Abstract
Objectives
The aggrecanase ADAMTS-5 and the collagenase MMP-13 are constitutively secreted by chondrocytes in normal cartilage, but rapidly endocytosed via the cell surface endocytic receptor low-density lipoprotein receptor-related protein 1 (LRP1) and subsequently degraded. This endocytic system is impaired in osteoarthritic (OA) cartilage due to increased ectodomain ‘shedding’ of LRP1. The aim of this study is to identify the LRP1 sheddase(s) in human cartilage, and to test whether inhibition of LRP1 shedding prevents the cartilage degradation in OA.
Methods
Cell-associated LRP1 and soluble LRP1 (sLRP1) released from human cartilage explants and chondrocytes were measured by Western blot analysis. LRP1 sheddases were identified by protease inhibitor profiling and gene silencing with siRNAs. Specific monoclonal antibodies were used to selectively inhibit the sheddases. Degradation of aggrecan and collagen in human OA cartilage was measured by Western blot analysis using an aggrecan neo-epitope antibody and hydroxyproline assay, respectively.
Results
The shedding of LRP1 was increased in OA cartilage compared to normal tissue. Shed sLRP1 bound to ADAMTS-5 and MMP-13 and prevented their endocytosis without interfering with their proteolytic activities. Two membrane-bound metalloproteinases, ADAM17 and MMP-14, have been identified as the LRP1 sheddases in cartilage. Inhibition of their activities restored the endocytic capacity of chondrocytes and reduced degradation of aggrecan and collagen in OA cartilage.
Conclusions
The shedding of LRP1 is a key link to OA progression. Local inhibition of LRP1 sheddase activities of ADAM17 and MMP-14 is a unique way to reverse matrix degradation in OA cartilage and could be effective as a therapeutic approach.
Keywords: Endocytosis, sheddase, metalloproteinases, aggrecan, collagen
INTRODUCTION
Osteoarthritis (OA) is the most prevalent age-related joint disorder, but there is no disease modifying treatment available, except joint replacement surgery (1). The main cause of the disease is degradation of articular cartilage due to elevated activities of matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinase with thrombospondin motifs (ADAMTSs). While both ADAMTS-4 and ADAMTS-5 have been considered to participate in aggrecan degradation in human OA (2, 3), recent studies by Larkin et al. with neutralising monoclonal antibodies have shown that ADAMTS-5 is more effective than ADAMTS-4 in aggrecan degradation in human OA cartilage and non-human primates in vivo (4). Collagen fibrils are mainly degraded by collagenolytic MMPs, and MMP-13 is considered as the major collagenase in OA cartilage (5–7).
We have recently found that both ADAMTS-5 and MMP-13 are constitutively produced in healthy human cartilage, but they are rapidly taken up by the chondrocytes via the endocytic receptor, low-density lipoprotein (LDL) receptor-related protein 1 (LRP1) and degraded intracellularly (8–10). These findings suggest that they probably function for a very short finite period of time to maintain normal homeostatic turnover of extracellular matrix (ECM) components of the tissue. Other proteins that are endocytosed by LRP1 include ADAMTS-4 (11) and tissue inhibitor of metalloproteinases 3 (TIMP-3) (12, 13), indicating that LRP1 is a key modulator of cartilage matrix-degradation systems. This endocytic pathway is impaired in OA cartilage because of the reduction of protein levels of LRP1 in chondrocytes without any significant changes in the mRNA for LRP1, resulting in increased extracellular activity of ADAMTS-5 (8). We thus proposed that the loss of LRP1 in OA cartilage is due to proteolytic shedding of the receptor, and that this process shifts normal homeostatic conditions of cartilage to a more catabolic environment, leading to the development of OA.
The aim of this study was to identify the “sheddase” activities that cleave LRP1 and releases soluble form of LRP1 (sLRP1) in human cartilage, and to test whether inhibition of the sheddase(s) prevents the degradation of cartilage in OA.
METHODS
Reagents and antibodies
The sources of materials used were as follows: mouse monoclonal anti-LRP1 α-chain antibody (8G1), mouse monoclonal anti-LRP1 β-chain antibody (5A6) that recognizes the ectodomain, BC-3 mouse monoclonal antibody that recognizes the N-terminal 374ARGSV aggrecan core protein fragments generated by aggrecanase, rabbit polyclonal anti-ADAM10 antibody (ab1997), rabbit polyclonal anti-ADAM17 antibody (ab2051), and rabbit monoclonal anti-MMP-14 antibody (ab51074) from Abcam (Cambridge, U.K.); mouse monoclonal anti-FLAG M2 antibody, chondroitinase ABC, endo-β-galactosidase, bovine nasal septum type II collagen, E-64 and 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF) from Sigma (Dorset, U.K.); human recombinant IL-1α and TNFα from Peprotech (London, U.K.); rabbit polyclonal anti-tubulin antibody (#2148) from Cell Signaling (Danvers, MA); goat polyclonal anti-actin antibody (I-19) from Santa Cruz Biotechnology (Dallas, TX); human plasma IgG (1-001-A) from R&D Systems (Minneapolis, MN); solubilized and purified full-length human LRP1 from BioMac (Leipzig, Germany); and a hydroxamate-based MMP inhibitor CT-1746 (N1-[2-(S)-(3,3-dimethylbutanamidyl)]-N4-hydroxy-2-(R)-[3-(4-chlorophenyl)-propyl]-succinamide) from UCB Celltech (Slough, U.K.). Anti-human ADAMTS-5 catalytic domain rabbit polyclonal antibody was raised in rabbits and characterized (14). Monoclonal inhibitory antibodies against human ADAM17 (D1A12) (15), MMP-14 (E2C6) and desmin (negative control antibody) (16), and ADAMTS-5 (2D3) (9), bovine nasal aggrecan (17), receptor associated protein (RAP) (8), recombinant human ADAMTS-5 lacking C-terminus thrombospondin domain with a FLAG-tag at C-terminus (14), MMP-13 with a FLAG tag between the signal and propeptide (18), TIMP-1 (19), TIMP-2 (20), TIMP-3 (21), and N-terminal domain of human TIMP-3 (22) were prepared as described previously. All other reagents used were of the highest analytical grade available.
Human cartilage tissue preparation and isolation of chondrocytes
Cartilage from human femoral condyles of the knee joints was used. Healthy normal articular cartilage was obtained from patients following knee amputation due to soft tissue sarcoma and osteosarcoma with no involvement of the cartilage. Tissues were obtained from 9 patients (6 males, ages 9–57 years, mean 35.5 years; 3 females, ages 13–19 years, mean 15.7 years). Human OA articular cartilage was obtained from patients following total knee replacement surgery. Tissues were obtained from 16 patients (8 males, ages 51–86 years, mean 75.1 years; 8 females, ages 50–82 years, mean 68.1 years). Dissected cartilage (~18 mm3, ~20 mg wet volume/weight) was placed in one well of a round-bottom 96-well plate and allowed to rest for 24 h in 200 μl of Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum (FCS) before use. The medium was replaced and the cartilage was rested for a further 24–96 h in 200 μl of DMEM at 37 °C before assays. Chondrocytes were isolated as described previously (12). Primary chondrocytes were used in the experiments to compare normal and OA chondrocytes and passaged cells were used in the experiments to identify the LRP1 sheddase.
Western blot analysis of LRP1 in cartilage
To analyze LRP1 in the cartilage, medium was removed after incubation for various periods of time and then total protein was extracted by adding 50 μl of 4× SDS-sampling buffer (200 mM Tris-HCl (pH 6.8)/8% SDS and 20% glycerol) to each explant in a 96-well plate. After 1 h incubation, the sample buffers were pooled from each condition (3 explants/condition) and 10 μl of samples were analyzed by SDS-PAGE under non-reducing conditions and Western blotting using anti-LRP1 α-chain, anti-LRP1 β-chain and anti-tubulin antibodies, respectively. Immune signals of LRP1 and tubulin were quantified using ImageJ, and the relative amounts of LRP1 α-and β-chains in the cartilage extracts were estimated using tubulin as an internal control.
Immunofluorescence staining of LRP1
OA and normal cartilage (n=3 each) was rested in culture with DMEM for 2 days. Explants were then snap-frozen and sectioned (5 μm sections) using a CM1900 cryostat (Leica Microsystems, Wetzlar, Germany). Each sample was fixed with methanol and incubated with anti-LRP1 β-chain antibody (3 h, room temperature). Alexa Fluor 568-conjugated anti-mouse IgG (Molecular Probes, Eugene, OR) were used to visualize the antigen signals (1 h, room temperature). Nuclei were stained with DAPI. Samples were viewed using a Nikon Eclipse TE2000-U confocal laser scanning microscope (Nikon, Tokyo, Japan). The data were collated using Volocity software (Improvision, Coventry, U.K.).
Quantitative reverse transcriptase-PCR (qPCR)
qPCR was carried out as described previously (8). Briefly, RNA was extracted and isolated using the RNeasy kit (Qiagen, Valencia, CA) from 50 mg of ground cartilage tissue and cDNA was then generated using a reverse-transcription kit (Applied Biosystems, Foster City, CA) following the manufacturer’s guidelines. cDNA was then used for real time PCR assays using TaqMan technology. The ΔΔ threshold cycle (ΔΔCt) method of relative quantitation was used to calculate relative mRNA levels for each transcript examined. The 60S acidic ribosomal protein P0 (RPLP0) gene was used to normalize the data. Predeveloped primer/probe sets for LRP1, ADAM12 and RPLP0 were purchased from Applied Biosystems.
Western blot analysis of cellular LRP1 and sLRP1
Chondrocytes (5 × 104) were cultured in 12-well plates in 2 ml of DMEM containing 10% FCS for 2 days. Cells were rested in 1 ml of DMEM for 24 h, the medium was replaced with 1 ml of DMEM and used in the experiments. After incubation of various period of time, the medium was collected, concentrated 20-fold using spin filters (Microcon YM-30, Merck Millipore, Darmstadt, Germany) and 20 μl of 4× SDS-sampling buffer was added to 50 μl of each concentrated medium. The cells were lysed with 200 μl of 2× SDS-sampling buffer and 10 μl of samples were analyzed by SDS-PAGE under non-reducing conditions and Western blotting using anti-LRP1 α-chain, anti-LRP1 β-chain and anti-tubulin antibodies, respectively. Immune signals of LRP1 and tubulin were quantified using ImageJ, and the relative amounts of LRP1 α-chain in the medium and LRP1 α-and β-chains in the cell lysate were estimated within the linear range of measurements (Figure S1, A and B) and normalized using tubulin and those in the standard cell lysates of chondrocytes as internal controls. An absolute number of LRP1 molecules released into medium were estimated in comparison of various concentrations of purified LRP1 within reasonable linear range.
Flow cytometry analysis of LRP1
Cells were plated in 6-well plates in DMEM containing 10% FCS and incubated until 80% confluent. Cells were rested in 2 ml of DMEM for 1 day and detached using a cell scraper and fixed for 5 minutes at 4°C with ice-cold methanol followed by 2 washes with FACS buffer (PBS containing 5% goat serum and 3% bovine serum albumin (BSA)). Cells were stained for 30 min at 25°C with anti-LRP1 β-chain antibody, washed with FACS buffer and then further incubated with allophycocyanin-conjugated goat anti-mouse IgG (BD Pharmingen) and isotype control in FACS buffer for 20 minutes at 25°C. Cells were then washed with FACS buffer and analyzed using a BD LSR II flow cytometer (BD Biosciences) and postacquisition data analysis was performed using FlowJo software version 7.6.1 (Tree Star).
Analysis of endocytosis of ADAMTS-5 and MMP-13
Cells (5 × 104) cultured in 24-well plates were rested in 500 μl of DMEM for 1 day. The medium was replaced with 500 μl of fresh DMEM with 10 nM of ADAMTS-5 or MMP-13 in the absence or presence of 2 nM or 10 nM sLRP1, or 500 nM RAP at 37 °C. After incubation for 0–4 h, media were collected and the protein was precipitated with 5% trichloroacetic acid and dissolved in 50 μl of 1× SDS-sampling buffer containing 5% 2-mercaptoethanol. All samples were analysed by SDS-PAGE under reducing conditions and Western blotting using anti-FLAG M2 antibody or anti-ADAMTS-5 antibody, respectively. Immune signals for exogenously added ADAMTS-5 and MMP-13 detected in the medium were quantified using ImageJ within the linear range of the measurements (Figure S1, C and D) and the amount of each recombinant protein remaining in the medium at each time point was calculated as a percentage of the amount of each recombinant protein at 0 h.
Analysis of aggrecanolytic activity of ADAMTS-5
Purified ADAMTS-5 (5 nM) was preincubated with 0–25 nM purified soluble LRP1 in TNCB buffer (50 mM Tris-HCl (pH 7.5)/150 mM NaCl/10 mM CaCl2/0.01% BSA) containing 0.01% Brij-35 for 10 min at 25 °C. The mixture was diluted 100-fold and incubated with 0.5 mg/ml purified bovine aggrecan for 0–4 h at 37 °C. The samples were deglycosylated as described previously (23). Briefly, aggrecan was deglycosylated in sodium acetate buffer with chondroitinase ABC and endo-β-galactosidase (each 0.01 unit/100 μg of aggrecan) for 24 h at 37 °C. Aggrecan was then precipitated using ice-cold acetone and analyzed by Western blotting using the antibody BC-3, which recognizes the N-terminal 374ARGS aggrecan core protein fragments generated by aggrecanase.
siRNA-mediated knockdown of membrane-bound metalloproteinases
siRNA oligonucleotides for ADAMs and MMP-14 (On-TargetPlus SMARTpool siRNA) and nontargeting oligonucleotide were purchased from Thermo Scientific Dharmacon (Lafayette, CO). Cells were plated at a density of 4 × 104 cells/well (12-well plate) in DMEM containing 10% FCS and incubated until 50% confluent. INTERFERin (peqlab, Erlangen, Germany) was used to transfect cells with siRNA at a final concentration of 20 nM in Opti MEM I. After 48-h incubation, the medium was replaced with fresh DMEM with or without 10 ng/ml IL-1 or 200 ng/ml TNFα and further incubated for 24 h. Cells were lysed with 200 μl of 2× SDS-sampling buffer containing 5% 2-mercaptoethanol and then the samples were analyzed by SDS-PAGE under reducing conditions and Western blotting using anti-ADAM10, anti-ADAM17, anti-MMP-14 and anti-actin antibodies, respectively. Immune signals of each enzyme and actin were quantified using ImageJ, and the relative amounts of each enzyme were estimated using actin as an internal control.
Analysis of the effect of inhibitory antibodies against ADAM17 and MMP-14 on LRP1 protein levels
Normal chondrocytes (5 × 104) cultured in 24-well plates were rested in 500 μl of DMEM for 1 day. The medium was replaced with 500 μl of fresh DMEM without or with 10 ng/ml IL-1 in the absence or presence of various concentrations of combinations of the control antibodies (human IgG for the anti-ADAM17 antibody (D1A12) and anti-Desmin intracellular domain antibody for the anti-MMP-14 antibody (E2C6)) or the anti-ADAM17 and the anti-MMP-14 antibodies. After 24 h incubation, the cells were lysed with 100 μl of 2× SDS-sampling buffer and then LRP1 α- and β-chains were detected as described above. To test the effect of inhibitory antibodies against ADAM17 and MMP-14 on LRP1 levels in OA chondrocytes and cartilage in culture, cells and cartilage explants were cultured with DMEM in the absence or presence of combinations of 250 nM each of the control antibodies, the anti-ADAM17 antibody and the anti-Desmin antibody, the anti-MMP-14 antibody and IgG or the anti-ADAM17 and the anti-MMP-14 antibodies for 24 h. LRP1 α- and β-chains were then detected as described above.
Analysis of aggrecan degradation in OA cartilage
OA cartilage was cultured in a round-bottom 96-well plate (one explant/well) and rested in DMEM for 1 day. The cartilage was further cultured in DMEM in the absence or presence of a combination of two antibodies (250 nM each) [i.e., combination of the anti-ADAM17 antibody and the anti-Desmin antibody (control), of the anti-MMP-14 antibody and IgG (control), of the anti-ADAM17 and the anti-MMP-14 antibodies, of the anti-Desmin antibody and IgG], or 250 nM of the anti-ADAMTS-5, or N-TIMP-3. After 12 h incubation, the medium was replaced with fresh DMEM containing the antibodies or TIMP and further incubated for 0–48 h. For Western blotting, the conditioned media were pooled from each condition (3 explants/condition) and deglycosylated as described above and immunoreactivity was measured within the linear range of the assay based on standard samples (Figure S1, E).
Analysis of collagen degradation in OA cartilage
OA cartilage was cultured with various antibodies or 250 nM of TIMP-1 as describe above for aggrecan degradation studies, and the media were harvested after 96 h. The extent of type II collagen degradation in OA cartilage were assessed by measuring the amount of hydroxyproline released into the media using a modification of the assay described by Bergman and Loxley (24). The hydroxyproline contents in cartilage explant remnants after culture were also determined by digesing them in 500 μl of papain digest solution (0.05 M phosphate buffet (pH 6.5)/2 mM N-acetyl cysteine/2 mM EDTA/10 μg/ml papain) at 65 °C for 24 h. The relative amount of collagen degradation was estimated by dividing the amount of hydroxyproline released into the medium by the summed amount of hydroxyproline in the medium and in the papain-digests.
Study approval
Normal human articular cartilage tissues were obtained from the Stanmore BioBank, Institute of Orthopaedics, Royal National Orthopaedic Hospital, Stanmore from patients following informed consent and approval by the Royal Veterinary College Ethics and Welfare Committee (Institutional approval URN 2012 0048H). Human OA cartilage tissues were obtained from the Oxford Musculoskeletal Biobank and were collected with informed donor consent in full compliance with national and institutional ethical requirements, the United Kingdom Human Tissue Act, and the Declaration of Helsinki (HTA Licence 12217 and Oxford REC C 09/H0606/11).
Statistics
All quantified data are represented as the mean ± SD where applicable. Significant differences between data sets were determined using a 2-tailed Student’s t test or one-way ANOVA followed by Dunnett’s multiple comparison test, where indicated.
RESULTS
Increased ectodomain shedding of LRP1 and reduced endocytic capacity in human OA cartilage
We first verified the loss of LRP1 protein in human OA cartilage. LRP1 consists of an extracellular 515-kDa α-chain and a 85-kDa β-chain that are processed from the precursor by furin. The α-chain contains the ligand-binding domains and β-chain has an extracellular, a transmembrane and a cytoplasmic domains. Western blotting analyses of α- and β-chains of cartilage extracts with antibodies that recognize the N-terminus of the α-chain and the extracellular domain of β-chain showed that both chains were reduced in OA cartilage by approx. 48% and 65%, respectively, compared with normal cartilage (Figure 1, A and B). The reduction of LRP1 was further confirmed by immunofluorescence staining of β-chain in the cartilage (Figure 1C). No significant change in mRNA level of LRP1 between normal and OA cartilage (Figure 1D) suggests that the loss of LRP1 in OA cartilage is due to proteolytic shedding of the receptor.
Figure 1. Increased ectodomain shedding of LRP1 and reduced endocytic capacity in human OA cartilage.
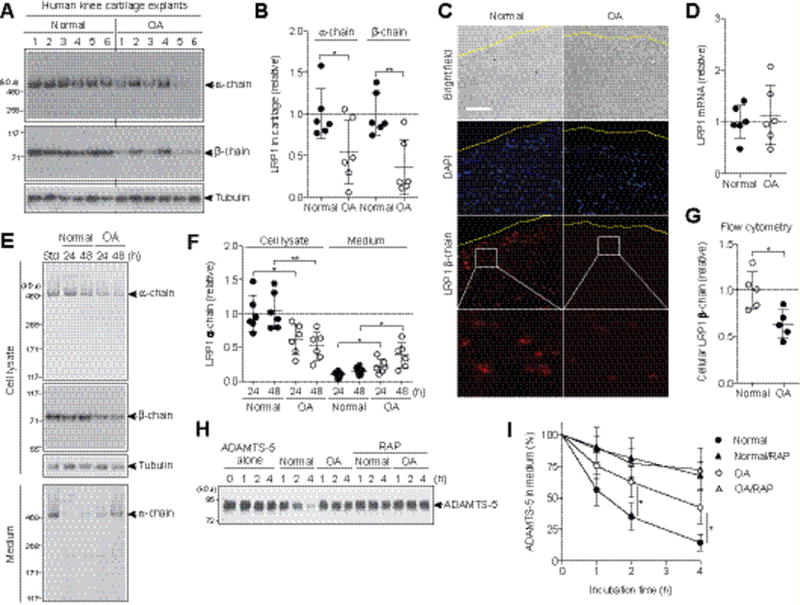
(A) The total proteins extracted from the cartilage explants of human knee joints of OA and non-arthritic patients (n=6 each) were subjected to Western blotting with antibodies against α- and β-chains of LRP1. (B) Densitometric analysis of LRP1 in A. Data were normalized against tubulin. (C) Immunoflourescence staining of LRP1 in frozen section of human knee cartilage. Dashed line, articular cartilage surface. Scale bar, 100 μm. (D) Relative LRP1 mRNA levels in cartilage measured by TaqMan qPCR. (E) Representative Western blotting of LRP1 protein in the cell lysates and medium of human normal and OA chondrocytes (n=6 each). std, standard cell lysates. (F) Quantification of LRP1 α-chain detected in E. (G) Flow cytometry analysis of LRP1 β-chain. Each point represents an individual cartilage donor. (H) Representative Western blotting for endocytosis of ADAMTS-5 (10 nM) by human normal and OA chondrocytes (n=3 each) without or with the LRP1 ligand antagonist RAP (500 nM). ADAMTS-5 in the medium was detected by Western blotting using anti-ADAMTS-5 antibody. (I) Quantified data of H. Data are expressed as the mean ± SD. *, p < 0.05, **, p < 0.01, by 2-tailed Student’s t test.
To further investigate the increased shedding of LRP1 in OA cartilage, chondrocytes were cultured and LRP1 proteins were analysed. Both α- and β-chains were reduced in OA cell lysates compared with normal chondrocytes, which was accompanied by an increased release of full-length α-chain into the medium (Figure 1, E and F). Flow cytometric analysis of the β-chain with anti-ectodomain antibody further confirmed the reduction of cell surface LRP1 including the β-chain in OA chondrocytes (Figure 1G), suggesting that the primary shedding site is located in the ectodomain of the β-chain. We estimated that a single normal chondrocyte released approx. 3.9 (±2.7) × 103 LRP1 molecules/hour, while a single OA chondrocyte released 9.6 (±5.4) × 103 LRP1 molecules/hour. As anticipated, the endocytic capacity of human OA chondrocytes was significantly reduced: The half-life of ADAMTS-5 was ~2.8-fold longer in OA (~210 min) than in normal chondrocytes (~75 min) (Figure 1, H and I).
Soluble LRP1 ectodomain (sLRP1) prevents endocytosis of ADAMTS-5 and MMP-13 without interfering their activities
We then evaluated whether sLRP1 alters half-lives of cartilage-degrading metalloproteinases. As shown in Figure 2 (A and B), endocytosis of ADAMTS-5 and MMP-13 was reduced partially with 2 nM sLRP1 and almost completely inhibited with 10 nM sLRP1 to the level that was attained with the LRP ligand antagonist, RAP. We also found that sLRP1-bound ADAMTS-5 and MMP-13 retained activity against their natural substrates, aggrecan and collagen, respectively (Figure 2, C and D). It is notable that ADAMTS-5 bound to sLRP1 was about ~3-times more active on aggrecan cleavage compared with free ADAMTS-5 (Figure 2C). Thus, shedding of the LRP1 ectodomain impairs the endocytic capacity of the cell by not only reducing the level of cell-surface LRP1 but also by converting membrane-anchored LRP1 into soluble decoy receptors, leaving excess matrix-degrading proteinases extracellularly.
Figure 2. Soluble LRP1 ectodomain (sLRP1) prevents endocytosis of ADAMTS-5 and MMP-13 without interfering their activities.
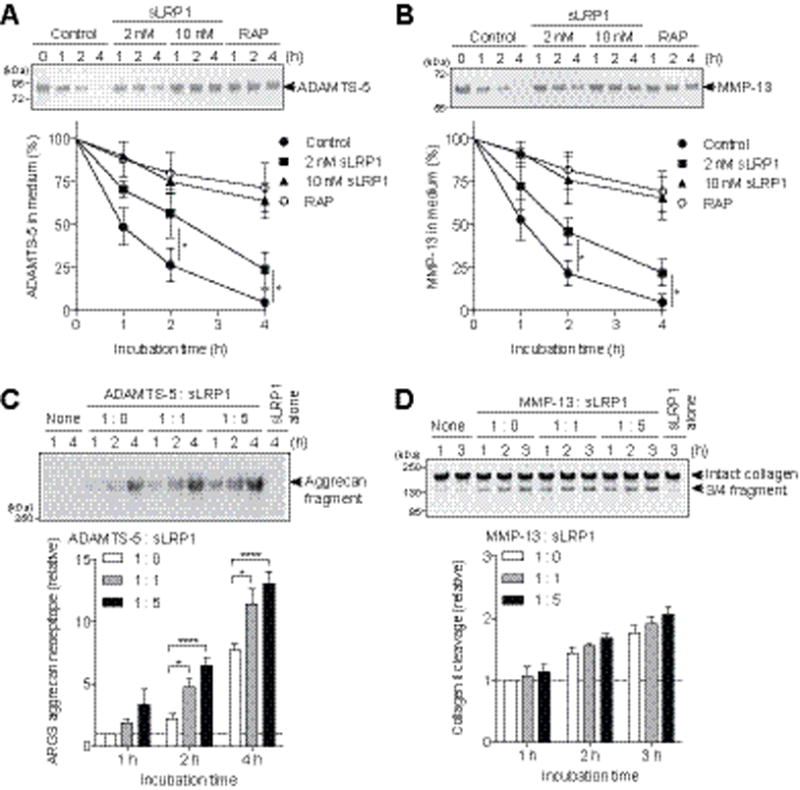
(A and B) Human normal chondrocytes (n=3) were cultured with DMEM containing 10 nM ADAMTS-5 (A) or 10 nM MMP-13 (B) in the presence of 0–10 nM sLRP1, or 500 nM RAP for 0–4 h. ADAMTS-5 and MMP-13 in the medium were detected by Western blotting using anti-FLAG M2 antibody. Upper panel, representative Western blotting. Lower panel, quantified data. (C) Bovine aggrecan (0.5 mg/ml) was incubated with 0.05 nM ADAMTS-5 in the presence of 0–0.25 nM sLRP1 for 0–4 h at 37 °C. The reactions were stopped by 10 mM EDTA and the reaction products were deglycosylated and subjected to Western blotting using anti-ARGS aggrecan neoepitope antibody. Upper panel, representative Western blotting. Lower panel, quantified data. (D) Collagen II (1 mg/ml) was incubated with 5 nM MMP-13 in the presence of 0–25 nM sLRP1 for 0–3 h at 25 °C. The reactions were stopped by 10 mM EDTA and the reaction products were analyzed by SDS-PAGE staining with Coomassie Brilliant Blue. Upper panel, representative SDS-PAGE. Lower panel, quantified data. Data were expressed as the mean ± SD. *, p < 0.05, ****, p < 0.0001, by 2-tailed Student’s t test (A and B) or one-way ANOVA followed by Dunnett’s multiple comparison test (C).
ADAM17 and MMP-14 are responsible LRP1 sheddases in human chondrocytes
To identify the LRP1 sheddase in human chondrocytes, we first examined whether pro-inflammatory cytokines such as interleukin 1 (IL-1) and tumour necrosis factor α (TNFα) that stimulate cartilage matrix degradation increase LRP1 shedding, as this might facilitate characterization of the sheddase in the cartilage. As shown in Figure 3 (A–C), these cytokines increased LRP1 shedding in normal human chondrocytes ~4.0-fold. The cytokine-stimulated LRP1 shedding was inhibited by the hydroxamate metalloproteinases inhibitor CT1746, but not by a serine protease inhibitor (AEBSF) or a cysteine protease inhibitor (E-64) (Figure 3D). Among the three TIMPs tested, TIMP-1 was not effective, but TIMP-2 and TIMP-3 were, and TIMP-3 showed the strongest inhibition (Figure 3E). Thus, we postulated that the responsible enzyme is likely to be a membrane-anchored ADAM or MMP. So far, ADAM10, ADAM12, ADAM17 and MMP-14 have been reported as LRP1 sheddases in other cell types (25). We therefore ablated each enzyme individually using a specific siRNA. The ADAM10 protein level was reduced by approx. 90% and the ADAM12 mRNA level by approx. 88% (Figure S2, A–C), but their knockdown did not affect LRP1 shedding (Figure S2D). siRNAs targeting ADAM17 and MMP-14 reduced their protein levels by 76% and 84%, respectively (Figure S2, E and F), and knockdown of each partially inhibited LRP1 shedding (Figure 3F). However, double knockdown of both MMP-14 and ADAM17 exhibited a stronger, additive effect, to the level achieved by TIMP-3 (Figure 3F), suggesting that these two proteinases function as LRP1 sheddases. A low level of LRP1 shedding occurs in non-stimulated chondrocytes and it is mainly due to MMP-14.
Figure 3. ADAM17 and MMP-14 are responsible LRP1 sheddases in human chondrocytes.
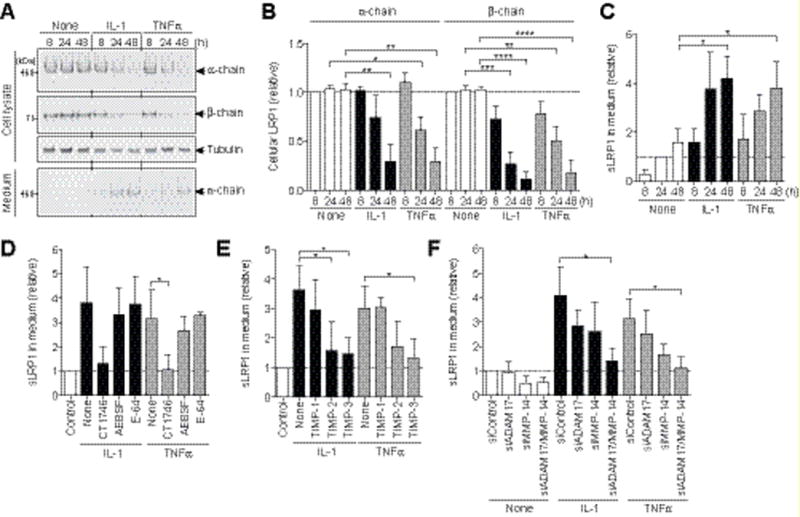
(A) Human normal chondrocytes (n=3) were cultured with DMEM in the presence of 10 ng/ml IL-1 or 200 ng/ml TNFα for 0–48 h and LRP1 proteins in the cell lysate and the medium were analysed as in Figure 1A. (B and C) Quantification of LRP1 protein in the cell lysate (B) and sLRP1 released into the medium (C). (D) Effect of metalloproteinase inhibitor CT1746 (20 μM), serine protease inhibitor AEBSF (50 μM) or cysteine protease inhibitor E-64 (10 μM) on cytokine-induced LRP1 shedding. (E) Effect of TIMP-1 (500 nM), TIMP-2 (500 nM) or TIMP-3 (300 nM) on cytokine-induced LRP1 shedding. (F) Effect of siRNA-mediated knockdown of ADAM17 or/and MMP-14 on cytokine-induced LRP1 shedding. Data are expressed as the mean ± SD. *, p < 0.05, **, p < 0.002, ***, p < 0.0002, ****, p < 0.0001, by one-way ANOVA followed by Dunnett’s multiple comparison test.
Combination of the inhibitory antibodies against ADAM17 and MMP-14 blocks LRP1 shedding in OA cartilage
To verify the role of ADAM17 and MMP-14 in LRP1 shedding, we used the recently developed specific antibodies against ADAM17 (antibody D1A12) (15) and against MMP-14 (antibody E2C6) (16), which inhibit the target enzymes with inhibition constants of 0.46 nM and 0.11 nM, respectively. The IL-1-induced loss of LRP1 was partially inhibited by a single antibody, but combination of the two antibodies blocked LRP1 shedding to the level of IL-1-untreated cells (Figure 4A). They were similarly effective at blocking LRP1 shedding in OA chondrocytes (Figure 4B). The anti-MMP-14 antibody increased cellular levels of the LRP1 α- and β-chains by 2.2- and 1.9-fold, respectively, whereas the anti-ADAM17 antibody increased them by 1.4- and 1.3-fold, respectively. Combination of the two antibodies increased both α- and β-chains by 2.3-and 2.6-fold, respectively (Figure 4B). The restoration of LRP1 in OA chondrocytes by combination of the two antibodies was time-dependent and it reached a plateau at 24 h (Figure 4C). Addition of the two antibodies to OA cartilage blocked LRP1 shedding and increased α- and β-chains by 2.6- and 2.3-fold, respectively (Figure 4D). This indicates that the antibodies can penetrate the tissue and inhibit the LRP1 sheddases in OA cartilage.
Figure 4. Combination of the inhibitory antibodies against ADAM17 and MMP-14 blocks LRP1 shedding in OA cartilage.
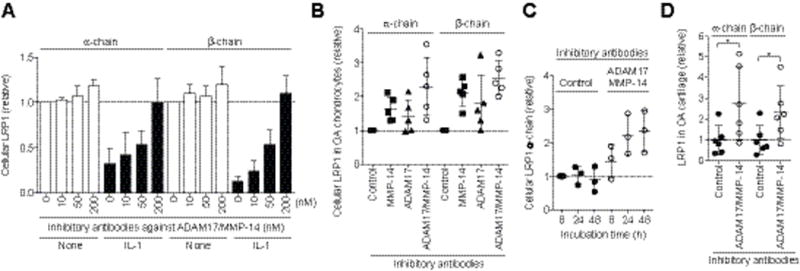
(A) Human normal chondrocytes (n=3) were cultured with DMEM containing 10 ng/ml IL-1 in the absence or presence of combination of the inhibitory antibodies against ADAM17 and MMP-14 (0–200 nM each) for 0–48 h and LRP1 proteins in the cell lysate were analysed as in Figure 1A. Data are expressed as the mean ±SD. (B) Effect of combination of anti-MMP-14 and anti-ADAM17 antibodies (200 nM each) on LRP1 shedding in human OA chondrocytes (n=5). (C) Time-course analysis of the effect of the combination of anti-MMP-14 and the anti-ADAM17 antibodies on the recovery of cellular LRP1. (D) Effect of combination of anti-MMP-14 and anti-ADAM17 antibodies on LRP1 shedding in human knee OA cartilage explants (n=6 each). Each point represents an individual donor (mean ±SD). *, p < 0.05, by 2-tailed Student’s t test.
Blocking of LRP1 sheddases restores endocytic capacity and reduces the degradation of aggrecan and collagen in OA cartilage
We then tested effect of combination of the two antibodies on the endocytic capacity of OA chondrocytes. The antibody-treated OA chondrocytes cleared exogenously added ADAMTS-5 from the medium about 2.4-fold faster (half-life: ~95 min) than the non-treated OA chondrocytes (half-life: ~210 min) (Figure 5A), indicating that blocking LRP1 sheddases restored the endocytic capacity of OA chondrocytes close to that of normal chondrocytes.
Figure 5. Blocking of LRP1 sheddases restores endocytic capacity and reduces the degradation of aggrecan and collagen in OA cartilage.
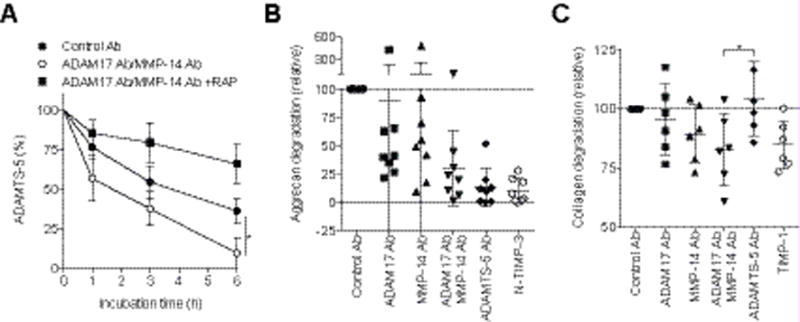
(A) Recovery of endocytic capacity in OA chondrocytes upon treatment with anti-ADAM17 and anti-MMP-14 antibodies. Endocytosis of ADAMTS-5 (10 nM) was measured as in Figure 1 (H and I). Data are expressed as the mean ± SD (n=3). (B and C) Inhibition of aggrecan and collagen degradation in human OA cartilage upon treatment with anti-ADAM17 and anti-MMP-14 antibodies, respectively. OA knee cartilage explants were incubated with antibodies or TIMPs at the concentration of 250 nM. (B) Aggrecan degradation detected after 48 h by an aggrecanase-cleavage specific anti-374ARGS neoepitope antibody. The mean value in medium of the cartilage incubated with the control antibodies was taken as 100 (n=8). (C) Collagen degradation of cultured OA cartilage after 96 h incubation was measured by hydroxyproline assay. The amount of hydroxyproline released with control antibodies was taken as 100. Each point represents an individual cartilage donor (mean ±SD). *, p < 0.05, by 2-tailed Student’s t test (A) or one-way ANOVA followed by Dunnett’s multiple comparison test (C).
Remarkably, the combined antibody treatment reduced the degradation of aggrecan and collagen in OA cartilage. Analysis of the same conditioned media for the aggrecanase specific cleavage motif using the anti-374ARGS neoepitope antibody indicated that the aggrecanase activity was markedly inhibited by blocking LRP1 sheddases (Figure 5B, Figure S3). Potent inhibition with the anti-ADAMTS-5 antibody and TIMP-3 indicates that the primary aggrecanase in OA cartilage is ADAMTS-5. Effective inhibition of aggrecan degradation by the combination treatment with anti-ADAM17 and anti-MMP-14 antibodies was further confirmed by Safranin O staining of the cartilage (Figure S4).
Collagen degradation was also inhibited in the presence of anti-ADAM17 and anti-MMP-14 antibodies by 5% and 10%, respectively, and more effective inhibition (17 %) was observed upon combination of the two antibodies (Figure 5C). In general, the MMP-14 antibody showed a stronger effect than the ADAM17 antibody, but there was considerable patient-to-patient variation in the effect of each antibody, which may reflect the multifactorial nature of OA. Inhibition was also detected with TIMP-1 treatment, but not with the anti-ADAMTS-5 antibody, indicating that collagen degradation is specific to collagenase. Cell viability analysis indicated that none of these treatments was toxic to chondrocytes (data not shown).
DISCUSSION
In this study, we have shown that the ectodomain shedding of LRP1 may be an important regulator of the development of human OA. The specific inhibitory antibodies that we recently developed for human MMP-14 and ADAM17 have allowed us to evaluate the role of LRP1 shedding in cartilage matrix degradation of human subjects and thus provided clinically relevant information.
Numerous membrane-anchored proteins are released from the cell surface by the process of regulated proteolysis called ectodomain shedding and the enzymes responsible for shedding are primarily membrane-anchored proteinases. This process regulates a wide variety of cellular and physiological functions and dysregulated shedding is linked to numerous diseases, such as Alzheimer’s disease, inflammation, rheumatoid arthritis (RA), cancer, chronic kidney disease, cardiac hypertrophy and heart failure (26, 27). LRP1 shedding is increased in inflammatory conditions such as in RA and systemic lupus erythematosus (28), and in cancer (29, 30), but the exact pathological role of LRP1 shedding in these diseases has not been clearly understood. We propose that LRP1 shedding in the local tissues under inflammatory or chronic pathological conditions dysregulates normal turnover of the ECM and cellular homeostasis leading to slowly progressing chronic diseases such as in OA.
LRP1 is widely expressed in different cell types and controls extracellular levels of numerous biologically active molecules to maintain tissue homeostasis (31). Currently, more than 50 ligands have been characterized including lipoproteins, ECM proteins, growth factors, cell surface receptors, proteinases, proteinase inhibitors and secreted intracellular proteins (31). In cartilage, LRP1 controls not only ECM-degrading proteinases, but also the WNT-β-catenin signalling pathway by interacting with frizzled-1 (32) and connective tissue growth factor (CCN2), and both regulate endochondral ossification and articular cartilage regeneration (33), emphasising the importance of LRP1 in skeletal development and the maintenance of cartilage homeostasis. Thus, the impairment of LRP1 function due to increased shedding of the receptor is detrimental to healthy cartilage as demonstrated here. This is triggered by increased activity of ADAM17 and MMP-14, but their protein levels were not significantly changed between healthy and OA cartilage (data not shown), suggesting that the activation of these enzymes is regulated post-translationally. The additive, but not synergistic effect of anti-ADAM17 and anti-MMP-14 antibodies further suggests that these proteinases may be activated by different mechanisms and act independently as LRP1 sheddases. In addition, MMP-14 and ADAM17 cleave a number of cell membrane proteins including growth factors, cytokines, cell-adhesion molecules and mechanosensors (26, 34). Therefore, their activation may also affects the integrity of other cell surface molecules in cartilage and affect cellular behaviour. We are currently investigating how ADAM17 and MMP-14 are activated and their substrate selectivity in cartilage as it may indicate additional molecular mechanisms for the development of OA, particularly in the early stages.
Another notable finding of this study is that anti-ADAM17 and anti-MMP14 antibodies reduced both aggrecanolytic and collagenolytic activities of human OA cartilage in culture and this effect was due to restoration of the lost LRP1 function by blocking their LRP1 sheddase activities (Figure 6). These results suggest that inhibition of elevated LRP1 sheddase activities in OA cartilage may be an effective way to prevent cartilage matrix degradation. Although the systematic inhibition of ADAM17 and MMP-14 as OA therapy may be problematic as these enzymes are biologically important in the release of growth factors and cell surface receptors in many cell types (27, 34), local administration of these antibodies or small molecule inhibitors of ADAM17 and MMP-14 may be worthy of investigating as disease-modifying OA drugs. This approach is an attractive option for OA therapy, as the reversal of the lost endocytic function of chondrocytes would help to maintain cartilage homeostasis. We are currently testing whether this approach is beneficial at early and advanced OA using pre-clinical animal models.
Figure 6. LRP1-mediated endocytic pathways in normal and OA cartilage.
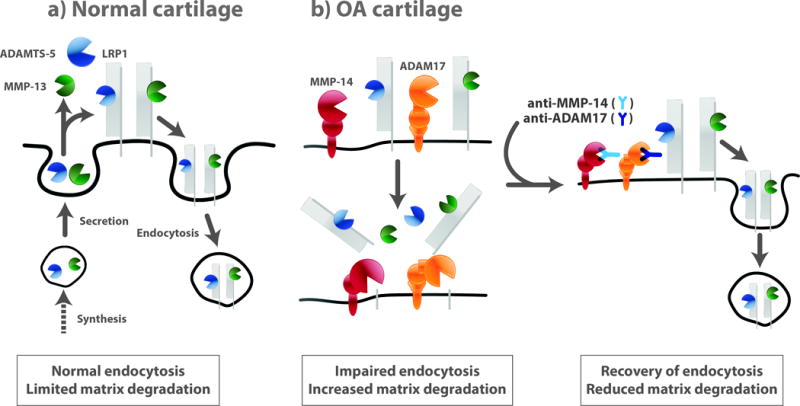
(a) ADAMTS-5 and MMP-13 secreted are largely endocytosed by LRP1 in normal cartilage. Thus, their activities in the extracellular milieu are limited. (b) In OA cartilage, the ectodomain of LRP1 is shed by ADAM17 and MMP-14, resulting in impairment of endocytic capacity of chondrocytes. Blocking of ADAM17 and MMP-14 activities reverses the lost endocytic function of OA chondrocytes, reduce cartilage matrix degradation, and restores cartilage homeostasis.
Supplementary Material
Acknowledgments
The authors thank Yasuyuki Shitomi for valuable discussion on the siRNA studies, Bryony Stott, Marcia Curtinha and Ida Parisi for the histology of knee sections, and Katherine Groves and the Oxford Musculoskeletal Biobank for supplying human OA tissues. The work was supported by grants from Arthritis Research UK [20563 (to Kazuhiro Yamamoto), 19466 (to Linda Troeberg)], Arthritis Research UK Centre for Osteoarthritis Pathogenesis (20205), the Kennedy Trust for Rheumatology Research, Orthopaedic Research UK [483 (to Jayesh Dudhia)], the Cancer Research UK [C100/A8243 (to Gillian Murphy)] and the National Institute of Arthritis and Musculoskeletal and Skin Diseases [AR40994 (to Hideaki Nagase)].
Footnotes
AUTHOR CONTRIBUTION
Kazuhiro Yamamoto conceived the project, performed the experiments and wrote the manuscript. Salvatore Santamaria prepared anti-ADAMTS-5 (2D3) antibody and performed the experiments. Kenneth Botkjaer prepared the anti-MMP-14 antibody (E2C6). Jayesh Dudhia procured normal human cartilage tissue. Linda Troeberg, Yoshifumi Itoh and Gillian Murphy oversaw the project and analyzed the data. Hideaki Nagase conceived and oversaw the project, and wrote the manuscript. All authors reviewed the results and approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
References
- 1.Goldring MB, Berenbaum F. Emerging targets in osteoarthritis therapy. Curr Opin in Pharmacol. 2015;22:51–63. doi: 10.1016/j.coph.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naito S, Shiomi T, Okada A, Kimura T, Chijiiwa M, Fujita Y, Yatabe T, Komiya K, Enomoto H, Fujikawa K, et al. Expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic cartilage. Pathol Int. 2007;57:703–711. doi: 10.1111/j.1440-1827.2007.02167.x. [DOI] [PubMed] [Google Scholar]
- 3.Song RH, Tortorella MD, Malfait AM, Alston JT, Yang Z, Arner EC, Griggs DW. Aggrecan degradation in human articular cartilage explants is mediated by both ADAMTS-4 and ADAMTS-5. Arthritis Rheum. 2007;56:575–585. doi: 10.1002/art.22334. [DOI] [PubMed] [Google Scholar]
- 4.Larkin J, Lohr TA, Elefante L, Shearin J, Matico R, Su JL, Xue Y, Liu F, Genell C, Miller RE, et al. Translational development of an ADAMTS-5 antibody for osteoarthritis disease modification. Osteoarthritis Cartilage. 2015;23:1254–1266. doi: 10.1016/j.joca.2015.02.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell PG, Magna HA, Reeves LM, Lopresti-Morrow LL, Yocum SA, Rosner PJ, Geoghegan KF, Hambor JE. Cloning, expression, and type II collagenolytic activity of matrix metalloproteinase-13 from human osteoarthritic cartilage. J Clin Invest. 1996;97:761–768. doi: 10.1172/JCI118475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiani C, Chen L, Wu YJ, Yee AJ, Yang BB. Structure and function of aggrecan. Cell research. 2002;12:19–32. doi: 10.1038/sj.cr.7290106. [DOI] [PubMed] [Google Scholar]
- 7.Little CB, Barai A, Burkhardt D, Smith SM, Fosang AJ, Werb Z, Shah M, Thompson EW. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60:3723–3733. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto K, Troeberg L, Scilabra SD, Pelosi M, Murphy CL, Strickland DK, Nagase H. LRP-1-mediated endocytosis regulates extracellular activity of ADAMTS-5 in articular cartilage. FASEB J. 2013;27:511–521. doi: 10.1096/fj.12-216671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Santamaria S, Yamamoto K, Botkjaer K, Tape C, Dyson MR, McCafferty J, Murphy G, Nagase H. Antibody-based exosite inhibitors of ADAMTS-5 (aggrecanase-2) Biochem J. 2015;471:391–401. doi: 10.1042/BJ20150758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto K, Okano H, Miyagawa W, Visse R, Shitomi Y, Santamaria S, Dudhia J, Troeberg L, Strickland DK, Hirohata S, et al. MMP-13 is constitutively produced in human chondrocytes and co-endocytosed with ADAMTS-5 and TIMP-3 by the endocytic receptor LRP1. Matrix Biol. 2016 doi: 10.1016/j.matbio.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto K, Owen K, Parker AE, Scilabra SD, Dudhia J, Strickland DK, Troeberg L, Nagase H. Low density lipoprotein receptor-related protein 1 (LRP1)-mediated endocytic clearance of a disintegrin and metalloproteinase with thrombospondin motifs-4 (ADAMTS-4): functional differences of non-catalytic domains of ADAMTS-4 and ADAMTS-5 in LRP1 binding. J Biol Chem. 2014;289:6462–6474. doi: 10.1074/jbc.M113.545376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Troeberg L, Fushimi K, Khokha R, Emonard H, Ghosh P, Nagase H. Calcium pentosan polysulfate is a multifaceted exosite inhibitor of aggrecanases. FASEB J. 2008;22:3515–3524. doi: 10.1096/fj.08-112680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scilabra SD, Troeberg L, Yamamoto K, Emonard H, Thogersen I, Enghild JJ, Strickland DK, Nagase H. Differential regulation of extracellular tissue inhibitor of metalloproteinases-3 levels by cell membrane-bound and shed low density lipoprotein receptor-related protein 1. J Biol Chem. 2013;288:332–342. doi: 10.1074/jbc.M112.393322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gendron C, Kashiwagi M, Lim NH, Enghild JJ, Thogersen IB, Hughes C, Caterson B, Nagase H. Proteolytic Activities of Human ADAMTS-5: COMPARATIVE STUDIES WITH ADAMTS-4. J Biol Chem. 2007;282:18294–18306. doi: 10.1074/jbc.M701523200. [DOI] [PubMed] [Google Scholar]
- 15.Tape CJ, Willems SH, Dombernowsky SL, Stanley PL, Fogarasi M, Ouwehand W, McCafferty J, Murphy G. Cross-domain inhibition of TACE ectodomain. Proc Natl Acad Sci U S A. 2011;108:5578–5583. doi: 10.1073/pnas.1017067108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Botkjaer KA, Kwok HF, Terp MG, Karatt-Vellatt A, Santamaria S, McCafferty J, Andreasen PA, Itoh Y, Ditzel HJ, Murphy G. Development of a specific affinity-matured exosite inhibitor to MT1-MMP that efficiently inhibits tumor cell invasion in vitro and metastasis in vivo. Oncotarget. 2016;7:16773–16792. doi: 10.18632/oncotarget.7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hascall VC, Sajdera SW. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969;244:2384–2396. [PubMed] [Google Scholar]
- 18.Yu Z, Visse R, Inouye M, Nagase H, Brodsky B. Defining the requirements for collagenase cleavage in collagen type III using a bacterial collagen system. J Biol Chem. 2012;287:22988–22997. doi: 10.1074/jbc.M112.348979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang W, Suzuki K, Nagase H, Arumugam S, Van Doren SR, Brew K. Folding and characterization of the amino-terminal domain of human tissue inhibitor of metalloproteinases-1 (TIMP-1) expressed at high yield in E. coli. FEBS letters. 1996;384:155–161. doi: 10.1016/0014-5793(96)00304-3. [DOI] [PubMed] [Google Scholar]
- 20.Troeberg L, Tanaka M, Wait R, Shi YE, Brew K, Nagase H. E. coli expression of TIMP-4 and comparative kinetic studies with TIMP-1 and TIMP-2: insights into the interactions of TIMPs and matrix metalloproteinase 2 (gelatinase A) Biochemistry. 2002;41:15025–15035. doi: 10.1021/bi026454l. [DOI] [PubMed] [Google Scholar]
- 21.Troeberg L, Fushimi K, Scilabra SD, Nakamura H, Dive V, Thogersen IB, Enghild JJ, Nagase H. The C-terminal domains of ADAMTS-4 and ADAMTS-5 promote association with N-TIMP-3. Matrix Biol. 2009;28:463–469. doi: 10.1016/j.matbio.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kashiwagi M, Tortorella M, Nagase H, Brew K. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5) J Biol Chem. 2001;276:12501–12504. doi: 10.1074/jbc.C000848200. [DOI] [PubMed] [Google Scholar]
- 23.Kashiwagi M, Enghild JJ, Gendron C, Hughes C, Caterson B, Itoh Y, Nagase H. Altered Proteolytic Activities of ADAMTS-4 Expressed by C-terminal Processing. J Biol Chem. 2004;279:10109–10119. doi: 10.1074/jbc.M312123200. [DOI] [PubMed] [Google Scholar]
- 24.Bergman I, Loxley R. Lung tissue hydrolysates: studies of the optimum conditions for the spectrophotometric determination of hydroxyproline. Analyst. 1969;94:575–584. doi: 10.1039/an9699400575. [DOI] [PubMed] [Google Scholar]
- 25.Etique N, Verzeaux L, Dedieu S, Emonard H. LRP-1: a checkpoint for the extracellular matrix proteolysis. Biomed Res Int. 2013:152163–152170. doi: 10.1155/2013/152163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartmann M, Herrlich A, Herrlich P. Who decides when to cleave an ectodomain? Trends Biochem Sci. 2013;38:111–120. doi: 10.1016/j.tibs.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Peschon JJ, Slack JL, Reddy P, Stocking KL, Sunnarborg SW, Lee DC, Russell WE, Castner BJ, Johnson RS, Fitzner JN, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281–1284. doi: 10.1126/science.282.5392.1281. 1998. [DOI] [PubMed] [Google Scholar]
- 28.Gorovoy M, Gaultier A, Campana WM, Firestein GS, Gonias SL. Inflammatory mediators promote production of shed LRP1/CD91, which regulates cell signaling and cytokine expression by macrophages. J Leukoc Biol. 2010;88:769–778. doi: 10.1189/jlb.0410220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rozanov DV, Hahn-Dantona E, Strickland DK, Strongin AY. The low density lipoprotein receptor-related protein LRP is regulated by membrane type-1 matrix metalloproteinase (MT1-MMP) proteolysis in malignant cells. J Biol Chem. 2004;279:4260–4268. doi: 10.1074/jbc.M311569200. [DOI] [PubMed] [Google Scholar]
- 30.Selvais C, D’Auria L, Tyteca D, Perrot G, Lemoine P, Troeberg L, Dedieu S, Noel A, Nagase H, Henriet P, et al. Cell cholesterol modulates metalloproteinase-dependent shedding of low-density lipoprotein receptor-related protein-1 (LRP-1) and clearance function. FASEB J. 2011;25:2770–2781. doi: 10.1096/fj.10-169508. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zilberberg A, Yaniv A, Gazit A. The low density lipoprotein receptor-1, LRP1, interacts with the human frizzled-1 (HFz1) and down-regulates the canonical Wnt signaling pathway. J Biol Chem. 2004;279:17535–17542. doi: 10.1074/jbc.M311292200. [DOI] [PubMed] [Google Scholar]
- 33.Kawata K, Kubota S, Eguchi T, Aoyama E, Moritani NH, Kondo S, Nishida T, Takigawa M. Role of LRP1 in transport of CCN2 protein in chondrocytes. J Cell Sci. 2012;125:2965–2972. doi: 10.1242/jcs.101956. [DOI] [PubMed] [Google Scholar]
- 34.Itoh Y. Membrane-type matrix metalloproteinases: Their functions and regulations. Matrix Biol. 2015;44–46:207–223. doi: 10.1016/j.matbio.2015.03.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


