Abstract
Helicobacter pylori infects the human stomach and causes a spectrum of disease that includes gastritis, peptic ulcers, and gastric adenocarcinoma. A chronic, neutrophil-rich inflammatory response characterizes this infection. It is established that H. pylori stimulates neutrophil chemotaxis and a robust respiratory burst, but other aspects of this interaction are incompletely defined. We demonstrate here that H. pylori induces N1-like subtype differentiation of human neutrophils as indicated by profound nuclear hypersegmentation, a CD62Ldim, CD16bright, CD11bbright, CD66bbright, CD63bright surface phenotype, proinflammatory cytokine secretion, and cytotoxicity. Hypersegmentation requires direct neutrophil-H. pylori contact as well as transcription and both host and bacterial protein synthesis, but not urease, NapA, VacA, CagA or CagT. The concept of neutrophil plasticity is new, and to our knowledge these data are the first evidence that neutrophils can undergo subtype differentiation in vitro in response to bacterial pathogen infection. We hypothesize that these changes favor H. pylori persistence and disease.
Keywords: hypersegmentation, neutrophil subsets, plasticity, TANs, Helicobacter pylori
Introduction
Polymorphonuclear leukocytes (PMNs, neutrophils) are the most abundant leukocyte in humans, and their ability to be rapidly recruited to sites of infection and kill ingested microbes is unequivocal (1). Though traditionally thought of as a homogenous, terminally differentiated population of cells, this view has been radically altered by the recent discovery of neutrophil subsets, which revealed their potential to exhibit phenotypic plasticity and undergo subtype differentiation in tissue microenvironments in vivo (2, 3). Although this field is in its infancy, PMNs with distinct properties have been identified in the circulation of humans with systemic inflammation and following trauma, in the joints of persons with rheumatoid arthritis, in the lungs, and in the murine tumor microenvironment (4–12). Attempts to recapitulate these phenotypes in vitro have not been successful, suggesting that in vivo cues may be essential, and leaving researchers to question whether PMN subsets arise during neutrophil development, or whether mature neutrophils have the capacity to differentiate into subtypes upon encountering specific stimuli.
Helicobacter pylori is a Gram-negative bacterial pathogen of humans that resides in the mucus layer over the gastric epithelium and causes gastritis, peptic ulcers, and gastric cancer (13). A chronic neutrophil-dominant inflammatory response characterizes this infection, and PMN density correlates with disease severity and tissue destruction (14). H. pylori uses multiple mechanisms to recruit and activate PMNs, but bacterial killing is inefficient (14). In particular, NADPH oxidase-derived targeting is manipulated such that toxic reactive oxygen species (ROS) are released into the extracellular space rather than into bacterial phagosomes, which contributes to epithelial damage and sustains infection (14, 15). Although PMN chemotaxis and activation have been studied in detail, other aspects of H. pylori-neutrophil interactions are understudied. Herein, we advance understanding of neutrophil plasticity by demonstrating that subtype differentiation can be induced in mature human neutrophils by in vitro infection with an important human pathogen, and provide insight into the underlying mechanisms.
Materials and Methods
Isolation of human neutrophils
Human neutrophils were isolated from heparinized venous blood drawn from healthy adult volunteers in accordance with a protocol approved by the Institutional Review Board for human subjects at the University of Iowa. Neutrophils were purified using sequential dextran sedimentation, density gradient separation, and hypotonic lysis of erythrocytes (16). Neutrophil purity was routinely 95–98%, with eosinophils as the major contaminant. Replicate experiments utilized PMNs from different donors.
H. pylori strains and infection of neutrophils
Helicobacter pylori strains NCTC11637 (hereafter 11637) and 60190 were grown under microaerophilic conditions, washed, and quantified by measurement of the absorbance at 600 nm (15, 17). Neutrophils were diluted in Hepes-buffered RPMI-1640 (Lonza, Walkersville, MD) containing 10% heat-inactivated FBS (HyClone Laboratories, Pittsburgh, PA) to 1×106 cells/ml. H. pylori were added at a ratio of 5 bacteria/PMN, and cell suspensions were incubated at 37°C under microaerophilic conditions for up to 48 h. Some experiments used 0.4 µm Transwell inserts (Corning, Oneonta, NY), or included neutrophils that were pretreated with 5 µg/ml actinomycin D or 10 µg/ml cycloheximide, or H. pylori that were pretreated with 50 µg/ml chloramphenicol for 30 min, and drugs were maintained in the medium for the duration of the experiment.
Quantitation of bacterial load and nuclear morphology
Neutrophils were cytocentrifuged onto coverslips and fixed and stained using a Hema 3 Stat Pack (Fisher Scientific, Kalamazoo, MI) (18). Nuclear morphology and bacterial load were examined by light microscopy, and at least 200 neutrophils were analyzed per coverslip and condition. Each experiment was performed at least three times.
Cell viability and cytokine secretion
Neutrophil apoptosis was quantified by flow cytometry after Annexin V-FITC (Invitrogen, Camarillo, CA) and propidium iodide (PI) staining (18). AGS cell viability was quantified by exclusion of 0.2% trypan blue. Cytokines in PMN supernatants were detected using a human cytokine array kit (R&D Systems, Minneapolis, MN) and IL-8 was quantified by ELISA (18).
Cell surface marker flow cytometry
Control and H. pylori-infected neutrophils, and neutrophils treated with 10 ng/ml ultrapure E. coli LPS (InvivoGen, San Diego, CA) were pelleted, resuspended in HBSS with divalent cations containing 0.2% BSA and 0.2% sodium azide, and then stained with either Alexa Fluor® 647, Alexa Fluor® 488, FITC, or PE conjugated isotype control antibodies, or conjugated primary antibodies specific for CD16, CD62L, CD11b, active CD11b, CD66b, CD35, CD63, or CD54 (from BD, Franklin Lakes, NJ or BioLegend, San Diego, CA) for 30 min in the dark on ice. Following washing and resuspension, cells were analyzed on an Accuri C6 Cytometer.
Statistical Analysis
Control and single experimental groups were compared using two-tailed Student’s t tests. For multiple comparisons, data were analyzed by one-way ANOVA followed by a Tukey’s posttest. GraphPad Prism version 6.0 software was used, and P<0.05 was considered statistically significant.
Results and Discussion
H. pylori infects human neutrophils and induces nuclear hypersegmentation
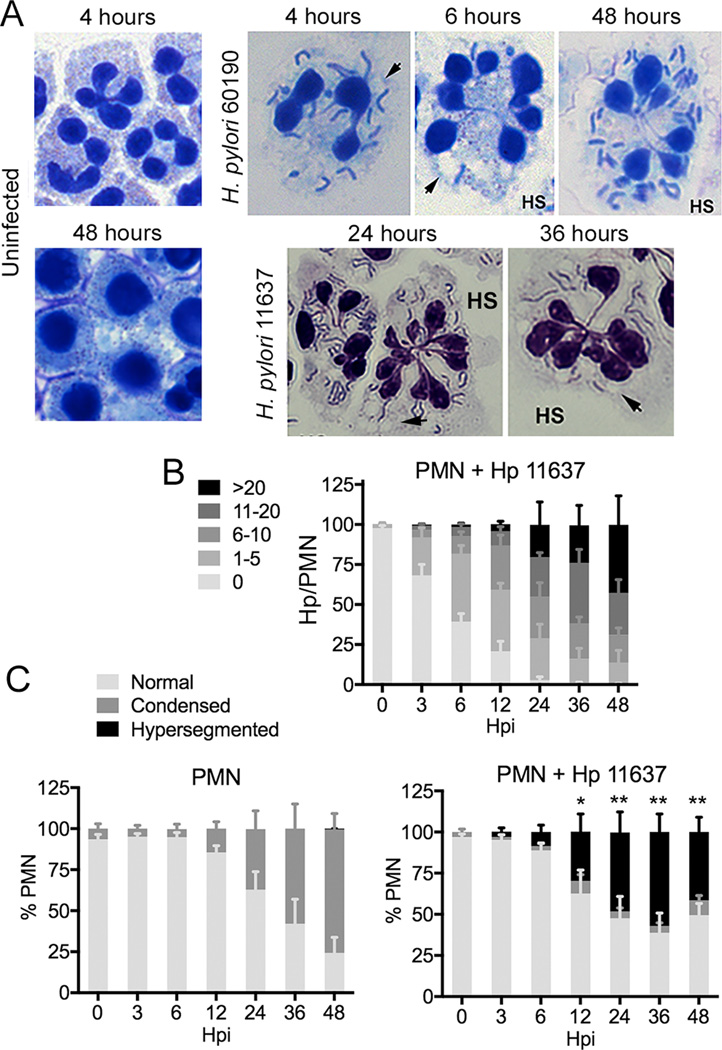
Human neutrophils were incubated in the presence and absence of two H. pylori strains, 60190 and 11637 (15, 17, 19), at a ratio of 5 bacteria/PMN under microaerophilic conditions for up to 48 h. Our data demonstrate that the majority of neutrophils were infected by 6 hpi and that bacterial load continued to increase out to 48 hpi (Figs. 1A, 1B and Supplemental Fig. 1A). Notably, the bacterial load we observed resembles infected PMNs in patient biopsy samples (20), and is therefore physiologically relevant. Moreover, in our system and in vivo uptake occurs by lectinophagocytosis, and the majority of intracellular H. pylori retained their characteristic morphology, which is a hallmark of bacterial health as these organisms undergo rapid conversion from spiral bacilli to coccoid forms in response to stress (Fig. 1A)(14, 20, 21). Infected neutrophils also contained small cytoplasmic vacuoles (Fig. 1A, arrowheads) that were often in close proximity to H. pylori phagosomes. The nature of these vacuoles and the composition of H. pylori phagosomes in PMNs are unknown. Nevertheless, H. pylori phagosomes exclude flavocytochrome b558 and lactoferrin (15), which suggests a defect in phagosome-secondary granule fusion.
FIGURE 1.
H. pylori accumulates in human neutrophils and induces nuclear hypersegmentation. (A) Microscopy images of control PMNs and cells that were infected with H. pylori strains 11637 or 60190 for the indicated amounts of time. Data shown are representative of 8 or more independent experiments. Arrowheads indicate vacuoles. HS indicates hypersegmented cells. (B) Bacterial load in infected PMNs. Data are the mean + SEM (n = 4). (C) PMN nuclear morphology was scored as normal (3–5 lobes), condensed (1–2 lobes), or hypersegmented (6 or more lobes). Data are the mean + SEM (n = 4). *P<0.05, **P<0.01 vs. 0 h hypersegmentation.
A distinctive feature of mature human neutrophils is their nuclei. These structures contain 3–4 interconnected lobes that merge and condense as cells age and undergo apoptosis (18, 22) (Figs. 1A and 1C). In sharp contrast, we found that H. pylori-infected PMNs acquired a hypersegmented morphology (Fig. 1A, HS), which is defined conservatively as six or more nuclear lobes/cell (4). We analyzed nuclear morphology over 48 h (Figs. 1A and 1C and Supplemental Figure 1B), and confirmed that although control PMNs acquired an apoptotic nuclear morphology over this time course, the infected PMNs did not. Rather, these cells exhibited marked nuclear hypersegmentation that was apparent by 3 hpi, and increased thereafter, yet was absent in the controls. Each hypersegmented nucleus exhibited a ‘cloverleaf’ arrangement of interconnected segments, but individual cells differed with respect to total lobe number (range of 6–17/cell) and size (homogeneous or heterogeneous) (Fig. 1A). This phenotype appears to be neutrophil-specific, as the few eosinophils in our samples were not affected (data not shown), and hypersegmentation of PMNs in the infected gastric mucosa demonstrates in vivo relevance (23).
As fewer than 4% of infected PMNs contained condensed nuclei, we hypothesized that these cells may not undergo apoptosis over 48 hpi. Indeed, Annexin V-FITC/PI staining and flow cytometry demonstrated that 84 ± 5% of control PMNs were apoptotic at 48 h versus only 20 ± 5% and 26 ± 5% of cells infected with H. pylori 11637 or 60190, respectively, (n=4, P<0.001) (not illustrated), and DNA release was not detected (data not shown). Thus, human neutrophils support robust H. pylori infection that is characterized by profound nuclear hypersegmentation and extended lifespan.
Hypersegmentation requires neutrophil-bacteria contact and protein synthesis
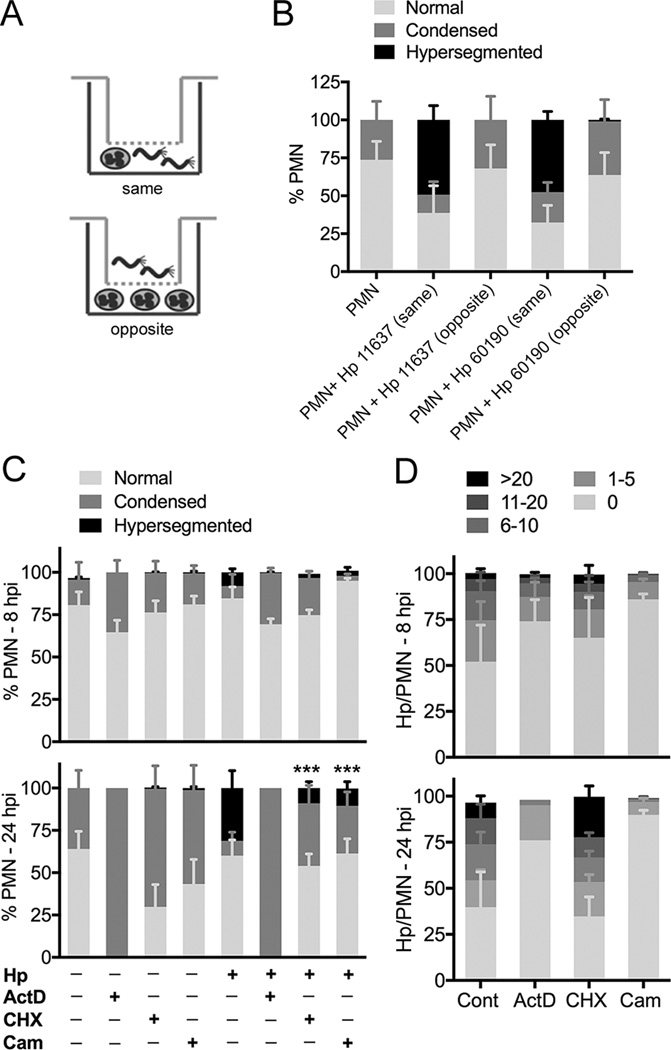
We utilized Transwells (Fig. 2A) to determine if manipulation of PMN phenotype required H. pylori binding and/or phagocytosis. The data in Figure 2B confirm the results shown in Figure 1, and demonstrate that direct H. pylori-PMN contact is essential for hypersegmentation, as neutrophils that were separated from bacteria were indistinguishable from the uninfected controls.
FIGURE 2.
Direct contact and metabolic activity are required for H. pylori-induced neutrophil hypersegmentation. (A–B) Transwell infections. PMNs were left untreated or were incubated in the presence of H. pylori that were added to the same or opposite chamber of a Transwell for 24 h. PMN nuclear morphology was assessed by microscopy. Data are the mean + SEM, n = 3. **P<0.01. (C–D) The effects of actinomycin D, cycloheximide and chloramphenicol on nuclear morphology (C) and bacterial load (D) were assessed after 8 h and 24 h, as indicated. Data are the mean + SEM, n = 3–4. ***P<0.001. Hp, Helicobacter pylori 11637. ActD, actinomycin D. CHX, cycloheximide. Cam, chloramphenicol.
Next, we tested a requirement for metabolic activity using inhibitors of host and bacterial transcription and translation, with analyses at 8 and 24 h. Blocking transcription with actinomycin D ablated hypersegmentation, and 100% of neutrophils had condensed nuclei by 24 h, suggesting rapid apoptosis induction (Fig. 2C). Blocking host or bacterial protein synthesis also caused significant inhibition of hypersegmentation by 24 hpi (Fig. 2C). Thus, whereas cycloheximide reduced hypersegmentation by 66.1 ± 24.1% (n=4, P<0.01), chloramphenicol reduced hypersegmentation by 81.3 ± 7.3% (n=3, P<0.001) relative to the no drug controls.
With respect to bacterial load, cycloheximide did not adversely affect infection (Fig. 2D). On the other hand, chloramphenicol markedly reduced bacterial load, as only 14 ± 5% and 10 ± 5% of cells contained H. pylori at 8 and 24 hpi, respectively, and the cells that were infected contained relatively few bacteria (Fig. 2D). Actinomycin D also impaired infection, as bacterial load was slightly diminished at 8 hpi, and was reduced further at 24 hpi, likely as a result of apoptosis induction (Fig. 2D). Thus, manipulation of PMN phenotype is an active process that is driven by both host and microbe and may be initiated at the earliest stages of infection.
The bacterial factors required for this phenotype remain unknown, as our studies of mutants generated in H. pylori 11637 (Supplemental Fig. 2A) show that hypersegmentation does not require bacterial urease (ΔureAB), the neutrophil activating protein (ΔnapA), or factors that disrupt epithelial cell function such as VacA, the type IV secretion system (ΔcagE, ΔcagT) or its effector CagA (14, 19, 24). Having excluded roles for several established virulence factors, a group of recently identified H. pylori proteins with functional nuclear localization sequences but unknown function merit further study (25).
H. pylori-infected neutrophils exhibit surface markers and functions indicative of subtype differentiation
Hypersegmentation is rare, was described first in persons with folate or vitamin B12 deficiency, and more recently as a hallmark of two distinct PMN subsets (5, 8, 26, 27). As a fivefold excess of folate and/or vitamin B12 had no effect on nuclear morphology in our system (Supplemental Fig. 1C), we hypothesized that H. pylori may induce PMN subtype differentiation.
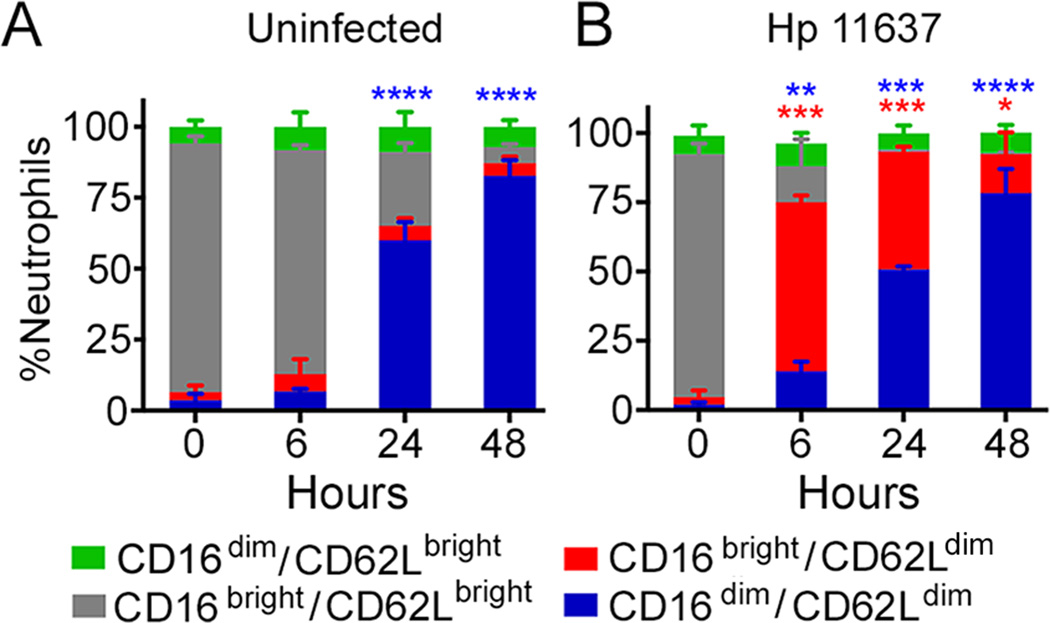
The first subset of interest was described by Pillay et al., is defined by hypersegmentation and a CD16bright/CD62Ldim surface phenotype, and is present at low frequency (15% of neutrophils) in the circulation of persons with endotoxemia along with CD16bright/CD62Lbright mature (segmented) PMNs and CD16dim/CD62Lbright immature (banded) cells (5). We analyzed CD16 (FcγRIII) and CD62L (L-selectin) by flow cytometry, and as expected the vast majority of freshly isolated PMNs were CD16bright/CD62Lbright (Fig. 3A, gray bars). However, this pattern changed markedly in response to H. pylori, as 61–68% of cells acquired a CD16bright/CD62Ldim phenotype by 6 hpi (Fig. 3B and Supplemental Figs. 1D–1E, red bars) that resembles the atypical PMN subset described above (5). This phenotype was nearly absent in the uninfected controls (Fig. 3A, red bars) (n=3, P<0.0001), and remained abundant in the infected PMNs until at least 24 hpi (Fig. 3B and Supplemental Figs. 1D–1E). Certain activating stimuli, including some bacterial pathogens, induce CD62L shedding via ADAM17 activation (28). Although effects of H. pylori on ADAM17 are unknown, our data are strong evidence that a distinct PMN subset has been induced.
FIGURE 3.
H. pylori-infected neutrophils have high surface CD16 and low surface CD62L. Control, uninfected (A), and H. pylori 11637-infected (B) PMNs were stained for CD16 and CD62L at the noted time points and analyzed by flow cytometry. The percentage of neutrophils in each category is shown as the mean + SEM, n=3. *P<0.05, **P<0.01, ***P<0.001, ****P≤0.0001 vs. 0 h.
CD16 mediates opsonophagocytosis and localizes to the Golgi, intracellular vesicles, and cell surface of healthy PMNs (29). Concomitant downregulation of CD16 and CD62L on aged, uninfected neutrophils by 48 h (Fig. 3A, blue bars) indicates progression to apoptosis (18, 22) and is consistent with the nuclear condensation shown in Figure 1. In contrast, loss of CD16 in the absence of apoptosis, as occurs in H. pylori infection, indicates sustained neutrophil activation, and ensues when surface shedding exceeds the capacity to replenish CD16 via new synthesis and secretion (28–30).
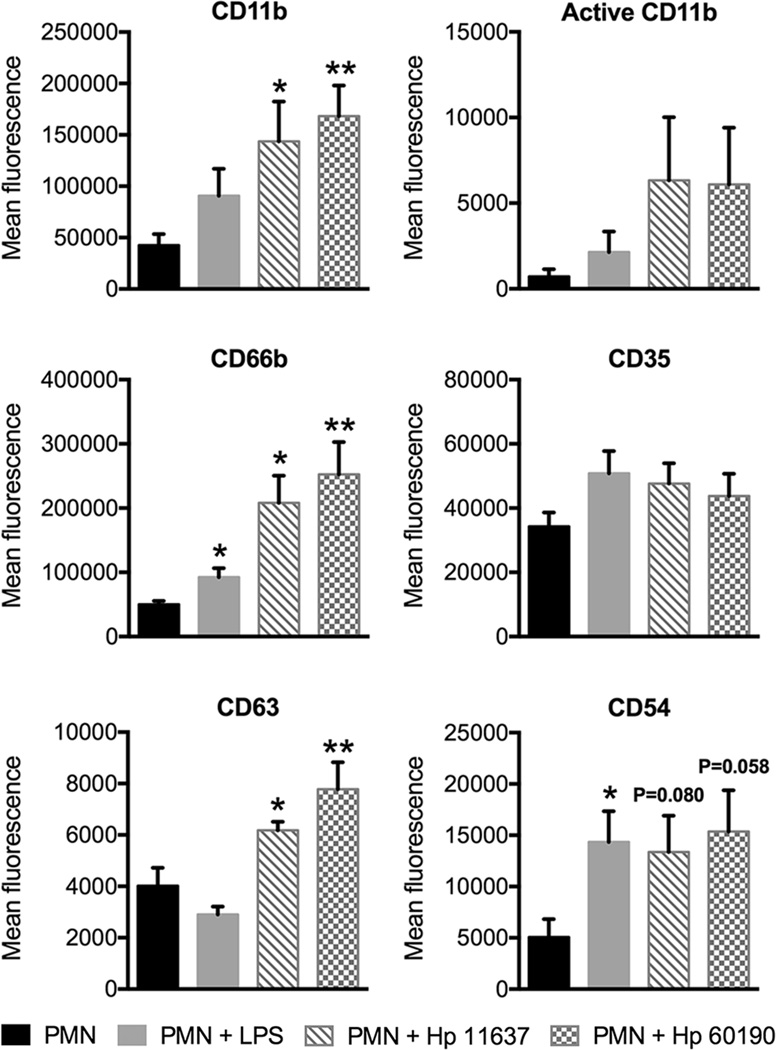
During neutrophil activation intracellular granules are mobilized and fuse with the plasma membrane. We analyzed the secretory vesicle marker CD35, the secondary granule marker CD66b, and the primary granule marker CD63, as well as adhesion molecules CD54 (ICAM-1) and CD11b (31) on untreated and H. pylori-infected PMNs, and included cells stimulated with E. coli LPS as an additional control. All these markers were present on H. pylori-infected PMNs (Fig. 4) and on the cells described by Pillay et al. (5). However, in our system, changes CD11b, CD66b, and CD63 were statistically significant, whereas only CD11b reached significance on the Pillay et al., subset (5). Metalloproteases and serine proteases released by mobilization of tertiary and primary granules mediate CD16 shedding (32) and likely contribute to the CD16dim phenotype that arises in late H. pylori infection. In contrast, E. coli LPS elicited ‘conventional’ activation, defined as moderate upregulation of all markers except CD63 (Fig. 4), and did not elicit hypersegmentation (data not shown), confirming published data (5, 33). Thus, H. pylori-infected PMNs are characterized by nuclear hypersegementation and a CD62Ldim, CD16bright, CD11bbright, CD66bbright, CD63bright surface phenotype.
FIGURE 4.
H. pylori-infected neutrophils display additional surface markers indicative of subtype differentiation. Mean fluorescence of CD11b, active CD11b, CD66b, CD35, CD63, and CD54 was assessed by flow cytometry at 24 h. H. pylori-infected neutrophils and neutrophils treated with 10ng/ml E. coli LPS were compared to untreated controls. n ≥ 4 donors. *P ≤ 0.05, **P ≤ 0.01.
Like myeloid-derived suppressor cells, the PMN subset described by Pillay et al. inhibits T-cell proliferation via a contact-dependent mechanism (5, 6). H. pylori arginase (RocF) suppresses T-cells directly via arginine depletion (34), and whether infected PMNs synergize with RocF to disrupt T-cell function remains to be determined.
We demonstrate instead that H. pylori-infected PMNs share many phenotypic and functional properties with a subset of murine tumor-associated neutrophils that are proinflammatory and cytotoxic, N1 TANs (8, 10). Both PMN populations are hypersegmented, CD11bright and CD54positive, and are notable for robust extracellular oxidant production (8, 11, 12, 14, 15)(Figs. 1 and 4). ROS released by N1 TANs and PMA-activated PMNs are cytotoxic (8, 11, 12), and we show here that H. pylori-infected neutrophils killed gastric epithelial cells, whereas untreated PMNs and bacteria alone did not (Supplemental Fig. 2B). H. pylori-infected PMNs also resembled N1 TANs (8, 12) in their capacity to secrete multiple cytokines and chemokines including MIP-1, IL-6, IL-8, CXCL1 and IL-1β that can reinforce neutrophil recruitment and activation (Supplemental Fig. 2C–2D).
Our findings are noteworthy as there is convincing evidence that PMNs have multiple, potentially paradoxical roles in the development and progression of gastric disease. Sustained recruitment and activation of neutrophils is a defining feature of H. pylori infection, and the results presented here extend prior studies of the NADPH oxidase to include granule mobilization and PMN cytokine secretion (14, 15) (Fig. 4 and Supplemental Figs. 2C–2D). In addition to permitting bacterial survival and replication, neutrophil ROS induce DNA damage, and synergize with virulence factors such as CagA and VacA to undermine the integrity, polarity and viability of the gastric epithelium (35) (Supplemental Fig. 2B).
As N1 TANs are cytotoxic and anti-tumorigenic, it is tempting to speculate that H. pylori-infected PMNs may curtail gastric cancer progression. This hypothesis is consistent with the fact that only 1–3% of infected individuals develop gastric adenocarcinoma despite classification of H. pylori as a carcinogen and clear evidence that its effects on the epithelium favor cancer initiation (35). Additionally, there is evidence that abundant TANs are beneficial in human gastric cancer patients (10, 36–38). Thus, induction of PMN subtype differentiation may aid in explaining how H. pylori is able to chronically infect humans, inducing gastritis and peptic ulceration that rarely progresses to gastric adenocarcinoma (36, 37).
In summary, the concept of neutrophil phenotypic plasticity is new, and although recent studies have begun to identify neutrophil subsets that differ with respect to maturation state, density, nuclear morphology, surface markers, and functional properties (3, 4, 9, 39), we are only beginning to understand the range of phenotypes that these cells can exhibit. In discovering the ability of H. pylori to induce PMN subtype differentiation in vitro, we reinforce the notion that mature human neutrophils retain a capacity for phenotypic plasticity that can be evoked in response to specific stimuli in the absence of in vivo cues, and argue against models which propose that these cells only arise from a distinct developmental lineage (6). The PMNs we describe are hypersegmented, proinflammatory and cytotoxic, and exhibit changes in surface markers that are indicative of strong and sustained activation. Accordingly, these ‘N1-Hp’ cells share many features with, yet appear distinct from, other PMN subtypes described to date. At the same time, the requirement for H. pylori-PMN contact and both host and bacterial protein synthesis, but not urease, NapA, VacA or CagA, suggests a complex underlying mechanism that merits further study and may reveal new aspects of bacterial pathogenesis. Collectively, our data advance the rapidly evolving field of neutrophil plasticity, and we hypothesize that manipulation of PMN phenotype and function is inherently linked to H. pylori persistence and its ability to elicit a spectrum of human disease.
Supplementary Material
Acknowledgments
This work was supported by funds from the National Institutes of Health including R01 AI119965 awarded to L-A.H.A. L.C.W. is a postdoc supported in part by T32 AI007260. M.N.W. received a medical student summer research fellowship via T32 AI007343.
Abbreviations
- hpi
hours post-infection
- PI
propidium iodide
- PMN
polymorphonuclear leukocyte
- TAN
tumor associated neutrophil
- ROS
reactive oxygen species
Footnotes
Disclosures
The authors declare that no conflicts of interest exist.
References
- 1.Nauseef WM, Borregaard N. Neutrophils at work. Nat. Immunol. 2014;15:602–611. doi: 10.1038/ni.2921. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 3.Scapini P, Cassatella MA. Social networking of human neutrophils. Blood. 2014;124:710–719. doi: 10.1182/blood-2014-03-453217. [DOI] [PubMed] [Google Scholar]
- 4.Beyrau M, Bodkin JV, Nourshargh S. Neutrophil hetergeneity in health and disease: a revitalized avenue in inflammation and immunity. Open Biol. 2012;2:120134. doi: 10.1098/rsob.120134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers JW, Ulfman LH, Leenen LP, Pickkers P, Koenderman L. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J. Clin. Invest. 2012;122:327–336. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pillay J, Tak T, Kamp V, Koenderman L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: similarities and differences. Cell. Mol. Life Sci. 2013;70:3813–3827. doi: 10.1007/s00018-013-1286-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fortunati E, Kazemier KM, Grutters JC, Koenderman L, Van den Bosch VJMM. Human neutrophils switch to an activated phenotype after homing to the lung irrespective of inflammatory disease. Clin. Exp. Immunol. 2009;155:559–566. doi: 10.1111/j.1365-2249.2008.03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piccard H, Muschel RJ, Opdenakker G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit. Rev. Oncol. Hematol. 2012;82:296–309. doi: 10.1016/j.critrevonc.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Kruger P, Saffarzadeh M, Weber AN, Rieber N, Radsak M, von Bernuth H, Benarafa C, Roos D, Skokowa J, Hartl D. Neutrophils: Between host defence, immune modulation, and tissue injury. PLoS Pathog. 2015;11:e1004651. doi: 10.1371/journal.ppat.1004651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33:949–955. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- 11.Fridlender ZG, Sun J, Kinm S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sagiv JY, Michaeli J, Assi S, Mishalian I, Kisos H, Levy L, Damti P, Lumbroso D, Polyansky L, Sionov RV, Ariel A, Hovav AH, Henke E, Fridlender ZG, Granot Z. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015;10:562–573. doi: 10.1016/j.celrep.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 13.Ernst PB, Gold BD. The disease spectrum of Helicobacter pylori: the immunopathogenesis of gastroduodenal ulcer and gastric cancer. Annu. Rev. Microbiol. 2000;54:615–640. doi: 10.1146/annurev.micro.54.1.615. [DOI] [PubMed] [Google Scholar]
- 14.Allen L-AH. Phagocytosis and persistence of Helicobacter pylori. Cell. Microbiol. 2007;9:817–828. doi: 10.1111/j.1462-5822.2007.00906.x. [DOI] [PubMed] [Google Scholar]
- 15.Allen L-AH, Beecher BR, Lynch JT, Rohner OV, Wittine LM. Helicobacter pylori disrupts NADPH oxidase targeting in human neutrophils to induce extracellular superoxide release. J. Immunol. 2005;174:3658–3667. doi: 10.4049/jimmunol.174.6.3658. [DOI] [PubMed] [Google Scholar]
- 16.Nauseef WM. Isolation of human neutrophils from venous blood. Methods Mol. Biol. 2007;412:15–20. doi: 10.1007/978-1-59745-467-4_2. [DOI] [PubMed] [Google Scholar]
- 17.Allen L-AH, Schlesinger LS, Kang B. Virulent strains of Helicobacter pylori demonstrate delayed phagocytosis and stimulate homotypic phagosome fusion in macrophages. J. Exp. Med. 2000;191:115–127. doi: 10.1084/jem.191.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz JT, Barker JH, Kaufman J, Fayram DC, McCracken JM, Allen L-AH. Francisella tularensis inhibits the intrinsic and extrinsic pathways to delay constitutive apoptosis and prolong human neutrophil lifespan. J. Immmunol. 2012;188:3351–3363. doi: 10.4049/jimmunol.1102863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghiara P, Marchetti M, Blaser MJ, Tummuru MK, Cover TL, Segal ED, Tompkins LS, Rappuoli R. Role of the Helicobacter pylori virulence factors vacuolating cytotoxin, CagA, and urease in a mouse model of disease. Infect. Immun. 1995;63:4154–4160. doi: 10.1128/iai.63.10.4154-4160.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Necchi V, Manca R, Ricci V, Solcia E. Evidence for transepithelial dendritic cells in human H pylori active gastritis. Helicobacter. 2009;14:208–222. doi: 10.1111/j.1523-5378.2009.00679.x. [DOI] [PubMed] [Google Scholar]
- 21.Andersen LP, Wadstrom T. Basic Bacteriology and Culture. In: Mobley HLT, Mendz GL, Hazell SL, editors. Helicobacter pylori: physiology and genetics. Washington DC: ASM Press; 2001. pp. 27–38. [Google Scholar]
- 22.Kennedy A, DeLeo F. Neutrophil apoptosis and the resolution of infection. Immunol. Res. 2009;43:25–61. doi: 10.1007/s12026-008-8049-6. [DOI] [PubMed] [Google Scholar]
- 23.Keenan JI, Peterson Ii RA, Hampton MB. NADPH oxidase involvement in the pathology of Helicobacter pylori infection. Free Radic. Biol. Med. 2005;38:1188. doi: 10.1016/j.freeradbiomed.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz J, Allen L-AH. Role of urease in megasome formation and Helicobacter pylori survival in macrophages. J. Leukoc. Biol. 2006;79:1214–1225. doi: 10.1189/jlb.0106030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JH, Jun SH, Baik SC, Kim DR, Park JY, Lee YS, Choi CH, Lee JC. Prediction and screening of nuclear targeting proteins with nuclear localization signals in Helicobacter pylori. J. Microbiol. Methods. 2012;91:490–496. doi: 10.1016/j.mimet.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Bills T, Spatz L. Neutrophilic hypersegmentation as an indicator of incipient folic acid deficiency. Am. J. Clin. Pathol. 1977;68:179–184. doi: 10.1093/ajcp/68.2.263. [DOI] [PubMed] [Google Scholar]
- 27.Thompson WG, Cassino C, Babitz L, Meola T, Berman RM, Lipkin MJ, Freedman M. Hypersegmented neutrophils and vitamin B12 deficiency. Acta Haematol. 1989;81:186–191. doi: 10.1159/000205559. [DOI] [PubMed] [Google Scholar]
- 28.Walcheck B, Herrera AH, St Hill C, Mattila PE, Whitney AR, DeLeo FR. ADAM17 activity during human neutrophil activation and apoptosis. Eur. J. Immunol. 2006;36:968–976. doi: 10.1002/eji.200535257. [DOI] [PubMed] [Google Scholar]
- 29.Jost CR, Gaillard ML, Fransen JAM, Daha MR, Ginsel LA. Intracellular localization of glycosyl-phosphatidylinositol-anchored CD67 and FcRIII (CD16) in affected neutrophil granulocytes of patients with paroxysmal nocturnal hemoglobinuria. Blood. 1991;178:3030–3036. [PubMed] [Google Scholar]
- 30.Wang Y, Zhang AC, Ni Z, Herrera A, Walcheck B. ADAM17 activity and other mechanisms of soluble L-selectin production during death receptor-induced leukocyte apoptosis. J. Immunol. 2010;184:4447–4454. doi: 10.4049/jimmunol.0902925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borregaard N, Sorensen OE, Theilgaard-Monch K. Neutrophil granules: a library of innate immunity proteins. Trends Immunol. 2007;28:340–345. doi: 10.1016/j.it.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Moldovan I, Galon J, Maridonneau-Parini I, Roman Roman S, Mathiot C, Fridman WH, Sautes-Fridman C. Regulation of production of soluble Fc gamma receptors type III in normal and pathological conditions. Immunol. Lett. 1999;68:125–134. doi: 10.1016/s0165-2478(99)00041-3. [DOI] [PubMed] [Google Scholar]
- 33.Tirouvanziam R, Gernez Y, Conrad CK, Moss RB, Schrijver I, Dunn CE, Dvies ZA, Herzenberg LA, Herzenberg LA. Profound functional and signaling changes in viable inflammatory neutrophils homing to cystic fibrosis ariways. Proc. Natl. Acad. Sci., USA. 2008;105:4335–4339. doi: 10.1073/pnas.0712386105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zabaleta J, McGee DJ, Zea AH, Hernandez CP, Rodriguez PC, Sierra RA, Correa P, Ochoa AC. Helicobacter pylori Arginase Inhibits T Cell Proliferation and Reduces the Expression of the TCR-zeta Chain (CD3zeta) J. Immunol. 2004;173:586–593. doi: 10.4049/jimmunol.173.1.586. [DOI] [PubMed] [Google Scholar]
- 35.Peek RM, Jr, Fiske C, Wilson KT. Role of innate immunity in Helicobacter pylori-induced gastric malignancy. Physiol. Rev. 2010;90:831–858. doi: 10.1152/physrev.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang WJ, Du Y, Zhao X, Ma LY, Cao GW. Inflammation-related factors predicting prognosis of gastric cancer. World J. Gastroenterol. 2014;20:4586–4596. doi: 10.3748/wjg.v20.i16.4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang F, Sun G, Zou Y, Zhong F, Ma T, Li X. Protective role of Helicobacter pylori infection in prognosis of gastric cancer: evidence from 2,454 patients with gastric cancer. PLoS One. 2013;8:e62440. doi: 10.1371/journal.pone.0062440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caruso RA, Bellocco R, Pagano M, Bertoli G, Rigoli L, Inferrera C. Prognostic value of intratumoral neutrophils in advanced gastric carcinoma in a high-risk area in northern Italy. Mod. Pathol. 2002;15:831–837. doi: 10.1097/01.MP.0000020391.98998.6B. [DOI] [PubMed] [Google Scholar]
- 39.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat. Immunol. 2011;12:1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.