Abstract
Social deficits in autism spectrum disorder (ASD) are linked to amygdala functioning and functional connection between the amygdala and subgenual anterior cingulate cortex (sACC) is involved in the modulation of amygdala activity. Impairments in behavioral symptoms and amygdala activation and connectivity with the sACC seem to vary by serotonin transporter-linked polymorphic region (5-HTTLPR) variant genotype in diverse populations. The current preliminary investigation examines whether amygdala-sACC connectivity differs by 5-HTTLPR genotype and relates to social functioning in ASD. A sample of 108 children and adolescents (44 ASD) completed an fMRI face-processing task. Youth with ASD and low expressing 5-HTTLPR genotypes showed significantly greater connectivity than youth with ASD and higher expressing genotypes as well as typically developing (TD) individuals with both low and higher expressing genotypes, in the comparison of happy vs. baseline faces and happy vs. neutral faces. Moreover, individuals with ASD and higher expressing genotypes exhibit a negative relationship between amygdala-sACC connectivity and social dysfunction. Altered amygdala-sACC coupling based on 5-HTTLPR genotype may help explain some of the heterogeneity in neural and social function observed in ASD. This is the first ASD study to combine genetic polymorphism analyses and functional connectivity in the context of a social task.
1. Introduction
Autism spectrum disorder (ASD) is a complex neurodevelopmental condition characterized by heterogeneous deficits in social interaction and communication (APA, 2013). Social deficits in ASD are linked to abnormalities in the functioning of the amygdala, a limbic structure involved in processing salient, often emotionally charged, stimuli (Baron-Cohen et al. 1999; Critchley et al. 2000; Kleinhans et al. 2009; Monk et al. 2010; Weng et al. 2011). For example, Kleinhans et al. (2009) showed that lower rates of amygdala habituation to emotion are related to more severe social impairment based on social affect subscale scores from the Autism Diagnostic Observation Schedule (ADOS; Lord et al. 2000), and Swartz et al. (2013) found that lower habituation rates are related to greater impairment based on the Social Responsiveness Scale (SRS; Constantino et al. 2003), a more general measure of impairment (Hus et al. 2013). Early fMRI studies on amygdala functioning reported that ASD groups display reduced activation in this region relative to TD groups in response to emotional faces (Baron-Cohen et al. 1999; Critchley et al. 2000; Pierce et al. 2001), whereas more recent ASD studies found amygdala hyperarousal with a shorter stimulus presentation time (Monk et al. 2010; Weng et al. 2011; Kliemann et al. 2012). Such discrepant findings have warranted further investigation into the structures modulating amygdala activity.
The subgenual anterior cingulate cortex (sACC) has dense cortical connections to the amygdala, and is involved in amygdala inhibition (McDonald, 1998; Milad and Quirk, 2012; Ray and Zald, 2012), possibly through excitatory signaling to inhibitory regions of the amygdala (Rosenkranz and Grace, 2001). Connectivity between the sACC and the amygdala is implicated in the extinction of negative affect (Milad et al., 2002; Maren and Quirk, 2004) and is linked to various mood disorders (Pezawas et al. 2005; Drevets, 2008).
Studies focusing on typically developing individuals (Bertolino et al. 2005; Canli et al. 2005; Hariri et al. 2005; Pezawas et al. 2005; Wiggins et al. 2014b) link variation in amygdala functioning and amygdala-sACC connectivity to serotonin transporter-linked polymorphic region (5-HTTLPR) genotypes. If 5-HTTLPR genotype affects amygdala functioning, amygdala-sACC connectivity, and socioemotional behavior across diagnoses, it may also affect connectivity, and as a possible consequence, variation in amygdala activity and social behavior in ASD.
5-HTTLPR is a functional polymorphism found in the promoter region of the gene that encodes the serotonin transporter (SERT), a protein that modulates serotonin function (Hu et al. 2006). The 5-HTTLPR polymorphism directly affects SERT expression and consequently serotonin function in that individuals with the short variant (S) produce less SERT mRNA and protein than individuals with the long (L) variant, thus creating increased levels of serotonin in the synaptic cleft. TD individuals who are carriers of 5-HTTLPR short (S) alleles exhibit increased anxiety related traits (Canli and Lesch, 2007), depressive symptoms (Caspi et al. 2003), as well decreased functional connectivity between the amygdala and the sACC when looking at fearful faces (Pezawas et al. 2005).
Whereas studies have found inconclusive evidence of an association between 5-HTTLPR and susceptibility to ASD (e.g. Cook et al. 1997; Devlin et al. 2005; Zhong et al. 1999; Ramoz et al. 2006), various reports highlight the effect of genotype on the severity of social dysfunction in ASD, which may be related to amygdala functioning and its connectivity with the sACC. For example, Tordjman et al. (2001) found evidence of increased severity in the Combined Social and Communication domain subset of the Autism Diagnostic Interview-Revised (ADI-R; Lord et al. 1994) in participants with the S allele compared to those homozygous for the L allele. Similarly, Brune et al., (2006) found an effect of 5-HTTLPR genotype on non-verbal social interaction severity where participants with S/S or S/L genotypes displayed more severity compared to those with the L/L genotype; individuals with L/L genotypes exhibited increased severity of restricted and repetitive behaviors. There are reports however, pointing to more severe social deficits in participants homozygous for the L allele (Gadow et al. 2012), which contradicts previous data. In sum, 5-HTTLPR seems to affect aspects of social dysfunction in ASD, despite a lack of association between 5-HTTLPR and susceptibility to ASD.
5-HTTLPR can be subdivided into further allelic variants (Nakamura et al. 2000). Short (S) alleles and long (L) alleles with genotypes that contain the G (i.e. SG and LG) variants are associated with decreased SERT expression compared to individuals with long alleles and genotypes that contain the A variant (i.e. LA; Hu et al. 2006). Recent studies have grouped S/S, S/LG and LG/LG genotypes as low expressing genotypes of the 5-HTTLPR polymorphism (Gadow et al, 2012; Wiggins 2014a; 2014b). Wiggins et al. (2013b) examined the influence of 5-HTTLPR on resting connectivity in ASD and found that youth with the low expressing genotypes exhibited stronger connectivity in the default network than higher expressing genotype groups, whereas the opposite was true for the TD group. In addition, youth with low expressing genotypes show decreased amygdala habituation (i.e., more sustained activation over time) in response to emotional faces (Wiggins et al. 2014a).
In sum, data do not point to 5-HTTLPR as a susceptibility gene for ASD; nevertheless, impairments in behavioral symptoms and differences in brain function vary by genotype within individuals with ASD as well as in TD individuals. Thus, we examined whether amygdala-sACC connectivity differs by genotype and relate to social functioning in ASD. We hypothesized a diagnosis (ASD vs. TD) by 5-HTTLPR genotype (low vs. higher) interaction on amygdala-sACC connectivity to specific emotional faces. We further tested whether amygdala-sACC connectivity was related to social symptoms within ASD.
2. Method
2.1 Participants
We recruited 187 participants. The final sample consisted of 43 individuals with ASD (Mean age=13.5, SD=3.26) and 65 TD individuals (Mean age = 14.7, SD = 3.73), ranging from 8 to 19 years of age. Reasons for data exclusion were: excessive head motion (maximum displacement >2.25 mm translation or 2.25° rotation) in any direction compared to the initial position, lack of amygdala or sACC coverage (below 90%), insufficient data after motion censoring (<60% of total time points censored), scoring below our accuracy cutoff (70%) in the gender identification task performed in the scanner, absent or incomplete magnetic resonance imaging (MRI) scan due to discomfort, fMRI technical problems, or because participants did not provide a saliva sample for genotyping (See Supplementary Materials section 1.1 for details). Participants considered for the study did not have metal in their bodies and other physical or neurological conditions that are contrary to the MRI safety guidelines. Preliminary analyses yielded no significant differences in verbal and non-verbal cognitive function tests between diagnosis groups (ASD and TD, low and higher expressing genotypes; see Table 1 in Supplementary Materials for additional detail). These data overlap with prior studies from our research group (Swartz et al. 2013, 2014; Weng et al. 2010, 2011; Wiggins et al. 2014a, 2014b).
Individuals with ASD were diagnosed at the University of Michigan Autism and Communication Disorders Center (UMACC). Diagnoses were based on the ADOS, ADI-R, and clinical expertise (Lord et al. 2006). TD individuals were recruited using flyers posted in approved posting areas around Ann Arbor, Michigan. The Institutional Review Boards of the University of Michigan Medical School oversaw and approved the methods and procedures conducted by this study. Adult participants and parents of minors signed informed consent forms. Participants under the age of 18 also gave assent.
Participant demographic and behavioral measures were obtained in a data-collection visit prior to their MRI scan. Pubertal development was measured using the Pubertal Development Scale (Petersen et al. 1988). Verbal and non-verbal cognitive function tests were measured using the Peabody Picture Vocabulary Test (Dunn et al. 1997), the Differential Ability Scales (DAS), the Stanford-Binet Intelligence Scales, the Wechsler Intelligence Scale for Children, or the Ravens Progressive Matrices (Raven, 1960). Aside from MRI safety exclusion, we also assessed for developmental, emotional, and behavioral conditions using the Child Depression Inventory (CDI; Kovacs, 1992), Multidimensional Anxiety Scale for Children (MASC; March et al. 1997), Child Behavior Checklist (CBCL; Achenbach, 1991), and Spence Children’s Anxiety Scale (Spence, 1998). Preliminary tests looking at these behavioral measures among diagnosis groups (ASD vs. TD; see Table 1 in Supplementary Materials) yielded no significant differences.
2.2 5-HTTLPR Procedures
Genetic analyses were performed using saliva samples collected with Genotek Oragene DNA kits (DNA Genotek, Kanata, Canada). The first step in the analysis was to differentiate between S and L alleles of 5-HTTLPR. For this, we used polymerase chain reaction (PCR) and agarose gel electrophoresis. To find whether the L allele presented an A or a G SNP, we used Sanger sequencing (Hu et al. 2006). Individuals with ASD and TD individuals were subdivided into low expressing and higher expressing genotype groups. Low expressing genotype groups were composed of individuals with S/S, S/LG and LG/LG genotypes. The higher expressing genotype groups were composed of individuals with medium and high expressing alleles LA/LA, S/LA, and LA/LG genotypes. S and LG alleles were grouped as they have been shown to drive 5-HTTLPR expression nearly equivalently (Hu et al. 2006). These groups were also chosen to address sample-size concerns that stem from subdividing by L alleles into smaller groups, similar to past studies (Cicchetti et al. 2007) as well to correspond to the data from our previous studies (Wiggins et al. 2013; Wiggins et al. 2014a). Fifteen individuals with ASD displayed low expressing genotypes and 29 displayed higher expressing genotypes. Within the TD group, 23 individuals displayed low expressing genotypes and 42 displayed higher expressing genotypes. Hardy-Weinberg Equilibrium tests were performed on 5-HTTLPR genotypes (S/S, S/L, L/L) within each group. Hardy-Weinberg equilibrium was not met in the TD group, χ2 (1, N=65) = 5.53, p=0.02). However, Hardy-Weinberg equilibrium was met in the ASD group, χ2 (1, N=44) = 0.36, p=0.55. In analyses containing all participants who provided a saliva sample for genotyping, both ASD, χ2 (1, N=89) = 0.0112, p=0.9156, and TD groups, χ2 (1, N=79) = 3.59, p=0.0581, met Hardy-Weinberg equilibrium.
2.3 Procedural Tasks
A face-processing task (Fig. 1) was presented to the participants inside the scanner. Faces used were part of the NimStim Set (Tottenham et al. 2009). Faces presented were model numbers: 1, 7, 10, 12, 15, 16, 17, 20, 23, 25, 30, 34, 38, 40, and 42 of this stimulus set. Each expression was presented 15 times, each time by a different model in a randomized order, for a total of 60 faces. The expressions presented were fearful, happy, neutral, or sad faces. Half of the faces presented were male and half were female. Eight models were European-American, four were African-American, and the remaining three models were Asian-American.
Figure 1.
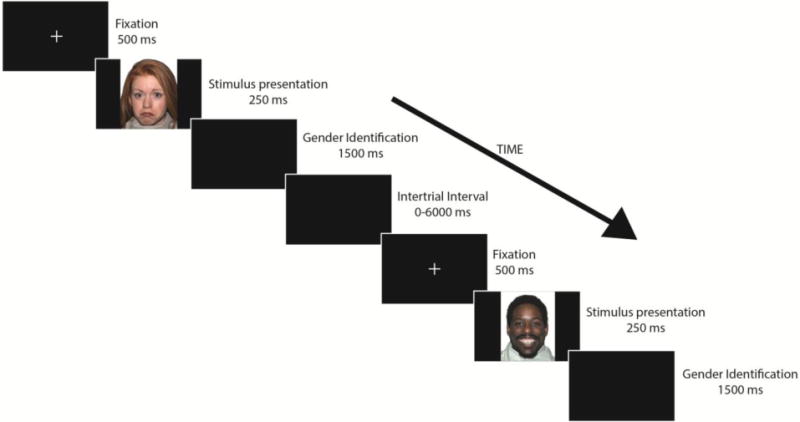
Depiction of face-processing task displays the order and duration of each stage in the procedure. Gender identification stage was the time participants were given to indicate the gender of the person displayed in the stimulus presentation. Intertrial interval ranged from 0 to 6000 ms with intervals of 2000 ms. A total of 60 faces (15 per emotion) were presented. The stimuli used were obtained from the NimStim Set (Tottenham et al. 2009).
Faces were presented for 250 ms to avoid group differences in attention found in studies where stimuli were presented for longer periods of time (Klin et al. 2002; Pelphrey et al. 2002). We used E-prime (Psychological Software Tools, Pittsburgh, PA) to display the stimuli and record the responses. Before every face presentation, a black screen with a white fixation cross was presented for 500 ms. Participants were given 1500 ms after every face presentation to identify the gender of the person in the stimulus via button press. A randomized intertrial interval (ITI) followed the response and ranged from 0 to 6000 ms with intervals of 2000 ms. The ITI represented the implicit baseline. Univariate ANOVAs were used to test for differences in reaction time and accuracy in our face-processing task among the four diagnosis-by-genotype subgroups.
After the scanning procedure, we acquired emotion recognition accuracy data by presenting participants with 120 trials showing the same expressions as in the fMRI task. Each emotion was shown 30 times. Participants were given a laptop equipped with E-Prime and were instructed to indicate what emotion they saw in every trial. Each trial consisted of the same fixation cross screen presented for 500 ms followed by the emotional stimulus for 250 ms. Participants were then given a multiple choice screen where they were asked to indicate whether the emotion they saw was fearful, happy, neutral or sad. A repeated-measures genotype × diagnosis × emotion tested for differences in emotion recognition accuracy.
2.4 fMRI Data Acquisition
Imaging data were acquired using a 3-T GE Signa scanner at the University of Michigan Functional MRI Laboratory. A reverse spiral sequence was used to acquire a total of 300 T2* weighted blood oxygen level dependent (BOLD) images (Glover and Law, 2001;TR=2000 ms, TE=30 ms, flip angle=90°, FOV=22 cm, 64° × 64 matrix, 40 contiguous axial 3 mm slices). Slices were obtained parallel to the AC-PC line. A 3D T1 axial overlay (TR=8.9, TE=1.8, flip angle=15°, FOV=26 cm, slice thickness=1.4mm, 124 slices; matrix=256 ×160) acquired for anatomical localization, and a sagitally acquired high-resolution spoiled gradient-recalled acquisition in steady state (SPGR) image (flip angle=15°, FOV=26 cm, 1.4 mm slice thickness, 110 slices) acquired for coregistration of the functional images, were used for the structural images.
2.5 fMRI Data Analysis
Imaging data were preprocessed as part of the standard processing procedure at the University of Michigan. K-space outliers greater than two standard deviations from the mean were removed from the raw data and were replaced with the average of the contiguous time points. K-space data were also reconstructed using field map correction to remove magnetic field inhomogeneity distortions. Slice timing differences were corrected for using local sinc interpolation (Oppenheim et al. 1989) with the middle slice as the temporal reference point. Finally, images were realigned and corrected for motion with MCFLIRT in FMRIB Software Library (Jenkinson et al. 2002) using the 10th functional images as reference.
The SPM8 Matlab toolbox (Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk) was used to further preprocess the data. Co-registration to the functional images was performed on the anatomical images. These were then smoothed using an isotropic 8 mm full width at half maximum (FWHM) Gaussian kernel. Individual subject level condition effects were run through the general linear model using SPM. Canonical hemodynamic response function (HRF) and HRF temporal derivative were modeled to each time emotional face stimulus presentation (Friston et al. 1997). A separate regressor was computed for each emotion, yielding 4 regressors of interest at the individual level of analysis. Incorrect responses to the gender of the face stimulus as well as the six motion parameters (x, y, z, roll, pitch, yaw directions) were treated as separate regressors and excluded in this process. To further address head motion, volumes with more than 1mm framewise displacement were censored. A high pass filter of 128 seconds was also used to remove the influence of linear drift. Using SPM, we conducted a psychophysiological interaction (PPI) analysis to investigate whether there were differences in connectivity in response to facial stimuli displaying the four chosen emotions (fearful, happy, sad, and neutral). We created a 6 mm sphere around a voxel located in the left amygdala (xyz −30, −6, −14), as the seed region to generate functional connectivity images. The sphere was centered around the left amygdala and not the right because of amygdala regulation interactions found in the left amygdala, between diagnosis (ASD vs. TD) and genotype (low vs. higher) in this same sample (Wiggins et al. 2014a). The mask chosen for small volume correction as sACC was Left Brodmann Area 25 (Left BA 25), defined structurally by Wake Forest University Pickatlas (Maldjian et al. 2003); this area was previously used in human and non-human animal research linking amygdala functioning to sACC connectivity (Swartz et al. 2013; Fisher et al. 2009).
Group analyses were conducted using SPM8 to create statistical models that tested for differences in the patterns of connectivity between the groups. Initial models included accuracy as a covariate to account for variations in attention to the stimuli presented. Analyses were performed for fearful, happy, sad, and neutral each compared to baseline condition, as well as to fearful, happy, and sad each compared to neutral stimuli. We tested for differences in connectivity using a small volume correction in Left BA 25, at a voxelwise threshold of p=0.05. This small-volume correction searched within BA25 and applied a family-wise error correction based on the size of the left BA 25 mask (Worsley et al. 1996). We then controlled for multiple comparisons of stimuli by setting the voxelwise threshold for significance at p < 0.007 (p=0.05/7). These analyses compared connectivity between the four diagnosis by genotype groups (ASD low, ASD higher, TD low, and TD higher). In order to understand how connectivity is related to heterogeneity in social function deficits commonly observed in ASD (Pelphrey et al. 2011), we conducted analyses testing the relationship between amygdala-sACC connectivity and the social subscale of the ADOS. To do so, we entered ADOS Social Affect scores as a covariate in an SPM8 multiple regression analysis. These analyses were conducted within the low and higher expressing 5-HTTLPR genotype groups with ASD, using a small volume correction in Left BA 25, at a voxelwise threshold of p=0.05 .
3. Results
3.1 Behavioral Results
Participant ability to identify emotions was tested outside the scanner. There were no group differences in the accuracy to identify each of the emotions presented (Diagnosis × Genotype × Emotion; F(1, 101) = .435, p= 0.76). There were no group differences in the accuracy of gender identification for our face-processing task inside the scanner (Diagnosis × Genotype interaction; F(1, 105) = 1.68, p = 0.18). Analyses of reaction time in response to the face-processing task showed no significant differences in reaction time. However, the p value suggested a trend towards faster reaction time in the TD Low expressing genotype group relative to the remaining 3 groups, F(1, 103) = 2.61, p = 0.06. To better understand this trend, we conducted an analysis of whether face processing reaction time differed among genotypes. Reaction times were lower in TD individuals with low expressing genotypes compared to all other groups in response to fearful faces (F(3, 103) = 3.16 p = 0.03). There were also trends for lower reaction time to happy (p = 0.078) and neutral faces (p = 0.107) in TD individuals with low expressing genotypes. There were no significant differences among the four groups in reaction time to sad faces (p = .257). Our sample differed in age (F(1, 105) = 4.73, p = 0.04) and pubertal development (F(1, 105) = 4.08 p = 0.01) among the four diagnosis-by-genotype subgroups (See Table 1 in Supplementary Materials for means). The effects of age differences on connectivity are discussed in section 3.4.
3.2 Connectivity Results
A significant diagnosis-by-genotype interaction on amygdala – sACC connectivity was observed in the contrast of happy vs. baseline after controlling for multiple comparisons of emotion (xyz = −4, 22, −12, t103 = 3.67, p = 0.006, Fig. 2). Specifically, individuals with ASD and low expressing 5-HTTLPR genotypes showed significantly greater amygdala-sACC connectivity than ASD higher expressing genotype group and both TD 5-HTTLPR genotype groups. Individuals with ASD and low expressing 5-HTTLPR genotypes yielded significantly greater connectivity than the three comparison groups in response to the happy vs. neutral contrast, xyz = −6, 24, −12, t103 = 3.59, p = 0.008. This difference did not survive Bonferroni correction at a voxelwise threshold of p=0.007. Sad, fearful, and neutral vs. baseline contrasts; and sad, fearful vs. neutral contrasts, did not show diagnosis-by-genotype connectivity interactions in Left BA 25.
Figure 2.
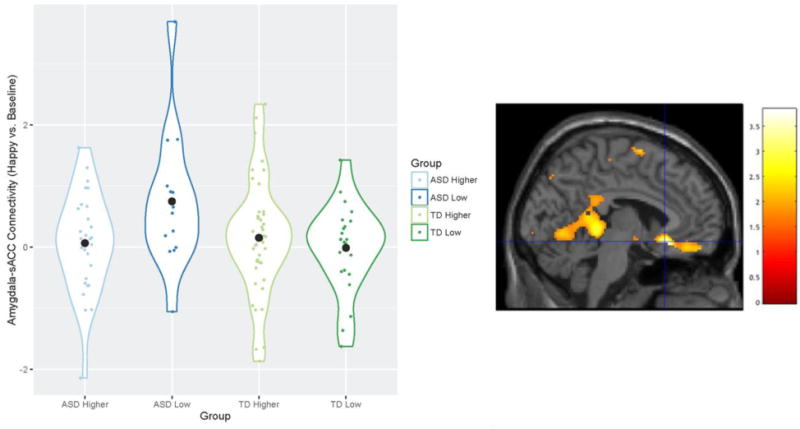
Differences in left amygdala-sACC (Left BA 25) connectivity across groups contrasting happy versus baseline conditions. Violin plots (meant for visualization purposes only) display the kernel probability density of connectivity values, individual connectivity values, and mean connectivity by group (marked by a black circle-shaped point). Brain image displays area where differences were observed (peak xyz = −4, 22, −12) in Left BA 25 (small-volume corrected), at a voxelwise threshold of .05. The difference in connectivity between the ASD low expressing group and the TD low expressing group remained significant after Bonferroni correction for multiple comparisons. Values for graph were extracted from left BA 25, defined structurally by the Wake Forest University Pickatlas (Maldjian et al. 2003).
3.3 Connectivity Differences and Behavior
A significant negative relationship between amygdala-sACC connectivity in the happy vs. baseline contrast and scores on the ADOS social affect subscale was found in the ASD higher expressing genotype group (t(27) = 3.31, p = 0.038; voxelwise threshold: p = 0.05), but not in the ASD low expressing genotype group, (t(12) = 0.79, p = 0.81; voxelwise threshold raised to p =0.5 to report values; Fig. 3).
Figure 3.
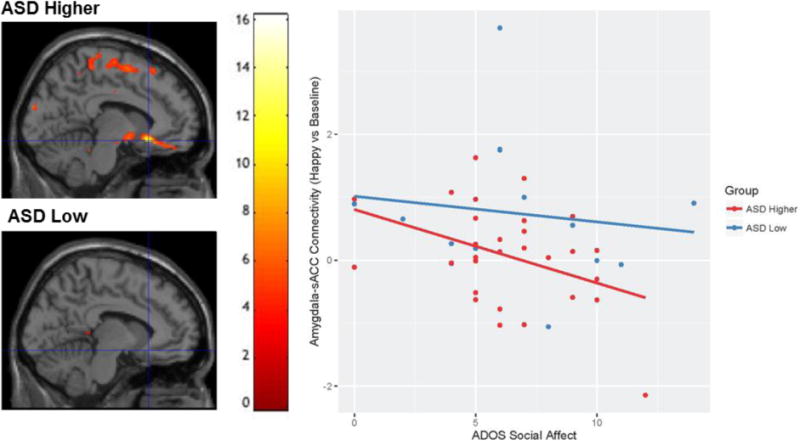
The relationship between ADOS Social Affect scores and Amygdala – sACC connectivity in ASD low and higher expressing genotypes. Brain images displays area (xyz = −8, 22, −12) where the ADOS Social Affect scores showed a negative relationship with amygdala-sACC connectivity (happy vs. baseline) for individuals with ASD and higher expressing genotypes, but not for individuals with ASD and low expressing genotypes. Scatterplot is used for visualization purposes only. Values for graph were extracted from structural left BA 25, defined structurally by the Wake Forest University Pickatlas (Maldjian et al. 2003).
3.4 Post-hoc Results
Weng et al. (2011) showed that individuals with ASD yielded increased amygdala activation using this same task. To rule out the possibility that the present result was observed due to amygdala activity differences and not connectivity between the amygdala and the sACC, we included amygdala activation values extracted from the left amygdala (defined structurally by WFU pickatlas) as a covariate. Connectivity continued to be significantly greater for individuals with ASD and low expressing genotype in response to happy versus baseline faces (xyz = −4, 22, −12, t102 = 3.60, p = 0.008) and neutral faces (xyz = −6, 24, −12, t102 = 3.53, p = 0.01).
In separate analyses, we tested the influence of age on connectivity, as the average age was found to be significantly different between the four ASD/TD 5-HTTLPR genotype groups. Without the variance associated to age, connectivity continued to be significantly greater for individuals with ASD and low expressing genotype in response to happy versus baseline faces, xyz = −4, 22, −12, t102= 3.61, p = 0.005 and to happy versus neutral faces, xyz = −6, 24, −12, t102= 3.69, p = 0.006.
Finally, there was a substantial difference in the percentage of Caucasian participants between the four groups (see Supplementary Materials: Table 3). Given differences in processing other-race faces (Golby et al. 2001), we conducted a Caucasian only test (n=89) to see if the results remained. A diagnosis by genotype interaction where individuals with ASD and low expressing genotypes yielded higher connectivity to happy faces was observed using a 6 mm region of interest around the peak voxel of our main result (xyz = −6, 24, −14, t83=3.07, p=0.012).
4. Discussion
The present preliminary investigation examined whether 5-HTTLPR genotype may help to explain heterogeneity in terms of neural function within ASD by examining patterns of amygdala-sACC connectivity in response to specific faces between 5-HTTLPR genotype groups in individuals with ASD and TD individuals. We hypothesized that there would be a diagnosis (ASD vs. TD) by 5-HTTLPR genotype (low vs. higher) interaction on amygdala-sACC connectivity to specific emotional faces. Supporting our hypothesis, individuals in the low expressing genotype ASD group showed significantly greater connectivity than the ASD higher expressing genotype group, as well as both TD groups, in response to happy faces relative to a baseline, and to happy relative to neutral faces.
We further tested whether amygdala-sACC connectivity to happy faces was related to symptoms of social dysfunction within the two ASD 5-HTTLPR groups. We found that ADOS social affect scores negatively predicted amygdala-sACC connectivity to happy faces in the ASD higher expressing genotype group but this relationship was not present in the low expressing ASD group. That is, in individuals with ASD and higher expressing 5-HTTLPR genotypes, less severe social dysfunction is related to more connectivity. On the other hand, connectivity in the ASD low expressing genotype group does not significantly predict changes in SRS social motivation scores, despite the presence of dysfunction in social symptoms.
If replicated in a larger sample, the relationship between lower levels of social dysfunction and higher connectivity in participants with ASD and higher expressing genotypes points to the possibility that connectivity between the amygdala and the sACC, at rates comparable to TD individuals, may help modulate social function when individuals view happy faces (e.g. positive social interactions). This possibility is supported by studies showing that the areas of the ventral prefrontal cortex modulate amygdala activity (Milad and Quirk, 2012; Ray and Zald, 2012), and that greater connectivity between the amygdala and the sACC is related to greater amygdala habituation to sad faces in controls (Swartz et al. 2013). Contrastingly, individuals with ASD and low expressing genotypes showed hyperconnectivity at all levels of social dysfunction. We speculate that functional connectivity at abnormally high rates may impede a modulatory relationship between connectivity and social dysfunction.
Moreover, these findings add to the body of literature documenting that individuals with ASD and low expressing 5-HTTLPR genotypes exhibit marked differences in brain function in comparison to other groups. Using a sample overlapping with that of the present study, Wiggins and colleagues found that participants with ASD and low expressing genotypes displayed decreased amygdala habituation (i.e., more sustained activation over time) during the same face-processing task as the present study (2014a) and stronger age-related increases in default network connectivity values during rest (Wiggins et al. 2013) relative to those with ASD and higher expressing genotypes as well as controls.
There are many complex steps between the functioning of a gene variant and brain function. Therefore, it is currently not possible to identify the neurochemical mechanisms that affect amygdala-sACC connectivity throughout development. Furthermore, the reason for differential effects of 5-HTTLPR genotypes between individuals who are typically developing and those with autism spectrum disorders is yet unknown. Nevertheless, one possible mechanism may be that amygdala-sACC connections are affected in individuals with low expressing 5-HTTLPR genotypes through an alteration in SERT early in development, possibly affecting emotion regulation. Disruptions in SERT function during central nervous system development affect adult emotional responses to novelty and stress in mice (Ansorge et al. 2004; Rebello et al. 2014) as well as the development of pyramidal neurons involved in inhibition and excitability of prelimbic and infralimbic neurons in the mice homolog of the medial prefrontal cortex (Rebello et al., 2014). It may be that the connection between amygdala and sACC is affected by abnormal serotonin levels in individuals with ASD and low expressing genotypes, as individuals with this variant show abnormal serotonin levels (Canli et al. 2007) and 30% of individuals with ASD exhibit hyperserotonemia (i.e. increased levels of serotonin; Veenstra-VanderWeele et al. 2012). Whereas it has been found that 5-HTTLPR genotype is not related to the level of total blood serotonin in ASD (Betancur et al. 2002; Anderson et al. 2002) and that there is inconclusive evidence for the overall link between 5-HTTLPR genotype and susceptibility to ASD (e.g. Cook et al. 1997; Devlin et al. 2005; Zhong et al. 1999; Ramoz et al. 2006), we believe that heterogeneity in social function in ASD may be partially explained by a possible combination of decreased serotonin reuptake (not specific to ASD), increased prevalence of hyperserotonemia in ASD, and their effects on cortical organization. In line with this possibility, SERT knockout rats show reduced social play behavior (Homberg et al. 2007) and selective serotonin reuptake inhibitors (SSRIs) as well as SERT agonists (5-HT1A) affect sociability traits in BTBR T+tf/J (BTBR) mice, a mouse strain that exhibits behavior reminiscent of ASD characteristics (Gould et al. 2011). Future studies may benefit from monitoring the effect of 5-HTTLPR genotype and serotonin levels on the development of structural connectivity in individuals with ASD.
Along with the present results, the findings of Wiggins et al. (2014a), showing decreased amygdala habituation (i.e., more sustained activation over time) to sad faces but not happy, fearful, or neutral faces in individuals with ASD and low expressing genotype, signals the possibility of a compromised amygdala inhibition network. However, our study does not explain how individuals who are showing hyperconnectivity between the amygdala and the sACC in response to happy faces are able to habituate to happy faces at a similar rate to other groups with normative connectivity. One possibility is that, whereas studies point to the sACC as one of the major inhibitory structures, there are other structures and processes contributing to amygdala habituation that still need to be examined. Indeed, Etkin, Egner, and Kalisch (2011) found that dorsal areas of the ACC and the mPFC are involved in appraisal and expression of emotions and that both the sACC as well as ventral areas of the mPFC are involved in inhibition. Studies that simultaneously examine the appraisal and inhibitory effects of multiple brain structures on amygdala activity in response to diverse social situations may help explain the complexity of emotional response in ASD.
The present preliminary investigation has limitations. First, sample size was relatively small for testing the effects of genetics on behavior. Whereas the first studies linking 5-HTTLPR genotype to the amygdala found effects with sample sizes as modest as 23 (Falgatter et al. 1999) and two independent groups of 14 participants each (Hariri et al. 2002), these results have replicated in a larger sample (N=92; Hariri et al. 2005) as well as by independent groups (Bertolino et al. 2005; Canli et al. 2005; Dannlowski et al. 2007). It is possible that imaging the effect of endophenotypes such as neural activation or connectivity may be more sensitive in detecting the effects of genotype than directly relating genotype to self-reported behavioral measures, as stated by Canli et al. (2007). Meta-analyses such as Murphy et al. (2013) and Flint and Munafo (2007), however, highlight the need for replication studies and collaboration, as no single study has been able to gather enough data to be sufficiently powered to reliably demonstrate effects. As such, preliminary studies with modest effect sizes serve the purpose of offering routes of investigation for future replication within and between research groups. Second, the sphere chosen as the seed was selected based on the result of a previous 5-HTTLPR/ASD study conducted by our group (Wiggins et al. 2014a). This is a limitation because artifacts (e.g. scanner, motion) that could have affected Wiggins et al. (2014a) may have affected the present investigation as well. Amygdala dropout in data from certain participants made us unable to conduct PPI analyses using a larger, independent seed (structural left amygdala). Nevertheless, the present study corrected for possible motion artifacts by censoring volumes with more than 1mm framewise displacement; a step that may help to avoid shared false positive results due to motion. Third, our sample differed in age, gender, and pubertal development among the four diagnosis-by-genotype subgroups. To control for this, we tested whether including age, gender, and puberty as a covariate affected our group analysis. Results continued to show that the ASD group with low expressing genotypes had significantly greater amygdala-sACC connectivity than ASD higher expressing genotypes and both TD genotype groups. Fourth, our sample’s ethnic background consisted, largely, of Caucasian individuals (see Supplementary Materials: Table 1). This may be a limitation given differences in processing other-race faces (Golby et al. 2001). However, the results of a Caucasian-only analysis yielded the same interaction pattern around a 6 mm sphere located in BA 25. Fifth, developmental transitions from childhood into adolescence have been shown to be important components in determining whether individuals with ASD display amygdala over-connectivity or under-connectivity in comparison to TD individuals (Nomi and Uddin, 2015). Analyses that consider the effects of genotype on connectivity across development will require a larger sample. Such an approach would provide a more complete understanding of neural changes occurring during this important developmental transition. Sixth, Hardy-Weinberg Equilibrium was not met in the TD sample. Whereas equilibrium was met in our ASD sample, our data were collected using convenience sampling and are not representative of a population with an expected distribution of 5-HTTLPR alleles. Participants included in this test were those who provided data for genotyping and successfully completed our fMRI scan. However, in analyses including all participants who provided data for genotyping (scanned and not-scanned), both groups meet Hardy-Weinberg Equilibrium. These data are preliminary and these limitations should be considered in replication attempts.
Future research may wish to investigate participant interpretation of the emotions presented. Our construal of the participants’ reaction in response to happy faces (i.e. an aversion to happy faces) was not tested. Future studies may add to the present results by testing the relationship between distress to social contact and happy faces in ASD using various sources. This could be assessed through skin conductance response to test for physiological arousal and eye tracking to test for avoidance of gaze to the eyes. Moreover, polymorphisms do not operate alone in their effect on brain function. Researchers may benefit from examining the additive effect of genotype variants along the same metabolic pathway; a method used by Hernandez et al. (2016) in their investigation on OXTR. This would give us a more informed picture of genetic influences on particular brain networks. Finally, a diffusion tensor imaging (DTI) study would inform our understanding of structural connectivity between the amygdala and the prefrontal cortex and its relationship to 5-HTTLPR genotype. Such research may highlight the relevance of the present finding to the differences in cortical connection size and number observed between ASD and TD individuals linked to serotonin (Casanova et al. 2002; Janušonis et al. 2004).
4.1 Conclusions
The present study provides preliminary evidence for an interaction between diagnosis (ASD vs. TD) and 5-HTTLPR genotype (low vs. higher expressing genotypes), where the ASD low expressing genotype group exhibits greater amygdala-sACC connectivity in response to happy faces than individuals with ASD and higher expressing genotypes and TD individuals of both low and higher expressing genotypes. The present findings are consistent with previous research showing that individuals with ASD and low expressing 5-HTTLPR genotypes display differences in brain function compared to higher expressing genotypes and TD individuals. Disparities in neural functioning have been found in terms of decreased amygdala habituation (Wiggins et al. 2014a) and increased default network connectivity with age (Wiggins et al. 2013) in a ASD low expressing genotype group. Moreover, the relationship between amygdala-sACC connectivity and aspects of social function in ASD may be better explained when considering 5-HTTLPR genotype. Sample size for this study is modest and thus present results should be considered preliminary until replicated with larger sample sizes and/or independent groups. This study is the first ASD-focused investigation that combines genetic polymorphism analyses and functional connectivity in the context of a social task. The current findings point to imaging as useful method for bridging the relationship between genetic contributions and social dysfunction in ASD.
Supplementary Material
Contributor Information
Francisco Velasquez, Department of Psychology, University of Michigan; Department of Radiology, University of Washington.
Jillian Lee Wiggins, Department of Psychology, University of Michigan; Department of Psychology, San Diego State University and San Diego State University/University of California San Diego Joint Doctoral Program in Clinical Psychology.
Whitney I. Mattson, Department of Psychology, University of Michigan
Donna M. Martin, Department of Human Genetics, University of Michigan
Catherine Lord, Center for Autism and the Developing Brain, Weill Cornell Medicine.
Christopher S. Monk, Department of Psychology, Neuroscience Program, Department of Psychiatry, Center for Growth and Human Development, University of Michigan
References
- Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 profile. Burlington, VT: Department of Psychiatry, University of Vermont; 1991. p. 288. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- Anderson GM, Gutknecht L, Cohen DJ, Brailly-Tabard S, Cohen JH, Ferrari P, Roubertoux PL, Tordjman S. Serotonin transporter promoter variants in autism: functional effects and relationship to platelet hyperserotonemia. Molecular psychiatry. 2002;7(8):831–836. doi: 10.1038/sj.mp.4001099. [DOI] [PubMed] [Google Scholar]
- Ansorge MS, Zhou M, Lira A, Hen R, Gingrich JA. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science. 2004;306(5697):879–881. doi: 10.1126/science.1101678. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, Williams SC. Social intelligence in the normal and autistic brain: an fMRI study. European journal of neuroscience. 1999;11(6):1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Arciero G, Rubino V, Latorre V, De Candia M, Mazzola V, Blasi G, Caforio G, Hariri A, Kolachana B, Nardini M. Variation of human amygdala response during threatening stimuli as a function of 5′ HTTLPR genotype and personality style. Biological psychiatry. 2005;57(12):1517–1525. doi: 10.1016/j.biopsych.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Betancur C, Corbex M, Spielewoy C, Philippe A, Laplanche JL, Launay JM, Gillberg C, Mouren-Simeoni MC, Hamon M, Giros B, Nosten-Bertrand M. Serotonin transporter gene polymorphisms and hyperserotonemia in autistic disorder. Molecular psychiatry. 2002;7(1):67. doi: 10.1038/sj/mp/4000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune CW, Kim SJ, Salt J, Leventhal BL, Lord C, Cook EH., Jr 5-HTTLPR genotype-specific phenotype in children and adolescents with autism. American Journal of Psychiatry. 2006 doi: 10.1176/appi.ajp.163.12.214. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nature neuroscience. 2007;10(9):1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Canli T, Omura K, Haas BW, Fallgatter A, Constable RT, Lesch KP. Beyond affect: a role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(34):12224–12229. doi: 10.1073/pnas.0503880102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002;58(3):428–432. doi: 10.1212/WNL.58.3.428. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1176/foc.3.1.156. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Sturge-Apple ML. Interactions of child maltreatment and serotonin transporter and monoamine oxidase A polymorphisms: depressive symptomatology among adolescents from low socioeconomic status backgrounds. Development and psychopathology. 2007;19(04):1161–1180. doi: 10.1017/S0954579407000600. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, Bullmore ET, Williams SC, Van Amelsvoort T, Robertson DM, Rowe A, Phillips M, McAlonan G, Howlin P, Murphy DG. The functional neuroanatomy of social behaviour. Brain. 2000;123(11):2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, Metzger LM, Shoushtari CS, Splinter R, Reich W. Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. Journal of autism and developmental disorders. 2003;33(4):427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- Cook EH, Jr, Leventhal BL. The serotonin system in autism. Current opinion in pediatrics. 1996;8(4):348–354. doi: 10.1097/00008480-199608000-00008. [DOI] [PubMed] [Google Scholar]
- Dannlowski U, Ohrmann P, Bauer J, Kugel H, Baune BT, Hohoff C, Kersting A, Arolt V, Heindel W, Deckert J, Suslow T. Serotonergic genes modulate amygdala activity in major depression. Genes, Brain and Behavior. 2007;6(7):672–676. doi: 10.1111/j.1601-183x.2006.00297.x. [DOI] [PubMed] [Google Scholar]
- Devlin B, Cook EH, Coon H, Dawson G, Grigorenko EL, McMahon W, Minshew N, Pauls D, Smith M, Spence MA, Rodier PM. Autism and the serotonin transporter: the long and short of it. Molecular psychiatry. 2005;10(12):1110–1116. doi: 10.1038/sj.mp.4001724. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The Subgenual Anterior Cingulate Cortex in Mood Disorders. CNS Spectrums. 2008;13(08):663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Examiner’s manual for the PPVT-III peabody picture vocabulary test: Form IIIA and Form IIIB. AGS; 1997. [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in cognitive sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallgatter AJ, Jatzke S, Bartsch AJ, Hamelbeck B, Lesch KP. Serotonin transporter promoter polymorphism influences topography of inhibitory motor control. The International Journal of Neuropsychopharmacology. 1999;2(2):115–120. doi: 10.1017/s1461145799001455. [DOI] [PubMed] [Google Scholar]
- Fisher PM, Meltzer CC, Price JC, Coleman RL, Ziolko SK, Becker C, Moses-Kolko EL, Berga SL, Hariri AR. Medial prefrontal cortex 5-HT2A density is correlated with amygdala reactivity, response habituation, and functional coupling. Cerebral cortex. 2009 doi: 10.1093/cercor/bhp022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J, Munafò MR. The endophenotype concept in psychiatric genetics. Psychological Medicine. 2006;37(02):163. doi: 10.1017/s0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gadow KD, DeVincent CJ, Siegal VI, Olvet DM, Kibria S, Kirsch SF, Hatchwell E. Allele-specific associations of 5-HTTLPR/rs25531 with ADHD and autism spectrum disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2013;40:292–297. doi: 10.1016/j.pnpbp.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic resonance in medicine. 2001;46(3):515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Golby AJ, Gabrieli JD, Chiao JY, Eberhardt JL. Differential responses in the fusiform region to same-race and other-race faces. Nature neuroscience. 2001;4(8):845–850. doi: 10.1038/90565. [DOI] [PubMed] [Google Scholar]
- Gould GG, Hensler JG, Burke TF, Benno RH, Onaivi ES, Daws LC. Density and function of central serotonin (5-HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+ tf/J mouse social behavior. Journal of neurochemistry. 2011;116(2):291–303. doi: 10.1111/j.1471-4159.2010.07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297(5580):400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Munoz KE, Kolachana BS, Mattay VS, Egan MF, Weinberger DR. A susceptibility gene for affective disorders and the response of the human amygdala. Archives of general psychiatry. 2005;62(2):146–152. doi: 10.1001/archpsyc.62.2.146. [DOI] [PubMed] [Google Scholar]
- Hernandez LM, Krasileva K, Green SA, Sherman LE, Ponting C, McCarron R, Lowe JK, Geschwind DH, Bookheimer SY, Dapretto M. Additive effects of oxytocin receptor gene polymorphisms on reward circuitry in youth with autism. Molecular Psychiatry. 2016 doi: 10.1038/mp.2016.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homberg JR, Schiepers OJ, Schoffelmeer AN, Cuppen E, Vanderschuren LJ. Acute and constitutive increases in central serotonin levels reduce social play behaviour in peri-adolescent rats. Psychopharmacology. 2007;195(2):175–182. doi: 10.1007/s00213-007-0895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. The American Journal of Human Genetics. 2006;78(5):815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus V, Bishop S, Gotham K, Huerta M, Lord C. Factors influencing scores on the social responsiveness scale. Journal of Child Psychology and Psychiatry. 2013;54(2):216–224. doi: 10.1111/j.1469-7610.2012.02589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janušonis S, Gluncic V, Rakic P. Early serotonergic projections to Cajal-Retzius cells: relevance for cortical development. The Journal of neuroscience. 2004;24(7):1652–1659. doi: 10.1523/JNEUROSCI.4651-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1006/nimg.2002.1132. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Johnson LC, Richards T, Mahurin R, Greenson J, Dawson G, Aylward E. Reduced neural habituation in the amygdala and social impairments in autism spectrum disorders. American Journal of Psychiatry. 2009 doi: 10.1176/appi.ajp.2008.07101681. [DOI] [PubMed] [Google Scholar]
- Kliemann D, Dziobek I, Hatri A, Baudewig J, Heekeren HR. The role of the amygdala in atypical gaze on emotional faces in autism spectrum disorders. The Journal of Neuroscience. 2012;32(28):9469–9476. doi: 10.1523/JNEUROSCI.5294-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual fixation patterns during viewing of naturalistic social situations as predictors of social competence in individuals with autism. Archives of general psychiatry. 2002;59(9):809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Kovacs M. Children’s depression inventory. North Tonawanda, NY: Multi-Health System; 1992. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of autism and developmental disorders. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of autism and developmental disorders. 2000;30(3):205–223. doi: 10.1023/A:1005592401947. [DOI] [PubMed] [Google Scholar]
- Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism from 2 to 9 years of age. Archives of general psychiatry. 2006;63(6):694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/S1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- March JS. Manual for the multidimensional anxiety scale for children (MASC) Toronto: Multi-Health Systems; 1997. [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nature Reviews Neuroscience. 2004;5(11):844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Progress in neurobiology. 1998;55(3):257–332. doi: 10.1016/S0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annual review of psychology. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk CS, Weng S, Wiggins JL, Kurapati N, Louro HMC, Carrasco M, Maslowski J, Risi S, Lord C. Neural circuitry of emotional face processing in autism spectrum disorders. Journal of Psychiatry & Neuroscience: JPN. 2010;35(2):105–14. doi: 10.1503/jpn.090085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy SE, Norbury R, Godlewska BR, Cowen PJ, Mannie ZM, Harmer CJ, Munafo MR. The effect of the serotonin transporter polymorphism (5-HTTLPR) on amygdala function: a meta-analysis. Molecular psychiatry. 2013;18(4):512–520. doi: 10.1038/mp.2012.19. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Ueno S, Sano A, Tanabe H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Molecular psychiatry. 2000;5(1):32–38. doi: 10.1038/sj.mp.4000698. [DOI] [PubMed] [Google Scholar]
- Nomi JS, Uddin LQ. Developmental changes in large-scale network connectivity in autism. NeuroImage: Clinical. 2015;7:732–741. doi: 10.1016/j.nicl.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim AV, Schafer RW, Buck JR. Discrete-time signal processing. Vol. 2. Englewood Cliffs, NJ: Prentice hall; 1989. [Google Scholar]
- Pierce K, Müller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiformface area’in autism: evidence from functional MRI. Brain. 2001;124(10):2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Sasson NJ, Reznick JS, Paul G, Goldman BD, Piven J. Visual scanning of faces in autism. Journal of autism and developmental disorders. 2002;32(4):249–261. doi: 10.1023/A:1016374617369. [DOI] [PubMed] [Google Scholar]
- Pelphrey KA, Shultz S, Hudac CM, Vander Wyk BC. Research review: constraining heterogeneity: the social brain and its development in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2011;52(6):631–644. doi: 10.1111/j.1469-7610.2010.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, Boxer A. A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence. 1988;17(2):117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature neuroscience. 2005;8(6):828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pierce K, Müller RA, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiformface area’in autism: evidence from functional MRI. Brain. 2001;124(10):2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Ramoz N, Reichert JG, Corwin TE, Smith CJ, Silverman JM, Hollander E, Buxbaum JD. Lack of evidence for association of the serotonin transporter gene SLC6A4 with autism. Biological psychiatry. 2006;60(2):186–191. doi: 10.1016/j.biopsych.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Raven JC. Guide to the standard progressive matrices: sets A, B, C, D and E. HK Lewis; 1960. [Google Scholar]
- Ray RD, Zald DH. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neuroscience & Biobehavioral Reviews. 2012;36(1):479–501. doi: 10.1016/j.neubiorev.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebello TJ, Yu Q, Goodfellow NM, Cagliostro MKC, Teissier A, Morelli E, Demireva EY, Chemiakine A, Rosoklija GB, Dwork AJ, Lambe EK. Postnatal day 2 to 11 constitutes a 5-HT-sensitive period impacting adult mPFC function. The Journal of Neuroscience. 2014;34(37):12379–12393. doi: 10.1523/jneurosci.1020-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. The Journal of Neuroscience. 2001;21(11):4090–4103. doi: 10.1111/j.1749-6632.2003.tb07107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Wolf DH, Loughead J, Ruparel K, Elliott MA, Hakonarson H, Gur RC, Gur RE. Impact of in-scanner head motion on multiple measures of functional connectivity: relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60(1):623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence SH. A measure of anxiety symptoms among children. Behaviour research and therapy. 1998;36(5):545–566. doi: 10.1016/S0005-7967(98)00034-5. [DOI] [PubMed] [Google Scholar]
- Swartz JR, Wiggins JL, Carrasco M, Lord C, Monk CS. Amygdala habituation and prefrontal functional connectivity in youth with autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52(1):84–93. doi: 10.1016/j.jaac.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz JR, Carrasco M, Wiggins JL, Thomason ME, Monk CS. Age-related changes in the structure and function of prefrontal cortex–amygdala circuitry in children and adolescents: A multi-modal imaging approach. NeuroImage. 2014;86:212–220. doi: 10.1016/j.neuroimage.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordjman S, Gutknecht L, Carlier M, Spitz E, Antoine C, Slama F, Carsalade V, Cohen DJ, Ferrari P, Roubertoux PL, Anderson GM. Role of the serotonin transporter gene in the behavioral expression of autism. Molecular psychiatry. 2001;6(4):434–439. doi: 10.1038/sj.mp.4000873. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, Cohen J, Mannangatti P, Jessen T, Thompson BJ, Ye R. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proceedings of the National Academy of Sciences. 2012;109(14):5469–5474. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng SJ, Wiggins JL, Peltier SJ, Carrasco M, Risi S, Lord C, Monk CS. Alterations of resting state functional connectivity in the default network in adolescents with autism spectrum disorders. Brain research. 2010;1313:202–214. doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng SJ, Carrasco M, Swartz JR, Wiggins JL, Kurapati N, Liberzon I, Risi S, Lord C, Monk CS. Neural activation to emotional faces in adolescents with autism spectrum disorders. Journal of Child Psychology and Psychiatry. 2011;52(3):296–305. doi: 10.1016/j.brainres.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Peltier SJ, Bedoyan JK, Carrasco M, Welsh RC, Martin DM, Lord C, Monk CS. The impact of serotonin transporter genotype on default network connectivity in children and adolescents with autism spectrum disorders. NeuroImage: Clinical. 2013;2:17–24. doi: 10.1016/j.nicl.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Swartz JR, Martin DM, Lord C, Monk CS. Serotonin transporter genotype impacts amygdala habituation in youth with autism spectrum disorders. Social cognitive and affective neuroscience. 2014a;9(6):832–838. doi: 10.1093/scan/nst039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins JL, Bedoyan JK, Carrasco M, Swartz JR, Martin DM, Monk CS. Age-related effect of serotonin transporter genotype on amygdala and prefrontal cortex function in adolescence. Human brain mapping. 2014b;35(2):646–658. doi: 10.1002/hbm.22208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human brain mapping. 1996;4(1):58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Zhong N, Ye L, Ju W, Tsiouris J, Cohen I, Brown WT. 5-HTTLPR variants not associated with autistic spectrum disorders. Neurogenetics. 1999;2(2):129–131. doi: 10.1007/s100480050064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


