SUMMARY
Infective endocarditis caused by Proteus mirabilis is a rare and poorly reported disease, with no well-defined effective antibiotic regimen. Here, we present a case of P. mirabilis aortic valve endocarditis. We reviewed prior cases and treatment regimens, and devised effective treatment, which was guided by in vitro sensitivity and synergy testing on the pathogen. Our patient survived without complications or the need for a surgical intervention.
BACKGROUND
Because of its exceeding rarity, there is not a well-defined antimicrobial regimen with which to treat Proteus mirabilis endocarditis. Our report describes the failure of an antibiotic regimen to cure a man with P. mirabilis endocarditis, despite initial antibiotic susceptibilities that were well within the sensitive range of this pathogen. This prompted us to perform extensive in vitro testing on the blood isolate with 24 hour time-kill studies that led to devising an effective antibiotic regimen that resulted in cure, without surgical intervention. Furthermore, our experience suggests that this entity may have been overlooked previously and may be more common than has been reported. We offer our experience to highlight the value of devising a treatment regimen aided by in vitro bacterial killing experiments and to alert clinicians to the potential presence of P. mirabilis endocarditis.
CASE PRESENTATION
A man aged 59 years with a history of prior cerebrovascular accident with left hemiparesis presented to the hospital with fever, diarrhoea and vomiting. On presentation, the patient had a low-grade fever (38°C), and was alert with left hemiparesis and partial expressive aphasia. He was diaphoretic with clear lungs and a normal cardiac examination without any murmurs. The remainder of the physical examination was unremarkable. On admission, he was found to have a white cell count of 9300 cells/μL with 76% segmented neutrophils and 20% banded neutrophils. All serum chemistries and liver function tests were within normal limits. Urine and two sets of blood cultures collected on the day of admission all grew pure isolates of P. mirabilis that were uniformly resistant to ciprofloxacin, ampicillin and trimethoprim/sulfamethoxazole. The patient was initially started on intravenous meropenem (2 g every 12 hours). His serum creatinine was acutely elevated at this time to 2.38 mg/dL corresponding to a creatinine clearance of 33 mL/min. After the third dose of meropenem, he was still febrile and two more sets of blood cultures were drawn. Both again grew P. mirabilis with identical sensitivities to the earlier blood isolate. Treatment was continued with ceftriaxone, 1 g every 24 hours, and a renal ultrasound was performed, which showed right hydronephrosis. A nephrostomy tube was inserted, and sanguineous fluid was drained and it was free of pus. The nephrostomy fluid had no white cells on Gram stain and yielded sterile cultures. On hospital day 6, repeat blood cultures were sterile and ceftriaxone was continued for 10 days, followed by oral cefdinir for another 5 days. A cystoscopy found a bladder stone, but there was no residual ureteral dilation or bladder abnormalities and the nephrostomy tube was removed.
Ten days after the completion of antibiotics, the patient again became febrile with a peak temperature of 39°C. Two sets of blood cultures again grew P. mirabilis with the same sensitivity profile as the previous blood isolate, while the urine culture was sterile. The patient’s physical examination was unchanged from earlier. Meropenem was initially restarted, before the bloodborne pathogen’s identity and susceptibilities were known, and an abdominal CT scan revealed no hydronephrosis, hepatobiliary abnormalities or intra-abdominal pathology. However, a transthoracic echocardiogram demonstrated a 5 mm independently mobile echogenic vegetation associated with the ventricular aspect of the aortic valve (figure 1). There was no associated aortic regurgitation or other signs of valve dysfunction. A careful review indicated the absence of any indwelling intravascular devices.
Figure 1.
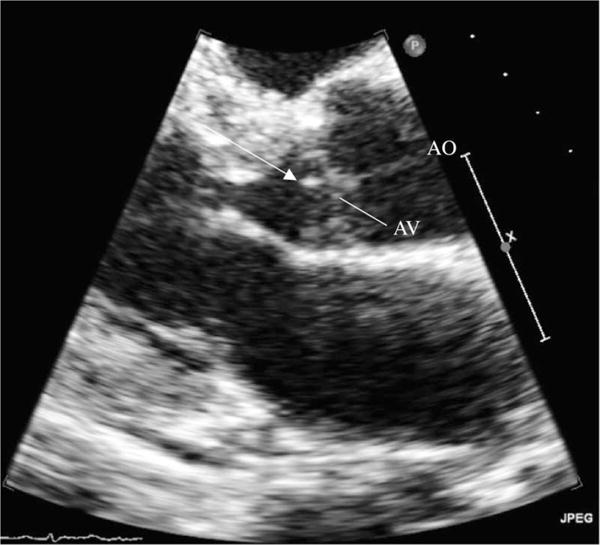
Transthoracic echocardiogram. A view of the aortic valve shows a large attached vegetation (white arrow) on the ventricular aspect of the aortic valve. AO, aorta; AV, aortic valve.
INVESTIGATIONS
Our original antibiotic regimen failed despite minimum inhibitory concentrations (MICs) that were well within the sensitive range of this pathogen to a wide array of antibiotics (table 1).
Table 1.
Minimum inhibitory concentrations of antibiotics for Proteus mirabilis blood isolate
| Antibiotic | Minimum inhibitory concentration (μg/mL) |
|---|---|
| Gentamicin | ≤4 |
| Piperacillin/tazobactam | ≤16 |
| Ceftazidime | ≤1 |
| Ceftriaxone | ≤1 |
| Cefepime | ≤4 |
| Aztreonam | ≤4 |
| Meropenem | ≤1 |
Prompted by this, and by the absence of a classically defined regimen for P. mirabilis endocarditis, we performed extensive in vitro testing on the blood isolate with 24 hour time-kill studies to assess the pharmacodynamic activity of clinically relevant agents and the potential value of a two-antibiotic regimen, as described previously.1 Bacteria were grown in LB broth (Fisher Bioreagents; Fair Lawn, New Jersey, USA) supplemented with 25 mg/L calcium and 12.5 mg/L magnesium. Previous data have supported in vitro killing as a predictor of clinical outcomes for a variety of infections.2–4 However, more robust translational studies are warranted to fully elucidate its value for patients with Gram-negative endocarditis, which has not been well explored in this context. An inoculum of 108 CFU/mL was used to replicate the high bacterial density in endocarditis.5,6 In vitro antibiotic concentrations were chosen based on the package insert pharmacokinetic data, and account for protein binding to carefully parallel the unbound, free fraction maximum concentration (fCmax) of a clinical dose. The following static antimicrobial concentrations were used in time-kill studies (simulated clinical dose): ceftriaxone, fCmax=25.7 μg/mL (2 g); gentamicin sulfate, fCmax=4 μg/mL (1 mg/kg); gentamicin sulfate, fCmax=15 μg/mL (5 mg/kg); ampicillin, fCmax=100 μg/mL (2 g); meropenem, fCmax=48 μg/mL (1 g, 30 min infusion). Viable bacterial concentrations were assessed at 0, 1, 2, 4, 6, 8 and 24 hours after the introduction of the antibiotics by plating 100 μL aliquots on trypticase soy agar with 5% sheep blood (TSA II) plates (BD Diagnostic Systems; Franklin Lakes, New Jersey, USA).
Although MIC testing supported the susceptibility of this P. mirabilis strain to meropenem (table 1), time-kill results more closely paralleled our patient’s failure to respond to meropenem, with only a −0.92 log10 CFU/mL reduction from baseline at 24 hours (figure 2). Ceftriaxone performed similarly to meropenem, causing a −0.94 log10 CFU/mL reduction from baseline at 24 hours. Neither meropenem nor ceftriaxone as a single agent was bactericidal at 24 hours (as defined by ≥99.9% reduction in CFU/mL from baseline). For this P. mirabilis isolate, ampicillin did not cause viable bacterial counts to drop below baseline, while the combination of ampicillin+gentamicin (gentamicin simulating 1 mg/kg) resulted in a maximal log reduction of −1.06 log10 CFU/mL at 6 hours followed by regrowth towards growth control levels by 24 hours. Ceftriaxone combinations with gentamicin concentrations simulating 1 and 5 mg/kg doses resulted in the greatest bacterial killing with 24 hour reductions of −2.84 and −8.27 log10 CFU/mL, respectively (figure 2), demonstrating the most efficient bacterial killing. Additional conditions were tested, and did not demonstrate improved time-kill, including cefepime alone and in combination with gentamicin or ciprofloxacin, as well as ciprofloxacin and gentamicin (data not shown).
Figure 2.
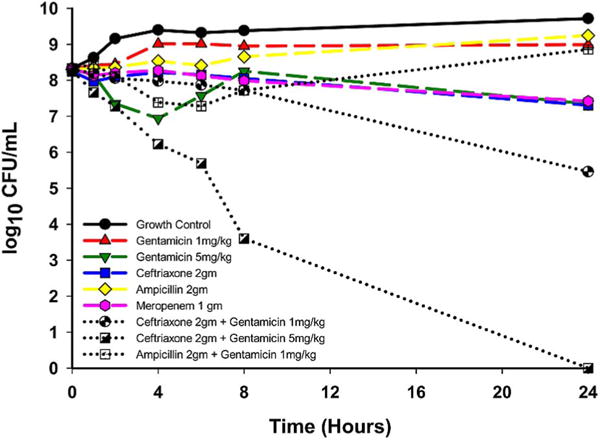
Twenty-four-hour time-kill study. Studies were performed to determine the pharmacodynamic activity of antimicrobial agents. Our patient’s Proteus mirabilis blood isolate was grown with antibiotics to parallel clinical dosing (concentration detailed in text) and bacterial survival compared with control growth. Ceftriaxone, combined with gentamicin, at 1and 5 mg/kg equivalents, demonstrated the most efficient synergistic bactericidal activity.
TREATMENT
On the basis of these results, ceftriaxone (2 g every 24 hours) and gentamicin (100 mg every 12 hours) were started. Gentamicin doses were adjusted to maintain peaks of 4.0–5.5 μg/mL and troughs of <1.0 μg/mL. A 6-week course of antibiotics was completed.
OUTCOME AND FOLLOW-UP
To further establish a proof of cure and to help validate the value of our methodology, surveillance blood cultures obtained 2 weeks after the discontinuation of antibiotics were sterile, and he remains well 6 months later.
DISCUSSION
Only a handful of cases of P. mirabilis endocarditis have been reported in the past literature. Of these, most caused strikingly severe illness. The first instance of survival without a surgical intervention was reported in 2007 in a patient treated with ceftriaxone for 4 weeks.7 More recently, a successful outcome was reported with ampicillin and gentamicin for an ampicillin-sensitive strain of P. mirabilis.8 In this instance, echocardiography was prompted after a murmur was detected on examination. To date, only six cases of native valve P. mirabilis endocarditis have been reported (table 2), two of which predated modern echocardiography.11–12
Table 2.
Previously reported cases of native valve Proteus mirabilis endocarditis
| Year | Antibiotic therapy | Surgical intervention | Outcome |
|---|---|---|---|
| 20118 | Ampicillin+gentamicin | None | Cure |
| 20067 | Ceftriaxone | None | Cure |
| 20059 | Ceftriaxone | MV replacement* | Cure |
| 200010 | Cefotaxime+ofloxacin | MV replacement* | Cure |
| 197711 | Ampicillin+gentamicin followed by carbenicillin+kanamycin | None | Death |
| 196112 | Chloramphenicol | None | Death |
The year listed in the first column corresponds with the occurrence of each case.
MV, mitral valve.
In contrast to our patient, all patients had mitral valve infections. In addition, there have been three reported cases of P. mirabilis infection of prosthetic valves, and four reports of mural or peri-valvular abscesses.13–19
Bacteraemia with P. mirabilis does not typically warrant a work-up for endocarditis, and our patient initially presented with no clinical findings to suggest this diagnosis. However, several factors ultimately prompted us to entertain this diagnosis. In both febrile episodes, bacteraemia was sustained, with multiple sets of positive blood cultures. Furthermore, our patient had ‘break-through’ bacteraemia despite treatment with broad-spectrum antibiotics to which the pathogen was exquisitely susceptible. The absence of pyonephrosis with nephrostomy tube placement failed to provide a plausible source for sustained bacteraemia. Finally, recurrence of P. mirabilis bacteraemia 10 days after completing a 2-week course of intravenous antibiotics and absence of a clearly defined source prompted investigation for an endovascular source. In retrospect, we suspect that he may have originally had urosepsis due to a transient ureteral obstruction with subsequent seeding to his aortic valve, perpetuating the bacteraemia even after the obstruction was relieved.
It is intriguing that our patient’s course contrasts dramatically with the majority of reported cases of P. mirabilis endocarditis that had high mortality or required surgical intervention (table 2). In fact, the overall mortality rate for non-HACEK Gram-negative bacillus endocarditis, in one large series, was 24%.20 However, very few of these cases were caused by P. mirabilis. We speculate that less severe instances of P. mirabilis endocarditis may be more prevalent than have been reported, and that the previous reported cases may have been diagnosed as endocarditis owing to the severity of disease. It is also possible that many undetected cases may have responded to shorter antibiotic courses. In fact, had our patient not had recurrence of his bacteraemia, his diagnosis might not have come to light.
We present our experience to highlight the value of devising a treatment regimen aided by in vitro bacterial killing experiments that resulted in clinical and microbiological cure, without the need for surgical intervention at least in this instance. This adds validation to evaluating the pathogen-specific pharmacodynamic profile of an administered antibiotic regimen. This approach may apply in similar clinical situations that lack definitive antibiotic regimens, although further clinical experiences will be necessary for confirmation, as well as for determining optimal duration. We also offer our experience to alert clinicians to the potential presence of P. mirabilis endocarditis and to encourage prompt aggressive investigation when warranted by clinical factors.
Learning points.
-
▸
Native valve infective endocarditis caused by Proteus mirabilis is a rare and poorly reported disease, for which there is no well-defined effective antibiotic regimen.
-
▸
While bacteraemia with P. mirabilis does not typically warrant consideration of endocarditis, persistent high-grade bacteraemia and ‘break-through’ bacteraemia, despite appropriate antibiotics, supports investigation for endocarditis.
-
▸
Whereas reported cases of P. mirabilis endocarditis have had high mortality and morbidity, our experience suggests that less severe instances of P. mirabilis endocarditis may be more common than has been reported.
-
▸
Our experience highlights the advantages of devising the treatment directed by the pathogen-specific pharmacodynamic profile for infectious entities that do not have well-defined treatment regimens.
Acknowledgments
Funding National Institute of Allergy and Infectious Diseases (R01AI111990).
Footnotes
Contributors CRB is responsible for manuscript composition and lead author. KAM is responsible for directed clinical and pharmacological research. ZPB is responsible for time-kill and synergy studies. BTT is responsible for time-kill and synergy studies. CSB is responsible for directed manuscript composition and patient care. All five authors contributed to conceptions and design, analysis and interpretation of data, drafting and revising of the article. All gave final approval and all are in agreement with accountability and accuracy of the data.
Competing interests None declared.
Patient consent Obtained.
Provenance and peer review Not commissioned; externally peer reviewed.
References
- 1.Bulitta JB, Ly NS, Yang JC, et al. Development and qualification of a pharmacodynamic model for the pronounced inoculum effect of ceftazidime against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2009;53:46–56. doi: 10.1128/AAC.00489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Widmer AF, Frei R, Rajacic Z, et al. Correlation between in vivo and in vitro efficacy of antimicrobial agents against foreign body infections. J Infect Dis. 1990;162:96–102. doi: 10.1093/infdis/162.1.96. [DOI] [PubMed] [Google Scholar]
- 3.Hilf M, Yu VL, Sharp J, et al. Antibiotic therapy for Pseudomonas aeruginosa bacteremia: outcome correlations in a prospective study of 200 patients. Am J Med. 1989;87:540–6. doi: 10.1016/s0002-9343(89)80611-4. [DOI] [PubMed] [Google Scholar]
- 4.Chadwick EG, Shulman ST, Yogev R. Correlation of antibiotic synergy in vitro and in vivo: use of an animal model of neutropenic gram-negative sepsis. J Infect Dis. 1986;154:670–5. doi: 10.1093/infdis/154.4.670. [DOI] [PubMed] [Google Scholar]
- 5.Hershberger E, Coyle EA, Kaatz GW, et al. Comparison of a rabbit model of bacterial endocarditis and an in vitro infection model with simulated endocardial vegetations. Antimicrob Agents Chemother. 2000;44:1921–4. doi: 10.1128/aac.44.7.1921-1924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrison PK, Freedman LR. Experimental endocarditis I. Staphylococcal endocarditis in rabbits resulting from placement of a polyethylene catheter in the right side of the heart. Yale J Biol Med. 1970;42:394–410. [PMC free article] [PubMed] [Google Scholar]
- 7.Claassen DO, Batsis JA, Orenstein R. Proteus mirabilis: a rare cause of infectious endocarditis. Scand J Infect Dis. 2007;39:373–5. doi: 10.1080/00365540600981652. [DOI] [PubMed] [Google Scholar]
- 8.Kalra A, Cooley C, Tsigrelis C. Treatment of endocarditis due to Proteus species: a literature review. Int J Infect Dis. 2011;15:e222–5. doi: 10.1016/j.ijid.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Lloyd M, Satterwhite L, Lerakis S. Successfully treated mitral valve Proteus mirabilis endocarditis. Am J Med Sci. 2005;329:267–9. doi: 10.1097/00000441-200505000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Vandenbos F, Hyvernat H, Lucas P, et al. Enterobacterial native valve endocarditis in the intensive care unit: report of two cases. Rev Med Interne. 2000;21:560–1. doi: 10.1016/s0248-8663(00)89236-x. [DOI] [PubMed] [Google Scholar]
- 11.Caruthers MM. Endocarditis due to enteric bacilli other than Salmonellae: case reports and literature review. Am J Med Sci. 1977;273:203–11. doi: 10.1097/00000441-197703000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Rosen P, Armstrong D. Infective endocarditis in patients treated for malignant neoplastic diseases: a post mortem study. Am J Clin Pathol. 1973;60:241–50. doi: 10.1093/ajcp/60.2.241. [DOI] [PubMed] [Google Scholar]
- 13.Ananthasubramaniam K, Karthikeyan V. Aortic ring abscess and aortoatrial fistula complicating fulminant prosthetic valve endocarditis due to Proteus mirabilis. J Ultrasound Med. 2000;19:63–6. doi: 10.7863/jum.2000.19.1.63. [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi T, Murphy FD. Mural bacterial endocarditis produced by Proteus. J Am Med Assoc. 1950;143:427–8. doi: 10.1001/jama.1950.82910400001006. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Jaunatey JR, Garcia-Acuna JM, Garcia-Bengoechea J, et al. Endocarditis with pericardial bioprosthesis: clinic-pathologic characteristics, immediate and long-term prognosis. J Heart Valve Dis. 1994;3:172–8. [PubMed] [Google Scholar]
- 16.Venezio FR, Thompson JE, Sullivan H, et al. Infection of a ventricular aneurism and cardiac mural thrombus. Survival after surgical resection. Am J Med. 1984;77:551–4. doi: 10.1016/0002-9343(84)90119-0. [DOI] [PubMed] [Google Scholar]
- 17.Abela GS, Majmudar B, Felner JM. Myocardial abscesses unassociated with endocarditis. South Med J. 1981;74:432–4. doi: 10.1097/00007611-198104000-00016. [DOI] [PubMed] [Google Scholar]
- 18.Aguado JM, González-Vílchez F, Martín-Durán R, et al. Perivalvular abscesses associated with endocarditis. Clinical features and diagnostic accuracy of 2-dimensional echocardiography. Chest. 1993;104:88–93. doi: 10.1378/chest.104.1.88. [DOI] [PubMed] [Google Scholar]
- 19.Arnett EN, Roberts WC. Prosthetic valve endocarditis: clinicopathologic analysis of 22 necropsy patients with comparison observations in 74 necropsy patients with active infective endocarditis involving natural left-sided cardiac valves. Am J Cardio. 1976;38:281–92. doi: 10.1016/0002-9149(76)90169-7. [DOI] [PubMed] [Google Scholar]
- 20.Morpeth S, Murdoch D, Cabell CH, et al. International Collaboration on Endocarditis Prospective Cohort Study (ICE-PCS) Investigators Non-HACEK Gram-negative bacillus endocarditis. Ann Intern Med. 2007;147:829–35. doi: 10.7326/0003-4819-147-12-200712180-00002. [DOI] [PubMed] [Google Scholar]


