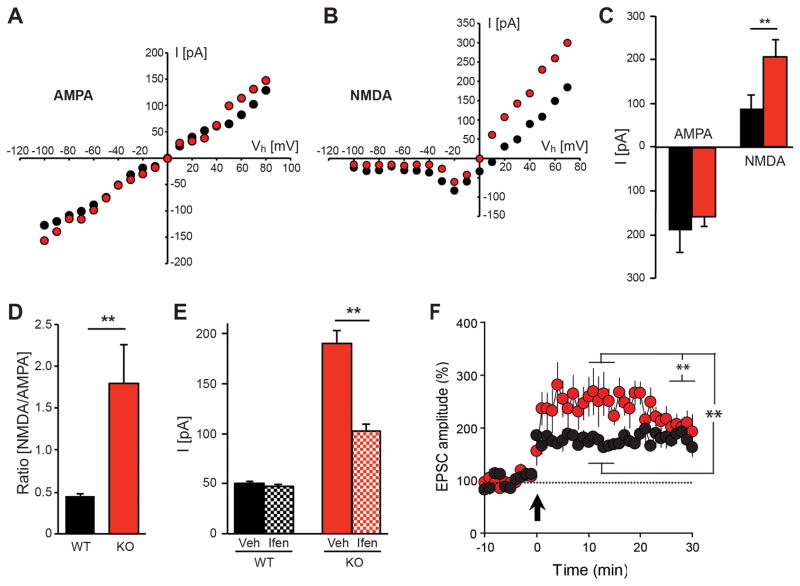
We began by analyzing current-voltage (I/V) relationships for AMPARs and NMDARs in adult acute hippocampal slices by recording excitatory postsynaptic currents (EPSCs) at SC-CA1 synapses. Whereas AMPAR I/V curves for NRG2 KOs and controls were linear and indistinguishable (Fig. 5A), we found a profound difference in the NMDAR I/V relationship at positive potentials ranging from +20 to +80 mV (Fig. 5B). In separate experiments, NMDAR EPSCs were recorded at +40 mV holding potential (Vh) in the presence of the AMPAR inhibitor CNQX (10 μM), and AMPAR were measured at −70 mV in the presence of the NMDAR inhibitor APV (50 μM) to avoid potential contaminating currents (Fig. 5C). Peak NMDAR currents in NRG2 KOs were 2.3-fold higher than in littermate controls (206.4±30.7 vs. 88.3±39.5 pA; p<0.01), whereas AMPAR currents did not differ between genotypes (−157.3±51.0 vs. −190.8±21.1 pA; p>0.05). Therefore, the dramatic increase in the NMDAR/AMPAR ratio in NRG2 KOs relative to controls (Fig. 5D; 1.80±0.46 vs. 0.45±0.03; p<0.001) is driven by an increase in NMDAR EPSCs.
Figure 5. Altered NMDAR function and enhanced GluN2B activity in NRG2 KO mice.
I–V curves of AMPAR- (A) and NMDAR-mediated currents (B) showing respective peak amplitudes obtained for voltage steps ranging from −100 to +80 mV in slices from NRG2 KO (red) and littermate control (black) mice (N=5 mice; n=5 slices each for WT and NRG2 KOs). (C) Average amplitudes of AMPAR EPSCs at −70 mV and NMDAR EPSCs at +40 mV (WT: N=5, n=7; KO: N=6, n=9). (D) NMDAR/AMPAR ratios in NRG2 KOs and littermate controls (plotted from data shown in C). E) Effects of the GluN2B-selective inhibitor ifenprodil on NMDAR-mediated currents in slices from NRG2 KOs (red) and WT littermate (black) mice. Ifenprodil did not significantly affect NMDAR currents in WT slices (N=5; n=9), but greatly reduced NMDAR currents in slices from NRG2 null mice (N=5; n=6; mean ±SEM,**p<0.01). F) Hippocampal slices from NRG2 KO mice exhibit transiently increased LTP compared to slices from littermate controls (NRG2 KO: N=4, n=6; WT: N=4, n=7, mean ±SEM, **p<0.001).
Amplitude and kinetics of NMDAR-mediated postsynaptic currents are largely dependent on GluN2 subunit composition, which is regulated during development and by dopamine signaling55. Whereas perinatal hippocampal SC-CA1 synapses mostly contain heterodimeric GluN1-GluN2B receptors, they are replaced during postnatal development by GluN1-GluN2A or heterotrimeric GluN1-GluN2A/2B receptors that have faster inactivation kinetics. To test whether increased NMDAR-mediated currents in NRG2 KOs are accompanied by an increase of GluN2B-containing receptors, we investigated the effects of two structurally independent antagonists (ifenprodil and Ro 25-6891) selective for heterodimeric GluN2B-containing receptors. We found that 3 μM ifenprodil (Fig. 5E) did not significantly alter NMDAR peak currents in adult slices of WT control mice (50.5±1.9 vs. 47.5±2.2 pA), consistent with the predominance of GluN2A-containing NMDAR in the adult hippocampus, whereas it dramatically reduced NMDAR EPSCs in slices from NRG2 KOs (190.0±12.8 vs. 102.0±7.6 pA, p<0.01). Essentially the same results were obtained with 0.3 μM Ro 25-6891 (Fig. S4). Therefore, the increased NMDAR synaptic currents in NRG2 KOs result, at least in part, from a larger proportion of GluN2B-containing postsynaptic NMDARs. Interestingly, as shown previously for NRG119, application of a peptide encompassing the NRG2 EGF-like domain 20 min following LTP induction also effectively depotentiates LTP at SC-CA1 synapses (Fig. S5) and, consistent with increased NMDAR currents in NRG2 KOs, we observed that during the initial 20 minutes following LTP induction it was transiently augmented in slices from mutant mice relative to WT littermate controls (Fig. 5F; NRG2 KO: 245 ± 33% vs. WT, 175 ± 11%; p<0.0001, Student’s t-test).
DISCUSSION
Here we report for the first time that NRG2 KO mice exhibit a marked dopamine dysbalance in the dorsal striatum and mPFC, altered glutamatergic transmission in the hippocampus and display numerous behavioral impairments, responsive to clozapine treatment, that phenocopy animal models for psychiatric disorders. As discussed below, these findings are relevant to identify NRG2 functions in the adult brain and have important implications for our understanding of how the NRG2-ErbB signaling pathway regulates dopamine balance, synaptic mechanisms and behaviors that underlie psychiatric disorders. As discussed below, the extensive overlap of behaviors altered in NRG2 and ErbB4 KO mice, strongly suggests that NRG2 is a major ErbB4 ligand in the adult brain affecting dopaminergic and glutamatergic functions.
Association of the NRG/ErbB4 signaling pathway with psychiatric disorders
There is accumulating genetic and functional evidence for an involvement of NRG/ErbB4 signaling pathway receptor in the etiology of schizophrenia and other psychiatric disorders1–4. Postmortem brain studies from subjects with schizophrenia and matched controls reported increased NRG1 and NRG3 mRNA levels56, 57, altered expression of ErbB4 splice variants58, 59 and enhanced NRG1-ErbB4 signaling60. Polymorphic variants of NRG1, NRG3 and ErbB4 are also associated with heritable schizophrenia endophenotypes, including reduced PPI, altered frontal and temporal lobe activity and working memory deficits5, 61. Genome-wide scans have identified a region that encompasses the NRG2 locus (5q31.2) that is linked to multigenerational extended families with attention-deficit hyperactivity disorder (ADHD)29, and associated with schizophrenia25–28 and bipolar disorder62. A novel syndrome identified in seven children with de novo overlapping 5q31 microdeletions that encompass three genes (including NRG2) share clinical features that include white matter abnormalities affecting frontal lobes, severe developmental and intellectual delays, difficulty feeding and seizures23, 24. Of note, the NRG/ErbB signaling pathway regulates peripheral myelination, and two independent NRG2 KO lines display early growth retardation (38; Fig. S2); we also observed that NRG2 KO pups suckle less effectively than littermates and may experience transient neonatal seizures (unpublished observations).
Some of the behavioral deficits observed in NRG2 KOs overlap with those of NRG1 hypomorphs (NRG1 KOs die at E10.5), including locomotor hyperactivity and PPI deficits responsive to clozapine7, 9; in addition, NRG1 (type III) heterozygotes have reduced short-term memory and spatial memory that are improved by nicotine8. However, our NRG2 null mice exhibit additional behavioral anomalies that were not reported in NRG1 hypomorphs, such as reduced anxiety-like behavior and increased sensitivity to psychostimulants. These phenotypic differences are consistent with the distinct regional and temporal expression patterns of NRG1 and NRG2. In contrast to NRG1, NRG2 expression increases postnatally and is detected at relatively high levels in striatal medium spiny and neocortical neurons (Fig. 1). Importantly, ErbB4 is expressed in mesencephalic dopaminergic neurons that project to the striatum, mPFC and hippocampus that are regulated by NRG 15–17. Interestingly, ErbB4 null mice exhibit many of the behavioral abnormalities that we have observed in NRG2 KOs10 and that are absent in NRG1 mutants (see above), suggesting that these behaviors are regulated by NRG2-ErbB4 signaling. Moreover, NRG2 KOs phenocopy the augmented striatal dopamine levels, Amph hypersensitivity and behavioral deficits reported for rodent lesion models63, 64 and other genetically-targeted models of schizophrenia endophenotypes65–70, suggesting a potential convergence of mechanisms that regulate neuronal circuits underlying core disease traits.
Dysbalance of dopaminergic and glutamatergic pathways in NRG2 KOs and its relevance to psychiatric disorders
NRG2 deficiency affects mPFC and striatal extracellular dopamine concentrations in opposite directions. This finding is reminiscent of functional imaging studies in persons with schizophrenia reporting pathway-specific dopamine dysfunction in the striatal pathway (hyperdopaminergia) and subcortical-extrastriatal and cortical pathways (hypodopaminergia)39, as well as reduced dopamine release in the DLPFC during a working memory task42. Consistent with increased striatal dopamine levels, NRG2 KO mice have reduced PPI and exhibit hyperactivity in the open field that is ameliorated by clozapine. Interestingly, acute clozapine administration also improved performance in the reward T-maze task, which requires working memory, and correlated with increased mPFC dopamine levels in NRG2 KOs. Improved T-maze performance may appear paradoxical, based on the ineffectiveness of clozapine to improve negative and cognitive deficits in schizophrenia71. However, early improvements (24h) in core schizophrenia symptoms in response to antipsychotics have been reported72 (also see 32), and in animal studies acute, but not chronic, clozapine administration increased mPFC dopamine levels40. Based on prior rodent and non-human primate studies, clozapine may have improved NRG2 KO performance on the rewarded T-maze either by indirectly augmenting D1R signaling via the increased dopamine levels55, by directly blocking excessive activity of D4Rs21, 73, 74 (which are functionally coupled to ErbB4 receptors on PV-positive interneurons and regulate gamma oscillations21), or by simultaneously activating of D1- and D2-type receptors to optimize temporal gating73, 74.
NRG2 deficiency also dramatically increases hippocampal NMDAR currents (Fig. 5). The observation that NMDAR antagonists phencyclidine and ketamine can elicit behaviors in healthy volunteers that resemble positive, negative and cognitive symptoms initially led to the NMDAR hypofunction hypothesis in schizophrenia. However, the effects of NMDAR antagonists and their cellular site of action are paradoxical33. Chronic NMDAR antagonist treatment facilitates synaptic NMDAR currents54 and increases GluN1 and GluN2B mRNA expression65. Acutely, NMDAR antagonists increase, rather than decrease, cortical excitation and the power of unevoked gamma oscillations - a type of neuronal network activity associated with working memory33. NMDAR antagonists could affect cortical excitability directly by diminishing excitatory drive onto GABAergic interneurons75, or indirectly by increasing the activity of cortico-mesoencephalic pathways that augment mPFC dopamine levels76. The latter mechanism is important because dopamine is known to modulate NMDAR kinetics and synaptic levels55, as we observe in NRG2 KOs. Moreover, chronic administration of NMDAR antagonists elicits many behavioral changes that phenocopy those observed in NRG2 KOs, such as increased sensitivity to stress and amphetamine-induced hyperactivity, working memory impairments, decreased social interactions and increased locomotor activity (the latter can however occur independently of dopamine changes77). Therefore, the dysregulation of dopamine and NMDAR signaling and altered behaviors in NRG2 KO mice could result from perturbations of the intricate bi-directional interactions between the glutamatergic and dopaminergic signaling pathways that may converge on common neuronal circuits that modulate these behaviors (see32). Recently, Rosen et al 70 used the term “electrophysiological endophenotypes” to describe how a variety of rodent models of psychosis and schizophrenia targeting widely different risk factors nevertheless exhibit similar core electrophysiological properties.
While the question of a direct role of NRG2 in the etiology of psychiatric disorders remains to be determined, the NRG2 KO mice reported here constitute an excellent model to dissect the roles of neurotransmitters and microcircuits that have been implicated in behavioral and electrophysiological endophenotypes relevant to several neuropsychiatric disorders, as well as drug abuse and addiction78. This is especially pertinent considering the behavioral phenotypes and alterations in dopamine and glutamate transmission reported here, the fact that ErbB4 signaling in the hippocampus and mPFC is restricted to GABAergic neurons and regulates plasticity and neuronal network oscillations through fast-spiking parvalbumin-positive interneurons, and our recent finds that identified a negative feedback loop between NMDARs and NRG2/ErbB4 signaling in GABAergic interneurons31. The fact that NRG2 regulates glutamatergic transmission in GABAergic neurons and selectively affects GluN2B-containing NMDARs (this study,31), potentially an important extrasynapatic receptor subtype affecting tonic glutamatergic transmission and a major proposed target of ketamine34, 79, raises the intriguing possibility that alterations in NRG2 levels could regulate numerous pathways and circuits implicated in several neuropsychiatric disorders.
Supplementary Material
Acknowledgments
The authors thank Miss Joanna Wang for helping with the reward seeking experiment. We are grateful for the financial support from the Eunice Shriver Kennedy NICHD Intramural Research Program. We are grateful to Drs. Vincent Schram and Carolyn Smith from the NICHD and NINDS imaging cores, to Daniel Abebe for help with behavior experiments, and to Dr. Marieke van der Hart from Brains On-Line for discussions about dopamine measurements.
Footnotes
Conflict of Interest:
The authors declare no conflict of interest.
References
- 1.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Molecular psychiatry. 2005 Jan;10(1):40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- 2.Buonanno A. The neuregulin signaling pathway and schizophrenia: from genes to synapses and neural circuits. Brain research bulletin. 2010 Sep 30;83(3–4):122–131. doi: 10.1016/j.brainresbull.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mei L, Nave KA. Neuregulin-ERBB signaling in the nervous system and neuropsychiatric diseases. Neuron. 2014 Jul 2;83(1):27–49. doi: 10.1016/j.neuron.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mostaid MS, Lloyd D, Liberg B, Sundram S, Pereira A, Pantelis C, et al. Neuregulin-1 and schizophrenia in the genome-wide association study era. Neuroscience and biobehavioral reviews. 2016 Jun 6;68:387–409. doi: 10.1016/j.neubiorev.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 5.Greenwood TA, Light GA, Swerdlow NR, Radant AD, Braff DL. Association analysis of 94 candidate genes and schizophrenia-related endophenotypes. PLoS One. 2012;7(1):e29630. doi: 10.1371/journal.pone.0029630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011 May 12;473(7346):221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stefansson H, Sigurdsson E, Steinthorsdottir V, Bjornsdottir S, Sigmundsson T, Ghosh S, et al. Neuregulin 1 and susceptibility to schizophrenia. American journal of human genetics. 2002 Oct;71(4):877–892. doi: 10.1086/342734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen YJ, Johnson MA, Lieberman MD, Goodchild RE, Schobel S, Lewandowski N, et al. Type III neuregulin-1 is required for normal sensorimotor gating, memory-related behaviors, and corticostriatal circuit components. J Neurosci. 2008 Jul 2;28(27):6872–6883. doi: 10.1523/JNEUROSCI.1815-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Tuathaigh CM, Babovic D, O’Sullivan GJ, Clifford JJ, Tighe O, Croke DT, et al. Phenotypic characterization of spatial cognition and social behavior in mice with ‘knockout’ of the schizophrenia risk gene neuregulin 1. Neuroscience. 2007 Jun 15;147(1):18–27. doi: 10.1016/j.neuroscience.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 10.Shamir A, Kwon OB, Karavanova I, Vullhorst D, Leiva-Salcedo E, Janssen MJ, et al. The importance of the NRG-1/ErbB4 pathway for synaptic plasticity and behaviors associated with psychiatric disorders. J Neurosci. 2012 Feb 29;32(9):2988–2997. doi: 10.1523/JNEUROSCI.1899-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neddens J, Fish KN, Tricoire L, Vullhorst D, Shamir A, Chung W, et al. Conserved interneuron-specific ErbB4 expression in frontal cortex of rodents, monkeys, and humans: implications for schizophrenia. Biological psychiatry. 2011 Oct 1;70(7):636–645. doi: 10.1016/j.biopsych.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corfas G, Rosen KM, Aratake H, Krauss R, Fischbach GD. Differential expression of ARIA isoforms in the rat brain. Neuron. 1995 Jan;14(1):103–115. doi: 10.1016/0896-6273(95)90244-9. [DOI] [PubMed] [Google Scholar]
- 13.Longart M, Liu Y, Karavanova I, Buonanno A. Neuregulin-2 is developmentally regulated and targeted to dendrites of central neurons. The Journal of comparative neurology. 2004 Apr 26;472(2):156–172. doi: 10.1002/cne.20016. [DOI] [PubMed] [Google Scholar]
- 14.Vullhorst D, Neddens J, Karavanova I, Tricoire L, Petralia RS, McBain CJ, et al. Selective expression of ErbB4 in interneurons, but not pyramidal cells, of the rodent hippocampus. J Neurosci. 2009 Sep 30;29(39):12255–12264. doi: 10.1523/JNEUROSCI.2454-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steiner H, Blum M, Kitai ST, Fedi P. Differential expression of ErbB3 and ErbB4 neuregulin receptors in dopamine neurons and forebrain areas of the adult rat. Experimental neurology. 1999 Oct;159(2):494–503. doi: 10.1006/exnr.1999.7163. [DOI] [PubMed] [Google Scholar]
- 16.Abe Y, Namba H, Zheng Y, Nawa H. In situ hybridization reveals developmental regulation of ErbB1-4 mRNA expression in mouse midbrain: implication of ErbB receptors for dopaminergic neurons. Neuroscience. 2009 Jun 16;161(1):95–110. doi: 10.1016/j.neuroscience.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Ledonne A, Nobili A, Latagliata EC, Cavallucci V, Guatteo E, Puglisi-Allegra S, et al. Neuregulin 1 signalling modulates mGluR1 function in mesencephalic dopaminergic neurons. Molecular psychiatry. 2015 Aug;20(8):959–973. doi: 10.1038/mp.2014.109. [DOI] [PubMed] [Google Scholar]
- 18.Kwon OB, Paredes D, Gonzalez CM, Neddens J, Hernandez L, Vullhorst D, et al. Neuregulin-1 regulates LTP at CA1 hippocampal synapses through activation of dopamine D4 receptors. Proceedings of the National Academy of Sciences of the United States of America. 2008 Oct 7;105(40):15587–15592. doi: 10.1073/pnas.0805722105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon OB, Longart M, Vullhorst D, Hoffman DA, Buonanno A. Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J Neurosci. 2005 Oct 12;25(41):9378–9383. doi: 10.1523/JNEUROSCI.2100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisahn A, Neddens J, Yan L, Buonanno A. Neuregulin-1 modulates hippocampal gamma oscillations: implications for schizophrenia. Cereb Cortex. 2009 Mar;19(3):612–618. doi: 10.1093/cercor/bhn107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andersson RH, Johnston A, Herman PA, Winzer-Serhan UH, Karavanova I, Vullhorst D, et al. Neuregulin and dopamine modulation of hippocampal gamma oscillations is dependent on dopamine D4 receptors. Proceedings of the National Academy of Sciences of the United States of America. 2012 Aug 7;109(32):13118–13123. doi: 10.1073/pnas.1201011109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Burgos G, Cho RY, Lewis DA. Alterations in Cortical Network Oscillations and Parvalbumin Neurons in Schizophrenia. Biological psychiatry. 2015 Jun 15;77(12):1031–1040. doi: 10.1016/j.biopsych.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosoki K, Ohta T, Natsume J, Imai S, Okumura A, Matsui T, et al. Clinical phenotype and candidate genes for the 5q31.3 microdeletion syndrome. American journal of medical genetics Part A. 2012 Aug;158A(8):1891–1896. doi: 10.1002/ajmg.a.35439. [DOI] [PubMed] [Google Scholar]
- 24.Shimojima K, Isidor B, Le Caignec C, Kondo A, Sakata S, Ohno K, et al. A new microdeletion syndrome of 5q31.3 characterized by severe developmental delays, distinctive facial features, and delayed myelination. American journal of medical genetics Part A. 2011 Apr;155A(4):732–736. doi: 10.1002/ajmg.a.33891. [DOI] [PubMed] [Google Scholar]
- 25.Lewis CM, Levinson DF, Wise LH, DeLisi LE, Straub RE, Hovatta I, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. American journal of human genetics. 2003 Jul;73(1):34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwab SG, Eckstein GN, Hallmayer J, Lerer B, Albus M, Borrmann M, et al. Evidence suggestive of a locus on chromosome 5q31 contributing to susceptibility for schizophrenia in German and Israeli families by multipoint affected sib-pair linkage analysis. Molecular psychiatry. 1997 Mar;2(2):156–160. doi: 10.1038/sj.mp.4000263. [DOI] [PubMed] [Google Scholar]
- 27.Sklar P, Pato MT, Kirby A, Petryshen TL, Medeiros H, Carvalho C, et al. Genome-wide scan in Portuguese Island families identifies 5q31-5q35 as a susceptibility locus for schizophrenia and psychosis. Molecular psychiatry. 2004 Feb;9(2):213–218. doi: 10.1038/sj.mp.4001418. [DOI] [PubMed] [Google Scholar]
- 28.Straub RE, MacLean CJ, O’Neill FA, Walsh D, Kendler KS. Support for a possible schizophrenia vulnerability locus in region 5q22-31 in Irish families. Molecular psychiatry. 1997 Mar;2(2):148–155. doi: 10.1038/sj.mp.4000258. [DOI] [PubMed] [Google Scholar]
- 29.Arcos-Burgos M, Castellanos FX, Pineda D, Lopera F, Palacio JD, Palacio LG, et al. Attention-deficit/hyperactivity disorder in a population isolate: linkage to loci at 4q13.2, 5q33.3, 11q22, and 17p11. American journal of human genetics. 2004 Dec;75(6):998–1014. doi: 10.1086/426154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell RM, Janssen MJ, Karavanova I, Vullhorst D, Furth K, Makusky A, et al. ErbB4 reduces synaptic GABAA currents independent of its receptor tyrosine kinase activity. Proceedings of the National Academy of Sciences of the United States of America. 2013 Nov 26;110(48):19603–19608. doi: 10.1073/pnas.1312791110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vullhorst D, Mitchell RM, Keating C, Roychowdhury S, Karavanova I, Tao-Cheng JH, et al. A negative feedback loop controls NMDA receptor function in cortical interneurons via neuregulin 2/ErbB4 signalling. Nature communications. 2015;6:7222. doi: 10.1038/ncomms8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coyle JT, Basu A, Benneyworth M, Balu D, Konopaske G. Glutamatergic synaptic dysregulation in schizophrenia: therapeutic implications. Handb Exp Pharmacol. 2012;(213):267–295. doi: 10.1007/978-3-642-25758-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moghaddam B, Krystal JH. Capturing the angel in “angel dust”: twenty years of translational neuroscience studies of NMDA receptor antagonists in animals and humans. Schizophrenia bulletin. 2012 Sep;38(5):942–949. doi: 10.1093/schbul/sbs075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balu DT, Coyle JT. The NMDA receptor ‘glycine modulatory site’ in schizophrenia: D-serine, glycine, and beyond. Current opinion in pharmacology. 2015 Feb;20:109–115. doi: 10.1016/j.coph.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Busfield SJ, Michnick DA, Chickering TW, Revett TL, Ma J, Woolf EA, et al. Characterization of a neuregulin-related gene, Don-1, that is highly expressed in restricted regions of the cerebellum and hippocampus. Molecular and cellular biology. 1997 Jul;17(7):4007–4014. doi: 10.1128/mcb.17.7.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carraway KL, 3rd, Weber JL, Unger MJ, Ledesma J, Yu N, Gassmann M, et al. Neuregulin-2, a new ligand of ErbB3/ErbB4-receptor tyrosine kinases. Nature. 1997 May 29;387(6632):512–516. doi: 10.1038/387512a0. [DOI] [PubMed] [Google Scholar]
- 37.Rocco BR, Sweet RA, Lewis DA, Fish KN. GABA-Synthesizing Enzymes in Calbindin and Calretinin Neurons in Monkey Prefrontal Cortex. Cereb Cortex. 2016 May;26(5):2191–2204. doi: 10.1093/cercor/bhv051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Britto JM, Lukehurst S, Weller R, Fraser C, Qiu Y, Hertzog P, et al. Generation and characterization of neuregulin-2-deficient mice. Molecular and cellular biology. 2004 Sep;24(18):8221–8226. doi: 10.1128/MCB.24.18.8221-8226.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weinstein JJ, Chohan MO, Slifstein M, Kegeles LS, Moore H, Abi-Dargham A. Pathway-Specific Dopamine Abnormalities in Schizophrenia. Biological psychiatry. 2016 Mar 31; doi: 10.1016/j.biopsych.2016.03.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen JP, Ruan D, Paredes W, Gardner EL. Effects of acute and chronic clozapine on dopaminergic function in medial prefrontal cortex of awake, freely moving rats. Brain research. 1992 Feb 7;571(2):235–241. doi: 10.1016/0006-8993(92)90660-2. [DOI] [PubMed] [Google Scholar]
- 41.Geyer MA, Ellenbroek B. Animal behavior models of the mechanisms underlying antipsychotic atypicality. Progress in neuro-psychopharmacology & biological psychiatry. 2003 Oct;27(7):1071–1079. doi: 10.1016/j.pnpbp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Slifstein M, van de Giessen E, Van Snellenberg J, Thompson JL, Narendran R, Gil R, et al. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA psychiatry. 2015 Apr;72(4):316–324. doi: 10.1001/jamapsychiatry.2014.2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aalto S, Bruck A, Laine M, Nagren K, Rinne JO. Frontal and temporal dopamine release during working memory and attention tasks in healthy humans: a positron emission tomography study using the high-affinity dopamine D2 receptor ligand [11C]FLB 457. J Neurosci. 2005 Mar 9;25(10):2471–2477. doi: 10.1523/JNEUROSCI.2097-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winterer G, Weinberger DR. Genes, dopamine and cortical signal-to-noise ratio in schizophrenia. Trends in neurosciences. 2004 Nov;27(11):683–690. doi: 10.1016/j.tins.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000 Sep;23(3):223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 46.Lieberman JA, Kane JM, Sarantakos S, Gadaleta D, Woerner M, Alvir J, et al. Prediction of relapse in schizophrenia. Archives of general psychiatry. 1987 Jul;44(7):597–603. doi: 10.1001/archpsyc.1987.01800190013002. [DOI] [PubMed] [Google Scholar]
- 47.Koreen AR, Lieberman JA, Alvir J, Chakos M. The behavioral effect of m-chlorophenylpiperazine (mCPP) and methylphenidate in first-episode schizophrenia and normal controls. Neuropsychopharmacology. 1997 Jan;16(1):61–68. doi: 10.1016/S0893-133X(96)00160-1. [DOI] [PubMed] [Google Scholar]
- 48.Yui K, Goto K, Ikemoto S, Ishiguro T, Angrist B, Duncan GE, et al. Neurobiological basis of relapse prediction in stimulant-induced psychosis and schizophrenia: the role of sensitization. Molecular psychiatry. 1999 Nov;4(6):512–523. doi: 10.1038/sj.mp.4000575. [DOI] [PubMed] [Google Scholar]
- 49.Gowrishankar R, Hahn MK, Blakely RD. Good riddance to dopamine: roles for the dopamine transporter in synaptic function and dopamine-associated brain disorders. Neurochemistry international. 2014 Jul;73:42–48. doi: 10.1016/j.neuint.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 50.Braff DL. Prepulse inhibition of the startle reflex: a window on the brain in schizophrenia. Curr Top Behav Neurosci. 2010;4:349–371. doi: 10.1007/7854_2010_61. [DOI] [PubMed] [Google Scholar]
- 51.Kohl S, Heekeren K, Klosterkotter J, Kuhn J. Prepulse inhibition in psychiatric disorders--apart from schizophrenia. Journal of psychiatric research. 2013 Apr;47(4):445–452. doi: 10.1016/j.jpsychires.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 52.Bourin M, Petit-Demouliere B, Dhonnchadha BN, Hascoet M. Animal models of anxiety in mice. Fundamental & clinical pharmacology. 2007 Dec;21(6):567–574. doi: 10.1111/j.1472-8206.2007.00526.x. [DOI] [PubMed] [Google Scholar]
- 53.Chen YJ, Zhang M, Yin DM, Wen L, Ting A, Wang P, et al. ErbB4 in parvalbumin-positive interneurons is critical for neuregulin 1 regulation of long-term potentiation. Proceedings of the National Academy of Sciences of the United States of America. 2010 Nov 24; doi: 10.1073/pnas.1010669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu B, Wang C, Liu J, Johnson KM, Gallagher JP. Adaptation to chronic PCP results in hyperfunctional NMDA and hypofunctional GABA(A) synaptic receptors. Neuroscience. 2002;113(1):1–10. doi: 10.1016/s0306-4522(02)00163-x. [DOI] [PubMed] [Google Scholar]
- 55.Ladepeche L, Dupuis JP, Groc L. Surface trafficking of NMDA receptors: gathering from a partner to another. Seminars in cell & developmental biology. 2014 Mar;27:3–13. doi: 10.1016/j.semcdb.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 56.Hashimoto R, Straub RE, Weickert CS, Hyde TM, Kleinman JE, Weinberger DR. Expression analysis of neuregulin-1 in the dorsolateral prefrontal cortex in schizophrenia. Molecular psychiatry. 2004 Mar;9(3):299–307. doi: 10.1038/sj.mp.4001434. [DOI] [PubMed] [Google Scholar]
- 57.Kao WT, Wang Y, Kleinman JE, Lipska BK, Hyde TM, Weinberger DR, et al. Common genetic variation in Neuregulin 3 (NRG3) influences risk for schizophrenia and impacts NRG3 expression in human brain. Proceedings of the National Academy of Sciences of the United States of America. 2010 Aug 31;107(35):15619–15624. doi: 10.1073/pnas.1005410107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. The involvement of ErbB4 with schizophrenia: association and expression studies. Am J Med Genet B Neuropsychiatr Genet. 2006 Mar 5;141B(2):142–148. doi: 10.1002/ajmg.b.30275. [DOI] [PubMed] [Google Scholar]
- 59.Joshi D, Fullerton JM, Weickert CS. Elevated ErbB4 mRNA is related to interneuron deficit in prefrontal cortex in schizophrenia. Journal of psychiatric research. 2014 Jun;53:125–132. doi: 10.1016/j.jpsychires.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 60.Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nature medicine. 2006 Jul;12(7):824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- 61.Hall J, Whalley HC, Job DE, Baig BJ, McIntosh AM, Evans KL, et al. A neuregulin 1 variant associated with abnormal cortical function and psychotic symptoms. Nature neuroscience. 2006 Dec;9(12):1477–1478. doi: 10.1038/nn1795. [DOI] [PubMed] [Google Scholar]
- 62.Zhao Z, Xu J, Chen J, Kim S, Reimers M, Bacanu SA, et al. Transcriptome sequencing and genome-wide association analyses reveal lysosomal function and actin cytoskeleton remodeling in schizophrenia and bipolar disorder. Molecular psychiatry. 2015 May;20(5):563–572. doi: 10.1038/mp.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lodge DJ, Grace AA. Hippocampal dysfunction and disruption of dopamine system regulation in an animal model of schizophrenia. Neurotoxicity research. 2008 Oct;14(2–3):97–104. doi: 10.1007/BF03033801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tseng KY, Chambers RA, Lipska BK. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behavioural brain research. 2009 Dec 7;204(2):295–305. doi: 10.1016/j.bbr.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rujescu D, Bender A, Keck M, Hartmann AM, Ohl F, Raeder H, et al. A pharmacological model for psychosis based on N-methyl-D-aspartate receptor hypofunction: molecular, cellular, functional and behavioral abnormalities. Biological psychiatry. 2006 Apr 15;59(8):721–729. doi: 10.1016/j.biopsych.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 66.Dzirasa K, Ramsey AJ, Takahashi DY, Stapleton J, Potes JM, Williams JK, et al. Hyperdopaminergia and NMDA receptor hypofunction disrupt neural phase signaling. J Neurosci. 2009 Jun 24;29(25):8215–8224. doi: 10.1523/JNEUROSCI.1773-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gainetdinov RR, Mohn AR, Bohn LM, Caron MG. Glutamatergic modulation of hyperactivity in mice lacking the dopamine transporter. Proceedings of the National Academy of Sciences of the United States of America. 2001 Sep 25;98(20):11047–11054. doi: 10.1073/pnas.191353298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lipina TV, Niwa M, Jaaro-Peled H, Fletcher PJ, Seeman P, Sawa A, et al. Enhanced dopamine function in DISC1-L100P mutant mice: implications for schizophrenia. Genes, brain, and behavior. 2010 Oct;9(7):777–789. doi: 10.1111/j.1601-183X.2010.00615.x. [DOI] [PubMed] [Google Scholar]
- 69.Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through neuregulin-1-ErbB4 and DISC1. Trends in neurosciences. 2009 Sep;32(9):485–495. doi: 10.1016/j.tins.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosen AM, Spellman T, Gordon JA. Electrophysiological endophenotypes in rodent models of schizophrenia and psychosis. Biological psychiatry. 2015 Jun 15;77(12):1041–1049. doi: 10.1016/j.biopsych.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Swartz MS, Stroup TS, McEvoy JP, Davis SM, Rosenheck RA, Keefe RS, et al. What CATIE found: results from the schizophrenia trial. Psychiatric services. 2008 May;59(5):500–506. doi: 10.1176/ps.2008.59.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kapur S, Arenovich T, Agid O, Zipursky R, Lindborg S, Jones B. Evidence for onset of antipsychotic effects within the first 24 hours of treatment. The American journal of psychiatry. 2005 May;162(5):939–946. doi: 10.1176/appi.ajp.162.5.939. [DOI] [PubMed] [Google Scholar]
- 73.Xu TX, Yao WD. D1 and D2 dopamine receptors in separate circuits cooperate to drive associative long-term potentiation in the prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2010 Sep 14;107(37):16366–16371. doi: 10.1073/pnas.1004108107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Furth KE, Mastwal S, Wang KH, Buonanno A, Vullhorst D. Dopamine, cognitive function, and gamma oscillations: role of D4 receptors. Frontiers in cellular neuroscience. 2013;7:102. doi: 10.3389/fncel.2013.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rotaru DC, Yoshino H, Lewis DA, Ermentrout GB, Gonzalez-Burgos G. Glutamate receptor subtypes mediating synaptic activation of prefrontal cortex neurons: relevance for schizophrenia. J Neurosci. 2011 Jan 5;31(1):142–156. doi: 10.1523/JNEUROSCI.1970-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997 Apr 15;17(8):2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cornish JL, Nakamura M, Kalivas PW. Dopamine-independent locomotion following blockade of N-methyl-D-aspartate receptors in the ventral tegmental area. J Pharmacol Exp Ther. 2001 Jul;298(1):226–233. [PubMed] [Google Scholar]
- 78.Javitt DC, Schoepp D, Kalivas PW, Volkow ND, Zarate C, Merchant K, et al. Translating glutamate: from pathophysiology to treatment. Sci Transl Med. 2011 Sep 28;3(102):102mr102. doi: 10.1126/scitranslmed.3002804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miller OH, Yang L, Wang CC, Hargroder EA, Zhang Y, Delpire E, et al. GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. eLife. 2014;3:e03581. doi: 10.7554/eLife.03581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.