Abstract
Neuropsychiatry represents a field of medicine situated at the crossroads of neurology and psychiatry and deals with the interface of behavioral phenomena driven by brain dysfunction. Psychiatric symptoms are highly prevalent in these conditions, are a major source of disability and diminished quality of life, and potentially represent the target of treatment interventions that stand to significantly decrease the suffering they generate. In this article, the disease paradigm is explained with particular attention to its role as an organizing principle for the field. Specific diseases including traumatic brain injury, stroke, Parkinson’s disease, Alzheimer’s disease, Multiple Sclerosis, and epilepsy are explored in relation to the presentation of multiple psychiatric phenotypes in each, associations with underlying brain pathology, and existing treatment approaches. Finally, the article explores the inherent complexities in this area of research and proposes a framework for future work based on the understanding of phenomenology and associated risk factors, the involvement of the rapidly advancing field of neuroscience, and targeted treatment development to serve as a road map for advancement in the field.
Introduction
Clinical neurologists and psychiatrists have long recognized the frequent occurrence of psychiatric conditions in the context of neurologic (brain) disease. Indeed this frequent co-occurrence of psychiatric with neurologic symptoms should come as no surprise since psychiatric disorders, such as schizophrenia and the mood disorders, can be induced by structural brain disease. Presumably, brain dysfunction from conditions that cause neurologic symptoms—such as impairments in movement, sensation, seizures, speech or language—also affects areas of the brain that regulate mood, emotion, cognition and perception. For the most part, this branch of psychiatry, neuropsychiatry1, has laid relatively unexplored until experiencing resurgence in the last several decades. A major reason for this lack of exploration was the use of psychological explanations such as “reactions” to conceptualize why psychiatric symptoms occurred in the presence of neurologic symptoms. For example, it was asked, “How could a person with hemiparesis not also feel depressed?” Or, “How could someone with aphasia not also be cognitively impaired?” More recently, it has been recognized that it is the diseased brain in many instances that causes the psychiatric symptoms. This appreciation has opened up new avenues for understanding of these symptoms and by extension of brain-behavior relationships in this context. That is, the traditional “lesion approach” that so significantly advanced our understanding of neurologic disease is now being increasingly applied to the psychiatric conditions seen in patients with neurologic disease.
Neuropsychiatry exists at the interface between neurology and psychiatry. the traditional approaches of these two fields underpin its potential for leading to a better understanding of brain-behavior relationships. Recent developments also emphasize the growing public health significance of neuropsychiatry given the rapid increase in the number of patients living with the consequences of chronic brain disease such as stroke, traumatic brain injury, Alzheimer’s, Parkinson’s disease, epilepsy, multiple sclerosis, and related conditions. Indeed, it has become clear that there is a high frequency of psychiatric symptoms in almost all neurologic diseases involving the central nervous system, such that the vast majority of patients with neurologic diseases will develop psychiatric disturbances ranging from affective disorders (e.g., depression, mania) to cognitive impairments (e.g., dementia, milder cognitive syndromes) to disturbances of perception (e.g., hallucinations, delusions) over the lifetime of their illness. These disturbances typically run parallel to the classical neurologic symptoms such as seizure, involuntary vocalization, motor weakness, sensory loss or language disorder, and tend to cause disability and impair quality of life as much or even more than the neurologic symptoms.
While the underlying causes of brain disease are often difficult to treat, there is emerging evidence that the psychiatric symptoms of brain disease are often amenable to treatment with existing therapies, both pharmacologic and non-pharmacologic. Since tens of millions of individuals now suffer from chronic neurologic disease, the public health importance of neuropsychiatry as a therapeutic area of psychiatry should be obvious. With the above in mind, approaching neuropsychiatry as an integrative field that teaches mechanistic aspects of brain-behavior relationships while being an active—and growing—clinical field of great public health importance, this synthetic overview will attempt to provide a brief conceptual overview of what is known and to make recommendations regarding future directions.
The Disease Paradigm
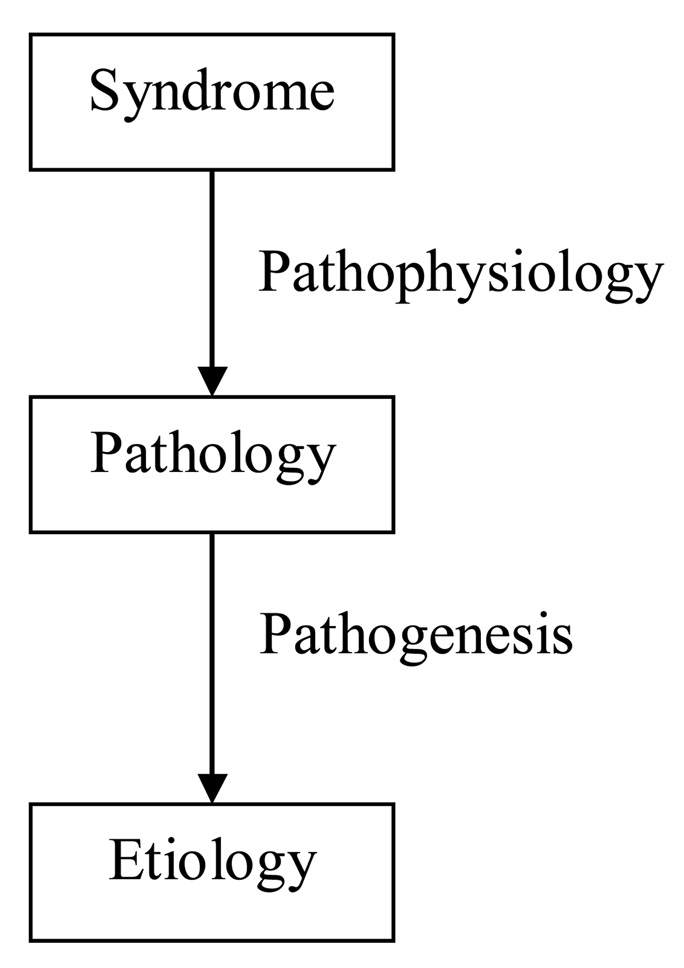
Neuropsychiatry general operates using the disease paradigm2 to explain the phenomena with which it is concerned. As shown in Figure 1, this is a top down approach, which begins by defining clinical signs, symptoms, and syndromes in mental state and behavior (a.k.a., “psychopathology”), linking them to an underlying pathology in the organ of interest, in this case, the brain, and then attempting to understand the etiology that brings about the pathology. Pathophysiology is the understanding of the how the clinical phenomena link mechanistically to the brain pathology. In neuropsychiatry, pathophysiology is approached by carefully describing the clinical phenomena of interest and their relationship to the neurologic phenomena, and then linking these up to the location, type, and degree of the pathology. This exercise is more complex than the one used by neurologists since one-to-one relationships between region and pathology are uncommon in neuropsychiatry, whereas they are common in neurology where clinical phenomena can generally be linked to specific pathologic areas in rather straightforward ways. Pathogenesis is concerned with understanding how the pathology itself comes about. Increasingly the pathogenesis of brain pathology is being understood, at least in common brain diseases, although much remains to be done in this area. In its present state, neuropsychiatry is more concerned with pathophysiology, and less concerned with pathogenesis, now increasingly in the realm of applied neuroscience as it becomes more interested in brain disease.
FIGURE 1.
The Disease Paradigm
The brain diseases of interest to neuropsychiatry occur in several pathogenetic groups, being the result of acute mechanical trauma, (traumatic brain injury with both regional and diffuse effects on the brain), vascular injury (acute and chronic)demyelination, and neurodegeneration. Genes influence all of the above, in some cases deterministically (i.e., through classical Mendelian inheritance), more often through more complex gene-environment risk relationships. While neuropsychiatry approaches the disease paradigm from above in a top down fashion, behavioral and general neurology tend to operate bottom up, beginning with the emergence of pathology in the brain, and attempting to understand the emergence of clinical syndromes out of this pathology.
Neuropsychiatry faces several common challenges worthy of discussion. A first challenge relates to the assessment and definition of psychiatric signs and symptoms in patients with neurologic disease. While in the past, many general psychiatrists expressed the concern that mental state and behavior could not be quantified, it has been shown consistently that it is possible to quantify disturbances in mental life and behavior with high reliability. However, in the context of brain disease there are additional challenges in ascertaining and defining clinical phenomena. Brain damaged patients frequently suffer impairments that affect ability to communicate. Cognitive impairment, memory loss in particular, might limit a patient’s ability to describe his mental life or remember it; nosoagnosia may impair a patient’s ability to appreciate his impairments. Thus, neuropsychiatrists must be careful about how they characterize the clinical phenomena they study and frequently need to involve informants, such as family members and caregivers, in ascertaining the clinical picture more carefully. Introducing outside informants introduces biases since the mental state of the informants, as well as the degree of burden they might experience in caring for the patient, can significantly influence their reporting of the patient’s state. As a result, mental status examinations in neuropsychiatry take longer, but have high degrees of reliability.
A second challenge for neuropsychiatry has to do with time frame. For the most part, both the ‘psychiatric’ and the ‘neurologic’ condition are chronic diseases brain. While regeneration is not an option at this point, the plasticity of the brain enables it to recover from or compensate for many injuries, at least in part. Thus, the organ out of which these psychiatric symptoms emerge is plastic even in the context of brain disease. Consequently, experienced clinicians are aware that the phenotype of psychiatric conditions changes over time in individual patients and across patients. Since the vast majority of research in neuropsychiatry has not taken time frame into account, but rather reported on cross-sectional findings, we know very little about the temporal course of psychopathology and brain disease.
A third challenge relates to the strong influence exerted by the patient’s premorbid state upon the emergence of psychopathology after the onset of neurologic disease. This depends in part on the condition. For example, with traumatic brain injury (TBI), the patient’s premorbid behavior influences whether their brain will be traumatized; many TBI patients bring premorbid psychiatric conditions, such as alcoholism, impulsivity, depression, or personality disorder, to the injury, which further affects their post-injury behavior. Since it is difficult to carefully dissect and ascertain premorbid state after the onset of neurologic disease, both clinical and research efforts are affected by this limitation.
A fourth challenge relates to environment and social support. While brain diseases can lead to the expression of a range of new behaviors and mental states, their expression is frequently dependent on the environment that surrounds the patient. A consistent theme is that patients with good social supports who reside in environments that are tailored to their condition are less likely to express problematic behaviors or other forms of psychopathology. This has clinical and mechanistic relevance. On the one hand it implies that manipulation of the environment is a critical aspect of care. On the other hand it poses interesting mechanistic questions about the interaction between environmental influences and particular types of brain damage that result in specific kinds of psychopathology.
A fifth and final challenge has to do with the common application of two, at times competing, explanatory paradigms when attempting to explain the occurrence of psychiatric symptoms in patients with brain disease. The disease paradigm has already been mentioned in which the psychopathology is primarily seen as a symptom of brain disease as with paralysis, language loss, or blindness. In addition, however, psychopathologic phenomena, even extreme ones such as mania and hallucinations, can be understood through meaningful connections1 as the reaction of human beings to what is happening to them, how their disease is affecting their plans, expectations, and the way they lead their lives. This effort to explain psychological states using narratives, a very powerful method widely used in Western society, sometimes interferes with explanations that see these same symptoms as cold and impersonal consequences of damage to the brain. This is not to say that these two types of explanation are always mutually exclusive because both types of explanation can lead to therapeutic approaches that can be applied concurrently and be of help to the patient from the point of view of a practical clinician. For example, if a patient develops depression after a stroke to the frontal lobes and the primary explanation is that the brain damage caused the depression, there is no doubt that the human side of the patient is greatly be helped by developing through psychotherapy a narrative that helps him tie together his adjustment to both the stroke and the depression, while he moves forward with his life.
Specific Neurologic Diseases
Attention now shifts to discussion of psychopathology in the context of specific diseases. The diseases discussed here are chosen both because they are the most common and for paradigmatic purposes, because they demonstrate the emergence of psychopathology in diseases of different pathogenetic origins. Thus, the discussion focuses on the following conditions:
Traumatic brain injury, an example of acute trauma to the brain with both focal and diffuse effects
Stroke, typically unexpected occurring in someone with significant risk factors such as hypertension, diabetes and heart disease, causing primarily focal damage although often against the backdrop of chronic vascular insufficiency
Parkinson’s disease, an example of a neurodegenerative disease with origins in the subcortex
Alzheimer’s disease, an example of a neurodegenerative disease with origins in the cortex
Multiple sclerosis, a demylenating condition, usually episodic, affecting the white matter diffusely in the brain and spinal cord; and
Epilepsy, in which repetitive abnormal electrical discharges occur but in which there is likely additional brain pathology, typically unknown, so that psychiatric disturbance might arise both in relationship to the seizures, or in relationship to underlying brain damage
While an overview is provided here in the context of the current synthetic discussion, the reader is referred to a recent textbook for a more comprehensive discussion3 or to a practical clinical volume4 that provides guidance of the clinical care of the psychiatric conditions seen in patients with these neurological diseases.
Traumatic Brain Injury (TBI)5
TBI has an annual incidence of about 1.5 million cases in the United States and is associated with both neurological and psychiatric consequences. Typically the neurologic consequences stabilize with time but the psychiatric disturbances tend to remit and relapse for many years after the injury. Patients who suffer TBI frequently have premorbid histories of alcohol use, impulsive behavior, lack of social support, drug use, and other psychiatric disturbances. Major depression is the most common psychiatric disturbance after TBI; the depressive phenotype is fairly typical with persistent sadness, anhedonia, poor sleep, appetite and energy, guilty feelings, thoughts of worthlessness, helplessness, and, at times suidicidality. Pre-TBI social functioning and left dorso-lateral frontal and/or left basal ganglia lesions seen on imaging soon after the TBI are risk factors for post TBI major depression.
While depression is common after TBI, little is known about the effectiveness of therapies for depression, so that approaches imported from general psychiatry such as the prescription of antidepressants is common although few randomized control trials in this context have conclusively shown efficacy. Psychotherapy is less well studied for the treatment of depression after traumatic brain injury but, anecdotally, appears to be helpful to patients.
Manic episodes are much less common after TBI than major depression but are associated with the atypical phenotype of irritability, agitation, impulsivity, violence and at times persecutory delusions or auditory hallucinations. Manic episodes must be distinguished from personality changes associated with traumatic brain injury. The latter consist primarily of impulsivity and disinhibition without associated sleep or appetite changes, psychotic features or driven aggression. Given the lack of specific therapeutic studies, the management of mania and personality change after TBI is comparable to the management of mania in any other context or the management of primary mania.
Anxiety disorders common in TBI patients include post traumatic stress disorder, obsessive compulsive disorder, and generalized anxiety disorder (GAD) which is by far the most common anxiety disorder. Panic disorder is rare, and probably no more common than its prevalence in the general population. In at least one study, however, GAD has been associated with post-TBI right hemispheric cortical lesions. Again, little is known about the management of anxiety disorders after TBI, but most commonly patients are treated in the same way as anxious patients without TBI.
Apathy is also common after TBI and is characterized by loss of interest in day to day activities, poor engagement in interpersonal relationships, lack of initiation of new activities, reduced motivation, and diminished emotional responsiveness. Typically apathy emerges as a new disturbance and does not always occur in the context of depression. Damage to the mesial frontal lobe and subcortical structures has generally been implicated in the development of apathy after TBI, although research in this area is limited. Stimulants, dopaminenergic agents (e.g., amantadine or buproprion) and cholinesterase inhibitors have been considered and used empirically for the treatment of apathy after TBI, but clinical experience suggests they are of rather limited effectiveness. Caregiver education is very important when apathy is present because caregivers can consider apathetic post TBI patients to be lazy, and this can lead to difficult interactions between patients and caregivers.
A range of cognitive impairments including problems with arousal, attention, concentration, memory, language and other forms of executive function has been reported after traumatic brain injury. Different impairments appear to occur at different stages of recovery after injury. Immediately post-injury, many patients are unconscious or have impaired attention or a mild delirium manifested by poor concentration, confusion, and disorientation. Later in recovery, typically past the 6–12 month mark, more permanent cognitive sequelae affecting memory, executive function, and in some cases language emerge. Cognitive deficits are primarily the result of cumulative effects of focal and diffuse brain damage, in particular, related to the axonal injury that occurs with TBI as the brain moves inside the skull bumping back and forth on the boney inside. While several medication therapies have been used for to treat these cognitive symptoms, their effectiveness appears limited. Cognitive rehabilitation, in which patients are taught a variety of new cognitive strategies, appears to be effective in some cases. This rehabilitation can be as simple as helping patients develop schedules, checklists and other ways of organizing their lives, or more complex using computer guided methods to improve functional memory and teach new words. Nevertheless, cognitive rehabilitation, while widely used, has not been systematically studied in control trials and is thus controversial.
Specific behavior problems are common after TBI and tend to interfere with rehabilitation. Most common are social inappropriateness, impulsivity, aggression, and poor judgment, at times leading to unsafe behaviors. These syndromes are thought to be reflective of executive dysfunction6 involving damage to frontal-subcortical loops critical to the regulation of complex social and interpersonal behavior. The management of these behaviors is complex, and requires careful assessment for the presence of other psychiatric syndromes such as mania, psychosis, or depression. In their absence, these behaviors are typically managed empirically with pharmacologic and non-pharmacologic interventions that are poorly studied. Environmental manipulations combined with the use of empirical pharmacologic therapy such as amantadine7, bromocriptine, psychostimulants, antipsychotics, or antidepressants may be successful.
The “Post Concussive Syndrome” (PCS) associated with traumatic brain injury comprises a cluster of clinical phenomena, more often seen after mild TBI as opposed to more severe TBI. PCS has been associated with physical, cognitive and emotional symptoms such as headaches, dizziness, fatigue, sensitivity to noise, memory lapses, poor concentration, sadness, anger, anxiety, and mood lability. As many as 90% of patients who develop PCS recover spontaneously in the first three months of the injury, which leads most experts to believe that this syndrome is the result of a diffusely battered brain adjusting to injury. However, a subgroup of 10–15% of patients have chronic residual PCS that can last years. Diffuse axonal injury is implicated in the emergence of the latter. However, patients with PCS have a lot of trouble adjusting and getting back to work and often require development of structured day-to-day lives, supervision and a lot of social support in order to function successfully.
Brain Vascular Disease8
With an annual incidence of more than 600,000 cases, stroke is the third leading cause of death in the U.S. Advances in modern medicine,have greatly increased the post-stroke survival rate. Currently about 4.5 million American adults are living with complications of stroke. Psychiatric syndromes associated with stroke lead to significant psychological distress, functional impairments, poor rehabilitation outcomes, and excess mortality9.
The most common psychiatric disturbances seen after stroke include cognitive impairment and dementia, depression, mania, anxiety disorders, and pathological laughing and crying—now referred to as involuntary emotion expression disorder or IEED10. Cognitive deficits of several types have been reported typically in relationship to the location of brain injury. Left hemisphere strokes frequently cause aphasia whereas right hemisphere strokes are associated with nosoagnosia, inattention, impaired spatial reasoning, and neglect syndromes. Motivation, memory, judgment, and impulse control may be affected after frontal stroke. Additionally, brain vascular disease is associated with the emergence of dementia. This can be the result of one stroke affecting a single critical area, such as the thalamus, several strokes affecting areas important to cognition, or chronic vascular insufficiency leading to white matter changes associated cognitive problems (“Vascular Cognitive Impairment”11). Finally, brain vascular disease and vascular risk factors have been associated with greater risk for, and acceleration of the progression of Alzheimer’s dementia12.
Post-stroke depression (PSD) characterized primarily through the work of Robinson and his collaborators13 can be differentiated from demoralization related to stroke based on its severity and enduring nature. Both major and minor depressive syndromes have been associated with stroke with major depression being better characterized. Twenty five percent of patients hospitalized with an acute stroke develop major depression which is phenomenologically indistinguishable from idiopathic major depression14. Left untreated, post stroke major depression appears to persist for one year in most cases, but then often attenuates into a minor depression without fully remitting. Longitudinal studies suggest that post-stroke major depression, and possibly minor depression, are major determinants of disability, failure to return to work, impaired interpersonal functioning and mortality15.
The causes of post stroke depression have been controversial although the balance of the evidence indicates that anterior and possibly left sided lesions are more likely to bring about depression16. Prevention of PSD is now an important priority. Randomized trials have suggested that antidepressants are effective in prevention and might reverse impairments in disability and possibly reduce mortality associated with PSD17,18. For this reason, an effort is underway to understand whether pharmacologic therapy should be initiated after certain types of stroke to prevent the onset of depression.
Post stroke generalized anxiety disorder has been described in as many as a quarter of acute stroke patients. Patients exhibit worry, restlessness, fatigue, poor concentration, and sleep disturbance without sadness, depression or anhedonia. These anxiety symptoms can be very debilitating and empirically respond well to traditional anti-anxiety therapies. However, few randomized trials have been conducted and much more knowledge is needed in this area.
IEED is a disorder of emotional expression seen in a range of neurologic diseases but perhaps best described in its occurrence after stroke19. Patients are prone to emotional displays provoked by nonspecific or inappropriate stimuli; in some cases, inappropriate emotional expression is spontaneous and without provocation. The classic description is for an emotional display such as laughing or crying with the patient describing a lack of feeling a congruent mood change. These episodes are uncontrollable and irresistible, slow to resolve, and can be severe and disabling. Sometimes laughter and crying occur together. The frequency of IEED after stroke is on the order of 10 to 20%. No clear relationship has been found with specific hemispheric lesions and IEED after stroke can persist for many months. Randomized trials have suggested that nortriptyline and SSRI antidepressants can lead to reduction of these debilitating symptoms20. More recently, randomized trial evidence suggests that dextromethorphan, combined with quinidine to reduce dextromethorphan metabolism, is also effective for IEED.21 The reason for this benefit with dextromethorphan is unclear but it may have to do with the known activity of the dug as a sigma receptor agonist. This also supports the idea that IEED may not be an affective disturbance but may be indeed a regulatory problem, a form of executive dysfunction where regulatory control of emotions by the frontal subcortical loops is lost.
Parkinson’s Disease22
Parkinson’s Disease (PD) has been associated with cognitive disorders, affective disorders, psychotic phenomena, impulse control disorders, and problematic repetitive behaviors. In an era where the motor symptoms can be relatively well controlled with L-dopa in the early and middle stages of PD, the psychiatric syndromes are often a major sources of disability, distress, and quality of life impairment for both patients and caregievrs.
Most patients with PD experience some cognitive impairment with 25 to 40% developing dementia over the course of their illness. Longitudinal studies suggest that the type and severity of cognitive disturbances is stage dependent. In early stages, patients primarily develop problems with memory and information processing, probably as a result of the disease’s primary involvement of subcortical structures. In later stages, impairments in cortical functions, such as apraxia and amnesia, emerge in many patients. A subgroup of patients, who may have co-morbid Alzheimer’s disease (AD), develop pronounced language deficits. Pathologic studies have shown mixed results with some studies suggesting that the primary pathology relates to dopominergic loss and associated cortical connection loss23, whereas other studies report that at least a subgroup of patients with PD also have Alzheimer’s pathology, while others have disseminated Lewy bodies in the cortex (“Dementia with Lewy Bodies”). Thus, the pathologic substrate of dementia in PD patients remains uncertain and likely represents several etiologies.
Depressive disturbances are common in PD with a prevalence of 40–50% over the course of the illness. Fewer than half have major depression; most patients have milder forms of depression referred to as dysthymia or subsyndromal depression.24 These episodes are poorly understood in their temporal characteristics and may have different phenotypes than idiopathic depression, with prominent anxiety and irritability.25 Anhedonia is common as is a reduced level of interest and engagement in day-to-day functioning. Depression is commonly not detected or treated in PD, and this compounds its persistence and associated disability. No clear risk factors for the occurrence of depression in PD have been described at this point. IEED has also been associated with the occurrence of depression, although it occurs independently in PD patients as well.
Anxiety is very common in PD, but is understudied. Up to 40% of PD patients have anxiety symptoms. Panic disorder is very common, with a prevalence as high as 25%. Panic attacks are fairly typical in their form, in that they are of sudden onset with apprehension and anxiety, associated fears of having a heart attack or dying, and a range of uncomfortable accompanying physical symptoms. The comorbidity of depressive and anxiety disorders in PD is common; most of the time neither occurs alone. Fluctuations in L-dopa levels, referred to as “on-off” states have been associated with depression but especially with anxiety. Patients frequently describe the onset of anxious symptoms during an off period that persist even after the motor function improves. Over time this gives rise to more sustained, at times severe, situational anxiety. The course of anxiety disorders in PD has not been well described.
Hallucinations occur in as many as 50% of PD patients, with 30% experiencing delusions over the course of the illness. Visual hallucinations are most typically of single images or complex scenes of well-formed people. Other hallucinations include a sensation of presence, or brief visions passing sideways in the visual field. Delusions tend to be persecutory in nature with highly elaborated themes of persecution, frequently tied in with the hallucinatory experiences. The development of such “psychotic” phenomena in PD has been linked to dopominergic therapy but it may predate the use of agents with these. The association between the dose of therapy and occurrence of symptoms is weak, and many patients have such symptoms either before they go on L-dopa or after it has been stopped. Disease factors other than dopaminergic therapy are also likely involved in their development.
Impulse control disorders have recently been described as fairly common in Parkinson’s patients although their exact prevalence is unknown.26 Hypersexuality, excessive spending, pathological gambling, and over-eating have been described separate from occurring in the context of a manic state. These can be very problematic in the clinical context and may put patients or caregivers at risk. Similar symptoms of executive dysfunction reported in as many as 14% PD patients include repetitive behaviors such as dissembling and reassembling mechanical items in the home (referred to as ‘punding’), shelving and reshelving books, and repetitive entering of sums in a calculator. These behaviors are obsessive-compulsive in their presentation, fairly stereotyped, and their execution is associated with relief of the anxious feeling.
Alzheimer’s Disease27
Alzheimer’s disease (AD) is the prototypical cortical dementia characterized with amnesia, aphasia, agnosia, and apraxia unfolding over a decade or longer. While dementia is the most prominent psychiatric disturbance, other neuropsychiatric symptoms occur in almost all Alzheimer’s patients over the lifetime of their condition.28 Most common are affective symptoms such as depression, apathy, and anxiety although 40–50% of patients also develop delusions or hallucinations. The cognitive syndrome is primarily linked to the occurrence of a cortical brain disease that begins in the entorhinal cortex and hippocampus, spreads into temporal, parietal, and frontal areas in early stages and over time involves almost the whole brain. Pathologically Alzheimer’s involves the deposition of amyloid plaques which through poorly understood mechanisms eventually translates into neuronal injury, neuronal damage with the formation of neurofibrillary tangles, and eventual neuronal death which ultimately gives rise to symptoms.
Affective symptoms are atypical in presentation with prominent anhedonia and loss of interest as well as irritability and anxiety, but less prominent guilty feelings or suicidal ideation.29 Depression in Alzheimer’s disease is frequently accompanied by delusions but less often by hallucination.30 This atypical presentation has given rise to proposals for specific diagnostic criteria to define depression in Alzheimer’s disease including the NIMH consensus panel criteria of “Depression of Alzheimer’s disease”31,32 as well as the Cache County criteria for Alzheimer’s Associated Affective Disorder.33 Depression is associated with significant disability and quality of life impairments in Alzheimer’s patients. The treatment of depression in Alzheimer’s is uncertain.34,35 Randomized trials of antidepressants have been mixed with some suggesting that selective serotonin reuptake inhibitors (SSRI’s) are superior to placebo, but others not finding efficacy for these or other antidepressants.
Alzheimer patients also frequently develop sleep disturbances, which have been associated with damage to the suprachiasmatic nucleus; however, little is known about the pathogenesis of these sleep problems.
Delusions and hallucinations affect 30–40% of Alzheimer’s patients.36 Delusions in particular are often associated with affective symptoms and in many cases are thought to be their consequence. Hallucinations are a phenomenon of later stage dementia and in many cases are associated with visual disturbances such as macular degeneration.
Apathy is very common in AD patients, although it often co-occurs with affective symptoms and anxiety.30 In later stages of the dementia, patients with Alzheimer’s are more prone to agitation, a syndrome characterized by emotional distress, physical overactivity such as pacing, irritability, and anxiety.37 In many cases, this can be differentiated from depression and has sometimes been associated with aggression and violence. It is a major source of disability and quality of life impairment. In even later stages, patients develop a range of unprovoked disinhibited behavior such as pacing and wandering, unprovoked hitting, and uncooperativeness with care. These are thought to be manifestations of the extensive brain damage caused by neurodegeneration.
Multiple Sclerosis38
MS is characterized by demyelination, axonal injury, inflammation, and gliosis involving the brain, spinal cord, and optic nerves. It can be characterized by episodic exacerbations separated by quiescence, or be relentless progressive. It typically involves multiphasic, multi-focal neurologic insults. By conservative estimates, 350,000 individuals in the U.S. have MS which is diagnosed typically between ages 20 and 40, and is twice as common in women than men. MS is the second most common cause of brain disease in early to middle adulthood.
Psychiatric syndromes seen in MS include demoralization, major depression, mania, IEED, cognitive impairment, and psychosis. Demoralization is particularly complex in the context of MS because of the intermittent nature of the condition which can make it particularly difficult to cope with. Patients usually have more difficulty adapting to acute rather than gradual changes in disease course. They can become increasingly demoralized in a condition that remits, remains quiescent for a while, and then returns often with more severe symptoms. Several studies suggest that over time many MS patients find it increasingly difficult to adapt psychologically to new episodes and that this can adversely impact their relationships and psychosocial functioning.39
The high prevalence of depression was recognized in aCharcot’s early characterization of MS. Over the course of MS, the prevalence of major depression ranges between 40 to 60%. Diagnosing depression in an MS patient can be difficult because many symptoms such as sleep disorder, fatigue, and apathy overlap with the primary disease. Nevertheless, with careful clinical assessment, depression can be confidently diagnosed. It is a major source of disability and quality of life impairment. Suicidal ideation is fairly prominent in MS patients with the prevalence across the disease on the order of 30%.40 6–12% percent of MS patients make suicide attempts, a very high rate for this age group. In at least one study, suicide was the third leading cause of death in MS patients following malignancy and pneumonia.41 Depression is the major cause of suicidal ideation.
Depression has not been correlated with severity of disability in MS but rather is thought to be a result of the pathogenesis of the brain disease in which the immune system plays a major role. Specifically, immune activation that damages neuronal cells through demyelination is thought to involve pro-inflammatory cytokines such as IL6 and TNF-alpha, which are then secreted in large amounts locally in the brain. It is hypothesized that immune mechanisms also lead to the occurrence of depressive symptoms. This innovative hypothesis is in the process of being tested and has potential for advancing not only the treatment of depression in MS but also a better understanding of brain immune mechanisms and their involvement in depression in general and in other neurologic diseases. The paper by Kaplin in this volume details this hypothesis further.
Euphoria and other manic symptoms have been reported in MS patients back to the days of Charcot. Up to 10% of patients develop euphoria or more severe forms of mania. Additionally, euphoria and mania can be the result of MS treatments and in particular steroid use. Brain imaging studies have suggested links between the emergence of euphoria and loss of brain matter in the pre-frontal cortex although these have not been replicated. For the most part, treatment of euphoria and mania in the context of MS is comparable to their treatment in other settings.
IEED occurs in as many as 10% of MS patients; and it is a later phenomenon since most patients who develop it have had the disease for a decade or longer. Treatment of IEED is complex although a few encouraging clinical trials have been reported. Dextromethorphan has been shown to have both safety and efficacy for the treatment of IEED associated MS.
Cognitive dysfunction is under recognized in MS even though up to 48% of patients fail four or more cognitive tests in a 31 test battery.42 Most commonly, MS patients manifest impairments in memory, sustained attention, verbal fluency, conceptual reasoning, and visuospatial perception. These impairments are not associated with illness duration after the first several years of the disease. They are associated with physical disability and with rapidity of progression. Few treatments exist for the cognitive impairments associated with MS.
Epilepsy43
Up to 50% of patients with epilepsy have psychiatric syndromes. Cognitive, mood, anxiety, and psychotic disturbances are most common. Since the epilepsies are heterogeneous and chronic conditions, this complexity is also reflected in the associated psychiatric disturbances. Epileptic syndromes are now classified using a disease approach according to seizure type including both focal and generalized epilepsies. For the most part, psychiatric disturbances have been categorized according to whether they are direct expressions of a seizure, features of a post-ictal state, or phenomena that occur during the interictal period. While this classification makes intuitive sense and is important because at least some psychiatric phenomena are in fact direct consequences of having a seizure, it runs the risk of taking the focus away from the damaged brain and putting it on the occurrence of the seizures. The majority of psychiatric syndromes in epilepsy occur in the interictal period, and thus probably have more to do with the state of the brain in the absence of excessive electrical discharge rather than with a discharge itself.
Cognitive dysfunction in epilepsy is manifested through mental slowness, memory dysfunction, and attentional problems in 30–50% of patients. If the age of onset of epilepsy is in childhood, learning disability and language deficits may develop because of the effects of the primary disease on brain maturation. The causes of cognitive dysfunction in epilepsy patients are complex and include the underlying brain disease, the effects of chronic repetitive seizures on the functioning of the brain, and the short term and long term effects of antiepileptic drug treatments.
Depressive disturbances are the most common psychiatric condition seen in patients with epilepsy, but tend to be underdetected and undertreated despite their significant effects on patients. Up to 50% may develop major depression although population based studies report much lower rates of lifetime depression in patients with epilepsy on the order of 6 to 30%.44 Depression rates are higher in patients who are surgical candidates for epilepsy treatment. The clinical presentation of depressive disturbances is for the most part typical for idiopathic depression. However, about a third of patients with epilepsy present with atypical features of depression that tend to be intermittent. They also resemble dysthymia and include anhedonia, fatigue, anxiety, and irritability with less prominent impairments in self attitude, self depreciative ideas, or suicidal ideation. However, overall, suicide rates are four times higher in patients with epilepsy and 25 times higher in patients with temporal lobe epilepsy than the general population.45 Little is known about the course, prognosis, or treatment of depression in epilepsy although antidepressants are frequently used. Of note is that some anti-epileptic drugs, such as levetiracitam (Keppra®)46, can induce mood changes and therefore should be used with care in patients with epilepsy and depression.
The rate of manic syndromes appear to be higher in epilepsy47, and these usually are atypical in presentation and more likely to present with irritability and overactivity than idiopathic bipolar disorder which itself does not appear to be more prevalent in epilepsy relative to the general population. This has led to the belief that the epilepsy associated brain damage is a major component in the occurrence of mania and temporal lobe epilepsy.
The prevalence of psychotic symptoms in interictal periods is on the order of 5–7% in patients with epilepsy. In patients with temporal lobe epilepsy, these disturbances are often schizophrenia-like in their presentation,. Paranoid or persecutory delusions and both visual and auditory hallucinations have been reported. Also “negative symptoms” of schizophrenia such as amotivation, apathy, flattened affect, and disorganized behavior have been reported in association with delusions and hallucinations. This has given rise to the hypothesis of the “schizophrenia-like psychoses of epilepsy”48, which remains controversial.
Pulling it all together
Several common themes emerge from this brief review of individual neurologic diseases and their psychiatric manifestations. First, regardless of the cause of the neurologic disease, these psychiatric disturbances have common features across diseases and fall into several definable and recognizable groups including cognitive disorders (dementia and non-dementia in severity), affective disorders (including major depression, atypical depressions, mania, and other bipolar disorders), anxiety disorders (in particular generalized anxiety and panic disorders), and a range of phenomena indicative of executive dysfunction including apathy, disinhibitive or compulsive behaviors, personality change, and aggression-agitation.
However, even though there are recognizable groupings that occur, across disorders there is considerable variability, which remains poorly characterized. For example, in some conditions, including stroke and traumatic brain injury, classical conditions such as major depression can be seen, whereas in other conditions such as AD and to a lesser extent PD, classical major depression is less common than atypical mood disorders, In epilepsy, a mixture of typical and atypical disorders is seen.
Another source of variability relates to the comorbidity of different psychiatric syndromes with each other. Most of the literature to date consists of efforts to describe individual psychiatric syndromes whose phenomenology comes from the DSM-IV, or other a priori criteria sets, which are then investigated in individual brain diseases, though without much concern as to comorbidity. For example, the most common problem is frequent comorbidity between depressive and anxiety syndromes. This is a broader problem in psychiatry, especially with the DSM-IV. Classification has now moved to the application of a priori criteria derived from panels of experts with a limited evidence base, as opposed to a more empirical approach investigating the occurrence and clustering of individual psychiatric symptoms as a way of defining psychiatric syndromes. This approach is illustrated by recent efforts in Alzheimer’s disease, which suggest that in neurologic disease empirical classification of psychiatric disorders is more appropriate.37 Such approaches are more replicable across patient populations, better account for the various forms of comorbidity, and appear to “breed true” over time. In an era where therapy for individual syndromes is critical in the context of neurologic disease, empirical classification of nosologic entities is more appropriate than the unthoughtful importation of diagnostic entities of DSM-IV, which were created for a different purpose.1
A second common theme is that there appear to be consistent links between specific types of psychopathology and specific brain areas no matter what the pathology of the disease. For example, depressive disturbances are most closely linked to the frontal lobes, the basal ganglia, and the nuclei that produce ascending mono-amines such as dopamine, serotonin, and norepinephrine. Delusions appear linked to temporal and to some extent parietal lobes. Cognitive disturbances correlate to more diffuse damage to several areas at once with variation of the cognitive phenotype depending on whether the picture at a given time point is mostly cortical or subcortical. Syndromes such as apathy and other forms of executive dysfunction appear to reflect injury in frontal subcortical loop circuits. Thus, psychopathology in neurologic disease seems to have to do more with the specifically affected brain circuits, rather than the pathology causing the dysfunction in those circuits.1
A more troubling common theme is how little is known in this area and what little guidance clinicians have for the detection, treatment, and management of psychopathologic conditions in neurologic disease. This leads to several recommendations that are critical for the advancement of the field:
1) Phenomenology
Further empirical study of psychiatric phenotypes across brain diseases, and over the course of these diseases is critical. Such study should be broad minded and attempt to derive disease specific empirical classifications of psychiatric syndromes rather than importing classifications from DSM-IV or ICD-10, which were not developed for this purpose. It will be particularly important to conduct this work in population based samples since samples presenting in other context are biased. For example, in the AD field, much research that has been conducted in clinically derived samples from either from neurology clinics or psychiatry clinics. Data derived from such clinical series are dependent on the biases of selection; if they come out of psychiatric clinics they tend to have more severe forms of psychiatric symptoms, or even only select forms of psychopathology if the psychiatric clinic subspecializes in certain areas such as depression or psychosis. It is also critical that descriptive effort takes into account the progression of the brain disease since stage specific description may be important. Of course, this implies that the staging of the neurologic disease itself is available and reliable. Different staging approaches exist for conditions with acute insults followed by recovery periods (e.g., traumatic brain injury, stroke), intermittent conditions (e.g., MS or epilepsy), or progressive conditions (e.g., AD and PD).
2) Risk factors
Risk factor studies in neurologic and brain disease have been conducted around the phenotypes discussed above. These have limited value and have generally not revealed consistent patterns. This may reflect the lack of systematic approaches or the lack of collaboration across groups of investigators or across diseases of the brain. Nevertheless, once the phenomenologic approach is nailed down, well thought out and disease stage specific risk factor studies need to be conducted. In general, several groups of factors should be investigated with emphasis placed on the status of the brain at the time of the emergence of the psychiatric phenomena, the premorbid history of the patient, and the current personal and environmental circumstances. Such studies should investigate risk factors for the occurrence of the psychiatric phenomenon, but also should carefully be examining the longitudinal impact of the psychiatric phenomenon on the patient’s functioning quality of life and the progression of the neurologic disease. One of the most complicated problems faced by neuropsychiatry that such risk factor studies must address is whether the occurrence of psychiatric phenomena reveal a more severe form of the brain disease or whether these phenomenon themselves contribute specifically to the worsening of the state of the brain.
3) Involving neuroscience to understand pathophysiology and pathogenesis
Powerful new methods are coming into play: brain imaging and genetics. Novel imaging techniques will bring strong explanatory abilities by offering tools that can image the structure and function of the brain in real time. Neuropsychiatrists will face significant challenges here because many neuropsychiatric patients are difficult to image although this barrier is being steadily overcome with time. Innovative paradigms are developing, in particular, through functional MRI that allow for imaging of patients under different states such as asleep, awake but resting, or being challenged through mental tasks to image functioning in key brain areas. Other relevant innovative methods based on MRI are diffusion tensor imaging, which facilitates imaging of linked brain structures (circuits), as well as magnetic resonance spectroscopy, which facilitates imaging of the metabolic state of brain cells. As more powerful magnetic imaging tools become available such as 7 Tesla MRI machines, opportunities for increased resolution down to the level of large proteins may create the possibility of imaging brain amyloid in AD for example. Similarly, positron emission tomography (PET) offers great opportunities since molecular imaging is likely to be a powerful way of imaging where the action is with regard to psychopathology. As PET ligands imaging specific molecules in the living brain become more available, opportunities will emerge to image specific neurotransmitters alongside other important molecules. The same is true of genetics. Genes interact with the environment and have a role in the genesis and maintenance of many neuropsychiatric syndromes. Well designed genetic association studies, and possibly family studies, will reveal genetic factors associated with the emergence of psychopathology in brain disease.
4) Treatment development
A lesson learned repeatedly in neuropsychiatry is that therapeutic strategies developed in other settings need to be tested again in this context. Disease specific efforts building upon phenomenology and risk factor studies as described above will be critical to developing specific therapies for the psychiatric syndromes seen in brain disease. Many of these initially will be symptomatic but eventually the effort should be targeted at developing therapies that address the underlying brain disease and the reasons for which the neuropsychiatric symptoms develop.
Conclusion
In recent decades the field of Neuropsychiatry has re-emerged as a branch of medicine well suited to address the intricate crossroads of brain dysfunction and behavioral phenomena. As this discussion highlighta, conditions such as TBI, stroke, Parkinson’s disease, Alzheimer’s disease, MS, and epilepsy demonstrate high rates of psychopathology despite varied pathophysiologic and pathogenetic origins. Armed with clinical expertise alongside the latest advances in neuroscience Neuropsychiatrists stand ready to utilize a pragmatic and methodological approach to understanding these myriad and complex conditions. The thoughtful application of the disease paradigm provides a reasoned tool to drive this process. Improved characterization of behavioral phenomenology will set the stage for the clarification of relevant risk factors, inform the application of the emerging methods of brain imaging and genetics, and ultimately lead to the development of optimized treatment approaches. The end result of this process will be witnessed in a steadily advancing understanding of the diseases that constitute this challenging field and, most importantly, improved strategies to ease the burden of patients and caregivers who struggle daily with these devastating conditions.
References
- 1.Lyketsos CG. Lessons from Neuropsychiatry. J Neuropsychiatry and Clin Neurosci. doi: 10.1176/jnp.2006.18.4.445. In press. [DOI] [PubMed] [Google Scholar]
- 2.McHugh PR, Slavney PR. The Concept of Diseases. In: McHugh PR, Slavney PR, editors. The Perspectives of Psychiatry: second edition. Baltimore, MD: The Johns Hopkins University Press; 1998. pp. 45–98. [Google Scholar]
- 3.Jeste DV, Friedman JH. Psychiatry for Neurologists. Totowa, NJ: Humana Press; 2005. [Google Scholar]
- 4.Slavney PR, Lyketsos CG, Pabins PV, Lipsey JR, editors. Psychiatric Aspects of Neurologic Diseases: Practical Approaches to Patient Care. Oxford University Press; in press. [Google Scholar]
- 5.Rao V. Psychiatric Aspects of Traumatic Brain Injury. In: Slavney PR, Rabins PV, Lyketsos CG, Lipsey JR, editors. Psychiatric Aspects of Neurologic Disease: Practical Approaches to Patient Care. Oxford University Press; in press. [Google Scholar]
- 6.Lyketsos CG, Rosenblatt A, Ravins PV. Forgotten frontal lobe syndrome or “Executive Dysfunction Syndrome”. Psychosomatics. 2004;45(3):247–255. doi: 10.1176/appi.psy.45.3.247. [DOI] [PubMed] [Google Scholar]
- 7.Leone H, Polsnetti BW. Amantadine for traumatic brain injury: does it improve cognition and reduce agitation? J Clin Pharm Ther. 2002;30(2):101–104. doi: 10.1111/j.1365-2710.2005.00628.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee HB. Psychiatric Disorders Following Stroke. In: Slavney PR, Rabins PV, Lyketsos CG, Lipsey JR, editors. Psychiatric Aspects of Neurologic Disease: Practical Approaches to Patient Care. Oxford University Press; in press. [Google Scholar]
- 9.Morris PL, Robinson RG, Andrezejewski P, Samuels J, Price TR. Association of depression with 10-year post-stroke mortality. Am J Psychiatry. 1993a;150:124–129. doi: 10.1176/ajp.150.1.124. [DOI] [PubMed] [Google Scholar]
- 10.Cummings JL, Arciniegas DB, Brooks BR, et al. Defining and diagnosing involuntary emotional expression disorder. CNS Spectr. 2006;11(6):1–7. doi: 10.1017/s1092852900026614. [DOI] [PubMed] [Google Scholar]
- 11.Roman GC, Sachdev P, Royall DR, et al. Vascular cognitive disorder: a new diagnsotic category updating vascular cognitive impairment and vascular dementia. J Neurol Sci. 2004;226:81–87. doi: 10.1016/j.jns.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Regan C, Katona C, Walker Z, et al. Relationship of vascular risk to the progression of Alzheimer’s disease. Neurology. 2006;67(8):1326–1327. doi: 10.1212/01.wnl.0000240129.46080.53. [DOI] [PubMed] [Google Scholar]
- 13.Robinson RG. The Clinical Neuropsychiatry of Stroke: Cognitive, Behavioral, and Emotional disorders following Vascular Brain Injury. Cambridge, UK: Cambridge University Press; 1998. [Google Scholar]
- 14.Fedoroff JP, Lipsey JR, Starkstein SE, et al. Phenomenologic comparisons of major depression following stroke, myocardial infarction, or spinal cord lesions. J Affective Disord. 1991;22(1–2):83–89. doi: 10.1016/0165-0327(91)90088-a. [DOI] [PubMed] [Google Scholar]
- 15.Robinson RG, Bolduc PL, Price TR. Two-year longitudinal study of post-stroke mood disorders: diagnosis and outcome at one and two years. Stroke. 1987;18:837–843. doi: 10.1161/01.str.18.5.837. [DOI] [PubMed] [Google Scholar]
- 16.Morris PL, Robinson RG, Raphael B, Hopwood MJ. Lesion location and poststroke depression. J Neuropsychiatry Clin Neurosci. 1996;8:399–403. doi: 10.1176/jnp.8.4.399. [DOI] [PubMed] [Google Scholar]
- 17.Andersen G, Vestergaard K, Lauritzen L. Effective treatment of poststroke depression with the selective serotonin reuptake inhibitor citalopram. Stroke. 1994;25:1099–1104. doi: 10.1161/01.str.25.6.1099. [DOI] [PubMed] [Google Scholar]
- 18.Robinson RG, Schultz SK, Castillo C, et al. Nortriptyline versus fluoxetine in the treatment of depression and in short-term recovery after stroke: a placebo-controlled double-blind study. Am J Psychiatry. 2000;57:351–359. doi: 10.1176/appi.ajp.157.3.351. [DOI] [PubMed] [Google Scholar]
- 19.Schiffer R, Pope LE. Review of pseudobulbar affect including a novel and potential therapy. J Clin Neurosci. 2005 Fall;17(4):447–454. doi: 10.1176/jnp.17.4.447. [DOI] [PubMed] [Google Scholar]
- 20.Robinson RG, Parikh RM, Lipsey JR, Starkstein SE, Price TR. Pathological laughter and crying following stroke: validation of a measurement scale and a double-bind treatment study. Am J Psychiatry. 1993;150:286–293. doi: 10.1176/ajp.150.2.286. [DOI] [PubMed] [Google Scholar]
- 21.Panitch HS, Thisted RA, Smith RA, et al. Randomized, controlled trial of dextromethorphan/quinidine for pseudobulbar affect in multiple sclerosis. Ann Neurol. 2006;59(5):780–787. doi: 10.1002/ana.20828. [DOI] [PubMed] [Google Scholar]
- 22.Marsh L. Parkinson’s Disease. In: Slavney PR, Rabins PV, Lyketsos CG, Lipsey JR, editors. Psychiatric Aspects of Neurologic Disease: Practical Approaches to Patient Care. Oxford University Press; in press. [Google Scholar]
- 23.Aarsland D, Perry R, Brown A, Larsen JP, Ballard C. Neuropathology of dementia in Parkinson's disease: a prospective, community-based study. Ann Neurol. 2005;58(5):773–776. doi: 10.1002/ana.20635. [DOI] [PubMed] [Google Scholar]
- 24.Marsh L, McDonald WM, Cummings J, Ravina B. Provisional diagnostic criteria for depression in Parkinson's disease: Report of an NINDS/NIMH Work Group. Mov Disord. 2005b;21:148–158. doi: 10.1002/mds.20723. [DOI] [PubMed] [Google Scholar]
- 25.Marsh L. Anxiety disorders in Parkinson's disease. Int Rev Psychiatry. 2000;12:307–318. [Google Scholar]
- 26.Weintraub D, Potenza MN. Impulse control disorders in Parkinson’s disease. Curr Neurol Neurosci Rep. 2006;6(4):302–306. doi: 10.1007/s11910-006-0022-y. [DOI] [PubMed] [Google Scholar]
- 27.Steinberg M. Alzheimer’s Disease. In: Slavney PR, Rabins PV, Lyketsos CG, Lipsey JR, editors. Psychiatric Aspects of Neurologic Disease: Practical Approaches to Patient Care. Oxford University Press; in press. [Google Scholar]
- 28.Steinberg M, Sheppard JME, Tschanz JT, et al. The incidence of mental and behavioral disturbances in dementia: the Cache County Study. J Neuropsychiatry Clin Neurosci. 2003;15:340–345. doi: 10.1176/jnp.15.3.340. [DOI] [PubMed] [Google Scholar]
- 29.Zubenko GS, Zubenko WN, McPherson S, et al. A collaborative study of the emergence and clinical features of the major depressive syndrome of Alzheimer's disease. Am J Psychiatry. 2003;160(5):857–866. doi: 10.1176/appi.ajp.160.5.857. [DOI] [PubMed] [Google Scholar]
- 30.Lyketsos CG, Sheppard JM, Steinberg M, et al. Neuropsychiatric disturbance in Alzheimer’s disease clusters into three groups: the Cache County Study. Int J Geriatr Psychiatry. 2001;16(11):1043–1053. doi: 10.1002/gps.448. [DOI] [PubMed] [Google Scholar]
- 31.Olin JT, Scneider LS, Katz IR, et al. Provisional diagnostic criteria for depression of Alzheimer disease. Am J Geraitr Psychiatry. 2002;10:125–128. [PubMed] [Google Scholar]
- 32.Rosenberg PB, Onyike CU, Katz IR, et al. Clinical application of operationalized criteria for 'Depression of Alzheimer's Disease'. Int J Geriatr Psychiatry. 2005;20(2):119–127. doi: 10.1002/gps.1261. [DOI] [PubMed] [Google Scholar]
- 33.Lyketsos CG, Rabins PV, Breitner JCS. An evidence-based proposal for the classification of neuropsychiatric disturbance in Alzheimer’s disease. Int J Ger Psychiatry. 2001;16(11):1037–1042. doi: 10.1002/gps.440. [DOI] [PubMed] [Google Scholar]
- 34.Lyketsos CG, Olin J. Depression in Alzheimer’s disease: overview and treatment. Biol Psychiatry. 2002;52:243–252. doi: 10.1016/s0006-3223(02)01348-3. [DOI] [PubMed] [Google Scholar]
- 35.Lyketsos CG, Lee HB. Diagnosis and treatment of depression in Alzheimer's disease. A practical update for the clinician. Dement Geriatr Cogn Disord. 2004;17(1–2):55–64. doi: 10.1159/000074277. [DOI] [PubMed] [Google Scholar]
- 36.Bassiony MM, Lyketsos CG. Delusions and hallucinations in Alzheimer's disease: review of the brain decade. Psychosomatics. 2003;44(5):388–401. doi: 10.1176/appi.psy.44.5.388. [DOI] [PubMed] [Google Scholar]
- 37.Lyketsos CG. Neuropsychiatric Symptoms (a.k.a. BPSD) and dementia treatment development. Int Psychoger. in press. [Google Scholar]
- 38.Kaplin A. Neuropsychiatric Aspects of Multiple Sclerosis. In: Slavney PR, Rabins PV, Lyketsos CG, Lipsey JR, editors. Psychiatric Aspects of Neurologic Disease: Practical Approaches to Patient Care. Oxford University Press; in press. [Google Scholar]
- 39.Mohr DC, Dick LP, Russo D, et al. The psychosocial impact of multiple sclerosis: exploring the patient's perspective. Health Psychol. 1999;18:376–382. doi: 10.1037//0278-6133.18.4.376. [DOI] [PubMed] [Google Scholar]
- 40.Feinstein A. An examination of suicidal intent in patients with multiple sclerosis. Neurology. 2002;59:674–678. doi: 10.1212/wnl.59.5.674. [DOI] [PubMed] [Google Scholar]
- 41.Sadovnick AD, Eisen K, Ebers GC, Paty DW. Cause of death in patients attending multiple sclerosis clinics. Neurology. 1991;41:1193–1196. doi: 10.1212/wnl.41.8.1193. [DOI] [PubMed] [Google Scholar]
- 42.Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. Frequency, patterns, and prediction. Neurology. 1991;41:685–691. doi: 10.1212/wnl.41.5.685. [DOI] [PubMed] [Google Scholar]
- 43.Marsh L. Epilepsy. In: Slavney PR, Rabins PV, Lyketsos CG, Lipsey JR, editors. Psychiatric Aspects of Neurologic Disease: Practical Approaches to Patient Care. Oxford University Press; in press. [Google Scholar]
- 44.Kanner AM. Depression in epilepsy: prevalence, clinical semiology, pathogenic mechanisms, and treatment. Biol.Psychiatry. 2003;54:388–398. doi: 10.1016/s0006-3223(03)00469-4. [DOI] [PubMed] [Google Scholar]
- 45.Harris EC, Barraclough B. Suicide as an outcome for mental disorders. A meta-analysis. Br.J Psychiatry. 1997;170:205–228. doi: 10.1192/bjp.170.3.205. [DOI] [PubMed] [Google Scholar]
- 46.Mula M, Trimble MR, Yuen A, Liu RSN, Sander JWAS. Psychiatric adverse events during levetiractem therapy. Neurology. 2003;61(5):704–706. doi: 10.1212/01.wnl.0000078031.32904.0d. [DOI] [PubMed] [Google Scholar]
- 47.Lyketsos CG, Stoline AM, Longstreet P, Lesser R, Fisher R, Folstein MF. Mania in temporal lobe epilepsy. Neuropsychiat Neuropsychol Behav Neurol. 1993;6:19–25. [Google Scholar]
- 48.Beard AW, Slater E. The schizophrenia-like psychosis of epilepsy. Proc R Soc Med. 1962;55:311–316. [PMC free article] [PubMed] [Google Scholar]