Abstract
Animals, including humans, require a highly coordinated and flexible system of social behavior and threat evaluation. However, trauma can disrupt this system, with the amygdala implicated as a mediator of these impairments in behavior. Recent evidence has further highlighted the context of infant trauma as a critical variable in determining its immediate and enduring consequences, with trauma experienced from an attachment figure, such as occurs in cases of caregiver-child maltreatment, as particularly detrimental. This review focuses on the unique role of caregiver presence during early-life trauma in programming deficits in social behavior and threat processing. Using data primarily from rodent models, we describe the interaction between trauma and attachment during a sensitive period in early life, which highlights the role of the caregiver’s presence in engagement of attachment brain circuitry and suppressing threat processing by the amygdala. These data suggest that trauma experienced directly from an abusive caregiver and trauma experienced in the presence of caregiver cues produce similar neurobehavioral deficits, which are unique from those resulting from trauma alone. We go on to integrate this information into social experience throughout the lifespan, including consequences for complex scenarios, such as dominance hierarchy formation and maintenance.
Introduction
We have known for half a century that the brain and behavior of altricial species, including humans and rodents, continues to develop after birth, and that genetics and experience interact to guide the intricate process of constructing the brain (Andersen & Teicher, 2008; De Bellis & Thomas, 2003; Fisher, 1955; Landers & Sullivan, 2012; Levine, 1957; Levine, 2005; Mainardi, Marsan, & Pasquali, 1965). This open system enables early-life experiences to sculpt the brain and optimize behaviors to more closely fit diverse environments to enhance survival (Bock, Rether, Groger, Xie, & Braun, 2014). However, this same open system can permit developmental perturbations to produce vulnerability to psychiatric disorders and maladaptive behaviors that reduce access to resources, especially during critical periods for programming complex cognition and behavior (Andersen & Teicher, 2008; Opendak & Sullivan, 2016). In particular, trauma experienced from a caregiver during a sensitive window in early life can produce life-long deficits in threat processing and social behavior across many species (Amaral, 2003; Callaghan, Sullivan, Howell, & Tottenham, 2014; McEwen, 2003; Moriceau, Wilson, Levine, & Sullivan, 2006; Tang, Reeb-Sutherland, Romeo, & McEwen, 2014; Tzanoulinou & Sandi, 2016; Zeanah, Keyes, & Settles, 2003). Modeling this in rodents suggests that repeated pairing of cues associated with the caregiver and with trauma can disrupt the typical developmental trajectory of brain areas important for both forming attachments and learning about threat (Opendak & Sullivan, 2016; Raineki, Cortes, Belnoue, & Sullivan, 2012). In particular, we have observed that trauma experienced in the presence of the caregiver has similar neurobehavioral consequences as trauma experienced directly from an abusive caregiver; these socially anchored traumas produce unique and profound effects that go beyond those of trauma alone.
Understanding trauma effects and the importance of a social context on brain development has been challenging, not only because the process of building a brain is complex, but because many of the effects of early life perturbations are often not expressed until a later stage of development (Ainsworth, 1969; Gunnar, Quevedo, De Kloet, Oitzl, & Vermetten, 2007; Landers & Sullivan, 2012; Raineki, et al., 2012). Threat processing has been a major focus in studying disordered attachment because of the link between trauma and aberrant detection of danger, vigilance, and regulation of emotion in mental illness in children and adults (Bremne & Vermetten, 2001; Caron, Weston-Lee, Haggerty, & Dozier, 2015; Cirulli et al., 2009; Drury et al., 2012; Elzinga, Schmahl, Vermetten, van Dyck, & Bremner, 2003; Jedd et al., 2015; Teicher et al., 2003; Tottenham & Sheridan, 2009). We rely on animal research to highlight mechanisms, which has shown that caregiver abuse in early life produces structural and functional changes in the amygdala and a changed threat system (Bagot et al., 2009; Bock et al., 2014; Caldji et al., 1998; Ivy, Brunson, Sandman, & Baram, 2008; Maestripieri, Tomaszycki, & Carroll, 1999; Raineki et al., 2012; Roth & Sullivan, 2005; Sanchez et al., 2001; Tang et al., 2014). Drury and colleagues (2015) have recently written a review comparing different animal models used to assess mechanisms of early life adversity (Drury, Sanchez & Gonzales, 2015).
Although there is a broad literature documenting multiple forms of early-life trauma across many species, the focus of this review will be species-atypical abusive maternal care using data from rodent models. While translating rodent data to humans can be challenging, the attachment system and its response to trauma have considerable convergence between species. Indeed, studies in rodents, primates, and humans have identified a sensitive period in early life during which abusive care from an attachment figure interacts with heightened neural plasticity to program long-term impairments in social behavior through effects on amygdala development (Opendak & Sullivan 2016; Humphreys & Zeanah 2015; Drury et al., 2015). We will begin to review attachment-trauma effects on amygdala development with an exploration of neurobiology of attachment in typical rearing conditions. We will then expand our discussion to disordered attachments that form following abusive caregiving in rodents. Finally, we will discuss the long-term consequences of these atypical attachment-trauma experiences for behavior in highly complex social scenarios in the wild.
Developmental trajectory of brain areas important for social behavior and threat processing
The effects of early life experience on the brain involve changes at nearly every level of analysis, from cellular signaling to behavioral expression. Indeed, through the decades, countless brain regions and nearly every neurotransmitter have been implicated in the etiology of psychopathology following early life experiences, including changes in receptors, epigenetics, brain structure, the microbiome, immune system, and homeostasis maintenance (Andersen & Teicher, 2008; Bale, 2015; Blakemore & Mills, 2014; Callaghan, Cowan, & Richardson, 2016; Drury et al., 2012; Gold et al., 2016; Green et al., 2016; Halevi, Djalovski, Vengrober, & Feldman, 2016; Hartley & Lee, 2015; Heim & Binder, 2012; Humphreys, Kircanski, Colich, & Gotlib, 2016; Kane et al., 2016; Kennedy et al., 2016; Knudsen, 2004; Lawler, Koss, Doyle, & Gunnar, 2016; Nelson, Lau, & Jarcho, 2014; Pechtel, Lyons-Ruth, Anderson, & Teicher, 2014; Poulos et al., 2014; Puetz et al., 2016; Reuben et al., 2016; Troller-Renfree, Nelson, Zeanah, & Fox, 2016; Umemori, Winkel, Castren, & Karpova, 2015; Werker & Hensch, 2015; Zannas & Binder, 2014; Zeanah & Sonuga-Barke, 2016). Research on rodents and nonhuman primates promptly identified the hypothalamic-pituitary-adrenal (HPA) axis as one mediator for how experience disrupts development (Rincon-Cortes & Sullivan, 2014; Sanchez, 2006). This system is involved in the body’s response to allostatic load; chronic over-activation of the HPA axis in response to early life trauma can produce long-term adaptations in the stress response, and these changes are thought to underlie the development of disorders such as PTSD, depression, and anxiety (Graham, Heim, Goodman, Miller, & Nemeroff, 1999). While the HPA axis has remained a focus as the mediator of early life trauma, additional mechanisms have been implicated in the complexity of early life experiences on brain programming, including a critical role for learning (Moriceau, Wilson, Levine, & Sullivan, 2006).
Although it is beyond the scope of this review to describe brain development in detail, a few basic concepts are helpful for the present discussion (for additional reading on brain development, see Casey, Tottenham, Liston, & Durston, 2005; Houston, Herting, & Sowell, 2014). First, the brain continues to develop throughout early life, with different brain areas each having their own trajectory of development and maturation (Berdel, Morys, & Maciejewska, 1997; Brummelte & Teuchert-Noodt, 2006; Chareyron, Lavenex, & Lavenex, 2012; Cunningham, Bhattacharyya, & Benes, 2002; Ehrlich, Ryan, & Rainnie, 2012; Knudsen, 2004; Van Eden & Uylings, 2004; Wakefield & Levine, 1985). Brain areas important for basic physiological functions are certainly mature at birth, but continue to mature and develop more complex connections with other brain areas and within themselves (Rinaman, Banihashemi, & Koehnle, 2011). Regions involved in complex behaviors and higher-order functioning, including the amygdala, hippocampus, and prefrontal cortex (PFC), are more delayed in maturation, although recent evidence suggests specific functions of each of these brain regions have their own developmental trajectories. For instance, hippocampus-dependent contextual fear learning emerges around postnatal (PN) day 23 in rodents (Raineki, Holman, et al., 2010), while other hippocampal dependent learning behaviors emerge either before or after this age (Ainge & Lanston, 2012; Moye & Rudy, 1987; Pugh & Rudy 1996; Stanton, 2000). Traditional measures of neural maturation, such as long-term potentiation (LTP), a presumed measure of synaptic plasticity, emerge over a week earlier than contextual fear learning (Ainge & Langston, 2012; Bekenstein & Lothman, 1991; Harris & Teyler, 1983; Swann, Smith, & Brady, 1990; Wilson, 1984) and do not highlight the distinct behavioral trajectories of specific hippocampal-dependent behaviors. Furthermore, some brain areas likely encode information at one age but affect behavioral expression at another (Moye & Rudy, 1987; Pattwell et al., 2012; Poulos et al., 2014).
Emerging evidence also suggests brain areas can have unique function in early life, such as the important role of locus coeruleus (LC) norepinephrine (NE) in attachment that is described below (Landers & Sullivan, 2012). Neurochemicals, too, can have age-specific effects, such as the switch between excitation and inhibition by GABA at birth (Ben-Ari, 2014). The changing roles of other molecules across development are less clear: while oxytocin has a demonstrable role in prosocial behavior and maternal care in adults (Calcagnoli et al., 2015; Frijling et al., 2016; Johnson & Young, 2015; Lee, Brady, Shapiro, Dorsa, & Koenig, 2007; Marlin & Froemke, 2016; Nelson & Panksepp, 1998; Shamay-Tsoory & Abu-Akel, 2016), its role in very early life and the sensitive period for attachment remains unclear (Nelson & Panksepp, 1998; Parr et al., 2016; Sannino, Chini, & Grinevich, 2016). Finally, the numerous connections among brain areas can be further delayed in maturation so that feedback systems that form complex loops of bidirectional information processing become functional at a later age. Thus, as we consider how early life trauma can impact a child, it is important to consider when brain areas implicated in adult trauma processing are functionally mature and functionally connected to other brain areas. As will be shown below, processing of trauma in early life is different from processing such information in adulthood (Gunnar et al., 2007; Opendak & Sullivan, 2016; Teicher et al., 2003).
The amygdala-hippocampus-PFC circuit is crucial for threat detection and many forms of social behavior, but the maturation and connectivity between these brain regions develops slowly over early life in humans (Casey et al., 2005; Gee et al., 2013; Graham et al., 1999; Malter Cohen et al., 2013; Skuse, Morris, & Lawrence, 2003; Tottenham, 2012; Tottenham & Sheridan, 2009), nonhuman primates (Bachevalier & Loveland, 2006; Chareyron, Lavenex, Amaral, & Lavenex, 2012; Lavenex & Banta Lavenex, 2013; Sanchez, Ladd, & Plotsky, 2001; Skuse et al., 2003) and rodents (Brummelte & Teuchert-Noodt, 2006; Holland & Gallagher, 2004). The amygdala is considered to be the critical structure involved in the formation and storage of conditioned fear associations (Davis, Rainnie, & Cassell, 1994; Phelps & LeDoux, 2005), but it has a role in many emotional functions, including those unrelated to fear, such as social odor processing and assessing hedonic value of stimuli (Holland & Gallagher, 2004; Maren & Fanselow, 1996; Phelps & LeDoux, 2005; Royet et al., 2000). It has been suggested that neonatal amygdala connectivity correlates with fear responses at six months of age in human infants (Graham et al., 2016). Furthermore, volumetric analysis indicates that amygdala volume peaks in pre-adolescence (Uematsu et al., 2012). The PFC works with the amygdala to regulate complex decision-making, particularly in functions relevant to threat processing and social behavior, but the developmental trajectory of this region is not fully understood. The PFC subarea orbitofrontal cortex (OFC), which plays an important role in assessing the hedonic value of odors (Anderson et al., 2003; Gottfried, Deichmann, Winston, & Dolan, 2002; Rolls, 2015; Zald & Pardo, 1997), is postulated as functional by the time a child is two or three years old, while the anterior cingulate (ACC) and medial PFC (mPFC) are thought to possibly become functional around four months and as early as four years, respectively (Allman, Hakeem, Erwin, Nimchinsky, & Hof, 2001; Gee et al., 2013; Graham et al., 2015).
The hippocampus is a region with diverse functions, including the ability to remember specific information about events, such as where and when events occurred. These functions appear to develop around two years old in children, but show great improvement over the next four years (Gomez & Edgin, 2015; Lavenex & Banta Lavenex, 2013). Since the child’s hippocampus is difficult to image using brain scanning techniques, data on hippocampal functional emergence does not exist, although hippocampal growth rates appear to peak around 9–11 years of age (Uematsu et al., 2012). Evidence from rodent studies on development of the amygdala and hippocampus show a similar developmental time course; both regions demonstrate considerable growth during the initial postnatal period and become functionally mature in normal rearing conditions at PN10 and PN23, respectively (Chareyron, Lavenex, & Levenex, 2012; Raineki, Holman, et al., 2010). These ages reflect significant developmental milestones in rats: although no consensus exists mapping rodent age onto human age, weaning around PN23 is considered a marker of early or peri-adolescence in infant rats, while PN0-PN9 is thought to represent infancy (Andersen, 2003; Sullivan & Holman, 2010). As will be discussed further, these first ten days represent a sensitive period for forming attachments. Although the amygdala, hippocampus, and PFC are robustly involved in trauma processing during adulthood, their involvement in processing early-life trauma is complex, due to their limited functional unavailability and/or immaturity at this age.
Infant social interaction: Importance of the caregiver as regulator of brain and behavior
Understanding the role of a caregiver relies on a rich historical literature that has demonstrated the importance of the relationship between a child and a caregiver across a variety of species-- a relationship primarily understood in terms of attachment. Animal research has provided clear evidence of the critical role of early life attachment in programming cognitive and emotional health. Specifically, seminal works by Niko Tinbergen, Konrad Lorenz, and John Hinde characterized how the newly hatched chick attached (imprinted) to the parent (Hess, 1962) within a temporally limited sensitive period. Around the same time, Harry Harlow and his colleagues were working with rhesus monkeys and assessing the effects of being reared without a mother but providing basic food, water and warmth (Harlow & Harlow, 1965). This work clearly highlighted the importance of the infant’s social interactions with the mother during a sensitive period in development since, without the caregiver, infants showed emotional and cognitive disabilities that were reminiscent of human children reared in inadequate orphanages without an attachment figure (Gunnar, Hostinar, Sanchez, Tottenham, & Sullivan, 2015; Humphreys & Zeanah, 2015; Levin, Fox, Zeanah, & Nelson, 2015; Teicher, Samson, Anderson, & Ohashi, 2016).
Attachment to a caregiver during a sensitive window is of paramount importance to altricial infant survival due to the infant’s greatly reduced ability to acquire food, protection, and warmth. Furthermore, during early life, the infant relies on the caregiver for regulation of basic physiology, ranging from vital functions, such as heart rate and respiration, to emotional regulation. Caregiver regulation of the infant’s emotional state is seen during perfunctory caregiving, such as by soothing a crying infant or by smiling at or tickling an infant to increase arousal. In turn, this stimulation of the infant’s sensory systems changes physiology; for example, soothing a stressed infant can lower stress hormone levels (Gunnar & Quevedo, 2007; Gunnar et al., 2007; Hofer, 1994; Sarro, Wilson, & Sullivan, 2014). In typically developing children, this caregiver regulation of infant physiology has been shown to be critical for a child’s interaction with the world, including reduction of fear, response to novelty, and learning (Humphreys & Zeanah, 2015; Gee et al., 2013; Nachmias, Gunnar, Mangelsdorf, Parritz, & Buss, 1996). In turn, this system appears to be compromised in children with early life trauma. Specifically, neglectful and/or abusive caregiving has been associated with poor regulation of infant physiology and is correlated with disrupted developmental trajectories related to social behavior and threat processing (Gunnar et al., 2007; Mikics et al., 2008; Nemeroff, 2004; Rincon-Cortes & Sullivan, 2014). These effects have little to do with the level of sensory stimulation of the infant, as this varies across cultures without producing deficits in caregiver regulation (Choi, 1995; Welles-Nystrom, New, & Richman, 1994).
The effects of inadequate and abusive care on the etiology of neurobehavioral deficits remain poorly understood, although animal models have attempted to add some insight into this complex issue (Gunnar et al., 2015; Hennessy, Hornschuh, Kaiser, & Sachser, 2006; Hennessy, Kaiser, & Sachser, 2009; Hostinar, Gunnar, & Sullivan, 2014; Opendak & Sullivan, 2016; Raineki, Moriceau, & Sullivan, 2010; Roth et al., 2013; Sarro et al., 2014; Sullivan, Perry, Sloan, Kleinhaus, & Burtchen, 2011; Sullivan & Holman, 2010). When drawing parallels between work in humans and animal models, it is important to clarify that animal studies typically model this attachment–trauma within the framework of either harmful or species-atypical input (e.g. abusive caregiving) or absence of expected input (e.g. maternal deprivation, decreased maternal care) (Champagne et al., 2008; Francis, Diorio, Plotsky, & Meaney, 2002; Tottenham, 2012; Tottenham & Sheridan, 2009). Similarly, trauma studies in children distinguish between the neurobehavioral effects of abuse, neglect, and trauma that is not associated with a caregiver (Bowlby, 1984; Bremner, 2003; Maestripieri & Carroll, 1998; Neigh, Gillespie, & Nemeroff, 2009). For instance, data from children raised in institutional care typically reflects the consequences of neglect, rather than abuse (Humphreys & Zeanah, 2015). Although these various subtypes of early-life trauma produce divergent adult outcomes, research using animal models and clinical populations highlights the amygdala’s involvement in the etiology of psychopathology (Raineki et al., 2012; Sitko, Bentall, Shevlin, O’Sullivan, & Sellwood, 2014; Teicher et al., 2016; Tzanoulinou & Sandi, 2016). By modeling attachment in normal and abusive circumstances, we can explore some of the neural mechanisms by which early life attachment programs social and threat processing throughout the lifespan.
The infant attachment circuit
The infant learns about the smell, sight, touch, sound and taste of the caregiver during social interactions. Once learned, these sensory cues from the caregiver are preferred and help regulate infant behavior and physiology. How the infant learns about the caregiver relies upon the unique neurobiology of the infant brain for attachment learning, which is describe below as identified in infant rodents. A useful framework in which to understand the changing learning circuitry of the young brain is to understand that the brain must continuously morph to accommodate the specific behavioral niche at each stage of development. For example, the young infant does not need a brain that supports learning behaviors to gather food from the environment or procure a receptive mate; rather, the brain is designed to learn about the caregiver and show prosocial behaviors toward the caregiver that will engage the caregiver to provide those resources needed for survival (Bowlby, 1978; Hess, 1962). As the child matures, he or she gains adult-like functioning for different tasks at different ages and this is presumably supported by transitions in brain morphology and function.
Given that an altricial infant’s attachment to a caregiver is critical for survival, the attachment circuit was likely shaped by evolutionary pressures to ensure attachment formation occurred, regardless of the quality of caregiving received. Indeed, John Bowlby’s Attachment Theory described the infant brain of altricial species as designed to support attachment to the caregiver, even when the quality of care is compromised or abusive, during a temporally defined sensitive period (Bowlby, 1965, 1978). This feature of attachment formation is supported by clinical and epidemiological literature indicating that abused children frequently long to be reunited with their abusive attachment figure after separation and placement in a safe home (Ainsworth, 1969; Perry & Sullivan, 2014). This has been modeled in a variety of species including birds, non-human primates, dogs, and rodents (Cirulli et al., 2009; Goursaud & Bachevalier, 2007; Harlow & Harlow, 1965; Malkova, Mishkin, Suomi, & Bachevalier, 2010; O’Connor & Cameron, 2006; Raper, Stephens, Sanchez, Bachevalier, & Wallen, 2014; Sanchez, 2006; Sanchez, McCormack, & Howell, 2015; Suomi, 2003).
A rich literature has identified a unique learning circuit involved in the formation of attachment across a variety of species, although the neurobiology of attachment has mostly been described using rodents. Infant rodents, called pups, can neither hear nor see until the third week of life, and olfaction is the main sensory system used for interactions with the caregiver (in contrast to newborn humans, who use all of their sensory systems) (Ehret, 1976; Weber & Olsson, 2008). Specifically, maternal odor is of paramount importance to pups’ survival, as they rely on this odor cue for nipple attachment, proximity seeking, and social behavior. Without these, pups cannot access nourishment, thermoregulation, or maternal care. Indeed, pups without the ability to smell rarely survive as they frequently fail to nipple attach and can become malnourished (Landers & Sullivan, 2012).
The incredibly complex process of caregiver-infant interaction was long considered to be innate and guided by a pheromone (Blass & Teicher, 1980; Distel & Hudson, 1985; Leon, 1983). However, research has indicated that learning is of major importance for activating behavioral systems that are age-relevant and biologically predisposed towards preference for maternal odor. In rat pups, this learning process begins in the prenatal environment, where amniotic odors can guide nipple attachment as soon as pups are born. Even a neutral odor can acquire the valence of a maternal odor if placed into the amniotic fluid a few days before birth, suggesting the odor itself is arbitrary for both rats and mice (Hepper & Cleland, 1998; Leon, 1992; Logan et al., 2012; Pedersen & Blass, 1982; Smotherman & Robinson, 1987; Sullivan & Leon, 1986; Sullivan & Wilson, 1991). Once pups are born, a new maternal odor can be rapidly learned; a novel odor (e.g. peppermint) placed either on the mother or in the air surrounding her will readily take on the properties of maternal odor (Cheslock, Varlinskaya, Petrov, & Spear, 2000; Roth & Sullivan, 2005a; Sullivan, Wilson, Wong, Correa, & Leon, 1990). Outside the nest, if a neutral odor is paired with warmth, milk, or stroking -- stimuli designed to mimic maternal behavior-- this odor acquires the value of a new maternal odor that is not only preferred, but can support nipple attachment and prosocial behavior in the absence of a natural maternal odor (Roth et al., 2013; Roth & Sullivan, 2005; Roth & Sullivan, 2006; Sullivan, Hofer, & Brake, 1986). This is especially important to ensure a robust attachment, given the fact that a dam’s odor can change with her diet and is dependent on gut bacteria (Leon, 1983; Leon, 1992).
During the first ten days of life, the learning process for new maternal odors in rat pups occurs through a relatively simple neurobiological substrate. At this early age, learning-associated plasticity occurs within the olfactory bulb, the first relay station for olfactory processing. This process requires that an odor is paired with copious amounts of NE (Sullivan, Stackenwalt, Nasr, Lemon, & Wilson, 2000; Sullivan, Zyzak, Skierkowski, & Wilson, 1992; Yuan, Harley, Bruce, Darby-King, & McLean, 2000). The sole source of the NE to the olfactory bulb is the LC, and this structure’s unique physiology during early life is essential for neonatal odor approach learning. In particular, the large amounts of NE required for this attachment-related plasticity results from the failure of the infant LC to show habituation or to turn itself off via auto-inhibition (as occurs in older pups and adults) (Nakamura, Kimura, & Sakaguchi, 1987; Winzer-Serhan, Raymon, Broide, Chen, & Leslie, 1996). In addition, the olfactory bulb undergoes a host of anatomical and physiological changes reflecting enhanced responding to the learned maternal odor (Raineki, Shionoya, Sander, & Sullivan, 2009; Roth & Sullivan, 2006; Sullivan et al., 1990; Yuan, Harley, McLean, & Knöpfel, 2002). It is important to note that both natural maternal odor and a learned artificial maternal odor generate the same responses from the olfactory bulb (Raineki, Pickenhagen, et al., 2010; Roth & Sullivan, 2005). The olfactory bulb axons of mitral cells project directly to the piriform cortex (Haberly, 2001; Schwob & Price, 1984; Swanson & Petrovich, 1998; Wilson & Stevenson, 2003); this region plays a key role in assigning the hedonic value to a learned odor stimuli in a region-specific manner. In particular, the anterior piriform is activated by odors learned during this sensitive period, while the posterior piriform is engaged in response to learned odor in older pups and adults (Moriceau & Sullivan, 2006; Moriceau et al., 2006; Roth & Sullivan, 2005). The sensitive period terminates when pups are around 10 days old, as the LC becomes more adult-like: NE release is greatly restricted due to the development of recurrent collaterals that quickly self-inhibit the LC’s response. After the sensitive period, NE takes on a modulatory role in odor learning that is more similar to what has been described in adult rats (Ferry & McGaugh, 2000).
Human infants also show learning of caregiver cues across multiple sensory modalities (DeCasper & Fifer, 1980; Sullivan et al., 2011), which enables them to form attachments to adoptive parents and caregivers of either sex. Although it remains unclear whether learning in human infants is the same as the rodent, NE plays a critical role in bond formation in numerous species (Nelson & Panksepp, 1998; Numan & Young, 2016), suggesting it is a phylogenetically conserved system. Notably, NE levels are very high in humans at birth and over the first two years of life (Lagercrantz & Bistoletti, 1977). Further work will be necessary to determine whether humans engage the same neural circuitry as rodents in forming attachments to a caregiver and whether the child’s brain is predisposed towards forming odor preferences at this age.
Maternal control over stress hormones: Social buffering
Once the attachment figure’s smell, sight, sound, touch and taste are learned, these cues take on the role of regulating the brain and behavior. A crucial factor mediating the effects of early-life attachment on adult outcomes is how well the caregiver can regulate stress reactivity in the infant. As noted above, maternal cues regulate a wide variety of neurobehavioral functions (Hofer, 1994). Social buffering is a phenomenon that has been observed in myriad species and throughout the lifespan and describes the reduction of both the stress response and release of stress hormones (Ditzen & Heinrichs, 2014; Hennessy et al., 2009; Hostinar, Johnson, & Gunnar, 2015; Kikusui, Winslow, & Mori, 2006; Sanchez et al., 2015; Sullivan & Perry, 2015; Takahashi et al., 2013). This has been demonstrated in children, for whom maternal presence dampens cortisol reactivity to threats even when they behaviorally exhibit fear (Nachmias et al., 1996).
Social buffering of the infant is a dynamic process that wanes as individuals across species mature and become independent (Gee et al., 2014; Levine, 2001; McCormack, Newman, Higley, Maestripieri, & Sanchez, 2009; Sanchez, 2006; Stanton & Levine, 1990; Suchecki, Nelson, Van Oers, & Levine, 1995; van Oers, de Kloet, Whelan, & Levine, 1998) (Figure 1). For example, at birth, rat pups have a functional HPA axis, although it soon becomes hypo-responsive and not activated by most painful stimuli, a period of life termed the stress hypo-responsive period (SHRP) (Dallman, 2000; Stanton & Levine, 1985). Since no stress response occurs, we have traditionally viewed social buffering as nonfunctional or irrelevant in infants. Although the specific time-course is unknown, there appears to be a similar stress hypo-responsive period in human children. Studies show that infants begin to exhibit dampened cortisol reactivity during the first year of life (6–12 months) (Gunnar & Donzella, 2002; Gunnar, Hostinar, Sanchez, Tottenham, & Sullivan, 2015). Although the duration of this period is unknown, basal cortisol remains at low levels through the preschool period (Grunau, Weinberg, & Whitfield, 2004; Watamura, Donzella, Kertes, & Gunnar, 2004).
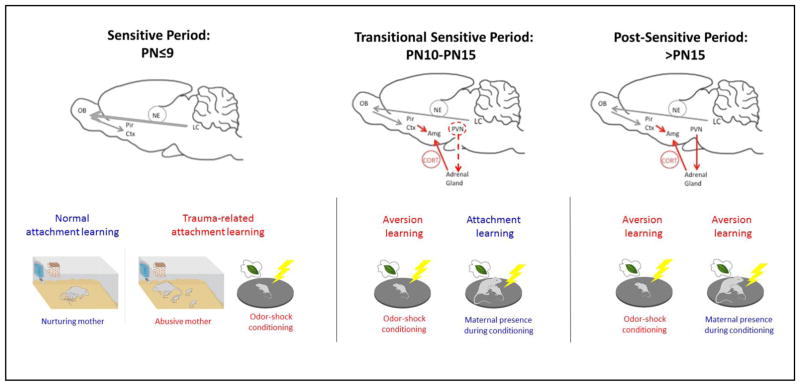
Figure 1.
Transitions in learning across early development. Using a fear conditioning paradigm of odor-shock presentations has enabled us to uncover a developmentally unique learning system in pups that typically supports attachment learning. Data indicate that during the sensitive period for attachment learning (PN<9), low CORT levels block amygdala plasticity to prevent pups from learning amygdala-dependent fear/threat. Instead, this learning paradigm activates the attachment learning neural circuit involving elevated NE (thick gray arrow) to produce approach responses to the odor (Moriceau et al., 2006). The odor also takes on qualities of the maternal odor to support nipple attachment and enhance prosocial behaviors to the mother. In pups older than PN9, this fear conditioning paradigm accesses the amygdala to support fear/threat learning if the pup is alone. A critical feature of this learning is that shock induces activation of the HPA axis and CORT release, which is necessary for the young amygdala to learn. However, if the mother is present, she socially buffers the pup’s stress response, and pups revert to sensitive period learning and learn an odor preference (red dashed line). This mother-controlled switch between fear and attachment learning is mediated through the mother’s ability to control pups CORT (Sullivan, in press). A more adult-like fear learning system, which cannot be switched on/off by CORT develops by PN15. Environmental variables that control pups’ CORT level, such as receiving CORT from a stressed mother via milk, environmental manipulations that increase pups’ CORT (abusive rearing) or the mother’s ability to socially buffer the pups (compromised in abusive mothers), have the potential to modify the age of these transitions and whether a pup learns fear or attachment (Moriceau et al., 2006; Perry & Sullivan, 2014; Raineki, Cortés, et al., 2012; Shionoya et al., 2007; Sullivan & Holman, 2010).
At PN10 in rodents, the SHRP begins to wane and we begin to see increases in CORT release in response to shock and other stressful stimuli in pups (Sullivan & Holman, 2010). It is also the age at which pups transition from crawling to walking and begin to leave the nest and nibble solid foods (Galef, 1981). As mentioned above, the LC takes on more adult-like functioning at this age (Moriceau & Sullivan, 2004; Nakamura & Sakaguchi, 1990; Sullivan & Wilson, 1994). The mother also begins to socially buffer the stress response, in a manner similar to that seen in adults. Specifically, at PN10, stressful stimuli begin to produce a more immediate increase in pups’ CORT levels, and the presence of the mother completely blocks its release (Moriceau & Sullivan, 2006; Stanton & Levine, 1990; Suchecki, Rosenfeld, & Levine, 1993). Research has begun to explore the specific neural mechanisms involved in social buffering (Hennessy et al., 2006; Hennessy et al., 2009; Hennessy et al., 2015; Moriceau & Sullivan, 2006; Shionoya, Moriceau, Bradstock, & Sullivan, 2007). It has been shown that in infant rodents, social buffering by the mother greatly attenuates stress hormone release by the HPA axis at the level of the hypothalamic paraventricular nucleus (PVN), through suppression of NE afferents from medullary A1/A2 noradrenergic neurons (Shionoya et al., 2007)--a system identified in adults (Ziegler & Herman, 2002). This social buffering by the mother has profound effects on whether pups have access to the attachment learning neural circuitry: the maternal social buffering can reopen pups sensitive period for attachment between the ages of PN10–15. In particular, during this transitional sensitive period, maternal presence can modulate whether pups learn to avoid or prefer an odor paired with shock; this process depends on amygdala serotonin and CORT levels (Moriceau et al., 2006) (Figure 2).
Figure 2.
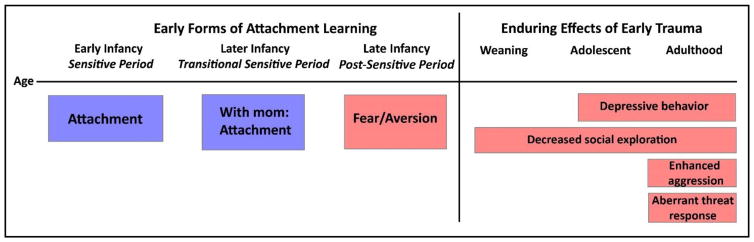
Timeline of attachment learning and the effects of early life maltreatment on later-life social and emotional behavior in the rat model of trauma associated with attachment. Early infants will learn attachment regardless of the quality of care, while slightly older infants (PN10-PN15) will either learn to fear a traumatic associated stimulus when away from the mother or learn an attachment if acquisition takes place with the mother. Testing later in life shows that only the early life trauma associated with attachment will lead to lifelong amygdala-dependent behavioral deficits, such as poor social behavior (onset prior to weaning) and depressive-like behaviors (onset post- weaning (Raineki, Cortes, et al., 2012; Raineki, 2010; Sevelinges et al., 2011; Sullivan, Landers, Yeaman, & Wilson, 2000). In adulthood, early trauma produces enhanced aggression (Marquez et al., 2013) and impaired threat response (Perry, in press).
After PN15, pups show a rapid transition to independence and by weaning age (~PN23), they show stress-induced activation of the HPA axis similar to adult-like levels. At this age, the relative ability of the mother to decrease the pups’ adult level stress hormone response is greatly reduced and leaves pups with significant CORT levels (Levine, Stanton, & Gutierrez, 1988; Stanton & Levine, 1990; Suchecki et al., 1993; Upton & Sullivan, 2010). The human literature also suggests that, with further maturation, maternal presence loses some value to socially buffer children beginning to enter adolescence (Gee et al., 2014; Hostinar et al., 2015; Sanchez et al., 2015; Sandi & Haller, 2015) which is consistent with the animal literature (Barr et al., 2009; Ditzen & Heinrichs, 2014; Hennessy et al., 2015; Kiyokawa, Kikusui, Takeuchi, & Mori, 2004; Sanchez et al., 2015; Shionoya et al., 2007; Sullivan & Perry, 2015; Takahashi et al., 2013).
Attachments formed to abusive caregivers
The quality of care an infant receives from the caregiver, while preserving attachment, does alter how well the caregiver can regulate the infant’s physiology. For instance, highly stressed caregivers have a reduced capacity to socially buffer children (Ainsworth & Bell, 1970; Gunnar et al., 2007; Gunnar, Brodersen, Nachmias, Buss, & Rigatuso, 1996; Nachmias et al., 1996). In spite of this, throughout the animal kingdom, young, including humans, form attachments to abusive caregivers (Harlow & Harlow, 1965; Hess, 1962; Rajecki, Lamb, & Obmascher, 1978; Salzen, 1970; Stanley, 1962). This abuse-related attachment appears phylogenetically conserved across species, including chicks that form attachments after being shocked during imprinting (Hess, 1962; Rajecki et al., 1978; Salzen, 1970), dogs (Stanley, 1962), and monkeys raised with a wire surrogate that inflict pain (Harlow & Harlow, 1965). More recent work has modeled abusive caregiving in nonhuman primates and again shows that infants retain strong preferences for the abusive caregiver (Maestripieri et al., 1999; O’Connor & Cameron, 2006; Sanchez et al., 2001; Suomi, 2003). Attachments to an abusive or negligent caregiver may have short-term advantages, e.g. there is neonatal access to care, but long-term consequences associated with compromised threat processing and emotion expression.
Due to the unique neurobiology of the infant brain that is biased towards forming attachments, traumatic cues during the sensitive period for attachment are processed within the attachment circuitry rather than the threat processing circuitry. Again, the rodent literature provides some clues to understanding why this occurs. Our lab employs an abuse paradigm in which the mother rat is provided insufficient bedding to build a nest for the pups. Under these circumstances, the mother becomes highly agitated and frequently builds and re-builds her nest. In the process, she mistreats the pups, behaviors that include stepping on pups, dragging them across the cage floor, and transporting them inappropriately, inducing pain-related vocalizations in pups (Roth & Sullivan, 2005; Moriceau, et al., 2009). Pairing this painful maternal care with a novel peppermint odor does not activate the important survival circuit within the brain to support aversion learning. Rather, pups not only learn to approach this odor, but this odor takes on the qualities of maternal odor to support nipple attachment and prosocial behaviors (Roth & Sullivan, 2005; Sullivan et al., 1986). This learning can occur rapidly, within as little as 10–30 minutes. While the behavioral output of attachment formation with pain appears similar to typical attachment within the nest, as noted below, stress can uncover infant neurobehavioral problems consistent with disordered attachment (Raineki et al., 2010).
Although this naturalistic maternal abuse paradigm is highly informative, its complexity makes it difficult to assess the mechanisms linking low resources and neurobehavioral pathology. Therefore, we complement this model with a classical conditioning paradigm to mimic abusive attachment. Just as new maternal odors can be learned when a previously neutral odor is paired with stimuli evoking maternal care, such as milk or stroking, we paired an odor with a moderately painful foot-shock (0.5 mA) or tail-pinch; this produced an odor preference in young pups (Camp & Rudy, 1988; Haroutunian & Campbell, 1979; Spear, 1978; Sullivan et al., 1986); we later showed this procedure also produces a new maternal odor that pups prefer (Raineki et al., 2012). The inability of the paired odor-pain procedure to produce fear learning is not due to pups’ inability to detect the aversive stimulus or feel pain. Noxious stimuli readily elicit <PN9 pup escape responses and the pain threshold does not appear to change as shock switches from supporting preference to supporting aversion learning (Barr, 1995; Collier & Bolles, 1980; Emerich, Scalzo, Enters, Spear, & Spear, 1985; Stehouwer & Campbell, 1978). As described above, the pup’s olfactory bulb, anterior piriform cortex, and a hyper-functioning LC work together to generate enhanced odor preference learning despite adversity. Critically, shock presentations that are not paired with an attachment odor fail to produce the same neurobehavioral sequelae as either abusive care or paired odor-shock treatment (Raineki et al., 2012).
As mentioned above, termination of the sensitive period for attachment at PN10 in typical rearing conditions is primarily due to increasing levels of CORT and functional emergence of the amygdala (Sullivan & Holman, 2010). While CORT levels naturally increase at PN10, the environment readily changes pups CORT levels, providing environmental control of the sensitive period for attachment termination. Specifically, increasing CORT during the sensitive period, either via rearing with an abusive mother, or through pharmaceutical manipulations (systemic injections or by intra-amygdala microinfusions), can prematurely end sensitive period learning. Indeed, the amygdala is mature enough to support threat learning in pups as young as PN6, provided sufficient levels of corticosterone are available within the amygdala (Debiec & Sullivan, 2014; Moriceau, Roth, Okotoghaide, & Sullivan, 2004; Moriceau, Shionoya, Jakubs, & Sullivan, 2009; Moriceau & Sullivan, 2004, 2006). Pups reared by an abusive mother during the SHRP receive CORT through her milk and her ability to socially buffer this CORT elevation is compromised; when these pups are trained on a peppermint odor-shock conditioning paradigm outside the nest at PN7, they will learn an aversion through amygdala-dependent mechanisms (Moriceau, Roth, & Sullivan, 2010; Raineki, Moriceau, & Sullivan, 2010). Similarly, pups reared with a normal nurturing mother but trained on a 5-day odor-shock conditioning procedure during the SHRP will learn an aversion to the odor if they receive CORT injections before each training session (Raineki et al., 2010). It is important to note that, although abused pups or pups injected with CORT can learn arbitrary odor aversions (eg. peppermint) via premature amygdala engagement, they will nevertheless always show a preference for the maternal odor. As will be discussed further, this preference accompanies a disordered, rather than typical, attachment.
Although abuse and repeated odor-shock fails to produce an aversion to maternal odor, these manipulations generate latent changes in amygdala function that emerge around weaning age. Importantly, direct abuse from the caregiver and pairing of the maternal odor with shock produce indistinguishable neurobehavioral outcomes. Amygdala-dependent deficits are expressed in a task-specific manner: amygdala hyperactivity underlies decreased social exploration in adolescence and depressive and anxiety-like behavior in adulthood (Figure 2), in parallel with impaired ability to learn aversions to threat and blunted amygdala activation during fear conditioning (Raineki et al., 2012; Sevelinges et al., 2007; Sevelinges et al., 2011; Sevelinges, Sullivan, Messaoudi, & Mouly, 2008). These results parallel clinical studies showing a role for amygdala dysfunction in psychiatric sequelae in adults with a history of attachment trauma, resulting from abuse and/or neglect, during childhood (Callaghan et al., 2014; Teicher et al., 2003).
Stress uncovers latent consequences of early-life trauma
Across many species, many of the effects of infant abuse remain latent until peri-adolescence (Adriani & Laviola, 2004; Amaral, 2003; Andersen & Teicher, 2008; Bauman, Toscano, Mason, Lavenex, & Amaral, 2006; Costello, Mustillo, Erkanli, Keeler, & Angold, 2003). For this reason, it can be difficult to identify abused children in the absence of physical evidence. In parallel with observations of latent abuse-related deficits in rodents and non-human primates, many of the effects of early life trauma on children’s mental health appear to be delayed until they begin to transition into adolescence (Bachevalier & Loveland, 2006; Bachevalier, Malkova, & Mishkin, 2001; Callaghan et al., 2014; Goursaud & Bachevalier, 2007; Goursaud, Wallen, & Bachevalier, 2014; Machado & Bachevalier, 2003; Raper et al., 2014; Tottenham & Sheridan, 2009). However, stress can uncover deficits associated with trauma that may otherwise be hidden, as demonstrated by Mary Ainsworth’s Strange Situation Test. In this procedure, repeatedly removing children’s caregiver and introducing a stranger was necessary to reveal behavioral impairments marking disordered attachment (Ainsworth, 1969; Crittenden, 1992; Nachmias et al., 1996). This can also be modeled in rodents: pups that were exposed to abuse in the low bedding procedure and repeated odor-shock pairings form a disordered attachment to the mother, expressed in decreased approach behavior towards the maternal odor, less nipple attachment, and amygdala hyperactivity if given CORT injections before testing (Raineki, Cortes, et al., 2012). The CORT injection in pups models a high-stress environment, which appears necessary to uncover neurobehavioral deficits before weaning. Importantly, this CORT injection did not disrupt social interaction or activate the amygdala in pups reared with a nurturing mother, nor was there evidence of disordered attachment in pups that received only shock trauma or unpaired odor-shock training. Taken together, these findings suggest that repeatedly experiencing trauma associated with caregiver cues during the sensitive period for attachment has a unique neural signature, producing latent changes in amygdala function to program emotionality and social behavior throughout the lifespan.
Amygdala involvement in social behavior throughout the lifespan
In humans, the amygdala is implicated in social behavior in adults (Thomas et al., 2001), as well as during development (Skuse et al., 2003; Tottenham & Sheridan, 2009). Furthermore, research has shown that humans with disorders associated with social behavior deficits, such as Autism and Williams Syndrome, show amygdala abnormalities (Baron-Cohen et al., 1999; Bachevalier et al., 2000; Critchley et al., 2000; Howard et al., 2000; Pierce et al., 2001; Haas et al., 2009; Paul et al., 2009). Moreover, studies in nonhuman primates demonstrate that adult monkeys without amygdalae display inappropriate social behavior (Amaral, 2003; Baron-Cohen et al., 2000; Bliss-Moreau, Bauman, & Amaral, 2011; Brothers, Ring, & Kling, 1990; Emery et al., 2001; Malkova et al., 2010).
While the amygdala is also implicated in social behavior in children (Tottenham & Sheridan, 2009), its role is less clear. Studies on abused and neglected children, as well as children with PTSD, indicates changes in the response to threatening faces (Pine et al., 2005; Pollak, Cicchetti, Hornung, & Reed, 2000). The most dramatic developmental differences were first observed in non-human primates, where infant amygdala lesions lead to decreased fear response to normally threatening stimuli and an enhanced response to novel social situations (Amaral, 2002, 2003). This parallels work showing impaired threat assessment in abused rats with amygdala dysfunction (Perry, Santiago, & Sullivan, in press) and rats that were stressed during peripuberty (Marquez et al., 2013).
The rodent literature allows for more precise manipulations of specific amygdala nuclei. Social behavior in adult rodents appears to rely on the medial amygdala (Rasia-Filho, Londero, & Achaval, 2000). It has been shown that c-Fos expression, an indirect marker of neural activation, increases in the medial amygdala following social encounters and maternal behavior in rodent models (Fleming, Suh, Korsmit, & Rusak, 1994; Kirkpatrick, Carter, Newman, & Insel, 1994). Medial amygdala activation is also associated with rodent parental behavior, which is blocked by lesioning this nucleus (Ferguson, Young, & Insel, 2002; Gobrogge, Liu, Jia, & Wang, 2007; Kirkpatrick et al., 1994). While the medial amygdala has a prominent role in social behavior, the basolateral, central and cortical amygdala nuclei have also been implicated (Katayama et al., 2009). As will be discussed below, these nuclei form part of a functional circuit with the hippocampus and vmPFC that is critically engaged in complex forms of social behavior.
It is important to note that while social behavior in adult rats involves the amygdala and can be a behavioral measure used to reveal those adults with early life trauma, in rat pups, it is only during periods of heightened stress (eg. heightened CORT levels) combined with early life trauma that social behavior deficits and heightened amygdala neural activity can be uncovered. This suggests that the amygdala is not involved with social behavior in infancy and its activation can, in fact, impair social behavior at this age.
Implications for complex social behavior
Aberrant processing of social and threatening cues stemming from amygdala dysfunction is a hallmark of psychiatric sequelae following early-life abuse and neglect (Levin et al., 2015; Teicher et al., 2003; Troller-Renfree et al., 2016; Zeanah & Gleason, 2015). As individuals mature, increasingly complex social demands may multiply the consequences of impaired social behavior, amplifying stress and even compromising welfare. Exploring these complex social arrangements in animal models can provide insight into the mechanisms underlying long-term effects of social deficits and identify therapeutic targets for attachment trauma in early life.
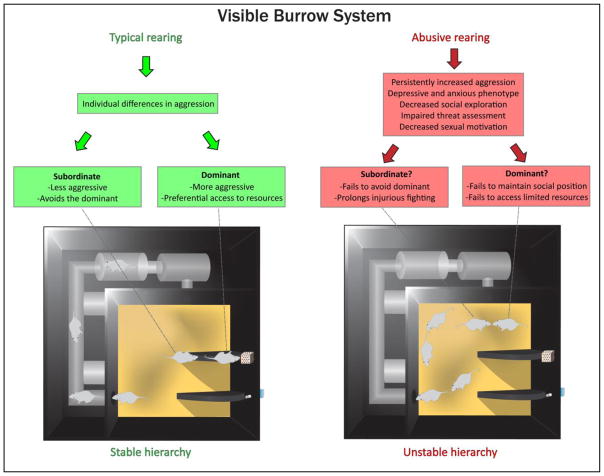
Dominance hierarchies provide one example of a complex social arrangement determined by individual differences that can result from early life experience. In the wild, rats are among a wide variety of species that naturally form dominance hierarchies as a result of competition for limited resources (Sapolsky, 2005). This can be simulated in the laboratory using a visible burrow system, a semi-naturalistic enclosure that replicates many of the challenges and opportunities of group-living in nature; in this setting, rats rapidly form stable dominance hierarchies (Figure 3)(Blanchard et al., 1995; Blanchard, Flannelly, & Blanchard, 1988; Opendak et al., 2016). A rich literature has described the effects of life in a dominance hierarchy on its members, including measures of behavior, hormones and physiology (Blanchard et al., 1995, Hardy et al., 2002). These studies have focused on differences between dominants and subordinates within the aggressive Long Evans (LE) rat strain. The factors that contribute to social position are complex, but the consequences of stratification can be dramatic for an animal’s quality of life. When LE rats form a dominance hierarchy within a laboratory enclosure, it has been shown that subordinate rats have elevated levels of CORT compared to dominants and can be prone to illness and weight loss due to chronic stress (Blanchard et al., 1995; Hardy et al., 2002). Subordinate rats show a decrease in overall activity and social behaviors, including aggression and sexual advances. In addition, they show an increase in a range of defensive responses to the dominant male (Blanchard et al., 1995, 2001). Furthermore, subordinates exhibit increases in the relative sizes of adrenal glands and spleen and decreases in the sizes of the thymus and testes than dominants. In contrast, dominants enjoy preferential access to resources, as well as increases in markers of adult-brain plasticity. Specifically, dominant rats show enhanced adult neurogenesis in the ventral dentate gyrus of the hippocampal formation, an effect that also has been shown in baboons (Kozorovitskiy & Gould, 2004; Peragine, Simpson, Mooney, Lovern, & Holmes, 2014; Wu et al., 2014).
Figure 3.
Schematic of a visible burrow system for use in rodents and putative effects of early-life abuse on dominance hierarchy formation. This enclosure, adapted from Blanchard et al (1995), allows for a semi-naturalistic setting in which rodents can be group-housed with limited access to resources including food, water, and receptive females. Social position can emerge from individual differences in aggression and stress reactivity. These factors may be compromised in cases of caregiver abuse in early life, which produces enhanced aggression (Marquez et al., 2013), impaired threat response (Perry, in press), depressive and anxiety-like behavior (Raineki, Cortes, et al., 2012; Raineki et al., 2015) and decreased social exploration (Raineki et al., 2015). As a result, abuse may affect formation and maintenance of a stable dominance hierarchy.
When animal size and previous agonistic experience are equivalent, social stratification emerges from small individual differences in several factors, including aggression, stress reactivity, social behavior, and “home-field advantage”, or benefits for residents versus intruders (Barnett, 1958; Sapolsky, 2005; So, Franks, Lim, & Curley, 2015). Although the neurobiology of forming a stable hierarchy is incredibly complex and involves myriad brain regions, several brain areas have been identified as key mediators of social dominance, such as the vmPFC, ventromedial hypothalamus, ventral hippocampus, and amygdala (Bauman et al., 2006; Rosvold, Mirsky, & Pribram, 1954; So et al., 2015; Watanabe & Yamamoto, 2015; Wong et al., 2016). These regions have been implicated in aggression-seeking behavior, social recognition, and memory for social position. Importantly, the basolateral amygdala inhibits the ventral hippocampus during social interaction (Felix-Ortiz, Burgos-Robles, Bhagat, Leppla, & Tye, 2016; Felix-Ortiz & Tye, 2014; Gunaydin et al., 2014). Interestingly, dominance has been linked with levels of corticotropin response factor (CRF) mRNA in the medial amygdala in mice (So et al., 2015).
Compromised amygdala function in animals with a history of caregiver abuse during the sensitive period for attachment may have highly maladaptive consequences with respect to social position (Figure 3). For instance, global impairments in social behavior may pre-dispose abused rats to a lifetime of subordination, resulting in not only a lack of rewards afforded to dominants, but also increased stress and illness in some types of hierarchies (Blanchard et al., 1995; Sapolsky, 2005). On the other hand, increased aggression may lead to dominance in some abused animals. However, heightened anxiety and/or depressive-like behavior may prevent these same animals from maintaining their social position, resulting in a chronically unstable hierarchy that can produce profound changes in adult brain plasticity for all members (Green, Barnes, & McCormick, 2013; Opendak & Gould, 2015; Opendak et al., 2016; Weathington, Arnold, & Cooke, 2012). Furthermore, caregiver abuse decreases sexual motivation in adult rodents (Raineki et al., 2015), suggesting that these animals may fail to engage receptive females, a benefit of dominance in typically-reared individuals (Blanchard et al., 1995; Blanchard et al., 1988; White, Fischer, & Meunier, 1986).
Rats with a history of caregiver abuse show impaired response to a salient predator odor threat and amygdala dysfunction, suggesting that maintenance of a social hierarchy may also be compromised in these animals (Perry et al., in press) (Figure 3). Specifically, these abused rats may fail to show subordination to an established dominant, leading to prolonged injurious fighting and precluding their timely access to limited resources (Blanchard et al., 1995; Blanchard & Blanchard, 1989). Indeed, rats that were stressed during adolescence show persistent aggression towards larger intruders in the resident-intruder paradigm, against whom a more optimal strategy may be to show submission (Marquez et al., 2013). Avoiding the dominant involves multiple learned cues, including social odor, and has been linked with adult-born neurons in the hippocampus (Lagace et al., 2010) and oxytocin receptor mRNA in the medial amygdala (Timmer, Cordero, Sevelinges, & Sandi, 2011). A role for the amygdala in this process is further supported by data in non-human primates showing impaired responses to fearful stimuli following infant amygdala lesions (Amaral, 2003; Bauman et al., 2006; Marquez et al., 2013).
Abuse-related impairments in amygdala function may have implications for other social behaviors as well. As mentioned previously, rats that were abused during the sensitive period for attachment show decreased social exploration in adulthood (Raineki et al., 2015). Specifically, they exhibit decreased preference for interacting with a conspecific over an empty container in a classic three-chamber test (Crawley, 2004). A variation on this test involves comparison between a novel conspecific and a familiar conspecific; at baseline, rats tend to prefer to interact with the novel rat. This behavior has been shown to be affected by specific social experiences within a dominance hierarchy. In particular, destabilization of a stable dominance hierarchy of rats by removal of the dominant reverses the social preference for novelty in all community members: in the three-chamber test, rats spend more time with a familiar conspecific. This behavior has been linked with changes in the number of adult-born neurons (Opendak et al., 2016). Work showing changes in novelty-seeking following early-life amygdala lesions in non-human primates (Amaral, 2003) suggests that social novelty preference may also be affected in animals with a history of abuse. Given the extensive connections between the amygdala and ventral hippocampus in social interaction and novelty-seeking (Felix-Ortiz & Tye, 2014; Gunaydin et al., 2014; Zeamer & Bachevalier, 2013), exploring these interactions in a model of amygdala dysfunction presents an exciting opportunity for further research.
Concluding remarks
Early life provides a sensitive window for programming lifelong emotional and cognitive processing, which has profound influence over threat-processing and social behaviors. In altricial species, this period of brain development favors forming attachments to a caregiver, regardless of the quality of care, in order to ensure the infant receives access to warmth, protection, and food. However, attachments formed in threatening or traumatic contexts have unique consequences for the development of social behavior and learning about threat. In the clinical literature, this has been demonstrated in individuals with a history of abuse or disordered attachment who exhibit compromised fear and social behavior, as well as vulnerability to later-life mental health disorders (Bowlby, 1984; Bremner, 2003; Famularo, Kinscherff, & Fenton, 1992; Graham et al., 1999; Nemeroff & Vale, 2005; Pechtel et al., 2014).
Animal models across a variety of species have allowed us to assess the mechanisms by which caregiver abuse engenders neurobehavioral deficits, with programming of the stress system as a point of convergence in adult outcomes (Andersen & Teicher, 2008; Denenberg, 1963; Famularo et al., 1992; Harlow & Harlow, 1965; Hofer, 1994; Levine, 1957; Levine, Johnson, & Gonzalez, 1985; Teicher et al., 2003). Here we have focused on two very selective models of caregiver abuse in rodents, namely, rough handling by the mother given low nesting resources, and repeated presentations of paired odor-shock during the sensitive period for attachment. Both of these manipulations produces latent changes in amygdala function and depressive-like behavior, aberrant fear expression, and social behavior deficits (Perry et al., in press; Raineki, Moriceau, & Sullivan, 2010; Raineki et al., 2015; Roth, Matt, Chen, & Blaze, 2014; Sevelinges et al., 2007; Sevelinges et al., 2011; Sevelinges et al., 2008). Importantly, these effects are not produced when a pup experiences shock without the mother (Sarro, Sullivan, & Barr, 2014; Tyler, Moriceau, Sullivan, & Greenwood-van Meerveld, 2007). These results suggest that caregiver abuse during the sensitive period for attachment produces a unique outcome, with the social dimension of early abuse, rather than pain alone, predisposing amygdala development towards an impaired phenotype.
In the clinical population, the amygdala has also been implicated in the pathogenesis of psychiatric sequelae. For instance, patients with depression also show alterations in amygdala function and its connectivity with other brain areas (Elzinga et al., 2003; Frijling et al., 2016; Jedd et al., 2015; McEwen, 2003; Ressler & Mayberg, 2007; Savitz & Drevets, 2009; Sibille et al., 2009; Teicher et al., 2003). In light of the relationship between attachment, the amygdala, and long-term mental health outcomes, we may predict that early life is not only a sensitive period for rodent amygdala development, but also for humans. The amygdala undergoes major development progress throughout the first seven years of life in children but continues to develop into adolescence (Giedd et al., 1996; Humphrey, 1968; Letcher, Smart, Sanson, & Toumbourou, 2009; Lupien, McEwen, Gunnar, & Heim, 2009; Tottenham & Sheridan, 2009; Uematsu et al., 2012; Ulfig, Setzer, & Bohl, 2003). This suggests that early life may be a period of rapid change and, likewise, heightened vulnerability of the amygdala to environmental influence, as well as multiple interconnected brain regions, including the hippocampus and PFC (Ehrlich & Josselyn, 2016; Ehrlich et al., 2012; Lupien et al., 2009). Future work will be necessary to fully uncover the unique role of the caregiver in programming brain regions relevant for adaptive threat processing and social behavior.
Acknowledgments
This work was supported by training grant T32MH019524 (supports MO), and NIH DC009910, MH091451, HD083217 (supports RMS)
Footnotes
Author Contributions: MO, EG and RMS wrote the review.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adriani W, Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behavioural Pharmacology. 2004;15(5–6):341–352. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Ainge JA, Langston RF. Ontogeny of neural circuits underlying spatial memory in the rat. Front Neural Circuits. 2012;6:8. doi: 10.3389/fncir.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth MD. Object relations, dependency, and attachment: a theoretical review of the infant-mother relationship. Child Dev. 1969;40(4):969–1025. [PubMed] [Google Scholar]
- Ainsworth MD, Bell SM. Attachment, exploration, and separation: illustrated by the behavior of one-year-olds in a strange situation. Child Dev. 1970;41(1):49–67. [PubMed] [Google Scholar]
- Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The Anterior Cingulate Cortex. Ann N Y Acad Sci. 2001;935(1):107–117. doi: 10.1111/j.1749-6632.2001.tb03476.x. [DOI] [PubMed] [Google Scholar]
- Amaral DG. The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biol Psychiatry. 2002;51(1):11–17. doi: 10.1016/s0006-3223(01)01307-5. [DOI] [PubMed] [Google Scholar]
- Amaral DG. The amygdala, social behavior, and danger detection. Ann N Y Acad Sci. 2003;1000:337–347. doi: 10.1196/annals.1280.015. [DOI] [PubMed] [Google Scholar]
- Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27(1–2):3–18. doi: 10.1016/s0149-7634(03)00005-8. S0149763403000058 [pii] [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31(4):183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, … Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 2003;6(2):196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Loveland KA. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neurosci Biobehav Rev. 2006;30(1):97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Malkova L, Mishkin M. The amygdala, social cognition, and autism. In: Aggleton J, editor. The amygdala. New York: Oxford University Press; 2000. pp. 509–543. [Google Scholar]
- Bachevalier J, Malkova L, Mishkin M. Effects of selective neonatal temporal lobe lesions on socioemotional behavior in infant rhesus monkeys (Macaca mulatta) Behavioral neuroscience. 2001;115(3):545–559. doi: 10.1037//0735-7044.115.3.545. [DOI] [PubMed] [Google Scholar]
- Bagot RC, van Hasselt FN, Champagne DL, Meaney MJ, Krugers HJ, Joels M. Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol Learn Mem. 2009;92(3):292–300. doi: 10.1016/j.nlm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Bale TL. Epigenetic and transgenerational reprogramming of brain development. Nat Rev Neurosci. 2015;16(6):332–344. doi: 10.1038/nrn3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett SA. An analysis of social behavior in wild rats. Proceedings of the Zoological Society of London. 1958;130(1):107–152. doi: 10.1111/j.1096-3642.1958.tb00565.x. [DOI] [Google Scholar]
- Baron-Cohen S, Ring HA, Bullmore ET, Wheelwright S, Ashwin C, Williams SC. The amygdala theory of autism. Neurosci Biobehav Rev. 2000;24(3):355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Barr G. Ontogeny of nociception and antinociception. NIDA Research Monograph. 1995;158:172–201. [PubMed] [Google Scholar]
- Barr GA, Moriceau S, Shionoya K, Muzny K, Gao P, Wang S, Sullivan RM. Transitions in infant learning are modulated by dopamine in the amygdala. Nat Neurosci. 2009;12(11):1367–1369. doi: 10.1038/nn.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Toscano JE, Mason WA, Lavenex P, Amaral DG. The expression of social dominance following neonatal lesions of the amygdala or hippocampus in rhesus monkeys (Macaca mulatta) Behavioral neuroscience. 2006;120(4):749–760. doi: 10.1037/0735-7044.120.4.749. [DOI] [PubMed] [Google Scholar]
- Bekenstein JW, Lothman EW. An in vivo study of the ontogeny of long-term potentiation (LTP) in the CA1 region and in the dentate gyrus of the rat hippocampal formation. Brain Res Dev Brain Res. 1991;63(1–2):245–251. doi: 10.1016/0165-3806(91)90084-v. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. The GABA excitatory/inhibitory developmental sequence: a personal journey. Neuroscience. 2014;279:187–219. doi: 10.1016/j.neuroscience.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Berdel B, Morys J, Maciejewska B. Neuronal changes in the basolateral complex during development of the amygdala of the rat. Int J Dev Neurosci. 1997;15(6):755–765. doi: 10.1016/s0736-5748(97)00022-1. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Mills KL. Is adolescence a sensitive period for sociocultural processing? Annu Rev Psychol. 2014;65:187–207. doi: 10.1146/annurev-psych-010213-115202. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20(2):117–134. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Antipredator defensive behaviors in a visible burrow system. J Comp Psychol. 1989;103(1):70–82. doi: 10.1037/0735-7036.103.1.70. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Flannelly KJ, Blanchard DC. Life-span studies of dominance and aggression in established colonies of laboratory rats. Physiol Behav. 1988;43(1):1–7. doi: 10.1016/0031-9384(88)90089-3. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Yudko E, Dulloog L, Blanchard DC. Defense changes in stress nonresponsive subordinate males in a visible burrow system. Physiology & Behavior. 2001;72(5):635–642. doi: 10.1016/s0031-9384(00)00449-2. [DOI] [PubMed] [Google Scholar]
- Blass EM, Teicher MH. Suckling. Science. 1980;210(4465):15–22. doi: 10.1126/science.6997992. [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau E, Bauman MD, Amaral DG. Neonatal amygdala lesions result in globally blunted affect in adult rhesus macaques. Behavioral Neuroscience. 2011;125(6):848–858. doi: 10.1037/a0025757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J, Rether K, Groger N, Xie L, Braun K. Perinatal programming of emotional brain circuits: an integrative view from systems to molecules. Front Neurosci. 2014;8:11. doi: 10.3389/fnins.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby J. Attachment. New York Basic Books; 1965. [Google Scholar]
- Bowlby J. Attachment theory and its therapeutic implications. Adolesc Psychiatry. 1978;6:5–33. [PubMed] [Google Scholar]
- Bowlby J. Violence in the family as a disorder of the attachment and caregiving systems. Am J Psychoanal. 1984;44(1):9–27. 29–31. doi: 10.1007/BF01255416. [DOI] [PubMed] [Google Scholar]
- Bremne JD, Vermetten E. Stress and development: behavioral and biological consequences. Dev Psychopathol. 2001;13(3):473–489. doi: 10.1017/s0954579401003042. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Long-term effects of childhood abuse on brain and neurobiology. Child Adolesc Psychiatr Clin N Am. 2003;12(2):271–292. doi: 10.1016/s1056-4993(02)00098-6. [DOI] [PubMed] [Google Scholar]
- Brothers L, Ring B, Kling A. Response of neurons in the macaque amygdala to complex social stimuli. Behav Brain Res. 1990;41(3):199–213. doi: 10.1016/0166-4328(90)90108-q. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Teuchert-Noodt G. Postnatal development of dopamine innervation in the amygdala and the entorhinal cortex of the gerbil (Meriones unguiculatus) Brain Res. 2006;1125(1):9–16. doi: 10.1016/j.brainres.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Calcagnoli F, Stubbendorff C, Meyer N, de Boer SF, Althaus M, Koolhaas JM. Oxytocin microinjected into the central amygdaloid nuclei exerts anti-aggressive effects in male rats. Neuropharmacology. 2015;90:74–81. doi: 10.1016/j.neuropharm.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Caldji C, Tannenbaum B, Sharma S, Francis D, Plotsky PM, Meaney MJ. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc Natl Acad Sci U S A. 1998;95(9):5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan BL, Cowan CS, Richardson R. Treating Generational Stress: Effect of Paternal Stress on Development of Memory and Extinction in Offspring Is Reversed by Probiotic Treatment. Psychol Sci. 2016;27(9):1171–1180. doi: 10.1177/0956797616653103. [DOI] [PubMed] [Google Scholar]
- Callaghan BL, Sullivan RM, Howell B, Tottenham N. The international society for developmental psychobiology Sackler symposium: early adversity and the maturation of emotion circuits--a cross-species analysis. Dev Psychobiol. 2014;56(8):1635–1650. doi: 10.1002/dev.21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camp LL, Rudy JW. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Developmental Psychobiology. 1988;21(1):25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- Caron EB, Weston-Lee P, Haggerty D, Dozier M. Community implementation outcomes of Attachment and Biobehavioral Catch-up. Child Abuse Negl. 2015 doi: 10.1016/j.chiabu.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. 2005;9(3):104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, … Krugers H. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J Neurosci. 2008;28(23):6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chareyron LJ, Lavenex PB, Amaral DG, Lavenex P. Postnatal development of the amygdala: A stereological study in macaque monkeys. J Comp Neurol. 2012;520(9):1965–1984. doi: 10.1002/cne.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chareyron LJ, Lavenex PB, Lavenex P. Postnatal development of the amygdala: a stereological study in rats. J Comp Neurol. 2012;520(16):3745–3763. doi: 10.1002/cne.23132. [DOI] [PubMed] [Google Scholar]
- Cheslock SJ, Varlinskaya EI, Petrov ES, Spear NE. Rapid and robust olfactory conditioning with milk before suckling experience: promotion of nipple attachment in the newborn rat. Behavioral Neuroscience. 2000;114(3):484–495. [PubMed] [Google Scholar]
- Choi EC. A contrast of mothering behaviors in women from Korea and the United States. J Obstet Gynecol Neonatal Nurs. 1995;24(4):363–369. doi: 10.1111/j.1552-6909.1995.tb02488.x. [DOI] [PubMed] [Google Scholar]
- Cirulli F, Francia N, Berry A, Aloe L, Alleva E, Suomi SJ. Early life stress as a risk factor for mental health: role of neurotrophins from rodents to non-human primates. Neurosci Biobehav Rev. 2009;33(4):573–585. doi: 10.1016/j.neubiorev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier A, Bolles R. The ontogensis of defensive reactions to shock in preweanling rats. Developmental Psychobiology. 1980;13:141–150. doi: 10.1002/dev.420130206. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Mustillo S, Erkanli A, Keeler G, Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch Gen Psychiatry. 2003;60(8):837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment Retard Dev Disabil Res Rev. 2004;10(4):248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- Critchley H, Daly E, Bullmore E, Williams S, Van Amelsvoort T, Robertson T, Rowe A, Phillips M, McAlonan G, Howlin P, Murphy D. The functional neuroanatomy of social behaviour: Changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123:2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Crittenden PM. Children’s strategies for coping with adverse home environments: an interpretation using attachment theory. Child Abuse Negl. 1992;16(3):329–343. doi: 10.1016/0145-2134(92)90043-q. [DOI] [PubMed] [Google Scholar]
- Cunningham MG, Bhattacharyya S, Benes FM. Amygdalo-cortical sprouting continues into early adulthood: Implications for the development of normal and abnormal function during adolescence. J Comp Neurol. 2002;453(2):116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Dallman MF. Moments in time--the neonatal rat hypothalamo-pituitary-adrenal axis. Endocrinology. 2000;141(5):1590–1592. doi: 10.1210/endo.141.5.7527. [DOI] [PubMed] [Google Scholar]
- Davis M, Rainnie D, Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994:17. doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Thomas LA. Biologic findings of post-traumatic stress disorder and child maltreatment. Current Psychiatric Reports. 2003;5:108–117. doi: 10.1007/s11920-003-0027-z. [DOI] [PubMed] [Google Scholar]
- Debiec J, Sullivan RM. Intergenerational transmission of emotional trauma through amygdala-dependent mother-to-infant transfer of specific fear. Proc Natl Acad Sci U S A. 2014;111(33):12222–12227. doi: 10.1073/pnas.1316740111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCasper AJ, Fifer WP. Of human bonding: newborns prefer their mothers’ voices. Science. 1980;208(4448):1174–1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- Denenberg V. Early experience and emotional development. Scientific American. 1963;208:138–146. doi: 10.1038/scientificamerican0663-138. [DOI] [PubMed] [Google Scholar]
- Distel H, Hudson R. The contribution of the olfactory and tactile modalities to the nipple-search behaviour of newborn rabbits. Journal of Comparative Physiology A-Sensory Neural & Behavioral Physiology. 1985;157(5):599–605. doi: 10.1007/BF01351354. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Heinrichs M. Psychobiology of social support: the social dimension of stress buffering. Restor Neurol Neurosci. 2014;32(1):149–162. doi: 10.3233/rnn-139008. [DOI] [PubMed] [Google Scholar]
- Drury SS, Sanchez MM, Gonzalez A. When mothering goes awry: Challenges and opportunities for utilizing evidence across rodent, nonhuman primate and human studies to better define the biological consequences of negative early caregiving. Horm Behav. 2015 doi: 10.1016/j.yhbeh.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury SS, Theall K, Gleason MM, Smyke AT, De Vivo I, Wong JY, … Nelson CA. Telomere length and early severe social deprivation: linking early adversity and cellular aging. Molecular Psychiatry. 2012;17(7):719–727. doi: 10.1038/mp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret G. Development of absolute auditory thresholds in the house mouse (Mus musculus) J Am Audiol Soc. 1976;1(5):179–184. [PubMed] [Google Scholar]
- Ehrlich DE, Josselyn SA. Plasticity-related genes in brain development and amygdala-dependent learning. Genes Brain Behav. 2016;15(1):125–143. doi: 10.1111/gbb.12255. [DOI] [PubMed] [Google Scholar]
- Ehrlich DE, Ryan SJ, Rainnie DG. Postnatal development of electrophysiological properties of principal neurons in the rat basolateral amygdala. J Physiol. 2012;590(Pt 19):4819–4838. doi: 10.1113/jphysiol.2012.237453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga BM, Schmahl CG, Vermetten E, van Dyck R, Bremner JD. Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology. 2003;28(9):1656–1665. doi: 10.1038/sj.npp.1300226. [DOI] [PubMed] [Google Scholar]
- Emerich D, Scalzo F, Enters E, Spear N, Spear L. Effects of 6-hydroxydopamine-induced catecholamine depletion on shock-precipitated wall climbing of infant rat pups. Developmental Psychobiology. 1985;18(3):215–227. doi: 10.1002/dev.420180303. [DOI] [PubMed] [Google Scholar]
- Emery NJ, Capitanio JP, Mason WA, Machado CJ, Mendoza SP, Amaral DG. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2001;115(3):515–544. [PubMed] [Google Scholar]
- Famularo R, Kinscherff R, Fenton T. Psychiatric diagnoses of maltreated children: preliminary findings. J Am Acad Child Adolesc Psychiatry. 1992;31(5):863–867. doi: 10.1097/00004583-199209000-00013. [DOI] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience. 2016;321:197–209. doi: 10.1016/j.neuroscience.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Tye KM. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J Neurosci. 2014;34(2):586–595. doi: 10.1523/jneurosci.4257-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front Neuroendocrinol. 2002;23(2):200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- Ferry B, McGaugh JL. Role of amygdala norepinephrine in mediating stress hormone regulation of memory storage. Acta Pharmacol Sin. 2000;21(6):481–493. [PubMed] [Google Scholar]
- Fisher AE. Ph.D. 1955. The effects of differential early treatment on the social and exploratory behavior of puppies. [Google Scholar]
- Fleming AS, Suh EJ, Korsmit M, Rusak B. Activation of Fos-like immunoreactivity in the medial preoptic area and limbic structures by maternal and social interactions in rats. Behavioral Neuroscience. 1994;108(4):724–734. doi: 10.1037//0735-7044.108.4.724. [DOI] [PubMed] [Google Scholar]
- Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J Neurosci. 2002;22(18):7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijling JL, van Zuiden M, Koch SB, Nawijn L, Veltman DJ, Olff M. Effects of intranasal oxytocin on amygdala reactivity to emotional faces in recently trauma-exposed individuals. Soc Cogn Affect Neurosci. 2016;11(2):327–336. doi: 10.1093/scan/nsv116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galef BG. The Ecology of Weaning: Parasitism and the Achievement of Independenceby Altricial Mammals. In: Gubernick DJ, Klopfer PH, editors. Parental care in mammals. Plenum Publishing Corporation; 1981. pp. 211–241. [Google Scholar]
- Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, … Tottenham N. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol Sci. 2014;25(11):2067–2078. doi: 10.1177/0956797614550878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, … Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci U S A. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, … Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J Comp Neurol. 1996;366(2):223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Jia X, Wang Z. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J Comp Neurol. 2007;502(6):1109–1122. doi: 10.1002/cne.21364. [DOI] [PubMed] [Google Scholar]
- Gold AL, Sheridan MA, Peverill M, Busso DS, Lambert HK, Alves S, … McLaughlin KA. Childhood abuse and reduced cortical thickness in brain regions involved in emotional processing. Journal of Child Psychology and Psychiatry. 2016;57(10):1154–1164. doi: 10.1111/jcpp.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez RL, Edgin JO. The extended trajectory of hippocampal development: Implications for early memory development and disorder. Dev Cogn Neurosci. 2015 doi: 10.1016/j.dcn.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, Deichmann R, Winston JS, Dolan RJ. Functional heterogeneity in human olfactory cortex: an event-related functional magnetic resonance imaging study. J Neurosci. 2002;22(24):10819–10828. doi: 10.1523/JNEUROSCI.22-24-10819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goursaud AP, Bachevalier J. Social attachment in juvenile monkeys with neonatal lesion of the hippocampus, amygdala and orbital frontal cortex. Behav Brain Res. 2007;176(1):75–93. doi: 10.1016/j.bbr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Goursaud AP, Wallen K, Bachevalier J. Mother recognition and preference after neonatal amygdala lesions in rhesus macaques (Macaca mulatta) raised in a semi-naturalistic environment. Dev Psychobiol. 2014;56(8):1723–1734. doi: 10.1002/dev.21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AM, Buss C, Rasmussen JM, Rudolph MD, Demeter DV, Gilmore JH, Styner M, Entringer S, Wadhwa PD, Fair DA. Implications of newborn amygdala connectivity for fear and cognitive development at 6-months-of-age. Dev Cogn Neurosci. 2016;18:12–25. doi: 10.1016/j.dcn.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AM, Pfeifer JH, Fisher PA, Lin W, Gao W, Fair DA. The potential of infant fMRI research and the study of early life stress as a promising exemplar. Dev Cogn Neurosci. 2015;12:12–39. doi: 10.1016/j.dcn.2014.09.005. doi: http://dx.doi.org/10.1016/j.dcn.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham YP, Heim C, Goodman SH, Miller AH, Nemeroff CB. The effects of neonatal stress on brain development: implications for psychopathology. Dev Psychopathol. 1999;11(3):545–565. doi: 10.1017/s0954579499002205. [DOI] [PubMed] [Google Scholar]
- Green MR, Barnes B, McCormick CM. Social instability stress in adolescence increases anxiety and reduces social interactions in adulthood in male Long-Evans rats. Dev Psychobiol. 2013;55(8):849–859. doi: 10.1002/dev.21077. [DOI] [PubMed] [Google Scholar]
- Green SA, Goff B, Gee DG, Gabard-Durnam L, Flannery J, Telzer EH, … Tottenham N. Discrimination of amygdala response predicts future separation anxiety in youth with early deprivation. Journal of Child Psychology and Psychiatry. 2016;57(10):1135–1144. doi: 10.1111/jcpp.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau RE, Weinberg J, Whitfield MF. Neonatal procedural pain and preterm infant cortisol response to novelty at 8 months. Pediatrics. 2004;114(1):e77–84. doi: 10.1542/peds.114.1.e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A, … Deisseroth K. Natural neural projection dynamics underlying social behavior. Cell. 2014;157(7):1535–1551. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27(1–2):199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar M, Quevedo KM, De Kloet ER, Oitzl MS, Vermetten E. Early care experiences and HPA axis regulation in children: a mechanism for later trauma vulnerability. Prog Brain Res. 2007;167:137–149. doi: 10.1016/S0079-6123(07)67010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Brodersen L, Nachmias M, Buss K, Rigatuso J. Stress reactivity and attachment security. Dev Psychobiol. 1996;29(3):191–204. doi: 10.1002/(SICI)1098-2302(199604)29:3<191::AID-DEV1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Hostinar CE, Sanchez MM, Tottenham N, Sullivan RM. Parental buffering of fear and stress neurobiology: Reviewing parallels across rodent, monkey, and human models. Soc Neurosci. 2015;10(5):474–478. doi: 10.1080/17470919.2015.1070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas B, Mills D, Yam A, Hoeft F, Bellugi U, Reiss A. Genetic Influences on Sociability: Heightened Amygdala Reactivity and Event-Related Responses to Positive Social Stimuli in Williams Syndrome. J Neurosci. 2009;29:1132–1139. doi: 10.1523/JNEUROSCI.5324-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses. 2001;26(5):551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- Halevi G, Djalovski A, Vengrober A, Feldman R. Risk and resilience trajectories in war-exposed children across the first decade of life. Journal of Child Psychology and Psychiatry. 2016;57(10):1183–1193. doi: 10.1111/jcpp.12622. [DOI] [PubMed] [Google Scholar]
- Hardy MP, Sottas CM, Ge R, McKittrick CR, Tamashiro KL, McEwen BS, … Sakai RR. Trends of reproductive hormones in male rats during psychosocial stress: role of glucocorticoid metabolism in behavioral dominance. Biology of Reproduction. 2002;67(6):1750–1755. doi: 10.1095/biolreprod.102.006312. [DOI] [PubMed] [Google Scholar]
- Harlow H, Harlow M. The affectional systems. In: Schrier A, Harlow H, Stollnitz F, editors. Behavior of nonhuman primates. Vol. 2. New York: Academic Press; 1965. pp. 287–334. [Google Scholar]
- Haroutunian V, Campbell BA. Emergence of interoceptive and exteroceptive control of behavior in rats. Science. 1979;205(4409):927–929. doi: 10.1126/science.472715. [DOI] [PubMed] [Google Scholar]
- Harris KM, Teyler TJ. Age differences in a circadian influence on hippocampal LTP. Brain Res. 1983;261(1):69–73. doi: 10.1016/0006-8993(83)91284-2. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Lee FS. Sensitive periods in affective development: nonlinear maturation of fear learning. Neuropsychopharmacology. 2015;40(1):50–60. doi: 10.1038/npp.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol. 2012;233(1):102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Hornschuh G, Kaiser S, Sachser N. Cortisol responses and social buffering: a study throughout the life span. Horm Behav. 2006;49(3):383–390. doi: 10.1016/j.yhbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Kaiser S, Sachser N. Social buffering of the stress response: diversity, mechanisms, and functions. Front Neuroendocrinol. 2009;30(4):470–482. doi: 10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Schiml PA, Willen R, Watanasriyakul W, Johnson J, Garrett T. Selective social buffering of behavioral and endocrine responses and Fos induction in the prelimbic cortex of infants exposed to a novel environment. Dev Psychobiol. 2015;57(1):50–62. doi: 10.1002/dev.21256. [DOI] [PubMed] [Google Scholar]
- Hepper PG, Cleland J. Developmental aspects of kin recognition. Genetica. 1998;104(3):199–205. doi: 10.1023/a:1026477724836. [DOI] [PubMed] [Google Scholar]
- Hess E. Ethology: an approach to the complete analysis of behavior. In: Brown R, Galanter E, Hess E, Mendler G, editors. New Directions in Psychology. New York: Holt, Rinehart and Winston; 1962. pp. 159–199. [Google Scholar]
- Hofer MA. Early relationships as regulators of infant physiology and behavior. Acta Paediatr Suppl. 1994;397:9–18. doi: 10.1111/j.1651-2227.1994.tb13260.x. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala-frontal interactions and reward expectancy. Curr Opin Neurobiol. 2004;14(2):148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Johnson AE, Gunnar MR. Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Dev Sci. 2015;18(2):281–297. doi: 10.1111/desc.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychol Bull. 2014;140(1):256–282. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston SM, Herting MM, Sowell ER. The neurobiology of childhood structural brain development: conception through adulthood. Curr Top Behav Neurosci. 2014;16:3–17. doi: 10.1007/7854_2013_265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M, Cowell P, Boucher J, Broks Mayes A, Farrant A, Roberts N. Convergent neuroanatomical and behavioural evidence of an amygdala hypothesis of autism. Neuroreport. 2000;11:2931–2935. doi: 10.1097/00001756-200009110-00020. [DOI] [PubMed] [Google Scholar]
- Humphrey T. The development of the human amygdala during early embryonic life. J Comp Neurol. 1968;132(1):135–165. doi: 10.1002/cne.901320108. [DOI] [PubMed] [Google Scholar]
- Humphreys KL, Kircanski K, Colich NL, Gotlib IH. Attentional avoidance of fearful facial expressions following early life stress is associated with impaired social functioning. Journal of Child Psychology and Psychiatry. 2016;57(10):1174–1182. doi: 10.1111/jcpp.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys KL, Zeanah CH. Deviations from the expectable environment in early childhood and emerging psychopathology. Neuropsychopharmacology. 2015;40(1):154–170. doi: 10.1038/npp.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 2008;154(3):1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd K, Hunt RH, Cicchetti D, Hunt E, Cowell RA, Rogosch FA, … Thomas KM. Long-term consequences of childhood maltreatment: Altered amygdala functional connectivity. Dev Psychopathol. 2015;27(4 Pt 2):1577–1589. doi: 10.1017/s0954579415000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, Young LJ. Neurobiological mechanisms of social attachment and pair bonding. Curr Opin Behav Sci. 2015;3:38–44. doi: 10.1016/j.cobeha.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane JC, Murray LK, Cohen J, Dorsey S, Skavenski van Wyk S, Galloway Henderson J, … Bolton P. Moderators of treatment response to trauma-focused cognitive behavioral therapy among youth in Zambia. Journal of Child Psychology and Psychiatry. 2016;57(10):1194–1202. doi: 10.1111/jcpp.12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama T, Jodo E, Suzuki Y, Hoshino KY, Takeuchi S, Kayama Y. Phencyclidine affects firing activity of basolateral amygdala neurons related to social behavior in rats. Neuroscience. 2009;159(1):335–343. doi: 10.1016/j.neuroscience.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Kennedy M, Kreppner J, Knights N, Kumsta R, Maughan B, Golm D, … Sonuga-Barke EJS. Early severe institutional deprivation is associated with a persistent variant of adult attention-deficit/hyperactivity disorder: clinical presentation, developmental continuities and life circumstances in the English and Romanian Adoptees study. Journal of Child Psychology and Psychiatry. 2016;57(10):1113–1125. doi: 10.1111/jcpp.12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, Mori Y. Social buffering: relief from stress and anxiety. Philos Trans R Soc Lond B Biol Sci. 2006;361(1476):2215–2228. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B, Carter CS, Newman SW, Insel TR. Axon-sparing lesions of the medial nucleus of the amygdala decrease affiliative behaviors in the prairie vole (Microtus ochrogaster): behavioral and anatomical specificity. Behavioral neuroscience. 1994;108(3):501–513. doi: 10.1037//0735-7044.108.3.501. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Partner’s stress status influences social buffering effects in rats. Behavioral neuroscience. 2004;118(4):798–804. doi: 10.1037/0735-7044.118.4.798. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16(8):1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y, Gould E. Dominance hierarchy influences adult neurogenesis in the dentate gyrus. J Neurosci. 2004;24(30):6755–6759. doi: 10.1523/jneurosci.0345-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O, … Eisch AJ. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci U S A. 2010;107(9):4436–4441. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagercrantz H, Bistoletti P. Catecholamine release in the newborn infant at birth. Pediatr Res. 1977;11(8):889–893. doi: 10.1203/00006450-197708000-00007. [DOI] [PubMed] [Google Scholar]
- Landers MS, Sullivan RM. The development and neurobiology of infant attachment and fear. Dev Neurosci. 2012;34(2–3):101–114. doi: 10.1159/000336732. 000336732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Banta Lavenex P. Building hippocampal circuits to learn and remember: insights into the development of human memory. Behav Brain Res. 2013;254:8–21. doi: 10.1016/j.bbr.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Lawler JM, Koss KJ, Doyle CM, Gunnar MR. The course of early disinhibited social engagement among post-institutionalized adopted children. Journal of Child Psychology and Psychiatry. 2016;57(10):1126–1134. doi: 10.1111/jcpp.12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PR, Brady DL, Shapiro RA, Dorsa DM, Koenig JI. Prenatal stress generates deficits in rat social behavior: Reversal by oxytocin. Brain Res. 2007;1156:152–167. doi: 10.1016/j.brainres.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon M. Chemical communication in mother-young interactions. In: Vandebergh JG, editor. Pheromones and Reproduction in Mammals. New York: Academic Press; 1983. p. 39. [Google Scholar]
- Leon M. The neurobiology of filial learning. Annual Review of Psychology. 1992;43:377–398. doi: 10.1146/annurev.ps.43.020192.002113. [DOI] [PubMed] [Google Scholar]
- Letcher P, Smart D, Sanson A, Toumbourou JW. Psychosocial Precursors and Correlates of Differing Internalizing Trajectories from 3 to 15 Years1. Social Development. 2009;18(3):618–646. doi: 10.1111/j.1467-9507.2008.00500.x. [DOI] [Google Scholar]
- Levin AR, Fox NA, Zeanah CH, Jr, Nelson CA. Social communication difficulties and autism in previously institutionalized children. J Am Acad Child Adolesc Psychiatry. 2015;54(2):108–15. e1. doi: 10.1016/j.jaac.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic--pituitary--adrenal axis in the rat. Physiol Behav. 2001;73(3):255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30(10):939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Levine S, Johnson DF, Gonzalez CA. Behavioral and hormonal responses to separation in infant rhesus monkeys and mothers. Behavioral neuroscience. 1985;99(3):399–410. doi: 10.1037//0735-7044.99.3.399. [DOI] [PubMed] [Google Scholar]
- Levine S, Stanton ME, Gutierrez YR. Maternal modulation of pituitary-adrenal activity during ontogeny. Adv Exp Med Biol. 1988;245:295–310. doi: 10.1007/978-1-4899-2064-5_24. [DOI] [PubMed] [Google Scholar]
- Logan DW, Brunet LJ, Webb WR, Cutforth T, Ngai J, Stowers L. Learned recognition of maternal signature odors mediates the first suckling episode in mice. Current Biology. 2012;22(21):1998–2007. doi: 10.1016/j.cub.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. Non-human primate models of childhood psychopathology: the promise and the limitations. J Child Psychol Psychiatry. 2003;44(1):64–87. doi: 10.1111/1469-7610.00103. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Carroll KA. Child abuse and neglect: usefulness of the animal data. Psychological bulletin. 1998;123(3):211–223. doi: 10.1037/0033-2909.123.3.211. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, Tomaszycki M, Carroll KA. Consistency and change in the behavior of rhesus macaque abusive mothers with successive infants. Developmental Psychobiology. 1999;34(1):29–35. doi: 10.1002/(sici)1098-2302(199901). [DOI] [PubMed] [Google Scholar]
- Mainardi D, Marsan M, Pasquali A. Causation of sexual preferences of the house mouse. The behaviour of mice reared by parents whose odour was artificially altered. Atti della Societa Italiana di scienze nationali e del museo civico di storia naturale di Milano. 1965;104:325–338. [Google Scholar]
- Malkova L, Mishkin M, Suomi SJ, Bachevalier J. Long-term effects of neonatal medial temporal ablations on socioemotional behavior in monkeys (Macaca mulatta) Behavioral Neuroscience. 2010;124(6):742–760. doi: 10.1037/a0021622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malter Cohen M, Jing D, Yang RR, Tottenham N, Lee FS, Casey BJ. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc Natl Acad Sci U S A. 2013;110(45):18274–18278. doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. The amygdala and fear conditioning: has the nut been cracked? Neuron. 1996;16(2):237–240. doi: 10.1016/s0896-6273(00)80041-0. [DOI] [PubMed] [Google Scholar]
- Marlin BJ, Froemke RC. Oxytocin Modulation of Neural Circuits for Social Behavior. Dev Neurobiol. 2016 doi: 10.1002/dneu.22452. [DOI] [PubMed] [Google Scholar]
- Marquez C, Poirier GL, Cordero MI, Larsen MH, Groner A, Marquis J, … Sandi C. Peripuberty stress leads to abnormal aggression, altered amygdala and orbitofrontal reactivity and increased prefrontal MAOA gene expression. Transl Psychiatry. 2013;3:e216. doi: 10.1038/tp.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack K, Newman TK, Higley JD, Maestripieri D, Sanchez MM. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Horm Behav. 2009;55(4):538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Early life influences on life-long patterns of behavior and health. Ment Retard Dev Disabil Res Rev. 2003;9(3):149–154. doi: 10.1002/mrdd.10074. [DOI] [PubMed] [Google Scholar]
- Mikics E, Toth M, Varju P, Gereben B, Liposits Z, Ashaber M, … Haller J. Lasting changes in social behavior and amygdala function following traumatic experience induced by a single series of foot-shocks. Psychoneuroendocrinology. 2008;33(9):1198–1210. doi: 10.1016/j.psyneuen.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Moye TB, Rudy JW. Ontogenesis of trace conditioning in young rats: dissociation of associative and memory processes. Dev Psychobiol. 1987;20(4):405–414. doi: 10.1002/dev.420200405. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Roth TL, Okotoghaide T, Sullivan RM. Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. International Journal of Developmental Neuroscience. 2004;22(5–6):415–422. doi: 10.1016/j.ijdevneu.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Shionoya K, Jakubs K, Sullivan RM. Early-life stress disrupts attachment learning: the role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. J Neurosci. 2009;29(50):15745–15755. doi: 10.1523/JNEUROSCI.4106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Unique neural circuitry for neonatal olfactory learning. J Neurosci. 2004;24(5):1182–1189. doi: 10.1523/JNEUROSCI.4578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Sullivan RM. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat Neurosci. 2006;9(8):1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S, Wilson DA, Levine S, Sullivan RM. Dual circuitry for odor-shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. Journal of Neuroscience. 2006;26(25):6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachmias M, Gunnar M, Mangelsdorf S, Parritz RH, Buss K. Behavioral inhibition and stress reactivity: the moderating role of attachment security. Child Dev. 1996;67(2):508–522. [PubMed] [Google Scholar]
- Nakamura S, Kimura F, Sakaguchi T. Postnatal development of electrical activity in the locus ceruleus. J Neurophysiol. 1987;58(3):510–524. doi: 10.1152/jn.1987.58.3.510. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Sakaguchi T. Development and plasticity of the locus coeruleus: a review of recent physiological and pharmacological experimentation. Prog Neurobiol. 1990;34(6):505–526. doi: 10.1016/0301-0082(90)90018-c. [DOI] [PubMed] [Google Scholar]
- Neigh GN, Gillespie CF, Nemeroff CB. The neurobiological toll of child abuse and neglect. Trauma, violence & abuse. 2009;10(4):389–410. doi: 10.1177/1524838009339758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Lau JY, Jarcho JM. Growing pains and pleasures: how emotional learning guides development. Trends Cogn Sci. 2014;18(2):99–108. doi: 10.1016/j.tics.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Panksepp J. Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci Biobehav Rev. 1998;22(3):437–452. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Neurobiological consequences of childhood trauma. J Clin Psychiatry. 2004;65(Suppl 1):18–28. [PubMed] [Google Scholar]
- Nemeroff CB, Vale WW. The neurobiology of depression: inroads to treatment and new drug discovery. J Clin Psychiatry. 2005;66(Suppl 7):5–13. [PubMed] [Google Scholar]
- Numan M, Young LJ. Neural mechanisms of mother-infant bonding and pair bonding: Similarities, differences, and broader implications. Horm Behav. 2016;77:98–112. doi: 10.1016/j.yhbeh.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor TG, Cameron JL. Translating research findings on early experience to prevention: animal and human evidence on early attachment relationships. American Journal of Preventive Medicine. 2006;31(6 Suppl 1):S175–181. doi: 10.1016/j.amepre.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Opendak M, Gould E. Adult neurogenesis: a substrate for experience-dependent change. Trends Cogn Sci. 2015;19(3):151–161. doi: 10.1016/j.tics.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Opendak M, Offit L, Monari P, Schoenfeld TJ, Sonti AN, Cameron HA, Gould E. Lasting Adaptations in Social Behavior Produced by Social Disruption and Inhibition of Adult Neurogenesis. J Neurosci. 2016;36(26):7027–7038. doi: 10.1523/jneurosci.4435-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opendak M, Sullivan RM. Unique Neurobiology during the Sensitive Period for Attachment Produces Distinctive Infant Trauma Processing. European Journal of Psychotraumatology. 2016;7(31279) doi: 10.3402/ejpt.v7.31276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr LA, Brooks JM, Jonesteller T, Moss S, Jordano JO, Heitz TR. Effects of chronic oxytocin on attention to dynamic facial expressions in infant macaques. Psychoneuroendocrinology. 2016;74:149–157. doi: 10.1016/j.psyneuen.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, … Lee FS. Altered fear learning across development in both mouse and human. Proceedings of the National Academy of Sciences. 2012;109(40):16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B, Snyder A, Haist F, Raichle M, Bellugi M, Stiles J. Amygdala response to faces parallels social behavior in Williams syndrome. Cogn Affec Neurosci. 2009;4:278–285. doi: 10.1093/scan/nsp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MH. Sensitive periods of amygdala development: the role of maltreatment in preadolescence. Neuroimage. 2014;97:236–244. doi: 10.1016/j.neuroimage.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen PE, Blass EM. Prenatal and postnatal determinants of the 1st suckling episode in albino rats. Dev Psychobiol. 1982;15(4):349–355. doi: 10.1002/dev.420150407. [DOI] [PubMed] [Google Scholar]
- Peragine DE, Simpson JA, Mooney SJ, Lovern MB, Holmes MM. Social regulation of adult neurogenesis in a eusocial mammal. Neuroscience. 2014;268:10–20. doi: 10.1016/j.neuroscience.2014.02.044. [DOI] [PubMed] [Google Scholar]
- Perry R, Sullivan RM. Neurobiology of attachment to an abusive caregiver: short-term benefits and long-term costs. Dev Psychobiol. 2014;56(8):1626–1634. doi: 10.1002/dev.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RE, Santiago AN, Sullivan RM. From trauma to safety: Rescuing adults from neurobehavioral impacts of infant abuse (in press) [Google Scholar]
- Phelps EA, LeDoux JE. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pierce K, Muller R, Ambrose J, Allen G, Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Pine DS, Mogg K, Bradley BP, Montgomery L, Monk CS, McClure E, … Kaufman J. Attention bias to threat in maltreated children: implications for vulnerability to stress-related psychopathology. Am J Psychiatry. 2005;162(2):291–296. doi: 10.1176/appi.ajp.162.2.291. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Cicchetti D, Hornung K, Reed A. Recognizing emotion in faces: developmental effects of child abuse and neglect. Dev Psychol. 2000;36(5):679–688. doi: 10.1037/0012-1649.36.5.679. [DOI] [PubMed] [Google Scholar]
- Poulos AM, Reger M, Mehta N, Zhuravka I, Sterlace SS, Gannam C, … Fanselow MS. Amnesia for early life stress does not preclude the adult development of posttraumatic stress disorder symptoms in rats. Biol Psychiatry. 2014;76(4):306–314. doi: 10.1016/j.biopsych.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puetz VB, Viding E, Palmer A, Kelly PA, Lickley R, Koutoufa I, … McCrory EJ. Altered neural response to rejection-related words in children exposed to maltreatment. Journal of Child Psychology and Psychiatry. 2016;57(10):1165–1173. doi: 10.1111/jcpp.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Cortes MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J Neurosci. 2012;32(22):7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Holman PJ, Debiec J, Bugg M, Beasley A, Sullivan RM. Functional emergence of the hippocampus in context fear learning in infant rats. Hippocampus. 2010;20(9):1037–1046. doi: 10.1002/hipo.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Moriceau S, Sullivan RM. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biol Psychiatry. 2010;67(12):1137–1145. doi: 10.1016/j.biopsych.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Pickenhagen A, Roth TL, Babstock DM, McLean JH, Harley CW, … Sullivan RM. The neurobiology of infant maternal odor learning. Braz J Med Biol Res. 2010;43(10):914–919. doi: 10.1590/s0100-879x2010007500090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Sarro E, Rincon-Cortes M, Perry R, Boggs J, Holman CJ, … Sullivan RM. Paradoxical neurobehavioral rescue by memories of early-life abuse: the safety signal value of odors learned during abusive attachment. Neuropsychopharmacology. 2015;40(4):906–914. doi: 10.1038/npp.2014.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Shionoya K, Sander K, Sullivan RM. Ontogeny of odor-LiCl vs. odor-shock learning: similar behaviors but divergent ages of functional amygdala emergence. Learn Mem. 2009;16(2):114–121. doi: 10.1101/lm.977909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajecki D, Lamb M, Obmascher P. Towards a general theory of infantile attachment: a comparative review of aspects of the social bond. Behavioral Brain Science. 1978;3:417–464. [Google Scholar]
- Raper J, Stephens SB, Sanchez M, Bachevalier J, Wallen K. Neonatal amygdala lesions alter mother-infant interactions in rhesus monkeys living in a species-typical social environment. Dev Psychobiol. 2014;56(8):1711–1722. doi: 10.1002/dev.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasia-Filho AA, Londero RG, Achaval M. Functional activities of the amygdala: an overview. J Psychiatry Neurosci. 2000;25(1):14–23. [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10(9):1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben A, Moffitt TE, Caspi A, Belsky DW, Harrington H, Schroeder F, … Danese A. Lest we forget: comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. Journal of Child Psychology and Psychiatry. 2016;57(10):1103–1112. doi: 10.1111/jcpp.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L, Banihashemi L, Koehnle TJ. Early life experience shapes the functional organization of stress-responsive visceral circuits. Physiol Behav. 2011;104(4):632–640. doi: 10.1016/j.physbeh.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon-Cortes M, Sullivan RM. Early life trauma and attachment: immediate and enduring effects on neurobehavioral and stress axis development. Front Endocrinol (Lausanne) 2014;5:33. doi: 10.3389/fendo.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET. Taste, olfactory, and food reward value processing in the brain. Prog Neurobiol. 2015;127–128:64–90. doi: 10.1016/j.pneurobio.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Rosvold HE, Mirsky AF, Pribram KH. Influence of amygdalectomy on social behavior in monkeys. J Comp Physiol Psychol. 1954;47(3):173–178. doi: 10.1037/h0058870. [DOI] [PubMed] [Google Scholar]
- Roth TL, Matt S, Chen K, Blaze J. Bdnf DNA methylation modifications in the hippocampus and amygdala of male and female rats exposed to different caregiving environments outside the homecage. Developmental Psychobiology. 2014 doi: 10.1002/dev.21218. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Raineki C, Salstein L, Perry R, Sullivan-Wilson TA, Sloan A, … Sullivan RM. Neurobiology of secure infant attachment and attachment despite adversity: a mouse model. Genes Brain and Behavior. 2013;12(7):673–680. doi: 10.1111/gbb.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psychiatry. 2005;57(8):823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Examining the role of endogenous opioids in learned odor–stroke associations in infant rats. Developmental Psychobiology. 2006;48(1):71–78. doi: 10.1002/dev.20107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royet JP, Zald D, Versace R, Costes N, Lavenne F, Koenig O, Gervais R. Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: a positron emission tomography study. J Neurosci. 2000;20(20):7752–7759. doi: 10.1523/JNEUROSCI.20-20-07752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzen E. Imprinting and environmental learning. In: Aronson L, Tobach E, Lehrman D, Rosenblatt J, editors. Development and evolution of behavior. San Francisco: W.H. Freeman; 1970. [Google Scholar]
- Sanchez M, Ladd C, Plotsky P. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sanchez MM. The impact of early adverse care on HPA axis development: nonhuman primate models. Horm Behav. 2006;50(4):623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, McCormack KM, Howell BR. Social buffering of stress responses in nonhuman primates: Maternal regulation of the development of emotional regulatory brain circuits. Soc Neurosci. 2015;10(5):512–526. doi: 10.1080/17470919.2015.1087426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C, Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci. 2015;16(5):290–304. doi: 10.1038/nrn3918. [DOI] [PubMed] [Google Scholar]
- Sannino S, Chini B, Grinevich V. Lifespan oxytocin signaling: Maturation, flexibility and stability in newborn, adolescent and aged brain. Dev Neurobiol. 2016 doi: 10.1002/dneu.22449. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308(5722):648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Sarro EC, Sullivan RM, Barr G. Unpredictable neonatal stress enhances adult anxiety and alters amygdala gene expression related to serotonin and GABA. Neuroscience. 2014;258(0):147–161. doi: 10.1016/j.neuroscience.2013.10.064. doi: http://dx.doi.org/10.1016/j.neuroscience.2013.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarro EC, Wilson DA, Sullivan RM. Maternal regulation of infant brain state. Curr Biol. 2014;24(14):1664–1669. doi: 10.1016/j.cub.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci Biobehav Rev. 2009;33(5):699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob JE, Price JL. The development of axonal connections in the central olfactory system of rats. Journal of Comparative Neurology. 1984;223(2):177–202. doi: 10.1002/cne.902230204. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y, Moriceau S, Holman P, Miner C, Muzny K, Gervais R, … Sullivan RM. Enduring effects of infant memories: infant odor-shock conditioning attenuates amygdala activity and adult fear conditioning. Biol Psychiatry. 2007;62(10):1070–1079. doi: 10.1016/j.biopsych.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y, Mouly AM, Raineki C, Moriceau S, Forest C, Sullivan RM. Adult depression-like behavior, amygdala and olfactory cortex functions are restored by odor previously paired with shock during infant’s sensitive period attachment learning. Dev Cogn Neurosci. 2011;1(1):77–87. doi: 10.1016/j.dcn.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevelinges Y, Sullivan RM, Messaoudi B, Mouly AM. Neonatal odor-shock conditioning alters the neural network involved in odor fear learning at adulthood. Learn Mem. 2008;15(9):649–656. doi: 10.1101/lm.998508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Abu-Akel A. The Social Salience Hypothesis of Oxytocin. Biol Psychiatry. 2016;79(3):194–202. doi: 10.1016/j.biopsych.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Shionoya K, Moriceau S, Bradstock P, Sullivan RM. Maternal attenuation of hypothalamic paraventricular nucleus norepinephrine switches avoidance learning to preference learning in preweanling rat pups. Horm Behav. 2007;52(3):391–400. doi: 10.1016/j.yhbeh.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille E, Wang Y, Joeyen-Waldorf J, Gaiteri C, Surget A, Oh S, … Lewis DA. A molecular signature of depression in the amygdala. Am J Psychiatry. 2009;166(9):1011–1024. doi: 10.1176/appi.ajp.2009.08121760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitko K, Bentall RP, Shevlin M, O’Sullivan N, Sellwood W. Associations between specific psychotic symptoms and specific childhood adversities are mediated by attachment styles: an analysis of the National Comorbidity Survey. Psychiatry Res. 2014;217(3):202–209. doi: 10.1016/j.psychres.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Skuse D, Morris J, Lawrence K. The amygdala and development of the social brain. Ann N Y Acad Sci. 2003;1008:91–101. doi: 10.1196/annals.1301.010. [DOI] [PubMed] [Google Scholar]
- Smotherman WP, Robinson SR. Prenatal expression of species-typical action patterns in the rat fetus (Rattus norvegicus) J Comp Psychol. 1987;101(2):190–196. [PubMed] [Google Scholar]
- So N, Franks B, Lim S, Curley JP. A Social Network Approach Reveals Associations between Mouse Social Dominance and Brain Gene Expression. PLoS One. 2015;10(7):e0134509. doi: 10.1371/journal.pone.0134509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear N. Processing memories: forgetting and retention. Hillsdale, New Jersey: Erlbaum; 1978. [Google Scholar]
- Stanley W. Differential human handling as reinforcing events and as treatments influencing later social behavior in basenji puppies. Psychological Reports. 1962;10:775–788. [Google Scholar]
- Stanton M, Levine S. Inhibition of infant glucocorticoid stress response: specific role of maternal cues. Developmental Psychobiology. 1990;23(5):411–426. doi: 10.1002/dev.420230504. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Levine S. Brief separation elevates cortisol in mother and infant squirrel monkeys. Physiol Behav. 1985;34(6):1007–1008. doi: 10.1016/0031-9384(85)90029-0. [DOI] [PubMed] [Google Scholar]
- Stehouwer D, Campbell B. Habituation of the forelimb-withdrawal response in neonatal rats. Journal of Experimental Psychology: Animal Behavior Processes. 1978;4(2):104–119. doi: 10.1037//0097-7403.4.2.104. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Nelson DY, Van Oers H, Levine S. Activation and inhibition of the hypothalamic-pituitary-adrenal axis of the neonatal rat: effects of maternal deprivation. Psychoneuroendocrinology. 1995;20(2):169–182. doi: 10.1016/0306-4530(94)00051-b. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Rosenfeld P, Levine S. Maternal regulation of the hypothalamic-pituitary-adrenal axis in the infant rat: the roles of feeding and stroking. Brain Research Developmental Brain Research. 1993;75(2):185–192. doi: 10.1016/0165-3806(93)90022-3. [DOI] [PubMed] [Google Scholar]
- Sullivan R, Perry R, Sloan A, Kleinhaus K, Burtchen N. Infant bonding and attachment to the caregiver: Insights from basic and clinical science. Clinics in perinatology. 2011;38(4):643–655. doi: 10.1016/j.clp.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Hofer MA, Brake SC. Olfactory-guided orientation in neonatal rats is enhanced by a conditioned change in behavioral state. Developmental Psychobiology. 1986;19(6):615–623. doi: 10.1002/dev.420190612. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Holman PJ. Transitions in sensitive period attachment learning in infancy: the role of corticosterone. Neurosci Biobehav Rev. 2010;34(6):835–844. doi: 10.1016/j.neubiorev.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Landers M, Yeaman B, Wilson DA. Good memories of bad events in infancy. Nature. 2000;407(6800):38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Leon M. Early olfactory learning induces an enhanced olfactory bulb response in young rats. Brain Res. 1986;392(1–2):278–282. doi: 10.1016/0165-3806(86)90256-7. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Perry RE. Mechanisms and functional implications of social buffering in infants: Lessons from animal models. Soc Neurosci. 2015;10(5):500–511. doi: 10.1080/17470919.2015.1087425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Stackenwalt G, Nasr F, Lemon C, Wilson DA. Association of an odor with activation of olfactory bulb noradrenergic beta-receptors or locus coeruleus stimulation is sufficient to produce learned approach responses to that odor in neonatal rats. Behavioral neuroscience. 2000;114(5):957–962. doi: 10.1037/0735-7044.114.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. The role of norepinephrine in the expression of learned olfactory neurobehavioral responses in infant rats. Psychobiology (Austin, Tex) 1991;19(4):308–312. doi: 10.3758/bf03332084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA. The locus coeruleus, norepinephrine, and memory in newborns. Brain Res Bull. 1994;35(5–6):467–472. doi: 10.1016/0361-9230(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Wilson DA, Wong R, Correa A, Leon M. Modified behavioral and olfactory bulb responses to maternal odors in preweanling rats. Brain Research Developmental Brain Research. 1990;53(2):243–247. doi: 10.1016/0165-3806(90)90013-o. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Zyzak D, Skierkowski P, Wilson DA. The role of olfactory bulb norepinephrine in early olfactory learning. Brain Research Developmental Brain Research. 1992;70(2):279–282. doi: 10.1016/0165-3806(92)90207-d. [DOI] [PubMed] [Google Scholar]
- Suomi SJ. Gene-environment interactions and the neurobiology of social conflict. Ann N Y Acad Sci. 2003;1008(1):132–139. doi: 10.1196/annals.1301.014. [DOI] [PubMed] [Google Scholar]
- Swann JW, Smith KL, Brady RJ. Neural networks and synaptic transmission in immature hippocampus. Adv Exp Med Biol. 1990;268:161–171. doi: 10.1007/978-1-4684-5769-8_19. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends in Neurosciences. 1998;21(8):323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Kiyokawa Y, Kodama Y, Arata S, Takeuchi Y, Mori Y. Olfactory signals mediate social buffering of conditioned fear responses in male rats. Behav Brain Res. 2013;240:46–51. doi: 10.1016/j.bbr.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Tang AC, Reeb-Sutherland BC, Romeo RD, McEwen BS. On the causes of early life experience effects: evaluating the role of mom. Front Neuroendocrinol. 2014;35(2):245–251. doi: 10.1016/j.yfrne.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27(1–2):33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. 2016;17(10):652–666. doi: 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, Casey BJ. Amygdala response to facial expressions in children and adults. Biol Psychiatry. 2001;49(4):309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Timmer M, Cordero MI, Sevelinges Y, Sandi C. Evidence for a role of oxytocin receptors in the long-term establishment of dominance hierarchies. Neuropsychopharmacology. 2011;36(11):2349–2356. doi: 10.1038/npp.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N. Human amygdala development in the absence of species-expected caregiving. Dev Psychobiol. 2012;54(6):598–611. doi: 10.1002/dev.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troller-Renfree S, Nelson CA, Zeanah CH, Fox NA. Deficits in error monitoring are associated with externalizing but not internalizing behaviors among children with a history of institutionalization. Journal of Child Psychology and Psychiatry. 2016;57(10):1145–1153. doi: 10.1111/jcpp.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler K, Moriceau S, Sullivan RM, Greenwood-van Meerveld B. Long-term colonic hypersensitivity in adult rats induced by neonatal unpredictable vs predictable shock. Neurogastroenterol Motil. 2007;19(9):761–768. doi: 10.1111/j.1365-2982.2007.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzanoulinou S, Sandi C. The Programming of the Social Brain by Stress During Childhood and Adolescence: From Rodents to Humans. Curr Top Behav Neurosci. 2016 doi: 10.1007/7854_2015_430. [DOI] [PubMed] [Google Scholar]
- Uematsu A, Matsui M, Tanaka C, Takahashi T, Noguchi K, Suzuki M, Nishijo H. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS One. 2012;7(10):e46970. doi: 10.1371/journal.pone.0046970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfig N, Setzer M, Bohl J. Ontogeny of the human amygdala. Ann N Y Acad Sci. 2003;985:22–33. doi: 10.1111/j.1749-6632.2003.tb07068.x. [DOI] [PubMed] [Google Scholar]
- Umemori J, Winkel F, Castren E, Karpova NN. Distinct effects of perinatal exposure to fluoxetine or methylmercury on parvalbumin and perineuronal nets, the markers of critical periods in brain development. Int J Dev Neurosci. 2015;44:55–64. doi: 10.1016/j.ijdevneu.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Upton KJ, Sullivan RM. Defining age limits of the sensitive period for attachment learning in rat pups. Dev Psychobiol. 2010;52(5):453–464. doi: 10.1002/dev.20448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eden C, Uylings H. Postnatal volumetric development of the prefrontal cortex in the rat. J Comp Neurol. 2004;241(3):268–274. doi: 10.1002/cne.902410303. [DOI] [PubMed] [Google Scholar]
- van Oers HJ, de Kloet ER, Whelan T, Levine S. Maternal deprivation effect on the infant’s neural stress markers is reversed by tactile stimulation and feeding but not by suppressing corticosterone. J Neurosci. 1998;18(23):10171–10179. doi: 10.1523/JNEUROSCI.18-23-10171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield CL, Levine MS. Early postnatal development of basolateral amygdala in kitten: a Golgi morphometric analysis. Brain Res Bull. 1985;14(2):159–167. doi: 10.1016/0361-9230(85)90075-9. [DOI] [PubMed] [Google Scholar]
- Watamura SE, Donzella B, Kertes DA, Gunnar MR. Developmental changes in baseline cortisol activity in early childhood: relations with napping and effortful control. Dev Psychobiol. 2004;45(3):125–133. doi: 10.1002/dev.20026. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Yamamoto M. Neural mechanisms of social dominance. Front Neurosci. 2015;9:154. doi: 10.3389/fnins.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathington JM, Arnold AR, Cooke BM. Juvenile social subjugation induces a sex-specific pattern of anxiety and depression-like behaviors in adult rats. Horm Behav. 2012;61(1):91–99. doi: 10.1016/j.yhbeh.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Weber EM, Olsson IAS. Maternal behaviour in Mus musculus sp.: An ethological review. Applied Animal Behaviour Science. 2008;114(1–2):1–22. doi: http://dx.doi.org/10.1016/j.applanim.2008.06.006. [Google Scholar]
- Welles-Nystrom B, New R, Richman A. The ‘good mother’--a comparative study of Swedish, Italian and American maternal behavior and goals. Scand J Caring Sci. 1994;8(2):81–86. doi: 10.1111/j.1471-6712.1994.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Werker JF, Hensch TK. Critical periods in speech perception: new directions. Annu Rev Psychol. 2015;66:173–196. doi: 10.1146/annurev-psych-010814-015104. [DOI] [PubMed] [Google Scholar]
- White PJ, Fischer RB, Meunier GF. Female discrimination of male dominance by urine odor cues in hamsters. Physiol Behav. 1986;37(2):273–277. doi: 10.1016/0031-9384(86)90232-5. [DOI] [PubMed] [Google Scholar]
- Wilson DA. A comparison of the postnatal development of post-activation potentiation in the neocortex and dentate gyrus of the rat. Brain Res. 1984;318(1):61–68. doi: 10.1016/0165-3806(84)90063-4. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Stevenson RJ. Olfactory perceptual learning: the critical role of memory in odor discrimination. Neuroscience & Biobehavioral Reviews. 2003;27(4):307–328. doi: 10.1016/s0149-7634(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan UH, Raymon HK, Broide RS, Chen Y, Leslie FM. Expression of α2 adrenoceptors during rat brain development—II. α2C messenger RNA expression and [3H]rauwolscine binding. Neuroscience. 1996;76(1):261–272. doi: 10.1016/s0306-4522(96)00369-7. doi: http://dx.doi.org/10.1016/S0306-4522(96)00369-7. [DOI] [PubMed] [Google Scholar]
- Wong LC, Wang L, D’Amour JA, Yumita T, Chen G, Yamaguchi T, … Lin D. Effective Modulation of Male Aggression through Lateral Septum to Medial Hypothalamus Projection. Curr Biol. 2016;26(5):593–604. doi: 10.1016/j.cub.2015.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MV, Shamy JL, Bedi G, Choi CW, Wall MM, Arango V, … Hen R. Impact of social status and antidepressant treatment on neurogenesis in the baboon hippocampus. Neuropsychopharmacology. 2014;39(8):1861–1871. doi: 10.1038/npp.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, Bruce JC, Darby-King A, McLean JH. Isoproterenol increases CREB phosphorylation and olfactory nerve–evoked potentials in normal and 5-HT-depleted olfactory bulbs in rat pups only at doses that produce odor prference learning. Learning & Memory. 2000;7(6):413–421. doi: 10.1101/lm.35900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Harley CW, McLean JH, Knöpfel T. Optical imaging of odor preference memory in the rat olfactory bulb. 2002;87(6):3156–3159. doi: 10.1152/jn.00917.2001. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci U S A. 1997;94(8):4119–4124. doi: 10.1073/pnas.94.8.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannas AS, Binder EB. Gene-environment interactions at the FKBP5 locus: sensitive periods, mechanisms and pleiotropism. Genes Brain Behav. 2014;13(1):25–37. doi: 10.1111/gbb.12104. [DOI] [PubMed] [Google Scholar]
- Zeamer A, Bachevalier J. Long-term effects of neonatal hippocampal lesions on novelty preference in monkeys. Hippocampus. 2013;23(9):745–750. doi: 10.1002/hipo.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah CH, Gleason MM. Annual research review: Attachment disorders in early childhood--clinical presentation, causes, correlates, and treatment. J Child Psychol Psychiatry. 2015;56(3):207–222. doi: 10.1111/jcpp.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah CH, Keyes A, Settles L. Attachment relationship experiences and childhood psychopathology. Ann N Y Acad Sci. 2003;1008:22–30. doi: 10.1196/annals.1301.003. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Sonuga-Barke EJS. Editorial: The effects of early trauma and deprivation on human development–from measuring cumulative risk to characterizing specific mechanisms. Journal of Child Psychology and Psychiatry. 2016;57(10):1099–1102. doi: 10.1111/jcpp.12642. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Herman JP. Neurocircuitry of stress integration: anatomical pathways regulating the hypothalamo-pituitary-adrenocortical axis of the rat. Integr Comp Biol. 2002;42(3):541–551. doi: 10.1093/icb/42.3.541. [DOI] [PubMed] [Google Scholar]