Abstract
The trans-Golgi network (TGN) is a major secretory pathway sorting station that directs newly synthesized proteins to different subcellular destinations. The TGN also receives extracellular materials and recycled molecules from endocytic compartments. In this review, we summarize recent progress on understanding TGN structure and the dynamics of trafficking to and from this compartment. Protein sorting into different transport vesicles requires specific interactions between sorting motifs on the cargo molecules and vesicle coat components that recognize these motifs. Current understanding of the various targeting signals and vesicle coat components that are involved in TGN sorting are discussed, as well as the molecules that participate in retrieval to this compartment in both yeast and mammalian cells. Besides proteins, lipids and lipid-modifying enzymes also participate actively in the formation of secretory vesicles. The possible mechanisms of action of these lipid hydrolases and lipid kinases are discussed. Finally, we summarize the fundamentally different apical and basolateral cell surface delivery mechanisms and the current facts and hypotheses on protein sorting from the TGN into the regulated secretory pathway in neuroendocrine cells.
Keywords: Trans-Golgi network, coat protein, sorting signal, lipid, lipid kinase, apical targeting, basolateral sorting, regulated secretion
Introduction
In our technology-laden era we are often surprised to realize that our ‘novel’ inventions are actually recapitulations of processes invented by biological systems eons ago. For example, in a recent copy of their in-flight magazine, Delta Airlines claimed to be the first company to implement the ‘hub-and-spoke’ system to sort the more than 500 million airline passengers that fly in the US alone. Passengers from around the world are collected into hub airports like that in Atlanta or Chicago and then sorted to their various destinations. The principal advantage claimed by this hub system is its ability to efficiently collect passengers in a central location and sort them to their final destinations in a timely, efficient, and energy-saving manner. Little did the worlds' airlines realize that they had stumbled on a hub system used for millions of years by the eukaryotic cell secretory pathway, the trans-Golgi network (TGN), to collect, package, and sort numerous molecules to their final cellular destinations. The seminal treatise published just 15 years ago by Griffiths and Simons [1] explained the TGN as a ‘specialized organelle on the trans side of Golgi stack that is responsible for the routing of proteins to lysosomes, secretory vesicles and the plasma membrane from the Golgi complex’. During the past several years, this simple concept has been expanded to include identification of the effectors – including proteins, lipids, various small molecules, and components of the cytoskeleton – that control the sorting capacity of the TGN. In this review, we summarize recent findings regarding new concepts of TGN structure and molecular dynamics and how they have affected our view of the TGN and the regulation of membrane traffic.
Structure and dynamics of the TGN
Newly synthesized proteins of the secretory pathway, which are not retained in the endoplasmic reticulum (ER), are targeted to the Golgi complex. The Golgi complex of most eukaryotic cells is a series of membrane-bound flattened cisternae arranged in a stack of usually four to six cisternae with numerous vesicles associated with the rims of the stack [2]. The cisternae are generally classified into cis, medial, or trans cisterna. Secretory pathway proteins transported from the ER enter the stack at the cis face, move through the various cisternae and then exit at the trans face. The stacked Golgi cisternae have two tubular-reticular structures associated with them. One is associated with the cis face and is varyingly know as the cis-Golgi network (CGN), ER-Golgi intermediate compartment (ERGIC), or the intermediate compartment (IC) [3-6]. This compartment is thought to be the entry site for cargo transported from the ER and may arise directly from the fusion of ER-derived transport intermediates known as vesicular-tubular clusters (VTCs) [7, 8]. The other tubular-reticular structure is known as the trans-Golgi network (TGN) and is thought to be the organelle responsible for sorting secretory pathway proteins to their final destinations [1].
The presence of a membranous compartment associated with the trans face of the Golgi stack was first suggested through the work of Novikoff and colleagues [9]. These studies demonstrated that membrane elements close to the Golgi apparatus were, like lysosomes, cytidine monophosphatase positive, and various data suggested that these structures were specialized regions of the smooth ER [10]. This led to the use of the acronym GERL to describe a structure ‘intimately related to the Golgi saccule, that is part of the ER, and that forms Lysosomes’ [9]. Subsequently, several lines of evidence argued strongly against the continuity of this compartment with the ER [reviewed in ref. 11], which led to a new acronym, the TGN, to describe this compartment [1].
The vast majority of structural and morphological information about the TGN has come from thin- and thick-section electron microscopy (EM) studies on chemically fixed cells. The first three-dimensional view of the TGN came from studies on Sertoli cells by Rambourg and colleagues [12] that revealed flattened saccules that were ‘peeling off’ from the trans face of the Golgi stack. These saccules were attached to an anastomotic network of membrane tubules, some of which were fragmented into smaller tubules, tubular networks, and vesicles [12].
Further analysis of the TGN structures from 14 different mammalian cell types has shown that TGNs vary widely in both size and configuration [comprehensively reviewed in ref. 13]. In general, cells that do not form large secretory granules but have extensive lysosomal systems also have extensive, multilayered TGN structures. Cells that form small- to medium-size secretory granules have small TGN structures that appear to be residual fragments associated with the trans cisternal elements of the Golgi stack. Cells producing very large secretory granules, on the other hand, do not appear to have TGN structures at all. These observations suggest that TGNs are not stable or permanent structures but are constantly undergoing renewal; moreover, the observed morphology and size of the TGN in a particular cell type appears to depend on the post-TGN intermediates that are formed in those cells.
More recent views of the three-dimensional structure of the entire Golgi complex have come from the elegant studies by Ladinsky and colleagues using dual-axis, high-voltage EM tomography of cryofixed, freeze-substituted normal rat kidney cells [14]. These studies failed to show the presence of any interconnected reticulum of tubules at the trans face of the Golgi stack. The Golgi structure elucidated by this technique is comprised of seven stacked, fenestrated cisternae with polymorphic membranous elements associated with the cis Golgi cisterna (suggested to correspond to the ERGIC). Interestingly, while the structure lacks a classical TGN-like compartment (in terms of morphology), all three of the trans-most cisternae demonstrate tubules that project into the trans space of the Golgi complex, many of which contain coat-like structures at the tubule tips. Each of these cisternae appears to produce only one type of coated vesicle structure, with clathrin-coated buds present only on the seventh cisterna. These data have raised the possibility that these three cisternae together could constitute what is commonly viewed as the TGN, and that the TGN is not actually an independent compartment; the tubular reticular structures previously observed could have resulted from changes in cisternal architecture due to events during the relatively slower fixation and staining procedures involved in more traditional EM. However, the data presented by Ladinsky and colleagues show the structure of only approximately 5% of the entire Golgi complex, and looked at only one cell type. Therefore, the possibility that TGN structures of the classical tubular-reticular morphology exist elsewhere in these cell types or in different cells cannot be discounted. Whether or not the TGN turns out to be an independent compartment has important consequences for how we understand sorting mechanisms for cargo moving through the secretory pathway. The model from Ladinsky et al. [14] suggests that sorting would occur in sequence with specific cargo budding from distinct cisternae (each of the three trans-most), whereas the traditional view of the TGN would predict parallel sorting with cargo budding into different carrier vesicles from the same ‘cisterna’ (fig. 1). It will be intriguing to see structural information on the Golgi complexes from the various cell types studied by Rambourg and colleagues, using the technique described by Ladinsky and colleagues. Interestingly Ladinsky and colleagues show an intimate association of ER elements with the trans cisterna of the Golgi, perhaps suggesting that the dismissal of the original GERL hypothesis may have been premature.
Figure 1.
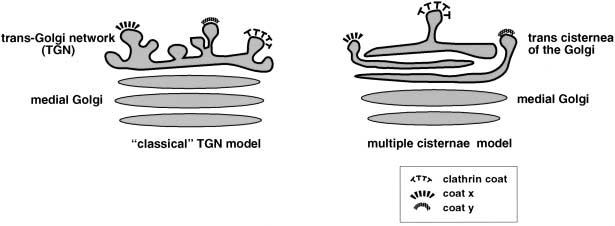
TGN structure. The left panel represents the classical view of the TGN as a separate tubular network at the trans side of the Golgi compartment, with clathrin-coated vesicles or other unknown coats budding from certain areas of the TGN. This model suggests that all proteins are sorted in parallel, in the same TGN compartment. The right panel represents a different view of the TGN: as the trans-most cisternae of the Golgi; each cisterna projects tubules and buds. This model suggests that protein sorting could occur in a sequential manner and in different cisternae of the TGN.
Much of the data that support the view of the TGN as a distinct compartment from the main Golgi stack have arisen from studies with the fungal metabolite brefeldin A (BFA). Incubation of cells with BFA was found to inhibit ER-to-Golgi transport, resulting in the rapid redistribution of Golgi markers into the ER [15-17]. This redistribution was correlated with the tubulation of the Golgi stack, with these tubules moving along microtubules and subsequently fusing with the ER [18]. The TGN was shown to undergo similar tubulation in the presence of BFA but these tubules fuse with the endosomal system, and markers of the TGN such as TGN38 are redistributed to a ‘collapsed’ area around the microtubule organizing center (MTOC) [19-21]. Thus, the different responses of proteins in the Golgi stack and TGN to BFA have been taken to demonstrate the functional and physical differences between these two compartments. However, all the markers of the TGN studied in the presence of BFA [such as TGN38, furin, and mannose-6-phosphate receptor (MPR)] are actively cycling proteins that exit the TGN and can reach the cell surface, after which they enter the endosomal system. All the Golgi stack markers studied in the presence of BFA (such as the numerous glycosylation enzymes), on the other hand, do not commonly exit the trans face of the Golgi and are more stable residents of the Golgi complex. In light of the findings of Ladinsky et al. it is conceivable that TGN marker proteins are actually present in the trans cisternae of the Golgi stack, and that BFA allowing their unregulated exit. Such events would allow all these TGN markers to leave the Golgi cisternae, and the subsequent accumulation around the MTOC could be due to an inhibition of endosome-to-TGN recycling pathways. One method to clear up some of the confusion about the true nature of the TGN would be to combine immunolocalization techniques with the structural determination techniques of Ladinsky et al. It would be of great interest to see which of the three trans cisternae hold the greatest concentration of TGN markers, such as TGN38 and furin, within the cryo-EM, three-dimensional structure of the Golgi.
Whatever the true nature of the TGN in mammalian cells, various elements of the cytoskeleton are known to play pivotal roles in the structure and function of the whole Golgi complex. The proximity of the Golgi complex to the MTOC demonstrates the close relationship of this organelle with the microtubule network. Microtubules are known to contribute to maintaining Golgi morphology, transport to and from this organelle, and equal partitioning of the Golgi into daughter cells during mitosis [reviewed in ref. 22]. There is also an emerging body of evidence showing the involvement of actin cytoskeleton components in Golgi morphology and function. One actin cytoskeleton protein, spectrin, is associated with many organelles; spectrin forms extensive multifunctional scaffolds by binding directly or indirectly to numerous membrane proteins, cytosolic proteins, phospholipids, and other cytoskeletal proteins [23]. One particular form of spectrin, β3 spectrin, has been shown to associate with the Golgi complex [24]. This Golgi-associated spectrin is thought to be involved in maintaining the structure of the Golgi complex and orchestrating protein traffic in the secretory pathway [comprehensively reviewed in ref. 25]. The association of spectrin with the Golgi complex is regulated by the small GTP-binding protein ARF, probably due in part to ARF-stimulated synthesis of phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2], a target of direct interaction for spectrin [26, 27]. Currently there are two compatible hypotheses for the involvement of spectrin in Golgi traffic. The first model, known as the mesh hypothesis, states that the Golgi-associated spectrin network forms a template for the Golgi structure and that the ability of the mesh to undergo rapid (ARF-dependent) remodeling allows pre- and post-Golgi transport intermediates to be released through the spectrin mesh [28]. The second hypothesis states that the Golgi spectrin network, including various adapter proteins, forms the basis for the physical capture of integral membrane proteins by direct interaction with their cytosolic domains, and the subsequent inclusion of such cargo into forming transport intermediates [29]. It will be interesting to assess the relative importance of these two models for the involvement of this intriguing Golgi scaffold in the morphology of the TGN and its effect on protein sorting.
Lysosomal sorting, coat components, and sorting signals
Proteins destined for endosomes or lysosomes are generally sorted away from the trafficking pathways followed by secreted proteins at the TGN and are instead targeted to the endocytic pathway compartments. Classical examples of proteins that follow this route are newly synthesized lysosomal hydrolases. These proteins are cotranslationally translocated into the ER and undergo glycosylation similar to normal secretory pathway proteins. However, certain oligosaccharide side chains on these lysosomal hydrolases are further modified in the Golgi apparatus by the addition of 6-phosphomannosyl residues. The post-translational addition of these mannose 6-phosphate (M6P) groups allows the newly synthesized lysosomal hydrolases to be recognized by transmembrane receptors, the MPRs. The MPR-lysosomal hydrolase complexes are then transported from the TGN to endosomal compartments. Lysosomal enzymes dissociate from the MPRs in endosomes due to their acidic environment. From these endosomal compartments, lysosomal hydrolases are subsequently delivered to the lysosomes, while the receptors are returned to the TGN for further rounds of transport [30]. The activity of MPR to cycle between the TGN and endosomal compartments is not unique to MPR, and other transmembrane proteins such as TGN38 and furin also follow similar pathways [31].
To achieve correct sorting of different TGN proteins, vesicle coat proteins can recognize different types of sorting signals contained in the cytoplasmic domain of cargo molecules and sort them into corresponding transport vesicles. Clathrin- and adaptor-coated vesicles have been characterized in the TGN and endosome system [32]. Clathrin/AP-1 coated vesicles are involved in vesicle formation on the TGN and are thought to mediate the sorting of lysosomal hydrolases (fig. 2). Clathrin/AP-2-coated vesicles form at the plasma membrane and mediate clathrin-dependent endocytosis. Two additional adaptor complexes have been identified: AP-3 [33, 34] and, most recently, AP-4 [33, 35, 36]. Whether AP-3 and AP-4 are also associated with clathrin is still unsettled. AP-3 is suggested to mediate the direct transport of some lysosomal proteins to the lysosomes from the TGN, while the function of AP-4 is not yet clear.
Figure 2.
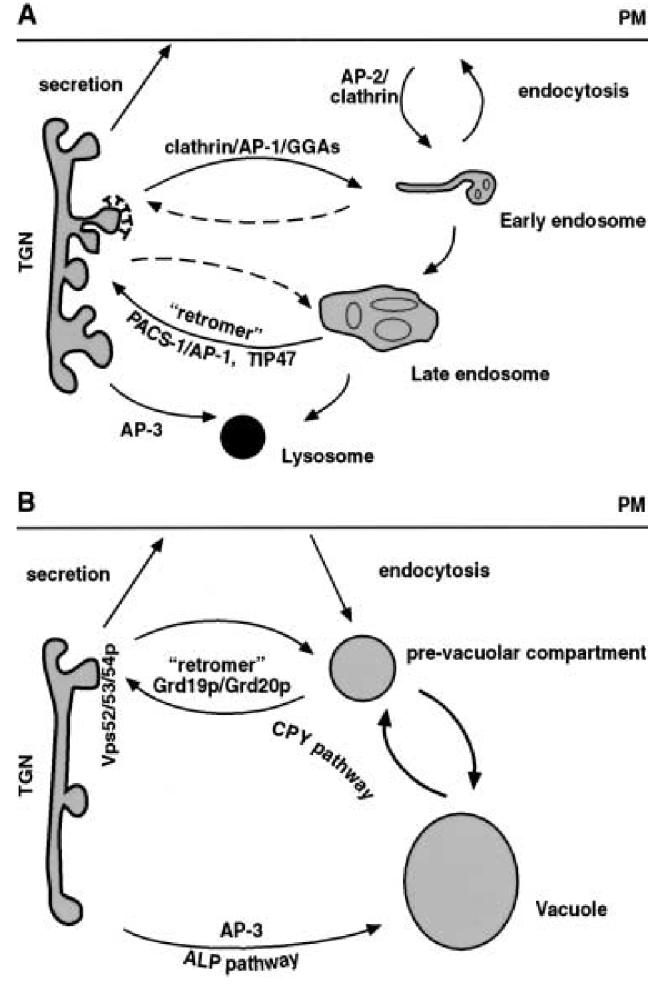
Protein sorting in the TGN/endosomal system. A In mammalian cells. From the TGN compartment, secretory proteins are transported to the plasma membrane and extracellular space via the constitutive and regulated secretory pathway. Proteins cycling in the TGN/endosomal system are transported from the TGN to endosomes with clathrin/AP-1-coated vesicles. From the endosomes, the retromer complex, PACS-1/AP1, and TIP47 are involved in retrograde transport. AP-3 vesicles represent a lysosomal delivery pathway that occurs for some proteins that are directly transported to the lysosomes. B In yeast cells. From the yeast TGN (late Golgi) compartment, secretory proteins are also transported directly to the cell surface. Similar to the trafficking pathway of lysosomal enzymes and MPR in mammalian cells, yeast CPY and Vps10p (the receptor of CPY) follow the ‘CPY pathway’ to the yeast vacuole. The retrieval of Vps10p to the TGN requires the yeast retromer complex. Grd19p and Grd20p are the two other proteins also involved in the retrieval pathway. In yeast, alkaline phosphatase (ALP) is transported in the ‘ALP pathway’ from the TGN directly to lysosomes using AP-3, bypassing the prevacuolar compartment.
Despite the different trafficking pathways in which adaptor complexes are involved, they are all heterotetramers that are composed of two large, one medium, and one small adaptin subunit [37]. AP-1 (composed of γ, β1, μ1, and σ1 adaptins) has been shown to mediate the transport of many proteins, including the MPRs, furin, some viral membrane glycoproteins such as the Varicella zoster virus glycoprotein E (VZV-gE), from the TGN to endosomes [38]. AP-1 is recruited onto the TGN membrane via the small GTP-binding protein ARF1. The interaction of AP-1 with TGN membranes is blocked by BFA and enhanced by GTPγS, a nonhydrolyzable analogue of GTP [39, 40]. The GTP-dependent ARF1 binding on the TGN membrane creates high-affinity binding sites for AP-1 and initiates clathrin/AP-1 coat assembly [41, 42]. The cytoplasmic domain of the major cargo molecule MPR is not thought to be involved in the initial ARF1-mediated AP-1 recruitment on the TGN membrane. However, the MPR cytoplasmic domain seems to affect the stability of ARF1-GTP on the membrane, thereby also affecting the formation of the clathrin-coated vesicles [42]. AP-1 has also been observed to be present on endosomes [43]. Although the functional role of endosomal-associated AP-1 is not yet known, the clathrin/AP-1 coat could be involved in endosomal recycling, a suggestion that is bolstered by the observation that clathrin is associated with tubular endosomes [44]. Recent studies on transgenic mice in which the μ1A gene locus was disrupted showed that the remaining AP-1 adaptins fail to bind to membrane (except in epithelial cells where the epithelial-specific μ1B is intact). In μ1 A-deficient cells, localization of MPRs is shifted to an endosomal compartment, while lysosomal enzyme delivery is normal [45]. These data suggest that AP-1 may have important functions in the retrograde transport of proteins from endosomes to the TGN [45].
In the search for answers to the question of the role of AP-1 in TGN sorting pathways, novel proteins have been discovered that are associated with AP-1 or are homologous to subunits of AP-1. For example γ-synergin has been shown to directly interact with γ adaptin by the yeast two-hybrid system [46]. Immunofluorescence analysis shows that γ synergin displays an extended perinuclear localization pattern similar to γ adaptin; γ synergin contains EH (Eps15 homology) domains, a domain involved in protein-protein interaction in intracellular trafficking [47, 48]. However, the role of γ synergin in clathrin/AP-1-coated vesicle assembly remains to be discovered. In the search for homologues to γ adaptin and the effectors of ARF1, five groups recently found a new family of proteins termed either Vear or GGA proteins (Golgi-localized, gamma ear-containing, ARF-binding proteins) [49-53]. The GGA protein was functionally identified in a yeast two-hybrid screen through interaction with an activated form of ARF3. GGA proteins contain an N-terminal VHS domain [a domain found in Vps27, Hrs (hepatocyte growth factor receptor-regulated tyrosine kinase) and STAM (signal-transducing adaptor molecules)], a domain found in a number of proteins implicated in membrane traffic, and a C-terminal domain with 70% identity to the C-terminal ‘ear’ domain of γ adaptin. GGA proteins are localized to the TGN and overexpression of GGA altered the localization of TGN38 and MPR [51]. The membrane-binding domain on GGA (termed GAT) interacts with ARF, and overexpression of the GAT region causes dissociation of a number of coat proteins including AP-1, AP-3, AP-4, and COP-I [52]. In yeast, two homologues of GGA have been found and the deletion of both genes results in the missorting of the vacuolar enzyme carboxypeptidase Y (CPY) [52, 53]. Taken together, these studies have demonstrated that in addition to AP-1 adaptor complexes, other proteins appear to be involved in the regulation of sorting from the TGN.
The AP-3 adaptor complex is another coat complex involved in transport to lysosomes (fig. 2). Studies in yeast reveal that yeast cells have two distinguished targeting pathways for transporting proteins to the degradative compartment (the vacuole in yeast). These two pathways are defined by the different trafficking itineraries of the proteins CPY and alkaline phosphatase (ALP) [54]. The delivery of CPY in yeast uses the classical Vps pathway (TGN-endosome-vacuole), similar to the transport of lysosomal enzymes in mammalian cells. However, the transport of ALP from the TGN to the yeast vacuole bypasses the endosomal compartments; instead, ALP is directly sorted from the TGN to the vacuole. The yeast homologue of the AP-3 complex has been shown to be involved in ALP transport to the vacuole. AP-3 is composed of δ, β3, μ3, and σ3 adaptins. The deletion of yeast AP-3 proteins prevents the appropriate localization of ALP and Vam3p (a vacuolar t-SNARE), two cargo molecules that have been so far found to follow the ALP pathway, but has no effect on the vacuolar delivery of CPY [55, 56]. Similar pathways might exist in higher eukaryotic cells. A number of naturally occurring mutations in AP-3 genes have been found in Drosophila, mouse, and human cells. They all show common defects in the biogenesis of lyso-some-related organelles such as melanosomes and platelet dense granules [54, 57]. In biochemical studies, AP-3 was shown to be involved in the lysosomal transport of several lysosomal integral membrane proteins such as LAMP1 and LIMP-II [57]. At the molecular level, AP-3 was shown to interact with the cytoplasmic tail of the LIMP-II and the melanosome-associated protein tyrosinase, and the binding is dependent on a dileucine sequence on both proteins [58]. Taken together, these data suggest that the mammalian AP-3 complex mediates transport from the TGN to lysosomes (or lysosome-like organelles) and that this pathway is different from the one followed by MPR.
The proper sorting of cargo molecules requires not only the coat proteins but also specific sorting signals on the cytoplasmic domain of these cargo molecules. Adaptor complexes are recruited to the cytoplasmic tails of itinerant membrane proteins through conserved amino acid sequences that constitute specific sorting motifs. Tyrosine-based motifs, a family of sorting signals based on the amino acid sequence YXXØ (X is any amino acid and Ø is an amino acid with a bulky hydrophobic side chain), is one of the best-characterized sorting motifs. It has been observed that these motifs direct endosomal and lysosomal targeting, internalization from the plasma membrane, and sorting at the TGN [59]. As mentioned the YXXØ motif has been shown to interact directly with the μ subunits of all known adaptor complexes by yeast two-hybrid analyses and by biochemical interactions [60-63]. However, given that each adaptor resides in distinct subcellular locations and that cargo-adaptor interactions are necessary for proper trafficking between various compartments, additional levels of specificity must exist that govern interactions between specific μ subunits and the various tyrosine-based motifs. Recent crystallographic studies have illustrated a possible mechanism by which the sequence of the tyrosine-based motif could mediate the sorting itineraries by favoring interaction with specific adaptors. Owen et al. [64] cocrystallized the C-terminal two-thirds of μ2 with peptides containing the tyrosine-based motifs of TGN38 (DYQRLN) and epidermal growth factor (EGF) receptor (FYRALM). Their studies have reiterated that tyrosine at postition 1 and a bulky hydrophobic residue at Y+3 are necessary for interaction with adaptors. Both these residues are buried in a hydrophobic binding pocket, while the hydroxyl group of the tyrosine participates in a series of hydrogen bonds within μ2 residues. Interestingly, the affinity of this interaction is modulated by the amino acids at positions Y+1 and Y+2 within the tyrosine-based motif. The arginine at position Y+2 in TGN38 donates a hydrogen bond to μ2 and exposes its guanadinium group to the solvent, whereas the arginine at Y+1 in the EGF receptor is highly unordered and makes no significant contacts with μ2. These preferences could suggest a mechanism by which the large group of tyrosine-based sorting motifs can be divided into specific signals that follow separate sorting itineraries. The μ chains of the different adaptor complexes could have varying affinities for amino acids at the Y+1 and Y+2 position of tyrosine motifs depending upon the nonconserved residues present within the various μ chains. This would allow the recruitment of the different adaptor complexes to different cytoplasmic tails. Indeed, μ1, μ2, and μ3a all have been shown to bind different subsets of YXXØ, favoring nonpolar, arginine-rich, and acidic amino acids at the X positions, respectively [60, 63].
Dileucine-based motifs form another class of sorting motifs present in the TGN/endosomal system. These motifs have been shown to be recognized by AP-1, AP-2, and AP-3 probably through the interaction with β adaptins [65]. A significant degree of overlap exists between the sorting itineraries directed by the presence of tyrosineand dileucine-based motifs: dileucine motifs also mediate internalization from the plasma membrane (PM) and sorting to endosomes [32]. One of the two leucines in the dileucine-based motif can be replaced by isoleucine, valine, or methionine and many dileucine-based motifs require surrounding acidic residues and phosphorylatable residues for their function [38]. For example, the CD3γ receptor uses SDXXXLI as an internalization signal, and both the aspartic acid and serine phosphorylation are important for efficient internalization [66, 67]. Acidic clusters, which are stretches of several acidic amino acids, can act in concert with dileucine- and tyrosine-based motifs to mediate protein trafficking. First identified in the cytoplasmic domain of furin and later found to be common to many other membrane proteins [68], acidic clusters often contain consensus casein kinase 2 (CK2) sites that serve as TGN retrieval signals [69]. Although furin contains both dileucine- and tyrosine-based motifs, efficient TGN localization of furin cannot occur unless the serines of the acidic cluster are phosphorylated [70, 71]. Acidic clusters contained in a number of other membrane proteins have also been shown to be important for their TGN localization and sorting, including MPR [72] and MHC-II [73]. Direct interaction between acidic clusters and adaptor complexes has not yet been reported. However a ‘connnector’ protein called PACS-1 has been shown to link phosphorylated acidic cluster sorting motifs to AP-1 adaptor complexes [74, 74a] (and see below).
Retrograde transport to the TGN
Lysosomal enzyme-MPR complexes dissociate in the low-pH environment of the late endosomes, allowing the lysosomal hydrolases to be transferred to the lysosomal compartment while the MPRs are recycled to the TGN for further rounds of transport. Other proteins that cycle through the TGN/endosomal system, such as furin and TGN38, are also thought to follow similar transport steps from endosomes to the TGN. Very little is known about the precise transport intermediates and proteins regulating these retrograde transport activities to the TGN. MPRs were shown to return to the TGN from the late endosomal compartment, and this transport is dependent on the small GTP-binding protein rab9 [75, 76]. Furin has also been reported to follow the same return pathway, whereas TGN38 seems to return from the early endosomal compartments directly to the TGN [31].
Recently, proteins that appear to be involved in retrograde transport to the TGN have begun to be identified in mammalian cells, including the AP-1 adaptor complex and two novel proteins, TIP47 and PACS-1. Studies on cell lines derived from transgenic mouse embryos with a disrupted μ1A gene have provided good evidence for the role of AP-1 in retrograde transport to the TGN [45]. However, the molecular mechanisms of AP-1 involvement in such transport have yet to be clearly demonstrated. A new cytosolic protein called TIP47 has been identified and shown to be required for MPR transport from late endosomes to the TGN. Using the cation-dependent MPR (CD-MPR) cytosolic domain as bait in a yeast two-hybrid screen, TIP47 was found to interact with a phenylalanine/tryptophan (FW) motif on CD-MPR [77]. TIP47 was shown to be associated with endosomes and required to mediate CD-MPR recycling from endosomes back to the TGN through a combination of in vitro endosome-TGN transport assays (with TIP47 immunodepletion) and antisense studies. These data suggest that TIP47 is directly required for the efficient sorting of CD-MPR into the transport intermediates destined for the TGN [77]. TIP47 also interacts with the cytosolic domain of the cation-independent MPR (CI-MPR) and is required for the transport of CI-MPR from endosomes back to the TGN [78, 79]. However, the CI-MPR cytosolic domain does not contain an FW motif as found in CD-MPR; the binding of TIP47 to CI-MPR seems to depend on the membrane-proximal region, as well as the secondary structure of the CI-MPR tail [78]. Interestingly, the binding of TIP47 to the CI-MPR cytosolic domain is competed by the binding of AP-2 but not AP-1 to CI-MPR, suggesting a mechanism that prevents TIP47s interference with MPR endocytosis but allows both TIP47 and AP-1 to act in concert [78]. Among all the cytosolic domains of potential protein cargo tested, TIP47 could interact only with CI- and CD-MPR but not with other TGN/endosomal cycling proteins, such as furin, TGN38, or carboxypeptidase D (CPD). These data suggest that the action of TIP47 may be specific to the MPRs [79].
To maintain its steady-state localization in the TGN, furin is actively retrieved from post-TGN compartments back to the TGN in a phosphorylation-dependent manner [69]. Using a yeast two-hybrid screen with a mutant mimicking the phosphorylated form of the furin cytosolic domain, a novel protein termed phosphofurin acidic cluster sorting protein-1 (PACS-1) was discovered. PACS-1 has been shown to interact specifically with the CK2-phosphorylated form of the furin cytosolic domain, and the presence of PACS-1 is also required for the correct TGN localization of furin [74]. While the phosphorylation of the furin acidic cluster motif by CK2 maintains furins TGN localization, conversely, the dephosphorylation of furin's acidic cluster by PP2A ACBα isoform is required for the transport of furin between endosomal compartments [80]. The interaction of PACS-1 specifically with the CK2-phosphorylated form of furin has revealed a new way by which cellular trafficking machinery can distinguish between phosphorylated and nonphosphorylated motifs within cargo molecules such as furin. PACS-1 behaves as a connector that forms a ternary complex with furin and AP-1, allowing the inclusion of phosphorylated furin into clathrin/AP-1 coated vesicles [74a]. Such a possibility correlates well with a role of AP-1 in retrieval pathways to the TGN. Furthermore, PACS-1 also recognizes acidic cluster-sorting motifs contained in other TGN/endosomal proteins, including CI-MPR, PC6B, CPD, and some viral proteins, such as the human cytomegalovirus glycoprotein B (HCMV-gB), and is required for their TGN retrieval [68, 74, 81]. Recently, PACS-1 was also found to interact with HIV-1 Nef protein, which contains a similar acidic cluster motif (EEEE65) and this acidic motif on Nef was shown to be required for the Nef-mediated down-regulation of MHC-I. Coimmunoprecipitation and immunofluorescence studies showed that cellular PACS-1 binds to the viral Nef acidic cluster and directs Nef to the TGN, where it is required for the down-regulation of MHC-I [82]. The function of PACS-1 is just beginning to be understood and the current knowledge on PACS-1 suggests an important role of this protein in TGN retrieval of cellular proteins as well as a role in viral pathogenesis (fig. 2).
In yeast, the sorting of CPY by Vps10p (the CPY receptor) is analogous to the sorting of lysosomal hydrolases by the MPR [83, 84]. The late Golgi compartment in yeast is defined by the presence of the proteolytic enzymes Kex1p, Kex2p, and dipeptidyl aminopeptidase A (DPAP A), and is considered to be the functional equivalent of the mammalian TGN. Similar to the MPR in mammalian cells, Vps10p cycles between the late Golgi compartment and the prevacuolar compartment (PVC; equivalent to late endosomes in mammalian cells). The retrieval of Vps10p from the PVC to the TGN has been shown to depend on the products of several yeast genes, termed the ‘retromer’ complex [85, 86]. The retromer complex is formed from the assembly of two subcomplexes: the Vps35p-Vps29p-Vps26p and the Vps5p-Vps17p complex. Cofractionation of Vps35p with Vps10p has suggested that the role of the Vps35p/29p/26p complex is cargo selection [85]. Furthermore, it has been recently shown that Vps35p could be coimmunoprecipitated with the cytosolic domain of DPAP A, which supports the model of Vps35p involvement in cargo sorting [87]. Vps5p bears self-assembly capacity and physically interacts with Vps17p [86, 88, 89]. The Vps5p/17p complex may be involved in further assembly of the retromer complex. In mammalian cells, Vps5p is homologous to sorting nexins, a family of proteins that have been shown to be involved in the retrieval of various cargo receptors [88-90]. The human orthologues of Vps26, Vps29p, and Vps35p have been recently cloned and this study further confirms that human Vps26p-Vps29p-Vps35p forms a complex, with the Vps35 protein serving as a core domain of assembly [91].
Through the study of A-ALP, a chimera of the cytosolic domain of DPAP A fused to the transmembrane and lumenal domains of ALP, a new gene grd19 was found to be required for the proper localization of A-ALP [92]. A grd19 null mutation causes mislocalization of A-ALP and Kex2p to the vacuole, but the sorting of Vps10p was largely unaffected. Grd19p is localized to the prevacuole compartment and interacts with the DPAP A cytosolic domain. It has therefore been suggested that Grd19p is involved in the retrieval of certain TGN proteins from the prevacuolar compartment. Another new gene, grd20, was also found to be involved in protein sorting in the TGN/endosomal system in yeast [93]. Interestingly, perturbation of grd20p function results in rapid mislocalization of Kex2p to the vacuole, while the trafficking of AALP and Vps10p remains unaffected. Taken together, it seems that while the retromer complex is involved in the retrieval of all cargo that recycle from endosomes back to the TGN, Grd19p is specifically involved in the retrieval of DPAP A and Kex2p, and Grd20p is specifically involved in the retrieval of Kex2p. These data suggest either that different TGN proteins follow different trafficking routes for their return to the TGN or that they require different mediators for their inclusion into the same retrograde transport vesicles.
In a recent screen of yeast mutants with vacuolar sorting defects, a new multimeric complex composes of Vps52p, Vps53p, and Vps54p has been found to be required for retrograde transport to the TGN [94]. These three proteins are associated in a 1:1:1 stoichiometry, and a vps52/53/54 triple mutant exhibits indistinguishable phenotype from single mutants of each of these three genes. The mutant strains all fail to transport a number of late Golgi proteins (including Vps10p and Kex2p) back to the TGN, resulting in the mislocalization of these proteins to the vacuole where they are cleaved by vacuolar proteases. These data suggest that Vps52p-Vps53p-Vps54p is yet another complex involved in TGN retrieval. However, it is unlikely that this complex is involved in either cargo sorting or budding from the PVC because it is localized to the late Golgi membrane. Therefore, it has been speculated that this Vps52p/53p/54p complex is involved in the docking and fusion of the retrograde transport vesicles with the late Golgi compartment (fig. 2).
Involvement of lipids and lipid-modifying enzymes in post-Golgi transport
In the complex process of vesicle formation, proteinaceous coats play important roles in initiating cargo sorting and deformation of the donor membranes. However, proteins are not the only constituents of the transport machinery. Lipids, the major component of membranes, have recently been discovered to play an active role in regulating membrane dynamics. Along the secretory pathway, various forms of phosphoinositides (PIs) and the hydrolyzed products from phosphatidylcholine (PC), such as phosphatidic acid (PA), play important roles in post-Golgi transport. The production and the balance of these lipids are controlled by various lipid kinases and lipid transfer proteins, which tightly regulate membrane transport.
The yeast protein Sec14p is a phosphatidylinositol transfer protein (PITP) that is required for post-Golgi transport in Saccharomyces cerevisiae [95-97]. A similar role for the mammalian PITP has also been demonstrated [98-100]. PITP is an abundant cytosolic protein that contains a single phospholipid-binding site per protein. This binding site can interact separately with PI and PC with high affinity and PITP functions to transfer PI and PC from one compartment to another. As mentioned, the yeast PITP (Sec14p) is essential for protein transport from the Golgi complex. The sec14-1ts mutant shows a Golgi secretory block at the restrictive temperature. Secretory pathway glycoproteins that are blocked in this mutant yeast strain are shown to have acquired their terminal glycosyl modifications, suggesting the block caused in sec14-1ts occurs at a late Golgi compartment. EM studies also show an accumulation of cisternate-like tubules at the restrictive temperature in the sec14-1ts yeast strain [95, 96]. In mammalian systems, PITP has been shown to be involved in many secretory pathway events. These include mediating the Ca2+-stimulated exocytosis of secretory granules in semipermeabilized neuroendocrine cells [101], the budding of the secretory vesicles and immature secretory granules (ISGs) from the TGN in neuroendocrine cells [102], and the formation of post-Golgi, polymeric IgA receptor (pIgR)-containing vesicles [103]. Recently, a PITP mutant that is incapable of PI transfer activity but is still capable of PC binding and transfer was shown to be able to rescue the secretory defect in sec14-1ts yeast [104]. This study suggests, then, that the PC binding and transfer activity, rather than that of PI, may be more directly involved in the control of vesicular trafficking. The exact mechanism of how PITP regulates secretory vesicle generation is not yet clear, but most likely it acts as a sensor of the balance between the PC and PI metabolic pathways.
Consistent with the hypothesis that PITP regulates post-Golgi transport by maintaining balanced levels of PC and PI along the secretory pathway, two groups of genes were discovered that are involved in PC or PI metabolism in yeast and are capable of rescuing the sec14 defects (fig. 3). One such gene is pld1, the gene encoding yeast phospholipase D (PId1p, PLD) [105, 106]. PLD is a lipid hydrolase that converts PC to PA and a hydrophilic head group choline. In eukaryotic cells, PLD has previously been shown to mediate intra-Golgi transport [107, 108] and post-Golgi secretion in the regulated secretory pathway [109]. In permeabilized pituitary GH3 cells, the release of nascent secretory vesicles can be measured following the secretion of growth hormone (GH) and prolactin. Using this system, the addition of mammalian PLD1 or plant PLD could stimulate GH-containing vesicle release. In mammalian cells, PLD1 is a PLD isoform that is activated by ARF1 and localizes to ER, Golgi, and endosomes. The enzymatic activity of PLD to hydrolyze PC to PA is required for GH release, and under conditions of enhanced budding of GH-containing vesicles, a concomitant stimulation of PLD activity is observed [109].
Figure 3.
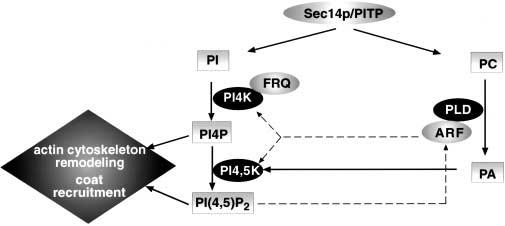
Lipids involved in TGN trafficking. Lipids from the PI and PC synthesis pathways are involved in post-TGN transport. Sec14/PITP regulates post-TGN transport probably by sensing and balancing between the two pathways. Along the PC pathway, ARF-activated phospholipase D (PLD) hydrolyzes PC to PA. PA may stimulate the recruitment of the protein machinery required for budding. Along the PI pathway, PI4P and PI(4,5)P2 are important lipids for recruiting coat proteins for budding and for regulating actin cytoskeletal proteins around the TGN. PI4 kinase further interacts with frequenin and is probably involved in sensing Ca2+ for regulated secretion. PI(4,5)P2 is known to be an activator of ARF and conversely, ARF and PA can stimulate the production of PI(4,5)P2. ARF was recently shown to directly recruit PI4 kinase and PI4,5 kinase to the TGN, an additional way of converging lipid kinases, coat machinery and actin cytoskeleton proteins in post-TGN transport.
As the increased levels of PA produced by PLD can be metabolized very quickly to diacylglycerol (DAG), the question was raised as to which of the two lipids is actually required for TGN budding. By adding PI-specific phospholipase C (PI-PLC) and DAG kinase to produce DAG and PA independently from PLD activity, Siddhanta and Shields [110] showed that the accumulation of PA, rather than DAG, seems to be the key requirement. To support this idea, when permeabilized GH3 cells are incubated with a primary alcohol, 1-butanol, to compete with PA production, the secretion of growth hormones from the TGN is inhibited [111]. Together, these data suggest a more important role for PA than for DAG in secretory vesicle release and that DAG might instead play a role in signaling events.
The other sec14 suppressors is a group of enzymes involved in the metabolism of PIs: phosphatidylinositol 4 phosphate (PI4P) and PI (4,5)P2. In yeast, Sac1 was recently shown to suppress the sec14-1ts defect [112]. The yeast Sac1 gene encodes a phosphatase that hydrolyzes PI4P and some other PIs. PI4P accumulates in the sac1 mutant, and the high level of PI4P can rescue the sec14-1ts secretory defect. These data suggest that PI4P is a critical lipid involved in secretion. Overexpression of Pik1p, one of the two yeast PI 4-kinases, can overcome the secretion defect in the sec14-ts strain by producing PI4P, thus bypassing the decreased level of PI4P that occurs at the restrictive temperature. In an independent screen to isolate yeast post-Golgi secretory mutants, Pik1p was also found to be involved in post-Golgi release of invertase, a secretory marker [113].
Pik1p is also found to be associated with Frq1, a homologue of frequenin in yeast. Frq1 binds to a conserved motif in Pik1p outside its catalytic domain, and stimulates the lipid kinase activity of PIK1 in vitro [114]. In mammalian cells, PI 4-kinase activity is associated with small synaptic vesicles and chromaffin granules and has been shown to be required for stimulated secretion [115, 116]. Both mammalian and Drosophila frequenin also function in neurotransmission. Drosophila frequenin is thought to modulate synaptic vesicle releases [117, 118], and the mammalian frequenin (also called neuronal calcium sensor-1) is thought to sense the local Ca2+ concentration and promote regulated secretion of dense-core granules in neuroendocrine cells [119]. Hence, the association of PI 4-kinase and Frq1 in yeast suggest that PI 4-kinase could act together with frequenin in sensing and regulating Ca2+-regulated secretion. Consistent with these suggestions, it has been recently reported that overex-pressed frequenin coimmunoprecipitates PI4 kinase (beta isoform) in polarized Madin-Darby canine kidney (MDCK) cells and inhibits the delivery of newly synthesized influenza HA from the TGN without affecting other transport steps. These data demonstrate a role of both frequenin and PI4 kinase in apical post-Golgi transport [120].
The phosphorylation and production of particular PIs are also regulated by the PLD and PA pathway, providing additional evidence for the interconnection of PC and PI pathways in the late secretory pathway. In the search for downstream effectors responsive to the raised level of PA, the level of the phospholipid PI(4,5)P2 was found to be increased [111]. The addition of recombinant human PLD1 stimulates PI(4,5)P2 synthesis due to an increased level of PA. This PA-stimulated PI(4,5)P2 synthesis was shown to be required for secretion, as well as for maintaining Golgi integrity and structure. From an independent report, ARF was also shown to stimulate PI4P and PI(4,5)P2 synthesis in the Golgi membrane by a new mechanism [27]. It was shown that ARF can directly recruit PI 4-kinase (beta isoform) and PI4P 5-kinase onto the Golgi membranes, where they become activated and cause an increased level of PI4P and PI(4,5)P2. It is suggested that the local production of PI(4,5)P2 could result in the formation of microdomains that can act as binding sites for spectrin, certain other actin-binding proteins or dynamin. This group of interactions prompts the local disassembly or rearrangement of the actin cytoskeleton, which in turn allows membrane deformation that facilitates the vesicle formation. The authors further show that a mutant form of the PI4 kinase (PI4Kβ D656A) lacking the kinase activity causes the Golgi to change into tubular and punctate structures, which is attributed to the disrupted interactions with the actin cytoskeleton proteins. Taken together, these data suggested that PI(4,5)P2 is an important lipid that plays a pivotal role in coordinating, the recruitment of coat proteins and various actin-binding proteins around the Golgi. Different signalling pathways through PLD and PA, ARF and PI-4 kinase, PI-5 kinase as well as PITP converge to produce and maintain the level of PI(4,5)P2 on the Golgi membrane, thereby maintaining the Golgi structure and regulating the formation of the secretory vesicles [28].
Polarized sorting from the TGN
As opposed to nonpolarized cells that have a continuous plasma membrane, a polarized cell surface is divided into separate domains that perform different physiological functions. In polarized epithelial cells such as MDCK cells, apical and basolateral cell surfaces have very different protein and lipid compositions, and the two domains are separated by tight junctions. To achieve and maintain this polarity, the majority of the newly synthesized apical and basolateral proteins are sorted in the TGN and transported to the appropriate surfaces. Indirect routes also exist to transport apical proteins, first to the basolateral surface and subsequently to the apical surface by transcytosis. In MDCK cells, polarity is primarily achieved by TGN sorting, whereas in hepatocytes or enterocytes, the indirect pathways are more frequently used [121-123].
Sorting of proteins to the basolateral surface in polarized cells relies on specific signals found within cytoplasmic domains of cargo molecules. Tyrosine, dileucine, and acidic sequences, as well as other unrelated amino acid motifs, have been shown to mediate the basolateral targeting of various proteins [for reviews see ref. 121-124]. Many of these basolateral-sorting signals are very similar to motifs that can interact with the clathrin-adaptor complexes involved in lysosomal sorting and endocytosis. Given the similarity of basolateral sorting signals to adaptor interaction motifs, the hypothesis that the clathrin-adaptor sorting machinery is involved in basolateral targeting naturally follows. To support this point of view, it has been recently shown that plgR could be coimmunoprecipitated with the AP-1 adaptor en route from TGN to the basolateral membrane [125]. However, it is not clear at which stage in the trafficking of plgR that the clathrin/AP-1 complex is acting. BFA-sensitive clathrin/AP-1 buds containing basolateral cargo have been observed on endosomal tubules, suggesting that AP-1 could be involved in basolateral cargo sorting from endosomal compartments [126]. However an interaction between pIgR and AP-1 could also be observed in the TGN [125]. These potential disparities may be explained by the possibility that basolateral cargo follows different routes from the TGN to the basolateral surface. An indirect route via endosomes has been shown to occur for a number of basolateral receptors such as pIgR [127], vesicular stomatitis virus-G (VSV-G) [128], transferrin receptor [129], asialoglycoprotein receptor H1 [130], and Semliki Forest virus p62 [131]. The possibility of direct transport routes that do not pass through endosomes, however, cannot be ignored. Evidence for such a direct pathway is suggested by the study of Orzech et al. [125], where a mutant form of the pIgR that failed to interact with AP-1 could be transported in a BFA-insensitive manner directly to the basolateral surface with slower kinetics. Transport vesicles that could mediate such a transport step are not known. Recently, an epithelial-specific μ1 adaptor isoform, μ1B, was identified [132]. In a few epithelial cell lines, such as LLCPK1, μ1B does not appear to be expressed, and several proteins such as low-density lipoprotein (LDL) receptor and transferrin receptor that are normally basolaterally located are missorted in these lines. The missorting of these proteins can be rescued with the expression of μ1B [133]. Similar to the μ chains from other adaptor complexes, μ1B recognizes tyrosine-based motifs [132]. However, it is unclear precisely where μ1B is acting and what is the molecular mechanism of μ1B action. It has been speculated that μ1B could function at both the TGN and the endosome due to the dramatic redistribution of LDL receptor and transferrin receptor [121].
Apical sorting mechanisms are fundamentally different from those involved in basolateral sorting. Apical membranes are highly enriched in glycosphingolipids, and apical sorting appears to be mainly based upon lipid-lipid and protein-lipid interactions [123]. One working model for apical sorting is that cholesterol-sphingolipid microdomains are formed in the Golgi and the TGN membrane. These microdomains then function as platforms for the inclusion of apical cargo proteins through high-affinity interactions with lipid and sugar modifications found on the apical-directed cargo molecules [123, 134-136]. However, it has been reported that the partitioning of proteins into rafts does not guarantee apical transport for such cargo, suggesting that other sorting machinery may well be involved [137, 138].
Cholesterol-sphingolipid microdomains are also called membrane rafts or detergent-insoluble glycolipid-rich (DIG) domains. They can be isolated on a sucrose gradient after a cold Triton X-100 extraction of the cell membrane. Using different microscopic techniques and following glycosylphosphatidylinositol-anchored proteins (GPI-APs), components of the rafts have been observed to be rather small (about 70 nm in diameter), but highly dynamic structures in vivo [135]. Various apical targeting signals have been identified over the years, including amino acid sequences located in the lumenal, transmembrane, and cytoplasmic domains of cargo molecules. Furthermore, the lipid modification of GPI-anchored proteins and sugar modifications of the N-linked or O-linked carbohydrates have also been identified as apical targeting signals [123, 139]. These sorting signals all have high affinity for lipid rafts.
Currently, no potential protein sorting machinery, such as a proteinaceous coat, has been identified for apical targeting. Some proteins, however, have high affinity for membrane rafts and could mediate raft clustering or protein sorting into the rafts. VIP21/caveolin-1 has a high affinity for cholesterol and has been proposed to form homo-oligomers that interact with GPI-anchored protein [140-142]. However, recent evidence showed that in Fisher rat thyroid cells (cells lacking caveolin), GPI-anchored proteins are mistargeted to the basolateral surface, and transfection of caveolin-1 into these cells does not rescue the apical sorting even though caveolae are formed under these conditions [143]. These data suggest that caveolin-1 probably plays a less direct role in apical sorting. VIP17/MAL, a tetraspanning membrane protein, has recently been found associated with apical transport vesicles, and the expression level of VIP17 affected apical targeting of influenza HA, giving good evidence for VIP17 involvement in apical targeting [144-146]. Annexin13b, a Ca2+-dependent phospholipid-binding protein has also been implicated in apical transport. This protein was recently shown to localize to the TGN, apical membranes, and vesicle carriers. Annexin13b is also associated with lipid rafts [147, 148]. Annexin13b interacts directly with an apically targeted protein, Nedd4 (a ubiquitin protein ligase), and the myristoylation of annexin13b was important for this apical transport function [147, 148].
The differential sorting of proteins to axons and dendrites in neuronal cells has often been compared to the basolateral and apical sorting pathways in polarized epithelial cells. As with the apical and basolateral membranes of polarized epithelial cells, axonal and dendritic membranes in neuronal cells have distinct protein compositions and cytoskeletal organization [149]. The sorting of neuronal proteins to dendrites is generally accepted to be similar to the basolateral sorting mechanisms discussed above, with targeting motifs such as the tyrosine-based motifs directing dendritic protein traffic [150]. However, to date, there seem to be few, if any. such similarities between apical and axonal sorting [for a review see ref. 151]. Recently a ‘smart motor’ model has been proposed to play an important role in controlling the sorting into axons and dendrites. This model postulates that the polarized trafficking of proteins and vesicles is mediated by corresponding microtubule-based motors that are segregated to either axons or dendrites [152]. Whether polarized epithelial cells also have different apical- and basolateral-destined motors remains to be investigated.
Sorting into the regulated secretory pathway
In endocrine and neuroendocrine cells, prohormone molecules are efficiently sorted into the regulated secretory pathway where they are processed and packaged into dense-core secretory granules. In response to physiological stimuli and an influx of extracellular calcium, secretory granules are induced to fuse with the plasma membrane and release their complement of stored molecules. Two models have been proposed for regulated pathway sorting – ‘sorting by entry’ and ‘sorting by retention’ [reviewed in ref. 153]. In entry-based models, sorting occurs in the TGN either by the intrinsic ability of regulated pathway proteins to aggregate or by the presence of a sorting signal on the prohormones themselves or by one or more TGN sorting receptors. By contrast, in the retention model, sorting is largely a post-TGN event, occurring in the clathrin- and AP-1-coated ISGs that support the proteolytic maturation of prohormone molecules. This model relies on the ability of granule proteins to condense into a core, leaving nongranule proteins in the lumenal periphery where they are removed from the maturing granule apparently by clathrin-based coats.
The basis for the entry model was founded on early studies showing that GH peptide sequence could target a fused reporter protein (a truncated VSV G molecule that is normally constitutively secreted) to secretory granules [154]. The ability of human GH (hGH) to target the reporter to the regulated pathway suggests the presence of a TGN-localized, regulated-pathway sorting receptor that operates similarly to the MPR or Vps10p. However, as the concentration of some granule components exceeds 100 mg/ml, and overexpression of prohormones fails to saturate regulated pathway sorting [155, 156], it is unlikely this targeting can be explained by a simple stoichiometric sortase.
The sorting of a large number of granule-destined molecules is simplified by a mechanism that allows such molecules to selectively coalesce into an ‘aggregate’ that can exclude constitutive pathway molecules. Such a phenomenon was first determined by analysis of amylase secretion in the exocrine pancreas [157] and is further supported by the elegant morphological analysis by Rambourg et al. [158]. Analysis of lactotrophs (endocrine cells that secret prolactin) from lactating rats showed the presence of electron-opaque nodular ‘progranules’ that became abundant in the fenestrated trans-most Golgi cisternae. Consistent with this model, exposure of a number of regulated-pathway proteins [including secretogranin II, chromogranins A and B, proPC2, carboxypeptidase E, prolactin, proopiomelanocortin (POMC), and proinsulin] to low pH (below pH 6.5) and millimolar calcium characteristic of the late Golgi results in the aggregation of the targeted molecules and the exclusion of constitutive secretory pathway markers [reviewed in ref. 159]. The ability of calcium ions to coordinate acidic amino acids near the isoelectric points of the studied proteins may explain the selective aggregation.
The regulated pathway targeting of the hGH/VSV-Gt suggests granule-targeted proteins likely contain a positive sorting signal that in some cases may act independent of aggregation. Although a single universal motif has yet to be identified, numerous studies support the presence of such signals. For example, regulated pathway sorting and aggregation of proatrial natriuretic hormone in AtT-20 cells requires the presence of a pair of glutamate residues within its proregion, raising the possibility that for this proprotein, the sorting and aggregation signals may be one and the same [160]. Sorting of chromogranin B in PC12 cells requires a different signal, one composed of an N-terminal disulfide-bonded loop structure [161]. A similar disulfide loop was reported for the N terminus of POMC [162] although this finding has been questioned [163]. One possible explanation for the conflicting results of the prohormone sorting signals may reside in the cell types studied. For example, in GH4C1 cells, the N-terminal loop of chromogranin B does not affect sorting. Rather, in these cells, an alternate sorting motif near the C terminus of the protein is recognized [164].
The entry model also predicts a sorting receptor that may recognize the aggregated cargo molecules. Intriguingly, one of the enzymes that catalyzes prohormone processing, carboxypeptidase E (CPE), was identified as a sorting receptor for entry to the regulated pathway. Binding studies showed that CPE associates with the N-terminal disulfide-rich segment of POMC and also binds proinsulin and proenkephalin [165]. Consistent with these binding studies, Cool et al. [166] reported that POMC is missorted in CPE −/− (fat/fat) mice. The role of CPE as a prohormone sortase is the center of much speculation, since others have reported that although prohormone processing is impaired in the mutant mice, prohormone sorting is unaffected [167]. As the Ser202 → Pro mutation in CPE in the fat/fat mice greatly inhibits stability of the enzyme [168], it is difficult to uncouple the enzymatic activity of the CPE mutation from its possible activity as a sortase. Additional studies into the role of CPE in prohormone sorting should address these questions.
Two recent reports investigating the sorting of cargo into the regulated pathway indicate that lipid rafts are membrane components that are used for such sorting. Characteristic of raft-associated molecules, both proPC2 and CPE resist extraction from membranes with 1% Triton X-100 at 4 °C [169, 170]. By contrast, chromogranin A was readily extracted under these conditions. Interestingly, treatment of AtT-20 cells with fumonisin 1B (a ceramide synthase inhibitor) caused the missorting of PC2 but not chromogranin A. The raft-independent sorting of chromogranin A is consistent with the CPE-independent sorting of this protein in the CPE (fat/fat) mice [166].
The retention model postulates that the initial sorting from the TGN into the regulated pathway is relatively permissive, with the ISG both housing the endoproteolytic processing of prohormone substrates and also selectively retaining some cargo molecules (i.e., peptide hormones) and membrane proteins (e.g., phogrin, [171]) while removing others (e.g., furin, MPR, and syntaxin 6) after the ISG is initially formed [71, 172]. In this way, the ISG is viewed as an extension of the TGN [153]. However, as TGN38 is not sorted into ISGs [71], the sorting of molecules from the TGN to the ISG to the mature secretory granule (MSG) is perhaps more accurately viewed as a selective and highly regulated distillation apparatus. Furthermore, these results support distinct roles for the ISG and TGN in protein sorting.
ISGs undergo a series of homotypic fusions and a net loss of membrane during their maturation to MSGs. The net loss of membrane is consistent with the transient decoration of the ISG with clathrin coats and suggests that the clathrin-based sorting machinery actively sorts membrane proteins from the ISG. A number of membrane proteins, including furin and the CI-MPR, are sorted into ISGs but are removed prior to granule maturation [71, 172]. Sorting of the CI-MPR through ISGs enables the removal of lysosomal hydrolases from this compartment and their transfer to endosomal compartments [173]. The functional role for furin in early compartments within the regulated pathway is not known but may include the activation of PC2 by cleavage of its escort protein, 7B2 [174], and also the processing of several granule proteins including proparathyroid hormone [175], chromogranin A [176], and pro-ELH [177].
Consistent with the morphological data, ISG-associated AP-1 appears to participate in removal of furin. Binding studies show that the AP-1 recruitment to ISG membranes is ARF1 dependent [178] and is enhanced by phosphorylation of ISGs with CK2 [71]. Consistent with this finding, furin constructs containing a mutation of the CK2 sites within the furin cytoplasmic domain acidic cluster fail to be efficiently removed from the ISG and are missorted into MSGs [71]. The effect suggests a role for PACS-1 in the retrieval of furin from ISGs; consistent with this model, expression of a PACS-1-dominant-negative construct in AtT-20 cells causes a missorting of co-expressed furin to MSGs [74a]. The presence of phosphorylatable acidic-cluster sorting motifs on the CI-MPR cytoplasmic domain raises the possibility of a similar mechanism contributing to the removal of this protein from ISGs along with lysosomal hydrolases bound to the CI-MPR lumenal domain. Whether retention of phogrin on ISGs is an active or a passive sorting step remains to be determined.
As well as the removal of selected membrane proteins from the ISG being a selective process, the ability to retain cargo molecules similarly appears to be an active process. For example, processing of proinsulin to insulin in the ISG facilitates the zinc-dependent condensation of the mature insulin hormone and enhances its selective retention in the MSG [179]. Thus aggregation-based events appear to contribute to aspects of both the entry- and retention-based models.
Conclusion
Our understanding of the TGN compartment has grown quickly over the past few years and new aspects of TGN sorting are constantly emerging. First, using new microscopy technology such as the dual-axis, high-voltage EM tomography of cryofixed cells, the three-dimensional structure of the Golgi compartment has been reconstructed. Second, these structural studies have shown that the last several stacks of the Golgi complex all project different types of transport vesicles, with the clathrin coats specifically localized on the last stack. These observations suggest that different TGN proteins might be sorted in physically distinct stacks, with the respective type of transport vesicle associated to each stack. This is an attractive model for TGN sorting and it will be interesting to localize TGN proteins in the three-dimensional structure to see whether different TGN proteins are indeed localized to different stacks.
As more studies are published, several hot spot areas in TGN sorting mechanisms have emerged. Along the classical lysosomal targeting pathway, new molecules related to adaptors, such as the new family of GGA proteins, have been discovered. The precise function of these GGAs needs to be determined, but their discovery suggests that the clathrin/AP-1 sorting machinery might include many other associated components. For lysosomal targeting, research on AP-3 has revealed a distinct transport route to the lysosomes that utilizes this adaptor complex. Whether AP-3 is associated with clathrin remains unsettled and the molecular composition of the AP-3-coated vesicles remains to be determined.
Very little is known about the transport machinery required for itinerant TGN membrane proteins to be transported from endosomes back to the TGN. Recently, a series of new molecules have been cloned both in mammalian cells and in yeast that appear to be involved in retrograde transport to the TGN. In yeast, the ‘retromer’ complex has been shown to mediate the retrograde transport of Vps10p, Kex2p, and DPAP A. It is the first potential ‘coat’ complex discovered for retrograde transport. While the ‘retromer’ complex seems to be a general vesicle coat-like protein complex for the transport of all cargo, molecules such as Grd19p and Grd20p were also found to be specifically involved in the retrieval of certain but not all cargo. This situation is similar to PACS-1 and TIP47 in mammalian cells. While both molecules were found to be involved in TGN retrieval, PACS-1 recognizes acidic-cluster TGN-sorting motifs contained in a number of molecules, including furin and MPR, but TIP47 appears to recognize signals only on CI- and CD-MPR and mediates their transport back to the TGN. The relative role of each of these molecules remains to be investigated. One speculation is that there are different retrieval pathways ‘toward’ the TGN and different categories of cargo might utilize different sorting proteins. Mammalian homologues of the yeast ‘retromer’ complex have been recently cloned [91] and it will be interesting to determine their function in mammalian cells.
Beside coat proteins, another emerging aspect in TGN sorting is the involvement of lipids, lipid transfer proteins, and lipid kinases. Although PITP has long been known to be involved in the late secretory pathway, more lipids and lipid kinases are now found to act synergistically with PITP to regulate secretion. Specific lipids are proposed to interact with different proteins, including coat proteins and PH-domain-containing proteins such as dynamin, spectrin, and certain other actin-binding proteins. PI(4,5)P2 appears to be a critical lipid on the Golgi membrane that can regulate local rearrangement of the Golgi membrane skeleton through interaction with PH domains [28]. All together, these studies show that sorting motifs, coat proteins, coat-related and associated proteins, lipids and actin cytoskeleton proteins act in concert to regulate the complex event of TGN sorting. Elucidating the interconnection of these components and their orchestration in TGN sorting is the challenge of the present. As new elements are clarified, we hope to have a more global understanding of the TGN and its sorting mechanisms.
Acknowledgements
F.G. is the recipient of an HFSP (Human frontiers of Science Program) postdoctoral fellowship and C.M.C. is the recipient of a Welcome Trust Travelling postdoctoral fellowship. This work was supported by grants from the NIH (G.T.). The authors thank Q. Justman for assistance in preparation of this manuscript.
References
- 1.Griffiths G, Simons K. The trans Golgi network: sorting at the exit site of the Golgi complex. Science. 1986;234:438–443. doi: 10.1126/science.2945253. [DOI] [PubMed] [Google Scholar]
- 2.Farquhar MG, Palade GE. The Golgi apparatus: 100 years of progress and controversy. Trends Cell Biol. 1998;8:2–10. doi: 10.1016/S0962-8924(97)01187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rambourg A, Clermont Y. Three-dimensional electron microscopy: structure of the Golgi apparatus. Eur. J. Cell Biol. 1990;51:189–200. [PubMed] [Google Scholar]
- 4.Schweizer A, Fransen JA, Matter K, Kreis TE, Ginsel L, Hauri HP. Identification of an intermediate compartment involved in protein transport from endoplasmic reticulum to Golgi apparatus. Eur. J. Cell Biol. 1990;53:185–196. [PubMed] [Google Scholar]
- 5.Balch WE, McCaffery JM, Plutner H, Farquhar MG. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;76:841–852. doi: 10.1016/0092-8674(94)90359-x. [DOI] [PubMed] [Google Scholar]
- 6.Hauri HP, Schweizer A. The endoplasmic reticulum-Golgi intermediate compartment. Curr. Opin. Cell Biol. 1992;4:600–608. doi: 10.1016/0955-0674(92)90078-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Presley JF, Cole NB, Schroer TA, Hirschberg K, Zaal KJ, Lippincott-Schwartz J. ER-to-Golgi transport visualized in living cells. Nature. 1997;389:81–85. doi: 10.1038/38001. [DOI] [PubMed] [Google Scholar]
- 8.Scales SJ, Pepperkok R, Kreis TE. Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell. 1997;90:1137–1148. doi: 10.1016/s0092-8674(00)80379-7. [DOI] [PubMed] [Google Scholar]
- 9.Novikoff AB. The endoplasmic reticulum: a cytochemist's view. Proc. Natl. Acad. Sci. USA. 1976;73:2781–2787. doi: 10.1073/pnas.73.8.2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novikoff PM, Novikoff AB, Quintana N, Hauw JJ. Golgi apparatus, GERL, and lysosomes of neurons in rat dorsal root ganglia, studied by thick section and thin section cytochemistry. J. Cell Biol. 1971;50:859–886. doi: 10.1083/jcb.50.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldfischer S. The internal reticular apparatus of Camillo Golgi: a complex, heterogeneous organelle, enriched in acid, neutral, and alkaline phosphatases, and involved in glycosylation, secretion, membrane flow, lysosome formation, and involved in glycosylation, secretion, membrane flow, lysosome formation, and intracellular digestion. J. Histochem. Cytochem. 1982;30:717–733. doi: 10.1177/30.7.6286754. [DOI] [PubMed] [Google Scholar]
- 12.Rambourg A, Clermont Y, Hermo L. Three-dimensional architecture of the Golgi apparatus in Sertoli cells of the rat. Am. J. Anat. 1979;154:455–476. doi: 10.1002/aja.1001540402. [DOI] [PubMed] [Google Scholar]
- 13.Clermont Y, Rambourg A, Hermo L. Trans-Golgi network (TGN) of different cell types: three-dimensional structural chracteristics and veriability. Anat. Rec. 1995;242:289–301. doi: 10.1002/ar.1092420302. [DOI] [PubMed] [Google Scholar]
- 14.Ladinsky MS, Mastronarde DN, McIntosh JR, Howell KE, Staehelin LA. Golgi structure in three dimensions: functional insights from the normal rat kidney cell. J. Cell Biol. 1999;144:1135–1149. doi: 10.1083/jcb.144.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Misumi Y, Miki K, Takatsuki A, Tamura G, Ikehara Y. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J. Biol. Chem. 1986;261:11398–11403. [PubMed] [Google Scholar]
- 16.Lippincott-Schwartz J, Yuan LC, Bonifacino JS, Klausner RD. Rapid redistribution of Golgi proteins into the ER in cells treated with brefeldin A: evidence for membrane cycling from Golgi to ER. Cell. 1989;56:801–813. doi: 10.1016/0092-8674(89)90685-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doms RW, Russ G, Yewdell JW. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J. Cell. Biol. 1989;109:61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lippincott-Schwartz J, Donaldson JG, Schweizer A, Berger EG, Hauri HP, Yuan LC. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990;60:821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- 19.Lippincott-Schwartz J, Yuan L, Tipper C, Amherdt M, Orci L, Klausner RD. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991;67:601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- 20.Wood SA, Park JE, Brown WJ. Brefeldin A causes a microtubule-mediated fusion of the trans-Golgi network and early endosomes. Cell. 1991;67:591–600. doi: 10.1016/0092-8674(91)90533-5. [DOI] [PubMed] [Google Scholar]
- 21.Reaves B, Banting G. Perturbation of the morphology of the trans-Golgi network following brefeldin A treatment: redistribution of a TGN-specific integral membrane protein, TGN38. J. Cell Biol. 1992;116:85–94. doi: 10.1083/jcb.116.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thyberg J, Moskalewski S. Role of microtubules in the organization of the Golgi complex. Exp. Cell Res. 1999;246:263–279. doi: 10.1006/excr.1998.4326. [DOI] [PubMed] [Google Scholar]
- 23.Morrow JS, Rimm DL, Kennedy SP, Cianci CD, Sinard JH, Weed SA. In: Handbook of Physiology of membrane stability and mosaics: the spectrin cytoskeleton. Hoffman J, Jamieson J, editors. Oxford University Press; Oxford: 1997. pp. 485–540. [Google Scholar]
- 24.Stankewich MC, Tse WT, Peters LL, Ch'ng Y, John KM, Stabach PR. A widely expressed betaIII spectrin associated with Golgi and cytoplasmic vesicles. Proc. Natl. Acad. Sci USA. 1998;95:14158–14163. doi: 10.1073/pnas.95.24.14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Matteis MA, Morrow JS. Spectrin tethers and mesh in the biosynthetic pathway. J. Cell Sci. 2000;113:2331–2343. doi: 10.1242/jcs.113.13.2331. [DOI] [PubMed] [Google Scholar]
- 26.Godi A, Santone I, Pertile P, Devarajan P, Stabach PR, Morrow JS. ADP ribosylation factor regulates spectrin binding to the Golgi complex. Proc. Natl. Acad. Sci. USA. 1998;95:8607–8612. doi: 10.1073/pnas.95.15.8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godi A, Pertile P, Meyers R, Marra P, Di Tullio G, Iurisci C. ARF mediates recruitment of PtdIns-4-OH kinase-beta and stimulates synthesis of PtdIns(4,5)P2 on the Golgi complex. Nat. Cell Biol. 1999;1:280–287. doi: 10.1038/12993. [DOI] [PubMed] [Google Scholar]
- 28.Lorra C, Huttner WB. The mesh hypothesis of Golgi dynamics. Nat. Cell Biol. 1999;1:E113–E115. doi: 10.1038/12939. [DOI] [PubMed] [Google Scholar]
- 29.De Matteis MA, Morrow JS. The role of ankyrin and spectrin in membrane transport and domain formation. Curr. Opin. Cell Biol. 1998;10:542–549. doi: 10.1016/s0955-0674(98)80071-9. [DOI] [PubMed] [Google Scholar]
- 30.Traub LM, Kornfeld S. The trans-Golgi network: a late secretory sorting station. Curr. Opin. Cell Biol. 1997;9:527–533. doi: 10.1016/s0955-0674(97)80029-4. [DOI] [PubMed] [Google Scholar]
- 31.Mallet WG, Maxfield FR. Chimeric forms of furin and TGN38 are transported with the plasma membrane in the trans-Golgi network via distinct endosomal pathways. J. Cell Biol. 1999;146:345–359. doi: 10.1083/jcb.146.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rohn WM, Rouille Y, Waguri S, Hoflack B. Bi-directional trafficking between the trans-Golgi network and the endosomal/lysosomal system. J. Cell Sci. 2000;113:2093–2101. doi: 10.1242/jcs.113.12.2093. [DOI] [PubMed] [Google Scholar]
- 33.Dell'Angelica EC, Ohno H, Ooi CE, Rabinovich E, Roche KW, Bonifacino JS. AP-3: an adaptor-like protein complex with ubiquitous expression. EMBO J. 1997;16:917–928. doi: 10.1093/emboj/16.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simpson F, Peden AA, Christopoulou L, Robinson MS. Characterization of the adaptor-related protein complex, AP-3. J. Cell Biol. 1997;137:835–845. doi: 10.1083/jcb.137.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirst J, Brigth NA, Rous B, Robinson MS. Characterization of a fourth adaptor-related protein complex. Mol. Biol. Cell. 1999;10:2787–2802. doi: 10.1091/mbc.10.8.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dell'Angelica EC, Mullins C, Bonifacino JS. AP-4, a novel protein complex related to clathrin adaptors. J. Biol. Chem. 1999;274:7278–7285. doi: 10.1074/jbc.274.11.7278. [DOI] [PubMed] [Google Scholar]
- 37.Kirchhausen T. Adaptors for clathrin-mediated traffic. Annu. Rev. Cell Dev. Biol. 1999;15:705–732. doi: 10.1146/annurev.cellbio.15.1.705. [DOI] [PubMed] [Google Scholar]
- 38.Le Borgne R, Hoflack B. Mechanisms of protein sorting and coat assembly: insights from the clathrin-coated vesicle pathway. Curr. Opin. Cell Biol. 1998;10:499–503. doi: 10.1016/s0955-0674(98)80065-3. [DOI] [PubMed] [Google Scholar]
- 39.Traub LM, Ostrom JA, Kornfeld S. Biochemical dissection of AP-1 recruitment onto Golgi membranes. J. Cell Biol. 1993;123:561–573. doi: 10.1083/jcb.123.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seaman MNJ, Sowerby PJ, Robinson MS. Cytosolic and membrane-associated proteins involved in the recruitment of AP-1 adaptors onto the trans-Golgi network. J. Biol. Chem. 1996;271:25446–25451. doi: 10.1074/jbc.271.41.25446. [DOI] [PubMed] [Google Scholar]
- 41.Zhu Y, Traub LM, Kornfeld S. ADP-ribosylation factor 1 transiently activates high-affinity adaptor protein complex AP-1 binding sites on Golgi membranes. Mol. Biol. Cell. 1998;9:1323–1337. doi: 10.1091/mbc.9.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Y, Traub LM, Kornfeld S. High-affinity binding of the AP-1 adaptor complex to trans-Golgi network membranes devoid of mannose 6-phosphate receptors. Mol. Biol. Cell. 1999;10:537–549. doi: 10.1091/mbc.10.3.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorkina T, Bild A, Tebar F, Sorkin A. Clathrin, adaptors and eps15 in endosomes containing activated epidermal growth factor receptors. J. Cell Sci. 1999;112:317–327. doi: 10.1242/jcs.112.3.317. [DOI] [PubMed] [Google Scholar]
- 44.Stoorvogel W, Oorschot V, Geuze HJ. A novel class of lathrin-coated vesicles budding from endosomes. J. Cell Biol. 1996;132:21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer C, Zizioli D, Lausmann S, Eskelinen EL, Hamann J, Saftig P, et al. mu1A-Adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 2000;19:2193–2203. doi: 10.1093/emboj/19.10.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Page LJ, Sowerby PJ, Lui WW, Robinson MS. Gamma-synergin: an EH domain-containing protein that interacts with gamma-adaptin. J. Cell Biol. 1999;146:993–1004. doi: 10.1083/jcb.146.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayer BJ. Endocytosis: EH domains lend a hand. Curr. Biol. 1999;9:R70–R73. doi: 10.1016/s0960-9822(99)80014-1. [DOI] [PubMed] [Google Scholar]
- 48.Di Fiore PP, Pelicci PG, Sorkin A. EH: a novel protein-protein interaction domain potentially involved in intracellular sorting. Trends Biochem. Sci. 1997;22:411–413. doi: 10.1016/s0968-0004(97)01127-4. [DOI] [PubMed] [Google Scholar]
- 49.Poussu A, Lohi O, Lehto VP. Vear, a novel Golgi-associated protein with VHS and gamma-adaptin ‘ear’ domains. J. Biol. Chem. 2000;275:7176–7183. doi: 10.1074/jbc.275.10.7176. [DOI] [PubMed] [Google Scholar]
- 50.Takatsu H, Yoshino K, Nakayama K. Adaptor gamma ear homology domain conserved in gamma-adaptin and GGA proteins that interact with gamma-synergin. Biochem. Biophys. Res. Commun. 2000;271:719–725. doi: 10.1006/bbrc.2000.2700. [DOI] [PubMed] [Google Scholar]
- 51.Boman AL, Zhang C, Zhu X, Kahn RA. A family of ADP-ribosylation factor effectors that can alter membrane transport through the trans-Golgi. Mol. Biol. Cell. 2000;11:1241–1255. doi: 10.1091/mbc.11.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dell'Angelica EC, Puertollano R, Mullins C, Aguilar RC, Vargas JD, Hartnell LM, et al. GGAs: a family of ADP ribosylation factor-binding proteins related to adaptors and associated with the Golgi complex. J. Cell Biol. 2000;149:81–94. doi: 10.1083/jcb.149.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirst J, Lui WW, Brigth NA, Totty N, Seaman MN, Robinson MS. A family of proteins with gamma-adaptin and VHS domains that facilitate trafficking between the trans-Golgi network and the vacuole/lysosome. J. Cell Biol. 2000;149:67–80. doi: 10.1083/jcb.149.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Odorizzi G, Cowles CR, Emr SD. The AP-3 complex: a coat of many colours. Trends Cell Biol. 1998;8:282–288. doi: 10.1016/s0962-8924(98)01295-1. [DOI] [PubMed] [Google Scholar]
- 55.Cowles CR, Odorizzi G, Payne GS, Emr SD. The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell. 1997;91:109–118. doi: 10.1016/s0092-8674(01)80013-1. [DOI] [PubMed] [Google Scholar]
- 56.Stepp JD, Huang K, Lemmon SK. The yeast adaptor protein complex, AP-3, is essential for the efficient delivery of alkaline phosphatase by the alternate pathway to the vacuole. J. Cell Biol. 1997;139:1761–1774. doi: 10.1083/jcb.139.7.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Le Borgne R, Alconada A, Bauer U, Hoflack B. The mammalian AP-3 adaptor-like complex mediates the intracellular transport of lysosomal membrane glycoproteins. J. Biol. Chem. 1998;273:29451–29461. doi: 10.1074/jbc.273.45.29451. [DOI] [PubMed] [Google Scholar]
- 58.Honing S, Sandoval IV, Figura K. von. A di-leucine-based motif in the cytoplasmic tail of LIMP-II and tyrosinase mediates selective binding of AP-3. EMBO J. 1998;17:1304–1314. doi: 10.1093/emboj/17.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kirchhausen T, Bonifacino JS, Riezman H. Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr. Opin. Cell Biol. 1997;9:488–495. doi: 10.1016/s0955-0674(97)80024-5. [DOI] [PubMed] [Google Scholar]
- 60.Ohno H, Aguilar RC, Yeh D, Taura D, Saito T, Bonifacino JS. The medium subunits of adaptor complexes recognize distinct but overlapping sets of tyrosine-based sorting signals. J. Biol. Chem. 1998;273:25915–25921. doi: 10.1074/jbc.273.40.25915. [DOI] [PubMed] [Google Scholar]
- 61.Ohno H, Fournier MC, Poy G, Bonifacino JS. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J. Biol. Chem. 1996;271:29009–29015. doi: 10.1074/jbc.271.46.29009. [DOI] [PubMed] [Google Scholar]
- 62.Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, et al. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- 63.Boll W, Ohno H, Songyang Z, Rapoport I, Cantley LC, Bonifacino JS, et al. Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin AP-2 complexes. EMBO J. 1996;15:5789–5795. [PMC free article] [PubMed] [Google Scholar]
- 64.Owen DJ, Evans PR. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science. 1998;282:1327–1332. doi: 10.1126/science.282.5392.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rapoport I, Chen YC, Cupers P, Shoelson SE, Kirchhausen T. Dileucine-based sorting signals bind to the beta chain of AP-1 at a site distinct and regulated differently from the tyrosine-based motif-binding site. EMBO J. 1998;17:2148–2155. doi: 10.1093/emboj/17.8.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dietrich J, Kastrup J, Nielsen BL, Odum N, Geisler C. Regulation and function of the CD3gamma DxxxLL motif: a binding site for adaptor protein-1 and adaptor protein-2 in vitro. J. Cell Biol. 1997;138:271–281. doi: 10.1083/jcb.138.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dietrich J, Hou X, Wegener AM, Geisler C. CD3 gamma contains a phosphoserine-dependent di-leucine motif involved in down-regulation of the T cell receptor. EMBO J. 1994;13:2156–2166. doi: 10.1002/j.1460-2075.1994.tb06492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Molloy SS, Anderson ED, Jean F, Thomas G. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 1999;9:28–35. doi: 10.1016/s0962-8924(98)01382-8. [DOI] [PubMed] [Google Scholar]
- 69.Jones BG, Thomas L, Molloy SS, Thulin CD, Fry MD, Walsh KA, et al. Intracellular trafficking of furin is modulated by the phosphorylation state of a casein kinase II site in its cytoplasmic tail. EMBO J. 1995;14:5869–5883. doi: 10.1002/j.1460-2075.1995.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takahashi S, Nakagawa T, Banno T, Watanabe T, Murakami K, Nakayama K. Localization of furin to the trans-Golgi network and recycling from the cell surface involves Ser and Tyr residues within the cytoplasmic domain. J. Biol. Chem. 1995;270:28397–28401. doi: 10.1074/jbc.270.47.28397. [DOI] [PubMed] [Google Scholar]
- 71.Dittie AS, Thomas L, Thomas G, Tooze SA. Interaction of furin in immature secretory granules from neuroendocrine cells with the AP-1 adaptor complex is modulated by casein kinase II phosphorylation. EMBO J. 1997;16:3859–4870. doi: 10.1093/emboj/16.16.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen HJ, Yuan J, Lobel P. Systematic mutational analysis of the cation-independent mannose 6-phosphate/insulin-like growth factor II receptor cytoplasmic domain: an acidic cluster containing a key aspartate is important for function in lysosomal enzyme sorting. J. Biol. Chem. 1997;272:7003–7012. doi: 10.1074/jbc.272.11.7003. [DOI] [PubMed] [Google Scholar]
- 73.Pond L, Kuhn LA, Teyton L, Schutze MP, Tainer JA, Jackson MR, et al. A role for acidic residues in di-leucine motif-based targeting to the endocytic pathway. J. Biol. Chem. 1995;270:19989–19997. doi: 10.1074/jbc.270.34.19989. [DOI] [PubMed] [Google Scholar]
- 74.Wan L, Molloy SS, Thomas L, Liu G, Xiang Y, Rybak SL, et al. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell. 1998;94:205–216. doi: 10.1016/s0092-8674(00)81420-8. [DOI] [PubMed] [Google Scholar]
- 74a.Crump CM, Xiang Y, Thomas L, Gu F, Austin C, Tooze SA, Thomas G. PACS-1 binding to adaptors is required for acidic cluster motif-mediated protein traffic. EMBO J. 2001;20:2191–2201. doi: 10.1093/emboj/20.9.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Diaz E, Schimmoller F, Pfeffer SR. A novel Rab9 effector required for endosome-to-TGN transport. J. Cell Biol. 1997;138:283–290. doi: 10.1083/jcb.138.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lombardi D, Soldati T, Riederer MA, Goda Y, Zerial M, Pfeffer SR. Rab9 functions in transport between late endosomes and the trans Golgi network. EMBO J. 1993;12:677–682. doi: 10.1002/j.1460-2075.1993.tb05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Diaz E, Pfeffer SR. TIP47: a cargo selection device for mannose b-phosphate receptor trafficking. Cell. 1998;93:433–443. doi: 10.1016/s0092-8674(00)81171-x. [DOI] [PubMed] [Google Scholar]
- 78.Orsel JG, Sincock PM, Krise JP, Pfeffer SR. Recognition of the 300-kDa mannose 6-phosphate receptor cytoplasmic domain by 47-kDa tail-interacting protein. Proc. Natl. Acad. Sci. USA. 2000;97:9047–9051. doi: 10.1073/pnas.160251397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krise JP, Sincock PM, Orsel JG, Pfeffer SR. Quantitative analysis of TIP47 (tail-interacting protein of 47kD)-receptor cytoplasmic domain interactions: implications for endosome-to-trans Golgi network trafficking. J. Biol. Chem. 2000;275:25188–25193. doi: 10.1074/jbc.M001138200. [DOI] [PubMed] [Google Scholar]
- 80.Molloy SS, Thomas L, Kamibayashi C, Mumby MC, Thomas G. Regulation of endosome sorting by a specific PP2A isoform. J. Cell Biol. 1998;142:1399–1411. doi: 10.1083/jcb.142.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiang Y, Molloy SS, Thomas L, Thomas G. The PC6B cytoplasmic domain contains two acidic clusters that direct sorting to distinct trans-Golgi network/endosomal compartments. Mol. Biol. Cell. 2000;11:1257–1273. doi: 10.1091/mbc.11.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Piguet V, Wan L, Borel C, Mangasarian A, Demaurex N, Thomas G, et al. HIV-1 Nef protein binds to the cellular protein PACS-1 to downregulate class I major histocompatibility complexes. Nat. Cell Biol. 2000;2:163–167. doi: 10.1038/35004038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kornfeld S. Structure and function of the mannose 6-phosphate/insulinlike growth factor II receptors. Annu. Rev. Biochem. 1992;61:307–330. doi: 10.1146/annurev.bi.61.070192.001515. [DOI] [PubMed] [Google Scholar]
- 84.Le Borgne R, Hoflack B. Protein transport from the secretory to the endocytic pathway in mammalian cells. Biochim. Biophys. Acta. 1998;1404:195–209. doi: 10.1016/s0167-4889(98)00057-3. [DOI] [PubMed] [Google Scholar]
- 85.Seaman MN, Marcusson EG, Cereghino JL, Emr SD. Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J. Cell Biol. 1997;137:79–92. doi: 10.1083/jcb.137.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seaman MN, McCaffery JM, Emr SD. A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J. Cell Biol. 1998;142:665–681. doi: 10.1083/jcb.142.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nothwehr SF, Ha SA, Bruinsma P. Sorting of yeast membrane proteins into an endosome-to-golgi pathway involves direct interaction of their cytosolic domains with Vps35p. J. Cell Biol. 2000;151:297–310. doi: 10.1083/jcb.151.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Horazdovsky BF, Davies BA, Seaman MN, McLaughlin SA, Yoon S, Emr SD. A sorting nexin-1 homologue, Vps5p, forms a complex with Vps17p and is required for recycling the vacuolar protein-sorting receptor. Mol. Biol. Cell. 1997;8:1529–1541. doi: 10.1091/mbc.8.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nothwehr SF, Hindes AE. The yeast VPS5/GRD2 gene encodes a sorting nexin-1-like protein required for localizing membrane proteins to the late Golgi. J. Cell Sci. 1997;110:1063–1072. doi: 10.1242/jcs.110.9.1063. [DOI] [PubMed] [Google Scholar]
- 90.Haft CR, Luz Sierra M. de la, Barr VA, Haft DH, Taylor SI. Identification of a family of sorting nexin molecules and characterization of their association with receptors. Mol. Cell. Biol. 1998;18:7278–7287. doi: 10.1128/mcb.18.12.7278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Haft CR, Sierra ML, Bafford R, Lesniak MA, Barr VA, Taylor SI. Human orthologs of yeast vacuolar protein sorting proteins vps26, 29, and 35: assembly into multimeric complexes. Mol. Biol. Cell. 2000;11:4105–4116. doi: 10.1091/mbc.11.12.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Voos W, Stevens TH. Retrieval of resident late-Golgi membrane proteins from the prevacuolar compartment of Saccharomyces cerevisiae is dependent on the function of Grd19p. J. Cell Biol. 1998;140:577–590. doi: 10.1083/jcb.140.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Spelbrink RG, Nothwehr SF. The yeast GRD20 gene is required for protein sorting in the trans-Golgi network/endosomal system and for polarization of the actin cytoskeleton. Mol. Biol. Cell. 1999;10:4263–4281. doi: 10.1091/mbc.10.12.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Conibear E, Stevens TH. Vps52p, Vps53p, and Vps54p form a novel multisubunit complex required for protein sorting at the yeast late Golgi. Mol. Biol. Cell. 2000;11:305–323. doi: 10.1091/mbc.11.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bankaitis VA, Aitken JR, Cleves AE, Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature. 1990;347:561–562. doi: 10.1038/347561a0. [DOI] [PubMed] [Google Scholar]
- 96.Bankaitis VA, Malehorn DE, Emr SD, Greene R. The Saccharomyces cerevisiae SEC14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J. Cell Biol. 1989;108:1271–1281. doi: 10.1083/jcb.108.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li X, Xie Z, Bankaitis VA. Phosphatidylinositol/phosphatidylcholine transfer proteins in yeast. Biochim. Biophys. Acta. 2000;1486:55–71. doi: 10.1016/s1388-1981(00)00048-2. [DOI] [PubMed] [Google Scholar]
- 98.Liscovitch M, Cantley LC. Signal transduction and membrane traffic: the PITP/phosphoinositide connection. Cell. 1995;81:659–662. doi: 10.1016/0092-8674(95)90525-1. [DOI] [PubMed] [Google Scholar]
- 99.Cunningham E, Tan SK, Swigart P, Hsuan J, Bankaitis V, Cockcroft S. The yeast and mammalian isoforms of phosphatidylinositol transfer protein can all restore phospholipase C-mediated inositol lipid signaling in cytosol-depleted RBL-2H3 and HL-60 cells. Proc. Natl. Acad. Sci. USA. 1996;93:6589–6593. doi: 10.1073/pnas.93.13.6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cockcroft S. Mammalian phosphatidylinositol transfer proteins: emerging roles in signal transduction and vesicular traffic. Chem. Phys. Lipids. 1999;98:23–33. doi: 10.1016/s0009-3084(99)00015-8. [DOI] [PubMed] [Google Scholar]
- 101.Hay JC, Martin TF. Phosphatidylinositol transfer protein required for ATP-dependent priming of Ca(2+)-activated secretion. Nature. 1993;366:572–575. doi: 10.1038/366572a0. [DOI] [PubMed] [Google Scholar]
- 102.Ohashi M, Jan de Vries K, Frank R, Snoek G, Bankaitis V, Wirtz K, et al. A role for phosphatidylinositol transfer protein in secretory vesicle formation. Nature. 1995;377:544–547. doi: 10.1038/377544a0. [DOI] [PubMed] [Google Scholar]
- 103.Jones SM, Alb JG, Jr, Phillips SE, Bankaitis VA, Howell KE. A phosphatidylinositol 3-kinase and phosphatidylinositol transfer protein act synergistically in formation of constitutive transport vesicles from the trans-Golgi network. J. Biol. Chem. 1998;273:10349–10354. doi: 10.1074/jbc.273.17.10349. [DOI] [PubMed] [Google Scholar]
- 104.Phillips SE, Sha B, Topalof L, Xie Z, Alb JG, Klenchin VA, et al. Yeast Sec14p deficient in phosphatidylinositol transfer activity is functional in vivo. Mol. Cell. 1999;4:187–197. doi: 10.1016/s1097-2765(00)80366-4. [DOI] [PubMed] [Google Scholar]
- 105.Sreenivas A, Patton-Vogt JL, Bruno V, Griac P, Henry SA. A role for phospholipase D (Pld1p) in growth, secretion, and regulation of membrane lipid synthesis in yeast. J. Biol. Chem. 1998;273:16635–16638. doi: 10.1074/jbc.273.27.16635. [DOI] [PubMed] [Google Scholar]
- 106.Xie Z, Fang M, Rivas MP, Faulkner AJ, Sternweis PC, Engebrecht JA, et al. Phospholipase D activity is required for suppression of yeast phosphatidylinositol transfer protein defects. Proc. Natl. Acad. Sci. USA. 1998;95:12346–12351. doi: 10.1073/pnas.95.21.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ktistakis NT, Brown HA, Sternweis PC, Roth MG. Phospholipase D is present on Golgi-enriched membranes and its activation by ADP ribosylation factor is sensitive to brefeldin A. Proc. Natl. Acad. Sci. USA. 1995;92:4952–4956. doi: 10.1073/pnas.92.11.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ktistakis NT, Brown HA, Waters MG, Sternweis PC, Roth MG. Evidence that phospholipase D mediates ADP ribosylation factor-dependent formation of Golgi coated vesicles. J. Cell Biol. 1996;134:295–306. doi: 10.1083/jcb.134.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen YG, Siddhanta A, Austin CD, Hammond SM, Sung TC, Frohman MA, et al. Phospholipase D stimulates release of nascent secretory vesicles from the trans-Golgi network. J. Cell Biol. 1997;138:495–504. doi: 10.1083/jcb.138.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Siddhanta A, Shields D. Secretory vesicle budding from the trans-Golgi network is mediated by phosphatidic acid levels. J. Biol. Chem. 1998;273:17995–17998. doi: 10.1074/jbc.273.29.17995. [DOI] [PubMed] [Google Scholar]
- 111.Siddhanta A, Backer JM, Shields D. Inhibition of phosphatidic acid synthesis alters the structure of the Golgi apparatus and inhibits secretion in endocrine cells. J. Biol. Chem. 2000;275:12023–12031. doi: 10.1074/jbc.275.16.12023. [DOI] [PubMed] [Google Scholar]
- 112.Hama H, Schnieders EA, Thorner J, Takemoto JY, De-Wald DB. Direct involvement of phosphatidylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 1999;274:34294–34300. doi: 10.1074/jbc.274.48.34294. [DOI] [PubMed] [Google Scholar]
- 113.Walch-Solimena C, Novick P. The yeast phosphatidylinositol-4-OH kinease Pik 1 regulates secretion at the Golgi. Nat. Cell Biol. 1999;1:523–525. doi: 10.1038/70319. [DOI] [PubMed] [Google Scholar]
- 114.Hendricks KB, Wang BQ, Schnieders EA, Thorner J. Yeast homologue of neuronal frequenin is a regulator of phosphatidylinositol-4-OH kinase. Nat. Cell Biol. 1999;1:234–241. doi: 10.1038/12058. [DOI] [PubMed] [Google Scholar]
- 115.Wiedemann C, Schafer T, Burger MM, Sihra TS. An essential role for a small synaptic vesicle-associated phosphatidylinositol 4-kinase in neurotransmitter release. J. Neurosci. 1998;18:5594–5602. doi: 10.1523/JNEUROSCI.18-15-05594.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wiedemann C, Schafer T, Burger MM. Chromaffin granule associated phosphatidylinositol 4-kinease activity is required for stimulated secretion. EMBO J. 1996;15:2094–2101. [PMC free article] [PubMed] [Google Scholar]
- 117.Rivosecchi R, Pongs O, Theil T, Mallart A. Implication of frequenin in the facilitation of transmitter release in Drosophila. J. Physiol. (Lond.) 1994;474:223–232. doi: 10.1113/jphysiol.1994.sp020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pongs O, Lindemeier J, Zhu XR, Theil T, Engelkamp D, Krah-Jentgens I, et al. Frequenin – a novel calcium-binding protein that modulates synaptic efficacy in the Drosophila nervous system. Neuron. 1993;11:15–28. doi: 10.1016/0896-6273(93)90267-u. [DOI] [PubMed] [Google Scholar]
- 119.McFerran BW, Graham ME, Burgoyne RD. Neuronal Ca2+ sensor 1, the mammalian homologue of freqenin, is expressed in chromaffin and PC12 cells and regulates neurosecretion from dense-core granules. J. Biol. Chem. 1998;273:22768–22772. doi: 10.1074/jbc.273.35.22768. [DOI] [PubMed] [Google Scholar]
- 120.Weisz OA, Gibson GA, Leung SM, Roder J, Jeromin A. Overexpression of frequenin, a modulator of phosphatidylinositol 4-kinase, inhibits biosynthetic delivery of an apical protein in polarized Madin-Darby canine kidney cells. J. Biol. Chem. 2000;275:24341–24347. doi: 10.1074/jbc.M000671200. [DOI] [PubMed] [Google Scholar]
- 121.Matter K. Epithelial polarity: sorting out the sorters. Curr. Biol. 2000;10:R39–R42. doi: 10.1016/s0960-9822(99)00256-0. [DOI] [PubMed] [Google Scholar]
- 122.Mostov KE, Verges M, Altschuler Y. Membrane traffic in polarized epithelial cells. Curr. Opin. Cell Biol. 2000;12:483–490. doi: 10.1016/s0955-0674(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 123.Ikonen E, Simons K. Protein and lipid sorting from the trans-Golgi network to the plasma membrane in polarized cells. Semin. Cell Dev. Biol. 1998;9:503–509. doi: 10.1006/scdb.1998.0258. [DOI] [PubMed] [Google Scholar]
- 124.Matter K, Mellman I. Mechanisms of cell polarity: sorting and transport in epithelial cells. Curr. Opin. Cell Biol. 1994;6:545–554. doi: 10.1016/0955-0674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- 125.Orzech E, Schlessinger K, Weiss A, Okamoto CT, Aroeti B. Interactions of the AP-1 Golgi adaptor with the polymeric immunoglobulin receptor and their possible role in mediating brefeldin A-sensitive basolateral targeting from the trans-Golgi network. J. Biol. Chem. 1999;274:2201–2215. doi: 10.1074/jbc.274.4.2201. [DOI] [PubMed] [Google Scholar]
- 126.Futter CE, Gibson A, Allchin EH, Maxwell S, Ruddock LJ, Odorizzi G, et al. In polarized MDCK cells basolateral vesicles arise from clathrin-gamma-adaptin-coated domains on endosomal tubules. J. Cell Biol. 1998;141:611–623. doi: 10.1083/jcb.141.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Orzech E, Cohen S, Weiss A, Aroeti B. Interactions between the exocytic and endocytic pathways in polarized Madin-Darby canine kidney cells. J. Biol. Chem. 2000;275:15207–15219. doi: 10.1074/jbc.275.20.15207. [DOI] [PubMed] [Google Scholar]
- 128.Chen W, Feng Y, Chen D, Wandinger-Ness A. Rab11 is required for trans-Golgi network-toplasma membrane transport and a preferential target for GDP dissociation inhibitor. Mol. Biol. Cell. 1998;9:3241–3257. doi: 10.1091/mbc.9.11.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Futter CE, Connolly CN, Cutler DF, Hopkins CR. Newly synthesized transferrin receptors can be detected in the endosome before they appear on the cell surface. J. Biol. Chem. 1995;270:10999–11003. doi: 10.1074/jbc.270.18.10999. [DOI] [PubMed] [Google Scholar]
- 130.Leitinger B, Hille-Rehfeld A, Spiess M. Biosynthetic transport of the asialoglycoprotein receptor H1 to the cell surface occurs via endosomes. Proc. Natl. Acad. Sci. USA. 1995;92:10109–10113. doi: 10.1073/pnas.92.22.10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sariola M, Saraste J, Kuismanen E. Communication of post-Golgi elements with early endocytic pathway: regulation of endoproteolytic cleavage of Semliki Forest virus p62 precursor. J. Cell Sci. 1995;108:2465–2475. doi: 10.1242/jcs.108.6.2465. [DOI] [PubMed] [Google Scholar]
- 132.Roush DL, Gottardi CJ, Naim HY, Roth MG, Caplan MJ. Tyrosine-based membrane protein sorting signals are differentially interpreted by polarized Madin-Darby canine kidney and LLC-PK1 epithelial cells. J. Biol. Chem. 1998;273:26862–26869. doi: 10.1074/jbc.273.41.26862. [DOI] [PubMed] [Google Scholar]
- 133.Folsch H, Ohno H, Bonifacino JS, Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99:189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- 134.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 135.Kurzchalia TV, Parton RG. Membrane microdomains and caveolae. Curr. Opin. Cell Biol. 1999;11:424–431. doi: 10.1016/s0955-0674(99)80061-1. [DOI] [PubMed] [Google Scholar]
- 136.Jacobson K, Dietrich C. Looking at lipid rafts? Trends Cell Biol. 1999;9:87–91. doi: 10.1016/s0962-8924(98)01495-0. [DOI] [PubMed] [Google Scholar]
- 137.Benting JH, Rietveld AG, Simons K. N-Glycans mediate the apical sorting of a GPI-anchored, raft-associated protein in Madin-Darby canine kidney cells. J. Cell Biol. 1999;146:313–320. doi: 10.1083/jcb.146.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lin S, Naim HY, Rodriguez AC, Roth MG. Mutations in the middle of the transmembrane domain reverse the polarity of transport of the influenza virus hemagglutinin in MDCK epithelial cells. J. Cell Biol. 1998;142:51–57. doi: 10.1083/jcb.142.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rodriguez-Boulan E, Gonzalez A. Glycans in post-Golgi apical targeting: sorting signals or structural props? Trends Cell Biol. 1999;9:291–294. doi: 10.1016/s0962-8924(99)01595-0. [DOI] [PubMed] [Google Scholar]
- 140.Dupree P, Parton RG, Raposo G, Kurzchalia TV, Simons K. Caveolae and sorting in the trans-Golgi network of epithelial cells. EMBO J. 1993;12:1597–1605. doi: 10.1002/j.1460-2075.1993.tb05804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lisanti MP, Tang ZL, Sargiacomo M. Caveolin forms a hetero-oligomeric protein complex that interacts with an apical GPI-linked protein: implications for the biogenesis of caveolae. J. Cell Biol. 1993;123:595–604. doi: 10.1083/jcb.123.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zurzolo C, Hof W. van't, Meer G. van, Rodriguez-Boulan E. VIP21/caveolin, glycosphingolipid clusters and the sorting of glycosylphosphatidylinositol-anchored proteins in epithelial cells. EMBO J. 1994;13:42–53. doi: 10.1002/j.1460-2075.1994.tb06233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lipardi C, Mora R, Colomer V, Paladino S, Nitsch L, Rodriguez-Boulan E, et al. Caveolin transfection results in caveolae formation but not apical sorting of glycosylphosphatidylinositol (GPI)-anchored proteins in epithelial cells. J. Cell Biol. 1998;140:617–626. doi: 10.1083/jcb.140.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zacchetti D, Peranen J, Murata M, Fiedler K, Simons K. VIP17/MAL, a proteolipid in apical transport vesicles. FEBS Lett. 1995;377:465–469. doi: 10.1016/0014-5793(95)01396-2. [DOI] [PubMed] [Google Scholar]
- 145.Puertollano R, Martin-Belmonte F, Millan J, Marco M. C. de, Albar JP, Kremer L, et al. The MAL proteolipid is necessary for normal apical transport and accurate sorting of the influenza virus hemagglutinin in Madin-Darby canine kidney cells. J. Cell Biol. 1999;145:141–151. doi: 10.1083/jcb.145.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Frank M. MAL, a proteolipid in glycosphingolipid enriched domains: functional implications in myelin and beyond. Prog. Neurobiol. 2000;60:531–544. doi: 10.1016/s0301-0082(99)00039-8. [DOI] [PubMed] [Google Scholar]
- 147.Plant PJ, Lafont F, Lecat S, Verkade P, Simons K, Rotin D. Apical membrane targeting of Nedd4 is mediated by an association of its C2 domain with annexin XIIIb. J. Cell Biol. 2000;149:1473–1484. doi: 10.1083/jcb.149.7.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lafont F, Lecat S, Verkade P, Simons K. Annexin XIIIb associates with lipid microdomains to function in apical delivery. J. Cell Biol. 1998;142:1413–1427. doi: 10.1083/jcb.142.6.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Craig AM, Banker G. Neuronal polarity. Annu. Ref. Neurosci. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- 150.Jareb M, Banker G. The polarized sorting of membrane proteins expressed in cultured hippocampal neurons using viral vectors. Neuron. 1998;20:855–867. doi: 10.1016/s0896-6273(00)80468-7. [DOI] [PubMed] [Google Scholar]
- 151.Winckler B, Mellman I. Neuronal polarity: controlling the sorting and diffusion of membrane components. Neuron. 1999;23:637–640. doi: 10.1016/s0896-6273(01)80021-0. [DOI] [PubMed] [Google Scholar]
- 152.Shah JV, Goldstein LS. Does motor protein intelligence contribute to neuronal polarity? Neuron. 2000;26:281–282. doi: 10.1016/s0896-6273(00)81158-7. [DOI] [PubMed] [Google Scholar]
- 153.Arvan P, Castle D. Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem. J. 1998;332:593–610. doi: 10.1042/bj3320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Moore HH, Kelly RB. Re-routing of a secretory protein by fusion with human growth hormone sequences. Nature. 1986;321:443–446. doi: 10.1038/321443a0. [DOI] [PubMed] [Google Scholar]
- 155.Thorne BA, Viveros OH, Thomas G. Expression and processing of mouse proopiomelanocortin in bovine adrenal chromaffin cells: a model system to study tissue-specific prohormone processing. J. Biol. Chem. 1991;266:13607–13615. [PubMed] [Google Scholar]
- 156.Kromer A, Glombik MM, Huttner WB, Gerdes HH. Essential role of the disulfide-bonded loop of chromogranin B for sorting to secretory granules is revealed by expression of a deletion mutant in the absence of endogenous granin synthesis. J. Cell Biol. 1998;140:1331–1346. doi: 10.1083/jcb.140.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Siekevitz P, Palade GE. Distribution of newly synthesized amylase in microsomal subfractions of guinea pigs pancreas. J. Cell Biol. 1966;30:519–530. doi: 10.1083/jcb.30.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rambourg A, Clermont Y, Chretien M, Olivier L. Formation of secretory granules in the Golgi apparatus of prolactin cells in the rat pituitary gland: a stereoscopic study. Anat. Rec. 1992;232:169–179. doi: 10.1002/ar.1092320202. [DOI] [PubMed] [Google Scholar]
- 159.Glombik MM, Gerdes H. Signal-mediated sorting of neuropeptides and prohormones: secretory granule biogenesis revisited. Biochimie. 2000;82:315–326. doi: 10.1016/s0300-9084(00)00195-4. [DOI] [PubMed] [Google Scholar]
- 160.Brechler V, Chu WN, Baxter JD, Thibault G, Reudelhuber TL. A protease processing site is essential for prorenin sorting to the regulated secretory pathway. J. Biol. Chem. 1996;271:20636–20640. doi: 10.1074/jbc.271.34.20636. [DOI] [PubMed] [Google Scholar]
- 161.Glombik MM, Kromer A, Salm T, Huttner WB, Gerdes HH. The disulfide-bonded loop of chromogranin B mediates membrane binding and directs sorting from the trans-Golgi network to secretory granules. EMBO J. 1999;18:1059–1070. doi: 10.1093/emboj/18.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cool DR, Fenger M, Snell CR, Loh YP. Identification of the sorting signal motif within pro-opiomelanocortin for the regulated secretory pathway. J. Biol. Chem. 1995;270:8723–8729. doi: 10.1074/jbc.270.15.8723. [DOI] [PubMed] [Google Scholar]
- 163.Roy P, Chevrier D, Fournier H, Racine C, Zollinger M, Crine P, et al. Investigation of a possible role of the amino-terminal pro-region of proopiomelanocortin in its processing and targeting to secretory granules. Mol. Cell. Endocrinol. 1991;82:237–250. doi: 10.1016/0303-7207(91)90037-s. [DOI] [PubMed] [Google Scholar]
- 164.Cowley DJ, Moore YR, Darling DS, Joyce PB, Gorr SU. N- and C-terminal domains direct cell type-specific sorting of chromogranin A to secretory granules. J. Biol. Chem. 2000;275:7743–7748. doi: 10.1074/jbc.275.11.7743. [DOI] [PubMed] [Google Scholar]
- 165.Cool DR, Loh YP. Carboxypeptidase E is a sorting receptor for prohormones: binding and kinetic studies. Mol. Cell. Endocrinol. 1998;139:7–13. doi: 10.1016/s0303-7207(98)00081-1. [DOI] [PubMed] [Google Scholar]
- 166.Cool DR, Normant E, Shen F, Chen HC, Pannell L, Zhang Y, et al. Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe(fat) mice. Cell. 1997;88:73–83. doi: 10.1016/s0092-8674(00)81860-7. [DOI] [PubMed] [Google Scholar]
- 167.Irminger JC, Verchere CB, Meyer K, Halban PA. Proinsulin targeting to the regulated pathway is not impaired in carboxypeptidase E-deficient Cpefat/Cpefat mice. J. Biol. Chem. 1997;272:27532–27534. doi: 10.1074/jbc.272.44.27532. [DOI] [PubMed] [Google Scholar]
- 168.Varlamov O, Leiter EH, Fricker L. Induced and spontaneous mutations at Ser202 of carboxypeptidase E: effect on enzyme expression, activity, and intracellular routing. J. Biol. Chem. 1996;271:13981–13986. doi: 10.1074/jbc.271.24.13981. [DOI] [PubMed] [Google Scholar]
- 169.Blazquez M, Thiele C, Huttner WB, Docherty K, Shennan KI. Involvement of the membrane lipid bi-layer in sorting prohormone convertase 2 into the regulated secretory pathway. Biochem. J. 2000;349:843–852. doi: 10.1042/bj3490843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Dhanvantari S, Loh YP. Lipid raft association of carboxypeptidase E is necessary for its function as a regulated secretory pathway sorting receptor. J. Biol. Chem. 2000;275:29887–29893. doi: 10.1074/jbc.M005364200. [DOI] [PubMed] [Google Scholar]
- 171.Wasmeier C, Hutton JC. Molecular cloning of phogrin, a protein-tyrosine phosphatase homologue localized to insulin secretory granule membranes. J. Biol. Chem. 1996;271:18161–18170. doi: 10.1074/jbc.271.30.18161. [DOI] [PubMed] [Google Scholar]
- 172.Klumperman J, Kuliawat R, Griffith JM, Geuze HJ, Arvan P. Mannose 6-phosphate receptors are sorted from immature secretory granules via adaptor protein AP-1, clathrin, and syntaxin 6-positive vesicles. J. Cell Biol. 1998;141:359–371. doi: 10.1083/jcb.141.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kuliawat R, Klumperman J, Ludwig T, Arvan P. Differential sorting of lysosomal enzymes out of the regulated secretory pathway in pancreatic beta-cells. J. Cell Biol. 1997;137:595–608. doi: 10.1083/jcb.137.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Paquet L, Bergeron F, Boudreault A, Seidah NG, Chretien M, Mbikay M, et al. The neuroendocrine precursor 7B2 is a sulfated protein proteolytically processed by a ubiquitous furin-like convertase. J. Biol. Chem. 1994;269:19279–19285. [PubMed] [Google Scholar]
- 175.Canaff L, Bennett HP, Hou Y, Seidah NG, Hendy GN. Proparathyroid hormone processing by the proprotein convertase-7: comparison with furin and assessment of modulation of parathyroid convertase messenger ribonucleic acid levels by calcium and 1,25-dihydroxyvitamin D3. Endocrinology. 1999;140:3633–3642. doi: 10.1210/endo.140.8.6882. [DOI] [PubMed] [Google Scholar]
- 176.Cohn DV, Fasciotto BH, Reese BK, Zhang JX. Chromogranin A: a novel regulator of parathyroid gland secretion. J. Nutr. 1995;125:2015S–2019S. doi: 10.1093/jn/125.suppl_7.2015S. [DOI] [PubMed] [Google Scholar]
- 177.Klumperman J, Spijker S, Minnen J. van, Sharp-Baker H, Smit AB, Geraerts WP. Cell type-specific sorting of neuropeptides: a mechanism to modulate peptide composition of large dense-core vesicles. J. Neurosci. 1996;16:7930–7940. doi: 10.1523/JNEUROSCI.16-24-07930.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Austin C, Hinners I, Tooze SA. Direct and GTP-dependent interaction of ADP-ribosylation factor 1 with clathrin adaptor protein AP-1 on immature secretory granules. J. Biol. Chem. 2000;275:21862–21869. doi: 10.1074/jbc.M908875199. [DOI] [PubMed] [Google Scholar]
- 179.Kuliawat R, Prabakaran D, Arvan P. Proinsulin endoproteolysis confers enhanced targeting of processed insulin to the regulated secretory pathway. Mol. Biol. Cell. 2000;11:1959–1972. doi: 10.1091/mbc.11.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]


