Abstract
Background
Stress and anxiety are widely considered to be causally related to alcohol craving and consumption, as well as development and maintenance of alcohol use disorders (AUD). However, numerous preclinical and human studies examining effects of stress or anxiety on alcohol use and alcohol-related problems have been equivocal. This study examined relationships between scores on self-report anxiety, anxiety sensitivity and stress measures and frequency and intensity of recent drinking, alcohol craving during early withdrawal, as well as laboratory measures of alcohol craving and stress reactivity among heavy drinkers with alcohol use disorder (AUD).
Methods
Media-recruited, heavy drinkers with AUD (N=87) were assessed for recent alcohol consumption. Anxiety and stress levels were characterized using paper-and-pencil measures, including the Beck Anxiety Inventory (BAI), the Anxiety Sensitivity Index-3 (ASI-3) and the Perceived Stress Scale (PSS). Eligible subjects (N=30) underwent alcohol abstinence on the Clinical Research Unit (CRU); twice daily measures of alcohol craving were collected. On day 4, subjects participated in the Trier Social Stress Test (TSST); measures of cortisol and alcohol craving were collected.
Results
In multivariate analyses, higher BAI scores were associated with lower drinking frequency and reduced drinks/drinking day; in contrast, higher ASI-3 scores were associated with higher drinking frequency. BAI anxiety symptom and ASI-3 scores also were positively related to AUDIT total scores and AUD symptom and problem subscale measures. Higher BAI and ASI-3 scores but not PSS scores were related to greater self-reported alcohol craving during early alcohol abstinence. Finally, BAI scores were positively related to laboratory stress-induced cortisol and alcohol craving. In contrast, the PSS showed no relationship with most measures of alcohol craving or stress reactivity.
Conclusions
Overall, clinically-oriented measures of anxiety compared with perceived stress were more strongly associated with a variety of alcohol-related measures in current heavy drinkers with AUD.
Keywords: Alcohol use disorder, alcohol craving, anxiety sensitivity, Trier Social Stress Test, cortisol
Introduction
Stress and anxiety are widely considered to be causally related to alcohol craving (Breese et al., 2011; Haass-Koffler et al., 2014; Spanagel et al., 2014) and consumption, as well as the development and maintenance of alcohol use disorders (AUD). Etiological explanations have been grounded in theories that alcohol is used to reduce tension/dampen stress responses (Sher and Levenson, 1982; Donovan and Marlatt, 1980), or self-medicate (Brady and Lydiard, 1993; Khantzian, 1985; Mueser et al., 1998). Alcohol is thought to reduce the unpleasant physiological and cognitive symptoms of stress and anxiety, thereby negatively reinforcing, or increasing, drinking behavior. However, numerous preclinical and human studies examining effects of stress or anxiety on alcohol use and alcohol-related problems (O'Grady et al., 2011; Wand et al., 1998; McCreary and Sadava, 2000; Sayette, 1999; Young et al., 1990) have been equivocal, showing positive, negative, or no relationship.
One potential source of the equivocal findings across studies may be the conflation of anxiety and stress as exposures. Stressors are generally defined as internal or external factors that initiate a cascade of hormonal, neurological and other biological “fight or flight” responses that disrupt homeostasis. Evidence from large epidemiological studies shows that stress from disasters and terrorism as well as work, family, legal and financial difficulties is associated with increased alcohol consumption and heavy drinking (Crum et al., 1995; San José et al., 2000; Keyes et al., 2011). Indeed, the number of stressors is positively correlated with the amount of alcohol consumption. Interestingly, there is some evidence that stress has differential effects on alcohol consumption in persons with and without an alcohol use disorder. Specifically, persons with as compared to those without an AUD were four times more likely to report drinking to cope with trauma-related emotions following disaster exposure (North et al., 2011), suggesting that drinking increases primarily among those individuals who have previously learned (Heilig et al., 2010; Spanagel et al., 2014) that alcohol is effective in relieving stress effects.
In contrast, anxiety is the anticipation of unpredictable, impending threats (American Psychiatric Association, 2013), and anxiety disorders are marked by persistent and exaggerated fear responses. Epidemiological findings indicate that there is a doubling or quadrupling of AUD risk for persons with some but not all anxiety disorders (Kessler et al., 1997; Regier et al., 1990). For example, persons with generalized anxiety disorder or post-traumatic stress disorder are at particularly high risk for AUD comorbidity (Hasin and Grant, 2015).
More recently, a third factor has been identified that may mediate the relationship between anxiety and stress and subsequent alcohol use. Anxiety sensitivity (AS) is the tendency to respond fearfully to one's own anxiety symptoms and is the belief that the experience of anxiety is itself harmful (Reiss et al., 1986). AS differs from trait anxiety which is a tendency to respond fearfully to stressors (McNally, 1989; DeMartini and Carey, 2011). High (versus low) AS has been associated with higher levels of weekly alcohol consumption (M=7.4 vs. 2.2 drinks/week) among nonalcoholic university women (Stewart et al., 1995). High AS, but not trait anxiety, also predicted AUD development over a 24-month follow up period among 440 young adults (Schmidt et al., 2007). A review of AS studies proposed a chained mediation model whereby anxiety symptoms help explain the relationship between AS and drinking motives (coping and social conformity) (Conrod et al., 1998; Stewart et al., 1997) and in turn drinking motives mediate the relationship between AS and drinking frequency (DeMartini and Carey, 2011). Thus, because AS influences both drinking motives and drinking frequency, it too can be a significant risk factor for alcohol misuse.
While stress, anxiety, and AS have a complex etiological role in alcohol consumption and AUD, a host of mediating and moderating factors influence these relationships. These include gender, alcohol expectancy effects, genetic differences in the regulation of hypothalamic pituitary adrenal (HPA) axis activity, acute versus chronic stress, and population characteristics, such as clinical, student or general population samples. Drinking history may also be significant as studies (McCreary and Sadava, 2000; McCreary and Sadava, 1998) that examine the influence of stress or anxiety on alcohol problems are more likely to find a positive relationship compared with studies that examine the influence of stress or anxiety on levels of alcohol consumption (Morris et al., 2005; Schry and White, 2013). In clinical studies, it has been challenging to sort out the etiologic role of anxiety or stress since data collection relies almost exclusively on self-report to define the relative onset of anxiety or stress and acceleration of alcohol consumption. Laboratory and preclinical studies can help to clarify some of these issues by directly examining the effects of anxiety and stress on alcohol consumption. In these studies, the anxiolytic effects of alcohol are well established (Gilman et al., 2008). However, several decades of research using a variety (Becker et al., 2011) of animal models and experimental procedures has yielded equivocal findings on effects of acute and chronic stress on alcohol use.
The purpose of this study was to examine the relationship between self-report measures of anxiety (Beck Anxiety Inventory (BAI, Beck et al., 1988)), anxiety sensitivity (Anxiety Sensitivity Index – 3 (ASI-3, Taylor et al., 2007)), and stress (Perceived Stress Scale (PSS, Cohen et al., 1983)) and the frequency and intensity of recent drinking as well as laboratory measures of alcohol craving and stress reactivity among heavy drinkers with alcohol use disorder (AUD).
Materials and Methods
Subjects
Media advertisements (radio, local papers, craigslist) were used to recruit persons who were current heavy drinkers with alcohol use disorder and were not seeking treatment. To qualify for study participation, subjects had to be 21 – 60 years old, currently drinking more than 10 drinks/week (women) or 20 drinks/week (men) (or more than 50% above the NIAAA definitions for moderate drinking), and meet DSM-5 criteria for alcohol use disorder. In addition, subjects had to have a positive result for phosphatidylethanol (PEth), an alcohol specific biomarker for recent heavy drinking (United States Drug Testing Laboratory) (Schröck et al., 2014). Persons were ineligible who were currently using illicit drugs as measured by self-report and on-site drug toxicology, met DSM-5 criteria for a current major mood or anxiety disorder, reported current psychiatric treatment or psychotropic medication, or had less than a 5th grade reading level. In addition, women could not have significant menstrual dysfunction, be pregnant or lactating, or be using hormonal birth control.
A total of 289 persons were screened by telephone for study eligibility; 137 provided informed consent and participated in the in-person assessment procedures. Of those, assessment data were obtained on 87 subjects, and a subset of 30 subjects participated in the CRU stay and human laboratory procedures.
The study was approved by the Johns Hopkins University Institutional Review Board.
Procedures
Assessment
Following informed consent, participants underwent a comprehensive baseline assessment that included the 90-day Timeline Follow-back (Sobell and Sobell, 1992) to quantify the intensity and frequency of recent drinking, the Alcohol Use Disorders Identification Test (AUDIT, Babor et al., 2001), the MINI International Neuropsychiatric Interview, Version 7.0 (Sheehan, 2014). Subjects completed several paper-and-pencil measures of stress and mood, including:
The Beck Anxiety Inventory (BAI, Beck et al., 1988) is a 21-item, paper-and-pencil self-report measure of anxiety symptoms. The instrument has been shown to have high internal consistency (Cronbach's alpha =0.92) and test-retest reliability (1 week = 0.75) (Beck et al., 1988). Using a scale of 0 (not at all) to 3 (severely), persons rate the severity of commonly experienced somatic (e.g., heart pounding/racing, dizzy/lightheaded, wobbliness in legs) and cognitive (e.g., fear of worst happening, terrified/afraid, fear of losing control) anxiety symptoms over the past month. A score of 22 and above is considered moderate to high anxiety severity. All assessed participants completed the BAI.
The Anxiety Sensitivity Index – 3 (ASI-3, Taylor et al., 2007) is a psychometrically sound, 18-item self-report questionnaire of the fear of anxiety-related sensations with high convergent validity with anxiety symptoms (Rifkin et al., 2015). Subjects rate the extent to which they agree with each item on a 5-point Likert scale ranging from 0 (very little) to 4 (very much). Allan and colleagues (2014) have established a three class model of AS, using a cut-off score of 17 for moderate and 23 for high AS. The ASI-3 was added to the assessment protocol after the study was already underway; thus scores were available on 55 participants.
The Perceived Stress Scale (PSS, Cohen et al., 1983) is a measure of the degree to which situations in one's life are evaluated as stressful. Ratings are believed to reflect how unpredictable, uncontrollable, and overloaded respondents find their lives and the degree to which life events exceed one's ability to cope. The PSS was added to the assessment protocol after the study was already underway; scores were available on 56 participants.
Clinical Research Unit (CRU)
Participants who met eligibility requirements and were generally healthy were admitted to the Johns Hopkins Bayview Medical Center Clinical Research Unit for a 6-night/7-day stay. On days 1 – 3, participants underwent supervised alcohol abstinence; vital signs and Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised (CIWA-Ar) scores were obtained by CRU nursing staff every 4 hrs while participants were awake. Subjects did not require medications to treat symptoms of alcohol withdrawal based on CIWA-Ar scores <12. On day 1, all subjects received an intravenous infusion of multivitamins, thiamine, folic acid and magnesium sulfate. On days 4 – 7, vital signs were obtained twice daily at 8 am and 8 pm. Throughout the CRU stay, over-the-counter medications were available upon request for pain and discomfort and stomach upset.
At 8 am and 8 pm each day, participants were prompted by nursing staff to complete the Alcohol Urges Questionnaire (AUQ, Bohn et al., 1995), Obsessive-Compulsive Drinking Scale (OCDS, Anton et al., 1995), and visual analog scale (VAS) for alcohol craving. Subjects were able to smoke ad lib on the CRU in a specially ventilated smoking room. On the day of discharge, subjects participated in a Brief Alcohol Intervention session with a trained member of staff under the supervision of Dr. McCaul.
Trier Social Stress Test (TSST)
On study days 4 and 5, subjects underwent the TSST and a neutral control session in random order. The TSST includes a public speaking phase followed by a mental arithmetic task; it has been shown to consistently activate the HPA axis and elevate cortisol levels (Kirschbaum et al., 1993). On session days, subjects received a standardized, caffeine-free, calorie-controlled breakfast and light lunch. Mood and anxiety self-report measures were completed before and after the TSST. The subject was taken to the testing room and seated in a chair facing a conference table; two chairs to seat the test panel were placed behind the table. The subject was asked to sit quietly, relax, and await instructions for the test protocol. During this 20-min waiting period, baseline saliva samples were collected for cortisol measurement. The subject then was told to listen carefully to the taped instructions for the first task, the job interview. The participant was told that she/he was interviewing for the position of a hospital administrator and that in a 5-min speech he/she had to convince the panel that he/she was the best candidate for the job. They were told that they must maintain eye contact with the panel throughout the interview. The participant was given 10 min to mentally prepare for the interview. The test period began (Time 0) when the two-member panel filed into the room and sat across the table from the subject. During the session, one of the confederates pretended to be filming the subject with a video camera. In actuality, the camera had no tape.
Following the speech, subjects were given instructions for the mental arithmetic test portion of the procedure. For the mental arithmetic test, the subject was told to repeat a four-digit number after the tester, subtract 13 from it, and call out the answer. The subject continued subtracting and calling out answers for 5 min. Throughout this challenge, the tester distracted the subject by commenting on the speed and accuracy of responses and urged the subject to look at the tester at all times. At the end of the mental arithmetic, the subject was asked to sit quietly for the remainder of the protocol. Saliva for cortisol measurement was collected three times prior to the start of the test protocol, immediately after the TSST, and at 10-minute intervals during the Alcohol Motivated Response (AMR) session. During the AMR session, subjects responded using a computer mouse on a progressive ratio (PR) schedule for alcohol or money. During each AMR session, there were 10 PR work cycles; subjects selected to work for either alcohol or money at the start of each PR cycle and also had the opportunity to switch reinforcers at any time during the cycle. Regularly during the AMR session, subjects rated their current level of alcohol craving on a visual analog scale (0 to 9). At the completion of the AMR session, the alcohol ingestion period began. Subjects were debriefed about the speech and arithmetic tasks at the end of their study participation, prior to CRU discharge. They were informed that the video camera did not contain film, that their performance was not actually being rated, and that the interviewers were actually confederates involved with the study.
The placebo session (no stress) was similar to above except subjects did not undergo the stressor but merely read magazines for the period of time corresponding to the stress period in the active session.
Statistical Analyses
We first summarized baseline characteristics, including demographics and drinking measures collected at assessment for the total assessment sample (N=87) and the subsample who completed the laboratory studies (N=30). The arithmetic mean and standard deviation of continuous variables and the percentage of counting variables were calculated. We summarized the distribution and statistics of anxiety/stress outcomes of interest, including BAI, ASI-3, and PSS. Depending on normality of distributions of these variables, two-sample t-test or Wilcoxon rank-sum test were performed to see if scores differed as a function of sex or race.
Next, we tested the correlation between each recent alcohol consumption measure and each anxiety/stress measure. The alcohol consumption variables were calculated using data collected in the 90-day Timeline-followback interview at assessment, including number of drinking days per week, number of binge drinking days per week, and number of drinks per drinking day. Then we constructed generalized linear models with each drinking measure as the dependent variable, and the BAI, ASI-3, and PSS scores as the independent variable in separate models. Assuming the probability of having a drinking day remains constant across the 90 TLFB period, and because the number of drinking days and number of heavy drinking/binge days are capped at 90, binomial distribution with logit link function was used in these two models; results are presented as odds ratios. Poisson distribution with log link function was used to model the number of drinks per drinking day; these results are presented as relative risks. We then tested the correlation between each recent alcohol consumption measure and each anxiety/stress measure, adjusting for scores on the remaining two anxiety/stress instruments and sex.
We also conducted a series of correlations between subjective measures and AUDIT total score and subscale scores using linear regression models with the AUDIT scores as the dependent variables. These results are presented as the beta coefficients with p values. Sex was added to all models as a covariate.
We tested the correlations between measures of alcohol craving on the CRU and the anxiety/stress measures using multiple linear regression models (N=30). The peak and average of VAS, AUQ, and OCDS scores were calculated and used as the dependent variables in the linear models, and anxiety/stress scores are the independent variables. We included sex as a covariate. These results are presented as the beta coefficients and p values. Because of the smaller sample size in the CRU and laboratory procedures, we did not complete multivariate analyses adjusting for the other anxiety/stress instruments.
Finally, we used multiple linear regression models to examine the correlation of stress-induced cortisol and alcohol craving measured during the alcohol-motivated response period following the TSST with each of the anxiety/stress measures (N=30). Due to the high skewness, cortisol values were first log-transformed. The area under the curve (AUC) subtracting baseline of the transformed cortisol values was calculated and used as the dependent variable in the regression, while each of the anxiety/stress measures, sex, and stressed versus neutral session order were independent variables. AUC of VAS craving scores during the stress session was calculated and tested in the model, but no transformation was needed to correct for skewness.
Results
Subject Characteristics
Of the 136 participants who provided informed consent, 87 completed assessment procedures. Reasons for rule out prior to completion of the assessment were positive urine toxicology (N=22), low reading levels (N=6), a current psychiatric disorder, most commonly major depressive disorder (N=16) or a medical exclusion (N=5). Assessed subjects who did not complete study laboratory procedures generally had become employed, relocated from the Baltimore area, withdrew from further participation; lost to follow-up (N=20) accounted for the largest proportion. A small number of participants were excluded for health reasons identified during the assessment. Mean age of assessed subjects was 33.3 years old, approximately 80% of participants were male and 60% were black. Mean AUDIT score was 19.9, reflecting high drinking severity. Subjects averaged 8.6 drinks per drinking day, 4.9 drinking days per week and approximately 3.4 binge drinking days per week (Table 1). Demographic and behavioral characteristics of the subset of participants who completed study procedures did not differ from the larger pool of assessment subjects (Table 1; all p>0.10).
Table 1. Demographic and Behavioral Characteristics of Assessment Participants and Subjects completing the inpatient and laboratory procedures.
| Assessment Participants (N=87) | Inpatient & Lab Participants (N=30) | |||
|---|---|---|---|---|
| Age (M, SD) | 33.3 | 10.4 | 35.1 | 11.0 |
| Sex (% male) | 79.3% | 70% | ||
| Race (%): | ||||
| White | 32.2% | 33.3% | ||
| Black | 60.9% | 56.7% | ||
| Other | 6.9% | 10% | ||
| AUDIT total score (M, SD) | 19.9 | 6.7 | 18.8 | 6.8 |
| Drinks/Drinking Day*(M, SD) | 8.6 | 4.4 | 8.2 | 3.7 |
| Drinking days (M, SD) * | 4.9 | 1.5 | 5.0 | 1.5 |
| Binge days (M, SD) * | 3.4 | 2.0 | 3.6 | 1.9 |
Drinking data based on 90-day Timeline Followback
Instrument score distributions – BAI, ASI-3, PSS
A total of 87 participants completed the BAI as part of their initial assessment. Of those, 97.7% scored below the clinical cut-off of 22 for moderate anxiety and only 2.3% were equal to or above the cut-off, reflecting clinically relevant levels of anxiety.
A total of 55 subjects completed the ASI-3 as part of their initial assessment. Of those, 69% scored below 17 reflecting low anxiety sensitivity. 14.5% scored in the moderate range of 17 – 22 and 16.4% obtained a high ASI-3 score greater than 22. Among those participants who were admitted to the CRU and completed the TSST procedures, ASI-3 scores were somewhat lower with 80% scoring in the low range and no subjects having a score > 22.
A total of 56 participants completed the PSS as part of their initial assessment. Using age- and gender-dependent tertile cutoff values (Cohen and Janicki-Deverts, 2012) for these subjects, 44.6% were in the low tertile, 35.7% were in the middle tertile and 19.6% were in the high tertile in the distribution of scores. Among subjects completing CRU study procedures, 38.5% were in the low tertile, 38.5% in the middle tertile and 23% were in the high tertile. Scores were normally distributed, with a median score of 16.
Mean BAI, ASI-3 and PSS scores did not differ between male and female participants nor as a function of race (all p > 0.10).
Relationships between recent alcohol consumption patterns and anxiety/stress measures
As shown in Table 2, there was a relationship between BAI scores and drinking frequency (OR = 1.01, P = 0.04), such that higher BAI scores were associated with increased drinking days/week on the TLFB interview. We did not observe a relationship between BAI scores and intensity of alcohol consumption as measured by binge drinking days or drinks per drinking day.
Table 2.
Generalized linear multiple regression models of the relationships between recent alcohol consumption patterns* and each anxiety/stress measure score for the Beck Anxiety Inventory (BAI), the Anxiety Sensitivity Index-3 (ASI-3), and the Perceived Stress Scale (PSS). Results are shown as odds ratio (OR) for drinking and binge drinking days and relative risk (RR) for number of drinks/drinking day. Sex is included as a covariate.
| BAI | ASI-3 | PSS | |
|---|---|---|---|
| Drinking days/week | 1.009 (0.999, 1.018) P = 0.052 | 0.992 (0.985, 0.999) P = 0.022 | 0.985 (0.977, 0.993) P <0.001 |
| Binge days/week | 1.000 (0.992, 1.008) NS | 0.985 (0.979, 0.992) P < 0.001 | 0.978 (0.971, 0.986) P <0.001 |
| Drinks/Drinking day | 1.002 (0.989, 1.015) NS | 0.999 (0.989, 1.010) NS | 0.998 (0.986, 1.010) NS |
Drinking data based on 90-day Timeline Followback
ASI-3 total scores were significantly associated with the number of drinking days (OR = 0.992, P = 0.022) and the number of binge drinking days (OR=0.985, P < 0.001) over the 90 days preceding study enrollment. Specifically, an increase in the ASI-3 total score was associated with lower odds of reporting any drinking and lower odds of reporting binge drinking on any given day within the 90 days prior to assessment. Higher anxiety sensitivity appears to be associated with a lower frequency of moderate and heavy alcohol consumption, although the cross-sectional nature of this analysis prevents us from making causal statements about these relationships. ASI-3 total scores were not related to the number of drinks per drinking day (Table 2).
Similarly, higher PSS scores also were significantly associated with lower odds of reporting any drinking days (OR= 0.985, P <0.001) and binge drinking days (0.978, P <0.001). PSS scores were not related to the number of drinks per drinking day (Table 2).
When each anxiety/stress measure was entered into a model that adjusted for the remaining instruments, there were several important changes in the overall pattern of outcomes for the BAI and the ASI-3 (Table 3). Specifically, BAI results changed in two important ways. First, increased BAI scores were now associated with lower odds of reporting any drinking days (OR=0.942, P <0.001) as well as lower drinks/drinking day (OR = 0.982, P=0.04). In contrast, increased ASI-3 scores were now associated with increased drinking frequency (OR = 1.009, P=0.011).
Table 3.
Multiple Linear Regression Analyses of the relationships between recent alcohol consumption patterns* and anxiety/stress measures, including the Beck Anxiety Inventory (BAI), the Anxiety Sensitivity Index-3 (ASI-3), and the Perceived Stress Scale (PSS). Analyses are adjusted for scores on the remaining anxiety/stress measures and sex. Results are shown as odds ratio (OR) for drinking and binge drinking days and relative risk (RR) for number of drinks/drinking day. Sex is included as a covariate.
| BAI | ASI-3 | PSS | |
|---|---|---|---|
| Drinking days/week | 0.942 (0.932, 0.952) <0.001 | 1.009 (1.002, 1.016) P=0.011 | 0.978 (0.969, 0.987) P <0.001 |
| Binge days/week | 1.002 (0.991, 1.012) NS | 0.974 (0.967, 0.981) P < 0.001 | 0.974 (0.966, 0.982) P <0.001 |
| Drinks/Drinking day | 0.982 (0.965, 0.999) 0.041 | 0.995 (0.984, 1.006) NS | 0.997 (0.983, 1.01) NS |
Drinking data based on 90-day Timeline Followback
Relationships between AUDIT total and subscale scores and anxiety/stress measures
As shown in Table 4, AUDIT total and subscale sores were primarily related to BAI scores. Specifically, as BAI scores increased, AUDIT total scores (β = 0.404, P=0.004), alcohol dependence subscales scores (β = 0.16, P=0.016), and alcohol-related problems subscale scores (β = 0.256, P=0.001) all increased. PSS scores were related only to alcohol consumption in a pattern similar to that observed for the TLFB measures. That is, as PSS score increased, alcohol consumption decreased (β = -0.086, P=0.018). There was no relationship between AUDIT scores and ASI-3 scores.
Table 4.
Multiple linear regression models* of the relationships between AUDIT total score and subscale scores and each anxiety/stress measure score, including the Beck Anxiety Inventory (BAI), the Anxiety Sensitivity Index-3 (ASI-3), and the Perceived Stress Scale (PSS). Results are the beta coefficient and p-value for each anxiety/stress measure score and each AUDIT measure analysis with sex included as a covariate.
| BAI | ASI-3 | PSS | |
|---|---|---|---|
| AUDIT Total Score | 0.404 (P = 0.004) | -0.09 (NS) | -0.084 (NS) |
| Alcohol consumption subscale | -0.017 (NS) | -0.022 (NS) | -0.082 (P = 0.013) |
| Alcohol dependence subscale | 0.16 (P = 0.016) | 0.016 (NS) | -0.037 (NS) |
| Alcohol-related problems subscale | 0.256 (P = 0.001) | -0.084 (NS) | 0.035 (NS) |
Sex was included in the models as a covariate.
When each anxiety/stress measure was entered into a model that adjusted for the remaining instruments, the overall pattern of outcomes was similar, except that two relationships were now observed between the ASI-3 and AUDIT scores (Table 5). There was a trend for an increase in ASI-3 scores to be associated with a decrease in total AUDIT score (β = -0.258, P=0.060) as well as a decrease in alcohol-related problem subscale scores (β = -0.196, P=0.013).
Table 5.
Multiple Linear Regression Analyses of the relationship between the AUDIT total score and subscale scores with anxiety/stress measures, including the Beck Anxiety Inventory (BAI), the Anxiety Sensitivity Index-3 (ASI-3), and the Perceived Stress Scale (PSS). Analyses are adjusted for scores on the remaining anxiety/stress measures and sex. Values are the beta coefficient and p-value.
| BAI | ASI-3 | PSS | |
|---|---|---|---|
| AUDIT Total Score | 0.592 (P=0.006) | -0.258 (P=0.060) | -0.069 (NS) |
| Alcohol consumption subscale | 0.058 (NS) | -0.011 (NS) | -0.086 (P=0.018) |
| Alcohol dependence subscale | 0.278 (P=0.005) | -0.051 (NS) | -0.064 (NS) |
| Alcohol-related problems subscale | 0.256 (P=0.034) | -0.196 (P=0.013) | 0.081 (NS) |
Sex was included in the models as a covariate.
Relationships between alcohol craving on the CRU and anxiety/stress measures
Three measures of alcohol urges and craving (VAS, AUQ and OCDS) were collected twice daily during the period of alcohol abstinence on days 1 – 4 of the CRU stay. BAI and ASI-3 scores were significantly related to mean and peak scores on all three measures (Table 6). In contrast, PSS scores were not related to VAS or AUQ average or peak scores, although there was a relationship with the OCDS average and peak. Across all three instruments, increased anxiety/stress scores were associated with greater alcohol craving during the early days of alcohol abstinence. Figure 2 illustrates the differences in the relationships to AUQ scores across the three anxiety/stress measures.
Table 6.
Multiple linear regression models* of the relationships between average and peak alcohol craving scores during inpatient abstinence on days 1 - 4 and each anxiety/stress measure score, including the Beck Anxiety Inventory (BAI), the Anxiety Sensitivity Index-3 (ASI-3), and the Perceived Stress Scale (PSS). Values are the beta coefficient and p-value. Sex is included as a covariate.
| BAI | ASI-3 | PSS | |
|---|---|---|---|
| VAS* average | 0.674 (P = 0.002) | 0.451 (P = 0.039) | 0.145 (P = NS) |
| VAS peak | 0.669 (P = 0.007) | 0.554 (P = 0.026) | 0.167 (P = NS) |
| AUQ** average | 0.816 (P = 0.004) | 0.616 (P = 0.021) | 0.127 (P = NS) |
| AUQ peak | 0.797 (P = 0.017) | 0.858 (P = 0.008) | 0.132 (P = NS) |
| OCDS*** average | 0.627 (P = 0.004) | 0.605 (P = 0.011) | 0.545 (P = 0.002) |
| OCDS peak | 0.629 (P = 0.005) | 0.645 (P = 0.009) | 0.581 (P = 0.002) |
Visual Analog Scale of alcohol craving (VAS)
Alcohol Urges Questionnaire (AUQ)
Obsessive Compulsive Drinking Scale (OCDS)
Figure 2.
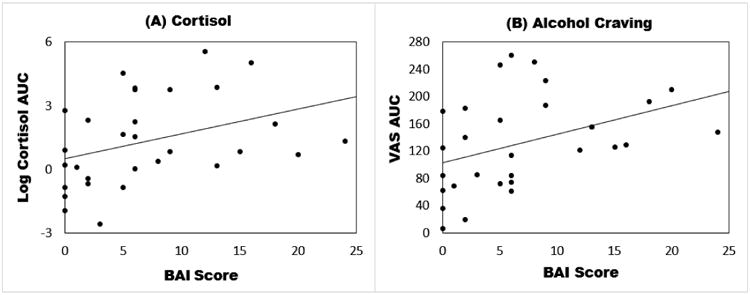
Scatter plots of the associations of Beck Anxiety Inventory Scores (BAI) against stress-induced cortisol (Panel A) and alcohol craving (Panel B) measured during the alcohol-motivated response period following the Trier Social Stress Test. Data points represent individual participants. Due to the high skewness, cortisol values were first log-transformed. The area under the curve (AUC) subtracting baseline of the transformed cortisol values is shown. Similarly the AUC of visual analog craving scores (VAS) was calculated with baseline subtracted and is shown in the plot. No transformation was performed on VAS AUC.
Relationships between laboratory stress-induced cortisol and anxiety/stress measure scores
There was a significant increase in peak cortisol following the TSST relative to the neutral session (p < 0.001). As shown in Table 7 and Figure 2, in analyses adjusted for sex and session order, BAI scores were positively associated with TSST-induced cortisol measured as area-under-the-curve (β=0.123, P = 0.058) and alcohol craving measured as area-under-the-curve on a visual analog scale (β=4.617, P = 0.022). Specifically, higher scores on the BAI were related to greater stress-induced cortisol levels and alcohol craving.
Table 7.
Multiple linear regression models of the relationships between stress-induced cortisol and alcohol craving measured during the alcohol-motivated response period following the Trier Social Stress Test and each anxiety/stress measure score, including the Beck Anxiety Inventory (BAI), the Anxiety Sensitivity Index-3 (ASI-3), and the Perceived Stress Scale (PSS) adjusted for sex and stressed versus neutral session order. Values are the beta coefficient and p-value.
| BAI | ASI-3 | PSS | |
|---|---|---|---|
| Cortisol AUC* | 0.123 (P = 0.058) | 0.101 (P = NS) | 0.0000 (P = NS) |
| VAS AUC | 4.617 (P = 0.022) | 4.169 (P = 0.061) | 1.431 (P = NS) |
AUC=area under the curve
There was a trend for ASI-3 scores to be associated with greater stress-induced alcohol craving (β=4.169, P = 0.061), but not cortisol levels. PSS scores were not related to either laboratory stress measure.
Discussion
Overall, clinically-oriented measures of anxiety compared with perceived stress were more strongly associated with a variety of alcohol-related measures in current heavy drinkers with AUD. We tested the correlations between the outcomes and anxiety/stress measures in both reduced models (only one anxiety stress measure and sex as independent variables) and full models (including all anxiety/stress measures and sex as covariates). In reduced models, higher BAI scores were associated with higher drinking frequency whereas higher ASI-3 and PSS scores were protective against the frequency of drinking episodes. Interestingly, when ASI-3 and PSS scores were included as covariates in the full model, higher BAI scores were associated with lower drinking frequency and reduced drinks/drinking day. The results for the ASI-3 also were reversed, with higher ASI-3 scores now positively associated with higher drinking frequency. In reduced models, BAI anxiety symptom scores also were positively related to AUDIT total scores, alcohol dependence subscale scores and alcohol-related problems; there was little relationship between the ASI-3 and PSS and these more AUD symptom-related measures. Multivariate analyses adjusting for the other anxiety/stress measures strengthened the positive associations of the BAI and negative associations of the ASI-3 to the AUDIT total and subscale scores. Additionally, higher BAI and ASI-3 scores but not PSS scores were related to greater self-reported alcohol craving and urges to drink on three different instruments during the early days of alcohol abstinence. Finally, BAI scores were positively related to laboratory stress-induced cortisol and alcohol craving, with a trend for a positive relationship between ASI-3 scores and alcohol craving. In contrast, the PSS, which measures recent physical manifestations of stress and stress coping, showed no relationship with most measures of alcohol craving reported during the CRU abstinence period or with these laboratory, stress-induced measures of cortisol and alcohol craving.
All three anxiety/stress measures were related to drinking frequency during the 90 days prior to study assessment. In contrast, none of our measures was related to the amount of alcohol consumed on a drinking day. Throughout the alcohol treatment literature, findings have suggested that drinking frequency as compared with daily drinking quantity is more readily modified by external influences such as alcohol interventions. Indeed, in our treatment study of HIV-infected, heavy drinking women, a brief alcohol intervention was effective in reducing frequency of drinking and binge drinking but was not effective in reducing the number of drinks on a drinking day (Chander et al., 2015). We speculate that drinking intensity may be biologically driven by one's alcohol tolerance, with the drinker targeting a “set-point” or a specific intoxication level. This set point may not be as easily influenced by stress or anxiety. Thus, interventions that target reductions in stress and anxiety may be expected to have a greater impact on drinking frequency than on drinking intensity.
Prior research has shown greater alcohol stress response dampening following a stressor in individuals with high compared with low anxiety sensitivity (Conrod et al., 1998; MacDonald et al., 2000; Stewart and Pihl, 1994). Our findings corroborate these earlier studies showing that the current level of clinical anxiety symptoms and the underlying trait of anxiety sensitivity were more robust predictors of alcohol craving than the more state-based measure of perceived stress. Our limited sample size prevented a direct test of the interaction of anxiety symptom severity and anxiety sensitivity on alcohol craving during alcohol withdrawal on the CRU. Nonetheless, our finding that ASI-3 scores were positively associated with higher craving during early alcohol withdrawal is consistent with the idea that high anxiety sensitivity people may be particularly intolerant of early withdrawal symptoms and strongly crave alcohol as a consequence (Stewart and Kushner, 2001). In the preclinical and much of the human literature, it has proven challenging to demonstrate a direct relationship between stress and drinking in the absence of chronic alcohol exposure (Lopez et al., 2016). It is plausible that, in individuals with AUD, stress alone in the absence of elevated anxiety symptoms does not drive alcohol craving or drinking. Rather underlying anxiety symptoms must also be present to amplify stress effects on drinking. Our study introduced two types of stressors for our AUD participants – first, the stress of alcohol withdrawal and second the social stressor of the TSST. In each of these procedures, there was a strong positive correlation between baseline anxiety symptom severity or anxiety sensitivity and subsequent alcohol craving scores. Interestingly, of the three craving measures that were assessed during CRU alcohol withdrawal, only the Obsessive-Compulsive Drinking Scale (Anton et al., 1995) was related to all three anxiety and stress measures. The OCDS was developed from the Yale-Brown Obsessive-Compulsive Scale (Goodman et al., 1989) and thus retains some of the anxiety-related elements of its parent instrument. Importantly, we identified these relationships in AUD individuals who did not meet diagnostic criteria for an anxiety disorder, thus demonstrating that even subdiagnostic anxiety can compel stress-induced alcohol craving. In the real world with a more diverse population of AUDs that includes persons with comorbid anxiety, these relationships may be even more robust. It will be of interest in future research to determine if these relationships persist in more severely anxious individuals or whether they dissipate at the extremes of the clinical anxiety spectrum.
Interestingly, the relationships of the BAI and the ASI-3 to measures of recent alcohol consumption observed in bivariate analyses (adjusted for sex) were reversed in multivariate analyses controlling for scores on the other instruments. For example, higher BAI scores were associated with higher drinking frequency in bivariate analyses whereas higher BAI scores were related to lower drinking frequency when controlling for anxiety sensitivity and perceived stress scores. In contrast, higher ASI-3 scores were associated with lower drinking frequency in bivariate analyses but with higher drinking frequency when controlling for the BAI and PSS scores. Importantly, these findings underscore the importance of both anxiety symptom severity and sensitivity on drinking behaviors but also suggests there may be important interactions between the two constructs that may modify their independent associations with drinking. These findings lend support to theories that posit that drinking is most highly motivated by anxiety reduction among anxious individuals who most fear experiencing anxiety symptoms (Stewart and Kushner, 2001). This highlights the need to include measures of anxiety sensitivity in future research examining the effects of anxiety/stress on drinking behaviors and may help to resolve the conflicting literature regarding anxiety/stress and alcohol consumption and problems.
Cortisol is an important biomarker for the intensity of the stress response (Stephens et al., 2016). Cortisol also is thought to increase dopamine production in the mesolimbic system and increase CRF expression within the amygdala. Drugs that block the glucocorticoid receptor are now under investigation for the treatment of AUD (Vendruscolo et al., 2015). Consistent with these findings, the BAI, which we showed to be correlated with craving, is also positively correlated with cortisol release during the social stress test in the laboratory. Our findings suggest that the current level of clinical anxiety symptoms is a robust predictor of stress responsivity and alcohol craving. In future research, it could be of interest to expand the assessment measures to include other state/trait factors such as negative affectivity. It also will be important to examine different types of stressors (e.g., social versus performance) to more fully understand these relationships.
This study has limitations. Participants were media recruited heavy drinkers who were not alcohol treatment seekers; they may have lower severity AUD and may not be representative of a clinically identified population. We also excluded individuals with current psychiatric disorders, potentially restricting the variability in the stress and anxiety measures that we studied and limiting their sensitivity as predictors of alcohol consumption, craving and symptoms. However, it is noteworthy that relationships emerged with alcohol craving and drinking, despite our nonclinical subject sample. Second, measures of recent drinking were based on self-report and therefore subject to recall bias. We attempt to reduce the impact of this limitation through several strategies: 1) use of the highly structured Timeline Followback Method that prompts more accurate recall through construction of a personalized 90-day calendar; 2) regular 6-month certification of all staff on the TLFB to promote accuracy and consistency among staff; 3) use of PEth to validate self-reported alcohol consumption. Third, we used a laboratory-based stress induction procedure, the Trier Social Stress Test, rather than a personalized, naturalistic procedure. The extensive literature on the TSST and its demonstrated ability to elevate cortisol levels in most subjects validates its use (Frisch et al., 2015). Finally, a larger sample size would have enabled a more comprehensive look at sex differences, although sex is included as a co-variate in our analyses. Findings by Zack and colleagues (2007) reported sex differences in the relationship between alcohol stress response dampening and anxiety sensitivity when the social stressor was the requirement to deliver a self-revealing speech. Specifically, male college students with high anxiety sensitivity had a significant alcohol priming effect on desire to drink, whereas no priming effect was observed in low AS males or high or low AS females. These results highlight the importance of examining sex effects in future research.
Anxiety, anxiety sensitivity and stress are often used interchangeably in the alcohol literature. These findings highlight the importance of maintaining the distinctions defined in the mental health field as we showed differential relationships across the three measures on alcohol consumption, craving and stress reactivity among persons with AUD. It will be of interest in future research to explore these relationships in persons without current AUD to further clarify the etiologic role of anxiety, anxiety sensitivity and stress in the development of AUD.
Figure 1.
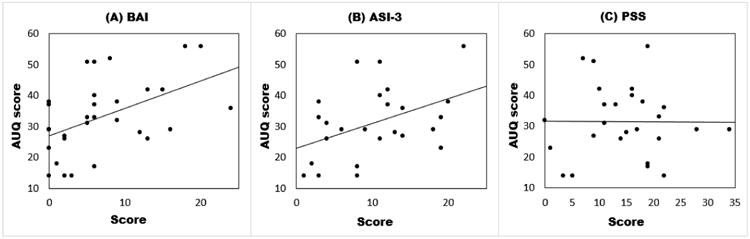
Scatter plots of the Alcohol Urges Questionnaire (AUQ) with the Beck Anxiety Inventory (Panel A, BAI), Anxiety Sensitivity Index-3 (Panel B, ASI-3), and Perceived Stress Scale (Panel C, PSS) during early alcohol abstinence on the Clinical Research Unit. Data points represent individual participants.
Acknowledgments
This research was supported by NIH grants U01-AA020890 (Co-PIs: Wand and McCaul), The Kenneth Lattman Foundation and K05AA020342 (PI: Wand). This publication also was made possible by the Johns Hopkins Institute for Clinical and Translational Research (ICTR) which is funded in part by Grant Number UL1 TR 001079 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, NCATS or NIH.
Footnotes
Authors have no conflicts of interest to report.
References
- Allan NP, Raines AM, Capron DW, Norr AM, Zvolensky MJ, Schmidt NB. Identification of anxiety sensitivity classes and clinical cut-scores in a sample of adult smokers: Results from a factor mixture model. J Anxiety Disord. 2014;28:696–703. doi: 10.1016/j.janxdis.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®) American Psychiatric Pub; 2013. [Google Scholar]
- Anton RF, Moak DH, Latham P. The Obsessive Compulsive Drinking Scale: A self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcoholism: Clinical and Experimental Research. 1995;19:92–99. doi: 10.1111/j.1530-0277.1995.tb01475.x. [DOI] [PubMed] [Google Scholar]
- Babor TF, Higgins-Biddle JC, Saunders JB, Monteiro MG. The Alcohol Use Disorders Identification Test (AUDIT): Guidelines for use in primary care. Department of Mental Health and Substance Dependence, World Health Organization; 2001. [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol. 1988;56:893. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: A review of animal studies. Psychopharmacology (Berl) 2011;218:131–156. doi: 10.1007/s00213-011-2443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn MJ, Krahn DD, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcoholism: Clinical and Experimental Research. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Brady KT, Lydiard RB. The association of alcoholism and anxiety. Psychiatr Q. 1993;64:135–149. doi: 10.1007/BF01065866. [DOI] [PubMed] [Google Scholar]
- Breese GR, Sinha R, Heilig M. Chronic alcohol neuroadaptation and stress contribute to susceptibility for alcohol craving and relapse. Pharmacol Ther. 2011;129:149–171. doi: 10.1016/j.pharmthera.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander G, Hutton HE, Lau B, Xu X, McCaul ME. Brief intervention decreases drinking frequency in HIV-infected, heavy drinking women: Results of a randomized controlled trial. J Acquir Immune Defic Syndr. 2015;70:137–145. doi: 10.1097/QAI.0000000000000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D. Who's stressed? Distributions of psychological stress in the United States in probability samples from 1983, 2006, and 2009. J Appl Soc Psychol. 2012;42:1320–1334. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- Conrod PJ, Pihl RO, Vassileva J. Differential sensitivity to alcohol reinforcement in groups of men at risk for distinct alcoholism subtypes. Alcoholism: Clinical and Experimental Research. 1998;22:585–597. doi: 10.1111/j.1530-0277.1998.tb04297.x. [DOI] [PubMed] [Google Scholar]
- Crum RM, Muntaner C, Eaton WW, Anthony JC. Occupational stress and the risk of alcohol abuse and dependence. Alcoholism: Clinical and Experimental Research. 1995;19:647–655. doi: 10.1111/j.1530-0277.1995.tb01562.x. [DOI] [PubMed] [Google Scholar]
- DeMartini KS, Carey KB. The role of anxiety sensitivity and drinking motives in predicting alcohol use: A critical review. Clin Psychol Rev. 2011;31:169–177. doi: 10.1016/j.cpr.2010.10.001. [DOI] [PubMed] [Google Scholar]
- Donovan D, Marlatt G. Assessment of expectancies and behaviors associated with alcohol consumption. A cognitive--behavioral approach. J Stud Alcohol. 1980;41:1153–1185. doi: 10.15288/jsa.1980.41.1153. [DOI] [PubMed] [Google Scholar]
- Frisch JU, Hausser JA, Mojzisch A. The Trier Social Stress Test as a paradigm to study how people respond to threat in social interactions. Front Psychol. 2015;6:14. doi: 10.3389/fpsyg.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW. Why we like to drink: A functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci. 2008;28:4583–4591. doi: 10.1523/JNEUROSCI.0086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. The Yale-Brown obsessive compulsive scale: I. Development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Haass-Koffler CL, Leggio L, Kenna GA. Pharmacological approaches to reducing craving in patients with alcohol use disorders. CNS Drugs. 2014;28:343–360. doi: 10.1007/s40263-014-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Grant BF. The national epidemiologic survey on alcohol and related conditions (NESARC) waves 1 and 2: Review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50:1609–1640. doi: 10.1007/s00127-015-1088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: Are they linked? Addict Biol. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Keyes KM, Hatzenbuehler ML, Hasin DS. Stressful life experiences, alcohol consumption, and alcohol use disorders: The epidemiologic evidence for four main types of stressors. Psychopharmacology (Berl) 2011;218:1–17. doi: 10.1007/s00213-011-2236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ. The self-medication hypothesis of addictive disorders: Focus on heroin and cocaine dependence. Am J Psychiatry. 1985;142:1259–1264. doi: 10.1176/ajp.142.11.1259. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’: A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Anderson RI, Becker HC. Effect of different stressors on voluntary ethanol intake in ethanol-dependent and nondependent C57BL/6J mice. Alcohol. 2016;51:17–23. doi: 10.1016/j.alcohol.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AB, Baker JM, Stewart SH, Skinner M. Effects of alcohol on the response to hyperventilation of participants high and low in anxiety sensitivity. Alcoholism: Clinical and Experimental Research. 2000;24:1656–1665. [PubMed] [Google Scholar]
- McCreary DR, Sadava SW. Stress, drinking, and the adverse consequences of drinking in two samples of young adults. Psychology of Addictive Behaviors. 1998;12:247. [Google Scholar]
- McCreary DR, Sadava SW. Stress, alcohol use and alcohol-related problems: The influence of negative and positive affect in two cohorts of young adults. J Stud Alcohol. 2000;61:466–474. doi: 10.15288/jsa.2000.61.466. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Is anxiety sensitivity distinguishable from trait anxiety? Reply to Lilienfeld, Jacob, and Turner (1989) Journal of abnormal psychology. 1989;98:193–194. doi: 10.1037//0021-843x.98.2.193. [DOI] [PubMed] [Google Scholar]
- Morris EP, Stewart SH, Ham LS. The relationship between social anxiety disorder and alcohol use disorders: A critical review. Clin Psychol Rev. 2005;25:734–760. doi: 10.1016/j.cpr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Mueser KT, Drake RE, Wallach MA. Dual diagnosis: A review of etiological theories. Addict Behav. 1998;23:717–734. [PubMed] [Google Scholar]
- North CS, Ringwalt CL, Downs D, Derzon J, Galvin D. Post-disaster course of alcohol use disorders in systematically studied survivors of 10 disasters. Arch Gen Psychiatry. 2011;68:173–180. doi: 10.1001/archgenpsychiatry.2010.131. [DOI] [PubMed] [Google Scholar]
- O'Grady MA, Cullum J, Armeli S, Tennen H. Putting the relationship between social anxiety and alcohol use into context: A daily diary investigation of drinking in response to embarrassing events. J Soc Clin Psychol. 2011;30:599–615. doi: 10.1521/jscp.2011.30.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK. Comorbidity of mental disorders with alcohol and other drug abuse: Results from the Epidemiologic Catchment Area (ECA) study. JAMA. 1990;264:2511–2518. [PubMed] [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Ther. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Rifkin LS, Beard C, Hsu KJ, Garner L, Björgvinsson T. Psychometric properties of the anxiety sensitivity index-3 in an acute and heterogeneous treatment sample. J Anxiety Disord. 2015;36:99–102. doi: 10.1016/j.janxdis.2015.09.010. [DOI] [PubMed] [Google Scholar]
- San José B, Mheen Hvd, Oers Jv, Mackenbach J, Garretsen H. Adverse working conditions and alcohol use in men and women. Alcoholism: Clinical and Experimental Research. 2000;24:1207–1213. doi: 10.1111/j.1530-0277.2000.tb02085.x. [DOI] [PubMed] [Google Scholar]
- Sayette MA. Does drinking reduce stress? Alcohol Research and Health. 1999;23:250–255. [PMC free article] [PubMed] [Google Scholar]
- Schmidt NB, Eggleston AM, Woolaway-Bickel K, Fitzpatrick KK, Vasey MW, Richey JA. Anxiety Sensitivity Amelioration Training (ASAT): A longitudinal primary prevention program targeting cognitive vulnerability. J Anxiety Disord. 2007;21:302–319. doi: 10.1016/j.janxdis.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Schröck A, Thierauf A, Wurst FM, Thon N, Weinmann W. Progress in monitoring alcohol consumption and alcohol abuse by phosphatidylethanol. Bioanalysis. 2014;6:2285–2294. doi: 10.4155/bio.14.195. [DOI] [PubMed] [Google Scholar]
- Schry AR, White SW. Understanding the relationship between social anxiety and alcohol use in college students: A meta-analysis. Addict Behav. 2013;38:2690–2706. doi: 10.1016/j.addbeh.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Sheehan DV. MINI International Neuropsychiatric Interview 7.0.0 for DSM-5 2014 [Google Scholar]
- Sher KJ, Levenson RW. Risk for alcoholism and individual differences in the stress-response-dampening effect of alcohol. J Abnorm Psychol. 1982;91:350. doi: 10.1037//0021-843x.91.5.350. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Timeline Follow-back: A technique for assessing self-reported ethanol consumption. In: Litten RZ, Allen JP, editors. Measuring Alcohol Consumption: Psychosocial and Biological Methods. Humana Press; Totowa, NJ: 1992. pp. 41–72. [Google Scholar]
- Spanagel R, Noori HR, Heilig M. Stress and alcohol interactions: Animal studies and clinical significance. Trends Neurosci. 2014;37:219–227. doi: 10.1016/j.tins.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Stephens MAC, Mahon PB, McCaul ME, Wand GS. Hypothalamic–pituitary–adrenal axis response to acute psychosocial stress: Effects of biological sex and circulating sex hormones. Psychoneuroendocrinology. 2016;66:47–55. doi: 10.1016/j.psyneuen.2015.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SH, Karp J, Pihl RO, Peterson RA. Anxiety sensitivity and self-reported reasons for drug use. J Subst Abuse. 1997;9:223–240. doi: 10.1016/s0899-3289(97)90018-3. [DOI] [PubMed] [Google Scholar]
- Stewart SH, Kushner MG. Introduction to the special issue on “Anxiety Sensitivity and Addictive Behaviors”. Addict Behav. 2001;26:775–785. doi: 10.1016/s0306-4603(01)00236-2. [DOI] [PubMed] [Google Scholar]
- Stewart SH, Peterson JB, Pihl RO. Anxiety sensitivity and self-reported alcohol consumption rates in university women. J Anxiety Disord. 1995;9:283–292. [Google Scholar]
- Stewart SH, Pihl RO. Effects of alcohol administration on psychophysiological and subjective-emotional responses to aversive stimulation in anxiety-sensitive women. Psychology of Addictive Behaviors. 1994;8:29. [Google Scholar]
- Taylor S, Zvolensky MJ, Cox BJ, Deacon B, Heimberg RG, Ledley DR, Abramowitz JS, Holaway RM, Sandin B, Stewart SH. Robust dimensions of anxiety sensitivity: Development and initial validation of the Anxiety Sensitivity Index-3. Psychol Assess. 2007;19:176. doi: 10.1037/1040-3590.19.2.176. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Estey D, Goodell V, Macshane LG, Logrip ML, Schlosburg JE, McGinn MA, Zamora-Martinez ER, Belanoff JK, Hunt HJ. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest. 2015;125:3193–3197. doi: 10.1172/JCI79828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wand GS, Mangold D, El Deiry S, McCaul ME, Hoover D. Family history of alcoholism and hypothalamic opioidergic activity. Arch Gen Psychiatry. 1998;55:1114–1119. doi: 10.1001/archpsyc.55.12.1114. [DOI] [PubMed] [Google Scholar]
- Young R, Oei TPS, Knight RG. The tension reduction hypothesis revisited: An alcohol expectancy perspective. Br J Addict. 1990;85:31–40. doi: 10.1111/j.1360-0443.1990.tb00621.x. [DOI] [PubMed] [Google Scholar]
- Zack M, Poulos CX, Aramakis VB, Khamba BK, MacLeod CM. Effects of drink- stress sequence and gender on alcohol stress response dampening in high and low anxiety sensitive drinkers. Alcoholism: Clinical and Experimental Research. 2007;31:411–422. doi: 10.1111/j.1530-0277.2006.00322.x. [DOI] [PubMed] [Google Scholar]


