Abstract
The mechanisms for atrial fibrillation (AF) are unclear in part because diverse mapping techniques yield diverse maps ranging from stable organized sources to highly disordered waves. We hypothesized that AF mechanisms may be clarified if mapping techniques were compared in the same patients, and referenced to a clinical endpoint. We compared 2 independent AF mapping techniques in patients in whom local ablation terminated persistent AF before pulmonary vein isolation (PVI).
Techniques and Results
We identified 12 patients with persistent AF (61.2±10.8 years, 4 female) in whom mapping with 64 pole baskets and technique 1 (activation/phase mapping, FIRM) identified rotational activation patterns during at least 50% of the 4-second mapping interval and targeted ablation at these rotational sites terminated AF to sinus rhythm (n=10) or atrial tachycardia. We analyzed the unipolar electrograms of these patients to determine phase maps of activation by an independent technique 2 (Kuklik, Schotten et al., IEEE Trans Biomed Eng 2015). Compared to technique 1, technique 2 revealed a source in 12/12 (100%) cases with spatial concordance in all cases (p<0.05) and similar rotational characteristics.
Discussion
At sites where ablation terminated persistent AF, 2 independent mapping techniques identified stable rotational activation for multiple cycles that drove peripheral disorder. Future comparative studies referenced to a clinical endpoint may help reconcile if discrepancies between AF mapping studies reports represent techniques, patient populations or models of AF, and improve mapping to better guide ablation.
Keywords: atrial fibrillation, catheter ablation, FIRM, phase mapping, rotor mapping, human
Introduction
Therapy for persistent atrial fibrillation (AF) is limited by uncertainty in its mechanisms, and even extensive ablation may not improve the moderate success of pulmonary vein isolation (PVI)1. However, mechanistic uncertainty stems in part from studies in diverse populations, using diverse mapping techniques with varying technical or clinical validation. For arrhythmias such as atrial macroreentry, the accuracy of mapping can be gauged by its ability to identify sites where arrhythmia terminates by ablation. Conversely, in AF, few studies have compared AF mapping techniques in the same patients, and even fewer are referenced to a defined clinical endpoint.
Some recent mapping studies2, 3 propose that rotational or focal drivers in localized regions maintain AF, with promising results by ablating such sites at independent centers4–8. This concurs with optical mapping of AF in human atria9. However, other studies disagree. First, AF mapping historically shows disorganized waves with no10 or very few11 drivers, typically in patients with accepted permanent AF at non-arrhythmia surgery. Second, some studies show organized drivers on dominant frequency analysis12 that may be unstable by activation13 or phase14–16 mapping. Third, AF-driver ablation outcomes are disappointing at some centers17–19. It is unresolved if conflicting results reflect patient selection, methodology or inter-center variations in the results of any approach to AF ablation20.
We hypothesized that AF mechanisms may be clarified if independent mapping techniques were compared in the same patients, referenced to the endpoint of AF termination. We report on an early cohort of patients at our Institution in whom limited ablation guided by one mapping technique (Focal Impulse and Rotor Mapping, FIRM) terminated persistent AF prior to pulmonary vein (PV) isolation, and compared the results with a distinct second mapping technique applied to the same clinical data.
Methods
Study Design
We studied patients ≥ 21 years of age referred for ablation of drug-refractory persistent AF to Stanford University. This report analyzes an early cohort of n=12 such patients in whom activation/phase (FIRM) mapping was performed in real-time, revealed rotational activity patterns for ≥50% of mapped periods (‘epochs’), and targeted ablation terminated AF before PVI commenced. We compared rotational activity in patients at first ablation, and those with prior PVI21 and PV reconnection. This study was approved by the Institutional committee on human research at Stanford University.
Electrophysiology Study
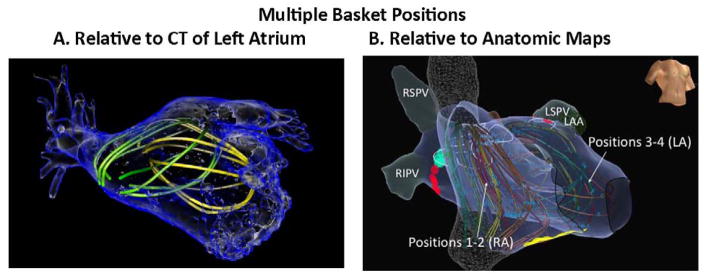
Electrophysiology (EP) study was performed after discontinuing antiarrhythmic medications for 5 half-lives. Catheters were advanced transvenously to the right atrium, coronary sinus and transseptally to left atrium. A 64-pole basket (FIRMap, Abbott Electrophysiology, Menlo park, CA) was advanced through an 8.5Fr SL1 sheath to map AF in right then left atria. Multiple basket positions were used routinely to cover the atria in successive mapping periods (‘epochs’) (figure 1). Electroanatomic shells were created (NavX, St Jude Medical, Sylmar, CA or Carto, Biosense-Webster, Diamond-Bar, CA) to relate basket electrodes to anatomic regions (Figures 2–4).
Figure 1. Multiple basket positions in each atrium.
are used to ensure that AF signals are recorded from the majority of both chambers, reconstructed here within (A) atrial computed tomography; (B) anatomic shell.
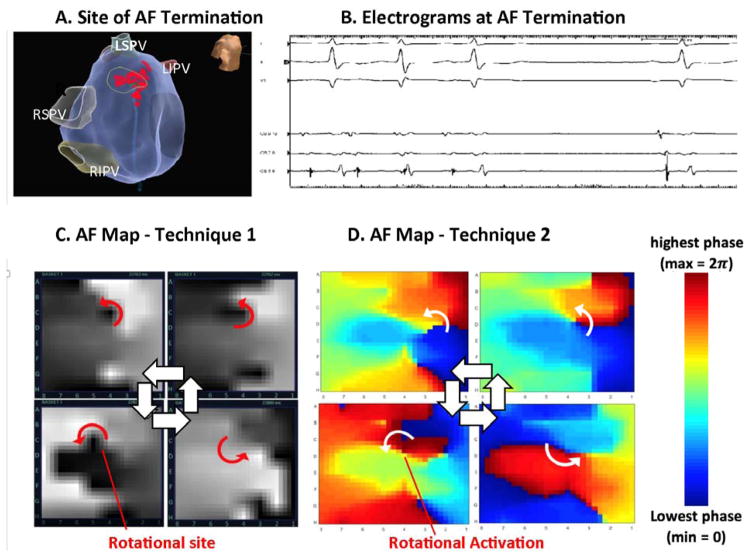
Figure 2. Identification of Rotations At Site of AF Termination Between Techniques (patient ID 1).
in a 78-year-old man with persistent AF. (A) Ablation at the inferior septal left atrium near the mitral annulus; (B) terminated AF. (C) Snapshots of AF map from technique 1 show clockwise activity for numerous cycles in 4 seconds at termination site (GH7; movie 1). (D) Snapshots of AF map from method 2 also show sustained clockwise activation at this AF termination site (movie 2). Both maps show fibrillatory complexity outside these sites.
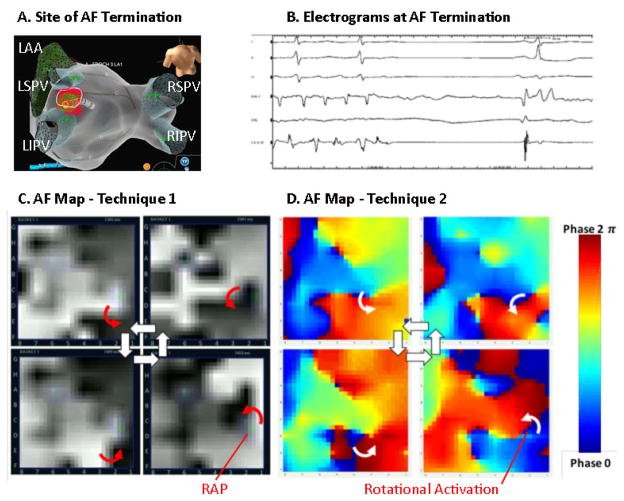
Figure 4. Identification of Rotational Activity at Site of AF Termination by Both Techniques (patient ID 3).
in a 67-year-woman with persistent AF. (A) Ablation site on posterior left atrial roof; (B) terminated AF. (C) Snapshots of AF map from technique 1 show a counterclockwise activation sequence (movie 5) and other CW rotations. (D) Snapshots of AF map from technique 2 also show counterclockwise rotation (movie 6) at termination site.
We exported the electrograms used for prospective mapping by technique 1 (FIRM, duration 1 minute) from our electrophysiological recorder (Prucka, GE Marquette, Milwaukee, WI), filtered at 0.05–500 Hz for independent mapping analysis by technique 2.
Index Mapping Approach (FIRM)
Real-time mapping in these cases was Focal Impulse and Rotor Mapping (FIRM)22, which was used as the index (technique 1) and served to generate cases with AF termination by localized ablation. Unipolar AF electrograms were recorded from multipolar basket catheters, with electroanatomic localization (e.g., NavX) inactive to avoid signal interference. FIRM creates maps of activation sequence which, for non-complex electrograms, may identify rotational activation23. However, AF electrograms often show far field deflections marked by traditional dV/dt criteria,24 which will alter map. FIRM filters out far-field activation using rate-related repolarization25, 26 and conduction27 to create activation maps, and also applies action potential data for phase analysis22. AF activation maps (gray scale maps) and singularities (colored rotational activity profiles, RAP) are used to identify organized regions during AF.
AF maps from FIRM were used prospectively to guide ablation. Rotational activation was defined as a stable spiral wave that drives disorder. A focal impulse was defined as an origin from where activation emerged centrifugally to cause disorder. Both patterns were used to guide ablation in this series if present for >50% of the mapped 4-second period (i.e., >10 cycles) within a limited spatial region (< 2–3 cm2) 28. Rotational activity profile (RAP) was used as an adjunct.
Ablation
Radiofrequency energy was delivered via an irrigated catheter (Thermocool™ or Smart-Touch™, Biosense-Webster; or Tacticath™, St Jude) at 25–35 Watts (10–15 Watts on posterior left atrium). FIRM-guided lesions were each applied for 15–30 seconds, typically requiring 10–20 lesions to cover each source area bounded by the projection of each electrode onto the shell.
This report focuses on cases in which AF terminated by targeted ablation alone. We excluded patients in whom PVI, FIRM or other ablation lesions were intermixed, for reasons of workflow or study protocol. Patients proceeded to wide area circumferential ablation of left and right PV pairs with verification of entrance and exit block by pacing without adenosine.
Independent AF Mapping Approach
Unipolar electrograms in AF from the precise segments used by technique 1 (FIRM) were exported for analysis by technique 2, an independent published algorithm14–16. Technique 2 differs from FIRM in several key respects. First, technique 2 does not create activation maps of AF, which is the principal output from FIRM (gray scale maps). Second, technique 2 does not apply algorithms to filter far-field timing data using action potential duration or conduction restitution, unlike FIRM. Third, unlike FIRM, technique 2 reconstructs AF signals using sinusoids14. We selected technique 2 because it shows very few (<1%) rotational sites in different patients and sheep models of AF14–16, i.e. it does not appear to falsely create rotational activation29.
We implemented this algorithm directly from its reports using the steps of Kuklik et al14–16 as follows. First, the QRS complex is removed on each channel by computing an average QRS complex and subtracting it from electrograms. Next, we applied a 1–30 Hz fourth-order Butterworth band pass filter and computed the dominant cycle length of each electrogram from the Welch Power Spectrum Density estimate of the signal, with a window size of 2000 ms, overlap of 1000 ms, and a cycle length cutoff between 130 and 280 ms. Finally, the recomposed signal was constructed as a sum of single-period sinusoidal waves with frequency equal to the computed dominant frequency and amplitude equal to the negative slope of the electrogram. For display we interpolated these recomposed signals to a grid, and applied the Hilbert Transform to compute phase maps (figures 2–4, even-numbered movies).
Phase maps generated by technique 2 were analyzed by 3 operators (MAH, GM, CK), blinded to clinical data. The number of rotations at each site and the location of these sites was compared to numbers and locations using technique 1. Rotational activity determined by both techniques were considered spatially concordant if locations differed by ≤1 electrode.
Statistical Analysis
Continuous data are represented as mean ± standard deviation (SD) or median and interquartile range (IQR) as appropriate. Comparisons between 2 groups were made with Student’s t-tests and summarized with means and standard deviations for independent samples if normally distributed or, if not normally distributed, with the Mann-Whitney U test and summarized with medians and quartiles. Nominal values were expressed as n (%) and compared with chi-square tests or the Fisher exact test when expected cell frequency was < 5. Multi-rater agreement was assessed using Fleiss’ Kappa score. A probability of < 0.05 was considered statistically significant throughout all analyses.
Results
Table 1 provides clinical details for patients in this cohort, in each of whom targeted ablation guided by mapping terminated persistent AF prior to PVI.
Mapping At Sites of AF Termination By Ablation
Index mapping using technique 1 detected 5.9±1.4 organized regions per patient (LA 3.4±1.2, RA 2.5±1.1). This report focuses on the region where targeted ablation (<2–3 cm2) terminated AF in each patient, which was the first source in 4 patients and the 2.1±1.0th source overall. All sources were ablated before PVI commenced in this series, and in no case did FIRM-guided ablation isolate a PV.
Figure 2 illustrates a 78-year-old man in whom targeted ablation (A) at the inferior septal left atrium (B) terminated persistent AF to sinus rhythm. A right atrial source had previously been ablated without termination. (C) AF maps from technique 1 show clockwise rotation at this site (coordinate GH7). Movie 1 shows that rotational activation (in white) was sustained for 18 cycles in 4 seconds, with rotational activity confirmed by phase analysis (colored repetitive activity profile, RAP). (D) AF maps using mapping technique 2 also showed clockwise activation at this site (GH7), sustained for many rotations (movie 2). Both techniques confirmed complex activation surrounding the rotational site of termination. AF continued until ablation at this site terminated AF to sinus rhythm.
Comparison of Mapping Techniques 1 and 2
Technique 1 and 2 both produced AF maps showing regional organization and surrounding disorder. As summarized in table 2, each site of termination by ablation exhibited rotational activation by index mapping and also by technique 2 (p<0.05).
Table 2.
Comparative Mapping in All Patients
| ID | Technique 1 (Index Mapping) | Technique 2 | ||
|---|---|---|---|---|
| No. cycles (4 seconds) | Comments | No. cycles (4 seconds) | Comments | |
| 1 | 18 | Figure 2, Movie 1. CW rotation at term site, minimal precession | 12 | Figure 2, movie 2. CW rotation with some precession; some foci |
| 2 | 12 | Figure 3, Movie 3. CCW rotation at term site; other transient rotations | 8 | Figure 3, Movie 4. CCW rotation at term site (2nd half), other rotations |
| 3 | 18 | Figure 4, Movie 5. CCW rotation at term site, also CW rotation sites. | 19 | Figure 4, movie 6. CCW rotation at term site, other rotational sites. |
| 4 | 19 | CCW rotation at term site, other transient rotations | 16 | CCW rotation at term site, other transient rotations |
| 5 | 12 | CCW rotation at term site | 12 | CCW rotation at term site |
| 6 | 13 | CCW rotation at term site | 9 | CCW rotation at term site with some precession |
| 7 | 18 | CCW rotation at term site, other transient rotations | 15 | CCW rotation at term site, other transient rotations |
| 8 | 15 | CW rotation at term site, other transient rotations | 12 | CW rotation at term site, other rotations |
| 9 | 11 | CCW rotation at term site | 5 | CCW rotation at term site, other transient rotations |
| 10 | 14 | CW rotation at term site | 10 | CW rotation at term site |
| 11 | 13 | CCW rotation at term site, other transient rotations | 16 | CCW rotation at term site, other transient rotations |
| 12 | 13 | CW rotation at term site, other transient rotations | 12 | CW rotation at term site, foci, other transient rotations |
| 14.7±2.8 | 12.2±3.9 | |||
Figure 3 illustrates persistent AF in 72 year old man in whom prospective ablation (A) at the carina of left superior pulmonary vein (B) terminated persistent AF to sinus rhythm prior to PVI. This was the first site targeted for ablation. (C) AF maps from technique 1 show counterclockwise rotation at this site, which are sustained for many cycles at site CD2 (movie 3; most apparent in second half). (D) AF maps from technique 2 also show counterclockwise activation, sustained in movie 4. Movies of both techniques show complex surrounding activity with competing wavefronts, and technique 2 showed slightly greater precession of rotational activation. AF continued until ablation at this site terminated AF to sinus rhythm.
Figure 3. Identification of Rotations at Site of Termination by Both Techniques (patient ID 2).
in a 72-year-old man with persistent AF. (A) Prospective guided ablation at the carina of left pulmonary vein (B) terminated persistent AF prior to PVI. (C) AF snapshots from technique 1 show counterclockwise rotation at termination site CD2 for > 10 cycles particularly in the second half of movie 3. (D) AF snapshots from technique 2 also show counter clockwise activation at this termination site (movie 4). Complex fibrillatory activity and competing wavefronts are also seen.
Figure 4 illustrates AF in a 67 year old woman in whom prospective targeted ablation (A) on the left atrial roof (B) organized then terminated persistent AF to sinus rhythm prior to PVI. A right atrial source had previously been ablated. (C) AF maps from technique 1 show counterclockwise rotational activation around a pivot (rotor precession area) that was targeted for ablation. In movie 5, rotational activation sustained for >10 cycles (19 cycles) in 4 seconds at site CD45, indicated by a computational index for rotational activity (colored markings, RAP). Applying mapping technique 2 to these data also shows (D) counterclockwise rotation at this location which, in movie 2, was sustained for >10 cycles at site CD45. Maps from technique 2 showed greater complexity than technique 1, with greater precession of the dominant rotation to locations CD67, intermittent foci and greater complexity of fibrillatory conduction surrounding the organized rotational site. AF continued until ablation at this site terminated AF to sinus rhythm.
Quantitative Comparison Between Techniques
Table 2 details activation at the site of AF termination by technique 1 (index approach) and technique 2 (validation method). Rotational activation was identified at each site of AF termination by technique 1 and by technique 2 (p<0.05) with a spatial concordance of less than 1 electrode (100% concordance, p<0.05) as illustrated in movies 1–6. The number of cycles detected in 4 seconds trended higher for technique 1 than technique 2 (14.7±2.8 versus 12.2±3.9, p=0.087).
Visual inspection uncovered qualitative differences between techniques, with technique 2 maps showing greater precession of rotational activation at sites of AF termination than technique 1, with greater surrounding complexity. Organized sites were intermittently obscured by competing wavefronts, either from another organized site or from disorganized waves (‘fibrillatory conduction’), before resuming in a very similar location. This phenomenon can be observed in movies 1–6.
Discussion
In this cohort of patients in whom localized ablation terminated persistent AF before pulmonary vein isolation, 2 independent mapping techniques revealed rotational activity at the site of AF termination for several cycles with surrounding complex fibrillatory activation. The approach of comparing mapping techniques referenced to a defined clinical endpoint provides a novel platform to study AF mechanisms. Future studies should investigate whether specific techniques under- or overestimate the presence of organized drivers in AF, referenced to sites of termination, to define the sensitivity and specificity of several methods for each endpoint, and finally examine sites where AF does not terminate by ablation.
Defined Clinical Endpoint
This study uses acute termination of persistent AF by targeted intervention as a reference for AF mapping – just as termination of atrial flutter by mapping-guided ablation can be used to validate that mapping. We acknowledge that acute AF termination is not equivalent to long-term freedom from AF, just as acute termination of atrial flutter is not equivalent to long-term freedom from atrial flutter, and may reflect the facts that mechanisms addressed acutely are not durably eliminated or because AF is later sustained by other mechanisms. In the absence of a clinical reference, it is possible that mapping of patients at non-arrhythmia surgery with permanent AF10, 11, 13 did not map regions of importance that were not identified, or studied patients whose mechanisms differ from patients referred for ablation because mapped patients did not receive AF therapy.
Alternative clinical reference endpoints that prove helpful for comparative mapping include AF termination to atrial tachycardia versus sinus rhythm, termination near versus remote from PVs, or sites where ablation terminates AF prior to isolation of PVs during ongoing PV ablation.
Comparative Mapping Techniques
The index method used to generate cases prospectively, method 1 (FIRM), and method 2 (Kuklik)14–16 produced similar maps in this cohort of patients with acute AF termination, showing organized rotational activity at sites of termination with peripheral disorder. This was true in patients at first and repeat ablation, suggesting similar AF mechanisms.
Despite showing similar results in this cohort, the mapping methods compared in this study are quite different. Method 1 (FIRM) creates activation maps of AF, using repolarization and conduction data to filter far-field, with additional phase mapping to reveal singularities22. Method 2 (Kuklik)14–16 avoids the proprietary algorithms used by FIRM and, while it uses phase, does not appear to create ‘false’ rotations because it showed only foci and conduction block and rare short-lived rotations in sheep AF16.
Method 1 (FIRM) has recently been correlated with optical mapping of human AF, with preliminary studies showing concordant rotational activity by both methods30. The spatiotemporal dynamics of sources in those human optical studies are similar to maps of AF termination sites by both methods in our patients: stable endocardial rotations intermittently obscured by competing waves31.
It is not clear why some techniques do not show rotational patterns in AF. In some cases, this may reflect epicardial-endocardial differences shown by bi-surface optical imaging of human atria, which may explain less stable drivers in body surface mapping3 or surgical13 studies of the epicardium. Other differences may reflect patient populations, differences between animal models (e.g., sheep) and human AF or algorithmic implementation.
Further studies comparing methodologies in the same patients with a defined clinical endpoint are required.
Inferring Mechanisms for Persistent AF
The current study provides a clinical reference of AF termination. However, studies are still needed to define how ablation at a rotational site may terminate AF32 or, by mapping techniques that do not show drivers at sites of AF termination, how ablation at disordered sites or sites with transient conduction block may terminate AF. Studies are needed to clarify if the lack of success of extensive ablation1, which limits critical mass, argues against the disordered wave hypothesis for AF.
Limitations
This study design used ‘true positives’ – cases in whom ablation terminated AF – and does not comment on sites where ablation does not terminate AF. Such studies are ongoing. Subjects in this study were enrolled on different protocols, but long-term outcomes studies in each protocol are ongoing. The method 1 algorithm has been described scientifically22, 26, 33 although its precise steps are undisclosed, but this limitation may be circumvented in this study because a second method showed similar results and AF termination provided a clinical reference to interpret mapping. Delayed termination of AF after ablation is an emerging concept, but may not alter our conclusions that ablation prior to PVI terminated AF at sources that appeared similar by both mapping techniques. Source ablation in this series typically preceded PVI by many minutes, giving time for delayed termination to occur. Patients with prior ablation were included by design to assess whether sources differed between groups. Finally, we did not perform concurrent epicardial mapping.
Conclusions
We demonstrate convergence of mechanisms in a cohort of patients with persistent AF, in whom 2 independent mapping techniques revealed rotational activity at sites where ablation terminated AF prior to PVI. The novel approach of comparing mapping techniques referenced to a defined clinical endpoint may provide a robust platform for future advances in AF mapping.
Table I.
Characteristics of Cohort
| ID | Age | Gender | Left atrial Size | LVEF | Prior Ablation | Terminate To | Where Ablated |
|---|---|---|---|---|---|---|---|
| 1 | 78 | M | 40 | 60 | Redo | Sinus Rhythm | Mitral Isthmus |
| 2 | 72 | M | 36 | 62 | 1st Ablation | Sinus Rhythm | Left PV Carina |
| 3 | 67 | F | 55 | 36 | Redo | Sinus Rhythm | Posterior LA Roof |
| 4 | 66 | M | 47 | 59 | Redo | Sinus Rhythm | Near LIPV |
| 5 | 53 | M | 52 | 36 | 1st Ablation | Atrial Tachycardia | Ant Septal Mitral |
| 6 | 50 | F | 40 | 59 | 1st Ablation | Sinus Rhythm | Near LA Appendage |
| 7 | 56 | M | 47 | 60 | Redo | Sinus Rhythm | Near LIPV |
| 8 | 49 | M | 53 | 51 | Redo | Sinus Rhythm | Post Lateral LA |
| 9 | 57 | M | 67 | 55 | 1st Ablation | Sinus Rhythm | Near RSPV |
| 10 | 79 | F | 47 | 69 | Redo | Sinus Rhythm | Near LA Appendage |
| 11 | 52 | M | 45 | 58 | Redo | Sinus Rhythm | Left PV Carina |
| 12 | 55 | F | 45 | 60 | 1st Ablation | Atrial Tachycardia | Infero-posterior to the LIPV |
| 61.2±10.8 | 4F | 47.8±8.2 | 55.4±10.0 | 7 Redos | 2 Atrial Tachycardia |
Acknowledgments
Mr. Vidmar is supported by a grant from the American Heart Association (16PRE30930015). Dr. Narayan is supported by the National Institutes of Health (R01 HL85537; K24 HL103800). Dr. Rappel is supported by the National Institutes of Health (R01 HL122384).
Footnotes
Drs. Narayan and Rappel are co-authors of intellectual property owned by the University of California Regents and licensed to Topera Inc, and have held equity in Topera inc. Dr. Narayan reports having received consulting fees from Abbott Electrophysiology, honoraria from Medtronic and St. Jude Medical. Dr. Wang reports honoraria and fellowship support from St. Jude Medical. Other authors: No disclosures.
References
- 1.Verma A, Jiang CY, Betts TR, Chen J, Deisenhofer I, Mantovan R, Macle L, Morillo CA, Haverkamp W, Weerasooriya R, Albenque JP, Nardi S, Menardi E, Novak P, Sanders P Investigators SAI. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372:1812–1822. doi: 10.1056/NEJMoa1408288. [DOI] [PubMed] [Google Scholar]
- 2.Narayan SM, Krummen DE, Shivkumar K, Clopton P, Rappel W-J, Miller J. Treatment of Atrial Fibrillation by the Ablation of Localized Sources: The Conventional Ablation for Atrial Fibrillation With or Without Focal Impulse and Rotor Modulation: CONFIRM Trial. J Am Coll Cardiol. 2012;60:628–636. doi: 10.1016/j.jacc.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haissaguerre M, Hocini M, Denis A, Shah AJ, Komatsu Y, Yamashita S, Daly M, Amraoui S, Zellerhoff S, Picat MQ, Quotb A, Jesel L, Lim H, Ploux S, Bordachar P, Attuel G, Meillet V, Ritter P, Derval N, Sacher F, Bernus O, Cochet H, Jais P, Dubois R. Driver Domains in Persistent Atrial Fibrillation. Circulation. 2014;130:530–538. doi: 10.1161/CIRCULATIONAHA.113.005421. [DOI] [PubMed] [Google Scholar]
- 4.Miller JM, Kowal RC, Swarup V, Daubert JP, Daoud EG, Day JD, Ellenbogen KA, Hummel JD, Baykaner T, Krummen DE, Narayan SM, Reddy VY, Shivkumar K, Steinberg JS, Wheelan KR. Initial Independent Outcomes from Focal Impulse and Rotor Modulation Ablation for Atrial Fibrillation: Multicenter FIRM Registry. J Cardiovasc Electrophys. 2014;25:921–929. doi: 10.1111/jce.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomassoni G, Duggal S, Muir M, Hutchins L, Turner K, McLoney AM, Hesselson A. Long-term Follow-up of FIRM-guided Ablation of Atrial Fibrillation: A Single-center Experience. J Innovations in Cardiac Rhythm Management. 2015:2145–2151. [Google Scholar]
- 6.Rashid H, Sweeney A. Approaches for Focal Impulse and Rotor Mapping in Complex Patients: A US Private Practice Perspective. J Innovations in Cardiac Rhythm Management. 2015;6:2193–2198. [Google Scholar]
- 7.Sommer P, Kircher S, Rolf S, John S, Arya A, Dinov B, Richter S, Bollmann A, Hindricks G. Successful Repeat Catheter Ablation of Recurrent Longstanding Persistent Atrial Fibrillation with Rotor Elimination as the Procedural Endpoint: A Case Series. J Cardiovasc Electrophysiol. 2016;27:274–280. doi: 10.1111/jce.12874. [DOI] [PubMed] [Google Scholar]
- 8.Spitzer SG, Karolyi L, Rammler C, Scharfe F, Weinmann T, Zieschank M, Langbein A. Treatment of Recurrent Non-Paroxysmal Atrial Fibrillation Using Focal Impulse and Rotor Mapping (FIRM)-Guided Rotor Ablation: Early Recurrence and Long-Term Outcomes. J Cardiovasc Electrophysiol. 2017;28:31–38. doi: 10.1111/jce.13110. [DOI] [PubMed] [Google Scholar]
- 9.Hansen BJ, Zhao J, Csepe TA, Moore BT, Li N, Jayne LA, Kalyanasundaram A, Lim P, Bratasz A, Powell KA, Simonetti OP, Higgins RS, Kilic A, Mohler PJ, Janssen PM, Weiss R, Hummel JD, Fedorov VV. Atrial fibrillation driven by micro-anatomic intramural re-entry revealed by simultaneous sub-epicardial and sub-endocardial optical mapping in explanted human hearts. Eur Heart J. 2015;36:2390–2401. doi: 10.1093/eurheartj/ehv233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allessie MA, de Groot NM, Houben RP, Schotten U, Boersma E, Smeets JL, Crijns HJ. ElectroPathological Substrate of Long-standing Persistent Atrial Fibrillation in Patients with Structural Heart Disease: Longitudinal Dissociation. Circ Arrhythm Electrophysiol. 2010;3:606–615. doi: 10.1161/CIRCEP.109.910125. [DOI] [PubMed] [Google Scholar]
- 11.Walters TE, Lee G, Morris G, Spence S, Larobina M, Atkinson V, Antippa P, Goldblatt J, Royse A, O’Keefe M, Sanders P, Morton JB, Kistler PM, Kalman JMK. Temporal Stability of Rotors and Atrial Activation Patterns in Persistent Human Atrial Fibrillation: A High Density Epicardial Mapping Study of Prolonged Recordings (Transient rotors in human persistent AF) J Am Coll Cardiol: Clinical Electrophysiology. 2015;1:18–25. doi: 10.1016/j.jacep.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Atienza F, Almendral J, Ormaetxe JM, Moya A, Martinez-Alday JD, Hernandez-Madrid A, Castellanos E, Arribas F, Arias MA, Tercedor L, Peinado R, Arcocha MF, Ortiz M, Martinez-Alzamora N, Arenal A, Fernandez-Aviles F, Jalife J Investigators R-A. Comparison of Radiofrequency Catheter Ablation of Drivers and Circumferential Pulmonary Vein Isolation in Atrial Fibrillation: A Noninferiority Randomized Multicenter RADAR-AF Trial. J Am Coll Cardiol. 2014;64:2455–2467. doi: 10.1016/j.jacc.2014.09.053. [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Sahadevan J, Khrestian CM, Cakulev I, Markowitz A, Waldo AL. Simultaneous Bi-Atrial High Density (510 – 512 electrodes) Epicardial Mapping of Persistent and Long-Standing Persistent Atrial Fibrillation in Patients: New Insights into the Mechanism of Its Maintenance. Circulation. 2015;132:2108–2117. doi: 10.1161/CIRCULATIONAHA.115.017007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuklik P, Zeemering S, Maesen B, Maessen J, Crijns HJ, Verheule S, Ganesan AN, Schotten U. Reconstruction of instantaneous phase of unipolar atrial contact electrogram using a concept of sinusoidal recomposition and Hilbert transform. IEEE Trans Biomed Eng. 2015;62:296–302. doi: 10.1109/TBME.2014.2350029. [DOI] [PubMed] [Google Scholar]
- 15.Kuklik P, Lau DH, Ganesan AN, Brooks AG, Sanders P. High-density mapping of atrial fibrillation in a chronic substrate: Evidence for distinct modes of repetitive wavefront propagation. Int J Cardiol. 2015;199:407–414. doi: 10.1016/j.ijcard.2015.07.057. [DOI] [PubMed] [Google Scholar]
- 16.Kuklik P, Zeemering S, van Hunnik A, Maesen B, Pison L, Lau D, Maessen J, Podziemski P, Meyer C, Schaffer B, Crijns H, Willems S, Schotten U. Identification of Rotors during Human Atrial Fibrillation using Contact Mapping and Phase Singularity Detection: Technical Considerations. IEEE Trans Biomed Eng. 2016 doi: 10.1109/TBME.2016.2554660. epub. [DOI] [PubMed] [Google Scholar]
- 17.Gianni C, Di Biase L, Deneke T, Tami Metz T, Halbfass P, Müller P, Schade A, Mohanty S, Trivedi C, Bai R, Al-Ahmad A, Burkhardt JD, Gallinghouse GJ, Horton RP, Hranitzky PM, Sanchez JE, Tomassoni GF, Natale A. Acute and Short-Term Outcomes in Persistent and Long-Standing Persistent Patients Undergoing Rotors Only Ablation (abstract) Heart Rhythm. 2015;12:PO01–58. [Google Scholar]
- 18.Buch E, Share M, Tung R, Benharash P, Sharma P, Koneru J, Mandapati R, Ellenbogen KA, Shivkumar K. Long-Term Clinical Outcomes of Focal Impulse and Rotor Modulation for Treatment of Atrial Fibrillation: A Multi-Center Experience. Heart Rhythm. 2016;13:636–641. doi: 10.1016/j.hrthm.2015.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinberg JS, Shah Y, Bhatt A, Sichrovsky T, Arshad A, Hansinger E, Musat D. Focal Impulse and Rotor Modulation: Acute Procedural Observations and Extended Clinical Follow-up. Heart Rhythm. 2016 doi: 10.1016/j.hrthm.2016.11.008. epub. [DOI] [PubMed] [Google Scholar]
- 20.Ganesan AN, Shipp NJ, Brooks AG, Kuklik P, Lau DH, Lim HS, Sullivan T, Roberts-Thomson KC, Sanders P. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. Journal of the American Heart Association. 2013;2:e004549. doi: 10.1161/JAHA.112.004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haissaguerre M, Hocini M, Sanders P, Takahashi Y, Rotter M, Sacher F, Rostock T, Hsu LF, Jonsson A, O’Neill MD, Bordachar P, Reuter S, Roudaut R, Clementy J, Jais P. Localized sources maintaining atrial fibrillation organized by prior ablation. Circulation. 2006;113:616–625. doi: 10.1161/CIRCULATIONAHA.105.546648. [DOI] [PubMed] [Google Scholar]
- 22.Narayan SM, Krummen DE, Enyeart MW, Rappel W. Computational Mapping Approach Identifies Stable and Long-Lived Electrical Rotors and Focal Sources in Human Atrial Fibrillation. PLos One. 2012;7:e46034. doi: 10.1371/journal.pone.0046034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaman JAB, Baykaner T, Swarup V, Kowal RC, Daubert JP, Day JD, Hummel J, Schricker AA, Krummen DE, Mansour M, Tomassoni GF, Wheelan KR, Vishwanathan M, Park S, Wang PJ, Narayan SM, Miller JM. Recurrent Post Ablation Paroxysmal Atrial Fibrillation Shares Substrates with Persistent Atrial Fibrillation: an 11 Center Study. JACC: Clinical Electrophysiology. 2016 doi: 10.1016/j.jacep.2016.10.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Narayan SM, Wright M, Derval N, Jadidi A, Forclaz A, Nault I, Miyazaki S, Sacher F, Bordachar P, Clementy J, Jais P, Haissaguerre M, Hocini M. Classifying Fractionated Electrograms in Human Atrial Fibrillation Using Monophasic Action Potentials and Activation Mapping: Evidence for Localized Drivers, Rate Acceleration and Non-Local Signal Etiologies. Heart Rhythm. 2011a;8:244–253. doi: 10.1016/j.hrthm.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narayan SM, Kazi D, Krummen DE, Rappel W-J. Repolarization and Activation Restitution Near Human Pulmonary Veins and Atrial Fibrillation Initiation: A Mechanism for the Initiation of Atrial Fibrillation by Premature Beats. J Am Coll Cardiol. 2008c;52:1222–1230. doi: 10.1016/j.jacc.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Narayan SM, Franz MR, Clopton P, Pruvot EJ, Krummen DE. Repolarization Alternans Reveals Vulnerability to Human Atrial Fibrillation. Circulation. 2011b;123:2922–2930. doi: 10.1161/CIRCULATIONAHA.110.977827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schricker A, Rostamian A, Lalani G, Krummen DE, Narayan SM. Human Atrial Fibrillation Initiates by Organized Not Disorganized Mechanisms. Circ Arrhythm Electrophysiol. 2014;7:816–824. doi: 10.1161/CIRCEP.113.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narayan SM, Shivkumar K, Krummen DE, Miller JM, Rappel W-J. Panoramic Electrophysiological Mapping But Not Individual Electrogram Morphology Identifies Sustaining Sites for Human Atrial Fibrillation (AF Rotors and Focal Sources Relate Poorly to Fractionated Electrograms) Circ Arrhythm Electrophysiol. 2013;6:58–67. doi: 10.1161/CIRCEP.111.977264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vijayakumar R, Vasireddi SK, Cuculich PS, Faddis MN, Rudy Y. Methodology Considerations in Phase Mapping of Human Cardiac Arrhythmias. Circ Arrhythm Electrophysiol. 2016:9. doi: 10.1161/CIRCEP.116.004409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansen BJ, Briggs C, Moore BT, Csepe TA, Li N, Zhao J, Garikipati NV, Janssen PM, Mohler PJ, Hummel JD, Fedorov VV. Human Atrial Fibrillation Drivers Seen Simultaneously by Focal Impulse and Rotor Mapping and High-resolution Optical Mapping [abstract] Circulation. 2015;132:A18402. [Google Scholar]
- 31.Li N, Csepe TA, Hansen BJ, Sul LV, Kalyanasundaram A, Zakharkin SO, Zhao J, Guha A, Van Wagoner DR, Kilic A, Mohler PJ, Janssen PM, Biesiadecki BJ, Hummel JD, Weiss R, Fedorov VV. Adenosine-Induced Atrial Fibrillation: Localized Reentrant Drivers in Lateral Right Atria due to Heterogeneous Expression of Adenosine A1 Receptors and GIRK4 Subunits in the Human Heart. Circulation. 2016;134:486–498. doi: 10.1161/CIRCULATIONAHA.115.021165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rappel WJ, Zaman JA, Narayan SM. Mechanisms for the Termination of Atrial Fibrillation by Localized Ablation: Computational and Clinical Studies. Circ Arrhythm Electrophysiol. 2015;8:1325–1333. doi: 10.1161/CIRCEP.115.002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lalani G, Schricker A, Gibson M, Rostamanian A, Krummen DE, Narayan SM. Atrial Conduction Slows Immediately Before the Onset of Human Atrial Fibrillation: A Bi-Atrial Contact Mapping Study of Transitions to Atrial Fibrillation. J Am Coll Cardiol. 2012;59:595–606. doi: 10.1016/j.jacc.2011.10.879. [DOI] [PMC free article] [PubMed] [Google Scholar]