Abstract
Introduction
In pre-dialysis chronic kidney disease (CKD), the association of muscle mass with mortality is poorly defined, and no study has examined outcomes related to the co-occurrence of low muscle mass and excess adiposity (sarcopenic-obesity). Methods: We examined abnormalities of muscle and fat mass in adult participants of the National Health and Nutrition Examination Survey 1999–2004. We determined whether associations of body composition with all-cause mortality differed between participants with CKD compared to those without.
Results
CKD modified the association of body composition with mortality (p=0.01 for interaction). In participants without CKD, both sarcopenia and sarcopenic obesity were independently associated with increased mortality compared with normal body composition (hazard ratio (HR) 1.44 (95%CI 1.07–1.93) and 1.64 (95%CI 1.26–2.13), respectively). These associations were not present among participants with CKD. Conversely, obese persons had the lowest adjusted risk of death, with an increased risk among those with sarcopenia (HR 1.43 (95%CI 1.05–1.95)) but not sarcopenic-obesity (p=0.003 for interaction by CKD status; HR 1.21 (95%CI 0.89–1.65)), compared with obesity.
Conclusion
Sarcopenia associates with increased mortality regardless of eGFR, but excess adiposity modifies this association among people with CKD. Future studies of prognosis and weight loss and exercise interventions in CKD patients should consider muscle mass and adiposity together rather than in isolation.
Keywords: body composition, lean body mass, skeletal muscle, appendicular skeletal muscle mass index, sarcopenic obesity, chronic kidney disease
INTRODUCTION
Muscle wasting is common among patients with end-stage renal disease (ESRD) receiving dialysis and associates with increased morbidity and mortality.1–3 In earlier stages of chronic kidney disease (CKD), the association of muscle mass with outcomes is less well-defined. There are few data on mortality associated with sarcopenia, or low muscle mass, although it is common among individuals with advanced pre-dialysis kidney disease.4 Studies that have examined urinary creatinine excretion and total lean body mass have yielded inconsistent results.5–7
Accurate prognostication may require simultaneously examining the muscle and fat compartments. BMI levels in the overweight and obese range are associated with the lowest mortality risk in CKD patients.8 However, people with CKD who have excess adiposity but are also sarcopenic – a not uncommon finding – are very unlikely to be classified as obese by BMI.9, 10 In hemodialysis patients, this phenotype, called sarcopenic-obesity, is associated with greater inflammation and increased mortality.11 The prognostic significance of sarcopenic-obesity in persons with CKD is not known.12
We hypothesized that sarcopenia and sarcopenic-obesity are associated with increased all-cause mortality among individuals with CKD who are not on dialysis. Our definition of CKD was restricted to people with estimated glomerular filtration rate (eGFR) <60 mL/min/1.73m2 because the pathophysiologic link between CKD and low muscle mass is greatest in this subgroup, and our prior work demonstrated that the prevalence of sarcopenia increased below this threshold.10 We tested this hypothesis using nationally representative data from the National Health and Nutrition Examination Survey (NHANES), including dual-energy x-ray absorptiometry (DEXA)-derived muscle and fat mass to classify participants into 4 mutually exclusive body composition categories: non-sarcopenic non-obese, obese, sarcopenic, and sarcopenic-obese.10 Our objective was to examine this question among people with CKD and to determine if associations differed from people without CKD in the same nationally representative dataset. We hypothesized that associations of both sarcopenia and sarcopenic-obesity with death would be stronger in persons with CKD than in those without, as CKD-induced muscle wasting is likely a poor prognostic factor.
When we initially conducted our analyses, linked mortality data were available with follow-up through December 31, 2006. Subsequently, we repeated our analyses when updated data became available with mortality status ascertained through December 31, 2011. We considered our findings from analyses conducted using the 2011 dataset to be our primary results, given the greater statistical power and longer follow-up time. However, we also compared the results obtained using each of these datasets to examine the impact of the duration of follow-up time on our findings.
METHODS
Study Population
NHANES 1999–2004 was a program of studies designed to assess the health and nutritional status of non-institutionalized civilians in the United States.13 The NHANES protocol was approved by the National Center for Health Statistics ethics review board and written informed consent was obtained from all participants. We examined adults ≥20 years old (n=15,332) with available body composition data (n=12,732), excluding participants with eGFR <15 mL/min/1.73 m2 (n=34) or missing data on covariates of interest (n=1,082). The resultant cohort had 11,616 participants.
Data collection
Information on race/ethnicity, education, smoking status, and comorbidities was based on self-report. Activity level was calculated as metabolic equivalents (MET-min/wk) based on questions regarding the frequency and duration of activities such as walking, cycling, home or yard work, and moderate or vigorous leisure activity performed within the past 30 days.14 Participants with diabetes mellitus were defined as those who had a physician diagnosis while not pregnant or were using insulin or oral hypoglycemic medications or had a glycohemoglobin level ≥6.5%. Hypertension was defined by systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, history of physician diagnosis, and/or antihypertensive medication use.15 Cardiovascular disease (CVD) was defined by self-report of a physician diagnosis of congestive heart failure, coronary heart disease, angina, myocardial infarction, or stroke.
Serum chemistries were measured using the Hitachi 917 multichannel analyzer (Roche Diagnostics, Indianapolis, IN) in 1999–2001 and the Beckman Synchron LX20 (Beckman Coulter Inc., Brea, CA) in 2002–2004. C-reactive protein (CRP) was quantified by latex-enhanced nephelometry. Serum albumin was measured by the bromocresol purple method. A modified kinetic Jaffe reaction was used to measure serum creatinine and the values from 1999–2000 were calibrated to the Cleveland Clinic laboratory standard by multiplying by 1.013 and adding 0.147. Values from 2001–2004 did not need correction. eGFR was calculated using the CKD-EPI equation.16 CKD was defined as an eGFR < 60mL/min/1.73m2.
Body Composition Data
Missing and invalid DEXA data were accounted for through multiple imputation by the National Center for Health Statistics.17 Details of the DEXA quality control, data validation, and multiple imputation procedures are available.17–20 DEXA data for at least one body region were imputed in 2,472 participants (21.3%). Muscle mass was quantified using the appendicular skeletal muscle mass index (ASMI, total lean mass of the four extremities divided by the square of the height).21 Sarcopenia was defined as ASMI <5.45 kg/m2 in women and <7.26 kg/m2 in men.21, 22 These cutoffs correspond to two standard deviations below the sex-specific means for healthy young adults 18–40 years of age and are recommended by the consensus guidelines of the European Working Group on Sarcopenia in Older People.23, 24 We examined ASMI rather total lean mass based on this recommendation, and also because appendicular lean mass is not confounded by changes in visceral lean mass due to chronic disease and is likely more relevant for functional status. Obesity was determined as % total body fat (TBF) > 42.1% for women and 29.6% for men, corresponding to the sex-specific 60th percentile for the study sample22 and to the current World Health Organization guidelines for BMI-defined obesity.25, 26
Outcome variables
All-cause mortality was determined primarily through probabilistic record matching with the National Death Index and was available using public-use linked mortality files.27, 28 Mortality status was initially ascertained through December 31, 2006,29 and then additional data became available with mortality status ascertained through December 31, 2011.30 Date and cause of death for selected records were subjected to data perturbation techniques due to concerns regarding participant anonymity, but vital status was not perturbed. The results of Cox proportional hazard models are not affected by these data perturbation techniques when compared with non-perturbed restricted-use data.31, 32
Statistical Analysis
Participant characteristics were examined within body composition categories, for the overall cohort and for those with eGFR <60 mL/min/1.73m2. Differences across categories were tested for statistical significance using linear regression, logistic regression, or multinomial regression as appropriate. Kaplan-Meier survivor functions were examined within body composition categories for the overall cohort and for those with eGFR <60 mL/min/1.73m2 and tested for statistical significance using the Cox test of equality and accounting for NHANES sampling weights. Cox proportional hazards models were created to examine associations of body composition categories with all-cause mortality. We examined unadjusted models, followed by adjustment for age, sex, and race/ethnicity, and then multivariable models which included potential confounders of the association of body composition with mortality, including education level, physical activity, smoking status, diagnosis of diabetes mellitus, hypertension, CVD, history of cancer excluding non-melanoma skin cancer, eGFR, and urine albumin-creatinine ratio. We then created separate models additionally adjusting for serum albumin and CRP, which as markers of inflammation may lie in the causal pathway with body composition and mortality. The functional form of continuous variables was tested for linearity using higher-order terms and categorical variables. We tested for interaction by CKD status by including multiplicative interaction terms in the models, and then repeated our analyses separately among participants with eGFR < and ≥ 60 mL/min/1.73m2. Effect modification by other covariates was tested using multiplicative interaction terms. The proportional hazards assumption was tested by visual inspection of log-log plots. All analyses used NHANES-appropriate sampling weights and all except the Kaplan-Meier analyses accounted for the complex multistage cluster design using the “survey” command in Stata 13.1 (Stata Corporation, College Station, TX, USA). A p-value <0.05 was considered statistically significant.
Sensitivity Analyses
We performed a matched-pair analysis in which we repeated our analyses in a cohort in which each participant with an eGFR <60 mL/min/1.73m2 was uniquely matched with a participant with eGFR ≥60 mL/min/1.73m2 based on age (±1 year), sex, and non-Hispanic black status. To determine if our findings were explained by early mortality related to sarcopenia, we repeated our analyses after excluding participants who died within 24 months of their NHANES examination. We explored confounding related to the use of creatinine-based eGFR by repeating our analyses using cystatin C-based eGFR33 in the subgroup with cystatin C measurements. To determine if similar findings were observed without using threshold definitions for body composition components, we examined associations with all-cause mortality when ASMI and %TBF were modeled as continuous variables.
RESULTS
Participant Characteristics
Overall 10.9% of participants were sarcopenic but not obese and 3.4% were sarcopenic-obese. Compared with the other body composition categories, sarcopenic-obese participants were older, more likely to be men and to be non-Hispanic white, current or former smokers, less active, and to have hypertension, CVD, eGFR <60 mL/min/1.73m2, and urine albumin-creatinine ratio >30 mg/g (Table 1). They also had a higher prevalence of diabetes, lower serum albumin and higher CRP than non-sarcopenic, non-obese and sarcopenic non-obese participants but not compared with obese participants. The mean BMI was similar between sarcopenic-obese and non-sarcopenic, non-obese participants, while for the former group, %TBF was similar to obese participants and ASMI was similar to sarcopenic non-obese participants.
Table 1.
Participant characteristics by body composition category in 11,616 participants of NHANES 1999–2004
| Characteristic | Non- sarcopenic, non-obese |
Sarcopenia only |
Obese only | Sarcopenic obese |
P |
|---|---|---|---|---|---|
| Proportion (%) | 49.4 (0.9) | 10.9 (0.4) | 36.2 (0.7) | 3.4 (0.2) | |
| Age (years) | 42.2 (0.3) | 47.9 (0.6) | 49.5 (0.3) | 63.4 (0.9) | <0.001 |
| Women (%) | 50.3 (0.6) | 52.6 (1.6) | 51.6 (0.7) | 42.3 (2.7) | 0.009 |
| Race/Ethnicity (%) | <0.001 | ||||
| Non-Hispanic White | 69.9 (1.6) | 76.5 (2.3) | 73.7 (1.8) | 81.7 (3.3) | |
| Mexican American | 7.2 (0.7) | 7.0 (1.0) | 7.5 (1.2) | 4.9 (1.3) | |
| Non-Hispanic Black | 12.1 (1.1) | 3.7 (0.5) | 10.2 (1.1) | 1.9 (0.5) | |
| Other | 10.8 (1.3) | 12.8 (1.8) | 8.6 (1.3) | 11.5 (2.7) | |
| BMI (kg/m2) | 25.8 (0.1) | 20.9 (0.1) | 33.7 (0.2) | 25.2 (0.1) | <0.001 |
| BMI ≥ 30 kg/m2 proportion (%) | 10.8 (0.5) | 0 | 69.8 (1.2) | 2.3 (0.6) | <0.001 |
| Education (% ≥ high school) | 81.2 (0.8) | 78.5 (1.5) | 78.9 (0.8) | 76.1 (3.0) | 0.02 |
| Smoking status | <0.001 | ||||
| Never | 51.1 | 43.3 | 50.8 | 40.9 | |
| Former | 21.6 | 22.4 | 30.0 | 36.5 | |
| Current | 27.4 | 34.2 | 19.2 | 22.5 | |
| Activity level (MET-min/wk, %) | <0.001 | ||||
| 0 | 13.1 (0.7) | 19.7 (1.3) | 19.6 (0.9) | 25.0 (2.8) | |
| <500 | 17.9 (1.3) | 20.7 (1.5) | 24.3 (0.8) | 27.2 (2.4) | |
| 500–2000 | 35.1 (0.7) | 36.0 (1.5) | 35.2 (0.8) | 30.4 (3.0) | |
| >2000 | 33.8 (0.9) | 23.6 (1.6) | 20.9 (1.1) | 17.5 (2.3) | |
| Hypertension (%) | 30.1 (1.0) | 32.0 (2.0) | 54.0 (1.1) | 66.7 (3.4) | <0.001 |
| Diabetes mellitus (%) | 5.5 (0.5) | 4.5 (0.6) | 14.2 (0.6) | 10.9 (1.7) | <0.001 |
| Cardiovascular disease (%) | 5.2 (0.5) | 9.0 (0.8) | 11.4 (0.6) | 22.0 (2.7) | <0.001 |
| History of cancer (%) | 4.7 (0.4) | 8.8 (0.7) | 7.4 (0.5) | 17.5 (2.0) | <0.001 |
| eGFR <60 ml/min/1.73 m2 (%) | 4.1 (0.3) | 7.3 (0.8) | 8.1 (0.4) | 17.8 (1.9) | <0.001 |
| UACR >30 mg/g (%) | 6.9 (0.4) | 11.2 (1.2) | 10.9 (0.7) | 17.0 (2.0) | <0.001 |
| Serum bicarbonate (mEq/L) | 24.2 (0.1) | 24.6 (0.1) | 23.9 (0.1) | 24.6 (0.2) | 0.001 |
| Serum albumin (g/dL) | 4.40 (0.01) | 4.40 (0.01) | 4.25 (0.01) | 4.31 (0.02) | <0.001 |
| Serum CRP >1 mg/dL (%) | 5.1 (0.4) | 6.1 (0.7) | 16.2 (0.7) | 12.6 (1.8) | <0.001 |
| % Total body fat | 30.2 (0.1) | 29.1 (0.2) | 40.4 (0.1) | 38.4 (0.4) | <0.001 |
| ASMI (kg/m2) | 7.6 (0.0) | 5.6 (0.0) | 8.4 (0.0) | 6.0 (0.1) | <0.001 |
Abbreviations: NHANES, National Health and Nutrition Examination Survey; UACR, urine albumin-creatinine ratio; CRP, C-reactive protein; ASMI, appendicular skeletal muscle mass index.
Data are expressed as mean (standard error (SE)) or percent (SE).
Of 11,616 participants, 1,101 had an eGFR <60 mL/min/1.73m2 (Table 2). Sarcopenia without obesity and sarcopenic-obesity were present in 12.5% and 9.7%, respectively. Differences in demographic characteristics across categories were similar to the full cohort, but there was less variation in the prevalence of comorbidities. Patterns of variation across categories for BMI, activity level, serum albumin, CRP, %TBF, and ASMI were similar to those in the full cohort. Compared with participants with eGFR ≥60 mL/min/1.73m2, those with eGFR <60 mL/min/1.73m2 had an increased risk of death (HR 1.59 (95% CI 1.36–1.85) after age, sex, and race/ethnicity adjustment; HR 1.25 (95% CI 1.06–1.48) after multivariable adjustment). There was a non-linear association of eGFR with mortality (Table S1).
Table 2.
Participant characteristics by body composition category in 1,101 participants of NHANES 1999–2004 with eGFR < 60 ml/min/1.73 m2
| Characteristic | Non- sarcopenic, non-obese |
Sarcopenia only |
Obese only | Sarcopenic obese |
P |
|---|---|---|---|---|---|
| Proportion (%) | 31.6 (1.8) | 12.5 (1.2) | 46.2 (2.1) | 9.7 (1.1) | |
| Age (years) | 70.6 (1.0) | 75.2 (1.1) | 70.8 (0.8) | 77.2 (1.0) | <0.001 |
| Women (%) | 69.0 (3.3) | 53.3 (5.1) | 61.3 (2.3) | 35.5 (5.0) | <0.001 |
| Race/Ethnicity (%) | <0.001 | ||||
| Non-Hispanic White | 79.5 (2.3) | 88.5 (3.0) | 82.4 (2.5) | 90.3 (3.4) | |
| Mexican American | 1.6 (0.5) | 1.6 (0.6) | 1.5 (0.4) | 2.0 (1.0) | |
| Non-Hispanic Black | 11.7 (1.7) | 2.1 (1.0) | 8.6 (1.2) | 0.6 (0.7) | |
| Other | 7.2 (2.2) | 7.9 (2.7) | 7.5 (2.3) | 7.2 (3.0) | |
| BMI (kg/m2) | 26.2 (0.2) | 21.7 (0.2) | 32.8 (0.4) | 25.5 (0.3) | <0.001 |
| BMI ≥ 30 kg/m2 proportion (%) | 10.6 (2.3) | 0 | 64.8 (2.2) | 2.6 (2.0) | <0.001 |
| Education (% ≥ high school) | 66.9 (3.1) | 64.4 (6.0) | 67.3 (2.5) | 68.6 (5.7) | 0.96 |
| Smoking status | 0.004 | ||||
| Never | 61.0 | 40.1 | 51.3 | 45.2 | |
| Former | 31.3 | 41.3 | 41.7 | 47.6 | |
| Current | 7.7 | 18.6 | 7.0 | 7.2 | |
| Activity level (MET-min/wk, %) | 0.03 | ||||
| 0 | 25.1 (2.6) | 39.1 (5.0) | 34.4 (2.3) | 33.9 (5.7) | |
| <500 | 20.7 (2.6) | 20.1 (4.3) | 24.3 (2.6) | 27.9 (5.7) | |
| 500–2000 | 33.8 (3.5) | 25.6 (4.2) | 26.8 (2.4) | 29.5 (5.6) | |
| >2000 | 20.4 (2.5) | 15.2 (4.4) | 14.5 (1.8) | 8.7 (2.8) | |
| Hypertension (%) | 83.3 (2.7) | 86.7 (4.1) | 91.4 (1.4) | 89.5 (3.8) | 0.03 |
| Diabetes mellitus (%) | 20.8 (3.0) | 17.5 (4.0) | 26.6 (2.5) | 18.1 (4.5) | 0.19 |
| Cardiovascular disease (%) | 30.9 (3.2) | 35.8 (4.3) | 37.7 (2.7) | 42.8 (5.7) | 0.26 |
| History of cancer (%) | 17.8 (2.7) | 19.9 (4.0) | 17.7 (2.0) | 30.9 (5.4) | 0.11 |
| UACR ≥30 mg/g (%) | 26.1 (3.5) | 31.9 (3.9) | 28.5 (2.1) | 32.1 (4.8) | 0.63 |
| Serum bicarbonate (mEq/L) | 24.4 (0.2) | 24.7 (0.3) | 24.3 (0.2) | 23.8 (0.4) | 0.21 |
| Serum albumin (g/dL) | 4.27 (0.02) | 4.15 (0.03) | 4.14 (0.02) | 4.17 (0.04) | <0.001 |
| Serum CRP ≥1 mg/dL (%) | 7.5 (1.9) | 10.6 (3.1) | 17.3 (1.7) | 12.6 (4.1) | 0.007 |
| % Total body fat | 34.2 (0.4) | 31.3 (0.6) | 41.7 (0.4) | 37.6 (0.6) | <0.001 |
| ASMI (kg/m2) | 7.1 (0.1) | 5.7 (0.1) | 7.8 (0.1) | 6.1 (0.1) | <0.001 |
Abbreviations: NHANES, National Health and Nutrition Examination Survey; UACR, urine albumin-creatinine ratio; CRP, C-reactive protein; ASMI, appendicular skeletal muscle mass index; CKD, Chronic Kidney Disease.
Data are expressed as mean (standard error (SE)) or percent (SE).
Associations of sarcopenia status with mortality
The median follow-up time for mortality status through 2006 and 2011 was 56 months (interquartile range (IQR) 39–73) (707 deaths) and 113 months (IQR 94–130) (1,695 deaths), respectively. When classifying body composition as either sarcopenic or non-sarcopenic, sarcopenic participants had an increased risk of mortality (Table 3A) after multivariable adjustment and after additional adjustment for CRP and serum albumin. We found significant effect modification by CKD status (p<0.001 for interaction). After multivariable adjustment, sarcopenia was associated with an increased risk of mortality in participants with eGFR ≥60 mL/min/1.73m2 (HR 1.34 (95% CI 1.15–1.57) but not those with eGFR <60 mL/min/1.73m2, although this nearly reached statistically significance (HR 1.25 (95% CI 1.00–1.57). Adjustment for CRP and serum albumin as markers of inflammation somewhat magnified this risk in participants with eGFR ≥60 mL/min/1.73m2 but not <60 mL/min/1.73m2. Analyses using the 2006 dataset yielded similar results (Table 3B).
Table 3.
Association of sarcopenia with all-cause mortality in 11,616 participants of NHANES 1999–2004*
|
A. Mortality status through 2011 | ||||
|
HR (95% CI) |
||||
|
Body Composition |
Model 1 | Model 2 | Model 3 | Model 4 |
| Entire cohort (n=11,616) | ||||
| Non-sarcopenic | Reference | Reference | Reference | Reference |
| Sarcopenic | 2.52 (2.24 to 2.83) | 1.43 (1.29 to 1.59) | 1.28 (1.15 to 1.43) | 1.32 (1.19 to 1.48) |
| Participants with eGFR ≥60 mL/min/1.73m2 (n=10,515) | ||||
| Non-sarcopenic | Reference | Reference | Reference | Reference |
| Sarcopenic | 2.48 (2.11 to 2.92) | 1.54 (1.34 to 1.78) | 1.34 (1.15 to 1.57) | 1.42 (1.21 to 1.66) |
| Participants with eGFR <60 mL/min/1.73m2 (n=1,101) | ||||
| Non-sarcopenic | Reference | Reference | Reference | Reference |
| Sarcopenic | 1.89 (1.54 to 2.32) | 1.33 (1.05 to 1.68) | 1.25 (1.00 to 1.57) | 1.24 (0.98 to 1.58) |
| B. Mortality status through 2006 | ||||
|
HR (95% CI) |
||||
| Body Composition | Model 1 | Model 2 | Model 3 | Model 4 |
| Entire cohort (n=11,616) | ||||
| Non-sarcopenic | Reference | Reference | Reference | Reference |
| Sarcopenic | 2.83 (2.37 to 3.38) | 1.51 (1.22 to 1.86) | 1.33 (1.06 to 1.67) | 1.38 (1.11 to 1.73) |
| Participants with eGFR ≥60 mL/min/1.73m2 (n=10,515) | ||||
| Non-sarcopenic | Reference | Reference | Reference | Reference |
| Sarcopenic | 2.89 (2.27 to 3.68) | 1.74 (1.35 to 2.25) | 1.47 (1.12 to 1.94) | 1.56 (1.19 to 2.04) |
| Participants with eGFR <60 mL/min/1.73m2 (n=1,101) | ||||
| Non-sarcopenic | Reference | Reference | Reference | Reference |
| Sarcopenic | 1.85 (1.36 to 2.52) | 1.21 (0.83 to 1.78) | 1.17 (0.84 to 1.68) | 1.19 (0.84 to 1.68) |
P-value for interaction by CKD status: A. Mortality through 2011: p<0.001; B. Mortality status through 2006: p=0.002
Abbreviations: HR, Hazard Ratio; CI, Confidence Interval; eGFR, estimated glomerular filtration rate; CKD, Chronic Kidney Disease.
Bold values indicate p<0.05.
Model 1: unadjusted
Model 2: adjusted for age, sex, race/ethnicity
Model 3: adjusted for age, sex, race/ethnicity, education level, activity level, smoking status, diagnosis of diabetes mellitus, hypertension, cardiovascular disease, history of cancer (other than non-melanoma skin cancer), eGFR categories and log-transformed urine albumin-creatinine ratio
Model 4: adjusted for age, sex, race/ethnicity, education level, activity level, smoking status, diagnosis of diabetes mellitus, hypertension, cardiovascular disease, history of cancer (other than non-melanoma skin cancer), eGFR categories, log-transformed urine albumin-creatinine ratio, serum albumin, log-transformed c-reactive protein
Associations of body composition categories with mortality
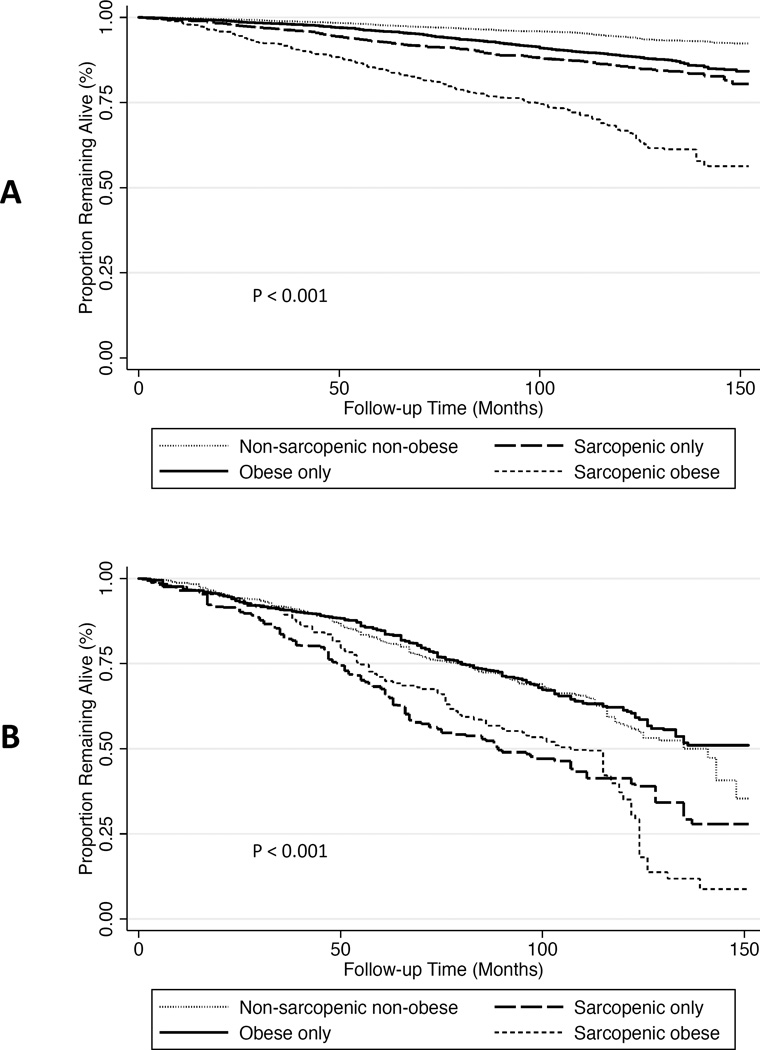
Sarcopenic-obese participants had the highest overall mortality (Figure 1A). After multivariable adjustment, sarcopenia without obesity and sarcopenic-obesity were significantly associated with increased mortality compared with the non-sarcopenic, non-obese phenotype (Table 4A, top panel). There was significant effect modification of these associations by CKD status (p=0.01). Among participants with eGFR ≥60 mL/min/1.73m2, both sarcopenia without obesity and sarcopenic-obesity were associated with an increased risk of mortality after multivariable adjustment (Table 4A, middle panel). The associations of all covariates with mortality in the multivariable-adjusted model are presented in Table S1. Further adjustment for CRP and serum albumin did not meaningfully change these findings.
Figure 1. Kaplan-Meier survival curves for all-cause mortality by body composition category in participants of NHANES 1999–2004.
(A) Kaplan-Meier survival curves by body composition category in 11,616 participants. (B) Kaplan-Meier survival curves by body composition category in 1,101 participants with eGFR < 60 ml/min/1.73 m2. P-values calculated using the Cox test of equality and accounting for NHANES sampling weights.
Table 4.
Association of body composition categories with all-cause mortality in 11,616 participants of NHANES 1999–2004*
|
A. Mortality status through 2011 | ||||||
|---|---|---|---|---|---|---|
|
HR (95% CI) |
||||||
|
Body Composition |
Model 1 | Model 2 | Model 3 | Model 4 | ||
| Entire cohort (n=11,616) | ||||||
| Non-sarcopenic non-obese |
Reference | Reference | Reference | Reference | ||
| Sarcopenic | 2.72 (2.25 to 3.29) | 1.62 (1.33 to 1.99) | 1.35 (1.10 to 1.67) | 1.32 (1.06 to 1.66) | ||
| Obese | 2.09 (1.74 to 2.50) | 1.27 (1.05 to 1.52) | 1.09 (0.90 to 1.32) | 0.98 (0.81 to 1.18) | ||
| Sarcopenic- obese |
6.99 (5.65 to 8.65) | 1.69 (1.38 to 2.06) | 1.35 (1.09 to 1.66) | 1.28 (1.04 to 1.57) | ||
| Participants with eGFR ≥60 mL/min/1.73m2 (n=10,515) | ||||||
| Non-sarcopenic non-obese |
Reference | Reference | Reference | Reference | ||
| Sarcopenic | 2.80 (2.14 to 3.66) | 1.79 (1.37 to 2.35) | 1.44 (1.07 to 1.93) | 1.45 (1.07 to 1.97) | ||
| Obese | 2.24 (1.78 to 2.81) | 1.41 (1.12 to 1.77) | 1.21 (0.96 to 1.54) | 1.09 (0.87 to 1.37) | ||
| Sarcopenic- obese |
7.45 (5.65 to 9.82) | 2.09 (1.61 to 2.70) | 1.64 (1.26 to 2.13) | 1.57 (1.19 to 2.05) | ||
| Participants with eGFR <60 mL/min/1.7 3m2 (n=1,101) | ||||||
| Non-sarcopenic non-obese |
Reference | Reference | Reference | Reference | ||
| Sarcopenic | 1.79 (1.32 to 2.44) | 1.46 (1.06 to 2.02) | 1.24 (0.89 to 1.71) | 1.14 (0.80 to 1.64) | ||
| Obese | 0.93 (0.72 to 1.19) | 1.00 (0.77 to 1.30) | 0.87 (0.67 to 1.12) | 0.77 (0.59 to 1.01) | ||
| Sarcopenic- obese |
1.83 (1.34 to 2.48) | 1.19 (0.88 to 1.62) | 1.05 (0.75 to 1.46) | 0.97 (0.70 to 1.35) | ||
| B. Mortality status through 2006 | ||||||
|
HR (95% CI) |
||||||
|
Body Composition |
Model 1 | Model 2 | Model 3 | Model 4 | ||
| Entire cohort (n=11,616) | ||||||
| Non-sarcopenic non-obese |
Reference | Reference | Reference | Reference | ||
| Sarcopenic | 2.99 (2.34 to 3.83) | 1.68 (1.29 to 2.19) | 1.39 (1.03 to 1.87) | 1.34 (1.00 to 1.83) | ||
| Obese | 1.93 (1.51 to 2.47) | 1.18 (0.92 to 1.50) | 1.00 (0.77 to 1.28) | 0.86 (0.67 to 1.12) | ||
| Sarcopenic- obese |
7.14 (5.43 to 9.40) | 1.65 (1.18 to 2.30) | 1.23 (0.83 to 1.84) | 1.17 (0.81 to 1.70) | ||
| Participants with eGFR ≥60 mL/min/1.73m2 (n=10,515) | ||||||
| Non-sarcopenic non-obese |
Reference | Reference | Reference | Reference | ||
| Sarcopenic | 3.13 (2.20 to 4.48) | 1.95 (1.38 to 2.75) | 1.52 (1.02 to 2.26) | 1.51 (1.00 to 2.30) | ||
| Obese | 2.11 (1.53 to 2.90) | 1.34 (0.97 to 1.85) | 1.15 (0.83 to 1.60) | 1.00 (0.71 to 1.40) | ||
| Sarcopenic- obese |
8.26 (5.51 to 12.38) | 2.30 (1.42 to 3.72) | 1.75 (1.07 to 2.87) | 1.63 (1.02 to 2.61) | ||
| Participants with eGFR <60 mL/min/1.73m2 (n=1,101) | ||||||
| Non-obese non- sarcopenic |
Reference | Reference | Reference | Reference | ||
| Sarcopenic | 1.79 (1.17 to 2.74) | 1.38 (0.87 to 2.18) | 1.24 (0.80 to 1.90) | 1.16 (0.74 to 1.81) | ||
| Obese | 0.87 (0.62 to 1.24) | 0.94 (0.68 to 1.29) | 0.77 (0.55 to 1.07) | 0.67 (0.47 to 0.95) | ||
| Sarcopenic- obese |
1.61 (1.03 to 2.50) | 0.95 (0.57 to 1.58) | 0.77 (0.48 to 1.23) | 0.73 (0.46 to 1.17) | ||
P-value for interaction by CKD status: A. Mortality through 2011: p=0.01; B. Mortality status through 2006: p=0.01
Abbreviations: HR, Hazard Ratio; CI, Confidence Interval; eGFR, estimated glomerular filtration rate; CKD, Chronic Kidney Disease.
Bold values indicate p<0.05.
Model 1: unadjusted
Model 2: adjusted for age, sex, race/ethnicity
Model 3: adjusted for age, sex, race/ethnicity, education level, activity level, smoking status, diagnosis of diabetes mellitus, hypertension, cardiovascular disease, history of cancer (other than non-melanoma skin cancer), eGFR categories and log-transformed urine albumin-creatinine ratio
Model 4: adjusted for age, sex, race/ethnicity, education level, activity level, smoking status, diagnosis of diabetes mellitus, hypertension, cardiovascular disease, history of cancer (other than non-melanoma skin cancer), eGFR categories, log-transformed urine albumin-creatinine ratio, serum albumin, log-transformed c-reactive protein
Among participants with eGFR <60 mL/min/1.73m2, sarcopenic non-obese and sarcopenic-obese participants had the highest overall mortality (Figure 1B). Compared with non-sarcopenic, non-obese participants, no body composition category was associated with an increased risk of death after multivariable adjustment (Table 4A, bottom panel). For obese and sarcopenic-obese individuals, these associations differed significantly from participants without CKD (p-value for interaction by CKD status: sarcopenia without obesity, p=0.22; obesity, p=0.01; sarcopenic-obesity, p=0.003). Furthermore, after accounting for inflammation, there was a non-significant trend toward reduced risk of death among obese participants (HR 0.77 (95% CI 0.59–1.01). Findings were similar using the 2006 dataset, except there was also a trend toward a reduced risk of death among sarcopenic-obese participants, similar to obese persons, in those with eGFR <60 mL/min/1.73m2 (Table 4B).
There was significant interaction between body composition and race/ethnicity (p interaction = 0.003 in the entire cohort); examination within the 2 eGFR subgroups suggested that the mortality risk of sarcopenia might be greater in non-Hispanic black participants compared to other race/ethnicity categories (Table S2), but this effect did not appear to differ based on eGFR.
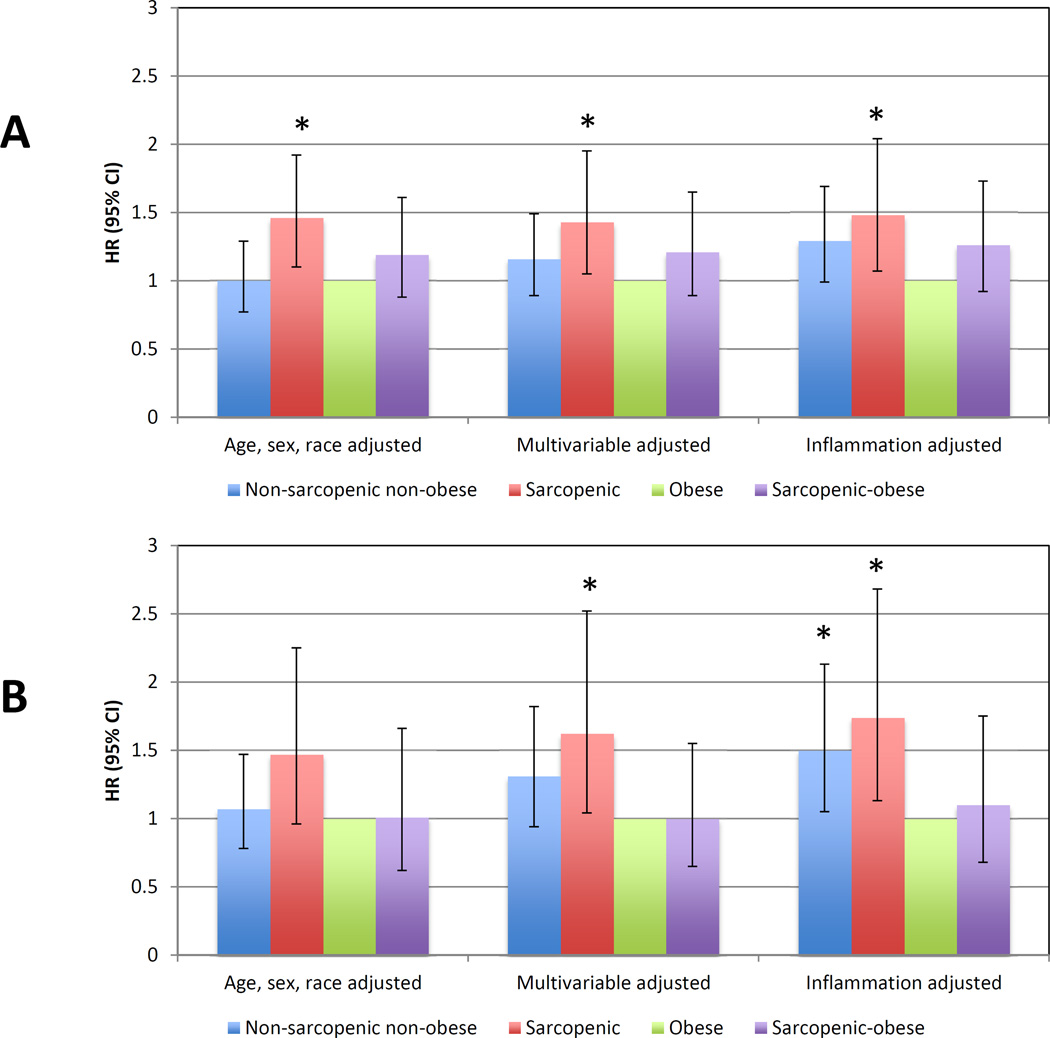
Because obesity was the most populous body composition category among participants with eGFR <60 mL/min/1.73m2, and because prior studies have suggested that obese dialysis patients may have the greatest survival, we also examined associations with mortality compared to obese persons (Figure 2A). When obese participants were considered the reference group, sarcopenia without obesity was significantly associated with increased mortality (HR 1.43 (95% CI 1.05–1.95)). With shorter duration of follow-up in the 2006 dataset, the risk of death was magnified for non-sarcopenic, non-obese participants and sarcopenic non-obese participants, but not for sarcopenic-obese persons (Figure 2B). Additional adjustment for serum albumin and CRP increased the risk of death relative to obesity for each of the other body composition categories.
Figure 2. All-cause mortality compared to obese persons among NHANES participants compared with eGFR < 60 ml/min/1.73 m2.
(A) Mortality ascertained through 2011. (B) Mortality ascertained through 2006. Multivariable-adjusted model adjusted for age, sex, race/ethnicity, education level, activity level, smoking status, diagnosis of diabetes mellitus, hypertension, cardiovascular disease, history of cancer (other than non-melanoma skin cancer), eGFR categories and log-transformed urine albumin-creatinine ratio. Inflammation-adjusted model additionally adjusted for serum albumin and log-transformed C-reactive protein.
Sensitivity Analyses
As the differences we observed between the 2 eGFR subgroups could have been related to underlying differences in demographic characteristics, we repeated our analyses in a cohort in which each participant with an eGFR <60 mL/min/1.73m2 was uniquely matched with a participant with eGFR ≥60 mL/min/1.73m2 based on age (±1 year), sex, and non-Hispanic black status. This resulted in 965 unique pairs (957/965 with age difference = 0 years). These results did not differ substantively from our main results, and there was a significant interaction by CKD status (p=0.04) (Table S3). After excluding participants who died in the first 2 years of follow-up, sarcopenia was significantly associated with mortality in participants with and without eGFR <60 mL/min/1.73m2 (Table S4). Compared with obese participants with eGFR <60 mL/min/1.73m2, the mortality HRs for those who were sarcopenic non-obese or sarcopenic-obese increased in magnitude (Table S5). Cystatin C was available in 3,949 participants, 755 of whom had an eGFR <60 mL/min/1.73m2. Repeating our analyses using cystatin C eGFR in this smaller sample yielded qualitatively similar results (Table S6, Table S7). Modeling ASMI and %TBF as continuous variables demonstrated an association of higher %TBF with lower risk of mortality only among participants with CKD (Table S8). There was a similar non-significant association when restricted to sarcopenic individuals.
DISCUSSION
In a nationally representative cohort, we found that the risk of death in people with CKD differs if muscle and fat mass are examined together rather than in isolation. BMI is of limited utility in this regard. Nearly 10% of participants with CKD and reduced eGFR were sarcopenic-obese, and their mean BMI was no different than people with normal body composition.
We hypothesized that associations of sarcopenia and sarcopenic-obesity with increased mortality would be greater in people with CKD than in those without. Our findings do not support this hypothesis. Whereas sarcopenia in the absence of obesity was an independent risk factor for increased mortality, this relationship was modified by eGFR. Among participants with CKD, the increased risk of death was present only when compared with obese persons but not those with normal body composition. Also contrary to our hypothesis, sarcopenic-obesity was not associated with higher mortality among people with CKD, in contrast to those with eGFR ≥60 mL/min/1.73m2. Rather, in our initial analyses of mortality status ascertained through 2006, the results suggested a reduced risk of death in these participants similar to that seen among obese individuals. Although this protective association seemed to be attenuated with greater duration of follow-up time (when mortality status was ascertained through 2011), the risk of death was still not significantly increased.
Whereas the association of body composition abnormalities with outcomes has been studied extensively in the ESRD population, it has been less well-examined in people with pre-dialysis CKD. Most studies have focused on obesity, using BMI as the defining metric.8, 34, 35 Muscle mass has been examined directly and indirectly in only a few studies, with conflicting results. One report of 287 CKD patients found increased mortality among those who were sarcopenic,4 while another using NHANES data found no association of total lean body mass with survival in people with CKD.6 Conversely, 2 studies reported that lower urinary creatinine excretion associates with an increased risk of death in CKD patients, suggesting that lower muscle mass is a poor prognostic factor.5, 7 However, it is not clear that these associations were due to differences in muscle mass. In the Chronic Renal Insufficiency Cohort study, fat-free mass measured by bioelectrical impedance analysis was only moderately correlated with creatinine excretion and did not similarly associate with mortality.7 Factors other than muscle mass, such as dietary intake and the ability to complete a 24-hour urine collection, may have mediated differences in measured urinary creatinine excretion in these studies.
Appendicular skeletal muscle mass may be more appropriate to examine in relation to mortality, as it is more directly related to functional ability and is not confounded by differences in visceral lean mass. Our report used a threshold for low appendicular muscle mass that is recommended by the European consensus guidelines for defining sarcopenia.23 Although the current guidelines also recommend including functional measures in the definition, we focused on muscle mass because of the limited number of individuals with functional assessments in this NHANES cohort. Despite this limitation, our findings demonstrate that low muscle mass is predictive of mortality risk in the general population and in people with CKD.
There are several possible explanations for the association of sarcopenia with mortality in people with CKD. Kidney disease may cause muscle wasting via accelerated muscle protein breakdown, possibly mediated by inflammation or insulin resistance.36–38 This could impact survival through several mechanisms. Better preserved muscle mass could help maintain functional status, decreasing the risk of falls, fractures, and the negative effects of a sedentary lifestyle.23 Greater skeletal muscle mass could improve metabolism, enhance peripheral glucose disposal, and increase energy stores.39 Intriguingly, inhibition of muscle proteolysis in a mouse model of CKD not only preserved muscle mass but improved survival as well.40 If the quantity of functional skeletal muscle indeed mediates mortality risk in CKD, this could also explain some of the early survival benefit attributed to obesity. Even in the CKD subgroup, the people in the obese-only category had greater muscle mass than those with normal body composition.
Accelerated aging likely also contributes to the development of sarcopenia in people with CKD. Adjustment for reduced activity level and comorbid conditions, markers of unsuccessful aging, attenuated the association with mortality but did not fully explain our findings. Similarly, inflammation has been linked with the development of sarcopenia,41, 42 but our results remained significant even after adjustment for serum albumin and CRP. Sarcopenia may be a marker of accelerated aging independent of these factors.43 On the other hand, although we attempted to account for differences in comorbidity and other characteristics among body composition categories, residual confounding may still explain some of our findings. In addition, sarcopenia could simply be a marker for sicker individuals with a greater burden of chronic illness. If this were the case, then our results could be due to an excess of early deaths among these sicker individuals. However, we did not see attenuation of risk with longer follow-up or when we excluded early mortality. It is possible that exclusion of death beyond the first 2 years might be required to detect such an effect. Regardless, we cannot exclude the possibility that differences in body composition are simply markers for other health-related characteristics.
Our findings illustrate the complexity of studying body composition in patients with CKD, even before the onset of ESRD. In the general population with eGFR ≥60 mL/min/1.73m2, sarcopenia was associated with mortality whether we classified people based on sarcopenia status alone or simultaneously accounted for adiposity. When classifying people with reduced eGFR based on sarcopenia status alone, it appeared there was a weaker association with mortality than in participants without CKD. More detailed classification of body composition was required to clarify this association. In people with reduced eGFR, whether sarcopenia associated with an increased risk of death depended on whether a person was also obese by %TBF criteria. The favorable prognostic value of obesity seemed to outweigh the mortality risk of sarcopenia, although this effect diminished with longer follow-up time. Surprisingly, excluding early mortality in our sensitivity analyses magnified the risk of death in people with CKD. This is in stark contrast to the general population cohort with eGFR ≥60 mL/min/1.73m2.
Most interventional studies in the general population have targeted either obesity or sarcopenia, but few have examined the sarcopenic-obese phenotype directly.44 Concerns have been raised about weight loss interventions in these individuals because muscle mass is lost along with fat mass. It has been suggested that exercise accompany supervised weight loss protocols in these circumstances.44 We are not aware of any interventional studies addressing this issue in CKD patients, and our data do not indicate whether interventions targeted at sarcopenic-obese CKD patients could be expected to improve survival. While some would argue that weight loss may not be appropriate given the reduced risk of death with obesity and sarcopenic-obesity in early follow-up, exercise including strength training seems a logical intervention to test. This could be expected to affect outcomes such as disability, falls, and quality-of-life, which may be adversely affected by both sarcopenia and obesity.45, 46
Several limitations of our study should be noted. The number of participants with eGFR<60 mL/min/1.73m2 was relatively small, thus limiting the power to detect significant associations. For example, in the CKD cohort the association of sarcopenia with mortality might have been statistically significant had a larger sample size been available for analysis. Similarly, when we further characterized body composition, the small sample of sarcopenic-obese individuals with CKD limited our ability to detect statistically significant associations. Nevertheless, there was significant effect modification by CKD status even within the smaller matched-pairs cohort, and the direction and magnitude of the point estimates were similar to our main results. Also, the definition of obesity did not account for the distribution of adipose tissue, and others have used different cutpoints for defining obesity based on adiposity. However, sarcopenic-obesity defined using this %TBF cutoff has been associated with disability in community-dwelling elderly,22 and our previous work found that American Society of Bariatric Physicians cutpoints (30% for women and 25% for men) would provide limited discriminatory power because they would classify >90% of individuals with CKD stage 3 and 4 as obese.10 Future studies should examine the interaction of abdominal obesity with sarcopenia in the CKD population. DEXA may overestimate lean mass in people with edema; this could have attenuated our risk estimates, as people with CKD would be most likely to have edema and to have overestimation of lean mass. As only cross-sectional laboratory data were available, we could not define CKD based on the presence of an eGFR<60 mL/min/1.73m2 for at least 3 months. Finally, given the observational nature of this study, we have determined associations but cannot comment on causality.
In conclusion, sarcopenia is associated with increased mortality in the CKD population, but the presence of obesity modifies this relationship. Unlike in the general population, obesity seems to blunt the mortality risk of sarcopenia in people with CKD. Future studies should assign supervised weight loss and different types of exercise interventions after accounting for the complex alterations in body composition that occur in CKD patients but are not detected by BMI.
Supplementary Material
Acknowledgments
This research was supported by K23 DK099438 from the National Institutes of Health (NIH) and an American Society of Nephrology Carl W. Gottschalk Research Scholar Grant (both to MKA), and by a New York Community Trust/National Medical Fellowships Medical Research Scholarship (LA). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None of the authors has any financial conflicts to disclose.
Supplementary Material
Table S1. Association of covariates with all-cause mortality through 2011 in 11,616 participants of NHANES 1999–2004
Table S2. Association of body composition categories with all-cause mortality through 2011 in 11,616 participants of NHANES 1999–2004 stratified by race/ethnicity
Table S3. Association of body composition categories with all-cause mortality in 1,930 participants of NHANES 1999–2004 matched on age, sex, and race/ethnicity
Table S4. Association of sarcopenia with all-cause mortality through 2011 in 11,191 participants of NHANES 1999–2004 after excluding mortality in the first 24 months
Table S5. Association of body composition categories with all-cause mortality through 2011 in 11,191 participants of NHANES 1999–2004 after excluding mortality in the first 24 months
Table S6. Association of sarcopenia with all-cause mortality through 2011 in 3,949 participants of NHANES 1999–2004 using cystatin C to determine eGFR
Table S7. Association of body composition categories with all-cause mortality through 2011 in 3,949 participants of NHANES 1999–2004 using cystatin C to determine eGFR
Table S8. Association of continuous ASMI and %TBF with all-cause mortality through 2011 by eGFR status in 11,616 participants of NHANES 1999–2004
REFERENCES
- 1.Fouque D, Kalantar-Zadeh K, Kopple J, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 2.Kopple JD. McCollum Award Lecture, 1996: protein-energy malnutrition in maintenance dialysis patients. Am J Clin Nutr. 1997;65:1544–1557. doi: 10.1093/ajcn/65.5.1544. [DOI] [PubMed] [Google Scholar]
- 3.Mehrotra R, Kopple JD. Nutritional management of maintenance dialysis patients: why aren’t we doing better? Annu Rev Nutr. 2001;21:343–379. doi: 10.1146/annurev.nutr.21.1.343. [DOI] [PubMed] [Google Scholar]
- 4.Pereira RA, Cordeiro AC, Avesani CM, et al. Sarcopenia in chronic kidney disease on conservative therapy: prevalence and association with mortality. Nephrology Dialysis Transplantation. 2015;30:1718–1725. doi: 10.1093/ndt/gfv133. [DOI] [PubMed] [Google Scholar]
- 5.Di Micco L, Quinn RR, Ronksley PE, et al. Urine creatinine excretion and clinical outcomes in CKD. Clin J Am Soc Nephrol. 2013;8:1877–1883. doi: 10.2215/CJN.01350213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navaneethan SD, Kirwan JP, Arrigain S, et al. Adiposity measures, lean body mass, physical activity and mortality: NHANES 1999–2004. BMC nephrology. 2014;15:108. doi: 10.1186/1471-2369-15-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson FP, Xie D, Anderson AH, et al. Urinary creatinine excretion, bioelectrical impedance analysis, and clinical outcomes in patients with CKD: the CRIC study. Clin J Am Soc Nephrol. 2014;9:2095–2103. doi: 10.2215/CJN.03790414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu JL, Kalantar-Zadeh K, Ma JZ, et al. Association of body mass index with outcomes in patients with CKD. J Am Soc Nephrol. 2014;25:2088–2096. doi: 10.1681/ASN.2013070754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal R, Bills JE, Light RP. Diagnosing obesity by body mass index in chronic kidney disease: an explanation for the “obesity paradox?”. Hypertension. 2010;56:893–900. doi: 10.1161/HYPERTENSIONAHA.110.160747. [DOI] [PubMed] [Google Scholar]
- 10.Sharma D, Hawkins M, Abramowitz MK. Association of sarcopenia with eGFR and misclassification of obesity in adults with CKD in the United States. Clin J Am Soc Nephrol. 2014;9:2079–2088. doi: 10.2215/CJN.02140214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honda H, Qureshi AR, Axelsson J, et al. Obese sarcopenia in patients with end-stage renal disease is associated with inflammation and increased mortality. Am J Clin Nutr. 2007;86:633–638. doi: 10.1093/ajcn/86.3.633. [DOI] [PubMed] [Google Scholar]
- 12.Tian S, Xu Y. Association of sarcopenic obesity with the risk of all-cause mortality: A meta-analysis of prospective cohort studies. Geriatrics & gerontology international. 2016;16:155–166. doi: 10.1111/ggi.12579. [DOI] [PubMed] [Google Scholar]
- 13.About the National Health and Nutrition Examination Survey. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; [Accessed March 25, 2016]. Available at: http://www.cdc.gov/nchs/nhanes/about_nhanes.htm. [Google Scholar]
- 14.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 15.Muntner P, Woodward M, Mann DM, et al. Comparison of the Framingham Heart Study hypertension model with blood pressure alone in the prediction of risk of hypertension: the Multi-Ethnic Study of Atherosclerosis. Hypertension. 2010;55:1339–1345. doi: 10.1161/HYPERTENSIONAHA.109.149609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schenker N, Borrud LG, Burt VL, et al. Multiple imputation of missing dual-energy X-ray absorptiometry data in the National Health and Nutrition Examination Survey. Stat Med. 2011;30:260–276. doi: 10.1002/sim.4080. [DOI] [PubMed] [Google Scholar]
- 18.The 1999–2006 Dual Energy X-ray Absorptiometry (DXA) Multiple Imputation Data Files and Technical Documentation. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; [Accessed March 25, 2016]. Available at: http://www.cdc.gov/nchs/nhanes/dxx/dxa.htm. [Google Scholar]
- 19.Heymsfield SB, Heo M, Thomas D, et al. Scaling of body composition to height: relevance to height-normalized indexes. Am J Clin Nutr. 2011;93:736–740. doi: 10.3945/ajcn.110.007161. [DOI] [PubMed] [Google Scholar]
- 20.Li C, Ford ES, Zhao G, et al. Estimates of body composition with dual-energy X-ray absorptiometry in adults. Am J Clin Nutr. 2009;90:1457–1465. doi: 10.3945/ajcn.2009.28141. [DOI] [PubMed] [Google Scholar]
- 21.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 22.Baumgartner RN, Wayne SJ, Waters DL, et al. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obes Res. 2004;12:1995–2004. doi: 10.1038/oby.2004.250. [DOI] [PubMed] [Google Scholar]
- 23.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age and ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gallagher D, Visser M, De Meersman RE, et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. Journal of applied physiology. 1997;83:229–239. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- 25.Gallagher D, Heymsfield SB, Heo M, et al. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72:694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 26.Nishida C, Ko GT, Kumanyika S. Body fat distribution and noncommunicable diseases in populations: overview of the 2008 WHO Expert Consultation on Waist Circumference and Waist-Hip Ratio. European journal of clinical nutrition. 2010;64:2–5. doi: 10.1038/ejcn.2009.139. [DOI] [PubMed] [Google Scholar]
- 27.National Health and Nutrition Examination Survey (NHANES) 1999–2004 Linked Mortality Files, Mortality follow-up through 2006: Matching Methodology. Hyattsville, MD: 2009. May, [Accessed July 27, 2010]. Available at: http://www.cdc.gov/nchs/data/datalinkage/nh99+_mortality_matching_methodology_final.pdf. [Google Scholar]
- 28.NCHS 2011 Linked Mortality Files Matching Methodology. National Center for Health Statistics; [Accessed March 16, 2016]. Available at: http://www.cdc.gov/nchs/data_access/data_linkage/mortality/linkage_methods_analytical_support/2011_linked_mortality_file_matching_methodology.pdf. [Google Scholar]
- 29.National Health and Nutrition Examination Survey Data. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; [Accessed July 27, 2010]. Available at: http://www.cdc.gov/nchs/data_access/data_linkage/mortality/nhanes_99_04_linkage_public_use.htm. [Google Scholar]
- 30.Public-use Linked Mortality File. [Accessed March 16, 2016];National Center for Health Statistics. 2015 Available at: http://www.cdc.gov/nchs/data_access/data_linkage/mortality.htm.
- 31.Comparative analysis of the NHANES (1999–2004) public-use and restricted-use linked mortality files: 2010 public-use data release. National Center for Health Statistics. Hyattsville, MD: 2010. May, [Accessed July 27, 2010]. Available at: http://www.cdc.gov/nchs/data_access/data_linkage/mortality/nhanes_99_04_linkage.htm. [Google Scholar]
- 32.Comparative analysis of the NHIS public-use and restricted-use linked mortality files: 2015 public-use data release. [Accessed March 16, 2016];National Center for Health Statistics. Available at: http://www.cdc.gov/nchs/data_access/data_linkage/mortality/linkage_methods_analytical_support.htm.
- 33.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ejerblad E, Fored CM, Lindblad P, et al. Obesity and risk for chronic renal failure. J Am Soc Nephrol. 2006;17:1695–1702. doi: 10.1681/ASN.2005060638. [DOI] [PubMed] [Google Scholar]
- 35.Hsu CY, McCulloch CE, Iribarren C, et al. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144:21–28. doi: 10.7326/0003-4819-144-1-200601030-00006. [DOI] [PubMed] [Google Scholar]
- 36.Carrero JJ, Stenvinkel P, Cuppari L, et al. Etiology of the protein-energy wasting syndrome in chronic kidney disease: a consensus statement from the International Society of Renal Nutrition and Metabolism (ISRNM) J Ren Nutr. 2013;23:77–90. doi: 10.1053/j.jrn.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Mitch WE, Du J. Cellular mechanisms causing loss of muscle mass in kidney disease. Semin Nephrol. 2004;24:484–487. doi: 10.1016/j.semnephrol.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Workeneh BT, Mitch WE. Review of muscle wasting associated with chronic kidney disease. Am J Clin Nutr. 2010;91:1128S–1132S. doi: 10.3945/ajcn.2010.28608B. [DOI] [PubMed] [Google Scholar]
- 39.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475–482. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Pan J, Dong Y, et al. Stat3 activation links a C/EBPdelta to myostatin pathway to stimulate loss of muscle mass. Cell Metab. 2013;18:368–379. doi: 10.1016/j.cmet.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bamman MM, Ferrando AA, Evans RP, et al. Muscle inflammation susceptibility: a prognostic index of recovery potential after hip arthroplasty? Am J Physiol Endocrinol Metab. 2015;308:E670–E679. doi: 10.1152/ajpendo.00576.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brinkley TE, Leng X, Miller ME, et al. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64:455–461. doi: 10.1093/gerona/gln038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buford TW, Anton SD, Judge AR, et al. Models of accelerated sarcopenia: critical pieces for solving the puzzle of age-related muscle atrophy. Ageing research reviews. 2010;9:369–383. doi: 10.1016/j.arr.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goisser S, Kemmler W, Porzel S, et al. Sarcopenic obesity and complex interventions with nutrition and exercise in community-dwelling older persons--a narrative review. Clinical interventions in aging. 2015;10:1267–1282. doi: 10.2147/CIA.S82454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lang IA, Llewellyn DJ, Alexander K, et al. Obesity, physical function, and mortality in older adults. J Am Geriatr Soc. 2008;56:1474–1478. doi: 10.1111/j.1532-5415.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- 46.Villareal DT, Banks M, Siener C, et al. Physical frailty and body composition in obese elderly men and women. Obes Res. 2004;12:913–920. doi: 10.1038/oby.2004.111. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.