Abstract
Objective
To estimate the incidence and lifetime risk (LTR) of Parkinson disease (PD) in a large cohort of men.
Background
Age is the strongest risk factor for PD, but whether its incidence continues to in-crease after age 80 years remains unclear.
Methods
Prospective cohort of 21,970 US male physicians aged 40–84 years at baseline who did not report PD before study entry. Participants self-reported PD on yearly follow-up questionnaires, and all deaths were confirmed. We calculated incidence rates and cumulative incidence using a modified Kaplan–Meier analysis. LTR was estimated by adjusting cumulative incidence for competing risks of death.
Results
Five hundred sixty-three cases of PD were identified over 23 years of follow-up. The crude incidence rate of PD was 121 cases/100,000 person-years. Age-specific incidence rates increased sharply beginning at age 60 years, peaked in those aged 85–89 years, and declined beginning at age 90 years. Cumulative incidence substantially overestimated the long-term risk of PD, particularly in those aged 80 years and older. Cumulative incidence was 9.9% (95% confidence interval [CI] 8.48%–11.30%) from ages 45 to 100 years, whereas LTR for the same period was 6.7% (95% CI 6.01%–7.43%). The incidence and LTR of PD decreased with increasing exposure to smoking.
Conclusions
Our study provides evidence that the incidence of Parkinson disease (PD) in men increases through age 89 years. Whether the subsequent decline represents a true decrease in risk remains to be established. A history of smoking substantially decreased the incidence and lifetime risk of PD. Incidence studies that do not adjust for competing risks of death may overestimate the true risk of PD in the elderly.
The burden of Parkinson disease (PD) in developing nations is expected to double over the next generation as the result of increasing life expectancy.1 People aged 85 years and older are currently the fastest growing segment of the US population.2 Age is the strongest risk factor for PD, with a nearly exponential increase in incidence between ages 55 and 79 years. However, the incidence of PD in advanced age remains controversial.3 The majority of prior studies have combined those aged 80 or 85 years and older together because of low numbers of participants in that age group, obscuring the relationship between PD and advanced age. Accurate predictions of the future population burden of PD depend on whether its incidence continues to increase, plateaus, or declines in those aged 80 years and older.
To appreciate the actual risk of an age-related neurodegenerative disease, it is necessary to ad-just for competing risks of death, as was first shown in Alzheimer disease (AD).4 By taking into account the mortality rate of the population, the lifetime risk method estimates the absolute risk of developing a disease before dying of some other cause. Such estimates are useful for predicting individual risk, health education, and population health planning.5 Only one prior estimate of lifetime risk in PD has been published.6 To further investigate the incidence of PD in advanced age, we estimated its age-specific incidence and remaining lifetime risk in a large prospective cohort of men with more than 23 years of follow-up.
METHODS
The Physicians’ Health Study (PHS) was a randomized trial of aspirin and β-carotene for the primary prevention of cardiovascular disease and cancer among 22,071 US male physicians. Detailed descriptions of the study design and findings have been published previously.7,8 All participants provided written informed consent, and the trial was approved by the institutional review board of the Brigham and Women’s Hospital. At study entry in 1982, participants were aged between 40 and 84 years and had no history of cardiovascular disease, cancer (with the exception of nonmelanoma skin cancer), or other serious illnesses. Ninety-two percent of the participants identified their race as white. Baseline information was self-reported and collected by a mailed questionnaire that asked about risk factors for disease as well as lifestyle variables. Participants were sent follow-up questionnaires asking about study outcomes and other medical information twice in the first year and then yearly. Post-trial follow up is ongoing.9 Follow-up information through March 30, 2007, was used in this analysis.
Ascertainment of PD
We identified all participants who reported a new diagnosis of PD on their annual survey between 1982 and 2006. To evaluate the accuracy of the physicians’ self-report of PD, we performed a validation study using the available medical records of 73 participants who indicated a new PD diagnosis, as previously reported.10 In the PHS, medical records were obtained for each reported study outcome (cardiac event, TIA, stroke, cancer, pulmonary embolism, or death). A physician (J.A.D.) reevaluated medical records of participants who self-reported PD before developing an outcome event. The records were then reviewed independently by two trained neurologists (T.K., G.L.).
The clinical diagnosis of PD was considered valid if record review revealed one or more of the following: 1) established diagnosis of PD in the medical record or PD as cause of death on the death certificate; 2) current use of PD medication such as DOPA or a DOPA agonist; 3) neurologic examination with physical findings consistent with parkinsonism (at least two of the following: rest tremor, rigidity, bradykinesia or postural instability) with no evidence of a secondary cause of parkinsonism such as stroke, history of encephalitis, brain tumor, or neuroleptic treatment in the year before disease onset; patients who developed dementia or severe dysautonomia within the first year of PD diagnosis were also not considered valid cases of PD; 4) patient followed up by a neurologist or a movement disorders specialist for PD.
Of the 73 patients with available medical records, the self-reported PD diagnosis was found to be valid in 90% (66 patients). Of these, 26 patients had an established diagnosis, had a confirmatory neurologic examination, and were taking PD medication. Thirty-six patients had an established diagnosis and were taking PD medication, and 8 had an established diagnosis or were taking PD medication. In 7% (5 patients), criteria for a clinical diagnosis of parkinsonism was present, but a secondary cause could not be ruled out. The diagnosis was found to be incorrect in only 3% (2 patients): one patient had intention tremor and the other did not have adequate evidence for a diagnosis of PD.
Statistical analysis
We used a modified life-table method to determine the crude and age-specific incidence rates of PD. An actuarial method was used in calculating person-time. A weight of 0.5 was given to all observations censored during a given age, and a weight of 1 was given to all other observations. Age (in years) was used as the time scale. Follow-up began at age at study entry, and we censored individuals at the age they developed PD, died, or reached the end of follow-up. Because few participants lived beyond their 10th decade, we censored data for those aged 100 years and older. We calculated 1-year crude incidence rates (per 100,000 person-years) for each age and then collapsed them into 5- or 10-year age groups. We calculated PD incidence in smokers and nonsmokers and then estimated age-adjusted incidence rates using direct standardization to the distribution of person-years in the overall group. We chose to stratify lifetime risk estimates by smoking because, with age, it is the best established factor associated with PD risk and a major predictor of mortality.
We estimated the cumulative incidence of PD conditional on survival to age 45 years using a modified Kaplan–Meier method.11 We calculated lifetime risk, an adjusted cumulative incidence for the competing risk of death from other causes, by a method described by Gaynor et al.12 We calculated the remaining lifetime risk of PD for those who reached the baseline ages of 45, 55, 65, 75, and 85 years free of the disease of interest.
Incidence estimates were produced using the Practical Incidence Estimators Macro, which has been described in detail else-where.13 All statistical calculations were performed using SAS statistical software (SAS Institute, Inc., version 9.1).
RESULTS
Over 23.1 years of median follow-up (437,316.5 person-years), participants reported 563 cases of incident PD. The median age of PD onset was 73.1 years (range 45.7–93.9 years). Men who developed PD during follow-up were older at baseline (median age 59.8 vs 52.3 years) than men without PD. The percentage of ever-smokers at baseline was slightly lower in those who developed PD (48.9% vs 50.4%). They were also less likely to be current smokers at baseline (6.8% vs 11.1%) and to smoke more than 2 packs per day (3.7% vs 7.2%).
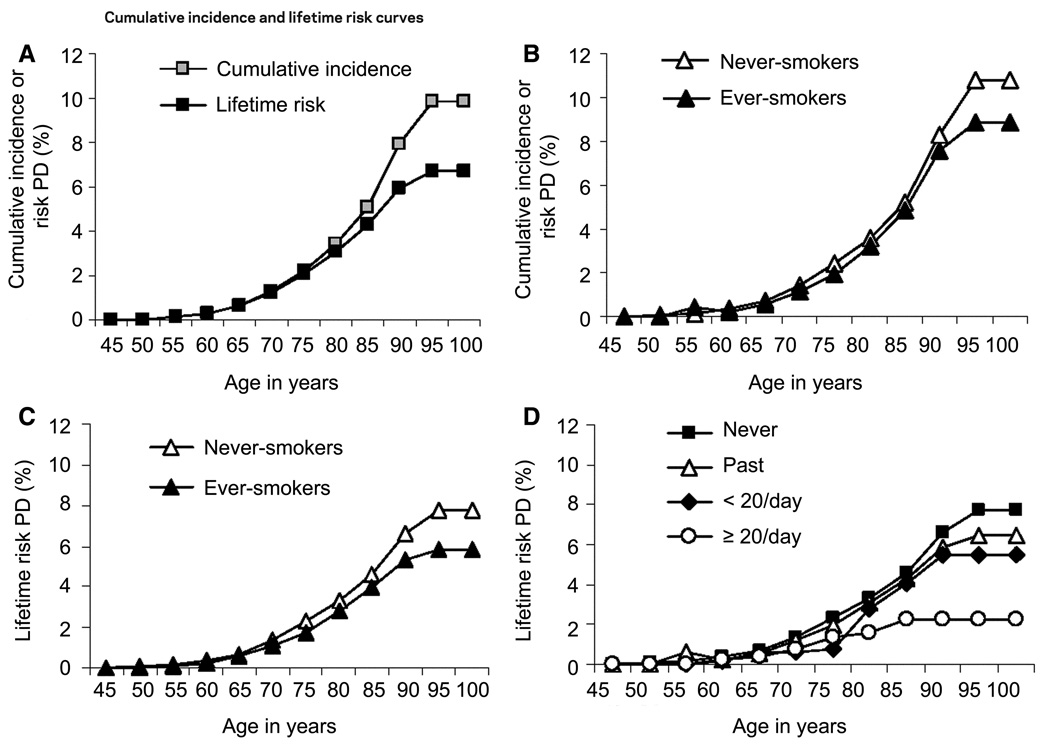
The crude annual incidence rate of PD in our population was 120.5 cases/100,000 person-years. The age-specific incidence rate increased sharply beginning at age 60 years, peaked in those aged 85–89 years at 614.7 cases/100,000 person-years, and declined in those aged 90–99 years. Overall and age-specific incidence rates are shown in table 1. The figure (A) shows the effect of adjustment for the competing risk of mortality on the cumulative incidence of PD. The curves begin to diverge after age 70 years, and the adjustment is greatest for those aged 85 years and older because of their high mortality rate. Cumulative incidence from ages 45 to 100 years is 9.89 (95% confidence interval [CI] 8.48–11.30), whereas lifetime risk for the same period is 6.72 (95% CI 6.01–7.43).
Table 1.
Age-specific and overall annual incidence rates of Parkinson disease per 100,000 person-years
| Age group, y | No. of PD cases | Person-years | Incidence rate |
|---|---|---|---|
| 40–44 | 0 | 12,553.0 | 0.0 |
| 45–49 | 2 | 34,324.0 | 5.83 |
| 50–54 | 9 | 54,448.5 | 16.53 |
| 55–59 | 28 | 72,172.0 | 38.80 |
| 60–64 | 57 | 84,889.0 | 67.15 |
| 65–69 | 101 | 75,356.5 | 134.03 |
| 70–74 | 111 | 58,443.0 | 189.93 |
| 75–79 | 101 | 39,660.0 | 254.67 |
| 80–84 | 80 | 22,512.5 | 355.36 |
| 85–89 | 59 | 9,597.5 | 614.74 |
| 90–99 | 15 | 3,361.0 | 446.30 |
| Total | 563 | 467,316.5 | 120.48 |
PD = Parkinson disease.
Figure 1.
(A) Unadjusted cumulative incidence vs mortality-adjusted lifetime risk of Parkinson disease (PD) for the entire cohort. (B) Cumulative incidence of PD in never- vs ever-smokers. (C) Lifetime risk of PD in never- vs ever-smokers. (D) Lifetime risk of PD by four categories of baseline smoking status (never, past, current <20 cigarettes/day, current ≥20 cigarettes/day).
Table 2 shows mortality-adjusted risk estimates for the development of PD from different ages reached free of the disease. The lifetime risk, which was 1 in 15 at age 45 years, remained relatively stable at ages 55 and 65 years and then declined to 1 in 18 at age 75 years and 1 in 25 at age 85 years.
Table 2.
Age-specific, mortality-adjusted risk estimates for the development of Parkinson disease in men of different ages reached free of Parkinson disease
| Age, y | Short-term, intermediate, and lifetime risks (95% CI) | |||
|---|---|---|---|---|
| 10-y risk | 20-y risk | 30-y risk | Lifetime risk | |
| Overall population | ||||
| 45 | 0.11 (0.04–0.17) | 0.62 (0.49–0.74) | 2.04 (1.81–2.27) | 6.72 (6.01–7.43) |
| 55 | 0.51 (0.41–0.63) | 1.96 (1.73–2.18) | 4.23 (3.83–4.63) | 6.72 (6.00–7.45) |
| 65 | 1.51 (1.30–1.71) | 3.87 (3.46–4.27) | 6.46 (5.72–7.21) | 6.46 (5.72–7.21) |
| 75 | 2.63 (2.24–3.01) | 5.52 (4.72–6.32) | 5.52 (4.72–6.32) | |
| 85 | 3.91 (2.96–4.86) | 3.91 (2.96–4.86) | ||
| Never-smokers | ||||
| 45 | 0.17 (0.05–0.28) | 0.68 (0.49–0.86) | 2.33 (1.97–2.68) | 7.76 (6.55–8.96) |
| 55 | 0.52 (0.36–0.67) | 2.19 (1.85–2.53) | 4.49 (3.87–5.10) | 7.70 (6.48–8.91) |
| 65 | 1.73 (1.41–2.05) | 4.09 (3.48–4.71) | 7.42 (6.17–8.66) | 7.42 (6.17–8.66) |
| 75 | 2.58 (2.00–3.17) | 6.20 (4.88–7.52) | 6.20 (4.88–7.52) | |
| 85 | 4.59 (3.07–6.10) | 4.59 (3.07–6.10) | ||
| Ever-smokers | ||||
| 45 | 0.04 (0.00–0.10) | 0.54 (0.38–0.71) | 1.76 (1.47–2.05) | 5.81 (4.98–6.64) |
| 55 | 0.51 (0.35–0.67) | 1.76 (1.47–2.05) | 3.99 (3.46–4.52) | 5.90 (5.05–6.74) |
| 65 | 1.31 (1.05–1.57) | 3.65 (3.13–4.18) | 5.66 (4.78–6.53) | 5.66 (4.78–6.53) |
| 75 | 2.66 (2.14–3.19) | 4.93 (3.98–5.88) | 4.93 (3.98–5.88) | |
| 85 | 3.23 (2.10–4.38) | 3.23 (2.10–4.38) | ||
CI = confidence interval.
The age-adjusted incidence rate in ever-smokers at baseline (113 cases/100,000 person-years) was lower than among never-smokers (127 cases/100,000 person-years). Table 2 displays mortality-adjusted short-term, intermediate, and lifetime risks for PD in never- and ever-smokers. Both incidence and lifetime risks of PD decreased as the level of smoking exposure increased (table 3). The figure (B and C) shows the cumulative incidence and lifetime risk curves for PD according to baseline smoking status. Further stratification of smoking exposure into four categories reveals a substantial difference in life-time risk between heavy smokers and never-smokers (figure, D).
Table 3.
Incidence, cumulative incidence, and remaining lifetime risk of Parkinson disease by baseline smoking status
| Smoking status | Participants (no. of PD cases) | Person-years | Crude incidence | Cumulative incidence (95% CI) | Lifetime risk (95% CI) |
|---|---|---|---|---|---|
| Never | 10,901 (288) | 229,772.5 | 125.34 | 10.79 (8.58–12.99) | 7.76 (6.55–8.96) |
| Past | 8,662 (237) | 178,238.0 | 132.97 | 9.35 (7.58–11.13) | 6.43 (5.44–7.40) |
| Current <1 pack/day | 847 (17) | 17,648.5 | 96.33 | 8.01 (3.32–12.70) | 5.45 (2.58–8.31) |
| Current ≥1 pack/day | 1,560 (21) | 29,626.5 | 70.88 | 3.47 (1.59–5.35) | 2.26 (1.21–3.31) |
PD = Parkinson disease; CI = confidence interval.
To better understand the relationship between smoking and lifetime risk for PD and to explore the possibility that the inclusion of cases of vascular parkinsonism influenced our results, we repeated the analysis after excluding 5,251 participants with confirmed cardiovascular disease. The study findings did not change substantially. Smokers still had a lower age-adjusted incidence than nonsmokers (101.5 cases/100,000 person-years vs 116.1 cases/100,000 person-years). Lifetime risk of PD for the overall population was 6.6% (95% CI 5.69%–7.57%), 7.8% among nonsmokers and 5.6% among smokers.
DISCUSSION
In this large prospective cohort study among male physicians, the average annual incidence of PD was 121 cases/100,000 person-years. The age-specific incidence increased steeply through age 89 years and then declined in the 10th decade of life. Unadjusted cumulative incidence substantially over-estimated the true risk of PD in those aged 80 years and older. The lifetime risk curve for PD reached a plateau by age 90 years. The cumulative incidence and lifetime risks of PD decreased substantially with increasing smoking exposure.
Our study has several strengths, including its large number of participants and outcome events, prospective design, and well-defined population with a long follow-up. We were able to adjust cumulative incidence for competing risks using mortality information from the same population and could perform stratified analyses by smoking status.
A number of limitations should be considered in the interpretation of our results. First, our diagnosis of PD was based on self-reports. However, prior work has shown the self-reported diagnosis of PD to be highly valid in a population of health professionals. 14 Direct validation of more than 10% of self-reported PD diagnoses using available medical records revealed an accuracy of 90%, which is similar to that found in validation studies of self-reported hypercholesterolemia and hypertension in the PHS.15 However, despite this good validation, misclassification of PD remains possible. A number of factors limit the generalizability of our results to other populations. Our cohort was composed of white men of the same educational level and profession who were motivated to modify their risks for disease. They also may have higher rates of disease diagnosis than nonphysicians because of easier access to medical care. However, our incidence estimates for PD were similar to that of a population-based study with direct ascertainment.16 We were unable to estimate the incidence and risk of PD in women.
Prior estimates of PD incidence have varied widely depending on nationality, sex, population age distribution, and case ascertainment methods. General populations have an average estimate of 13 cases/ 100,000 (range 1.5–26),17 whereas populations aged 65 years and older have an average of 180 cases/100,000 (range 89–332).16,18–22 We recalculated the incidence rates of studies that evaluated most or all population members using accepted diagnostic criteria and reported age-specific incidence in older white men (table 4). The rate obtained in our cohort (224 cases/100,000 person-years) was similar to that of the Rotterdam Study (261 cases/100,000 person-years),16 a primarily white population-based cohort in which the diagnosis of PD was obtained by direct screening followed by a detailed clinical workup. Thirty-nine percent of PD cases were newly diagnosed by study physicians, demonstrating the substantial number of patients with PD who are missed when case finding is based on medical records alone. Despite the fact that our participants are physicians with easy access to medical care, our rate was lower than that of two door-to-door studies of elders in Italy and Spain, in which PD was directly ascertained.18,19 Finally, our rate was higher than that in three US-based studies in which no population screening was performed and cases were found through health care providers or medical records systems.6,21,22
Table 4.
Comparison of the Physicians’ Health Study with studies using widely accepted diagnostic criteria that report crude annual incidence of Parkinson disease in men
| Reference | Population | Study years | Age range, y | Person- years | Unadjusted incidence rate per 100,000 person-years |
|---|---|---|---|---|---|
| Current article | US national | 1982–2007 | ≥65 | 208,931 | 224 |
| 15 | Rotterdam | 1990–1999 | ≥65 | 11,105 | 261 |
| 18 | Central Spain | 1994–1998 | ≥65 | 5,284 | 360 |
| 20 | US, Manhattan | 1988–1991 | ≥65 | 14,238 | 119 |
| 5 | US, Rochester, Minnesota | 1976–1991 | ≥65 | 49,723 | 113 |
| 21 | US, California | 1994–1995 | ≥60 | 261,074 | 100 |
| 17 | Italy | 1992–1996 | ≥65 | 6,285 | 461 |
In our cohort, PD incidence increases through age 89 years and then declines in the 10th decade of life. In the majority of studies reporting age-specific incidence in white men, incidence increases through the oldest age group,16,18,19,21–23 as it does in our study when those aged 85 years and older are combined. However, a number of well-conducted studies show a decline in PD incidence in men after age 75 or 79 years.6,23–25 Similar results have been found for AD in a cohort characterized by exceptional longevity in which the incidence of AD in men began to decline in their early 90s.26
It is unclear whether the observed decline represents a true decrease in the risk of PD or is simply due to underdiagnosis. Older patients may become lost to follow-up, particularly in studies with less-intensive cohort surveillance.23 Because parkinsonian signs as well as comorbidities increase markedly with age,27 the decline of PD in the oldest old may also reflect the difficulty of distinguishing idiopathic PD from other causes of parkinsonism.20 In the Rochester Epidemiology Project, the incidence of PD declined in men after age 79 years when strict diagnostic criteria were used, but continued to increase through age 99 years when broad criteria were used.28 When included PD patients with concurrent dementia, the incidence rate increased slightly but still declined after age 89 years (data not shown). Our study provides evidence that PD incidence increases at least to age 90 years, thus shifting to the right the age where we can be confident that incidence is still increasing.
Cumulative incidence is often used to provide a measure of long-term disease risk. However, it over-estimates the incidence when the disease has a prevalence of 10% or greater, or the competing risk of mortality is high.29 The lifetime risk method adjusts the incidence rate of a disease by the all-cause mortality rate in the population. It thus provides the actual risk of developing the disease before dying of some other cause, assuming one has survived free of that disease to a specified age. Lifetime risk estimates are helpful as an absolute measure of long-term individual risk and are easily understood by the lay public. They are increasingly being used by researchers and policy makers to predict population risks30 and develop clinical practice guidelines.31,32
Our study illustrates the importance of adjusting for the competing risk of death when estimating the incidence of a disease in an older population. The cumulative incidence of PD from ages 45 to 100 years was 9.9% (1 in 10), but mortality-adjusted life-time risk for the same period was 6.7% (1 in 15). Our estimate of lifetime cumulative incidence at age 40 years was remarkably similar to that of the Rochester Epidemiology Project (10.9%), but our estimates of lifetime risk were higher. This likely reflects the increased longevity of our cohort, which has a life expectancy of 49.3 additional years at age 40 years, 12 years longer than that of 40-year-old men in the general US population.33 Populations with a longer life expectancy have a longer period at risk; thus, life-time risk estimates cannot be compared across different populations unless they have similar mortality rates.
Although smoking is known to decrease PD risk,34 it increases the risk of many other major diseases and of death. The cumulative incidence of PD decreased as the level of smoking exposure increased, suggesting a dose–response relationship between smoking and protection from PD. The lifetime risk of PD followed the same pattern (figure, D), and lifetime risk in heavy smokers (2.3%) was substantially less than in never-smokers (7.8%). The difference in lifetime risk curves between ever- and never-smokers was accounted for by increased mortality from cardiovascular disease, cancer, and pulmonary disease among smokers (data not shown). Because we had relatively few current smokers in the cohort, smoking-associated mortality rates were lower than might be found in a general population. In a cohort with a higher smoking exposure, both cumulative incidence and lifetime risks of PD among smokers might be even lower. It is worth emphasizing that while smoking may decrease the incidence of PD by some protective factors, it decreases the lifetime risk of PD by substantially increasing the risk of death from smoking-related diseases.
We were unable to address the incidence and life-time risk of PD in women. Women are known to have a twofold decreased risk of PD compared with men,20 which seems to persist even to very old ages.18 On the other hand, older women have increased longevity compared with men and are less likely to be smokers. Thus, although incidence in men is higher at all ages, the difference in lifetime risk between men and women likely decreases with age.6 Further studies of lifetime risk in elderly women are needed.
Our results illustrate the sensitivity of PD incidence and risk estimates to a number of factors, including the age and longevity of the population, methods of case finding, the strictness of PD criteria, and the prevalence of smoking. In this population of health-conscious male physicians with exceptional longevity, PD incidence increased sharply to age 90 years, and lifetime risk was as high as 1 in 15 at age 40 years. As life expectancy increases worldwide, similar lifetime risks can be expected in general populations.
ACKNOWLEDGMENT
The authors thank the staff of the Physician’s Health Study and the 22,071 dedicated physicians who made this project possible.
The Physicians’ Health Study is supported by grants CA-34944, CA-40360, and CA-097193 from the National Cancer Institute and grants HL-26490 and HL-34595 from the National Heart, Lung, and Blood Institute, Bethesda, MD. J.A.D. is supported by a grant from the Parkinson’s Disease Foundation.
DISCLOSURE
While we believe that we have no conflict of interest that could inappropriately influence (or bias) our decisions, work, or writing of the manuscript with regard to the specific matter of the submitted paper, we report a full disclosure for the last 5 years for each of the authors below. J.A.D. has nothing to disclose. G.L. has received investigator-initiated research funding from the NIH and has received honoraria from Pfizer and Lilly Pharmaceutical for speaking engagements in 2003. J.M.G. has received investigator-initiated research funding and support as Principal Investigator from the NIH, BASF, DSM Pharmaceuticals, Wyeth Pharmaceuticals, McNeil Consumer Products, and Pliva; has received honoraria from Bayer and Pfizer for speaking engagements; and is a consultant for Bayer, McNeil Consumer Products, Wyeth Pharmaceuticals, Merck, Nutraquest, and GlaxoSmithKline. T.K. has received investigator-initiated research funding as Principal or Co-Investigator from the NIH, Bayer AG, McNeil Consumer and Specialty Pharmaceuticals, Merck, and Wyeth Consumer Healthcare; is a consultant to i3 Drug Safety; and has received honoraria from Genzyme for educational lectures and from Organon for contributing to an expert panel.
GLOSSARY
- AD
Alzheimer disease
- CI
confidence interval
- LTR
lifetime risk
- PD
Parkinson disease
- PHS
Physicians’ Health Study
REFERENCES
- 1.Dorsey ER, Constantinescu R, Thompson JP, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services. Washington, DC: Department of Health and Human Services; A Profile of Older Americans: 2005. 2005
- 3.Hirtz D, Thurman DJ, Gwinn-Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the “common” neurologic disorders? Neurology. 2007;68:326–337. doi: 10.1212/01.wnl.0000252807.38124.a3. [DOI] [PubMed] [Google Scholar]
- 4.Seshadri S, Wolf PA, Beiser A, et al. Lifetime risk of dementia and Alzheimer’s disease: the impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49:1498–1504. doi: 10.1212/wnl.49.6.1498. [DOI] [PubMed] [Google Scholar]
- 5.Seshadri S, Wolf PA. Lifetime risk of stroke and dementia: current concepts, and estimates from the Framingham Study. Lancet Neurol. 2007;6:1106–1114. doi: 10.1016/S1474-4422(07)70291-0. [DOI] [PubMed] [Google Scholar]
- 6.Elbaz A, Bower JH, Maraganore DM, et al. Risk tables for parkinsonism and Parkinson’s disease. J Clin Epidemiol. 2002;55:25–31. doi: 10.1016/s0895-4356(01)00425-5. [DOI] [PubMed] [Google Scholar]
- 7.Steering Committee of the Physicians’ Health Study Research Group. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 8.Hennekens CH, Buring JE, Manson JE, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–1149. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 9.Christen WG, Gaziano JM, Hennekens CH. Design of Physicians’ Health Study II: a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol. 2000;10:125–134. doi: 10.1016/s1047-2797(99)00042-3. [DOI] [PubMed] [Google Scholar]
- 10.Driver JA, Kurth T, Buring JE, Gaziano JM, Logroscino G. Prospective case-control study of nonfatal cancer pre-ceding the diagnosis of Parkinson’s disease. Cancer Causes Control. 2007;18:705–711. doi: 10.1007/s10552-007-9005-9. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 12.Gaynor J, Feuer E, Tan C, et al. On the use of cause-specific failure and conditional failure probabilities: examples from clinical oncology data. J Am Stat Assoc. 1993;88:400–409. [Google Scholar]
- 13.Beiser A, D’Agostino RB, Sr, Seshadri S, Sullivan LM, Wolf PA. Computing estimates of incidence, including lifetime risk: Alzheimer’s disease in the Framingham Study—the Practical Incidence Estimators (PIE) macro. Stat Med. 2000;19:1495–1522. doi: 10.1002/(sici)1097-0258(20000615/30)19:11/12<1495::aid-sim441>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 14.Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64:664–669. doi: 10.1212/01.WNL.0000151960.28687.93. [DOI] [PubMed] [Google Scholar]
- 15.Camargo CA, Jr, Hennekens CH, Gaziano JM, Glynn RJ, Manson JE, Stampfer MJ. Prospective study of moderate alcohol consumption and mortality in US male physicians. Arch Intern Med. 1997;157:79–85. [PubMed] [Google Scholar]
- 16.de Lau LM, Giesbergen PC, de Rijk MC, Hofman A, Koudstaal PJ, Breteler MM. Incidence of parkinsonism and Parkinson disease in a general population: the Rotterdam Study. Neurology. 2004;63:1240–1244. doi: 10.1212/01.wnl.0000140706.52798.be. [DOI] [PubMed] [Google Scholar]
- 17.Twelves D, Perkins KS, Counsell C. Systematic review of incidence studies of Parkinson’s disease. Mov Disord. 2003;18:19–31. doi: 10.1002/mds.10305. [DOI] [PubMed] [Google Scholar]
- 18.Baldereschi M, Di Carlo A, Rocca WA, et al. Parkinson’s disease and parkinsonism in a longitudinal study: two-fold higher incidence in men. ILSA Working Group. Italian Longitudinal Study on Aging. Neurology. 2000;55:1358–1363. doi: 10.1212/wnl.55.9.1358. [DOI] [PubMed] [Google Scholar]
- 19.Benito-Leon J, Bermejo-Pareja F, Morales-Gonzalez JM, et al. Incidence of Parkinson disease and parkinsonism in three elderly populations of central Spain. Neurology. 2004;62:734–741. doi: 10.1212/01.wnl.0000113727.73153.68. [DOI] [PubMed] [Google Scholar]
- 20.Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Incidence and distribution of parkinsonism in Olmsted County, Minnesota, 1976–1990. Neurology. 1999;52:1214–1220. doi: 10.1212/wnl.52.6.1214. [DOI] [PubMed] [Google Scholar]
- 21.Mayeux R, Marder K, Cote LJ, et al. The frequency of idiopathic Parkinson’s disease by age, ethnic group, and sex in northern Manhattan, 1988–1993. Am J Epidemiol. 1995;142:820–827. doi: 10.1093/oxfordjournals.aje.a117721. [DOI] [PubMed] [Google Scholar]
- 22.Van Den Eeden SK, Tanner CM, Bernstein AL, et al. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 23.Morens DM, Davis JW, Grandinetti A, Ross GW, Popper JS, White LR. Epidemiologic observations on Parkinson’s disease: incidence and mortality in a prospective study of middle-aged men. Neurology. 1996;46:1044–1050. doi: 10.1212/wnl.46.4.1044. [DOI] [PubMed] [Google Scholar]
- 24.Granieri E, Carreras M, Casetta I, et al. Parkinson’s disease in Ferrara, Italy, 1967 through 1987. Arch Neurol. 1991;48:854–857. doi: 10.1001/archneur.1991.00530200096026. [DOI] [PubMed] [Google Scholar]
- 25.Kuopio AM, Marttila RJ, Helenius H, Rinne UK. Changing epidemiology of Parkinson’s disease in southwestern Finland. Neurology. 1999;52:302–308. doi: 10.1212/wnl.52.2.302. [DOI] [PubMed] [Google Scholar]
- 26.Miech RA, Breitner JC, Zandi PP, Khachaturian AS, Anthony JC, Mayer L. Incidence of AD may decline in the early 90s for men, later for women: the Cache County Study. Neurology. 2002;58:209–218. doi: 10.1212/wnl.58.2.209. [DOI] [PubMed] [Google Scholar]
- 27.Bennett DA, Beckett LA, Murray AM, et al. Prevalence of parkinsonian signs and associated mortality in a community population of older people. N Engl J Med. 1996;334:71–76. doi: 10.1056/NEJM199601113340202. [DOI] [PubMed] [Google Scholar]
- 28.Bower JH, Maraganore DM, McDonnell SK, Rocca WA. Influence of strict, intermediate, and broad diagnostic criteria on the age- and sex-specific incidence of Parkinson’s disease. Mov Disord. 2000;15:819–825. doi: 10.1002/1531-8257(200009)15:5<819::aid-mds1009>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 29.Schouten LJ, Straatman H, Kiemeney LA, Verbeek AL. Cancer incidence: life table risk versus cumulative risk. J Epidemiol Community Health. 1994;48:596–600. doi: 10.1136/jech.48.6.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 31.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 32.Wood D, De Backer G, Faergeman O, Graham I, Mancia G, Pyorala K. Prevention of coronary heart disease in clinical practice: recommendations of the Second Joint Task Force of European and other Societies on Coronary Prevention. Atherosclerosis. 1998;140:199–270. doi: 10.1016/s0021-9150(98)90209-x. [DOI] [PubMed] [Google Scholar]
- 33.Arias E. United States life tables, 2003. Natl Vital Stat Rep. 2006;54:1–40. [PubMed] [Google Scholar]
- 34.Hernan MA, Takkouche B, Caamano-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson’s disease. Ann Neurol. 2002;52:276–284. doi: 10.1002/ana.10277. [DOI] [PubMed] [Google Scholar]