Abstract
Effective early disease modifying options for osteoarthritis remain lacking. Tissue engineering approach to generate cartilage in vitro has emerged as a promising option for articular cartilage repair and regeneration. Signaling molecules and matrix modifying agents, derived from knowledge of cartilage development and homeostasis, have been used as biochemical stimuli toward cartilage tissue engineering and have led to improvements in the functionality of engineered cartilage. Clinical translation of neocartilage faces challenges, such as phenotypic instability of the engineered cartilage, poor integration, inflammation, and catabolic factors in the arthritic environment; these can all contribute to failure of implanted neocartilage. A comprehensive understanding of signaling molecules involved in osteoarthritis pathogenesis and their actions on engineered cartilage will be crucial. Thus, while it is important to continue deriving inspiration from cartilage development and homeostasis, it has become increasing necessary to incorporate knowledge from osteoarthritis pathogenesis into cartilage tissue engineering.
Keywords: Articular cartilage, tissue engineering, osteoarthritis, signaling molecules, cartilage development
1. Introduction
Arthritis is a debilitating disease that currently affects more than 50 million of US adults; this number is projected to rise to ~67 million by 2030 [1–3]. Characterized by the destruction of joint cartilage, osteoarthritis is the most common type of arthritis. Changes in biomechanical characteristics of articular cartilage and chondrocyte metabolism, which are often associated with aging or injury, lead to matrix degradation, causing severe pain and disability. Due to the limited intrinsic regenerative capacity of articular cartilage, surgical and conservative therapies have been employed in attempts at tissue restoration and to relieve pain [4–6]. However, current treatment modalities are insufficient to modify the disease as they give poor long-term outcomes. Effective therapeutic options for osteoarthritis remain lacking despite its prevalence.
Tissue engineering has emerged as a promising treatment option for articular cartilage repair. Engineering cartilage tissue often involves the fabrication of three-dimensional (3D) tissues in vitro by seeding cells into scaffolds in the presence of biochemical and biomechanical stimuli. Scaffold-free cultures have also been investigated in tissue engineering to minimize the adverse effects of scaffold degradation and alternations in cell phenotype [7–9]. The goal is to replace articular cartilage defects in the patient with neocartilage formed in vitro to restore function. A variety of cell sources, including stem cells and primary cells, natural or synthetic scaffold materials, signaling molecules, and mechanical stimuli have been explored to improve biological and biomechanical functions of engineered cartilage.
Signaling molecules play major roles in modulating cell to cell signaling and cellular activities within developing and mature articular cartilage. Several growth and transcription factors that are involved in cartilage development and homeostasis have been examined in the application of cartilage tissue engineering. Transforming growth factor-β (TGFβ) subfamily members, bone morphogenetic proteins (BMPs), insulin-like growth factors (IGFs), fibroblast growth factors (FGFs), and sex determining region Y (SRY)-box (SOXs) are common molecules used to induce chondrogenesis in vitro. The role of these signaling molecules in engineering cartilage as biochemical stimuli and the signal transduction pathways involved have been well characterized in past years, signifying the importance of repurposing cues seen in cartilage development toward cartilage tissue engineering.
Successful cartilage tissue engineering relies not only on the functionality of the engineered articular cartilage to mimic properties of native tissue, but also on the clinical applicability of such tissue. Using soluble signals derived from our knowledge of cartilage development has led to significant strides in engineering cartilage, resulting in the maintenance of the cartilage phenotype in vitro and the production of cartilaginous matrices (Figure 1). Despite this, the clinical translation of engineered cartilage continues to face challenges. Engineered tissues with insufficient mechanical properties that do not replicate the properties of native tissue, phenotypic instability after implantation, and poor integration into surrounding native tissue remain major challenges. In addition, inflammation in the degenerative joint is another barrier to overcome for successful cartilage repair in the clinical setting. To resolve these challenges, it has become increasingly important for clinicians and researchers to derive insight from not only cartilage development, but also from cartilage homeostasis, repair, and degenerative processes, and to apply such new-found knowledge toward the clinical translation of engineered cartilage (Figure 1). In short, a continuum of states can be observed for articular cartilage in vivo, ranging from a mostly anabolic development phase to a mostly catabolic disease phase. Inspiration can be drawn from each state toward implementing engineering replacements.
Fig. 1. Cartilage tissue engineering inspired by the states of articular cartilage in vivo.
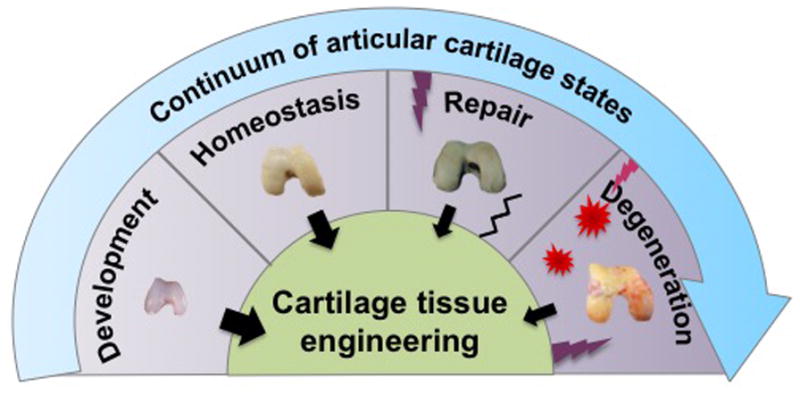
From cartilage development, a mostly anabolic phase, to degeneration, a mostly catabolic phase, inspiration drawn from each state has led to many advances in cartilage tissue engineering.
This review discusses the significant role of signaling molecules in engineering articular cartilage while placing them in the context of chondrogenesis during development. Special focus is placed on signaling molecules involved in differentiation and cartilaginous matrix production, such as TGF-βs, BMPs, IGFs, FGFs, and SOXs. Additionally, the role of biophysical agents, such as chondroitinase, that break down articular cartilage, is discussed to note how these, too, can have a role in engineering cartilage. Molecules that have shown potential for improving stability and integration of engineered cartilage, and for resisting inflammation, are also discussed.
2. Signaling molecules in chondrogenesis during cartilage development
Chondrogenesis, the process by which cartilage is formed, involves a plethora of different signaling pathways. Through a process termed condensation, undifferentiated mesenchymal stem cells (MSCs) derived from the lateral plate mesoderm migrate to the limb field region and aggregate [10] (Figure 2). The process of condensation, regulated by cell-cell and cell-matrix interactions, is critical for chondrocyte differentiation [11]. Stem cells in the condensation differentiate into chondrocytes that produce an abundance of extracellular matrix (ECM) (e.g., proteoglycans and collagen types II, IX, and XI) [12], forming the cartilaginous anlage. During the process known as endochondral ossification, chondrocytes in the center of the condensation zone become hypertrophic, producing type X collagen [12,10]. This is followed by interzone initiation and formation of the epiphyseal ossification center, which defines the developing joint. Subsequently, the cells at the edge of the cartilaginous anlage form the articular cartilage. For a more detailed description, please see reference [13].
Fig. 2. Formation of the cartilage anlage during development.
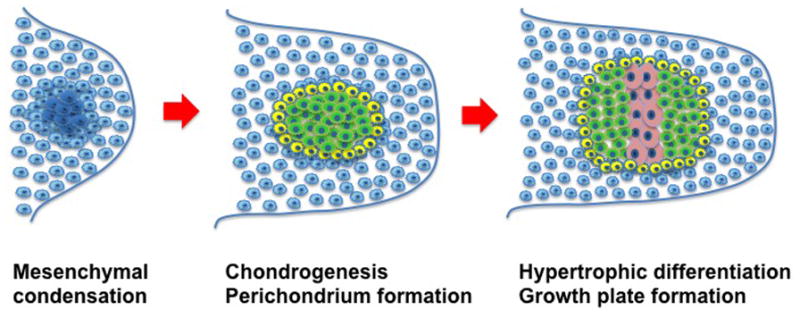
During the mesenchymal condensation phase, mesenchymal cells (blue) condense. Subsequently, during the chondrogenesis phase the cells differentiate into chondrocytes (green). Perichondrium cells are also formed (yellow). During the hypertrophic differentiation phase, chondrocytes in the central zone undergo hypertrophy (pink) to initiate the formation of the growth plate.
Various signals are involved in regulating the processes of chondrogenesis and hypertrophy. The roles of growth and transcription factors range from modulating the chondrocytic phenotype to stimulating cartilage matrix production. As discussed below, knowledge of signaling molecules ubiquitous in cartilage development has been the inspiration for many advances in cartilage tissue engineering. In order to provide a background of how signaling molecules are used in tissue engineering, their roles during chondrogenesis and chondrocyte hypertrophy are described below. Further details on signaling pathways involved in development and maintenance of articular cartilage can be found elsewhere [13].
2.1. Transforming growth factor-beta (TGF-β) superfamily
The members of the TGF-β superfamily consist of various ligands including activin/inhibin, TGF-βs, BMPs, and growth and differentiation factors (GDFs). Members of the TGF-β superfamily bind to type I and II receptors to activate downstream signaling pathways that are involved in development and homeostasis of a variety of tissues [14]. They are widely expressed in chondrocytes and play a crucial role in the process of chondrogenesis as well as maintenance of cartilage homeostasis.
2.1.1. TGF-β
TGF-βs are representative proteins involved in cartilage development. TGF-β signals are transduced through type II receptors, which recruit and phosphorylate type I receptors, resulting in activation of Smad proteins, Smad2 and 3. The phosphorylated Smad proteins interact with co-Smad (Smad4) to translocate to the nucleus, activating target gene expression [15]. Studies have shown that TGF-β signaling during chondrogenesis and chondrocyte maturation is mediated predominantly by Smad3 rather than Smad2 [16,17]. TGF-β can also activate mitogen-activated protein (MAP) kinase pathways, extracellular signal-regulated kinase (ERK) and p38 to regulate chondrogenesis [18]. TGF-β isoforms, TGF-β1, TGF-β2, and TGF-β3, are found in the perichondrium and periosteum, a fibrous cell layer derived from cells at the periphery of the mesenchymal condensation, as well as in the hypertrophic cartilage in the growth plate. TGF-β1 and TGF-β2 expression persists in adult articular cartilage, suggesting their involvement not only in the development but also in the maintenance of articular cartilage [19].
TGF-βs are important in all stages of chondrogenesis, including mesenchymal condensation, chondrocyte proliferation, ECM production, and terminal differentiation. During the first phase of condensation, MSCs express adhesion molecules to promote cell interactions. TGF-βs 1–3 are involved in the synthesis of adhesion molecules including N-cadherin and N-CAM, as well as ECM proteins such as fibronectin and tenascin to which the cells bind [20]. MAP kinases, ERK, p38, and c-Jun N-terminal kinase (JNK), as well as Wingless-Int (Wnt) signaling are involved in the modulation of N-cadherin expression mediated by TGF-β in mesenchymal progenitor cells [21]. Blocking of the adhesion molecules and ECM proteins disrupts cartilage differentiation both in vitro and in vivo by inhibiting condensation [22–24]. In addition to the role in condensation, TGF-β stimulates cell proliferation and synthesis of cartilage matrix such as glycosaminoglycans (GAG) as well as expression of cartilage-specific genes such as aggrecan and type II collagen [25,26]. TGF-β-activated Smad3/4 has been shown to stimulate SOX-9-mediated transcription; this involves the association of SOX-9 with the activated Smad3 and p300, a transcriptional co-activator, and their binding to the enhancer region of type II collagen gene [27]. Thus, TGF-βs contribute as stimulators in chondrogenesis, and their interaction with other signaling molecules modulates the chondrogenesis.
While TGF-βs have stimulatory effects in the early stages of chondrogenesis, they act as inhibitors in the later stages of chondrocyte differentiation. TGF-β inhibits differentiation into hypertrophic chondrocytes by inhibiting expression of type X collagen, matrix metalloproteinase 13 (MMP-13), vascular endothelial growth factor (VEGF), and osteocalcin [28]. Smad2/3 signaling mediates the inhibitory effect of TGF-β and is essential in inhibiting further progression into hypertrophy [16]. A study using homozygous mice with a targeted disruption of Smad3 has shown abnormally increased numbers of hypertrophic chondrocytes, suggesting the essential role of Smad signaling in suppressing chondrocyte terminal differentiation by TGF-β [29]. Furthermore, Smad3 activated by TGF-β interacts with runt-related transcription factor-2 (RUNX-2), a transcription factor involved in chondrocyte maturation and osteoblast differentiation [30], to inhibit RUNX-2 function [31]. The dual role of TGF-β signaling pathways in regulating chondrogenesis and hypertrophic differentiation, as well as controlling their levels at certain stages of development, may largely affect cartilage formation.
2.1.2. Bone morphogenetic protein (BMP)
BMPs are heavily involved in multiple stages of skeletal development. They play crucial roles in chondrogenesis and osteogenesis in vivo, including the commitment of mesenchymal cells to the chondrogenic lineage, induction of cell proliferation and maturation in the growth plates, and formation of joints and bones [19]. TGF-βs and BMPs are modulated by distinctly different signaling pathways to influence chondrogenesis, although these two categories of growth factors are members of the TGF-β superfamily. TGF-β signaling is mediated by Smad2/3, whereas Smad1/5/8 are responsible for transducing BMP signaling [15]. BMP-induced chondrogenesis has been shown to involve the p38 MAP kinase pathway [32].
The function of BMP signaling during chondrogenesis is mediated by the SOX family. Among the SOX family, SOX-9, L-SOX-5, and SOX-6 are well studied transcription factors involved in chondrogenesis. BMP induces expression of SOX-9, which acts downstream of BMP signaling to stimulate expression of cartilage markers [33]. Similarly, SOX-6 has been shown to be induced by BMP in a time-dependent manner; the binding of the SOX-6 to the type II collagen gene enhancer was increased with BMP treatment, indicating an important role of SOX-6 in mediating BMP signaling in chondrogenesis [34]. In addition, BMP signaling is essential for maintenance of SOX protein expression as demonstrated by a study where expressions of SOX-9, L-SOX-5, and SOX-6 were abolished during condensation in mice carrying double mutants of BMP receptor type 1A (BMPR1A) and 1B, where BMPs bind to transduce their signals [35]. Enhanced BMP-induced chondrogenic differentiation by overexpression of SOX-9, and synergistic effects on chondrocyte condensation and proliferation by both SOX-9 and BMP in ex vivo limb culture were reported [36]. Taken together, the relationship between BMP signaling and SOX expression is a key regulator of chondrogenesis.
In addition to the role in the early stages of chondrogenesis, BMPs reveal their roles in later stages in the growth plates by promoting chondrocyte proliferation and hypertrophy [19]. Addition of BMP increased longitudinal growth of metatarsal bone and stimulated chondrocyte proliferation and hypertrophy in the growth plate, while addition of noggin, an antagonist for BMP signaling, resulted in blocking these effects [37]. Transgenic mice expressing BMP under the control of the α2 (XI) collagen gene promoter/enhancer had an enlarged area of hypertrophic zone; this was possibly due to enhanced hypertrophic differentiation of chondrocytes [38]. Instead of mature hypertrophic chondrocytes, immature chondrocytes were observed in noggin over-expressing mice [38]. As noggin is also expressed during cartilage development, the level and function of BMP may be regulated by the action of its antagonist during the development of the growth plate cartilage.
Multiple BMP ligands including BMP-2, -4, -7, and GDF-5 have been shown to play roles during chondrogenesis and in the growth plate. BMP-2 is essential in the condensation process of MSCs and stimulates the synthesis of cartilage matrix proteins [19]. It also induces hypertrophic differentiation of proliferating chondrocytes in the growth plate by stimulating type X collagen. BMP-2-stimulated Smad1/5 in association with RUNX-2 has been shown to stimulate transcription of type X collagen expression gene to regulate chondrocyte hypertrophy [39]. Similarly, BMP-4 promotes cartilage matrix production by increasing type II collagen and aggrecan expression. However, BMP-4 suppresses expression of type X collagen, thus preventing chondrocyte hypertrophy [40]. BMP-7 is synthesized by proliferating chondrocytes present near the perichondrium [19]. In the presence of BMP-7, MSCs decrease their proliferative ability but increase the synthesis of cartilage matrix proteins [41]. BMP-7 exhibits not only anabolic activity, but also anti-catabolic activity such as expression inhibition of matrix proteases and cytokines [42]. GDF-5, also known as BMP-14, is expressed in the early condensation phase, while it is also involved in stimulating joint formation [43]. GDF-5 has been shown to stimulate survival of mesenchymal cells and maturation of chondrocytes [44,45]. Different BMP isoforms display similar as well as distinct roles, and thus their involvement during cartilage development in terms of levels and phases is different.
2.2. SRY (sex determining region Y)-box (SOX)
Among many transcription factors involved in cartilage development, SOX-9 is a key molecule that regulates chondrocyte differentiation and cartilage formation. It encodes a high mobility group DNA binding domain and associates with the SOX-9 binding sites on promoters/enhancers of cartilage specific genes such as type II, IX, and XI collagens, and aggrecan [46]. SOX-9 is necessary in the condensation phase of MSCs during chondrogenesis. Cells that express SOX-9 undergo aggregation and start differentiating into the chondrocyte lineage [12]. Loss of SOX-9 in limb buds resulted in disruption of the mesenchyme condensation and no cartilage and bone formation [47]. The essential roles of SOX-9 after mesenchymal condensation on chondrocyte differentiation have also been demonstrated; inactivation of SOX-9 after condensation phase led to severe chondrodysplasia, inhibited chondrocyte proliferation, and induced defects in joint formation [47].
SOX-9 induces and is necessary for the expression of SOX-5 and SOX-6. SOX-5 and 6 are also known transcription factors that guide MSCs into the chondrogenic lineage. These stimulate type II, IX, and XI collagens and aggrecan by cooperating with SOX-9 [48,49]. The activation of SOX-5 and 6 is needed for chondroprogenitor cells expressing SOX-9 to undergo appropriate chondrogenic differentiation. In the absence of SOX-5 and 6, chondroprogenitors shift their fate toward tendon and ligament lineage by expressing scleraxis (SCX), a tendon and ligament transcription factor [50]. Mutation of either or both genes results in mild skeletal defects and chondrodysplasia, respectively [49]. Thus, the interplay between SOX-5/-6 and SOX-9 plays a key role in chondrogenesis.
In addition to its role in promoting chondrogenesis, SOX-9 is also involved in the process of endochondral ossification. Loss of SOX-9 resulted in the maturation of immature chondrocytes into hypertrophic cells while overexpression of SOX-9 decelerates the processes of chondrocyte hypertrophy in immature chondrocytes [51,52]. SOX-9 has been shown to block the activity of RUNX-2 and to suppress genes such as type X collagen and VEGF-A, expressed by hypertrophic chondrocytes [53–55], suggesting the multiple roles of SOX-9 not only during chondrogenesis but also in the growth plate.
SOX-9 expression is modulated by several transcription and growth factors [46]. Sonic hedgehog, a molecule involved in patterning of the anterior-posterior limb axis, as well as hypoxia-inducible factor 1α, a positive regulator of chondrogenesis, have been shown to enhance the promoter activity of SOX-9 and increase its expression [46]. TGF-β and BMP-2 signals, and FGF-1/2 and IGF-1 are also involved in upregulating the expression of SOX-9 [46]. Thus, SOX-9 can be controlled by or interact with signaling pathways activated by other molecules to regulate cartilage development.
2.3. Insulin-like Growth Factor (IGF)
As with the factors discussed above, IGF also plays multiple roles in cartilage development. IGF is a key component for mesenchymal differentiation toward chondrocytes and also in the subsequent stages of development, such as the synthesis of cartilaginous matrix. In addition, IGF is involved in the chondrogenesis of mesenchymal cells as well as the maintenance and survival of differentiated articular chondrocytes via a phosphoinositide 3-kinase (PI3K) pathway, involving ERK, p38 kinase, and protein kinase C (PKC) signaling [56]. Further, it was demonstrated that IGF has a pivotal role in the relationship between chondrogenesis and osteogenesis.
IGF-1 is a regulatory factor in the process of chondrogenesis from MSCs. Both IGF isoforms, IGF-1 and IGF-2, have been shown to promote clonal growth of human adult and fetal chondrocytes, respectively [57,58]. Specifically, IGF-1 was shown to induce cell proliferation in MSC pellets and promoted the expression of chondrogenic markers, such as type II collagen and SOX-9 [59]. IGF appears to act independently during chondrogenic differentiation of MSCs, but its actions can be enhanced when acting in conjunction with TGF-β or BMP-2 [12,59]. IGF has proven to be a critical factor for chondrocyte proliferation during the early phases of chondrogenesis [12]. IGF-1 mediates type II collagen synthesis mainly via the increased binding of SOX-9 and specificity protein (Sp1)/Sp3 to their cis elements in the intron-specific enhancer region of type II collagen gene, and this involves a physical interaction with p300 [60].
Apart from chondrogenesis, IGF was proven to play a key role in hypertrophic maturation of chondrocytes. Recently, the dogma that considers chondrocytes and osteoblasts as entirely independent lineages derived from a common progenitor [61] came into question when hypertrophic chondrocytes were observed to have the ability to become osteoblasts and osteocytes during endochondral bone formation and during bone repair [62]. The strategic role of IGF as a regulator of the above processes was confirmed by the presence of specific cellular patterns of gene expression for the IGF system during both chondro- and osteogenesis [63]. Furthermore, mRNAs of IGF receptors (IGF-1R and IGF-2R) are expressed in great amounts in hypertrophic chondrocytes. The role of IGF in both mature chondrocytes and osteoblasts has been confirmed by similar actions of IGF and IGF receptors to growth plate chondrocytes. Specifically, IGF is a critical component of chondrocyte proliferation in growth plate [12]. In an in vitro study using embryonic and postnatal growth plate chondrocytes isolated from a IGF-1R gene knockout mice, the absence of IGF-1R was associated with suppressed cell proliferation and promoted apoptosis via increased PTHrP expression [64]. Thus, IGF has manifold functions not only during the early phases of chondrogenesis but also in hypertrophy and matrix synthesis.
2.4. Fibroblastic Growth Factor (FGF)
FGFs represent a family of 22 structurally related proteins that share common biochemical and functional properties [65]. The role of FGF signaling in skeletal development has been revealed from the discovery that a point mutation in the transmembrane domain of FGF receptor (FGFR) 3 is responsible for achondroplasia, the most common genetic form of dwarfism in humans. Subsequently, it was discovered that two major groups of skeletal developmental disorders were associated etiologically with specific mutations in the genes encoding FGFRs 1, 2, and 3: 1) the dwarfing chondrodysplasia syndromes, such as hypochondroplasia [66] and achondroplasia [67,68] and 2) the craniosynostosis syndromes, such as Apert syndrome [69], Crouzon syndrome [70–72], and Pfeiffer syndrome [73,72].
For the early chondrogenesis stages, FGFR expression is critical for further limb development. Signaling from mesenchymally expressed FGF-10 to FGFR2b is important for the formation of the apical ectodermal ridge. Afterwards, FGF-8 is expressed in the apical ectodermal ridge and initiates a type of reciprocal signaling to FGFR1c in the limb mesoderm [74]. During the mesenchymal condensation phase, FGFR2 is expressed in the ectoderm of the condensing mesenchyme, and FGFR1 is expressed in the periphery of the mesenchymal condensation [74]. Despite the fact that FGF-2 is the most common growth factor used in cartilage tissue engineering, its exact role during chondrogenesis remains unclear. This may be due to its indirect regulatory action during chondrogenesis, as it has been shown that FGF-2 inactivates signaling pathways involving IGF-1 and TGF-β [75].
During chondrogenesis, FGFR3 expression begins as chondrocytes differentiate and proliferate. Other FGF family members also contribute to chondrogenesis. [76,77]. Specifically, FGF-1, FGF-2, and FGF-7 have been shown to enhance SOX-9 expression via a MAP kinase, ERK1/2 pathway in mouse primary costal chondrocytes [78]. Further, in C3H10T1/2 cells, which are murine mesenchymal progenitor cells, members of the FGF family were able to induce their differentiation toward chondrocytes [78]. FGFR3/FGF-18 interaction suppressed cellular proliferation and promoted limb mesenchymal cell differentiation [79]. In later stages of chondrogenesis, different members of the FGF family are expressed, such as FGF-9, -10, -18, together with FGF-1, -2 and -3 as stated above [74]. FGF-8’s central role in chondrogenesis was confirmed by the central regulatory effect of FGF-8 in heterotopic ossification, a type of endochondral ossification [80]. FGF family acts via tyrosine kinase receptors, and their activations leads to mitogenic response in various cell types including chondrocytes [81].
During chondrocyte hypertrophy, both FGF and FGFRs play major roles. Specifically, in both pre-hypertrophic and hypertrophic chondrocytes, FGFR1 is expressed, and it contributes to the maintenance of hypertrophy and survival of hypertrophic chondrocytes [82,74,76]. FGFR3 was also expressed in hypertrophic chondrocytes [83,65,74]. Interestingly, FGFR1 and 3 seem to be expressed in different subpopulations of hypertrophic chondrocytes with minimum overlap [83,74]. FGFR1 was expressed initially in chondrocytes originating from mesenchymal condensation, but, subsequently, it is expressed mainly in chondrocytes in the peripheral mineralized zones and in the adjacent osteoblasts, suggesting a potential role in hypertrophy and in osteoblastic differentiation [83,74]. In contrast, FGFR3 is mainly expressed in proliferating chondrocytes, which implies a regulatory role in chondrocyte proliferation [83]. FGF-18 has been found to regulate early chondrocyte proliferation and differentiation through FGFR3 signaling [84]. Interestingly, FGF-9 also has a potential role in chondrocyte proliferation and hypertrophy. As demonstrated in mice that lack FGF-9 expression, the dearth of an adequate number of chondrocytes undergoing hypertrophy was responsible for a delay in type X collagen expression and simultaneous maintenance of type II collagen [85]. Despite the fact that several FGF ligands are involved in all stages of chondrogenesis, only FGF-9 and FGF-18 have been recognized so far as active molecules during the cartilage hypertrophy stage.
Several signaling pathways involving FGF have been shown to interact during chondrogenesis. Recently, it was demonstrated that FGF, TGF-β, and Wnt protein families control different differentiation stages during chondrogenesis via the presence of a signaling crosstalk [86]. FGFs increase the level of SOX-9 expression and enhance the activity of SOX-9- dependent-chondrocyte-specific enhancer elements in the gene for type II collagen [78]. From all these examples, it becomes obvious that multiple signaling factors interact in a well-balanced manner in order to promote chondrogenesis; better understanding of the processes that occur during cartilage development would further enrich applicable knowledge in tissue engineering.
3. Signaling molecules for cartilage tissue engineering
Cell sources and biochemical/biomechanical stimuli represent two of the main elements of cartilage tissue engineering. Articular chondrocytes, adult stem cells, embryonic stem cells (ESCs), and induced pluripotent stem cells (iPSCs) are all promising cell sources for articular cartilage repair and regeneration. The ability for the cells to differentiate into and maintain the chondrogenic phenotype is largely affected by biochemical and biomechanical stimuli (Figure 3). A variety of signaling molecules have been applied in cartilage tissue engineering to trigger chondrogenic differentiation and to stimulate synthesis of cartilage-specific matrix. To address the role of signaling molecules in a variety of cell types used in cartilage tissue engineering, the following sub-sections review 1) signaling factors used in expansion and differentiation of each cell type, and 2) biochemical molecules and biophysical agents employed for the improvement of biomechanical properties of engineered articular cartilage, including corresponding ECM components.
Fig. 3. Articular cartilage tissue engineering.
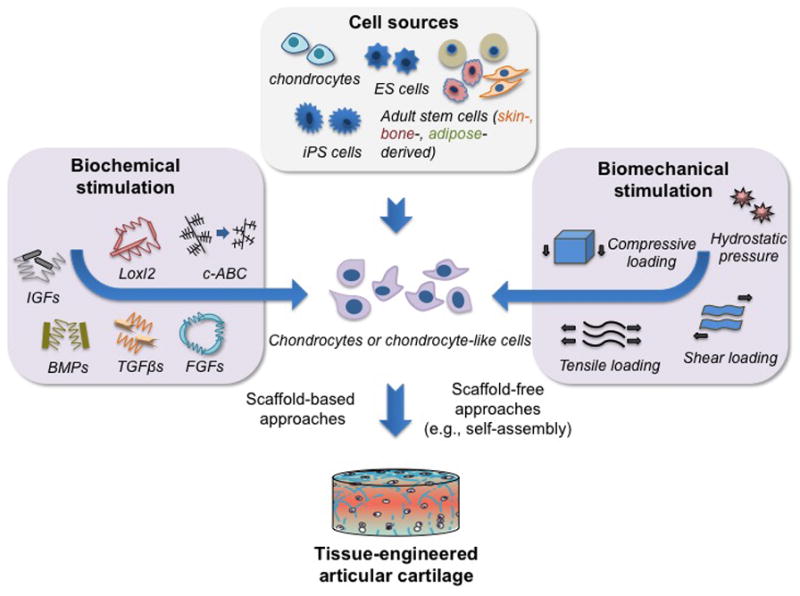
Engineering cartilage tissue often involves the formation of three-dimensional (3D) tissues in vitro by seeding chondrocytes or chondrocyte-like cells into scaffolds or through scaffold-free approaches in the presence of biochemical and biomechanical stimuli. A variety of cell sources, signaling molecules, and mechanical stimuli has been explored for cartilage tissue engineering.
3.1. Signaling molecules for expansion and differentiation of different cell types
3.1.1. Chondrocytes
Articular chondrocytes (e.g., autologous articular chondrocytes) are already used clinically to repair cartilage defects. However, due to the limited availability of donor tissue, obtaining a sufficient amount of cells for clinical application is challenging. Expansion of articular chondrocytes in monolayer causes dedifferentiation with increased type I collagen expression and potential loss of ability to revert to a chondrogenic phenotype.
The use of growth factors and their effects on 2D and 3D cultures (e.g., alginate beads, micromass, pellet, and scaffolds) have been investigated to improve the proliferative capacity and to restore the chondrogenic potential of articular chondrocytes. FGF-2 has shown to increase cell proliferation and to reduce apoptosis in human articular chondrocytes in monolayer, while it decreased the amount of type II collagen and aggrecan [87]. A combination of IGF-1 and BMP-7 has been shown to promote cell viability, cell proliferation, and matrix synthesis in both normal and osteoarthritic human articular chondrocytes in alginate beads in vitro [88]. When combined with IGF-1 and BMP-7, FGF-2 significantly stimulated cell proliferation in human articular chondrocytes cultured in alginate beads. However, a high dose, 100ng/ml of FGF-2 as compared to 1ng/ml, decreased proteoglycan levels and inhibited the ability of IGF-1 and BMP-7 to stimulate proteoglycan production [89]. A synergistic effect on the expression of type II collagen and aggrecan was also observed for TGF-β2 and IGF-1 when human articular chondrocytes were cultured in alginate beads [90]. BMP-2 sustained expression of type II collagen and increased aggrecan in chondrocytes cultured in monolayer [91,92], though no distinct changes in matrix production occurred when BMP-2 was applied in aggregate and pellet cultures [93]. Dedifferentiated rabbit articular chondrocytes, transduced with adenovirus expressing BMP-4, recovered their chondrogenic phenotype both in monolayer and in pellet cultures [94]. In vitro expansion of human articular chondrocytes in monolayer with a combination of TGF-β1 and FGF-2 has been shown to promote cell proliferation and express abundant cartilage matrix protein when redifferentiated in pellets [95]. Cell expansion with TGF-β1, FGF-2, and platelet-derived growth factor (PDGF)-bb, followed by a combination of TGF-β1, GDF-5, and BMP-2 during post expansion in human articular chondrocytes, resulted in heightened expression of chondrogenic genes and cartilage-specific matrix synthesis [96]. Culturing human articular chondrocytes with TGF-β2 in hypoxic conditions prominently elevated expression of type II collagen when compared to a condition without TGF-β2 [97]. Interestingly, hypoxic conditions and 3D environment, provided by methoxypolyethyleneglycol-block-co-poly (50:50 lactide-co-glycolide) (MPEG-PLGA) scaffold, resulted in higher expression of cartilage-specific genes, including SOX-9, aggrecan, and type II collagen in human articular chondrocytes when compared to hypoxic conditions in monolayer culture [98].
Taken together, the effects of growth factors known to play key roles in cartilage development, either alone or in combination, have been investigated for maintenance of the chondrogenic phenotype and for promoting cartilage formation in vitro. The effects of these growth factors on chondrocytes are dependent on both concentration and the presence of other growth factors. In addition, the effect of hypoxia on chondrogenic phenotype is also to be considered. Importantly, 2D versus 3D culture greatly influences cellular response. It is still unclear if the activity of growth factors used in vitro will persist after orthotopic implantation in vivo. Comprehensive understanding of the function of growth factors with respect to time, dose, and dosing regimen is critical for the clinical application of chondrocytes.
3.1.2. Adult mesenchymal stem cells (MSCs)
Mesenchymal stem cells (MSCs) constitute an alternative cell source in cartilage tissue engineering. A variety of adult tissues such as bone marrow, fat tissue, skeletal muscle, and skin are sources of MSCs. Due to their abundant proliferative capacity and multiple lineage differentiation potential, much attention has been paid to MSCs as an effective source of chondrocytes for cartilage repair. The most commonly used adult stem cells for cartilage tissue engineering are MSCs derived from bone marrow, adipose, and synovium.
TGF-β1 and TGF-β3 are the main chondrogenic inducers used for MSCs. In addition, BMPs, FGF-2, and IGF-1 have been studied on promoting chondrogenic differentiation of MSCs in vitro. FGF-2 treatment during in vitro expansion enhanced cell proliferation in monolayer and promoted chondrogenesis in both synovium and bone marrow-derived MSCs [99,100]. Adipose-derived MSCs treated with FGF-2 followed by chondrogenesis in the presence of TGF-β1 significantly increased cartilage-specific matrix gene expression and decreased type X collagen gene expression compared to cells expanded without FGF-2, suggesting possible crosstalk between FGF-2 and TGF-β1 for chondrogenesis [101]. The effects of combining growth factors have also been investigated for chondrogenic induction. A combination of TGF-β3 and BMP-6, or TGF-β3 and IGF-1 enhanced chondrogenesis in bone marrow MSCs when compared to treatment with TGF-β3, BMP-6, and IGF-1 alone [102]. BMP-2 was more effective than BMP-4 and -6 for the synthesis of proteoglycans and type II collagen in cartilage formation from bone marrow MSCs in the presence of TGF-β3 [103]. Chondrogenesis of adipose MSCs was most effectively induced by treatment with a combination of TGF-β2 and BMP-7 when compared to a combination of TGF-β2 and BMP-2 or TGF-β2 and BMP-6 [104]. Synovium MSCs synthesized more cartilage matrix in the presence of TGF-β3 and BMP-2, as compared to combinations of TGF-β3 and IGF-1, and TGF-β3 and FGF-2 [105]. Exposure of synovium MSCs to a combination of TGF-β1, IGF-1, and FGF-2 for the first 3 days of culture in pellets stimulated cell growth and enhanced chondrogenic differentiation by TGF-β1 [106]. Further, FGF-2 treatment for the first 3 days, followed by the continuous treatment with TGF-β1 and IGF-1, supported chondrogenesis using synovium MSCs [106]. Although the growth factors described above tend to exhibit similar effects, it is important to note that different combinations of growth factors, including variance in isoforms and timing of exposure, can influence chondrogenic differentiation of stem cells and the effects may vary depending on stem cell sources.
In addition to the use of various growth factors to induce chondrogenic differentiation, the effects of hypoxia toward chondrogenesis have also been investigated in MSCs. Hypoxia was shown to induce chondrogenesis in bone marrow MSCs in the absence of exogenous growth factors [107]. When compared to normoxia, hypoxia in the presence of GDF-5 increased expression of type II collagen and aggrecan, and decreased expression of type X collagen in self-assembled cartilage from bone marrow MSCs [108]. Thus, performance of various growth factors in terms of chondrogenic efficacy of adult stem cells, as a function of oxygen tension, continues to require additional evaluation.
While MSCs can be coaxed to progress toward the chondrogenic lineage by growth factors, this process is frequently accompanied by hypertrophic differentiation, as evident by the production of type X collagen, MMP13, and alkaline phosphatase (ALP). The subsequent potential for tissue mineralization remains an unsolved problem for tissue engineering articular cartilage. Parathyroid hormone related peptide (PTHrP) is a molecule present in the growth plate and acts as an inhibitor of chondrocyte hypertrophy. For marrow MSCs and adipose MSCs in pellet culture, expression of type X collagen and RUNX-2 were reduced significantly by PTHrP [109]. Parathyroid hormone (PTH) inhibited expression of type X collagen and increased expression of type II collagen in marrow-derived MSCs from patients with osteoarthritis [110]. Further, PTHrP/PTH’s ability to regulate hypertrophic differentiation may be useful for controlling MSC-chondrogenesis in vitro.
3.1.3. Embryonic stem cells
Embryonic stem cells (ESCs) provide infinite proliferative capacity and pluripotency [111]. However, complex ethical and political issues are associated with the derivation of these cells. For cartilage tissue engineering, ESCs are often first cultured as 3D embryoid bodies to differentiate them toward the chondrogenic phenotype. During this time, TGF-βs, BMPs, and IGF-1 to ESCs are often used. For example, ESCs treated with TGF-β3, followed by a combination of TGF-β1 and IGF-1, while being cultured as embryoid bodies yielded cells that, when used for engineering articular cartilage, produced no type I collagen [112]. In the same study, exposure to BMP-2 during the embryoid body phase produced a fibrocartilage-like phenotype [112]. In another study, embryoid bodies were plated and differentiated with TGF-β3 and BMP-2, resulting in positive staining for type II collagen and SOX-9 [113]. Instead of embryoid body formation, ESCs seeded in pellet culture showed the highest gene expression of aggrecan and type II collagen when treated with BMP-7 alone, as compared to TGF-β1 alone or a combination of TGF-β1 and BMP-7 [114].
The effect of hypoxia in chondrogenic differentiation of ESCs has also been reported. Exposure of ESCs to hypoxic conditions during embryonic body culture was shown to significantly enhance cartilage protein synthesis and mechanical properties of self-assembled neocartilage [115]. Similarly, pellets, derived from ESCs cultured in conditioned medium by primary chondrocytes in the presence of transient hypoxia during embryonic body culture, produced enhanced type II collagen and GAG when compared to pellets derived from ESCs in normoxia [116].
These findings indicate the ability of growth factors to induce chondrogenesis in ESCs and potentiate the application of ESCs in cartilage repair and regeneration. However, as compared to differentiated cells, ESCs are more sensitive to culture conditions. The studies discussed above applied tissue engineering techniques to cells dissociated from embryoid bodies, cells still in the embryoid body form, cells that migrated out of embryoid bodies, and cells that were not placed in embryoid bodies at all. The effects of growth factors on chondro-differentiation and on chondrogenesis can, thus, be different from one condition to the next. Systematic examinations comparing growth factor effects across different culture conditions are, therefore, needed for optimizing the use of signaling molecules in ESC culture.
3.1.4. Induced pluripotent stem cells
Induced pluripotent stem cells (iPSCs) do not face the same ethical issues as ESCs. iPSCs can be autologous, providing less risk of immune rejection and disease transmission. By introducing defined factors, Oct-3/4, SOX-2, KLF-4, and c-Myc, adult fibroblasts can be reprogrammed to iPSCs with pluripotent and self-renewal capacities [117]. To avoid the risk of tumor formation by transduction of the reprogramming factors, methods to generate iPSCs without genomic integration of transgenes have been reported [118–121]. For engineering articular cartilage, strategies using an adult’s own cells can start with the generation of iPSCs that are subsequently differentiated into chondrocytes, for example, using TGF-β3 and BMP-2, singly or in combination, as seen for iPSCs from various cell sources [122–125]. For the chondrogenic induction of iPSCs, the duration of the growth factor application is important. iPSCs treated with a combination of TGF-β1, BMP-2, and GDF-5 resulted in chondrogenesis, but removing the stimulus followed by another 4 weeks of culture significantly reduced type I collagen expression compared to continuous exposure [126]. Studies on the effects of growth factor stimulation on iPSC chondrogenesis are yet limited, and it is unclear if inducing pluripotency is necessary for engineering articular cartilage.
3.1.5. Other cell sources
Skin and costal cartilage may be viable cell sources for cartilage tissue engineering without induced pluripotency. While dermal fibroblasts have been recognized as a resource for iPSC applications, their capability for chondrogenesis through other means has also been investigated. Direct chondrogenic induction has been demonstrated using human dermal fibroblasts without going through a pluripotent state by transducing c-Myc, KLF-4, and SOX-9 to generate chondrogenic cells [127,128]. Whether these cells will respond similarly as iPSCs do to growth factors remains to be seen, and further comprehensive approaches using growth factors for chondrogenic differentiation of iPSCs are necessary. Without genetic manipulation, it has been shown that dermal fibroblasts pre-treated with IGF-1 can be chondro-induced through exposure to an aggrecan substrate, as seen by type II collagen and GAG expression [129]. Human dermal fibroblasts stimulated with GDF-5 expressed cartilage-specific markers including type II collagen, aggrecan, and SOX-9 both in monolayer and in micromass culture [130].
Various subpopulations of stem cells likely exist within skin [131]. Dermis includes multipotent adult stem cell populations that have the potential to differentiate into multiple cell types. Skin-derived precursors isolated from the whole dermis population by culturing them in suspension using FGF-2, epidermal growth factor (EGF), and B27™ showed differentiation potentials toward both neural and mesodermal cell types [132]. Chondrogenic differentiation of the skin-derived precursors was demonstrated in the presence of BMP-2 in micromass culture [133]. Clonally derived dermal fibroblasts can differentiate into multiple lineages including the chondrogenic lineage. Studies have shown chondrogenic differentiation of the clonally derived dermal fibroblasts in the presence of TGF-β1 or TGF-β1 plus IGF-1 in pellet cultures [134,135]. Stem cells isolated from dermis obtained by rapid adherence to tissue culture plates, termed dermis isolated adult stem (DIAS) cells, showed multiple lineage differentiation potentials; the chondrogenic potential, when cultured with TGF-β1 or BMP-2, was observed with increased GAG contents in self-assembled constructs [136]. Chondrogenesis of DIAS cells was enhanced with hypoxia in the presence of TGF-β1 and IGF-1 in micromass culture [137]. Studies are still needed to enhance the chondrogenic capacity of cells derived from the skin to reach the biochemical and biomechanical characteristics of native articular cartilage, and it remains to be seen whether tissues engineered from these cells possess lubricious properties.
Despite the promise of using the stem cell sources described above, there remain concerns that still need to be addressed. For example, human ESCs expanded in vitro have a tendency to undergo karyotypic aberrations, while undifferentiated ESCs have the capacity to form teratomas in vivo [138,139]. Furthermore, malignant transformation in bone marrow MSCs cultured for long-term ex vivo has also been reported [140]. Immunogenicity and biodistribution of stem cells are additional concerns [141]. The development of specific assays to identify and remove these concerns, toward evaluating safety and efficacy, is thus of paramount importance [141].
Costal cartilage, located at the anterior ends of the ribs, is not an articular cartilage. However, chondrocytes derived from costal cartilage have been used to engineer tissues that express lubricin, a protein that helps articular cartilage achieve low frictional properties. In this case, TGF-β1, FGF-2, and PDGF were applied to costochondral cells during monolayer expansion [142]. Not much research has been reported for the use of costochondral cells to tissue engineer articular cartilage, and further studies comparing various growth factors to improve the quality of the engineered tissue are necessary. Nonetheless, the potential of using costal cartilage opens up another autologous cell source for cartilage repair and regeneration.
3.2. Signaling molecules for improving biomechanical properties
Articular cartilage withstands compressive, tensile, and shear loads as part of its function, and it is also lubricious, resulting in a tissue with very low coefficient of friction. Compressive, tensile, and shear moduli have been measured or estimated to range from 0.08 to 2 MPa, 5 to 25 MPa, 0.05 to 0.25 MPa, respectively [13] and the coefficient of friction ranges from 0.03 to 0.06 [143]. Cartilage’s biochemical content, primarily water, collagen, and proteoglycans, and the organization of these molecules allow for appropriate load distribution and transmission. Therefore, one of the major design criteria in cartilage tissue engineering is the creation of neocartilage with biomechanical properties that will withstand the demanding mechanical environment in vivo. Proper organization of the neocartilage is anticipated to allow for long-term functionality and durability.
3.2.1. Compressive properties and corresponding ECM components
Through their effects on matrix metabolism, growth factors influence GAG and collagen synthesis. Specifically, addition of IGF-1 in chondrocytes seeded onto biodegradable polyglycolic acid (PGA) scaffolds resulted in an increase in the total GAG content of the tissue 5-fold [144]. In contrast, a supplement of PDGF decreased GAG production by 43%, with no effect on collagen content [144]. There is universal acceptance that TGF-β1 enhances collagen synthesis (e.g., up to 34-fold increase in monolayer culture [144,25], though its effects on GAG synthesis appears varied and dependent on 3D versus 2D culture and cell origin. In chondrocytes seeded in PGA scaffolds a decrease in GAG was recorded, while in monolayer culture an increase in total GAG content was demonstrated [144,25]. Compared to cells from other zones, superficial zone chondrocytes responded to TGF-β1 with the highest increase (220% compared to control) in GAG production [25]. Regarding BMPs, BMP-2, BMP-12, and BMP-13 were shown to increase GAG synthesis in chondrocytes, but only BMP-2 use was not associated with chondrocyte hypertrophy [145]. Growth factor-induced alterations in biochemical content not only provide insight to phenotype but are also important due to their direct linkage to biomechanical properties.
In a study comparing combinations of BMP-2, IGF-1, and TGF-β1 at various dosing regimens, all were shown to increase compressive properties (71%, 75%, and 73%, respectively) with increases in GAG content (39%, 41%, and 31%, respectively) [146]. In terms of dosing regimen, it was found that IGF-1 and BMP-2 were efficacious when applied either continuously or intermittently toward improving compressive properties by ∼90% and 70%, respectively, although TGF-β1 was only effective with continuous administration (89% increase in compressive properties compared to control). The combination of BMP-2 and IGF-1 was the most successful in improving the GAG content of the engineered tissue by 54%, which was translated to the highest improvement in compressive properties, a 119% increase [146]. Finally, it was shown that a lag time after TGF-β administration was required for the observed effect [146].
Signaling molecules have been successfully combined with compressive loading to improve the biomechanical properties of engineered cartilage. Moderate dynamic compressive loading between 0–12% strain of articular cartilage has been demonstrated to play an anabolic role on chondrocytes by promoting collagen and proteoglycan synthesis by 31-34% and 17-38%, respectively [147]. IGF-1 stimulation with dynamic compression at 3% and 0.1Hz showed a synergistic effect on collagen and proteoglycan synthetic activity, as demonstrated by an increase in H-proline incorporation by 180% and in S-sulfate incorporation by 290% compared to 30% and 120% increase, respectively, with dynamic compression only, and 90% and 160% increase with IGF-1 stimulation alone [148]. This difference was potentially due to changes in the transport of IGF-1 by different loading regimens, as demonstrated by the analysis of diffusivity of IGF-1 in cartilage [148]. Dynamic compression of chondrocyte-seeded agarose hydrogels at 10% strain and 1Hz combined with either TGF-β1 or IGF-1 resulted in a 3-4-fold increase in aggregate modulus for both growth factors, as well as in collagen (∼7-fold and 5-fold increases for TGF-β1 and IGF-1, respectively) and GAG synthesis (∼2-fold and ∼2.5-fold increases for TGF-β1 and IGF-1, respectively) [149]. It is noteworthy that, in general, changes in biomechanical properties do not scale linearly with changes in biochemical components, suggesting important roles for interaction among matrix components and matrix organization in determining biomechanical properties.
Similar to direct compression, hydrostatic pressure is a stimulus that improves the compressive and biochemical properties of engineered cartilage [150]. Combined with TGF-β3 in a chondrogenesis model using mesenchymal stem cells, intermittent hydrostatic pressure of 10 MP at 1Hz stimulated mRNA expression of SOX-9, type II collagen, and aggrecan by 1.9-fold, 3.3-fold, and 1.6-fold, respectively, compared to TGF-β3 application alone [151]. Combined with BMP-2 and IGF-1, 10 MPa static hydrostatic pressure in neocartilage, engineered with articular chondrocytes, increased aggregate and tensile modulus values by 17% and 30%, respectively [152]. The same hydrostatic pressure regimen improved aggregate and tensile modulus values by 41% and 40%, respectively, when applied with TGF-β1 [152]. Since hydrostatic pressure can be applied before engineered cartilage develops robust mechanical characteristics, hydrostatic pressure can be applied earlier than direct compression during tissue engineering of articular cartilage.
Better understanding of the exact mode of action and how synergism occurs among the different stimulatory signals above is the next step towards optimization of their potential. In a study where TGF-β3 was released from a poly(lactide-co-caprolacton) PLCL scaffold over a 12 week period, the compressive properties of the engineered cartilage gradually increased from ∼300 kPa at 4 weeks, to ∼400 kPa at 8 weeks, and to ∼550 kPa at 12 weeks [153]. In cartilage tissue engineering, the development and use of different anabolic stimuli, in combination with mechanical loading signals, is emerging as a promising approach to enhance the compressive properties of neocartilage.
3.2.2. Tensile properties and corresponding ECM components
Improving the tensile properties of neocartilage has been one of the major challenges in cartilage tissue engineering [154]. Among the different growth factors used in articular cartilage engineering, TGF-β is the one that has most prominently been shown to improve tensile properties. Application of 30 ng/ml TGF-β1 has been shown to increase tensile properties by 2-fold over controls when applied continuously as a result of increased collagen synthesis [146,149]. The stimulatory effect of the application of dynamic, unconfined, compressive loading on chondrocyte-seeded agarose hydrogels at 0-10% strain and 1Hz was further enhanced by TGF-β1 leading to increased collagen production by ∼2-fold. [149]. Despite these advancements, improvements in tensile properties due to stimulation by growth factors are still substantially lower than native tissue values. This is true even when combining growth factors with compressive loading and hydrostatic pressure [152,149]. As a result, biophysical agents, such as chondroitinase-ABC (c-ABC), and enzymes, such as lysyl oxidase-like 2 (LOXL2), and ECM proteins, such as superficial zone protein (SZP), have been investigated to further improve the tensile properties of neocartilage.
Application of c-ABC to native tissue and to tissue-engineered cartilage results in cleavage of chondroitin and dermatan sulfate and immediate depletion of GAGs [155,156]. The depleted GAGs recover upon culture, bringing the compressive mechanical properties back to levels of untreated samples [157,155]. Counterintuitively, however, persistent effects of c-ABC treatment were observed; collagen production was enhanced by approximately 300%, and an increase of ∼180% in tensile properties was observed [157]. Multiple c-ABC applications result in improvement over a single application [157–159]. It has been hypothesized that this effect may be due to matrix-bound growth factors that are released upon c-ABC’s loosening of the cartilage matrix [160]. Another reason may be that c-ABC treatment induces cell proliferation [155]. Genetic microarrays, however, showed that c-ABC’s effects are mostly biophysical [161]. Trimethylamine N-oxide (TMNO), an osmolyte that is found in sharks, has been used in combination with c-ABC to improve tensile properties by ∼100% [159]. Cell proliferation, osmotic-loading, and protein stabilization due to TMNO have been hypothesized as potential routes of action. Additional studies that elucidate how, for instance, protein stabilization may work in concert with c-ABC to result in improved tensile properties may help to identify other protein stabilizing molecules to be used in cartilage tissue engineering.
Collagen cross-links represent a fundamental component of collagen organization and contribute significantly to the tensile properties of cartilaginous tissues [162,163,154]. In a novel application of exogenous LOXL2 in neocartilage, it was shown that the tensile properties were significantly improved by 5-fold through pyridinoline (PYR) cross-linking (16-fold increase in PYR cross-linking compared to control) [154]. This improvement in tensile properties was both time- and dose-dependent. High dose LOLX2 application improved the tensile modulus by ∼2-fold compared to low dose application, and early LOXL2 application led to a ∼3-fold increase compared to late application [154]. The improved properties persisted in vivo, with LOXL2 increasing collagen fibril density that also helped improve tensile properties [154]. Interestingly, hypoxia induced upregulation of LOX gene expression, resulting in increases in PYR crosslinks and tensile properties in engineered cartilage [164].
SZP is a glycoprotein that is produced mainly by synoviocytes and chondrocytes of the superficial cartilaginous zone [165]. A recent study that used chondrocytes from middle and superficial zones in different ratios demonstrated that chondrocytes in the superficial zone were able to improve tensile properties of the neocartilage. By increasing the ratio of superficial zone cells from 0% to 100%, the tensile modulus increased from 1.1 MPa to 4.5 MPa [166]. This increase was hypothesized to be due to increased synthesis of SZP, as it was demonstrated that SZP media accumulation increased from approximately 0.5 μg/ml to 3.5 μg/ml, respectively [167].
A few soluble molecules have been identified to increase tensile properties of engineered cartilage, but these are not as numerous when compared with molecules identified to improve cartilage compressive properties. Further, it appears that mechanical stimuli used to improve compressive properties do not have the same magnitude of effect on tensile properties. Combinations of growth factors, biophysical agents, ECM components, and even new classes of mechanical stimuli need to be explored with respect to engineering robust tensile properties in articular cartilage.
3.2.3. Mechanotransduction of signals
Cells convert physical forces into biochemical signals through mechanotransduction. Mechanotransduction can occur through various mechanisms, linked by cellular components such as tyrosine kinase receptors, ion channels, and various cytoskeletal filaments; these are termed mechanosensors [168]. These mechanosensors have been investigated with regard to their roles in mechanotransducing signals in chondrocytes, as described below.
As noted previously, mechanical stimuli, such as direct compression and hydrostatic pressure, can lead to changes in cell proliferation and matrix synthesis that, in turn, manifest as changes in mechanical properties of the engineered cartilage. Understanding how mechanical stimuli can alter proliferation and matrix synthesis is, thus, of importance for obtaining finer control of mechanical stimuli. An example is studying the role of ERK in mechanotransduction. In cartilage explants compressive static loading at 40% strain induced phosphorylation of ERK, a MAP kinase, leading to increased cell proliferation [169]. Application of 10 MPa of static hydrostatic pressure in tissue-engineered cartilage activated the ERK signaling pathway and led to increases in tensile properties by 70% [170,169]. Inhibition of the ERK pathway during hydrostatic pressure loading abolished this enhancement [170]. The ERK signaling pathway has been recognized as a major component of mechanotransduction; understanding of its exact role, as well as recognition of other signaling pathways involved, can serve as an important addition to the tissue engineering of articular cartilage.
Ion channels are important mechanosensors. Hydrostatic pressure application of 10 MPa inhibited Na+/K+ ion channels and activated Na+/H+ channels in chondrocytes, as shown by the effect on the Na+ and H+ exchange activity [171,172]. Modulation of Na+/K+ and calcium ion channels using inhibitors of Na+ ion transporters, such as ouabain and bumetanide, and stimulators of intracellular Ca2+, such as histamine and ionomycin, have been shown to result in increases in the mechanical properties of neocartilage [173]. Tensile properties reached 185% and 130% as stimulated by ouabain and ionomycin, respectively [173]. Mechanical stress in a form of fluid flow shear was also shown to enhance chondrocyte proliferation via activation of ion channels through Indian hedgehog and BMP-dependent pathways [174]. The exact roles that ion channel signaling play in mechanical transduction remain to be determined toward improvement of engineered cartilage functional properties.
Mechanotransduction through the cytoskeleton can begin with integrin-mediated adhesions that transmits forces from the ECM to cytoskeletal filaments [168]. Cadherins may also have a role in mechanotransduction due to their close interaction with both integrins and kinase receptors [168]. Disturbances of microtubule organization prevented the stimulatory effect of hydrostatic pressure loading on proteoglycan synthesis [175]. Specifically, microtubule de-polymerization induced by nocodazole, an anti-polymerization agent that is used as an anti-neoplasmatic agent, inhibited the increase of ∼20% in proteoglycan synthesis that would normally been exhibited from application of 5 MP cyclic hydrostatic pressure at 0.5 Hz [175]. Despite the fact that the mechanotransduction pathways involved in articular cartilage and the exact role of mechanosensors is yet to be determined, current knowledge indicates the beginning of an exciting era where modulation of these signaling pathways would potentially result in significant improvement of engineered tissue.
4. Current challenges in cartilage tissue engineering and future perspectives
Despite the progress in engineering biologically functional cartilage tissue in vitro with the aid of signaling molecules, challenges remain for its successful clinical translation. Issues often arise after the transplantation of engineered tissue in vivo, and these include phenotypic instability and poor integration (Figure 4). Inflammatory responses against implanted engineered tissue are also problematic. In the following section, reviewed are the major challenges in the current cartilage tissue engineering and the bioactive agents that exhibit potential toward resolving these challenges.
Fig. 4. Challenges in cartilage tissue engineering.
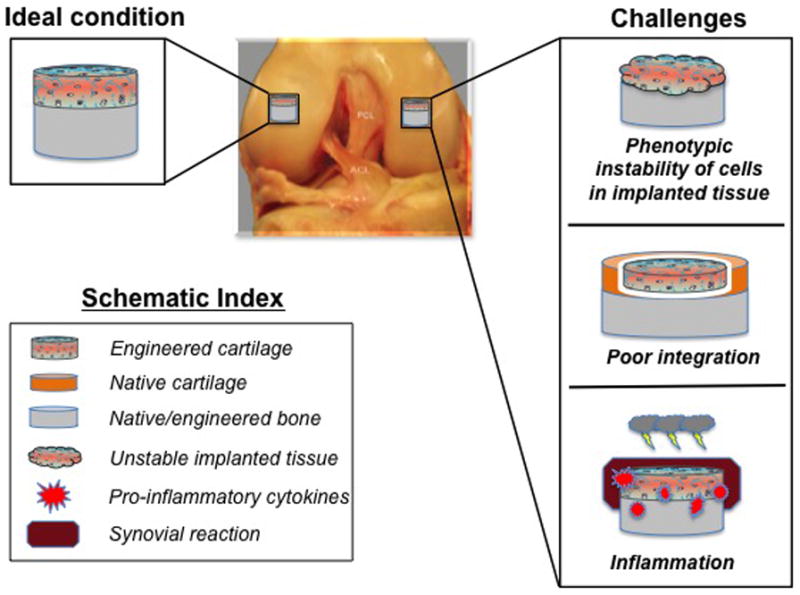
Phenotypic instability of engineered cartilage tissue, poor integration, and the inflammatory environment are disadvantages that need to be addressed toward successful cartilage tissue engineering for future clinical use.
4.1. Phenotypic instability
The phenotypic instability of engineered cartilage is a common problem. Implanted cells often contain undesired phenotypes that potentially lead to the formation of tissues that exhibit deficient biological and biomechanical functions. Frequently, cells with inferior chondrogenic ability form fibrous cartilage with properties that are not reminiscent of the physiological function of hyaline cartilage. Dedifferentiation of implanted cells with increased production of type I collagen leads to inferior cartilage matrix and mechanical function. Additionally, the appearance of hypertrophic phenotype in engineered tissue can also be problematic as it may promote mineralization. In stem cell-based treatments, in vitro chondrogenesis using adult stem cells is often accompanied by hypertrophic differentiation with increased expression of type X collagen. As an example, stem cells from different sources were shown to result in fibrous or hypertrophic phenotypes, when transplanted subcutaneously in vivo [176]. To suppress hypertrophic differentiation, several molecules have been explored. In addition to PTH and PTHrP described above, BMP-7 has been shown to inhibit type X collagen in human MSCs while also inducing chondrogenic differentiation [177]. A combination of SOX-5/-6/-9 was able to suppress hypertrophic markers and osteogenic markers in human MSCs [178]. Nkx3.2, a transcription factor involved in cartilage development, also inhibits RUNX-2 function [179], suggesting a potential use for preventing hypertrophy. With the increased desire of identifying and using stem cells for cartilage tissue engineering, retaining cells in a state of differentiated mature chondrocytes becomes of paramount importance and remains a main challenge for translation. Further improvement of current methodologies, as well as studies on potential bioactive agents to prevent these challenges, will be necessary.
Chondrocyte hypertrophy is commonly detected in osteoarthritis pathogenesis. Chondrocytes in osteoarthritis cartilage exhibit hypertrophic phenotypes by expressing type X collagen [180]. Although the characteristics of chondrocytes in osteoarthritis resemble those of chondrocytes in the growth plate, their pathways for controlling hypertrophy may be different [181]. The hypertrophic phenotype of chondrocytes in osteoarthritis is induced by many cytokines, growth factors, and ECM degradation products [181]. Thus, it is possible that the osteoarthritic environment can also contribute to hypertrophic differentiation of implanted cells for tissue engineering applications. Understanding hypertrophy processes in osteoarthritis, including signaling molecules and corresponding pathways, will be pivotal toward overcoming the challenge of phenotypic instability of engineered tissue.
4.2. Integration
Failure of integration between engineered tissue and surrounding native cartilage continues to be a fundamental problem in the field of cartilage tissue engineering. Cartilage lesions can also extend into the subchondral bone, making cartilage to cartilage, cartilage to bone, and bone to bone integration processes relevant for the clinical translation of cartilage implants. Several factors that influence the ability of the repair tissue to integrate with the native cartilage have been proposed, such as cell death at the wound edge, the phenotype of the cells in the implanted tissue, and donor age [182]. In addition, the degree of maturation of engineered constructs can affect integration [183].
Traditionally, cartilage tissue engineering has sought to stimulate the production of collagen and GAGs at levels similar to native tissue. Ironically, it has been shown that these matrix components can interfere with integration. The matrix of the native tissue can prevent adhesion and diffusion of cells and matrix proteins [184,185], which can impede the engineered tissue from integrating [185]. Strategies have, thus, been developed to disrupt the mature matrix in order to effect integration. Disruption of certain matrix molecules via enzymatic treatment has been shown to enhance integration. For example, collagenase and hyaluronidase applied to the wound site increased cell density as well as improved integration with implanted cartilage [186]. β-aminopropionitrile (BAPN), a blocker of lysyl oxidase, was used on tissue-engineered constructs to prevent crosslinks from forming in the engineered tissue, allowing for the presence of more crosslink sites on the sides of implanted tissue for enhanced integration [187]. Similarly, considering that the PYR crosslink in collagen can take weeks to complete forming, strategies have also included “priming” the implant with exogenous LOXL2 to accumulate collagen precursors. The concept is that, upon implantation, these crosslink precursors in the engineered cartilage will form mature crosslinks with the native cartilage [188].
Temporal depletion of GAG at cartilage surface by c-ABC or trypsin has also been shown to improve coverage by repair cells and integration of repair tissue, respectively [184,183]. These enzymes can be combined with the anabolic factors described previously to counteract their catabolic effects [161] or with factors and molecules that disrupt cartilage matrix formation, such as IGF-1, BAPN, and para-nitrophenyl-β-D-xyloside, which disrupts proteoglycan formation, to promote integration [189]. Although the presence of matrix components, such as GAG and collagen, in engineered cartilage is necessary to withstand stresses in vivo, their temporal absence may allow for robust integration. The dual roles that bioactive agents c-ABC and LOXL2 play in improving integration, as well as enhancing collagen production and tensile properties, signify that these agents should be part of the tissue engineering armamentarium toward resolving the as-of-yet intractable challenge of integration.
4.3. Inflammation
Inflammation is well-recognized as a main contributor in osteoarthritis development and progression, but the exact mechanisms of action of pro-inflammatory cytokines requires further clarification. Elevated levels of these cytokines in the osteoarthritic joint impair cartilage homeostasis by disrupting the balance between chondrocyte anabolic and catabolic activities. Inflammation mediated by interaction between the joint cartilage and surrounding tissues such as bone, muscle, adipose tissues, and synovium has also been suggested to contribute to the development and progression of osteoarthritis [190]. Notably, interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) are pro-inflammatory cytokines present in the arthritic joint and involved in the progression of cartilage destruction. Pro-inflammatory cytokine signals are transduced through the nuclear factor kappa B (NF-κB) pathway, resulting in the induction of nitric oxide (NO), cyclooxygenase2 (COX2), and prostaglandin E2 (PGE2) [191]. Several in vitro studies suggest the destructive effects of an inflammatory environment can influence implanted engineered cartilage tissue in vivo. Application of both IL-1β and TNF-α inhibited chondrogenesis of human MSCs in a dose-dependent manner through activation of the NF-κB pathway [192]. Osteoarthritic synovium-derived, conditioned medium has also shown to inhibit chondrogenesis of human MSCs [193]. Inflammation in the arthritic joint can potentially impede with neotissue growth and integration into the joint while also diminishing its functional properties. Thus, providing an environment controlling inflammation to reduce damage of the implanted tissue, as well as to maintain its functionality, may be necessary for successful cartilage repair.
In an attempt to prevent cartilage damage from inflammation, growth factors, GAG compounds, and platelet-rich plasma (PRP) have been investigated. Growth factors such as IGF-1 and PDGF-bb have shown to suppress IL-1β induced NF-κB activation and apoptosis in chondrocytes [194]. BMP-2 and BMP-9 recovered IL-1β-induced damage and partially blocked the suppressive effect of IL-1β on cartilage-specific matrix expression in human MSCs [195]. In addition to growth factors, some GAG compounds such as chondroitin sulfate, glucosamine, and hyaluronic acid have shown to have anti-inflammatory effects [196]. A synergistic action between hyaluronic acid and PRP has been observed in recovering the chondrogenic phenotype of osteoarthritic articular chondrocytes through activation of CD44 and TGF-β receptor II, and inhibiting expression of inflammation-related chemokines and cytokines [197].
PRP contains various growth factors, chemokines/cytokines, and adhesive proteins [198], and its use has been associated with reducing pain and restoring function in osteoarthritic joints [198,199]. Several reports demonstrated the potential use of PRP on cell proliferation and differentiation of cartilage cells and MSCs in vitro [199]. However, discrepancies within studies, possibly due to preparation methods and donor variance [199], means more studies should be performed to understand how PRP may influence engineered cartilage. Although anti-inflammatory products have been introduced to inhibit inflammation-induced damage, their exact roles and mechanisms are not clear. To effect successful cartilage regeneration, the role of degenerative changes on this process needs to be elucidated. Thus, comprehensive studies need to be conducted to establish the role of inflammation leading to osteoarthritis, as well as the role of anti-inflammatory agents as potential protectors of the implanted neotissue.
5. Conclusion
Current research in articular cartilage tissue engineering derives most of its inspiration from phenomena observed during cartilage development as well as homeostasis. Advances in bio-functionality of engineered cartilage, especially in terms of neotissue biochemical and biomechanical properties, in response to signaling molecules or matrix-modifying agents, have emanated from knowledge in development and normophysiology (Figure 5). However, we are still away from engineering neocartilage with biochemical and biomechanical properties on par with those of native articular cartilage. This continues to be a major challenge in cartilage tissue engineering, especially when one considers that the engineered tissue is frequently intended to operate in an inflammatory environment. Indeed, implanted engineered tissues face a complex plethora of stimuli in the arthritic joint, in addition to the highly strenuous environment. Furthermore, engineered tissues exhibit phenotypic instability and suffer from lack of integration as well in the recipient site.
Fig. 5. Future research in tissue engineering in vitro.
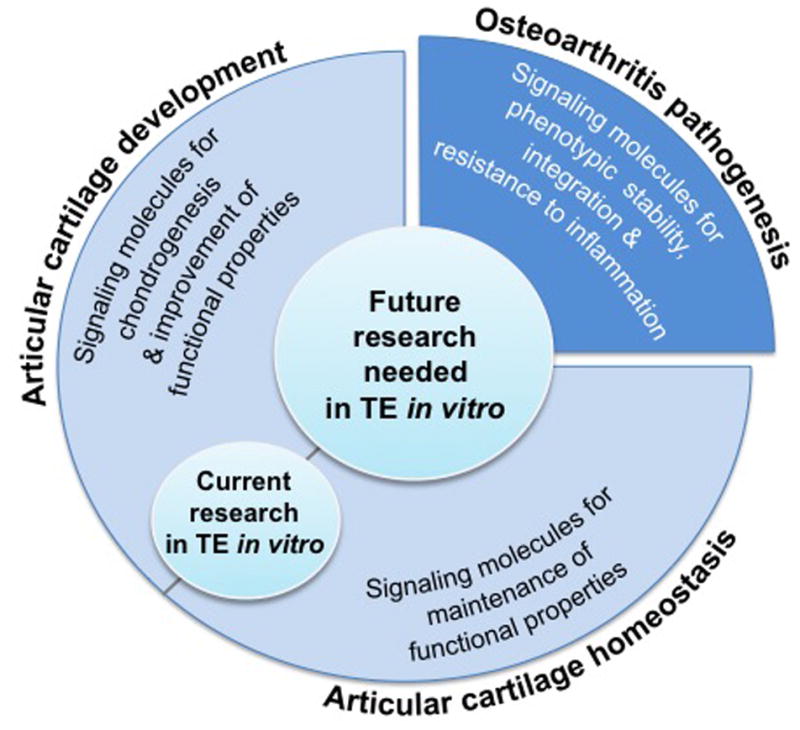
Current research is often informed by knowledge pertaining to articular cartilage development and homeostasis to tissue-engineer articular cartilage. Future research needs to continue to derive inspiration from these phases but also needs to incorporate information from OA pathogenesis to resolve the challenges in cartilage tissue engineering.
To address these challenges, it is informative to understand pathogenesis of osteoarthritis for two major reasons: 1) to identify catabolic agents or other pathological factors that can have a positive effect in the tissue engineering process, such as for example chondroitinase-ABC, and 2) to help us design an engineered tissue that, when implanted in vivo, can function in an inflammatory environment. Thus, for future research, it will be important not only to continue to derive inspiration from cartilage development and homeostasis but also to incorporate information from osteoarthritis pathogenesis.
Acknowledgments
We would like to acknowledge funding by NIH R01 AR067821, AR061496, and CIRM TR3-05709.
References
- 1.Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis and rheumatism. 2006;54(1):226–229. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease C, Prevention. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation--United States, 2010–2012. MMWR Morbidity and mortality weekly report. 2013;62(44):869–873. [PMC free article] [PubMed] [Google Scholar]
- 3.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F National Arthritis Data W. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis and rheumatism. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah MR, Kaplan KM, Meislin RJ, Bosco JA., 3rd Articular cartilage restoration of the knee. Bulletin of the NYU hospital for joint diseases. 2007;65(1):51–60. [PubMed] [Google Scholar]
- 5.Smith GD, Knutsen G, Richardson JB. A clinical review of cartilage repair techniques. The Journal of bone and joint surgery. 2005;87(4):445–449. doi: 10.1302/0301-620X.87B4.15971. [DOI] [PubMed] [Google Scholar]
- 6.Wieland HA, Michaelis M, Kirschbaum BJ, Rudolphi KA. Osteoarthritis - an untreatable disease? Nature reviews. 2005;4(4):331–344. doi: 10.1038/nrd1693. [DOI] [PubMed] [Google Scholar]
- 7.Elder SH, Cooley AJ, Jr, Borazjani A, Sowell BL, To H, Tran SC. Production of hyaline-like cartilage by bone marrow mesenchymal stem cells in a self-assembly model. Tissue engineering Part A. 2009;15(10):3025–3036. doi: 10.1089/ten.TEA.2008.0617. [DOI] [PubMed] [Google Scholar]
- 8.Hu JC, Athanasiou KA. A self-assembling process in articular cartilage tissue engineering. Tissue engineering. 2006;12(4):969–979. doi: 10.1089/ten.2006.12.969. [DOI] [PubMed] [Google Scholar]
- 9.Grande DA, Halberstadt C, Naughton G, Schwartz R, Manji R. Evaluation of matrix scaffolds for tissue engineering of articular cartilage grafts. Journal of biomedical materials research. 1997;34(2):211–220. doi: 10.1002/(sici)1097-4636(199702)34:2<211::aid-jbm10>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 10.DeLise AM, Fischer L, Tuan RS. Cellular interactions and signaling in cartilage development. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2000;8(5):309–334. doi: 10.1053/joca.1999.0306. [DOI] [PubMed] [Google Scholar]
- 11.Hall BK, Miyake T. Divide, accumulate, differentiate: cell condensation in skeletal development revisited. The International journal of developmental biology. 1995;39(6):881–893. [PubMed] [Google Scholar]
- 12.Demoor M, Ollitrault D, Gomez-Leduc T, Bouyoucef M, Hervieu M, Fabre H, Lafont J, Denoix JM, Audigie F, Mallein-Gerin F, Legendre F, Galera P. Cartilage tissue engineering: molecular control of chondrocyte differentiation for proper cartilage matrix reconstruction. Biochimica et biophysica acta. 2014;1840(8):2414–2440. doi: 10.1016/j.bbagen.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 13.Athanasiou KA, Darling Eric M, DuRaine Grayson D, Hu Jerry C, Reddi A Hari. Articular cartilage. CRC Press; Boca Raton, FL: 2013. [Google Scholar]
- 14.Umlauf D, Frank S, Pap T, Bertrand J. Cartilage biology, pathology, and repair. Cell Mol Life Sci. 2010;67(24):4197–4211. doi: 10.1007/s00018-010-0498-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Caestecker M. The transforming growth factor-beta superfamily of receptors. Cytokine & growth factor reviews. 2004;15(1):1–11. doi: 10.1016/j.cytogfr.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson CM, Schwarz EM, Reynolds PR, Puzas JE, Rosier RN, O’Keefe RJ. Smad2 and 3 mediate transforming growth factor-beta1-induced inhibition of chondrocyte maturation. Endocrinology. 2000;141(12):4728–4735. doi: 10.1210/endo.141.12.7848. [DOI] [PubMed] [Google Scholar]
- 17.Furumatsu T, Tsuda M, Taniguchi N, Tajima Y, Asahara H. Smad3 induces chondrogenesis through the activation of SOX9 via CREB-binding protein/p300 recruitment. The Journal of biological chemistry. 2005;280(9):8343–8350. doi: 10.1074/jbc.M413913200. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Zhao Z, Liu J, Huang N, Long D, Wang J, Li X, Liu Y. MEK/ERK and p38 MAPK regulate chondrogenesis of rat bone marrow mesenchymal stem cells through delicate interaction with TGF-beta1/Smads pathway. Cell proliferation. 2010;43(4):333–343. doi: 10.1111/j.1365-2184.2010.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pogue R, Lyons K. BMP signaling in the cartilage growth plate. Current topics in developmental biology. 2006;76:1–48. doi: 10.1016/S0070-2153(06)76001-X. [DOI] [PubMed] [Google Scholar]
- 20.Chimal-Monroy J, Diaz de Leon L. Expression of N-cadherin, N-CAM, fibronectin and tenascin is stimulated by TGF-beta1, beta2, beta3 and beta5 during the formation of precartilage condensations. The International journal of developmental biology. 1999;43(1):59–67. [PubMed] [Google Scholar]
- 21.Tuli R, Tuli S, Nandi S, Huang X, Manner PA, Hozack WJ, Danielson KG, Hall DJ, Tuan RS. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. The Journal of biological chemistry. 2003;278(42):41227–41236. doi: 10.1074/jbc.M305312200. [DOI] [PubMed] [Google Scholar]
- 22.Oberlender SA, Tuan RS. Expression and functional involvement of N-cadherin in embryonic limb chondrogenesis. Development. 1994;120(1):177–187. doi: 10.1242/dev.120.1.177. [DOI] [PubMed] [Google Scholar]
- 23.Widelitz RB, Jiang TX, Murray BA, Chuong CM. Adhesion molecules in skeletogenesis: II. Neural cell adhesion molecules mediate precartilaginous mesenchymal condensations and enhance chondrogenesis. Journal of cellular physiology. 1993;156(2):399–411. doi: 10.1002/jcp.1041560224. [DOI] [PubMed] [Google Scholar]
- 24.Frenz DA, Jaikaria NS, Newman SA. The mechanism of precartilage mesenchymal condensation: a major role for interaction of the cell surface with the amino-terminal heparin-binding domain of fibronectin. Dev Biol. 1989;136(1):97–103. doi: 10.1016/0012-1606(89)90133-4. [DOI] [PubMed] [Google Scholar]
- 25.Darling EM, Athanasiou KA. Growth factor impact on articular cartilage subpopulations. Cell and tissue research. 2005;322(3):463–473. doi: 10.1007/s00441-005-0020-4. [DOI] [PubMed] [Google Scholar]
- 26.Kulyk WM, Rodgers BJ, Greer K, Kosher RA. Promotion of embryonic chick limb cartilage differentiation by transforming growth factor-beta. Dev Biol. 1989;135(2):424–430. doi: 10.1016/0012-1606(89)90191-7. [DOI] [PubMed] [Google Scholar]
- 27.Furumatsu T, Ozaki T, Asahara H. Smad3 activates the Sox9-dependent transcription on chromatin. The international journal of biochemistry & cell biology. 2009;41(5):1198–1204. doi: 10.1016/j.biocel.2008.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Ziran N, Goater JJ, Schwarz EM, Puzas JE, Rosier RN, Zuscik M, Drissi H, O’Keefe RJ. Primary murine limb bud mesenchymal cells in long-term culture complete chondrocyte differentiation: TGF-beta delays hypertrophy and PGE2 inhibits terminal differentiation. Bone. 2004;34(5):809–817. doi: 10.1016/j.bone.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 29.Yang X, Chen L, Xu X, Li C, Huang C, Deng CX. TGF-beta/Smad3 signals repress chondrocyte hypertrophic differentiation and are required for maintaining articular cartilage. The Journal of cell biology. 2001;153(1):35–46. doi: 10.1083/jcb.153.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jonason JH, Xiao G, Zhang M, Xing L, Chen D. Post-translational Regulation of Runx2 in Bone and Cartilage. Journal of dental research. 2009;88(8):693–703. doi: 10.1177/0022034509341629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alliston T, Choy L, Ducy P, Karsenty G, Derynck R. TGF-beta-induced repression of CBFA1 by Smad3 decreases cbfa1 and osteocalcin expression and inhibits osteoblast differentiation. The EMBO journal. 2001;20(9):2254–2272. doi: 10.1093/emboj/20.9.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura K, Shirai T, Morishita S, Uchida S, Saeki-Miura K, Makishima F. p38 mitogen-activated protein kinase functionally contributes to chondrogenesis induced by growth/differentiation factor-5 in ATDC5 cells. Experimental cell research. 1999;250(2):351–363. doi: 10.1006/excr.1999.4535. [DOI] [PubMed] [Google Scholar]
- 33.Zehentner BK, Dony C, Burtscher H. The transcription factor Sox9 is involved in BMP-2 signaling. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 1999;14(10):1734–1741. doi: 10.1359/jbmr.1999.14.10.1734. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-Lloris R, Vinals F, Lopez-Rovira T, Harley V, Bartrons R, Rosa JL, Ventura F. Induction of the Sry-related factor SOX6 contributes to bone morphogenetic protein-2-induced chondroblastic differentiation of C3H10T1/2 cells. Molecular endocrinology. 2003;17(7):1332–1343. doi: 10.1210/me.2002-0254. [DOI] [PubMed] [Google Scholar]
- 35.Yoon BS, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(14):5062–5067. doi: 10.1073/pnas.0500031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao J, Hu N, Zhou N, Lin L, Zhao C, Yi S, Fan T, Bao W, Liang X, Chen H, Xu W, Chen C, Cheng Q, Zeng Y, Si W, Yang Z, Huang W. Sox9 potentiates BMP2-induced chondrogenic differentiation and inhibits BMP2-induced osteogenic differentiation. PloS one. 2014;9(2):e89025. doi: 10.1371/journal.pone.0089025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Luca F, Barnes KM, Uyeda JA, De-Levi S, Abad V, Palese T, Mericq V, Baron J. Regulation of growth plate chondrogenesis by bone morphogenetic protein-2. Endocrinology. 2001;142(1):430–436. doi: 10.1210/endo.142.1.7901. [DOI] [PubMed] [Google Scholar]
- 38.Tsumaki N, Nakase T, Miyaji T, Kakiuchi M, Kimura T, Ochi T, Yoshikawa H. Bone morphogenetic protein signals are required for cartilage formation and differently regulate joint development during skeletogenesis. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2002;17(5):898–906. doi: 10.1359/jbmr.2002.17.5.898. [DOI] [PubMed] [Google Scholar]
- 39.Leboy P, Grasso-Knight G, D’Angelo M, Volk SW, Lian JV, Drissi H, Stein GS, Adams SL. Smad-Runx interactions during chondrocyte maturation. The Journal of bone and joint surgery American volume. 2001;83-A(Suppl 1 Pt 1):S15–22. [PubMed] [Google Scholar]
- 40.Miljkovic ND, Cooper GM, Marra KG. Chondrogenesis, bone morphogenetic protein-4 and mesenchymal stem cells. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2008;16(10):1121–1130. doi: 10.1016/j.joca.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Danisovic L, Varga I, Polak S. Growth factors and chondrogenic differentiation of mesenchymal stem cells. Tissue & cell. 2012;44(2):69–73. doi: 10.1016/j.tice.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Chubinskaya S, Hurtig M, Rueger DC. OP-1/BMP-7 in cartilage repair. International orthopaedics. 2007;31(6):773–781. doi: 10.1007/s00264-007-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erlacher L, McCartney J, Piek E, ten Dijke P, Yanagishita M, Oppermann H, Luyten FP. Cartilage-derived morphogenetic proteins and osteogenic protein-1 differentially regulate osteogenesis. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 1998;13(3):383–392. doi: 10.1359/jbmr.1998.13.3.383. [DOI] [PubMed] [Google Scholar]
- 44.Takahara M, Harada M, Guan D, Otsuji M, Naruse T, Takagi M, Ogino T. Developmental failure of phalanges in the absence of growth/differentiation factor 5. Bone. 2004;35(5):1069–1076. doi: 10.1016/j.bone.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 45.Tsumaki N, Tanaka K, Arikawa-Hirasawa E, Nakase T, Kimura T, Thomas JT, Ochi T, Luyten FP, Yamada Y. Role of CDMP-1 in skeletal morphogenesis: promotion of mesenchymal cell recruitment and chondrocyte differentiation. The Journal of cell biology. 1999;144(1):161–173. doi: 10.1083/jcb.144.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Furumatsu T, Asahara H. Histone acetylation influences the activity of Sox9-related transcriptional complex. Acta medica Okayama. 2010;64(6):351–357. doi: 10.18926/AMO/41320. [DOI] [PubMed] [Google Scholar]
- 47.Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes & development. 2002;16(21):2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lefebvre V, Li P, de Crombrugghe B. A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. The EMBO journal. 1998;17(19):5718–5733. doi: 10.1093/emboj/17.19.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smits P, Li P, Mandel J, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B, Lefebvre V. The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Developmental cell. 2001;1(2):277–290. doi: 10.1016/s1534-5807(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 50.Brent AE, Braun T, Tabin CJ. Genetic analysis of interactions between the somitic muscle, cartilage and tendon cell lineages during mouse development. Development. 2005;132(3):515–528. doi: 10.1242/dev.01605. [DOI] [PubMed] [Google Scholar]
- 51.Bi W, Huang W, Whitworth DJ, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(12):6698–6703. doi: 10.1073/pnas.111092198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akiyama H, Lyons JP, Mori-Akiyama Y, Yang X, Zhang R, Zhang Z, Deng JM, Taketo MM, Nakamura T, Behringer RR, McCrea PD, de Crombrugghe B. Interactions between Sox9 and beta-catenin control chondrocyte differentiation. Genes & development. 2004;18(9):1072–1087. doi: 10.1101/gad.1171104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leung VY, Gao B, Leung KK, Melhado IG, Wynn SL, Au TY, Dung NW, Lau JY, Mak AC, Chan D, Cheah KS. SOX9 governs differentiation stage-specific gene expression in growth plate chondrocytes via direct concomitant transactivation and repression. PLoS genetics. 2011;7(11):e1002356. doi: 10.1371/journal.pgen.1002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou G, Zheng Q, Engin F, Munivez E, Chen Y, Sebald E, Krakow D, Lee B. Dominance of SOX9 function over RUNX2 during skeletogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(50):19004–19009. doi: 10.1073/pnas.0605170103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hattori T, Muller C, Gebhard S, Bauer E, Pausch F, Schlund B, Bosl MR, Hess A, Surmann-Schmitt C, von der Mark H, de Crombrugghe B, von der Mark K. SOX9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification. Development. 2010;137(6):901–911. doi: 10.1242/dev.045203. [DOI] [PubMed] [Google Scholar]
- 56.Oh CD, Chun JS. Signaling mechanisms leading to the regulation of differentiation and apoptosis of articular chondrocytes by insulin-like growth factor-1. The Journal of biological chemistry. 2003;278(38):36563–36571. doi: 10.1074/jbc.M304857200. [DOI] [PubMed] [Google Scholar]
- 57.Schoenle E, Zapf J, Humbel RE, Froesch ER. Insulin-like growth factor I stimulates growth in hypophysectomized rats. Nature. 1982;296(5854):252–253. doi: 10.1038/296252a0. [DOI] [PubMed] [Google Scholar]
- 58.Vetter U, Zapf J, Heit W, Helbing G, Heinze E, Froesch ER, Teller WM. Human fetal and adult chondrocytes. Effect of insulinlike growth factors I and II, insulin, and growth hormone on clonal growth. The Journal of clinical investigation. 1986;77(6):1903–1908. doi: 10.1172/JCI112518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Longobardi L, O’Rear L, Aakula S, Johnstone B, Shimer K, Chytil A, Horton WA, Moses HL, Spagnoli A. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-beta signaling. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2006;21(4):626–636. doi: 10.1359/jbmr.051213. [DOI] [PubMed] [Google Scholar]
- 60.Renard E, Poree B, Chadjichristos C, Kypriotou M, Maneix L, Bigot N, Legendre F, Ollitrault D, De Crombrugghe B, Mallein-Gerin F, Moslemi S, Demoor M, Boumediene K, Galera P. Sox9/Sox6 and Sp1 are involved in the insulin-like growth factor-I-mediated upregulation of human type II collagen gene expression in articular chondrocytes. Journal of molecular medicine. 2012;90(6):649–666. doi: 10.1007/s00109-011-0842-3. [DOI] [PubMed] [Google Scholar]
- 61.Karsenty G, Kronenberg HM, Settembre C. Genetic control of bone formation. Annual review of cell and developmental biology. 2009;25:629–648. doi: 10.1146/annurev.cellbio.042308.113308. [DOI] [PubMed] [Google Scholar]
- 62.Yang L, Tsang KY, Tang HC, Chan D, Cheah KS. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(33):12097–12102. doi: 10.1073/pnas.1302703111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang E, Wang J, Chin E, Zhou J, Bondy CA. Cellular patterns of insulin-like growth factor system gene expression in murine chondrogenesis and osteogenesis. Endocrinology. 1995;136(6):2741–2751. doi: 10.1210/endo.136.6.7750499. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y, Cheng Z, Elalieh HZ, Nakamura E, Nguyen MT, Mackem S, Clemens TL, Bikle DD, Chang W. IGF-1R signaling in chondrocytes modulates growth plate development by interacting with the PTHrP/Ihh pathway. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2011;26(7):1437–1446. doi: 10.1002/jbmr.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ornitz DM, Itoh N. Fibroblast growth factors. Genome biology. 2001;2(3):REVIEWS3005. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bellus GA, Gaudenz K, Zackai EH, Clarke LA, Szabo J, Francomano CA, Muenke M. Identical mutations in three different fibroblast growth factor receptor genes in autosomal dominant craniosynostosis syndromes. Nature genetics. 1996;14(2):174–176. doi: 10.1038/ng1096-174. [DOI] [PubMed] [Google Scholar]
- 67.Rousseau F, Bonaventure J, Legeai-Mallet L, Pelet A, Rozet JM, Maroteaux P, Le Merrer M, Munnich A. Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia. Nature. 1994;371(6494):252–254. doi: 10.1038/371252a0. [DOI] [PubMed] [Google Scholar]
- 68.Shiang R, Thompson LM, Zhu YZ, Church DM, Fielder TJ, Bocian M, Winokur ST, Wasmuth JJ. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994;78(2):335–342. doi: 10.1016/0092-8674(94)90302-6. [DOI] [PubMed] [Google Scholar]
- 69.Wilkie AO, Slaney SF, Oldridge M, Poole MD, Ashworth GJ, Hockley AD, Hayward RD, David DJ, Pulleyn LJ, Rutland P, et al. Apert syndrome results from localized mutations of FGFR2 and is allelic with Crouzon syndrome. Nature genetics. 1995;9(2):165–172. doi: 10.1038/ng0295-165. [DOI] [PubMed] [Google Scholar]
- 70.Jabs EW, Li X, Scott AF, Meyers G, Chen W, Eccles M, Mao JI, Charnas LR, Jackson CE, Jaye M. Jackson-Weiss and Crouzon syndromes are allelic with mutations in fibroblast growth factor receptor 2. Nature genetics. 1994;8(3):275–279. doi: 10.1038/ng1194-275. [DOI] [PubMed] [Google Scholar]
- 71.Reardon W, Winter RM, Rutland P, Pulleyn LJ, Jones BM, Malcolm S. Mutations in the fibroblast growth factor receptor 2 gene cause Crouzon syndrome. Nature genetics. 1994;8(1):98–103. doi: 10.1038/ng0994-98. [DOI] [PubMed] [Google Scholar]
- 72.Schell U, Hehr A, Feldman GJ, Robin NH, Zackai EH, de Die-Smulders C, Viskochil DH, Stewart JM, Wolff G, Ohashi H, et al. Mutations in FGFR1 and FGFR2 cause familial and sporadic Pfeiffer syndrome. Human molecular genetics. 1995;4(3):323–328. doi: 10.1093/hmg/4.3.323. [DOI] [PubMed] [Google Scholar]
- 73.Muenke M, Schell U, Hehr A, Robin NH, Losken HW, Schinzel A, Pulleyn LJ, Rutland P, Reardon W, Malcolm S, et al. A common mutation in the fibroblast growth factor receptor 1 gene in Pfeiffer syndrome. Nature genetics. 1994;8(3):269–274. doi: 10.1038/ng1194-269. [DOI] [PubMed] [Google Scholar]
- 74.Ornitz DM, Marie PJ. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes & development. 2002;16(12):1446–1465. doi: 10.1101/gad.990702. [DOI] [PubMed] [Google Scholar]
- 75.Ito T, Sawada R, Fujiwara Y, Tsuchiya T. FGF-2 increases osteogenic and chondrogenic differentiation potentials of human mesenchymal stem cells by inactivation of TGF-beta signaling. Cytotechnology. 2008;56(1):1–7. doi: 10.1007/s10616-007-9092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Peters KG, Werner S, Chen G, Williams LT. Two FGF receptor genes are differentially expressed in epithelial and mesenchymal tissues during limb formation and organogenesis in the mouse. Development. 1992;114(1):233–243. doi: 10.1242/dev.114.1.233. [DOI] [PubMed] [Google Scholar]
- 77.Deng CX, Wynshaw-Boris A, Shen MM, Daugherty C, Ornitz DM, Leder P. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes & development. 1994;8(24):3045–3057. doi: 10.1101/gad.8.24.3045. [DOI] [PubMed] [Google Scholar]
- 78.Murakami S, Kan M, McKeehan WL, de Crombrugghe B. Up-regulation of the chondrogenic Sox9 gene by fibroblast growth factors is mediated by the mitogen-activated protein kinase pathway. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(3):1113–1118. doi: 10.1073/pnas.97.3.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ellman MB, Yan D, Ahmadinia K, Chen D, An HS, Im HJ. Fibroblast growth factor control of cartilage homeostasis. Journal of cellular biochemistry. 2013;114(4):735–742. doi: 10.1002/jcb.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valta MP, Hentunen T, Qu Q, Valve EM, Harjula A, Seppanen JA, Vaananen HK, Harkonen PL. Regulation of osteoblast differentiation: a novel function for fibroblast growth factor 8. Endocrinology. 2006;147(5):2171–2182. doi: 10.1210/en.2005-1502. [DOI] [PubMed] [Google Scholar]
- 81.Raucci A, Laplantine E, Mansukhani A, Basilico C. Activation of the ERK1/2 and p38 mitogen-activated protein kinase pathways mediates fibroblast growth factor-induced growth arrest of chondrocytes. The Journal of biological chemistry. 2004;279(3):1747–1756. doi: 10.1074/jbc.M310384200. [DOI] [PubMed] [Google Scholar]
- 82.Luyten FP, Hascall VC, Nissley SP, Morales TI, Reddi AH. Insulin-like growth factors maintain steady-state metabolism of proteoglycans in bovine articular cartilage explants. Archives of biochemistry and biophysics. 1988;267(2):416–425. doi: 10.1016/0003-9861(88)90047-1. [DOI] [PubMed] [Google Scholar]
- 83.Delezoide AL, Benoist-Lasselin C, Legeai-Mallet L, Le Merrer M, Munnich A, Vekemans M, Bonaventure J. Spatio-temporal expression of FGFR 1, 2 and 3 genes during human embryo-fetal ossification. Mechanisms of development. 1998;77(1):19–30. doi: 10.1016/s0925-4773(98)00133-6. [DOI] [PubMed] [Google Scholar]
- 84.Liu Z, Lavine KJ, Hung IH, Ornitz DM. FGF18 is required for early chondrocyte proliferation, hypertrophy and vascular invasion of the growth plate. Developmental biology. 2007;302(1):80–91. doi: 10.1016/j.ydbio.2006.08.071. [DOI] [PubMed] [Google Scholar]
- 85.Hung IH, Yu K, Lavine KJ, Ornitz DM. FGF9 regulates early hypertrophic chondrocyte differentiation and skeletal vascularization in the developing stylopod. Developmental biology. 2007;307(2):300–313. doi: 10.1016/j.ydbio.2007.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cleary MA, van Osch GJ, Brama PA, Hellingman CA, Narcisi R. FGF, TGFbeta and Wnt crosstalk: embryonic to in vitro cartilage development from mesenchymal stem cells. Journal of tissue engineering and regenerative medicine. 2015;9(4):332–342. doi: 10.1002/term.1744. [DOI] [PubMed] [Google Scholar]
- 87.Schmal H, Zwingmann J, Fehrenbach M, Finkenzeller G, Stark GB, Sudkamp NP, Hartl D, Mehlhorn AT. bFGF influences human articular chondrocyte differentiation. Cytotherapy. 2007;9(2):184–193. doi: 10.1080/14653240601182846. [DOI] [PubMed] [Google Scholar]
- 88.Loeser RF, Pacione CA, Chubinskaya S. The combination of insulin-like growth factor 1 and osteogenic protein 1 promotes increased survival of and matrix synthesis by normal and osteoarthritic human articular chondrocytes. Arthritis and rheumatism. 2003;48(8):2188–2196. doi: 10.1002/art.11209. [DOI] [PubMed] [Google Scholar]
- 89.Loeser RF, Chubinskaya S, Pacione C, Im HJ. Basic fibroblast growth factor inhibits the anabolic activity of insulin-like growth factor 1 and osteogenic protein 1 in adult human articular chondrocytes. Arthritis and rheumatism. 2005;52(12):3910–3917. doi: 10.1002/art.21472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yaeger PC, Masi TL, de Ortiz JL, Binette F, Tubo R, McPherson JM. Synergistic action of transforming growth factor-beta and insulin-like growth factor-I induces expression of type II collagen and aggrecan genes in adult human articular chondrocytes. Experimental cell research. 1997;237(2):318–325. doi: 10.1006/excr.1997.3781. [DOI] [PubMed] [Google Scholar]
- 91.Claus S, Aubert-Foucher E, Demoor M, Camuzeaux B, Paumier A, Piperno M, Damour O, Duterque-Coquillaud M, Galera P, Mallein-Gerin F. Chronic exposure of bone morphogenetic protein-2 favors chondrogenic expression in human articular chondrocytes amplified in monolayer cultures. Journal of cellular biochemistry. 2010;111(6):1642–1651. doi: 10.1002/jcb.22897. [DOI] [PubMed] [Google Scholar]
- 92.Sailor LZ, Hewick RM, Morris EA. Recombinant human bone morphogenetic protein-2 maintains the articular chondrocyte phenotype in long-term culture. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 1996;14(6):937–945. doi: 10.1002/jor.1100140614. [DOI] [PubMed] [Google Scholar]
- 93.Stewart MC, Saunders KM, Burton-Wurster N, Macleod JN. Phenotypic stability of articular chondrocytes in vitro: the effects of culture models, bone morphogenetic protein 2, and serum supplementation. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2000;15(1):166–174. doi: 10.1359/jbmr.2000.15.1.166. [DOI] [PubMed] [Google Scholar]
- 94.Lin L, Zhou C, Wei X, Hou Y, Zhao L, Fu X, Zhang J, Yu C. Articular cartilage repair using dedifferentiated articular chondrocytes and bone morphogenetic protein 4 in a rabbit model of articular cartilage defects. Arthritis and rheumatism. 2008;58(4):1067–1075. doi: 10.1002/art.23380. [DOI] [PubMed] [Google Scholar]
- 95.Jakob M, Demarteau O, Schafer D, Hintermann B, Dick W, Heberer M, Martin I. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. Journal of cellular biochemistry. 2001;81(2):368–377. doi: 10.1002/1097-4644(20010501)81:2<368::aid-jcb1051>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 96.Murphy MK, Huey DJ, Hu JC, Athanasiou KA. TGF-beta1, GDF-5, and BMP-2 stimulation induces chondrogenesis in expanded human articular chondrocytes and marrow-derived stromal cells. Stem cells. 2015;33(3):762–773. doi: 10.1002/stem.1890. [DOI] [PubMed] [Google Scholar]
- 97.Das R, Timur UT, Edip S, Haak E, Wruck C, Weinans H, Jahr H. TGF-beta2 is involved in the preservation of the chondrocyte phenotype under hypoxic conditions. Annals of anatomy = Anatomischer Anzeiger: official organ of the Anatomische Gesellschaft. 2015;198:1–10. doi: 10.1016/j.aanat.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 98.Foldager CB, Nielsen AB, Munir S, Ulrich-Vinther M, Soballe K, Bunger C, Lind M. Combined 3D and hypoxic culture improves cartilage-specific gene expression in human chondrocytes. Acta orthopaedica. 2011;82(2):234–240. doi: 10.3109/17453674.2011.566135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Solchaga LA, Penick K, Porter JD, Goldberg VM, Caplan AI, Welter JF. FGF-2 enhances the mitotic and chondrogenic potentials of human adult bone marrow-derived mesenchymal stem cells. Journal of cellular physiology. 2005;203(2):398–409. doi: 10.1002/jcp.20238. [DOI] [PubMed] [Google Scholar]
- 100.Kim JH, Lee MC, Seong SC, Park KH, Lee S. Enhanced proliferation and chondrogenic differentiation of human synovium-derived stem cells expanded with basic fibroblast growth factor. Tissue engineering Part A. 2011;17(7–8):991–1002. doi: 10.1089/ten.TEA.2010.0277. [DOI] [PubMed] [Google Scholar]
- 101.Kabiri A, Esfandiari E, Hashemibeni B, Kazemi M, Mardani M, Esmaeili A. Effects of FGF-2 on human adipose tissue derived adult stem cells morphology and chondrogenesis enhancement in Transwell culture. Biochem Biophys Res Commun. 2012;424(2):234–238. doi: 10.1016/j.bbrc.2012.06.082. [DOI] [PubMed] [Google Scholar]
- 102.Indrawattana N, Chen G, Tadokoro M, Shann LH, Ohgushi H, Tateishi T, Tanaka J, Bunyaratvej A. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem Biophys Res Commun. 2004;320(3):914–919. doi: 10.1016/j.bbrc.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 103.Sekiya I, Larson BL, Vuoristo JT, Reger RL, Prockop DJ. Comparison of effect of BMP-2, -4, and -6 on in vitro cartilage formation of human adult stem cells from bone marrow stroma. Cell and tissue research. 2005;320(2):269–276. doi: 10.1007/s00441-004-1075-3. [DOI] [PubMed] [Google Scholar]
- 104.Kim HJ, Im GI. Combination of transforming growth factor-beta2 and bone morphogenetic protein 7 enhances chondrogenesis from adipose tissue-derived mesenchymal stem cells. Tissue engineering Part A. 2009;15(7):1543–1551. doi: 10.1089/ten.tea.2008.0368. [DOI] [PubMed] [Google Scholar]
- 105.Shirasawa S, Sekiya I, Sakaguchi Y, Yagishita K, Ichinose S, Muneta T. In vitro chondrogenesis of human synovium-derived mesenchymal stem cells: optimal condition and comparison with bone marrow-derived cells. Journal of cellular biochemistry. 2006;97(1):84–97. doi: 10.1002/jcb.20546. [DOI] [PubMed] [Google Scholar]
- 106.Pei M, He F, Vunjak-Novakovic G. Synovium-derived stem cell-based chondrogenesis. Differentiation; research in biological diversity. 2008;76(10):1044–1056. doi: 10.1111/j.1432-0436.2008.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Duval E, Bauge C, Andriamanalijaona R, Benateau H, Leclercq S, Dutoit S, Poulain L, Galera P, Boumediene K. Molecular mechanism of hypoxia-induced chondrogenesis and its application in in vivo cartilage tissue engineering. Biomaterials. 2012;33(26):6042–6051. doi: 10.1016/j.biomaterials.2012.04.061. [DOI] [PubMed] [Google Scholar]
- 108.Tian HT, Zhang B, Tian Q, Liu Y, Yang SH, Shao ZW. Construction of self-assembled cartilage tissue from bone marrow mesenchymal stem cells induced by hypoxia combined with GDF-5. Journal of Huazhong University of Science and Technology Medical sciences = Hua zhong ke ji da xue xue bao Yi xue Ying De wen ban = Huazhong keji daxue xuebao Yixue Yingdewen ban. 2013;33(5):700–706. doi: 10.1007/s11596-013-1183-y. [DOI] [PubMed] [Google Scholar]
- 109.Kim YJ, Kim HJ, Im GI. PTHrP promotes chondrogenesis and suppresses hypertrophy from both bone marrow-derived and adipose tissue-derived MSCs. Biochem Biophys Res Commun. 2008;373(1):104–108. doi: 10.1016/j.bbrc.2008.05.183. [DOI] [PubMed] [Google Scholar]
- 110.Mwale F, Yao G, Ouellet JA, Petit A, Antoniou J. Effect of parathyroid hormone on type X and type II collagen expression in mesenchymal stem cells from osteoarthritic patients. Tissue engineering Part A. 2010;16(11):3449–3455. doi: 10.1089/ten.TEA.2010.0091. [DOI] [PubMed] [Google Scholar]
- 111.Park S, Im GI. Embryonic stem cells and induced pluripotent stem cells for skeletal regeneration. Tissue engineering Part B, Reviews. 2014;20(5):381–391. doi: 10.1089/ten.TEB.2013.0530. [DOI] [PubMed] [Google Scholar]
- 112.Koay EJ, Hoben GM, Athanasiou KA. Tissue engineering with chondrogenically differentiated human embryonic stem cells. Stem cells. 2007;25(9):2183–2190. doi: 10.1634/stemcells.2007-0105. [DOI] [PubMed] [Google Scholar]
- 113.Bai HY, Chen GA, Mao GH, Song TR, Wang YX. Three step derivation of cartilage like tissue from human embryonic stem cells by 2D-3D sequential culture in vitro and further implantation in vivo on alginate/PLGA scaffolds. Journal of biomedical materials research Part A. 2010;94(2):539–546. doi: 10.1002/jbm.a.32732. [DOI] [PubMed] [Google Scholar]
- 114.Nakagawa T, Lee SY, Reddi AH. Induction of chondrogenesis from human embryonic stem cells without embryoid body formation by bone morphogenetic protein 7 and transforming growth factor beta1. Arthritis and rheumatism. 2009;60(12):3686–3692. doi: 10.1002/art.27229. [DOI] [PubMed] [Google Scholar]
- 115.Koay EJ, Athanasiou KA. Hypoxic chondrogenic differentiation of human embryonic stem cells enhances cartilage protein synthesis and biomechanical functionality. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2008;16(12):1450–1456. doi: 10.1016/j.joca.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 116.Yodmuang S, Marolt D, Marcos-Campos I, Gadjanski I, Vunjak-Novakovic G. Synergistic effects of hypoxia and morphogenetic factors on early chondrogenic commitment of human embryonic stem cells in embryoid body culture. Stem cell reviews. 2015;11(2):228–241. doi: 10.1007/s12015-015-9584-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 118.Okita K, Matsumura Y, Sato Y, Okada A, Morizane A, Okamoto S, Hong H, Nakagawa M, Tanabe K, Tezuka K, Shibata T, Kunisada T, Takahashi M, Takahashi J, Saji H, Yamanaka S. A more efficient method to generate integration-free human iPS cells. Nature methods. 2011;8(5):409–412. doi: 10.1038/nmeth.1591. [DOI] [PubMed] [Google Scholar]
- 119.Zhou W, Freed CR. Adenoviral gene delivery can reprogram human fibroblasts to induced pluripotent stem cells. Stem cells. 2009;27(11):2667–2674. doi: 10.1002/stem.201. [DOI] [PubMed] [Google Scholar]
- 120.Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science. 2009;324(5928):797–801. doi: 10.1126/science.1172482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, Kim KS. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell stem cell. 2009;4(6):472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nejadnik H, Diecke S, Lenkov OD, Chapelin F, Donig J, Tong X, Derugin N, Chan RC, Gaur A, Yang F, Wu JC, Daldrup-Link HE. Improved approach for chondrogenic differentiation of human induced pluripotent stem cells. Stem cell reviews. 2015;11(2):242–253. doi: 10.1007/s12015-014-9581-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Koyama N, Miura M, Nakao K, Kondo E, Fujii T, Taura D, Kanamoto N, Sone M, Yasoda A, Arai H, Bessho K, Nakao K. Human induced pluripotent stem cells differentiated into chondrogenic lineage via generation of mesenchymal progenitor cells. Stem cells and development. 2013;22(1):102–113. doi: 10.1089/scd.2012.0127. [DOI] [PubMed] [Google Scholar]
- 124.Guzzo RM, Gibson J, Xu RH, Lee FY, Drissi H. Efficient differentiation of human iPSC-derived mesenchymal stem cells to chondroprogenitor cells. Journal of cellular biochemistry. 2013;114(2):480–490. doi: 10.1002/jcb.24388. [DOI] [PubMed] [Google Scholar]
- 125.Medvedev SP, Grigor’eva EV, Shevchenko AI, Malakhova AA, Dementyeva EV, Shilov AA, Pokushalov EA, Zaidman AM, Aleksandrova MA, Plotnikov EY, Sukhikh GT, Zakian SM. Human induced pluripotent stem cells derived from fetal neural stem cells successfully undergo directed differentiation into cartilage. Stem cells and development. 2011;20(6):1099–1112. doi: 10.1089/scd.2010.0249. [DOI] [PubMed] [Google Scholar]
- 126.Yamashita A, Morioka M, Yahara Y, Okada M, Kobayashi T, Kuriyama S, Matsuda S, Tsumaki N. Generation of scaffoldless hyaline cartilaginous tissue from human iPSCs. Stem cell reports. 2015;4(3):404–418. doi: 10.1016/j.stemcr.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hiramatsu K, Sasagawa S, Outani H, Nakagawa K, Yoshikawa H, Tsumaki N. Generation of hyaline cartilaginous tissue from mouse adult dermal fibroblast culture by defined factors. The Journal of clinical investigation. 2011;121(2):640–657. doi: 10.1172/JCI44605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Outani H, Okada M, Yamashita A, Nakagawa K, Yoshikawa H, Tsumaki N. Direct induction of chondrogenic cells from human dermal fibroblast culture by defined factors. PloS one. 2013;8(10):e77365. doi: 10.1371/journal.pone.0077365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.French MM, Rose S, Canseco J, Athanasiou KA. Chondrogenic differentiation of adult dermal fibroblasts. Annals of biomedical engineering. 2004;32(1):50–56. doi: 10.1023/b:abme.0000007790.65773.e0. [DOI] [PubMed] [Google Scholar]
- 130.Yin S, Cen L, Wang C, Zhao G, Sun J, Liu W, Cao Y, Cui L. Chondrogenic transdifferentiation of human dermal fibroblasts stimulated with cartilage-derived morphogenetic protein 1. Tissue engineering Part A. 2010;16(5):1633–1643. doi: 10.1089/ten.TEA.2009.0570. [DOI] [PubMed] [Google Scholar]
- 131.Vapniarsky N, Arzi B, Hu JC, Nolta JA, Athanasiou KA. Concise Review: Human Dermis as an Autologous Source of Stem Cells for Tissue Engineering and Regenerative Medicine. Stem cells translational medicine. 2015 doi: 10.5966/sctm.2015-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Toma JG, McKenzie IA, Bagli D, Miller FD. Isolation and characterization of multipotent skin-derived precursors from human skin. Stem cells. 2005;23(6):727–737. doi: 10.1634/stemcells.2004-0134. [DOI] [PubMed] [Google Scholar]
- 133.Lavoie JF, Biernaskie JA, Chen Y, Bagli D, Alman B, Kaplan DR, Miller FD. Skin-derived precursors differentiate into skeletogenic cell types and contribute to bone repair. Stem cells and development. 2009;18(6):893–906. doi: 10.1089/scd.2008.0260. [DOI] [PubMed] [Google Scholar]
- 134.Junker JP, Sommar P, Skog M, Johnson H, Kratz G. Adipogenic, chondrogenic and osteogenic differentiation of clonally derived human dermal fibroblasts. Cells, tissues, organs. 2010;191(2):105–118. doi: 10.1159/000232157. [DOI] [PubMed] [Google Scholar]
- 135.Chen FG, Zhang WJ, Bi D, Liu W, Wei X, Chen FF, Zhu L, Cui L, Cao Y. Clonal analysis of nestin(−) vimentin(+) multipotent fibroblasts isolated from human dermis. Journal of cell science. 2007;120(Pt 16):2875–2883. doi: 10.1242/jcs.03478. [DOI] [PubMed] [Google Scholar]
- 136.Sanchez-Adams J, Athanasiou KA. Dermis isolated adult stem cells for cartilage tissue engineering. Biomaterials. 2012;33(1):109–119. doi: 10.1016/j.biomaterials.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 137.Kalpakci KN, Brown WE, Hu JC, Athanasiou KA. Cartilage tissue engineering using dermis isolated adult stem cells: the use of hypoxia during expansion versus chondrogenic differentiation. PloS one. 2014;9(5):e98570. doi: 10.1371/journal.pone.0098570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Baker DE, Harrison NJ, Maltby E, Smith K, Moore HD, Shaw PJ, Heath PR, Holden H, Andrews PW. Adaptation to culture of human embryonic stem cells and oncogenesis in vivo. Nature biotechnology. 2007;25(2):207–215. doi: 10.1038/nbt1285. [DOI] [PubMed] [Google Scholar]
- 139.Andrews PW, Matin MM, Bahrami AR, Damjanov I, Gokhale P, Draper JS. Embryonic stem (ES) cells and embryonal carcinoma (EC) cells: opposite sides of the same coin. Biochemical Society transactions. 2005;33(Pt 6):1526–1530. doi: 10.1042/BST20051526. [DOI] [PubMed] [Google Scholar]
- 140.Rosland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, Mysliwietz J, Tonn JC, Goldbrunner R, Lonning PE, Bjerkvig R, Schichor C. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer research. 2009;69(13):5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- 141.Heslop JA, Hammond TG, Santeramo I, Tort Piella A, Hopp I, Zhou J, Baty R, Graziano EI, Proto Marco B, Caron A, Skold P, Andrews PW, Baxter MA, Hay DC, Hamdam J, Sharpe ME, Patel S, Jones DR, Reinhardt J, Danen EH, Ben-David U, Stacey G, Bjorquist P, Piner J, Mills J, Rowe C, Pellegrini G, Sethu S, Antoine DJ, Cross MJ, Murray P, Williams DP, Kitteringham NR, Goldring CE, Park BK. Concise review: workshop review: understanding and assessing the risks of stem cell-based therapies. Stem cells translational medicine. 2015;4(4):389–400. doi: 10.5966/sctm.2014-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Murphy MK, Huey DJ, Reimer AJ, Hu JC, Athanasiou KA. Enhancing post-expansion chondrogenic potential of costochondral cells in self-assembled neocartilage. PloS one. 2013;8(2):e56983. doi: 10.1371/journal.pone.0056983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.DuRaine G, Neu CP, Chan SM, Komvopoulos K, June RK, Reddi AH. Regulation of the friction coefficient of articular cartilage by TGF-beta1 and IL-1beta. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2009;27(2):249–256. doi: 10.1002/jor.20713. [DOI] [PubMed] [Google Scholar]
- 144.Blunk T, Sieminski AL, Gooch KJ, Courter DL, Hollander AP, Nahir AM, Langer R, Vunjak-Novakovic G, Freed LE. Differential effects of growth factors on tissue-engineered cartilage. Tissue engineering. 2002;8(1):73–84. doi: 10.1089/107632702753503072. [DOI] [PubMed] [Google Scholar]
- 145.Gooch KJ, Blunk T, Courter DL, Sieminski AL, Vunjak-Novakovic G, Freed LE. Bone morphogenetic proteins-2, -12, and -13 modulate in vitro development of engineered cartilage. Tissue engineering. 2002;8(4):591–601. doi: 10.1089/107632702760240517. [DOI] [PubMed] [Google Scholar]
- 146.Elder BD, Athanasiou KA. Systematic assessment of growth factor treatment on biochemical and biomechanical properties of engineered articular cartilage constructs. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2009;17(1):114–123. doi: 10.1016/j.joca.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 1989;7(5):619–636. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 148.Bonassar LJ, Grodzinsky AJ, Frank EH, Davila SG, Bhaktav NR, Trippel SB. The effect of dynamic compression on the response of articular cartilage to insulin-like growth factor-I. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2001;19(1):11–17. doi: 10.1016/S0736-0266(00)00004-8. [DOI] [PubMed] [Google Scholar]
- 149.Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue engineering. 2003;9(4):597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 150.Elder BD, Athanasiou KA. Effects of temporal hydrostatic pressure on tissue-engineered bovine articular cartilage constructs. Tissue engineering Part A. 2009;15(5):1151–1158. doi: 10.1089/ten.tea.2008.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Miyanishi K, Trindade MC, Lindsey DP, Beaupre GS, Carter DR, Goodman SB, Schurman DJ, Smith RL. Effects of hydrostatic pressure and transforming growth factor-beta 3 on adult human mesenchymal stem cell chondrogenesis in vitro. Tissue engineering. 2006;12(6):1419–1428. doi: 10.1089/ten.2006.12.1419. [DOI] [PubMed] [Google Scholar]
- 152.Elder BD, Athanasiou KA. Synergistic and additive effects of hydrostatic pressure and growth factors on tissue formation. PloS one. 2008;3(6):e2341. doi: 10.1371/journal.pone.0002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kim SH, Kim SH, Jung Y. TGF-beta3 encapsulated PLCL scaffold by a supercritical CO2-HFIP co-solvent system for cartilage tissue engineering. Journal of controlled release: official journal of the Controlled Release Society. 2015;206:101–107. doi: 10.1016/j.jconrel.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 154.Makris EA, Responte DJ, Paschos NK, Hu JC, Athanasiou KA. Developing functional musculoskeletal tissues through hypoxia and lysyl oxidase-induced collagen cross-linking. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(45):E4832–4841. doi: 10.1073/pnas.1414271111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Natoli RM, Revell CM, Athanasiou KA. Chondroitinase ABC treatment results in greater tensile properties of self-assembled tissue-engineered articular cartilage. Tissue engineering Part A. 2009;15(10):3119–3128. doi: 10.1089/ten.TEA.2008.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Asanbaeva A, Masuda K, Thonar EJ, Klisch SM, Sah RL. Mechanisms of cartilage growth: modulation of balance between proteoglycan and collagen in vitro using chondroitinase ABC. Arthritis and rheumatism. 2007;56(1):188–198. doi: 10.1002/art.22298. [DOI] [PubMed] [Google Scholar]
- 157.Natoli RM, Responte DJ, Lu BY, Athanasiou KA. Effects of multiple chondroitinase ABC applications on tissue engineered articular cartilage. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2009;27(7):949–956. doi: 10.1002/jor.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.O’Connell GD, Nims RJ, Green J, Cigan AD, Ateshian GA, Hung CT. Time and dose-dependent effects of chondroitinase ABC on growth of engineered cartilage. European cells & materials. 2014;27:312–320. doi: 10.22203/ecm.v027a22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.O’Connell GD, Fong JV, Dunleavy N, Joffe A, Ateshian GA, Hung CT. Trimethylamine N-oxide as a media supplement for cartilage tissue engineering. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2012;30(12):1898–1905. doi: 10.1002/jor.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nandini CD, Sugahara K. Role of the sulfation pattern of chondroitin sulfate in its biological activities and in the binding of growth factors. Advances in pharmacology. 2006;53:253–279. doi: 10.1016/S1054-3589(05)53012-6. [DOI] [PubMed] [Google Scholar]
- 161.Responte DJ, Arzi B, Natoli RM, Hu JC, Athanasiou KA. Mechanisms underlying the synergistic enhancement of self-assembled neocartilage treated with chondroitinase-ABC and TGF-beta1. Biomaterials. 2012;33(11):3187–3194. doi: 10.1016/j.biomaterials.2012.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bastiaansen-Jenniskens YM, Koevoet W, de Bart AC, van der Linden JC, Zuurmond AM, Weinans H, Verhaar JA, van Osch GJ, Degroot J. Contribution of collagen network features to functional properties of engineered cartilage. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2008;16(3):359–366. doi: 10.1016/j.joca.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 163.Ahsan T, Harwood F, McGowan KB, Amiel D, Sah RL. Kinetics of collagen crosslinking in adult bovine articular cartilage. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2005;13(8):709–715. doi: 10.1016/j.joca.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 164.Makris EA, Hu JC, Athanasiou KA. Hypoxia-induced collagen crosslinking as a mechanism for enhancing mechanical properties of engineered articular cartilage. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2013;21(4):634–641. doi: 10.1016/j.joca.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Schumacher BL, Hughes CE, Kuettner KE, Caterson B, Aydelotte MB. Immunodetection and partial cDNA sequence of the proteoglycan, superficial zone protein, synthesized by cells lining synovial joints. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 1999;17(1):110–120. doi: 10.1002/jor.1100170117. [DOI] [PubMed] [Google Scholar]
- 166.Peng G, McNary SM, Athanasiou KA, Reddi AH. Surface zone articular chondrocytes modulate the bulk and surface mechanical properties of the tissue-engineered cartilage. Tissue engineering Part A. 2014;20(23–24):3332–3341. doi: 10.1089/ten.TEA.2014.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Peng G, McNary SM, Athanasiou KA, Reddi AH. The distribution of superficial zone protein (SZP)/lubricin/PRG4 and boundary mode frictional properties of the bovine diarthrodial joint. Journal of biomechanics. 2015 doi: 10.1016/j.jbiomech.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nature reviews Molecular cell biology. 2009;10(1):75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 169.Ryan JA, Eisner EA, DuRaine G, You Z, Reddi AH. Mechanical compression of articular cartilage induces chondrocyte proliferation and inhibits proteoglycan synthesis by activation of the ERK pathway: implications for tissue engineering and regenerative medicine. Journal of tissue engineering and regenerative medicine. 2009;3(2):107–116. doi: 10.1002/term.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.DuRaine GD, Athanasiou KA. ERK activation is required for hydrostatic pressure-induced tensile changes in engineered articular cartilage. Journal of tissue engineering and regenerative medicine. 2015;9(4):368–374. doi: 10.1002/term.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hall AC. Differential effects of hydrostatic pressure on cation transport pathways of isolated articular chondrocytes. Journal of cellular physiology. 1999;178(2):197–204. doi: 10.1002/(SICI)1097-4652(199902)178:2<197::AID-JCP9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 172.Browning JA, Walker RE, Hall AC, Wilkins RJ. Modulation of Na+ x H+ exchange by hydrostatic pressure in isolated bovine articular chondrocytes. Acta physiologica Scandinavica. 1999;166(1):39–45. doi: 10.1046/j.1365-201x.1999.00534.x. [DOI] [PubMed] [Google Scholar]
- 173.Natoli RM, Skaalure S, Bijlani S, Chen KX, Hu J, Athanasiou KA. Intracellular Na(+) and Ca(2+) modulation increases the tensile properties of developing engineered articular cartilage. Arthritis and rheumatism. 2010;62(4):1097–1107. doi: 10.1002/art.27313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wu Q, Zhang Y, Chen Q. Indian hedgehog is an essential component of mechanotransduction complex to stimulate chondrocyte proliferation. The Journal of biological chemistry. 2001;276(38):35290–35296. doi: 10.1074/jbc.M101055200. [DOI] [PubMed] [Google Scholar]
- 175.Jortikka MO, Parkkinen JJ, Inkinen RI, Karner J, Jarvelainen HT, Nelimarkka LO, Tammi MI, Lammi MJ. The role of microtubules in the regulation of proteoglycan synthesis in chondrocytes under hydrostatic pressure. Archives of biochemistry and biophysics. 2000;374(2):172–180. doi: 10.1006/abbi.1999.1543. [DOI] [PubMed] [Google Scholar]
- 176.Vinardell T, Sheehy EJ, Buckley CT, Kelly DJ. A comparison of the functionality and in vivo phenotypic stability of cartilaginous tissues engineered from different stem cell sources. Tissue engineering Part A. 2012;18(11–12):1161–1170. doi: 10.1089/ten.TEA.2011.0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Caron MM, Emans PJ, Cremers A, Surtel DA, Coolsen MM, van Rhijn LW, Welting TJ. Hypertrophic differentiation during chondrogenic differentiation of progenitor cells is stimulated by BMP-2 but suppressed by BMP-7. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2013;21(4):604–613. doi: 10.1016/j.joca.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 178.Ikeda T, Kamekura S, Mabuchi A, Kou I, Seki S, Takato T, Nakamura K, Kawaguchi H, Ikegawa S, Chung UI. The combination of SOX5, SOX6, and SOX9 (the SOX trio) provides signals sufficient for induction of permanent cartilage. Arthritis and rheumatism. 2004;50(11):3561–3573. doi: 10.1002/art.20611. [DOI] [PubMed] [Google Scholar]
- 179.Lengner CJ, Hassan MQ, Serra RW, Lepper C, van Wijnen AJ, Stein JL, Lian JB, Stein GS. Nkx3.2-mediated repression of Runx2 promotes chondrogenic differentiation. The Journal of biological chemistry. 2005;280(16):15872–15879. doi: 10.1074/jbc.M411144200. [DOI] [PubMed] [Google Scholar]
- 180.von der Mark K, Kirsch T, Nerlich A, Kuss A, Weseloh G, Gluckert K, Stoss H. Type X collagen synthesis in human osteoarthritic cartilage. Indication of chondrocyte hypertrophy. Arthritis and rheumatism. 1992;35(7):806–811. doi: 10.1002/art.1780350715. [DOI] [PubMed] [Google Scholar]
- 181.Pitsillides AA, Beier F. Cartilage biology in osteoarthritis--lessons from developmental biology. Nature reviews Rheumatology. 2011;7(11):654–663. doi: 10.1038/nrrheum.2011.129. [DOI] [PubMed] [Google Scholar]
- 182.Khan IM, Gilbert SJ, Singhrao SK, Duance VC, Archer CW. Cartilage integration: evaluation of the reasons for failure of integration during cartilage repair. A review. European cells & materials. 2008;16:26–39. doi: 10.22203/ecm.v016a04. [DOI] [PubMed] [Google Scholar]
- 183.Obradovic B, Martin I, Padera RF, Treppo S, Freed LE, Vunjak-Novakovic G. Integration of engineered cartilage. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2001;19(6):1089–1097. doi: 10.1016/S0736-0266(01)00030-4. [DOI] [PubMed] [Google Scholar]
- 184.Hunziker EB, Kapfinger E. Removal of proteoglycans from the surface of defects in articular cartilage transiently enhances coverage by repair cells. The Journal of bone and joint surgery. 1998;80(1):144–150. doi: 10.1302/0301-620x.80b1.7531. [DOI] [PubMed] [Google Scholar]
- 185.Rice MA, Homier PM, Waters KR, Anseth KS. Effects of directed gel degradation and collagenase digestion on the integration of neocartilage produced by chondrocytes encapsulated in hydrogel carriers. Journal of tissue engineering and regenerative medicine. 2008;2(7):418–429. doi: 10.1002/term.113. [DOI] [PubMed] [Google Scholar]
- 186.van de Breevaart Bravenboer J, In der Maur CD, Bos PK, Feenstra L, Verhaar JA, Weinans H, van Osch GJ. Improved cartilage integration and interfacial strength after enzymatic treatment in a cartilage transplantation model. Arthritis research & therapy. 2004;6(5):R469–476. doi: 10.1186/ar1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.McGowan KB, Sah RL. Treatment of cartilage with beta-aminopropionitrile accelerates subsequent collagen maturation and modulates integrative repair. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. 2005;23(3):594–601. doi: 10.1016/j.orthres.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 188.Makris EA, MacBarb RF, Paschos NK, Hu JC, Athanasiou KA. Combined use of chondroitinase-ABC, TGF-beta1, and collagen crosslinking agent lysyl oxidase to engineer functional neotissues for fibrocartilage repair. Biomaterials. 2014;35(25):6787–6796. doi: 10.1016/j.biomaterials.2014.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bastiaansen-Jenniskens YM, Koevoet W, Feijt C, Bos PK, Verhaar JA, Van Osch GJ, DeGroot J. Proteoglycan production is required in initial stages of new cartilage matrix formation but inhibits integrative cartilage repair. Journal of tissue engineering and regenerative medicine. 2009;3(2):117–123. doi: 10.1002/term.147. [DOI] [PubMed] [Google Scholar]
- 190.Rainbow R, Ren W, Zeng L. Inflammation and Joint Tissue Interactions in OA: Implications for Potential Therapeutic Approaches. Arthritis. 2012;2012:741582. doi: 10.1155/2012/741582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004;(427 Suppl):S27–36. doi: 10.1097/01.blo.0000144854.66565.8f. [DOI] [PubMed] [Google Scholar]
- 192.Wehling N, Palmer GD, Pilapil C, Liu F, Wells JW, Muller PE, Evans CH, Porter RM. Interleukin-1beta and tumor necrosis factor alpha inhibit chondrogenesis by human mesenchymal stem cells through NF-kappaB-dependent pathways. Arthritis and rheumatism. 2009;60(3):801–812. doi: 10.1002/art.24352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Heldens GT, Blaney Davidson EN, Vitters EL, Schreurs BW, Piek E, van den Berg WB, van der Kraan PM. Catabolic factors and osteoarthritis-conditioned medium inhibit chondrogenesis of human mesenchymal stem cells. Tissue engineering Part A. 2012;18(1–2):45–54. doi: 10.1089/ten.TEA.2011.0083. [DOI] [PubMed] [Google Scholar]
- 194.Montaseri A, Busch F, Mobasheri A, Buhrmann C, Aldinger C, Rad JS, Shakibaei M. IGF-1 and PDGF-bb suppress IL-1beta-induced cartilage degradation through down-regulation of NF-kappaB signaling: involvement of Src/PI-3K/AKT pathway. PloS one. 2011;6(12):e28663. doi: 10.1371/journal.pone.0028663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Majumdar MK, Wang E, Morris EA. BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. Journal of cellular physiology. 2001;189(3):275–284. doi: 10.1002/jcp.10025. [DOI] [PubMed] [Google Scholar]
- 196.Henrotin Y, Lambert C, Richette P. Importance of synovitis in osteoarthritis: evidence for the use of glycosaminoglycans against synovial inflammation. Seminars in arthritis and rheumatism. 2014;43(5):579–587. doi: 10.1016/j.semarthrit.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 197.Chen WH, Lo WC, Hsu WC, Wei HJ, Liu HY, Lee CH, Tina Chen SY, Shieh YH, Williams DF, Deng WP. Synergistic anabolic actions of hyaluronic acid and platelet-rich plasma on cartilage regeneration in osteoarthritis therapy. Biomaterials. 2014;35(36):9599–9607. doi: 10.1016/j.biomaterials.2014.07.058. [DOI] [PubMed] [Google Scholar]
- 198.Andia I, Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nature reviews Rheumatology. 2013;9(12):721–730. doi: 10.1038/nrrheum.2013.141. [DOI] [PubMed] [Google Scholar]
- 199.Zhu Y, Yuan M, Meng HY, Wang AY, Guo QY, Wang Y, Peng J. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: a review. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2013;21(11):1627–1637. doi: 10.1016/j.joca.2013.07.017. [DOI] [PubMed] [Google Scholar]


