Abstract
Background/Aims
People who inject drugs (PWID) experience high incarceration rates, and previous incarceration is associated with elevated hepatitis C virus (HCV) transmission risk. In Scotland, national survey data indicate lower HCV incidence in prison than the community (4.3 vs 7.3 per 100py), but a 2.3-fold elevated transmission risk amongst recently released (<6 months) PWID. We evaluated the contribution of incarceration to HCV transmission amongst PWID, and the impact of prison-related prevention interventions, including scaling-up direct-acting antivirals (DAAs) in prison.
Design
Dynamic mathematical modelling of incarceration and HCV transmission, using approximate Bayesian computation for model calibration.
Setting
Scotland, UK.
Participants
A simulated cohort of 1000 PWID.
Measurements
Population attributable fraction of incarceration (PAF) to HCV transmission among PWID. Decrease in HCV incidence and chronic prevalence due to current levels of prison opiate substitution therapy (OST; 57% coverage) and HCV treatment; as well as scaling-up DAAs in prison and/or preventing the elevated risk associated with prison-release.
Findings
Incarceration contributes 27.7% (PAF; 95%CrI −3.1–51.1%) of HCV transmission amongst PWID in Scotland. Over the next 15 years, current HCV treatment rates (10.4/6.8 per 1000 incarcerated/community PWID annually), with existing prison OST, could reduce incidence and chronic prevalence among all PWID by a relative 10.7% (95%CrI 8.4–13.3%) and 9.7% (95%CrI 7.7–12.1%), respectively. Conversely, without prison OST, HCV incidence and chronic prevalence would decrease by 3.1% (95%CrI −28.5–18.0%) and 4.7% (95%CrI −11.3–14.5%). Additionally, preventing the heightened risk among recently released PWID could reduce incidence and chronic prevalence by 45.0% (95%CrI 19.7–57.5%) and 33.3% (95%CrI 15.6–43.6%) or scaling-up prison HCV treatments to 80% of chronic PWID prison entrants with sufficient sentences (>16 weeks) could reduce incidence and prevalence by 45.6% (95%CrI 38.0–51.3%) and 45.5% (95%CrI 39.3–51.0%), respectively.
Conclusions
Incarceration and the elevated transmission risk following prison-release can contribute significantly to hepatitis C virus transmission amongst people who inject drugs. Scaling-up hepatitis C virus treatment in prison can provide important prevention benefits.
INTRODUCTION
Hepatitis C virus (HCV) is a blood-borne disease causing considerable morbidity(1). Injecting drug use is the primary mode of HCV transmission in many developed countries(2), with approximately half of people who inject drugs (PWID) infected with HCV(3). Strategies to control HCV transmission amongst PWID, therefore, are critical to preventing HCV in the population.
Globally, PWID experience high incarceration rates (56–90% ever being incarcerated(4)), and previous incarceration is frequently associated with HCV infection(5) and increased injecting risk in the community(6, 7). Recent prison release is also associated with heightened transmission risk(8). HCV incidence amongst incarcerated PWID (4.3–34 per 100py(9, 10)) varies greatly worldwide.
Prison could be an important setting to deliver HCV prevention interventions, although few countries currently do this(4, 11, 12). In Spanish prisons, PWID experience five-fold lower incidence if on OST(13). Similarly, after introducing prison OST in Scotland, evidence suggests HCV incidence amongst incarcerated PWID reduced(9, 14), and is now lower than amongst community PWID(15). HCV treatment for incarcerated PWID, especially with shorter direct-acting antivirals (DAAs(16)), could reduce HCV transmission in prison and the community. However, although modelling suggests testing and treatment with DAAs could be cost-effective in UK prisons(17), HCV treatment in prison remains low(11).
In this study we evaluate the importance of prison as a setting to undertake HCV prevention interventions for PWID in Scotland. Specifically, we aim to:
estimate the contribution of incarceration to the Scottish HCV epidemic among PWID
estimate the 15-year impact of existing prison-based prevention and HCV treatment interventions on HCV incidence and chronic prevalence among PWID
estimate the 15-year impact of potential future prison-associated prevention and HCV treatment interventions on HCV incidence and chronic prevalence
METHODS
Setting
Scotland; where 61% of PWID have ever been incarcerated with an average sentence length of 5.6 months, and HCV incidence amongst PWID is lower in prison than community, but PWID released in the last 6 months have a greater risk of HCV acquisition than other community PWID.
Design
To address these aims, we developed a mathematical model of HCV transmission and incarceration amongst PWID (see Model Description below) which, where possible, was fitted to detailed data from Scotland. Model parameterization and calibration comprised of two stages. In stage 1, incarceration dynamics were parameterized and calibrated to self-reported data from community PWID on their incarceration history, using Bayesian methodology that incorporated uncertainty in both the inputs and the outputs (approximate Bayesian computation sequential Monte Carlo scheme (18)). In stage 2, the HCV transmission component was parameterised, utilizing results from the relevant literature, and calibrated to recent data on the HCV incidence and prevalence amongst PWID in Scotland (see Model Parameterisation and Calibration for the further details).
The model was used to estimate the contribution of incarceration to the Scottish HCV epidemic amongst PWID, the “population attributable fraction” (PAF). The world health organization defines PAF as the “proportional reduction in population disease or mortality that would occur if exposure to a risk factor were reduced to an alternative ideal exposure scenario”(19). We estimate this PAF by considering the relative reduction in endemic HCV incidence if there were no differences in HCV transmission risk during incarceration or post-release; by considering endemic HCV incidence, our estimate incorporates the impact that these differences in risk have on elevating the whole epidemic. The model also estimates the 15-year impact on HCV incidence and prevalence of existing prison-based interventions; HCV treatment and OST. The model is used to project the impact of potential future prison-associated prevention and HCV treatment interventions; preventing future incarceration of PWID, the scaling-up of HCV treatment on entry into prison and/or the prevention of the elevated risk following prison release (See Model Analyses for further details).
Model Description
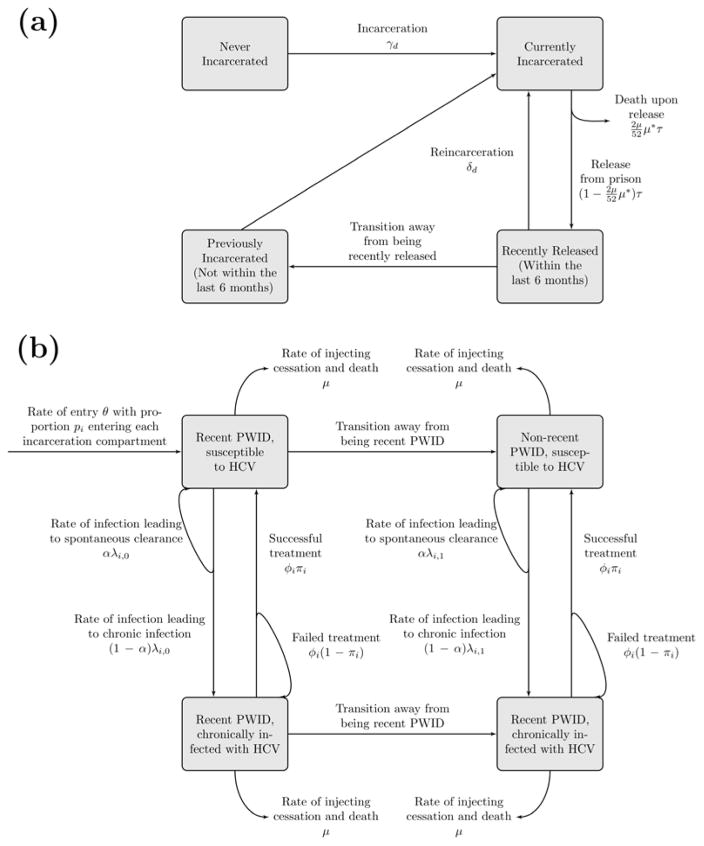
We developed a dynamic, deterministic model of incarceration and HCV transmission amongst current PWID (schematic in figure 1; model equations in section 1 of the supplementary materials). The PWID population was stratified by incarceration state (never, currently, recently and non-recently released from prison; within the last 6 months or not, respectively), HCV infection state (susceptible and chronically infected) and injecting duration (recent (<5 years) and non-recent (>5 years) injector). The model is open; PWID enter through drug use initiation, and leave either through permanent cessation of injecting or death, with excess mortality following prison release(20).
Figure 1.
Schematic of model components for (a) PWID incarceration and (b) HCV transmission.
PWID are incarcerated or reincarcerated at rates which vary by duration of injecting, and are released from prison at a constant rate. A small proportion of those released from prison leave the model due to mortality on release, whilst the remainder enter the recently released compartment where they experience elevated HCV acquisition risk for 6 months before transitioning to the non-recent previously incarcerated compartment.
All PWID can acquire and transmit HCV in their given setting (prison or community). Susceptible PWID become infected at a rate proportional to the chronic prevalence in their setting and the infection rate. The infection rate varies by setting, by whether a PWID has recently initiated injecting or not, and is elevated if a PWID has been recently released from prison. A proportion of those acutely infected spontaneously clear infection and remain in the susceptible compartment, whilst the remainder proceed to chronic infection. The model does not include a compartment for acutely infected PWID because previous modelling indicates it contributes little to transmission(21, 22).
A fixed number of chronically infected PWID are treated in the community and prison annually. If prison HCV treatment rates exceed the number of eligible chronically infected incarcerated PWID, defined to be those infected PWID with long enough sentences to complete treatment, then all eligible PWID are treated. A proportion of treated PWID achieve sustained viral response (SVR) and become susceptible, while those failing treatment remain chronically infected. The SVR rates are time-dependent and setting-specific. We model HCV treatment as instantaneous, because of the short duration of DAA treatment regimens(16). PWID failing treatment are eligible for retreatment due to the wide range of HCV treatment options becoming available(16).
Model Parameterisation and Calibration
Where possible, the model was fitted to detailed data from Scotland. Data for parameterising and calibrating the models came either from a national cross-sectional sero-behavioural survey of Scotland’s closed prisons (2010/11, denoted as the ‘prison survey’)(9), or the Needle Exchange Surveillance Initiative (NESI); a series of four cross-sectional surveys of community PWID in Scotland between 2008 and 2014(8, 23).
Stage 1: Parameterising and calibrating the Incarceration sub-Model
We tracked a simulated cohort of 1000 PWID for 20 years from initiation of injecting to calibrate the model’s incarceration and re-incarceration rates, and proportion of new PWID initiating injecting in each incarceration state. An approximate Bayesian computation sequential Monte Carlo scheme(18) was used to obtain a sample of 10,000 incarceration-related parameter sets (prior distributions and posterior parameter ranges in Table 1) that sufficiently fit the NESI incarceration data on the proportions of community PWID who have never been incarcerated, incarcerated once or multiple times by duration injecting (data used shown in Supplementary Table 1), whilst also giving a total PWID population size within latest estimates(24). Full details of this calibration process are in section 2 of the supplementary materials.
Table 1.
Posterior model parameter ranges used in the full model, obtained through the incarceration sub-model calibration
| Parameter | Symbol | Prior distribution | Posterior parameter range | Source and Comments |
|---|---|---|---|---|
| Death rate (per year) 1 | μ1 | Sampled from a Poisson distribution with mean (10), with sampled values divided by 1000. | 0.006 – 0.014 | (25) |
| Average duration injecting (years) 1 | μ2 | Uniform on (5,20) | 5.1 – 17.7 | (26) |
| Factor increase in mortality rate for 2 weeks following prison release | μ* | Lognormal with parameters (2.0053,0.1393) truncated to 95% confidence interval [5.7,9.9] | 6.3–8.2 | (20) |
| Percentage of prison population that are current PWID | P | Normal with parameters (0.19, .006) truncated to 95% CI (0.18 – 0.21) | 18.7–21.0% | Scottish prison survey(9) |
| Current PWID population size | N | N/A | N/A | Parameter sets are rejected if model population size not within 11500–18600(24) |
| Percentage of PWID initiating injecting when 3 | Dirichlet distribution with parameters (10,1,1,1,1) | - | Obtained through model fitting. | |
| Never incarcerated | p1 | 72.2–92.5% | ||
| Incarcerated for first time | p2 | 1.6%–12.0% | ||
| Community, incarcerated once | p3 | 1.4–10.3% | ||
| Incarcerated for second or more time | p4 | 3.2–13.7% | ||
| Community, incarcerated twice or more | p5 | 0.2–8.3% | ||
| Incarceration rates per year | γ | Obtained through model fitting | ||
| Recent PWID (<5yrs injecting) | Uniform on (0,0.25) | 0.12–0.17 | ||
| Non-recent PWID (>5yrs injecting) | Uniform on (0,0.25) | 0.03–0.06 | ||
| Re-incarceration rates per year | δ | Obtained through model fitting | ||
| Recent PWID | Uniform on (0,1) | 0.63–0.88 | ||
| Non-recent PWID | Uniform on (0,1) | 0.08–0.17 | ||
| Release rate per year | τ | Normal with parameters (0.48, .019) truncated to 95% CI (0.44 – 0.52) | 0.47–0.51 | Scottish prison survey2. Corresponds to an average 5.7–6.1 months spent in prison per incarceration. |
The PWID leaving rate, μ, is given by: μ1 + 1/μ2
We used the weighted average time between date of incarceration and earliest date of liberty for current PWID, i.e. weighted by the reciprocal of these times to allow for the likely oversampling of prisoners with long sentences.
In the final model which does not stratify incarceration history into incarcerated once and twice or more, p2 and p4 are combined to give the proportion of PWID initiating injecting in prison. Whilst, p3 and p5 are combined to give the proportion of PWID initiating injecting in the community having been incarcerated – a random proportion of which have been recently released.
Stage 2: Parameterising and calibrating the Full Model
Parameters for the full model are shown in Tables 1 and 2. For each of the 10,000 parameter fits for the incarceration sub-model, the HCV transmission component of the full model was calibrated to sampled HCV incidences (from distribution ranges given in Table 2) for recent and non-recent community PWID (2008) and incarcerated PWID (2010) (more details in section 3 of the supplementary materials). Parameter sets were accepted as model fits if the resulting model projections for the HCV prevalence amongst community PWID and incarcerated PWID lay within the 95% confidence intervals of the corresponding data for NESI 2008 and the prison survey (2010/11), respectively. We assumed the HCV epidemic was stable (in steady-state) prior to the scale-up of HCV treatment in 2008. The model assumed a factor increase in community HCV acquisition risk amongst PWID recently released (<6 months) from prison (2.30, 95%CI 0.97–5.46; details in section 3.1 of the supplementary materials).
Table 2.
Full Model Parameters obtained from literature and data analyses.
| Parameter | Symbol | Range of Parameter Values | Source and Comments |
|---|---|---|---|
| Inflow of new injectors per year | θ | - | Fit to PWID population size |
| HCV incidence among PWID per 100 person years (2008 unless stated otherwise) | Vary infection rate, λ, to fit. | Estimated from NESI data(23) and prison survey(9). See section 3.2 of the supplementary details. HCV incidences are sampled from the distributions obtained by a bootstrapping method to estimate the 95% confidence intervals. | |
| Recent community PWID (<5 years injecting) | 11.9–40.6 | ||
| Non-recent community PWID (>5yrs injecting) | 4.8–19.5 | ||
| Incarcerated PWID with OST (2010/11) | 0.9–10.2 | Incidence amongst incarcerated PWID in absence of OST is sampled from lognormal(2.3,0.6) which is truncated to the 95% confidence interval (4.5,31.8) found in previous prison survey before OST was introduced(14). | |
| Incarcerated PWID without OST | 4.5–31.8 | ||
| HCV antibody prevalence | |||
| Community PWID (2008) | 49.7–54.0% | (23) | |
| Incarcerated PWID (2010/11) | 51.0–55.9% | (9) | |
| Proportion of new infections that spontaneously clear | α | 0.22–0.29 | (30) Sampled from uniform distribution |
| Annual PWID treatments in community (Average rate per 1000 Community PWID) | Φc | (27, 28) | |
| 2008–2014 | 66–103 (4.4–6.8) | ||
| 2015–2030 | 103 (6.8) | ||
| Annual PWID treatments in Prison (Average rate per 1000 Incarcerated PWID) | Φp | (27, 28) | |
| 2008–2014 | 4–16 (2.6–10.4) | ||
| 2015–2030 | Varied | ||
| Sustained viral response | π | ||
| PEG-IFN/RBV in community | 60–66% | (29) Sampled from uniform distribution | |
| PEG-IFN/RBV in prison | 55–66% | (29) Sampled from uniform distribution | |
| DAAs (2015–2030) | 90% | (16) | |
| Percentage of incarcerated PWID with sentences: | |||
| >16 weeks | ε | 39.9–46.0% | Estimated from the prison survey. Both sampled from normal distribution |
| >12 weeks | ε2 | 57.3–63.3% | |
| Increased risk amongst recently released PWID (<6 months since release) | η | 0.97–5.46 | Estimated from NESI data (see section 3.1 of the supplementary details). Sampled from lognormal distribution |
Annual rates of HCV treatment for incarcerated and community PWID in Scotland were estimated for 2008–2014 from their national treatment database(27–29). Community and prison SVR rates for HCV treatment with pegylated interferon and ribavirin over 2008–2014 were parameterised based on recent analyses of HCV treatment outcomes amongst Scottish patients(29), which found lower (albeit not significantly lower) SVR rates amongst incarcerated patients initiating treatment. From 2015, we assume HCV treatment with DAAs and assume only those with sufficiently long sentences are treated, with no difference in the SVR rates between prison and community.
Model analyses
Contribution of incarceration to the Scottish HCV epidemic among PWID
Using the calibrated model, we projected the contribution or population attributable fraction (PAF) of incarceration to current HCV transmission amongst PWID in Scotland. We compared the endemic HCV incidence in the baseline epidemic (reduced HCV transmission in prison compared to community, but elevated HCV acquisition risk in the 6-month period post-release) with the projected HCV incidence resulting from a scenario where there is no effect of incarceration on HCV transmission risk. This was modelled by increasing the prison HCV transmission risk to the same as the community and assuming no excess risk amongst recently released PWID. In both scenarios, the model was run to the stable endemic state, with the relative difference between the endemic HCV incidence for the ‘no effect of incarceration’ scenario and the baseline scenario being defined as the PAF of incarceration to HCV transmission.
Impact of existing prevention and HCV treatment interventions
Firstly, we projected the ‘status quo’ HCV epidemic among PWID until 2030 including existing interventions (current levels of in-prison and community HCV treatment, with IFN-free DAAs being used from start of 2015, and lower incidence in prison compared to the community). Secondly, we projected how the impact would change if in-prison HCV treatment were ceased from 2015. Thirdly, we projected the impact of the existing OST program in Scottish prisons, by assuming an HCV incidence amongst incarcerated PWID observed prior to initiation of prison OST in a long-stay prison in Scotland from 2015 onwards.
Impact of potential future prison-associated prevention and HCV treatment interventions
We additionally projected the impact of future prevention and HCV treatment interventions. First we evaluate the potential impact of decriminalization by considering a theoretical scenario where there are no new incarcerations of PWID from 2015. We model this by turning off incarceration and re-incarceration in the model (i.e. set the rates to 0) but with people still initiating injecting in prison, whilst HCV treatment of community PWID continues at the same rate per 1000 PWID as in the ‘status quo’ scenario, i.e. an increased number of annual treatments. Second, we simulated a potential new intervention strategy that prevents the elevated transmission risk post-release, estimated by comparing the ‘status quo’ epidemic with a scenario where there is no elevated risk post-release. Then, we projected the impact of scaling-up in-prison HCV treatment from 2015. Specifically, the following scenarios were modelled:
Immediate scale-up of HCV treatment to 80% of chronically infected PWID with at least 16-week sentences treated immediately on prison entry (43% of imprisoned PWID). A 16-week sentence was assumed to be the minimum time needed to diagnose, assess and treat someone with a 12-week DAA treatment course.
Immediate scale-up of HCV treatment to 80% of chronically infected PWID with at least 12-week sentences treated immediately on prison entry (60% of imprisoned PWID). This assumes an 8-week DAA treatment course.
We projected the impact of these scaled-up HCV treatment scenarios with and without the immediate prevention of the elevated transmission risk post-release.
Uncertainty Analysis
We undertook a linear regression analysis of covariance to determine which parameter uncertainties contribute most to uncertainty in the 15-year impact of scaling-up annual prison HCV treatment rates so that 80% of chronically infected PWID with at least 16-week sentences are treated on prison entry from 2015. The proportion of each model outcome’s sum-of-squares contributed by each parameter was calculated to estimate the importance of individual parameters to the overall uncertainty.
RESULTS
Contribution of Incarceration to the Scottish HCV epidemic
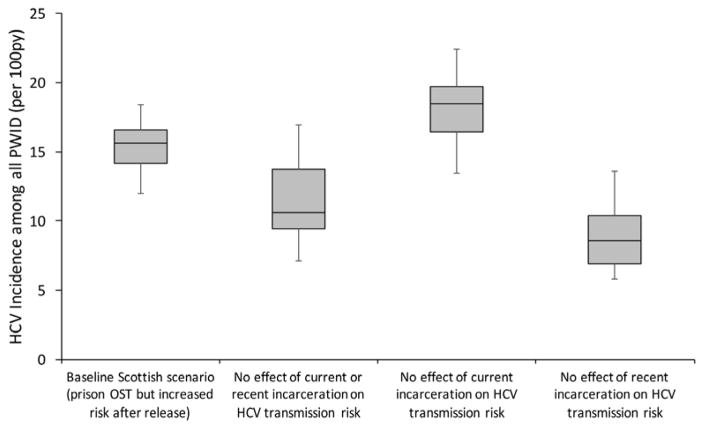
Our baseline projections for Scotland predicted an overall HCV incidence among PWID of 15.6 per 100py (95%CrI 12.0–18.4) in 2008 (figure 2). HCV incidence would be 27.7% (95%CrI −3.1–51.1%) lower at 10.6 per 100py (95%CrI 7.1–17.0) if incarceration had no effect on HCV transmission. Hence, despite lower HCV incidence in prison than the community, incarceration of PWID contributes nearly a third of all current HCV transmission amongst PWID (i.e. the PAF of incarceration is 27.7%). This is purely due to the heightened risk post-release, as shown in figure 2.
Figure 2. Endemic HCV chronic incidence amongst all PWID with various effects of incarceration removed.
Boxes indicate the interquartile range, with the lines inside indicating the median incidence, with whiskers representing 95% CrI for the simulations.
Impact of existing prevention and HCV treatment interventions
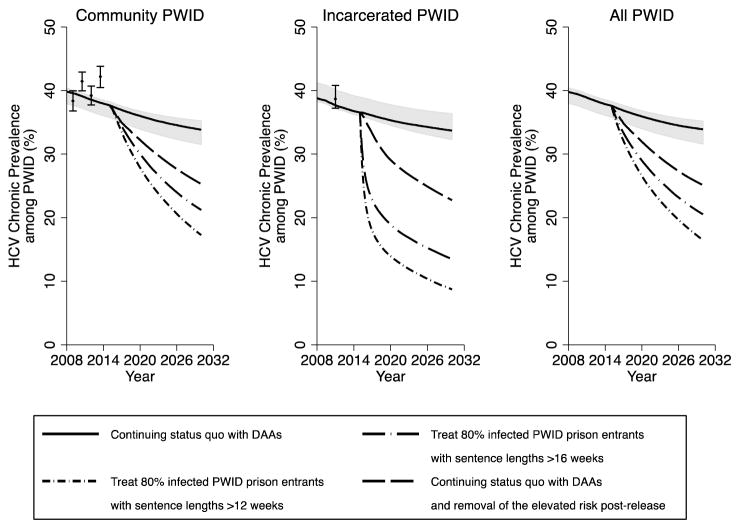
In the status quo scenario, maintaining current HCV treatment rates (average annual rates of 10.4 and 6.8 per 1000 incarcerated and community PWID, respectively) with DAAs decreases overall HCV incidence and chronic prevalence among PWID over 15 years by a relative 10.7% (95%CrI 8.4–13.3%) and 9.7% (95%CrI 7.7–12.1%), respectively (figures 3–6), with prevalence decreasing from 37.6% (95%CrI 35.8–38.3%) in 2015 to 33.9% (95%CrI 31.6–35.2%) in 2030. Conversely, if no prison HCV treatment occurred from 2015 onwards, then incidence and chronic prevalence would still decrease due to continued community DAA treatment, 10.0% (95%CrI 8.4–11.6%) and 8.9% (95%CrI 7.2–11.2%), respectively, over 15 years. Additionally, without current coverage levels of prison OST, HCV incidence and chronic prevalence would still decrease, but by only a relative 3.1% (95%CrI −28.5–18.0%) and 4.7% (95%CrI −11.3–14.5%), respectively over 15 years, with incidence being 9.3% (95%CrI −7.2–46.6%) higher than the status quo scenario in 2030.
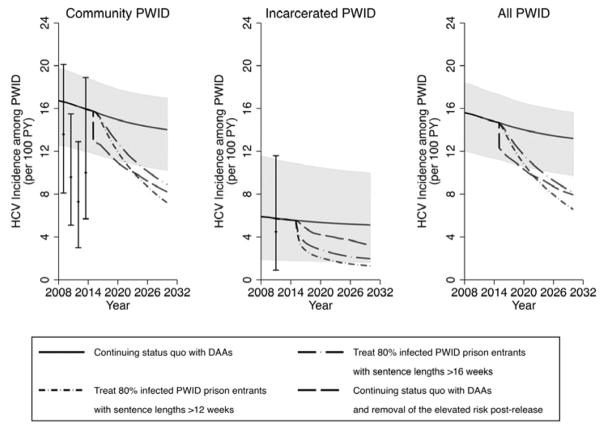
Figure 3. Impact of different prevention and treatment scenarios on chronic HCV prevalence over time in Scotland amongst community PWID, incarcerated PWID and all PWID.
Lines represent the median chronic HCV prevalence, with the shaded area representing the 95% CrI for the status quo projection (no scale-up) from 2015 onwards. HCV prevalence data points shown for comparison with 95% confidence intervals.
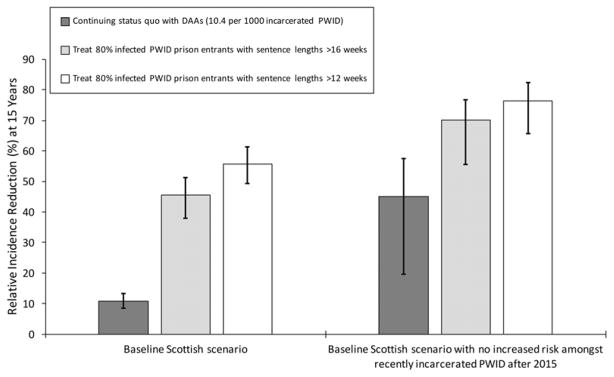
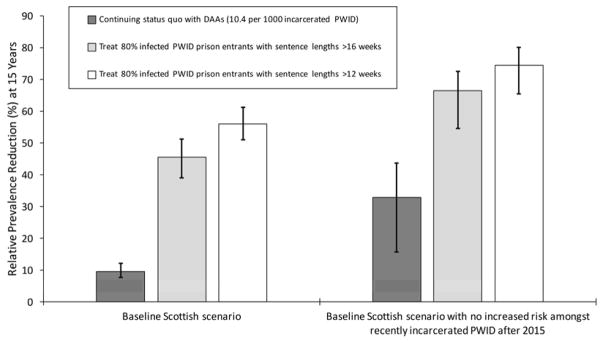
Figure 6. Relative incidence reduction among all PWID from 2015 to 2030 for different prison treatment scenarios, with or without the concurrent removal (from 2015) of the heightened HCV transmission risk amongst recently released PWID.
Bars indicate median incidence reduction, with whiskers representing the 95% CrI for the projections.
Impact of potential future prison-associated prevention and HCV treatment interventions
Preventing future incarceration of PWID from 2015, along with current HCV treatment rates, could reduce HCV incidence and chronic prevalence by 21.9% (95%CrI 4.8–38.5%) and 16.9% (95%CrI 6.1–27.9%), respectively by 2030. Conversely, pairing current HCV treatment and prison OST with an intervention that prevented the heightened risk among recently released PWID could further decrease incidence and chronic prevalence, by 45.0% (95%CrI 19.7–57.5%) and 33.3% (95%CrI 15.6–43.6%), respectively (figures 5 and 6) over 15 years. Alternatively, if prison treatment rates are scaled-up so that 80% of all chronically infected PWID with sentences longer than 16 weeks are treated on prison entry then HCV incidence and chronic prevalence (figures 5 and 6) would reduce by 45.6% (95%CrI 38.0–51.3%) and 45.5% (95%CrI 39.3–51.0%), respectively by 2030. If the heightened transmission risk amongst recently released PWID is also prevented, then incidence and chronic prevalence could reduce further, by up to 70.2% (95%CrI 55.0–77.4%) and 66.5% (95%CrI 51.4–70.1%), respectively. Conversely, if 80% of chronically infected PWID entering prison with sentences longer than 12 weeks could be treated then HCV incidence and chronic prevalence would reduce by 55.8% (95%CrI 49.3–61.4%) and 55.9% (95%CrI 51.1–61.3%), respectively over 15 years if there was no reduction in the HCV risk post-release, or 76.4% (95%CrI 65.6–82.2%) and 74.4% (95%CrI 61.8–77.3%) if this risk was also prevented.
Figure 5. Relative chronic prevalence reduction among all PWID from 2015 to 2030 for different prison treatment scenarios, with or without the concurrent removal (from 2015) of the heightened HCV transmission risk amongst recently released PWID.
Bars indicate median chronic prevalence reduction, with whiskers representing the 95% CrI for the projections.
Uncertainty Analysis
Analysis of covariance indicated that uncertainty in the heightened risk amongst recently released PWID (accounts for 17 and 12% of uncertainty, respectively), HCV transmission rate amongst non-recent community PWID (24 and 25%) and the proportion of incarcerated PWID eligible for HCV treatment (18 and 20%) contributed most to the uncertainty in the impact of scaling-up prison HCV treatment rates on overall PWID HCV incidence and chronic prevalence from 2015–2030. No other model parameters contributed more than 10% to the uncertainty (See section 4 of the supplementary materials).
DISCUSSION
Main Findings
Model projections suggest that despite lower HCV transmission risk during imprisonment than the community, nearly a third of current HCV transmission amongst Scottish PWID could be attributed to incarceration. This is primarily due to the elevated HCV risk post-release, with the model suggesting that HCV incidence could be reduced by 45% over the next 15 years if this risk was prevented. Less impact would be achieved by preventing future incarcerations of PWID (e.g. by decriminalization), a 22% reduction in HCV incidence over 15 years, due to current incarceration being associated with low HCV transmission risk. Conversely, continuing with current levels of HCV treatment among PWID over the next 15 years will have only a modest prevention impact; reducing HCV incidence and chronic prevalence by about one-tenth. In contrast, if 80% of infected prisoners and sentences longer than 16 weeks were treated, HCV incidence and chronic prevalence among PWID in Scotland could be almost halved in 15 years. If this scale-up in HCV treatment could also be combined with an intervention preventing the elevated HCV risk post-release, prevalence and incidence could reduce further by nearly three-quarters to 13.6% and 4.2 per 100py, respectively.
Limitations
Our study had several limitations. Firstly, our findings may not be directly generalisable to other settings as our model was parameterised to Scotland. Nonetheless, Scotland is one of few sites with detailed national data on community and prison HCV incidence and prevalence, as well as detailed incarceration data that enables such a detailed evaluation of the role of incarceration. In settings with higher HCV incidence in prison, greater incarceration rates and longer sentences than Scotland (61% of PWID have ever been incarcerated with average sentence length of 5.6 months), e.g. Thailand, incarceration is likely to contribute more to HCV transmission(31).
Secondly, our model projections assumed stable levels of HCV treatment amongst community PWID for 2015–2030(28). Although community treatment rates may increase in coming years with the greater availability of DAAs, which would achieve greater impact on HCV transmission, we did not consider this because it was not the focus of our study.
Thirdly, our analyses suggest the elevated HCV risk post-release may by an important contributor to the current HCV epidemic amongst PWID in Scotland, but uncertainty exists over the magnitude and duration of this risk. However, although the odds-ratio for the elevated HCV acquisition risk post-release is not statistically significant (p=0.059), other statistical analyses based on the same dataset give a consistent picture, with recent incarceration being associated with greater injecting risk (injecting daily and sharing needles or syringes in the last 6 months - unpublished analyses) and lower coverage levels of OST and NSP. Furthermore, the model’s posterior range for elevated transmission risk post-release is 1.17 – 5.24; suggesting that the model only agrees with observed prevalence data when there is an increased risk following release. It is also uncertain whether some of the heightened risk associated with recent release may occur during the period of incarceration. However, this is unlikely considering the low HCV incidence observed in Scottish prisons(9). Although other studies have observed similar heightened risks or behaviours amongst PWID recently released from prison(6, 7), it is important that further research better determines the magnitude and reasons for this heightened risk. Additionally, we model optimistic intervention scenarios where the elevated HCV risk post-release is fully prevented to show the potential benefit of prevention interventions targeting this important period of risk. Although studies have shown OST and needle and syringe programmes are highly effective at reducing an individual’s risk of acquiring HCV (in combination up to 80%(32, 33)), it is unclear whether all the elevated risk post-release could be prevented, even with intensive prevention efforts upon prison release. Indeed, it is likely that other structural factors may also need to be addressed, including high levels of homelessness following release(8, 23, 34), to fully prevent this period of elevated risk. However, our results suggest that efforts to reduce this risk, which may include linking PWID to harm reduction services and providing housing support on release from prison, could greatly reduce both HCV incidence and prevalence.
Fourthly, our estimates of the impact of ongoing in-prison OST for reducing HCV transmission may be under-estimated if HCV transmission risk without this intervention was higher than the historical estimate from a long-stay Scottish prison (11.9 per 100py in 1999/2000(14)) used in our counterfactual scenario. As reported in Australia(35), it is possible that individuals with shorter incarceration durations may have greater acquisition risk. Although our projections suggest existing prison OST may be having little impact on the overall epidemic, due to the low proportion (9%) of PWID in prison at any point in time, it is still likely to be cost-effective because of the large reduction in HCV incidence and other benefits achieved (e.g. reduction in drug-related deaths(36)). Importantly, prison OST is likely to have greater impact in other settings where PWID experience greater rates of incarceration and longer sentences(31), e.g. Ukraine.
Lastly, we explore the possible impact of decriminalization by considering a scenario in which there are no new incarceration of PWID. Although it is unlikely that all incarceration of PWID would be prevented by decriminalization alone, this scenario is used to demonstrate the potential impact that decriminalization could have in reducing HCV transmission amongst PWID.
Comparisons with existing studies
The work is consistent with previous modelling considering the impact of OST and HCV treatment as prevention among community PWID(22, 37, 38), and with models of the cost-effectiveness of HCV case-finding in prison(17, 39). Furthermore, our work is consistent with recent modelling which evaluated the impact of scaling-up HCV treatment in United States prisons(40). However, in contrast to the US study, our model is based on detailed empirical data on differences in transmission risk in community and prison, including increased risk post-release, as well as detailed data on the incarceration dynamics of PWID. Our study is the first to consider the implications of HCV transmission risk being elevated post-release, and the potential impact of interventions that prevent this risk. A review found that the high costs of DAAs is a key barrier in scaling-up HCV treatment for prevention in PWID and prisoners, whilst short prison sentences for PWID in many settings may have limited the prevention impact of HCV treatment in prisons(41). Our study indicates that in the DAA era, a substantial proportion of PWID prisoners in Scotland (>40%) have sufficiently long sentences for completing treatment (16 weeks), supporting the hypothesis that prison-based HCV treatment could now be highly effective and cost-effective.
Implications
It is widely recognized that the period immediately after prison poses an increased risk for drug-related deaths(20). Our findings raise the hypothesis that this is also a critical period of HCV transmission, contributing substantially to HCV risk in the community. Further research and syntheses of available evidence are required to better define this risk. The reasons for the increased risk post-release are likely to be multi-factorial, associated with injecting risk environment and individual behaviors; for example, relapse may be unplanned and not involve sterile equipment, and post-release PWID may be more likely to have unstable housing(8, 23, 34) or be unemployed(6). Additionally, they may experience changes in social networks and inadequate family and financial support(42, 43). This further highlights the detrimental effects associated with incarceration and the high societal costs of drug prohibition (31). Our findings also suggest that reduced incarceration amongst PWID is likely to reduce HCV prevalence and incidence. Policy changes that would reduce incarceration are likely to generate cost savings to the criminal justice system (UK estimates of the lifetime crime costs per person who uses drugs were £445,000 in 2009 (44)) which possibly could be used to finance further treatment of community PWID, further decreasing HCV prevalence and transmission.
There is emerging evidence that leaving prison on OST can increase OST uptake in the community(45), and in combination with community OST can reduce the risk of drug-related mortality(36). In addition, PWID in some prison settings are given naloxone upon release to reduce mortality risk(46), and sterile injecting equipment to reduce injecting risk(47). We show that it is important to determine whether these interventions can reduce HCV risk, with our modelling suggesting the scale-up of prison interventions could be an important part of comprehensive harm reduction programmes.
Supplementary Material
Figure 4. Impact of different prevention and treatment scenarios on HCV incidence over time in Scotland amongst community PWID, incarcerated PWID and all PWID.
Lines represent the median HCV incidence, with the shaded area representing the 95% CrI for the status quo projection (no scale-up) from 2015 onwards. HCV incidence data points shown for comparison with 95% confidence intervals.
Acknowledgments
Financial support: This work was supported through a research grant from Gilead Sciences. Gilead had no influence on the design, analysis and content of the study. JS acknowledges funding from a PhD scholarship from the Engineering and Physical Sciences Research Council (EPSRC). NKM, PV, and MH acknowledge funding from National Institute for Drug Abuse R01 DA037773-01A1. NKM acknowledges research funding from the National Institute for Drug Abuse (R01 DA037773-01A1) and the University of California San Diego Center for AIDS Research(CFAR), an NIH-funded program (P30 AI036214), which is supported by the following NIH Institutes and Centers: NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA, NIGMS, and NIDDK. MH and PV acknowledge funding by the National Institute for Health Research Health Protection Research Unit (NIHR HPRU) in Evaluation of Interventions at University of Bristol. The views expressed are those of the authors and not necessarily those of the UK National Health Service (NHS), NIHR, or the Department of Health.
Footnotes
Declaration of interests: JS has received a conference attendance sponsorship from Gilead. NKM has received research grants from Gilead, and honoraria from Merck, AbbVie, and Gilead. DJG has received honoraria for educational contributions (e.g. lectures, reports) and for providing advice on aspects of Hepatitis C and public health from AbbVie, Merck, Gilead, BMS, and Janssen. PV has received research grants from Gilead. MH has received honoraria from Merck, Gilead and Janssen and is a co-investigator on research grants from Gilead. SJH has received honoraria from AbbVie and Gilead. AM has received honoraria from Merck. PCH has received funding from Gilead, Roche, MSD, Abbvie, BMS and Jannsen.
References
- 1.Cooke GS, Lemoine M, Thursz M, Gore C, Swan T, Kamarulzaman A, et al. Viral hepatitis and the Global Burden of Disease: a need to regroup. Journal of viral hepatitis. 2013;20:600–601. doi: 10.1111/jvh.12123. [DOI] [PubMed] [Google Scholar]
- 2.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. The Lancet Infectious diseases. 2005;5:558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 3.Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378:571–583. doi: 10.1016/S0140-6736(11)61097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jurgens R, Nowak M, Day M. HIV and incarceration: prisons and detention. J Int AIDS Soc. 2011;14:26. doi: 10.1186/1758-2652-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Who/Unodc/Unaids. Effectiveness of interventions to address HIV in prisons (Evidence For Action Technical Paper) Geneva, Switzerland: World Health Organization; 2007. [Google Scholar]
- 6.Cepeda JA, Niccolai LM, Lyubimova A, Kershaw T, Levina O, Heimer R. High-risk behaviors after release from incarceration among people who inject drugs in St Petersburg, Russia. Drug and alcohol dependence. 2015;147:196–202. doi: 10.1016/j.drugalcdep.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milloy MJ, Buxton J, Wood E, Li K, Montaner JS, Kerr T. Elevated HIV risk behaviour among recently incarcerated injection drug users in a Canadian setting: a longitudinal analysis. BMC Public Health. 2009;9:156. doi: 10.1186/1471-2458-9-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen EJ, Palmateer NE, Hutchinson SJ, Cameron S, Goldberg DJ, Taylor A. Association between harm reduction intervention uptake and recent hepatitis C infection among people who inject drugs attending sites that provide sterile injecting equipment in Scotland. The International journal on drug policy. 2012;23:346–352. doi: 10.1016/j.drugpo.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Taylor A, Munro A, Allen E, Dunleavy K, Cameron S, Miller L, et al. Low incidence of hepatitis C virus among prisoners in Scotland. Addiction. 2013;108:1296–1304. doi: 10.1111/add.12107. [DOI] [PubMed] [Google Scholar]
- 10.Larney S, Kopinski H, Beckwith CG, Zaller ND, Jarlais DD, Hagan H, et al. Incidence and prevalence of hepatitis C in prisons and other closed settings: results of a systematic review and meta-analysis. Hepatology. 2013;58:1215–1224. doi: 10.1002/hep.26387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arain A, Robaeys G, Stover H. Hepatitis C in European prisons: a call for an evidence-informed response. BMC Infect Dis. 2014;14(Suppl 6):S17. doi: 10.1186/1471-2334-14-S6-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamarulzaman A, Reid SE, Schwitters A, Wiessing L, El-Bassel N, Dolan K, et al. Prevention of transmission of HIV, hepatitis B virus, hepatitis C virus, and tuberculosis in prisoners. Lancet. 2016;388:1115–1126. doi: 10.1016/S0140-6736(16)30769-3. [DOI] [PubMed] [Google Scholar]
- 13.Marco A, Gallego C, Cayla JA. Incidence of hepatitis C infection among prisoners by routine laboratory values during a 20-year period. PloS one. 2014;9:e90560. doi: 10.1371/journal.pone.0090560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Champion JK. Incidence of Hepatitis C Virus Infection and Associated Risk Factors among Scottish Prison Inmates: A Cohort Study. American journal of epidemiology. 2004;159:514–519. doi: 10.1093/aje/kwh061. [DOI] [PubMed] [Google Scholar]
- 15.University of the West of Scotland, Health Protection Scotland, Glasgow Caledonian University and the West of Scotland Specialist Virology Centre. The Needle Exchange Surveillance Initiative (NESI): Prevalence of HCV and injecting risk behaviours among people who inject drugs (PWID) attending injecting equipment provision services (IEPs) in Scotland, 2008/2009 – 2013/2014. University of the West of Scotland; Jan, 2015. [Google Scholar]
- 16.Dore GJ, Feld JJ. Hepatitis C virus therapeutic development: in pursuit of “perfectovir”. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015;60:1829–1836. doi: 10.1093/cid/civ197. [DOI] [PubMed] [Google Scholar]
- 17.Martin NK, Vickerman P, Brew IF, Williamson J, Miners A, Irving WL, et al. Is increased hepatitis C virus case-finding combined with current or 8-week to 12-week direct-acting antiviral therapy cost-effective in UK prisons? A prevention benefit analysis. Hepatology. 2016;63:1796–1808. doi: 10.1002/hep.28497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toni T, Welch D, Strelkowa N, Ipsen A, Stumpf MP. Approximate Bayesian computation scheme for parameter inference and model selection in dynamical systems. J R Soc Interface. 2009;6:187–202. doi: 10.1098/rsif.2008.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Metrics: Population Attributable Fraction (PAF) http://www.who.int/healthinfo/global_burden_disease/metrics_paf/en/ Archived at http://www.webcitation.org/6lkPD7FGJ on November 3rd 2016.
- 20.Merrall EL, Kariminia A, Binswanger IA, Hobbs MS, Farrell M, Marsden J, et al. Meta-analysis of drug-related deaths soon after release from prison. Addiction. 2010;105:1545–1554. doi: 10.1111/j.1360-0443.2010.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin NK, Vickerman P, Foster GR, Hutchinson SJ, Goldberg DJ, Hickman M. Can antiviral therapy for hepatitis C reduce the prevalence of HCV among injecting drug user populations? A modeling analysis of its prevention utility. Journal of hepatology. 2011;54:1137–1144. doi: 10.1016/j.jhep.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 22.Vickerman P, Martin N, Turner K, Hickman M. Can needle and syringe programmes and opiate substitution therapy achieve substantial reductions in hepatitis C virus prevalence? Model projections for different epidemic settings. Addiction. 2012;107:1984–1995. doi: 10.1111/j.1360-0443.2012.03932.x. [DOI] [PubMed] [Google Scholar]
- 23.Palmateer NE, Taylor A, Goldberg DJ, Munro A, Aitken C, Shepherd SJ, et al. Rapid decline in HCV incidence among people who inject drugs associated with national scale-up in coverage of a combination of harm reduction interventions. PloS one. 2014;9:e104515. doi: 10.1371/journal.pone.0104515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Overstall AM, King R, Bird SM, Hutchinson SJ, Hay G. Incomplete contingency tables with censored cells with application to estimating the number of people who inject drugs in Scotland. Stat Med. 2014;33:1564–1579. doi: 10.1002/sim.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hickman M, Hope V, Coleman B, Parry J, Telfer M, Twigger J, et al. Assessing IDU prevalence and health consequences (HCV, overdose and drug-related mortality) in a primary care trust: implications for public health action. Journal of public health. 2009;31:374–382. doi: 10.1093/pubmed/fdp067. [DOI] [PubMed] [Google Scholar]
- 26.Sweeting M, De Angelis D, Ades A, Hickman M. Estimating the prevalence of ex-injecting drug use in the population. Statistical methods in medical research. 2009;18:381–395. doi: 10.1177/0962280208094704. [DOI] [PubMed] [Google Scholar]
- 27.Innes H, Goldberg D, Dillon J, Hutchinson SJ. Strategies for the treatment of Hepatitis C in an era of interferon-free therapies: what public health outcomes do we value most? Gut. 2015;64:1800–1809. doi: 10.1136/gutjnl-2014-308166. [DOI] [PubMed] [Google Scholar]
- 28.Public Health England. Hepatitis C in the UK: 2014 report. 2014. [Google Scholar]
- 29.Aspinall EJ, Mitchell W, Schofield J, Cairns A, Lamond S, Bramley P, et al. A matched comparison study of hepatitis C treatment outcomes in the prison and community setting, and an analysis of the impact of prison release or transfer during therapy. Journal of viral hepatitis. 2016 doi: 10.1111/jvh.12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Micallef JM, Kaldor JM, Dore GJ. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. Journal of viral hepatitis. 2006;13:34–41. doi: 10.1111/j.1365-2893.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 31.Csete J, Kamarulzaman A, Kazatchkine M, Altice F, Balicki M, Buxton J, et al. Public health and international drug policy. Lancet. 2016;387:1427–1480. doi: 10.1016/S0140-6736(16)00619-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Turner KM, Hutchinson S, Vickerman P, Hope V, Craine N, Palmateer N, et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction. 2011;106:1978–1988. doi: 10.1111/j.1360-0443.2011.03515.x. [DOI] [PubMed] [Google Scholar]
- 33.Van Den Berg C, Smit C, Van Brussel G, Coutinho R, Prins M, Amsterdam C. Full participation in harm reduction programmes is associated with decreased risk for human immunodeficiency virus and hepatitis C virus: evidence from the Amsterdam Cohort Studies among drug users. Addiction. 2007;102:1454–1462. doi: 10.1111/j.1360-0443.2007.01912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Topp L, Iversen J, Baldry E, Maher L. Housing instability among people who inject drugs: results from the Australian needle and syringe program survey. J Urban Health. 2013;90:699–716. doi: 10.1007/s11524-012-9730-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teutsch S, Luciani F, Scheuer N, Mccredie L, Hosseiny P, Rawlinson W, et al. Incidence of primary hepatitis C infection and risk factors for transmission in an Australian prisoner cohort. BMC Public Health. 2010;10:633. doi: 10.1186/1471-2458-10-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Degenhardt L, Larney S, Kimber J, Gisev N, Farrell M, Dobbins T, et al. The impact of opioid substitution therapy on mortality post-release from prison: retrospective data linkage study. Addiction. 2014;109:1306–1317. doi: 10.1111/add.12536. [DOI] [PubMed] [Google Scholar]
- 37.Martin NK, Hickman M, Hutchinson SJ, Goldberg DJ, Vickerman P. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57(Suppl 2):S39–45. doi: 10.1093/cid/cit296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martin NK, Vickerman P, Grebely J, Hellard M, Hutchinson SJ, Lima VD, et al. Hepatitis C virus treatment for prevention among people who inject drugs: Modeling treatment scale-up in the age of direct-acting antivirals. Hepatology. 2013;58:1598–1609. doi: 10.1002/hep.26431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin NK, Hickman M, Miners A, Hutchinson SJ, Taylor A, Vickerman P. Cost-effectiveness of HCV case-finding for people who inject drugs via dried blood spot testing in specialist addiction services and prisons. BMJ Open. 2013:3. doi: 10.1136/bmjopen-2013-003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.He T, Li K, Roberts MS, Spaulding AC, Ayer T, Grefenstette JJ, et al. Prevention of Hepatitis C by Screening and Treatment in U.S Prisons. Ann Intern Med. 2016;164:84–92. doi: 10.7326/M15-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin NK, Vickerman P, Dore GJ, Hickman M. The hepatitis C virus epidemics in key populations (including people who inject drugs, prisoners and MSM): the use of direct-acting antivirals as treatment for prevention. Curr Opin HIV AIDS. 2015;10:374–380. doi: 10.1097/COH.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Binswanger IA, Nowels C, Corsi KF, Glanz J, Long J, Booth RE, et al. Return to drug use and overdose after release from prison: a qualitative study of risk and protective factors. Addict Sci Clin Pract. 2012;7:3. doi: 10.1186/1940-0640-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calcaterra SL, Beaty B, Mueller SR, Min SJ, Binswanger IA. The association between social stressors and drug use/hazardous drinking among former prison inmates. J Subst Abuse Treat. 2014;47:41–49. doi: 10.1016/j.jsat.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.NICE LGB18: Tackling Drug Use. https://www.nice.org.uk/advice/lgb18/chapter/Costs-and-savings Archived at http://www.webcitation.org/6noAVIAME on January 26th 2017.
- 45.Rich JD, Mckenzie M, Larney S, Wong JB, Tran L, Clarke J, et al. Methadone continuation versus forced withdrawal on incarceration in a combined US prison and jail: a randomised, open-label trial. Lancet. 2015;386:350–359. doi: 10.1016/S0140-6736(14)62338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bird SM, Mcauley A, Perry S, Hunter C. Effectiveness of Scotland’s National Naloxone Programme for reducing opioid-related deaths: a before (2006–10) versus after (2011–13) comparison. Addiction. 2016;111:883–891. doi: 10.1111/add.13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harm Reduction International. The global state of harm reduction 2012. Towards an integrated response. 2014 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.