Abstract
Objectives
To assess the prevalence, characteristics, and risk of sensorineural hearing loss (SNHL) through 18 years of age in children with congenital CMV infection identified through hospital-based newborn screening who were asymptomatic at birth compared to uninfected children.
Methods
We included 92 case-patients and 51 controls assessed using auditory brainstem response and behavioral audiometry. We used Kaplan-Meier survival analysis to estimate prevalence of SNHL, defined as ≥25 dB hearing level (HL) at any frequency, and Cox proportional hazards regression analyses to compare SNHL risk between groups.
Results
At the end of follow-up, SNHL prevalence was 25% (95% CI: 17–36%) among case-patients and 8% (95% CI: 3–22%) in controls (hazard ratio (HR): 4.0; 95% CI: 1.2–14.5; p-value=0.02). Among children without SNHL by age 5 years, the risk of delayed-onset SNHL was not significantly greater for case-patients than for controls (HR: 1.6; 95% CI: 0.4–6.1; P=0.5). Among case-patients, the risk of delayed-onset SNHL was significantly greater among those with unilateral congenital/early-onset loss than those without (hazard ratio: 6.9; 95% CI: 2.5–19.1; P<0.01). At the end of follow-up, the prevalence of severe to profound bilateral SNHL among case-patients was 2% (95% CI: 1–9%).
Conclusions
Delayed-onset and progression of SNHL among children with asymptomatic congenital CMV infection continued to occur throughout adolescence. However, the risk of developing SNHL after age 5 years among case-patients was not different than in uninfected children. An estimated 2% of case-patients developed SNHL severe enough to be candidates for cochlear implantation.
Keywords: congenital cytomegalovirus, sensorineural hearing loss
BACKGROUND
Congenital cytomegalovirus (CMV) infection causes a spectrum of impairments, including sensorineural hearing loss (SNHL), vision loss, and developmental delays. In the United States, an estimated 20,000 (0.5%) children are born with congenital CMV infection annually.1, 2 Although the majority (85–90%) appear asymptomatic at birth, SNHL may be present at birth, progress in severity, or develop later.3
The burden of SNHL in children with asymptomatic congenital CMV infection at birth remains incompletely characterized, and the extent to which these children remain at risk of SNHL throughout childhood and adolescence is not well described. Previous studies have documented delayed-onset SNHL among children with asymptomatic congenital CMV infection up to age 15 years.4–7 However, data from controlled studies with follow-up through adolescence are lacking. Studies that also included uninfected children had follow-up until 5–7 years and did not attempt to compare the risk of delayed-onset SNHL between children with asymptomatic congenital CMV infection and uninfected children.4–6, 8, 9 In this study, we assessed prevalence, characteristics, and risk of SNHL through age 18 years in children with congenital CMV infection identified through hospital-based newborn screening who were asymptomatic at birth compared to uninfected children.
PATIENTS AND METHODS
During 1982–1992, 32,543 newborns delivered at Women’s Hospital of Texas, Houston TX, were screened for congenital CMV infection via urine culture collected within 3 days of life, as described previously.10, 11 Of 135 (0.4%) CMV-positive newborns, 92 (68%) were enrolled in a longitudinal study as asymptomatic case-patients, e.g. they had no CMV-related signs at birth (purpura/petechiae, jaundice, hepatosplenomegaly, microcephaly, elevated liver enzymes, bilirubinemia, hemolytic anemia or thrombocytopenia). Fifty-one uninfected newborns whose parents agreed to participate in the study were enrolled as controls, 42 (82%) were among CMV-negative newborns randomly pre-selected within 6 days of birth of a CMV-positive newborn (n=298), and 9 (18%) were siblings of referred CMV-positive infants or born to women diagnosed with CMV infection during pregnancy. We analyzed data from serial audiological assessments from birth to 18 years of age. The Institutional Review Board for Human Subject Research for Baylor College of Medicine and Affiliated Hospitals approved the study protocol.
Audiologic assessments were conducted by audiologists unaware of subject CMV status and included auditory brainstem response (ABR), behavioral audiometry (0.25 to 8 kHz), and tympanometry.10 ABR testing included click and frequency-specific tone-burst stimuli. We combined the latter with frequency-specific pure-tone air conduction results obtained by behavioral audiometry after subtracting 10 dB for 0.5, 1 and 8 kHz, and 0 dB for 2 and 4 kHz from the tone-burst levels.12 We defined SNHL as ≥25 dB hearing level (HL) for the ABR click or at any frequency for the corrected tone-burst or pure-tone air conduction results. Because middle ear disorder can cause transient conductive hearing loss, we excluded assessments with tympanometry type B.
We analyzed SNHL by age at onset, laterality, and progression. We categorized SNHL among case-patients for each ear as congenital/early-onset when detected in the first ABR assessment at age ≤12 months and confirmed in subsequent assessments, or as delayed-onset when detected after one or more assessments with normal hearing. We classified SNHL as unilateral if it was present in one ear or bilateral if present in both ears. We defined SNHL as progressive when a change to worse hearing occurred between the first detection of SNHL and the last assessment, or stable when there was no change between these two assessments. We defined fluctuations as changes to worse or better hearing level between consecutive assessments: an absolute difference of ≥20 dB in one or more frequencies, ≥10 dB across any 2 or 3 adjacent frequencies, ≥10 dB in the average of the pure-tone thresholds at 0.5, 1, 2, and 4 kHz (four-frequency average), or a change from “hearing” to “no response” or vice-versa at 3 adjacent frequencies.13
We categorized SNHL severity for each ear using the ABR click result or the four-frequency average, as follows: slight (16 to 25 dB HL), mild (26 to 40 dB HL), moderate (41 to 55 dB HL), moderately severe (56 to 70 dB HL), severe (71 to 90 dB HL) and profound (91 dB HL or greater) hearing loss.14 We classified children with ≥25 dB HL in any frequency without affecting the four-frequency average as having SNHL at isolated frequencies. We described SNHL severity in the poorer- and better-hearing ears. For example, a child with unilateral SNHL could be categorized as having profound loss in the poorer-hearing ear but normal hearing in the better-hearing ear. Characterization by poorer-hearing ear provides a more complete description of SNHL burden as it includes children with unilateral loss. However, eligibility criteria for health insurance coverage for audiologic services may require bilateral loss15, which is described by better-hearing ear assessment. To estimate need for audiologic services, we assumed hearing aids would be recommended for children with unilateral or bilateral SNHL ≥40 db HL, and cochlear implants for those with bilateral SNHL ≥70 db HL.15
We compared demographic and birth characteristics among case-patients and controls using Chi-square or exact test. To deal with loss to follow-up at varying ages, we used Kaplan-Meier survival analysis to estimate the proportion of children with SNHL by age. We calculated hazard ratios (HR) using Cox proportional hazards regression analyses to compare SNHL risk between groups. We considered results with a p-value <0.05 statistically significant. For analyses, we used SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
RESULTS
The majority of the 92 case-patients and 51 controls were male (58% vs. 67%), born at ≥37 weeks gestation (88% vs. 98%) to mothers who were <30 years of age (63% vs. 53%), non-Hispanic White (82% vs. 86%), married (95% vs. 100%), and multipara (78% vs. 70%), with no statistically significant differences between the 2 groups (p>0.05 for all). A higher proportion of case-patients’ mothers had ≥1 living children at the time of birth than control’s mothers (68% vs. 49%; p<0.05).
Among the 92 case-patients, the median number of audiologic assessments was 7 (range: 1–17); the median age at first ABR evaluation was 2.4 months (range: 4 days-11.5 months), after which 6 (6%) case-patients without SNHL were lost to follow-up. The remaining 86 (94%) case-patients had a median of 8 assessments, with the last one at a median age of 17 years (range: 9 months-18 years); 3 (3%) at 0–3 years, 5 (6%) at 6–9 years, and 78 (91%) at 12–18 years. Among the 51 controls, the median number of audiologic assessments was 3 (range: 1–8); the median age at the first assessment was 3 years (range: 1 month-14 years). Among 41 controls with ≥2 assessments, the median age at last assessment was 17 (range: 1–18) years; 1 (2%) at 1 year, 2 (4%) at 6–9 years, and 38 (93%) at 12–18 years.
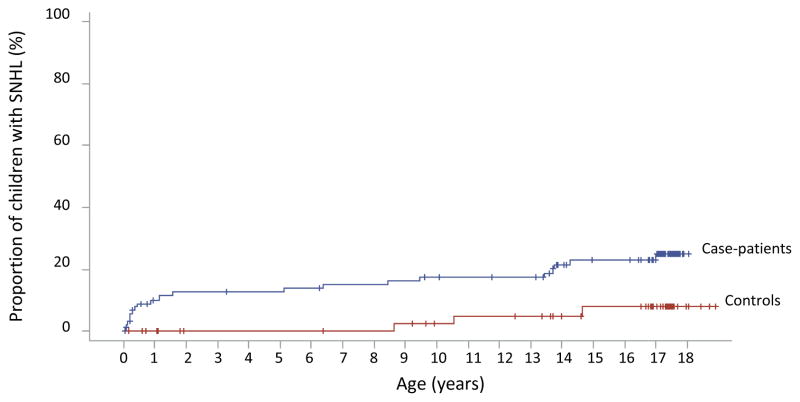
Using survival analysis, we estimated the proportion of children with SNHL increased from 7% at age 3 months to 14% at 5 years and 25% at 18 years, among case-patients, and from 0% at 5 years to 8% at 18 years, among controls (Table 1). SNHL risk from birth through 18 years was 4-fold greater among case-patients compared to controls (HR: 4.0; 95% CI: 1.2–14.5; p-value=0.02). SNHL risk from 3 months to 18 years was 3-fold greater among case-patients compared to controls (HR: 3.0; 95% CI: 0.9–10.5; p-value=0.08), but not statistically significant. SNHL risk from 6 to 18 years was 1.6-fold greater among case-patients compared to controls, but not statistically significant (HR: 1.6; 95% CI: 0.4–6.1; p-value=0.5) (Figure 1).
Table 1.
SNHL among children with asymptomatic congenital CMV infection and controls
| Age | Children with asymptomatic congenital CMV infection (n=92)
|
Proportion of controls with any SNHL (n=51) % (95% CI) | |||
|---|---|---|---|---|---|
| Total with SNHL n (%) | Total with Unilateral Loss n (%) | Total with Bilateral Loss n (%) | Proportion with any SNHL % (95% CI) | ||
| 3 months | 6 (30) | 6 (100) | 0 (0) | 7 (3–14) | 0 |
| 12 months | 9 (45) | 7 (78) | 2 (22) | 10 (5–19) | 0 |
| 24 months | 11 (55) | 9 (82) | 2 (18) | 13 (7–22) | 0 |
| 5 years | 12 (60) | 9 (75) | 3 (25) | 14 (8–23) | 0 |
| 10 years | 15 (75) | 8 (53) | 7 (47) | 17 (11–27) | 5 (1–18) |
| 14 years | 19 (95) | 12 (63) | 7 (37) | 23 (15–34) | 8 (3–22) |
| 18 years | 20 (100) | 10 (50) | 10 (50) | 25 (17–36) | 8 (3–22) |
Figure 1.
Sensorineural hearing loss among children with asymptomatic congenital CMV infection and controls
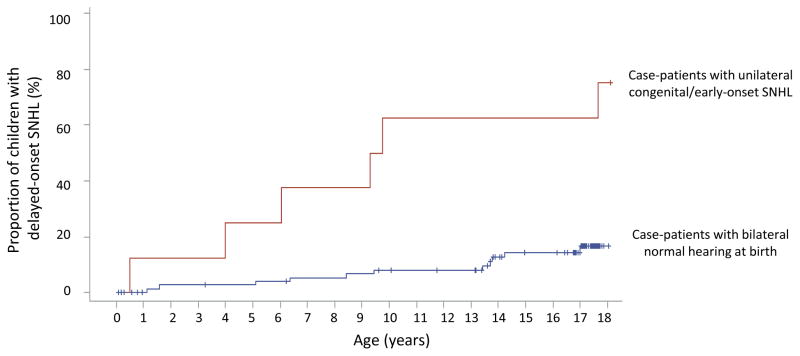
Among case-patients, 9 (10%) were ultimately classified with congenital/early-onset SNHL. Although 23 (25%) of 92 case-patients had had ≥25 dB HL detected at the first ABR (screening) assessment, 14 (61%) had normal hearing in both ears in subsequent assessments. Most (8/9) case-patients with confirmed congenital/early-onset SNHL presented with unilateral loss but the majority (6/8) subsequently developed delayed-onset SNHL in the contralateral ear. In contrast, only 11 (14%) of 77 case-patients without congenital/early-onset SNHL who had ≥2 assessments had delayed-onset SNHL (Figure 2). Among case-patients, the risk of delayed-onset SNHL was significantly greater among those with unilateral congenital/early-onset loss than those without (HR: 6.9; 95% CI: 2.5–19.1). Overall, the proportion of case-patients with SNHL that had bilateral loss increased from 22% at 12 months of age to 50% at last assessment (Table 1). The median interval from unilateral to bilateral SNHL was 4 years (range: 4 months–18 years).
Figure 2.
Delayed-onset sensorineural hearing loss among children with asymptomatic congenital CMV infection with and without unilateral congenital/early-onset hearing loss
Worsening of SNHL in affected ears was common; among 20 case-patients with SNHL, 13 (65%) had progressive loss in the poorer-hearing ear, 5 (25%) had stable loss, and 2 (10%) were indeterminate. Among 10 case-patients with bilateral SNHL, 4 (40%) had progressive loss in the better-hearing ear, 3 (15%) had stable loss without fluctuations, and 3 (15%) were indeterminate. Among 10 case-patients with SNHL who had fluctuations, progression occurred in all but one. In all case-patients with SNHL, the initially poorer-hearing ears remained the more severely affected ear throughout follow-up.
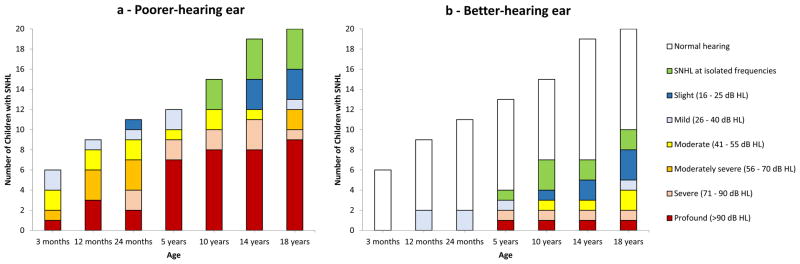
SNHL severity increased with age. At the last assessment, 12 (60%) of the 20 case-patients with SNHL had moderate or worse loss in the poorer-hearing ear and 4 of the 10 case-patients with bilateral SNHL had moderate or worse loss in the better-hearing ear (Figure 3). SNHL severity was greater among the 9 case-patients with congenital/early-onset SNHL, of whom 8 (89%) had profound loss in the poorer-hearing ear at last assessment. The 8 case-patients and 3 controls diagnosed with delayed-onset SNHL after age 5 years all had mild or lesser degree of loss in the poorer-hearing ear, among whom 3 (38%) and 1 (33%), respectively, had audiograms suggestive of noise-induced loss.
Figure 3.
Cumulative number of children with asymptomatic congenital CMV infection with sensorineural hearing loss (n=20) by age and sensorineural hearing loss severity in the poorer- and better-hearing ears
We estimated the proportion of case-patients that would require hearing aids increased from 10% at age 12 months to 14% at 18 years (Supplementary material). The proportion of case-patients that would meet current candidacy criteria for cochlear implants increased from 1% at age 25 months to 2% at 5 years, remaining unchanged after that age. In considering more expansive criteria for cochlear implantation, 5% of case-patients had SNHL ≥70 dB HL in the poorer-hearing ear by age 12 months, increasing to 13% at 18 years.
DISCUSSION
In this study of children with congenital CMV infection identified through hospital-based newborn screening who were asymptomatic at birth, prevalence and severity of SNHL increased throughout childhood. Children with asymptomatic congenital CMV infection who had unilateral congenital/early-onset SNHL were at greater risk of subsequent delayed-onset loss in the normal-hearing ear compared to those without any SNHL in the first year of life. Many children with unilateral loss present with bilateral loss later and/or experience progressive hearing loss, e.g. from mild/moderate to severe or profound hearing loss. Therefore, ongoing audiological monitoring is critical so that they can receive appropriate interventions in a timely manner.
From age 3 months to 5 years, the prevalence of SNHL doubled among case-patients, from 7% to 14% but remained at 0% among controls. From 6 years to 18 years, the changes in SNHL prevalence were similar between the case-patients and controls, 11% and 8%, respectively. This finding is consistent with the 13% prevalence reported nationally among children 6–19 years of age in the United States.16 Therefore, it appears that the risk of delayed-onset SNHL among school-aged case-patients was not appreciably higher than in the comparison group. Larger controlled studies will be important to confirm these findings and inform future guidance on optimal duration of audiologic monitoring for children with asymptomatic congenital CMV infection. The possibility that routine monitoring for SNHL among children with congenital CMV infection who have normal hearing may not be necessary beyond 5 years of age is of clinical importance.
We observed 65% of our case-patients with SNHL had progressive loss. Although we used strict criteria based on ototoxicity monitoring studies13 for categorizing SNHL as progressive, the proportion with progressive SNHL in our study was higher than the 20% estimated in a recent meta-analysis.17 Referral bias in the studies included in the meta-analysis likely contribute to this difference. Some studies were based on cohorts of children identified with asymptomatic congenital CMV infection due to diagnosis of SNHL at birth or primary maternal CMV infection during pregnancy.7, 18–20 Thus, some children were likely at greater risk of more severe SNHL at onset than the entire population of infants with asymptomatic congenital CMV infection. Consistent with this, the same meta-analysis17 estimated that a higher proportion of children with asymptomatic congenital CMV infection and SNHL have bilateral severe to profound loss compared to our study (42% vs. 16% by age 5 years). Studies in which a larger proportion of children present with profound loss when SNHL is detected would have relatively fewer children who could experience SNHL progression.
CMV-related SNHL in children with congenital CMV infection who passed hearing screening tests in the first month of life has been detected as early as 3 months of age.21 Newborn hearing screening programs will not detect all infants with CMV-related hearing loss. In our study, at least 25% of case-patients with SNHL by age 5 would not have been identified by newborn hearing screening. This proportion is lower than the 50% found in a large hospital-based newborn screening study with follow-up through age 6 years21, albeit higher than the 9% estimated in a recent meta-analysis.17 Comparisons of delayed-onset SNHL among children with asymptomatic congenital CMV infection across studies are complicated by heterogeneity in case ascertainment methods and duration of follow-up.22 Currently, an estimated one third of all children with bilateral SNHL ≥40 dB HL by age 4 years are not identified by newborn hearing screening.23 The ongoing CMV and Hearing Multicenter Screening Study will provide population-based estimates of prevalence of congenital CMV infection and CMV-related SNHL through age 4 years in the United States.24 These data will be useful to inform the potential benefit of newborn screening for congenital CMV infection in identifying children at risk of delayed-onset SNHL who are missed by newborn hearing screening.
Identifying the etiology of a hearing loss may affect clinical management and can provide reassurance to families.23 Diagnosis of CMV-related SNHL depends on diagnosis of congenital CMV infection which requires laboratory testing on a specimen collected within 3 weeks of life. However, full audiologic evaluation to confirm or rule out hearing loss may not be conducted until later in infancy when laboratory testing can no longer confirm congenital infection. Thus, targeted CMV testing among newborns who refer in newborn hearing screening has been explored.25–28 In the United Kingdom, this approach was found to be feasible and acceptable within the newborn hearing screening program, and did not appear to result in increased parental anxiety.28 In Utah, which implemented a policy in 2013 mandating CMV testing for all infants who fail newborn hearing screening26, an improvement in follow-up rates of all infants who fail the hearing screening was reported. This testing strategy has the potential to increase identification of newborns with SNHL with symptomatic congenital CMV infection who would be eligible for antiviral treatment who might otherwise have gone unrecognized as well as infants with asymptomatic infection.29, 30 However, the efficacy of antiviral treatment in preventing hearing deterioration among children with asymptomatic infection has not been systematically studied. Therefore, antiviral treatment is not currently recommended for routine use in this population.29 More data on the feasibility and benefits of targeted CMV testing are likely to become available as this approach is more widely adopted.
Our study had limitations. Our sample size was too small to detect statistically significant differences in SNHL risk after age 3 months. We may have underestimated SNHL risk among case-patients and controls because of loss of follow-up, particularly among children with only a single assessment at younger age. Not all CMV-positive newborns identified through screening were enrolled. However, universal newborn hearing screening was not routinely done during 1982–1992, thus, it is unlikely that there were systematic biases in the enrollment of participants by hearing status that would have affected our estimates. Although our control group had fewer audiological assessments and the first assessment at an older age compared to case-patients, it does not affect our estimates of delayed-onset SNHL because the age at last assessment was similar for both groups. Our control group included a small number of children selected among uninfected siblings of referred CMV-positive infants. Analyses including the control group consisting only of those selected among CMV-negative screened newborns resulted in similar findings. Thus, although the small number of controls may have limited the power of the study to detect some statistically significant differences, the control group appears to have been appropriately valid for comparisons. We were unable to precisely determine if SNHL was congenital or delayed-onset because not all case-patients had hearing evaluations in the first month of life. In addition, some infants only had click-evoked ABR without frequency-specific tone burst stimuli, which can result in false-negative results. Other than ruling out the administration of gentamicin to premature case-patients, we were unable to fully investigate other etiologies of SNHL. Data on genetic testing and noise exposure were not available and the audiologic assessments did not consistently include testing at 3 and 6 kHz, which could have aided in the evaluation of noise-induced SNHL.
CONCLUSIONS
The burden of CMV-related SNHL is substantial considering the impact of SNHL on children’s development and academic achievement, and their need for ongoing audiologic monitoring and interventions. We estimate that approximately 5% of children with asymptomatic congenital CMV infection, about 900 children annually, have SNHL ≥70 dB HL in at least one ear by age 12 months and half of these meet current candidacy criteria for cochlear implantation. As cochlear implant technologies and indications for their use continue to evolve, the number of children with asymptomatic congenital CMV infection and SNHL who might be considered candidates for cochlear implants could increase. Newborn screening for congenital CMV infection has the potential to identify children at risk for CMV-related SNHL who currently go unrecognized and who might benefit from earlier intervention.31 Further investigation of the age of onset and risk factors for SNHL in children with asymptomatic congenital CMV infection are needed to inform evaluation of the potential costs and benefits of CMV screening.
Supplementary Material
What’s Known on This Subject
The extent to which children with congenital CMV infection who are asymptomatic at birth remain at risk for delayed-onset and progressive sensorineural hearing loss (SNHL) throughout childhood is not well established.
What This Study Adds
SNHL progression occurred through adolescence. SNHL risk after age 5 years was not significantly higher than in uninfected children. Overall, 2% of children with asymptomatic congenital CMV infection have severe enough bilateral SNHL to potentially meet cochlear implantation candidacy.
Acknowledgments
Funding Source: The study was supported, in part, by the CMV Research Fund Donors at Baylor College of Medicine; the Woman’s Hospital of Texas Research Foundation; the Office of Research Resources and the General Clinical Research Center for Children at Texas Children’s Hospital and Baylor College of Medicine (NIH 5M0I RR00188-33); the Mental Retardation Research Center at Baylor College of Medicine (NIH-CHHD 5 P30 HD24064P); the Research to Prevent Blindness, Inc. New York, NY; the Deafness Foundation, Houston, TX; the Vale Ashe Foundation, Houston, TX; the Maddie’s Mission Foundation, Katy, TX; the Naymola Foundation, Beaumont, TX; the American Pediatric Society-Society for Pediatric Research Summer Student Research Program (NIH-CHHD); and the Centers for Disease Control and Prevention (Cooperative Agreement FOA IP 10-006).
We thank all the children who participated in the study, their families and physicians for their lifetime of dedication and support for this study.
The Congenital CMV Longitudinal Study Group through the years has included: Shahzad Ahmed, Hanna Baer, MD, Amit R. Bhatt, MD, Peggy Blum, AuD and Texas Children’s Hospital Audiology, Frank Brown, MD, Francis Catlin, MD, Alison C. Caviness, MD, PhD, MPH, David K. Coats, MD, Jane C. Edmonds, MD, Marily Flores, MS, Daniel Franklin, MD, Cindy Gandaria, Jewel Greer, Carol Griesser, RN, Mohamed A. Hussein, MD, Isabella Iovino, PhD, Allison Istas, MPH, Haoxing (Douglas) Jin, Mary K. Kelinske, OD, Joseph T. Klingen, Antone Laurente, PhD, Thomas Littman, PhD, Mary Murphy, MS, Jerry Miller, PhD, Christopher Nelson, MD, Daniel Noyola, MD, Evelyn A. Paysse, MD, Alan Percy, MD, Sara Reis, RN, Ann Reynolds, MD, Judith Rozelle, MS, O’Brien Smith, PhD, Paul Steinkuller, MD, Marie Turcich, MS, Sherry Sellers Vinson, MD, Robert G. Voigt, MD, Bethann Walmus, Jill Williams, MA, Daniel Williamson, MD, Kimberly G. Yen, MD, Martha D. Yow, MD, and Gail J. Demmler-Harrison MD.
Abbreviations
- CMV
cytomegalovirus
- SNHL
sensorineural hearing loss
- HL
hearing level
- HR
hazard ratio
Footnotes
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Contributor’s Statement:
Tatiana M. Lanzieri conceptualized and conducted the analysis contained in this report, interpreted the data, led the writing of the initial manuscript and revised versions, and approved the final version.
Winnie Chung conceptualized the analysis contained in this report, reviewed and interpreted individual audiological data, critically revised the manuscript and approved the final version.
Marily Flores and Jerry A. Miller assisted with data management and quality control for the Longitudinal Congenital CMV Study, critically revised the manuscript and approved the final version.
Peggy Blum conceptualized and provided audiological follow-up in the Longitudinal Congenital CMV Study, critically revised the manuscript and approved the final version.
A. Chantal Caviness was the co-Principal Investigator for the Longitudinal Congenital CMV Study, critically revised the manuscript and approved the final version.
Stephanie R. Bialek and Scott D. Grosse conceptualized the analysis contained in this report, interpreted the data, critically revised the manuscript and approved the final version.
Gail Demmler-Harrison was the Principal Investigator for the Longitudinal Congenital CMV Study, provided patient follow-up, conceptualized the analysis contained in this report, interpreted the data, critically revised the manuscript and approved the final version.
Potential Conflict of Interest: Dr. Demmler-Harrison’s institution received funding from Merck Sharpe & Dohme Corporation since July 2016 to assist with salary support for further analysis on long term outcomes of congenital CMV infection not included in this report. The other authors have no potential conflicts to disclose.
References
- 1.Boppana SB, Ross SA, Novak Z, Shimamura M, Tolan RW, Jr, Palmer AL, et al. Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA. 2010;303(14):1375–1382. doi: 10.1001/jama.2010.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boppana SB, Ross SA, Shimamura M, Palmer AL, Ahmed A, Michaels MG, et al. Saliva polymerase-chain-reaction assay for cytomegalovirus screening in newborns. N Engl J Med. 2011;364(22):2111–2118. doi: 10.1056/NEJMoa1006561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17(5):355–363. doi: 10.1002/rmv.544. [DOI] [PubMed] [Google Scholar]
- 4.Ahlfors K, Ivarsson SA, Harris S. Report on a long-term study of maternal and congenital cytomegalovirus infection in Sweden. Review of prospective studies available in the literature. Scand J Infect Dis. 1999;31(5):443–457. doi: 10.1080/00365549950163969. [DOI] [PubMed] [Google Scholar]
- 5.Saigal S, Lunyk O, Larke RP, Chernesky MA. The outcome in children with congenital cytomegalovirus infection. A longitudinal follow-up study. Am J Dis Child. 1982;136(10):896–901. doi: 10.1001/archpedi.1982.03970460026006. [DOI] [PubMed] [Google Scholar]
- 6.Townsend CL, Forsgren M, Ahlfors K, Ivarsson SA, Tookey PA, Peckham CS. Long-term outcomes of congenital cytomegalovirus infection in Sweden and the United Kingdom. Clin Infect Dis. 2013;56(9):1232–1239. doi: 10.1093/cid/cit018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahle AJ, Fowler KB, Wright JD, Boppana SB, Britt WJ, Pass RF. Longitudinal investigation of hearing disorders in children with congenital cytomegalovirus. J Am Acad Audiol. 2000;11(5):283–290. [PubMed] [Google Scholar]
- 8.Fowler KB, McCollister FP, Dahle AJ, Boppana S, Britt WJ, Pass RF. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J Pediatr. 1997;130(4):624–630. doi: 10.1016/s0022-3476(97)70248-8. [DOI] [PubMed] [Google Scholar]
- 9.Preece PM, Pearl KN, Peckham CS. Congenital cytomegalovirus infection. Arch Dis Child. 1984;59(12):1120–1126. doi: 10.1136/adc.59.12.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson WD, Demmler GJ, Percy AK, Catlin FI. Progressive hearing loss in infants with asymptomatic congenital cytomegalovirus infection. Pediatrics. 1992;90(6):862–866. [PubMed] [Google Scholar]
- 11.Yow MD, Williamson DW, Leeds LJ, Thompson P, Woodward RM, Walmus BF, et al. Epidemiologic characteristics of cytomegalovirus infection in mothers and their infants. Am J Obstet Gynecol. 1988;158(5):1189–1195. doi: 10.1016/0002-9378(88)90252-9. [DOI] [PubMed] [Google Scholar]
- 12.Stapells DR, Gravel JS, Martin BA. Thresholds for auditory brain stem responses to tones in notched noise from infants and young children with normal hearing or sensorineural hearing loss. Ear Hear. 1995;16(4):361–371. doi: 10.1097/00003446-199508000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Konrad-Martin D, James KE, Gordon JS, Reavis KM, Phillips DS, Bratt GW, et al. Evaluation of audiometric threshold shift criteria for ototoxicity monitoring. J Am Acad Audiol. 2010;21(5):301–314. doi: 10.3766/jaaa.21.5.3. quiz 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Speech-Language-Hearing Association. [Accessed April 26, 2016];Degree of Hearing Loss. http://www.asha.org/public/hearing/Degree-of-Hearing-Loss/
- 15.Centers for Medicare and Medicaid Services. Coverage with Evidence Development. [Accessed April 26, 2016];Cochlear Implantation. https://www.cms.gov/Medicare/Coverage/Coverage-with-Evidence-Development/Cochlear-Implantation-.html.
- 16.Niskar AS, Kieszak SM, Holmes AE, Esteban E, Rubin C, Brody DJ. Estimated prevalence of noise-induced hearing threshold shifts among children 6 to 19 years of age: the Third National Health and Nutrition Examination Survey, 1988–1994, United States. Pediatrics. 2001;108(1):40–43. doi: 10.1542/peds.108.1.40. [DOI] [PubMed] [Google Scholar]
- 17.Goderis J, De Leenheer E, Smets K, Van Hoecke H, Keymeulen A, Dhooge I. Hearing loss and congenital CMV infection: a systematic review. Pediatrics. 2014;134(5):972–982. doi: 10.1542/peds.2014-1173. [DOI] [PubMed] [Google Scholar]
- 18.Foulon I, Naessens A, Faron G, Foulon W, Jansen AC, Gordts F. Hearing thresholds in children with a congenital CMV infection: a prospective study. Int J Pediatr Otorhinolaryngol. 2012;76(5):712–717. doi: 10.1016/j.ijporl.2012.02.026. [DOI] [PubMed] [Google Scholar]
- 19.Royackers L, Christian D, Frans D, Ermelinde R. Hearing status in children with congenital cytomegalovirus: up-to-6-years audiological follow-up. Int J Pediatr Otorhinolaryngol. 2011;75(3):376–382. doi: 10.1016/j.ijporl.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Capretti MG, Lanari M, Tani G, Ancora G, Sciutti R, Marsico C, et al. Role of cerebral ultrasound and magnetic resonance imaging in newborns with congenital cytomegalovirus infection. Brain Dev. 2014;36(3):203–211. doi: 10.1016/j.braindev.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Fowler KB, Dahle AJ, Boppana SB, Pass RF. Newborn hearing screening: will children with hearing loss caused by congenital cytomegalovirus infection be missed? J Pediatr. 1999;135(1):60–64. doi: 10.1016/s0022-3476(99)70328-8. [DOI] [PubMed] [Google Scholar]
- 22.Grosse SD, Chung W, Lanzieri TM. [Accessed July 13th, 2016];Delayed onset hearing loss in children with congenital CMV infection: A critique. Available at: http://pediatrics.aappublications.org/content/134/5/972.comments#delayed-onset-hearing-loss-in-children-with-congenital-cmv-infection-a-critique.
- 23.Morton CC, Nance WE. Newborn hearing screening--a silent revolution. N Engl J Med. 2006;354(20):2151–2164. doi: 10.1056/NEJMra050700. [DOI] [PubMed] [Google Scholar]
- 24. [Accessed April 26, 2016];The CMV & Hearing Mulitcenter Screening (CHIMES) Study. http://www.uab.edu/medicine/chimesstudy/
- 25.Choi KY, Schimmenti LA, Jurek AM, Sharon B, Daly K, Khan C, et al. Detection of cytomegalovirus DNA in dried blood spots of Minnesota infants who do not pass newborn hearing screening. Pediatr Infect Dis J. 2009;28(12):1095–1098. doi: 10.1097/INF.0b013e3181af6230. [DOI] [PubMed] [Google Scholar]
- 26.Park AH, Duval M, McVicar S, Bale JF, Hohler N, Carey JC. A diagnostic paradigm including cytomegalovirus testing for idiopathic pediatric sensorineural hearing loss. Laryngoscope. 2014;124(11):2624–2629. doi: 10.1002/lary.24752. [DOI] [PubMed] [Google Scholar]
- 27.Stehel EK, Shoup AG, Owen KE, Jackson GL, Sendelbach DM, Boney LF, et al. Newborn hearing screening and detection of congenital cytomegalovirus infection. Pediatrics. 2008;121(5):970–975. doi: 10.1542/peds.2006-3441. [DOI] [PubMed] [Google Scholar]
- 28.Williams EJ, Kadambari S, Berrington JE, Luck S, Atkinson C, Walter S, et al. Feasibility and acceptability of targeted screening for congenital CMV-related hearing loss. Arch Dis Child Fetal Neonatal Ed. 2014;99(3):F230–236. doi: 10.1136/archdischild-2013-305276. [DOI] [PubMed] [Google Scholar]
- 29.Kimberlin DW, Jester PM, Sanchez PJ, Ahmed A, Arav-Boger R, Michaels MG, et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med. 2015;372(10):933–943. doi: 10.1056/NEJMoa1404599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimberlin DW, Lin CY, Sanchez PJ, Demmler GJ, Dankner W, Shelton M, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr. 2003;143(1):16–25. doi: 10.1016/s0022-3476(03)00192-6. [DOI] [PubMed] [Google Scholar]
- 31.Demmler GJ. Screening for congenital cytomegalovirus infection: a tapestry of controversies. J Pediatr. 2005;146(2):162–164. doi: 10.1016/j.jpeds.2004.11.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.