Abstract
Vestibular schwannomas are the most common cerebellopontine angle tumor. Over the past century, the management goals of vestibular schwannomas have shifted from total resection to functional preservation. Current treatment options include surgical resection, stereotactic radiosurgery and observation. Imaging has become a crucial part of the initial screening, evaluation and follow up assessment of vestibular schwannomas. Recognizing and understanding the management objectives, various treatment modalities, expected post-treatment findings and complications allows the radiologists to play an essential role in a multidisciplinary team by providing key findings that are relevant to the treatment planning and outcome assessment. The authors provide a comprehensive discussion of the surgical management, role of radiation therapy and observation, imaging differential, and pre- and post-treatment imaging findings of vestibular schwannomas.
Introduction
Vestibular schwannomas (VS) are benign neoplasms of the nerve sheath and account for 6–8% of all intracranial tumors and 80% of cerebellopontine angle (CPA) tumors1. VS may remain within the internal auditory canal (IAC) or extend into the CPA. Symptoms are typically related to compression of adjacent cranial nerves (CN), brainstem or posterior fossa (PF) structures.
Imaging plays a central role in the screening, initial and follow up assessment of VS. Imaging can often differentiate VS from other entities such as facial nerve schwannoma, meningioma, epidermoid cyst, arachnoid cyst, aneurysm and metastasis2. MRI is the preferred modality and can provide exquisite tumor characterization, surgical planning and post-therapeutic evaluation3–5. A contrast-enhanced CT of the temporal bones can serve as an alternative if the patient cannot undergo MRI.
The goals of VS management have shifted from total resection to functional preservation, particularly when the entire tumor cannot be safely resected with respect to cranial nerve preservation6–7. Studies have revealed sub-optimal post-surgical facial nerve function when performing gross total resection of large VS8–9. Depending on many factors, including patient age, tumor size and growth, and symptomatology, patients can choose surgery, radiation or conservative management. Patients with NF2, which is characterized by bilateral VS, other schwannomas, meningiomas, ependymomas and ocular abnormalities, are managed differently than sporadic unilateral VS10 and will not be further discussed due to the scope of this topic.
Advances in surgical management of VS over the past century have defined lateral skull base approaches that are now applied in the management of other PF and skull base pathologies. Each approach offers different surgical exposures, benefits and disadvantages. Stereotactic radiosurgery (SRS) is an acceptable option, with similar rates of tumor control and low risk for permanent facial nerve palsy. Observation is a reasonable option for smaller tumors, older patients and those with significant comorbidities.
This article will review the treatment objectives, surgical approaches and expected post-treatment findings and complications of VS management. Knowledge of these advances enhances the radiologist’s ability to participate in a multidisciplinary team by providing key information relevant to the treatment planning and outcome.
Background
VS, often referred to as “acoustic neuromas”, most commonly originate from the vestibular division of the vestibulocochlear nerve sheath, often at the transition from central to peripheral myelin near the vestibular ganglion at the IAC fundus. Inactivation of the neurofibromin 2 gene has been implicated in sporadic and NF 2 VS11. This gene is located on chromosome 22 and produces schwannomin(merlin), a tumor suppressor cell membrane-related protein. Perineural elements of Schwann cell, with areas of dense (Antoni A) and sparse (Antoni B) cellularity, are found histopathologically. Immunohistochemical staining for S100 protein is typically positive.
VS present at a median age of 50 years and are unilateral in >90% of patients, with an equal incidence on the left and right. Symptomatology is often related to cranial neuropathies. Patients more often present with chronic asymmetric sensorineural hearing loss than tinnitus or unsteadiness. True vertigo, sudden hearing loss, facial pain, numbness and weakness are uncommon due to slow tumor growth. Sensorineural hearing loss is confirmed by audiometry and brainstem-evoked response audiometry (AER/ABR), which are abnormal in >90–95% of VS patients12.
Historical Perspective
Charles McBurney performed the first sub-occipital plate removal in 189113, though the patient died soon after. A few years later, Sir Charles Balance in England was the first to successfully remove a VS via a sub-occipital plate and blunt dissection of a CPA mass14. Surgical outcome, in general, remained poor in the late 1800s, with a surgical mortality of 80% and high post-operative morbidity15.
By observing the radiographic properties of bowel gas, Walter Dandy injected air into the subarachnoid spaces, creating the first pneumoencephalographic images in the early 1900s16. Pneumoencephalography allowed the localization of intracranial masses by observing the mass effect upon the ventricles and direction of midline shift17.
Advances in neurosurgery in the early 1900s lead to a decrease in surgical mortality to 20%. Harvey Cushing promoted a bilateral sub-occipital approach and removal of the tumor core while leaving the tumor capsule in place to improve CN preservation18.
In 1961, William House introduced the operative microscope in temporal bone surgeries, allowing for exquisite visualization and improved preservation of the facial nerve19. William House fostered collaboration with the neurosurgeon William Hitselberger, establishing a multi-disciplinary approach to VS resections. William House re-introduced the translabyrinthine approach as an option for patients with non-serviceable hearing20. In 1979, Tomas Delgado performed the first intra-operative CN monitoring, which improved CN preservation21.
During the same period, Lars Leksell in Sweden invented the Gamma Knife in 1968 and performed the first SRS on VS in 196922–23. SRS was later confirmed as an effective alternative to surgery in the treatment of VS24.
Radiographically, positive-contrast cisternography in the 1960s improved delineation of PF structures25. Polytome Pantopaque allowed depiction of even smaller intracanalicular VS26. The advent of cross-sectional imaging in the 1970s now provides non-invasive means of detecting and evaluating small VS.
Natural History of VS
More than half of all VS grow at an average of 2–4 mm/year, whereas less than 10% regress27. One study revealed that extrameatal tumors (28.9%) were more likely to grow compared to intrameatal tumors (17%) and a larger percentage of tumors grew early on after detection28. VS >2 cm are more likely to grow compared to smaller VS29–30. Growth rates of >2 mm/year are associated with decreased rates of hearing preservation compared to slower growth rates31.
Surgical Management of VS
Surgical objectives have shifted from total resection to long-term functional preservation6–7. Subtotal resection followed by observation or SRS, particularly for large VS, can achieve long term tumor control with improved CN preservation6–7,32. In general, small to medium VS <3 cm are managed differently than large VS, as surgery is often favored over SRS for large VS. While some have experience in successfully treating large VS with SRS33, others believe that SRS may risk compressive ischemia of CN VII and brainstem compression in the treatment of large VS34–35. The optimal treatment of VS, particularly small to medium VS, remains controversial and treatment modality preference will vary from center to center.
Gross total resection is offered to younger patients with persistent dizziness, small anatomically favorable tumors with good hearing, cystic tumors and larger tumors with symptoms related to mass effect35. Surgery, as opposed to SRS, provides definitive histopathologic diagnosis. Due to the post-radiation effects on tissue, SRS following surgical resection is more favorable than surgical resection following SRS. Surgery, however, is associated with a greater risk for permanent facial nerve palsy compared to SRS35. Other risks of surgical resection include iatrogenic hearing loss, CSF leak, meningitis, headache and anesthesia related complications. Following gross total resection, the 5 year recurrence rate of VS has been reported in up to 10%, with 10-year tumor control rates of 78% and 82% for gross total and subtotal resection36.
Surgical Approaches
VS may be approached by a translabyrinthine (TL), retrosigmoid (RS) or middle fossa (MF) craniotomy. The indications, advantages and disadvantages of each are summarized in Table 1.
Table 1.
Indications, benefits and disadvantages of lateral skull base approaches for VS resection.
| Translybyrinthine | Retrosigmoid | Middle fossa | |
|---|---|---|---|
| Indications | Non-serviceable hearing; any IAC or CPA VS | VS with large CPA component; medial IAC VS | Small lateral IAC VS (<0.5 cm); small medial IAC VS with < 1 cm CPA component |
| Advantages | Minimal brain retraction | Panoramic CPA exposure; better facial nerve and hearing preservation for medial VS | Better exposure lateral IAC |
| Disadvantages | Complete hearing loss; difficult approach for CPA VS ventral to porus acusticus; risk for facial nerve injury | Limited access to lateral IAC; potential for cerebellar and brainstem injury | Limited PF access; temporal lobe retraction; risk for facial nerve injury |
Translabyrinthine Craniotomy
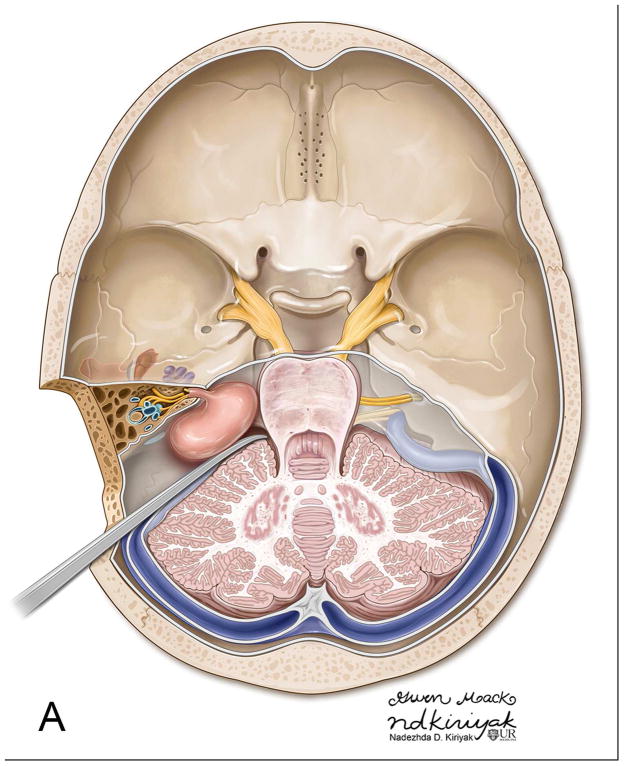
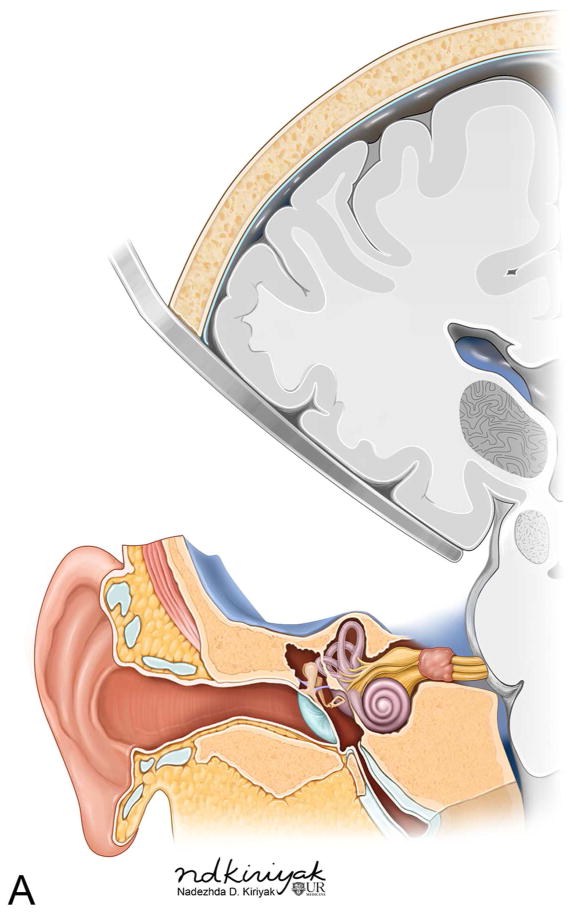
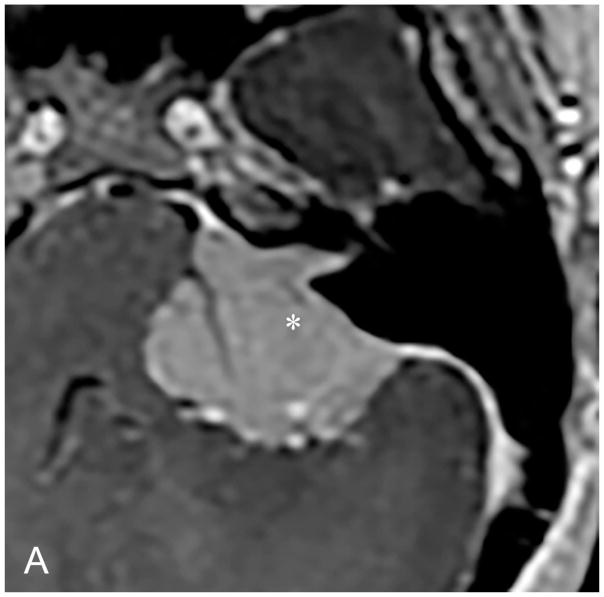
The TL is a posterior approach through the mastoid temporal bone, anterior to the sigmoid sinus [Figure 1]. Following a simple mastoidectomy, the vertical facial nerve canal is skeletonized and a labyrinthectomy is performed, allowing access to the IAC behind the vestibule37[Figure 1]. Access to the CPA can be gained by removing bone posterior to the porus acusticus. While performing facial nerve monitoring, the tumor is debulked and microdissected. The craniotomy is closed by placing temporalis fascia at the aditus ad antrum and abdominal fat packing within the mastoidectomy defect. Fat is preferred to muscle as fat is easily obtainable and associated with less morbidity. The fat signal can be advantageously suppressed on follow up contrast-enhanced MR imaging [Figure 2].
Figure 1.
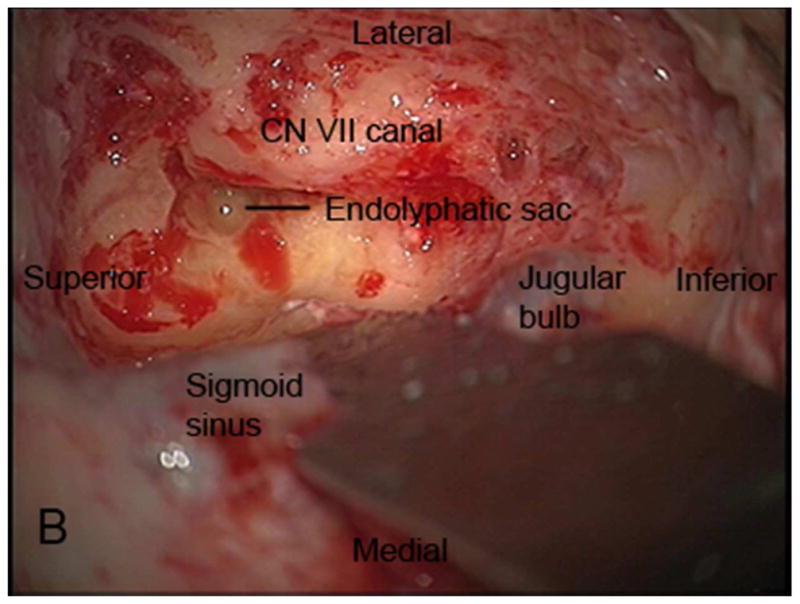
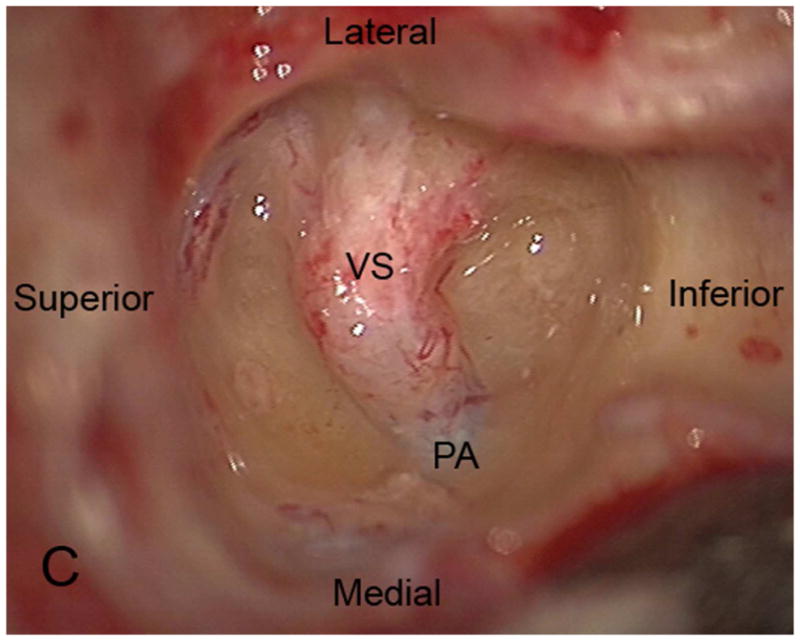
Axial illustration (A) of a translabyrinthine craniotomy demonstrates exposure of the IAC and CPA that may be performed with or without cerebellar retraction. Intra-operative images just prior to (B) and following (C) the labyrinthectomy demonstrate exposure to the intracanalicular vestibular schwannoma (VS). PA Porus acusticus.
Figure 2.
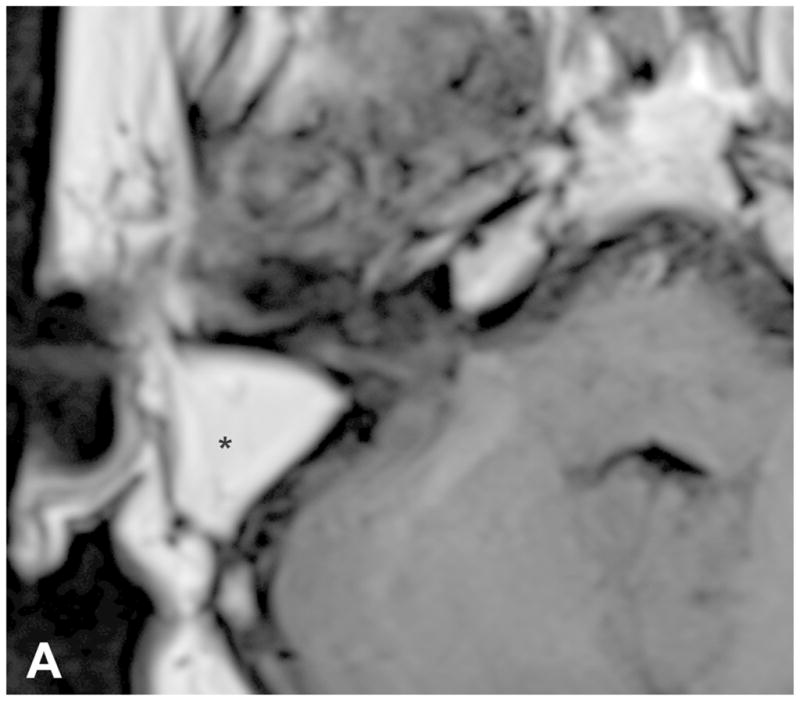
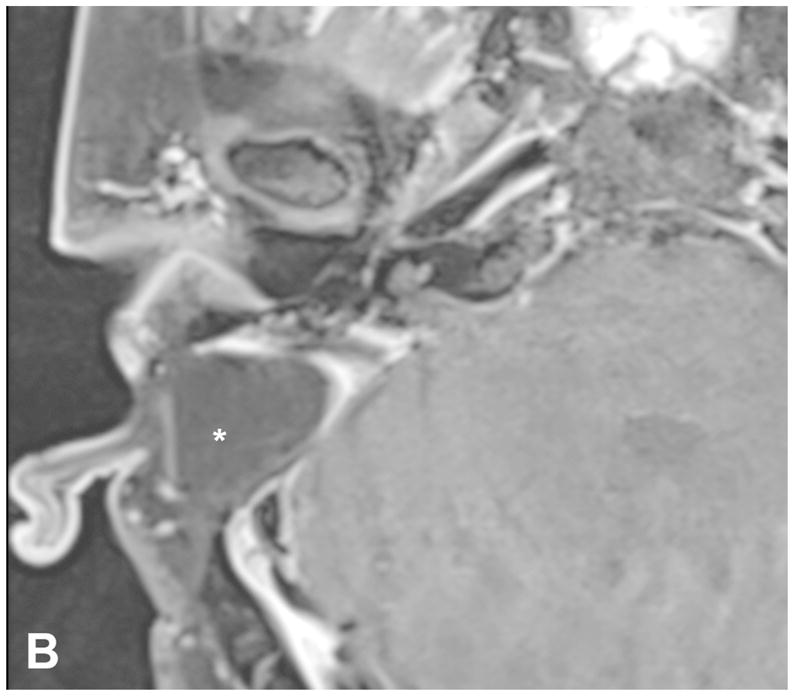
Pre-contrast axial T1-WI (A) and post-contrast axial T1-WI with fat suppression (B) images demonstrates typical post-operative findings following a translabyrinthine craniotomy, with abdominal fat packing within the mastoidectomy defect (*). Linear enhancement along the mastoidectomy bed reflects post-surgical changes, without evidence of recurrent tumor within the IAC.
The TL allows adequate exposure of the IAC and PF with minimal brain retraction. The RS approach may be preferred if a large PF component is present. Due to the complete loss of hearing, TL is reserved only for patients with non-serviceable hearing or poor hearing prognosis.
Retrosigmoid Craniotomy
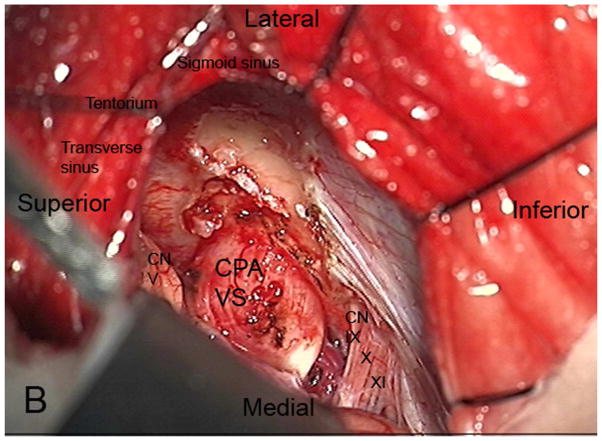
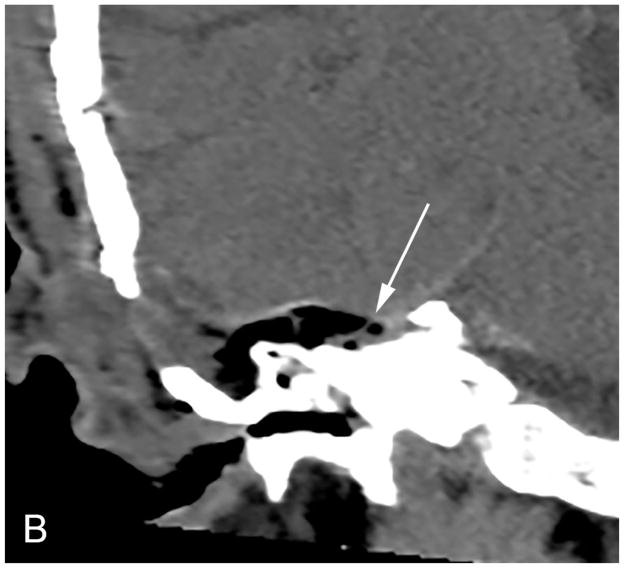
The RS is a posterior approach that allows panoramic visualization of the CPA [Figure 3]. Following a suboccipital craniotomy posterior to the sigmoid sinus, the cerebellum is retracted medially, exposing the CPA mass and neurovascular structures [Figure 3]. The facial nerve is identified and the CPA component dissected. The intrameatal component can then be accessed and removed by drilling the posterior meatal lip [Figure 3]. Tumor infiltration of the cochlear nerve, poor pre-operative hearing and larger tumor size decrease the likelihood of hearing preservation37.
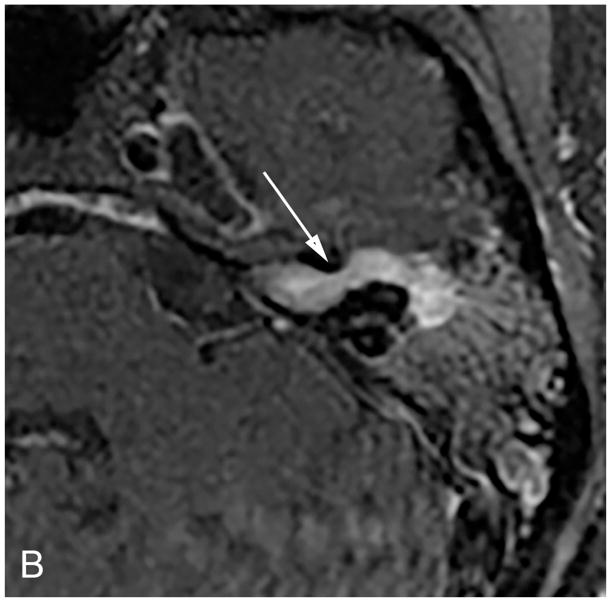
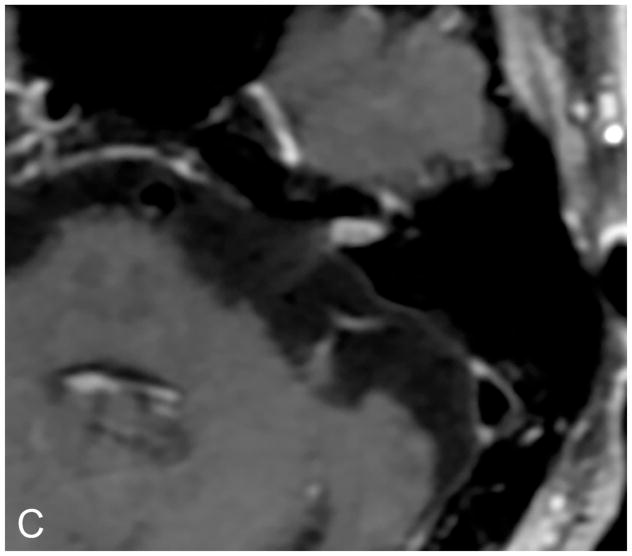
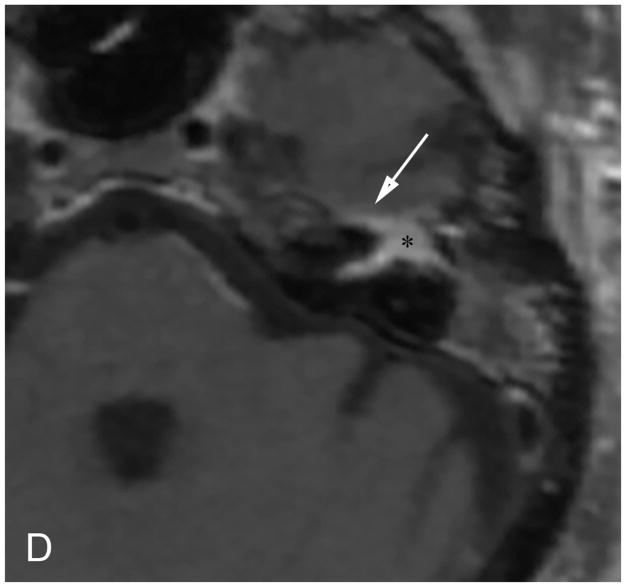
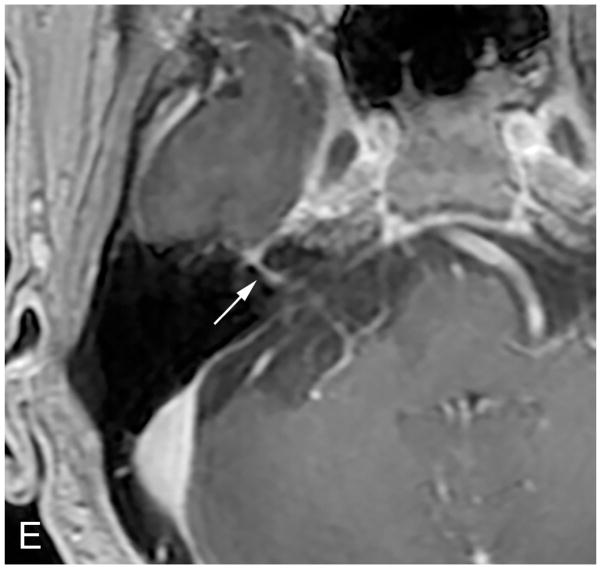
Figure 3.
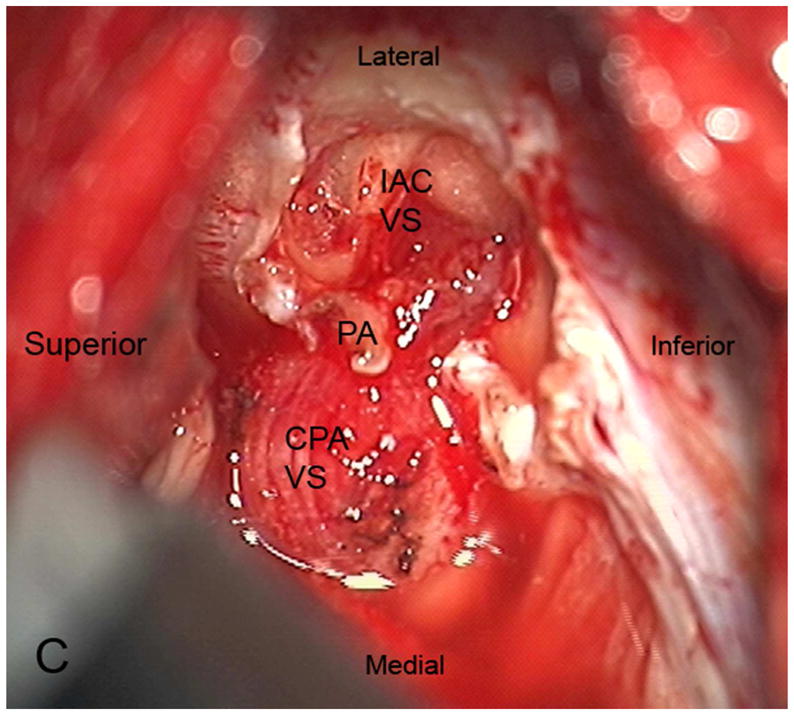
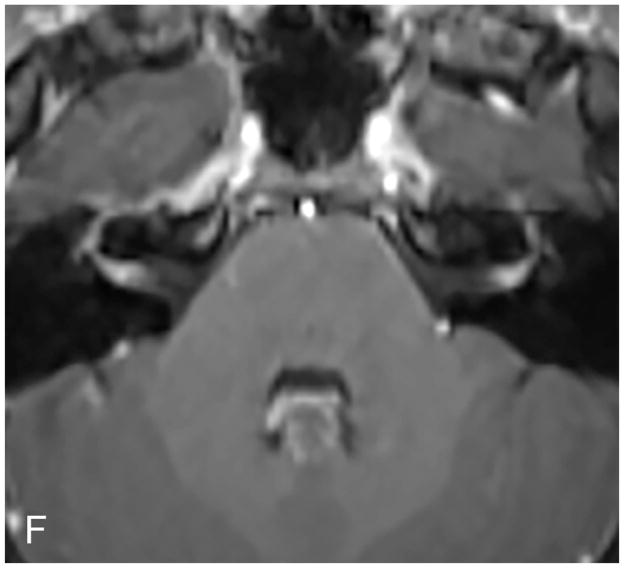
Axial illustration (A) of a retrosigmoid craniotomy reveals a typical exposure of the CPA and lateral IAC by drilling through the posterior meatal lip. Intra-operative image (B) reveals excellent exposure of the CPA VS and adjacent cranial nerves (CN V, IX-XI). A second intra-operative image (C) following removal of the posterior face of the IAC wall exposes the intra-meatal component of the VS (IAC VS). Immediate post-operative non-contrast axial CT (D) and a contrast-enhanced T1-WI with fat suppression (E) images demonstrate a retrosigmoid craniectomy with a defect in the posterior meatal lip (arrows) and a residual extrameatal enhancing VS on the CE T1-WI. PA Porus acusticus.
The RS permits resection of large extrameatal and small medial intrameatal tumors while allowing hearing preservation38–40. The RS approach to intrameatal VS can be limited by a high riding jugular bulb or obstructed by the labyrinth40. Cerebellum retraction may lead to parenchymal injury. Early post-operative headaches following RS may be higher than TL41, possibly secondary to subarachnoid bone dust dissemination or to the use of a titanium plate.
Middle Fossa Approach
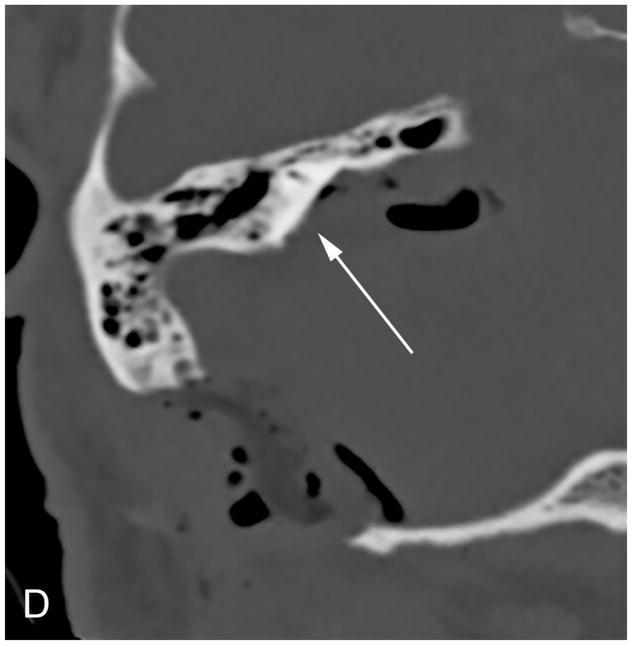
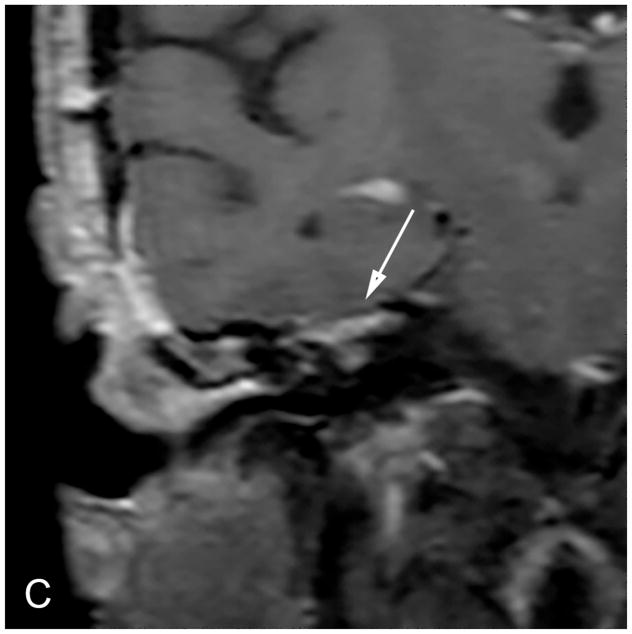
The MF is a lateral approach to the IAC [Figure 4]. A temporal craniotomy is performed above the external auditory canal [Figure 4]. The dura is elevated off the skull base and the temporal lobe is retracted superiorly. Landmarks for this approach include the arcuate eminence and the greater superficial petrosal nerve. The IAC can then be accessed from above [Figure 4] and the tumor resected following microdissection of the facial and cochlear nerves. Bone wax is used to fill exposed mastoid air cells.
Figure 4.
Coronal illustration (A) of a middle fossa craniotomy demonstrates retraction of the temporal lobe and drilling of the petrous apex over the superior semicircular canal to provide access to the IAC. Post-operative coronal reformation of a non-contrast CT (B) and a coronal T1-WI with fat suppression (C) images reveal a temporal craniotomy and absence of the IAC roof (arrow), through which the VS was accessed, and linear enhancement within the IAC that reflects expected post-surgical changes without evidence of residual tumor.
The MF is best for small lateral IAC tumors, particularly those that extend to the IAC fundus, when hearing preservation is a treatment objective. The MF is not typically attempted on tumors with a >1 cm CPA component due to the limited exposure to the PF37, although some surgeons have had success with larger tumors via this approach. Temporal lobe retraction is associated with a small risk for seizures, aphasia and stroke. The MF is optimal for VS arising from the superior division, which displaces the facial nerve anteriorly.
Radiation Therapy
Radiation can be performed using SRS, stereotactic radiotherapy and conventional fractionated radiation therapy. SRS is the most commonly used technique and converges multiple beams onto a delineated volume using cross-sectional imaging to minimize injury to adjacent tissues. Initial SRS dosage of 16–20 Gy marginal dose achieved a 98% tumor control rate but resulted in unacceptably high rates of early hearing loss (60%), and facial and trigeminal neuropathies (33%)24,42–44.
SRS dose reductions from 13–14 to 11–12 Gy in more recent years have resulted in >90% tumor control rates and <1% risk for permanent facial nerve palsies45–46. Slightly lower doses of 12–13 Gy can be preferentially given to patients with serviceable hearing and slightly higher doses of 13–14 Gy to patients with poor hearing prognosis44.
While hearing preservation rates of 60–70% were initially reported, longer-term follow up studies of up to 10 years revealed progressive hearing deterioration in a majority of patients. Serviceable hearing was preserved in only 23–24% patients at 10 years47–49. Older age, larger tumors and poorer pre-treatment hearing were found to be risk factors for progressive post-treatment hearing loss47,49–50. Reducing cochlear dose to improve hearing preservation continues to be controversial and has not been confirmed to reduce long-term hearing deterioration51.
Observation
Observation is offered to select patients who are typically followed with serial MR imaging every 6–12 months. Indications include patients >60 years with significant comorbidity, small tumor size, and absence of symptoms. Patients who are at risk for hearing loss from other causes or prefer observation may also be offered conservative management. Observation, however, is associated with progressive hearing loss, due to the slow growth of the majority of these tumors.
Tumor growth >2.5 mm/yr. is associated with higher rates of hearing deterioration compared to slower growing tumors31. If hearing preservation remains a treatment objective, earlier intervention may lead to a better outcome52.
Imaging
Differential
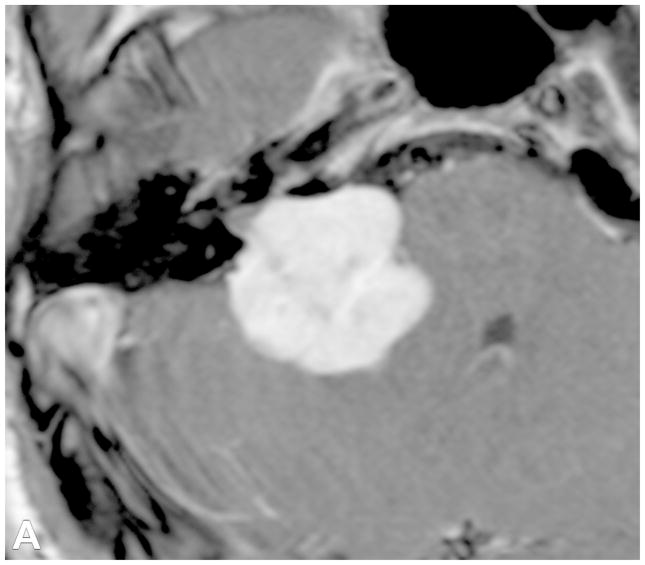
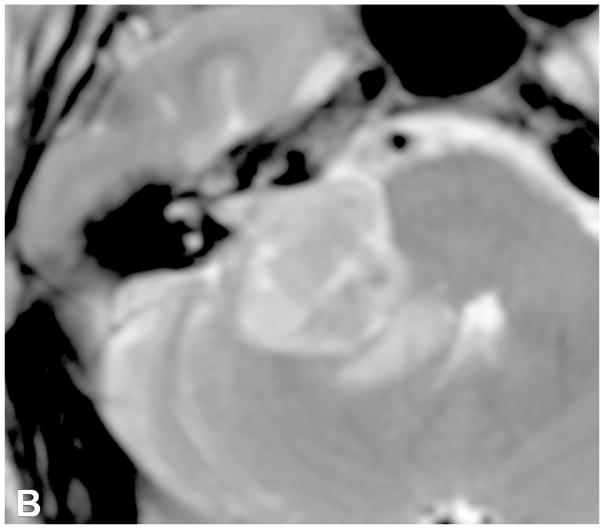
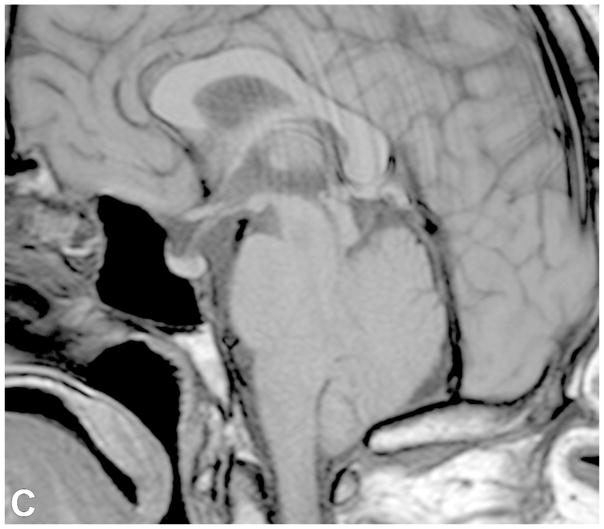
VS are the most common extra-axial CPA mass (70–80%), followed by meningiomas (10–15%) and epidermoid cysts (5%). CPA meningiomas are dural-based enhancing masses that grow along the petrous ridge and can extend into the IAC. Large meningiomas are often positioned asymmetric relative to the IAC [Figure 5]. Meningiomas may contain intralesional calcifications and a dural tail, and can result in changes of the underlying bone, as well as peritumoral vasogenic edema if mass effect is present.
Figure 5.
Examples of various enhancing IAC and CPA masses on contrast-enhanced T1-WI with fat suppression images (B-D, F) and 3D SPGR images (A,E). (A) A large CPA meningioma, located eccentric to the porus acusticus (* denotes tumor midline), extends into the IAC, without the associated bony expansion that is often seen with VS (See Figure 6). (B) An enhancing facial nerve schwannoma within the IAC extends into the labyrinthine (arrow), anterior genu and tympanic segments, which differentiates a facial nerve from a vestibular schwannoma. (C) A small enhancing metastatic lesion within the IAC, in a patient with non-small cell lung cancer, extends into the IAC fundus, labyrinthine, anterior genu and tympanic segments. (D) Perineural spread along the intratemporal and intracanicular segments of the facial nerve in a patient with squamous cell carcinoma of the periauricular skin (* anterior genu; arrow – greater superficial petrosal nerve). (E) Ill-defined tuft of enhancement within the IAC fundus, extending into the labyrinthine segment and anterior genu of the facial nerve, in a patient with right Bells palsy. (F) Bilateral ill-defined enhancement of the distal IAC bilaterally, extending into the labyrinthine segment and anterior genu of the facial nerve canal, in a patient with neurosarcoidosis.
Other enhancing lesions of the IAC and CPA include neoplastic etiologies, such as leptomeningeal metastasis, lymphoma, meningeal melanocytoma or malignant melanoma, and facial nerve perineural spread, inflammatory processes, such as Bell’s palsy and neurosarcoidosis, and aneurysms [Figure 5]. Identifying enhancement of the labyrinthine facial nerve can distinguish CN VII pathologies from a VS [Figure 5]. Aneurysms demonstrate nodular enhancement but are contiguous with vascular structures, and often exhibit flow voids, eccentric peripheral enhancement and pulsation artifact on MR.
Because VS can contain cystic components, the radiologist should also be aware of other cystic lesions of the CPA. The characteristic MR signal and enhancement patterns of these lesions, however, should not lead to any confusion between these entities. Epidermoid cysts are non-enhancing cysts of congenital ectodermal elements that encase or displace neurovascular structures and extend into the cerebellar fissures with ill-defined margins. Relative to CSF, these cysts demonstrate similar attenuation on CT, isointense to slightly hyperintense signal to CSF on T1-WI and T2-WI and incomplete suppression FLAIR signal. The presence of diffusion restriction differentiates epidermoid from arachnoid cysts, which follow CSF signal on all sequences. Arachnoid cysts do not enhance and displace rather than engulf adjacent structures. Other uncommon cysts include dermoid cysts, neurocysticercosis and neuroenteric cysts.
Initial assessment
CT can detect moderate-large VS, though small intracanalicular tumors can be missed. On CT, solid VS are isoattenuating relative to the cerebellar parenchyma and typically enhance. Unlike meningiomas, VS do not contain calcifications.
CT is advantageous in assessing bony anatomy and pathologic changes. Unlike meningiomas, moderate-large VS tend to expand the IAC [Figure 6], which may reflect tumor aggressiveness53. IAC expansion is associated with poorer pre-operative hearing and post-operative hearing function53. Because the cochlear nerve is often located anterior or inferior to the tumor, larger tumors extending in this direction may encapsulate, infiltrate or stretch the nerve38,53. The facial nerve can be affected by anterior extension of the tumor, though it appears to be more resilient than the cochlear nerve53.
Figure 6.
Contrast-enhanced axial T1-WI (A), axial T2-WI (B) and sagittal T1-WI (C) reveals a large right CPA VS with asymmetric enlargement of the IAC, brainstem and cerebellar compression, peritumoral edema and tonsillar herniation.
Due to superior contrast resolution, MR is now the standard of care in evaluating VS. A sample MR protocol used in the evaluation of CPA masses is included in Table 2. VS are typically T1 isointense relative to the cerebellar parenchyma and demonstrate avid enhancement on post-contrast T1-WI [Figure 6]. VS may contain intralesional hemorrhage, which may exhibit T1 hyperintense signal and susceptibility artifact on T2* gradient echo sequences. Larger VS often demonstrate inhomogeneous enhancement secondary to intralesional hemorrhage and cysts. Concerning features include larger size, brainstem or cerebellar compression, peritumoral edema, hydrocephalus and tonsillar herniation [Figure 6]. Enhancement may extend into the modiolus secondary to cochlear infiltration [Figure 7], which decreases the rate of hearing preservation.
Table 2.
A sample MR protocol for the evaluation of VS. Except for the axial 3D SPGR+C, all sequences are referenced to the AC PC line. An axial T2 FLAIR can be performed instead of the sagittal 3D T2 FLAIR. An axial and coronal T1 FS+C can be performed in lieu of a sagittal 3D T1 FS+C. FS fat suppression.
| Sequence | FOV (cm) | Thick- ness and spacing (mm) | TR (ms) [or flip angle] | TE (ms) | Matrix | Comments |
|---|---|---|---|---|---|---|
| Sag T1 FLAIR | 24 | 5x1 | 2800 | 9 | 320x224 | (TI 858 ms) |
| Ax DTI | 26 | 3x0 | 8000 | Min | 128x128 | |
| Sag 3D T2 FLAIR FS | 27 | 1.4 | 7600 | 120 | 256x256 | With axial and coronal reformations |
| Ax T2 FLAIR | 24 | 5x1 | 9500 | 125 | 352x224 | Alternative to 3D T2 FLAIR (TI 2250 ms) |
| Ax T2 FS | 18 | 3x0.5 | 4917 | 87 | 320x320 | Through posterior fossa only |
| Ax FIESTA | 18 | 0.8 | [45°] | Min | 300x300 | With axial and sagittal oblique reformations |
| Ax SWAN or SWI | 25 | 2 | Min [15°] | 25 | 320x224 | Optional |
| Sag 3D T1 FS+C | 25 | 1.2 | 600 | Min | 288x288 | With axial and coronal reformations |
| Ax 3D SPGR+C | 25 | 1.5 | [20°] | Min | 320x224 | For treatment planning. Non-angled orthogonal axial |
| Ax T1 FS+C | 18 | 3x0.5 | 2723.5 | 22 | 320x320 | Alternative to 3D T1 FS +C (TI 111 ms) |
| Cor T1 FS+C | 18 | 3x0.5 | 2475 | Min | 383x224 | Alternative to 3D T1 FS +C (TI 111 ms) |
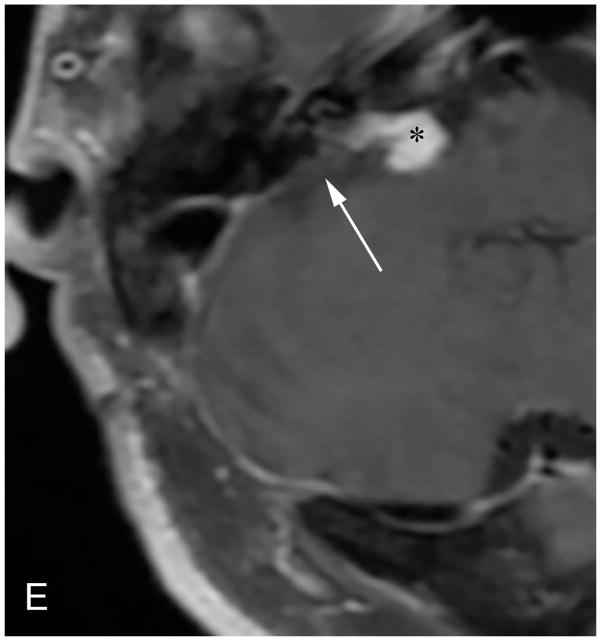
Figure 7.
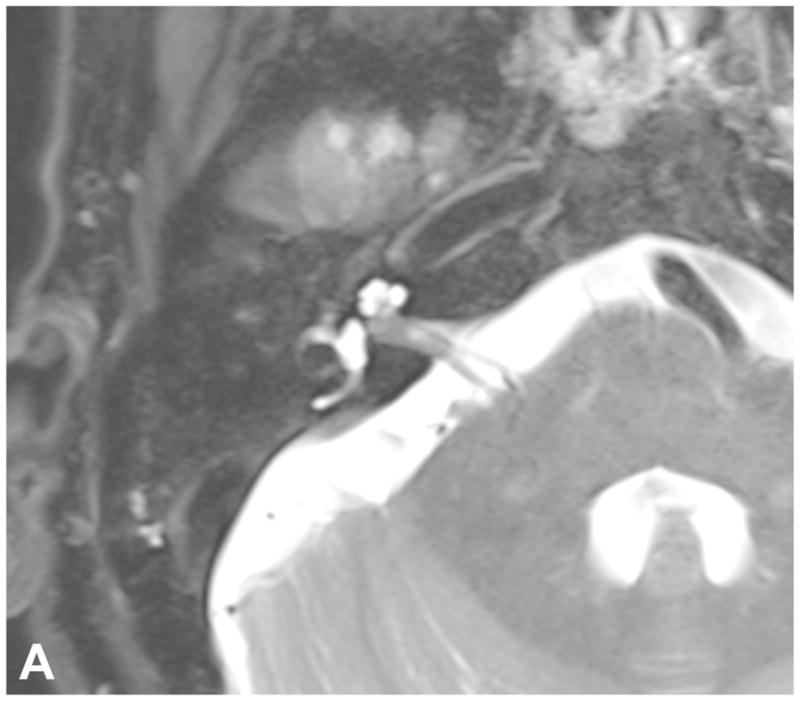
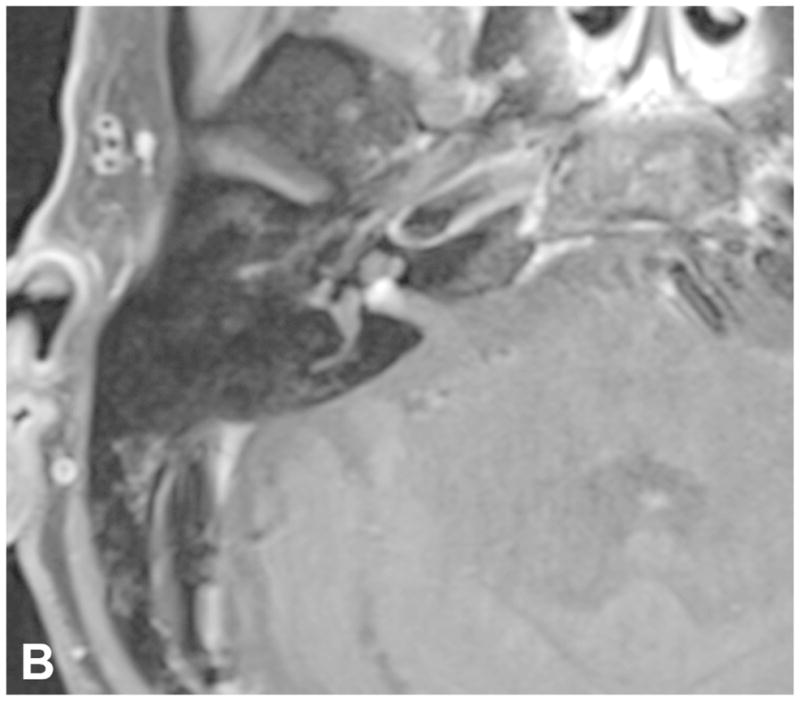
Pre-contrast axial T2-WI (A) and post-contrast axial T1-WI (B) demonstrate a small intracanalicular VS with lateral extension into the IAC fundus as well as the modiolus, which is associated with a decreased rate of hearing preservation.
Cystic VS are a sub-type that account for approximately 10% of all VS and are associated with higher degrees of hearing loss54. VS cysts are thought to arise from recurrent microbleeding or osmosis-induced expansion of CSF trapped in arachnoid tissue54, leading to T2 hyperintense signal and variable T1 signal. Enhancement of the cyst wall differentiates a cystic VS from an arachnoid or epidermoid cyst, the latter of which demonstrates diffusion restriction. Cystic VS may rapidly expand, leading to brainstem and cerebellar compression, edema and hydrocephalus55. Surgical intervention is favored over SRS in the management of cystic VS, as cystic VS may respond poorly and unpredictably to SRS56–57. In one study, 6.4% of cystic VS initially treated with radiation therapy required surgical intervention57. Cystic VS are considered more aggressive, with shorter symptomatic periods prior to presentation. They may surround and adhere to neurovascular structures as well the more hypervascular solid component of the mass, leading to a less favorable surgical outcome55. Subtotal resection of cystic VS is sometimes advocated, particularly if there are peripherally located thin walled cysts55, which should be emphasized in radiologic reporting [Figure 8].
Figure 8.
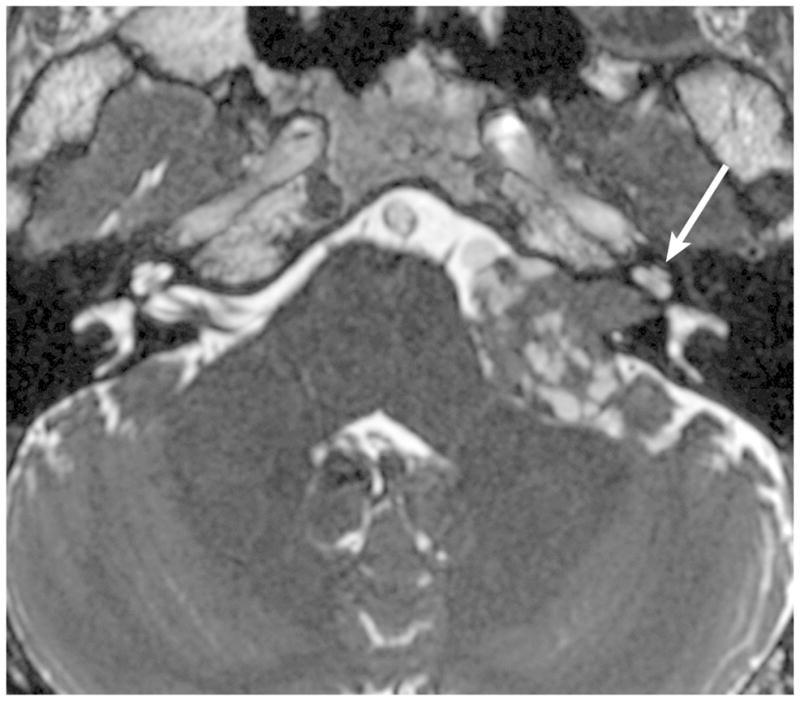
Axial FIESTA reveals a large left CPA VS with multiple superficial cysts, which may indicate increased adherence to neurovascular structures and lead to a more difficult surgical resection. Note asymmetric decreased T2 signal within the left cochlea (arrow) compared to the right.
High-resolution volumetrically acquired steady-state gradient echo (3D SS-GRE) sequences with heavily T2-weighted signal provide exquisite detail of the location and morphology of the mass, the presence of decrease labyrinthine signal, the course of neighboring CN in relation to the mass, and the relationship of the labyrinth to the posterior meatal lip. Identifying CSF lateral to an intracanalicular mass near the IAC fundus on 3D SS-GRE or CE-T1 WI is a favorable prognostic finding, as involvement of the IAC fundus is associated with decreased rates of hearing preservation58 [Figure 7]. Decreased labyrinthine signal 3D SS-GRE on initial imaging is associated with lower rates of post-treatment hearing preservation59[Figure 8].
Sagittal-oblique reformations of 3D SS-GRE sequences allow detailed assessment of the facial nerve course relative to mass. VS arising from the superior division of the vestibular nerve will often displace the facial nerve anteriorly, whereas those arising from the inferior division will displace the facial nerve more superiorly. The location of the facial nerve in relation to the VS influences the surgical approach chosen. Facial nerves that are displaced superiorly by the VS may be more easily injured with a TL or MF approach, leading the surgeon to favor the RS.
Because the posterior meatal lip is drilled to access the IAC in the RS approach, this region is carefully evaluated pre-operatively either by CT or MR. Pneumatized air cells in this region may lead to a post-surgical CSF fistula53. A high riding jugular bulb or jugular bulb diverticulum within the posterior meatal lip may potentially lead to vascular injury. Portions of the labyrinthine lying medial to the fundus-sinus line (line from the sigmoid sinus to the IAC fundus) pose a higher risk for fenestration than those located laterally53.
An abbreviated non-contrast MR using 3D SS-GRE has been proposed as an inexpensive screening exam to exclude an IAC mass60. This study reported 100% sensitivity with high specificity and advocated adding a coronal T2-WI to reduce false positive/negative exams secondary to volume averaging and banding artifacts that could occur if relying solely on 3D SS-GRE60. An abbreviated non-contrast screening MR, however, may not identify etiologies that are better depicted with a CE-MR, such as other neoplastic and inflammatory conditions discussed above.
Increased labyrinthine T2 FLAIR hyperintense signal has been detected in patients with various pathologies, including VS, meningiomas, Meniere’s disease, Ramsay Hunt syndrome, otosclerosis and sudden idiopathic sensorineural hearing loss61–63. The T2 FLAIR hyperintense cochlear signal in patients with VS is attributed to increased protein content within the perilymph61,64, which may be secondary to tumor compression of the cochlear nerve, resulting in interference with neuroaxonal transport of proteins61. 3D-FLAIR sequences can optimally detect cochlear T2 FLAIR hyperintense signal65–67. Kim et al65 reported a significant correlation between the T2 FLAIR hyperintense cochlear signal and degree of hearing impairment in patients with intracanalicular VS. This retrospective study, however, did not specify whether the 3D-FLAIR sequence was performed consistently before or following intravenous contrast administration. Two smaller retrospective studies reported no correlation and a weak correlation between post-contrast T2 FLAIR hyperintense signal and level of hearing impairment in patients with VS66–67. Additional studies should be performed to further clarify the significance of the T2 FLAIR hyperintense cochlear signal in VS.
Follow up assessment
Objectives of follow-up imaging include identification of residual/recurrent tumor, assessment of tumor size, response to radiation therapy and presence of post-therapeutic complications. Residual tumor is best assessed with a fat-suppressed contrast-enhanced T1-WI, as the signal from fat packing can be nullified [Figure 2]. As the goals of therapy have shifted from total resection to functional preservation, residual tumor is often intentionally left behind in areas near the facial nerve. The presence of residual enhancing tumor is not uncommon and may be followed with serial imaging and further treated with SRS [Figure 3]. A residual mass tends to contract and become more rounded within 6–12 months upon completion of SRS.
Standardized methods of tumor reporting and measurements have been promoted by national organizations, such as the American Academy of Otolaryngology-Head Neck Surgery (AAO-HNS) in 199568 and the Consensus Meeting on Systems for Reporting Results in Acoustic Neuroma in 200369, though no single method has been clearly adopted. VS should be described as intracanalicular, extrameatal, or intrameatal and extrameatal, and cross-sectional measurements should be specific for each component. Growth tends to be the greatest in the extrameatal component and recommendations have focused on the extrameatal measurements. The AAO-HNS has recommended the square root product of the extrameatal AP x ML diameters, with the AP diameter measured parallel to the petrous ridge68. The Consensus Meeting in 2003 favored using the maximum extrameatal diameter, which by itself sufficiently reflected growth of the tumor69. One study has found the AAO-HNS methodology to be preferable as tumors tend to grow in both AP and ML directions70.
Immediately following SRS, the tumor may increase in size due to intralesional edema, which rarely indicates treatment failure71. In one study, 5% of tumors enlarged following SRS but remained stable on subsequent imaging72. Most VS treated with SRS will subsequently decrease or remain stable in size, reflecting adequate tumor control44. Decreased enhancement centrally within the tumor is considered a positive response to therapy and is typically seen within 6 months following SRS44[Figure 9]. Radiation therapy may uncommonly induce cystic degeneration that may be secondary to microbleeding, increased vascular permeability or scarring of arachnoid adhesions73[Figure 9]. The potential for post-radiation cystic degeneration is one rationale for treating cystic VS initially with surgical resection.
Figure 9.
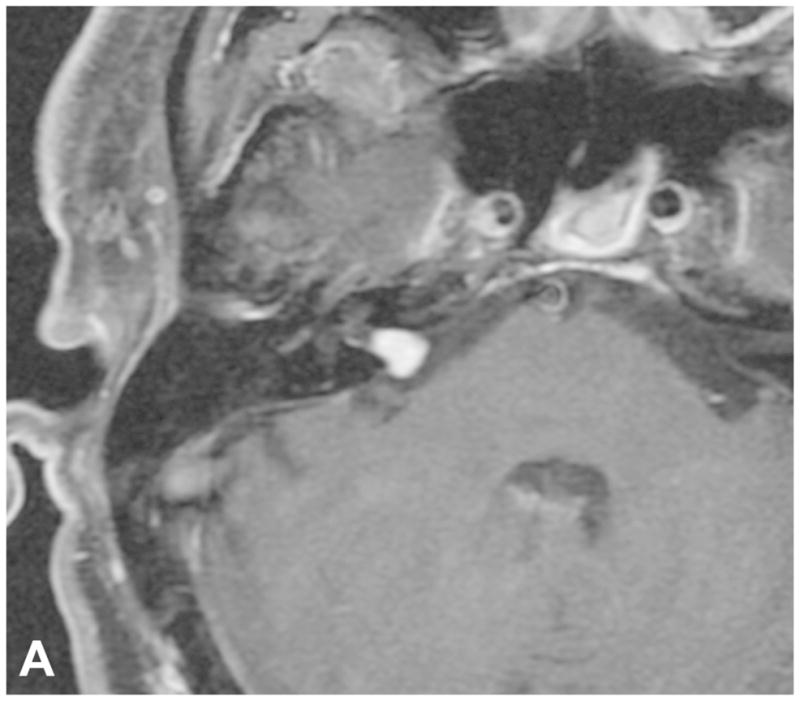
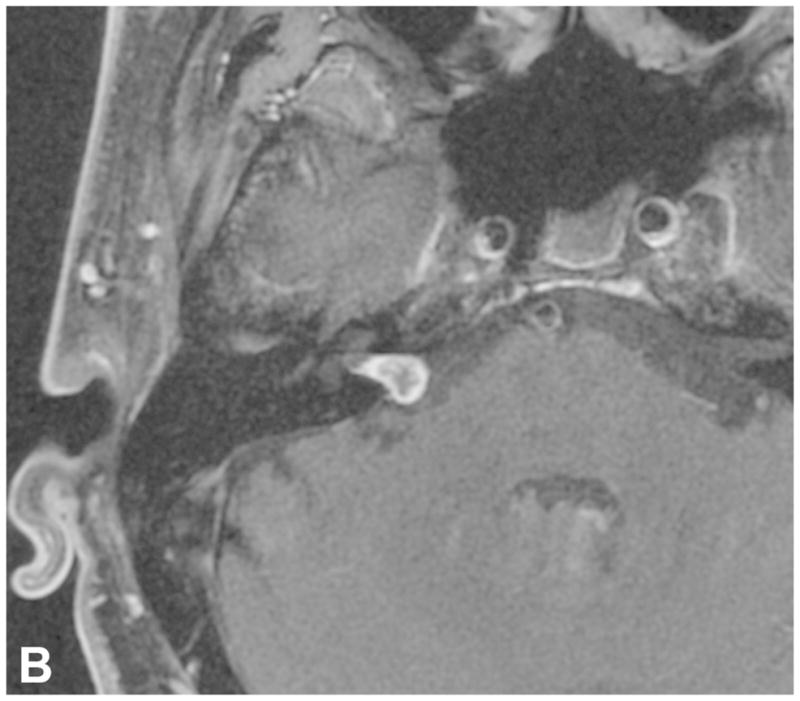
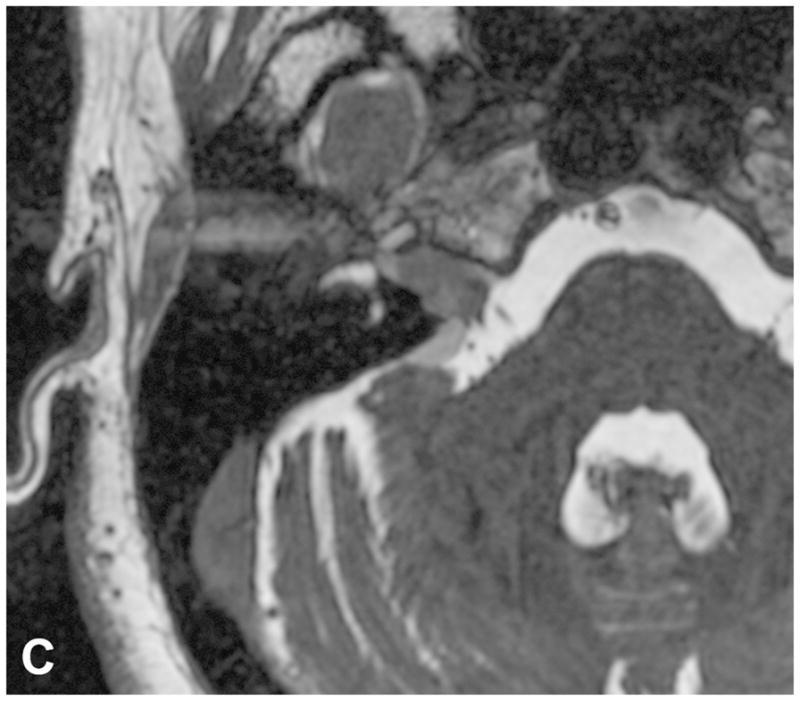

Two examples of post-SRS imaging. Post-contrast axial T1-WI with fat suppression in a patient before (A) and following (B) SRS reveal decreased enhancement centrally within the tumor on post-therapeutic imaging (B), confirming a positive response to SRS. Two axial FIESTA images (C and D) performed during two consecutive follow up exams in a two year period demonstrate interval enlargement of the cystic component within the right CPA associated with a predominantly intrameatal VS following radiation therapy. The cystic component was later resected (not shown).
While uncommon, dural sinus thrombus may be seen following a RS or TL approach secondary to injury of the sigmoid sinus and may result in venous congestion or infarction. Brain retraction during a RS or MF approach may result in edema or ischemia of the cerebellum or temporal lobe, respectively. Postoperative infection may result in a meningitis or, if severe, cerebritis. CSF leak can sometimes be detected by identifying the presence of a fluid collection within or subjacent to the craniotomy site. Other complications such as CN deficits are better assessed by clinical examination.
Labyrinthine fenestration may present with post-operative hearing loss and can be evaluated with a dedicated CT of the temporal bones. Bony labyrinthine dehiscence, however, may not always correlate with hearing loss or vestibular symptoms74. Decreased T2 signal within the vestibulocochlear complex on 3D SS-GRE imaging post-surgically may reflect membranous fenestration, microvascular injury to the cochlea or labyrinthitis ossificans. The decrease in T2 signal has been correlated with post-operative hearing loss74.
Conclusion
VS are benign neoplasms of the vestibulocochlear nerve sheath and are the most common CPA tumor. VS can be managed by surgical resection, radiation therapy and observation, though only select patients are followed conservatively due to its association with hearing loss. The treatment objectives of VS have shifted from total resection to long-term tumor control with maximum functional preservation. Larger tumors >3 cm are generally surgically resected, as radiation poses a risk of brainstem compression due to post-treatment edema. Smaller tumors may be treated with surgery or radiation. Lateral skull base approaches include the TL, RS and MF craniotomies and have been applied to other skull base and PF pathologies. Knowledge of the management options and objectives allows the radiologist to provide imaging findings pertinent to the initial management and recognize expected post-therapeutic findings and un-expected complications.
Acknowledgments
We would like to thank Nadezhda Kiriyak and Gwen Mack for their illustrations in this article.
Abbreviations
- VS
Vestibular schwannomas
- CPA
Cerebellopontine angle
- IAC
Internal auditory canal
- CN
Cranial nerve
- PF
Posterior fossa
- SRS
Stereotactic radiosurgery
- TL
Translabyrinthine craniotomy
- RS
Retrosigmoid craniotomy
- MF
Middle fossa craniotomy
- 3D SS-GRE
Volumetrically acquired steady-state gradient echo
- AAO-HNS
American Academy of Otolaryngology-Head Neck Surgery
Footnotes
Portions of this article were presented at the 7th Annual Midwest Head and Neck Imaging Meeting at the University of Wisconsin on 5 March 2016, entitled “The Lateral Skull Base: Historical, Surgical and Radiologic Perspectives”
Contributor Information
EP Lin, University of Rochester Medical Center, Department of Imaging Sciences, 601 Elmwood Ave. Box 648, Rochester, NY 14642.
BT Crane, University of Rochester Medical Center, Department of Otolaryngology.
References
- 1.Mahaley MS, Mettlin C, Natarajan N, et al. Analysis of patterns of care of brain tumor patients in the United States: a study of the brain tumor section of the AANS and the CNS and the commission on cancer of the ACS. Clin Neurosurg. 1990;36:347–52. [PubMed] [Google Scholar]
- 2.Gentry LR, Jacoby CG, Turski PA, Houston LW, Strother CM, Sackett JF. Cerebellopontine angle-petromastoid mass lesions: comparative study of diagnosis with MR imaging and CT. Radiology. 1987;162:513–520. doi: 10.1148/radiology.162.2.3492010. [DOI] [PubMed] [Google Scholar]
- 3.House JW, Waluch V, Jacker RK. Magnetic resonance imaging in acoustic neuroma diagnosis. Ann Otol Rhinol Laryngol. 1986;95:16–20. doi: 10.1177/000348948609500104. [DOI] [PubMed] [Google Scholar]
- 4.Curati WL, Graif M, Kingsley DPE, King T, Scholtz CL, Steiner RE. MRI in acoustic neuroma: a review of 35 patients. Neuroradiology. 1986;28:208–214. doi: 10.1007/BF00548194. [DOI] [PubMed] [Google Scholar]
- 5.Jackler RK, Shapiro MS, Dillon WP, Pitts L, Lanser MJ. Gadolinium-DTPA enhanced magnetic resonance imaging in acoustic neuroma diagnosis and management. Otolaryngol Head Neck Surg. 1990;102(6):670–7. doi: 10.1177/019459989010200608. [DOI] [PubMed] [Google Scholar]
- 6.Kemink JL, Langman AW, Niparko JK, Graham MD. Operative management of acoustic neuromas: the priority of neurologic function over complete resection. Otolaryngol Head Neck Surg. 1991;104:96–99. doi: 10.1177/019459989110400117. [DOI] [PubMed] [Google Scholar]
- 7.Sughrue ME, Kaur R, Rutkowski MJ, et al. Extent of resection and the long-term durability of vestibular schwannoma surgery. J Neurosurg. 2011;114:1218–1223. doi: 10.3171/2010.11.JNS10257. [DOI] [PubMed] [Google Scholar]
- 8.Samii M, Gerganov VM, Samii A. Functional outcome after complete surgical removal of giant vestibular schwannomas. J Neurosurg. 2010;112:860–7. doi: 10.3171/2009.7.JNS0989. [DOI] [PubMed] [Google Scholar]
- 9.Bloch O, Sughrue ME, Kaur R, et al. Factors associated with preservation of facial nerve function after surgical resection of vestibular schwannoma. J Neurooncol. 2011;102:281–6. doi: 10.1007/s11060-010-0315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slatery WH. Neurofibromatosis Type 2. Otolaryngol Clin N Am. 2015;48:443–460. doi: 10.1016/j.otc.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Sughrue ME, Yeung AH, Rutkowski MJ, et al. Molecular biology of familial and sporadic vestibular schwannomas: implications for novel therapeutics. J Neurosurg. 2011;114:359. doi: 10.3171/2009.10.JNS091135. [DOI] [PubMed] [Google Scholar]
- 12.Doyle KJ. Is there still a role for auditory brainstem response audiometry in the diagnosis of acoustic neuroma? Arch Otolaryngol Head Neck Surg. 1999;125:232. doi: 10.1001/archotol.125.2.232. [DOI] [PubMed] [Google Scholar]
- 13.McBurney C, Starr MA. A contribution to cerebral surgery: diagnosis, localization and operation for removal of three tumors of the brain. Am J Med Sci. 1893;55:361–87. [Google Scholar]
- 14.Ballance C. Some points in the surgery of the brain and its membranes. London, England: Macmillan & Co; 1917. p. 276. [Google Scholar]
- 15.Van Eiselsberg A. Uber die chirurgische Behandlung der Hirntumoren. Trans Intl Cong Med. 1913;7:209–17. [Google Scholar]
- 16.Dandy WE. Ventriculography following the injection of air into the cerebral ventricles. Ann Surg. 1918;68:5. doi: 10.1097/00000658-191807000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beller AJ, Schwartz A. Pneumoencephalographic studies following complete removal of supratentorial lesions. Radiol. 1953;61:200–207. doi: 10.1148/61.2.200. [DOI] [PubMed] [Google Scholar]
- 18.Cushing H. Intracranial tumors. Springfield, IL: Charles C. Thomas; 1932. pp. 85–92. [Google Scholar]
- 19.House WF. Surgical exposure of the internal auditory canal and its contents through the middle cranial fossa. Laryngoscope. 1961;71:1363–85. doi: 10.1288/00005537-196111000-00004. [DOI] [PubMed] [Google Scholar]
- 20.House WF. Transtemporal bone microsurgical removal of acoustic neuromas: Evolution of transtemporal bone removal of acoustic tumors. Arch Otolaryngol. 1964;80:731–42. [PubMed] [Google Scholar]
- 21.Delgado TE, Bucheit WA, Rosenholtz HR, et al. Intraoperative monitoring of facial muscle evoked responses obtained by intracranial stimulation of the facial nerve. Neurosurgery. 1979;4(5):418–21. doi: 10.1227/00006123-197905000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102(4):316–9. [PubMed] [Google Scholar]
- 23.Leksell L. A note on the treatment of acoustic tumours. Acta Chir Scand. 1971;137(8):763–5. [PubMed] [Google Scholar]
- 24.Kondziolka D, Lunsford LD, McLaughlin MR, et al. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med. 1998;339:1426–33. doi: 10.1056/NEJM199811123392003. [DOI] [PubMed] [Google Scholar]
- 25.Burrows EH. Positive contrast examination (cerebellopontine cisternography) in extrameatal acoustic neurofibromas. BJR. 1969;42(504):902–913. doi: 10.1259/0007-1285-42-504-902. [DOI] [PubMed] [Google Scholar]
- 26.Hitselberger WE, House WF. Polytome-pantopaque: a technique for the diagnosis of small acoustic tumors. J Neurosurg. 1968;29(2):214–7. doi: 10.3171/jns.1968.29.2.0214. [DOI] [PubMed] [Google Scholar]
- 27.Smouha EE, Yoo M, Mohr K, et al. Conservative management of acoustic neuroma: a meta-analysis and proposed treatment algorithm. Laryngoscope. 2005;115:450–4. doi: 10.1097/00005537-200503000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Stangerup SE, Caye-Thomasen P, Tos M, Thomsen J. Natural history of vestibular schwannoma. Otol Neurotol. 2006;27:547–552. doi: 10.1097/01.mao.0000217356.73463.e7. [DOI] [PubMed] [Google Scholar]
- 29.Nikolopoulos TP, Fortnum H, O’Donoghue G, et al. Acoustic neuroma growth: systematic review of the evidence. Otol Neurotol. 2010;31:478–85. doi: 10.1097/MAO.0b013e3181d279a3. [DOI] [PubMed] [Google Scholar]
- 30.Fucci MJ, Buchman CA, Brackmann DE, et al. Acoustic tumor growth: implications for treatment choices. Am J Otol. 1999;20:495–9. [PubMed] [Google Scholar]
- 31.Sughrue ME, Yang I, Aranda D, et al. The natural history of untreated sporadic vestibular schwannomas: comprehensive review of hearing outcomes. J Neurosurg. 2010;112:163–67. doi: 10.3171/2009.4.JNS08895. [DOI] [PubMed] [Google Scholar]
- 32.Huang MJ, Kano H, Mousavi SH, et al. Stereotactic radiosurgery for recurrent vestibular schwannoma after previous resection. J Neurosurg. 2016 doi: 10.3171/2016.5.JNS1645. Published online July 29, 2016. [DOI] [PubMed]
- 33.Chung WY, Pan DH, Lee CC, et al. Large vestibular schwannomas treated by Gamma Knife surgery: long-term outcomes. J Neurosurg. 2010;113(Suppl):112–21. doi: 10.3171/2010.8.GKS10954. [DOI] [PubMed] [Google Scholar]
- 34.Mandl ES, Meijer OW, Slotman BJ, Vandertop WP, Peedeman SM. Stereotactic radiation therapy for large vestibular schwannomas. Radiother Oncol. 2010;95:94–8. doi: 10.1016/j.radonc.2009.12.042. [DOI] [PubMed] [Google Scholar]
- 35.Carlson M, Tveiten Ø, Driscoll C, et al. Long-term quality of life in patients with vestibular schwannoma: an international multicenter, cross-sectional study comparing microsurgery, stereotactic radiosurgery, observation, and non-tumor controls. J Neurosurg. 2015;122(4):833–42. doi: 10.3171/2014.11.JNS14594. [DOI] [PubMed] [Google Scholar]
- 36.Sughrue ME, Kaur R, Rutkowski MJ, et al. Extent of resection and the long-term durability of vestibular schwannoma surgery. J Neurosurg. 2011;114:1218–1223. doi: 10.3171/2010.11.JNS10257. [DOI] [PubMed] [Google Scholar]
- 37.Oghalai JS, Driscoll CL. Atlas of Neurotologic and Lateral Skull Base Surgery. New York, NY: Springer; 2016. pp. 19–103. [Google Scholar]
- 38.Samii M, Matthies C. Management of 1000 vestibular schwannomas. Neurosurgery. 1997;40:248–262. doi: 10.1097/00006123-199702000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Samii M, Gerganov V, Samil A. Improved preservation of hearing and facial nerve function in vestibular schwannoma surgery via the retrosigmoid approach in a series of 200 patients. J Neurosurg. 2006;105:527–535. doi: 10.3171/jns.2006.105.4.527. [DOI] [PubMed] [Google Scholar]
- 40.Blevins NH, Jackler RK. Exposure of the lateral extremity of the internal auditory canal through the retrosigmoid approach. Otolaryngol Head Neck Surg. 1994;111(1):81–90. doi: 10.1177/019459989411100116. [DOI] [PubMed] [Google Scholar]
- 41.Ruckenstein MJ, Harris JP, Cueva RA, et al. Pain subsequent to resection of acoustic neuromas via suboccipital and translabyrinthine approaches. Am J Otol. 1996;17(4):620–4. [PubMed] [Google Scholar]
- 42.Flickinger JC, Lunsford LD, Linskey ME, et al. Gamma knife radiosurgery for acoustic tumors: multivariate analysis of four year results. Radiother Oncol. 1993;27(2):91–8. doi: 10.1016/0167-8140(93)90127-t. [DOI] [PubMed] [Google Scholar]
- 43.Foote RL, Coffey RJ, Swanson JW, et al. Stereotactic radiosurgery using the gamma knife for acoustic neuromas. J Radiat Oncol Biol Phys. 1995;32(4):1153–60. doi: 10.1016/0360-3016(94)00454-s. [DOI] [PubMed] [Google Scholar]
- 44.Jacob JT, Pollock BE, Carlson ML, et al. Sterotatctic Radiosurgery in the Management of Vestibular Schwannoma and Glomus Jugulare: Indications, Techniques and Results. Otolaryngol Clin N Am. 2015;48:515–526. doi: 10.1016/j.otc.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 45.Flickinger JC, Kondziolka D, Lunsford LD. Dose and diameter relationships for facial, trigeminal, and acoustic neuropathies following acoustic neuroma radiosurgery. Radiother Oncol. 1996;41(3):215–9. doi: 10.1016/s0167-8140(96)01831-2. [DOI] [PubMed] [Google Scholar]
- 46.Lunsford LD, Niranjan A, Flickinger JC, et al. Radiosurgery of vestibular schwannomas: summary of experience in 829 cases. J Neurosurg. 2013;119:195–9. [PubMed] [Google Scholar]
- 47.Roos DE, Potter AE, Zacest AC. Hearing preservation after low dose linac radiosurgery for acoustic neuroma depends on initial hearing and time. Radiother Oncol. 2011;101(3):420–4. doi: 10.1016/j.radonc.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 48.Hasegawa T, Kida Y, Kato T, et al. Long-term safety and efficacy of stereotactic radiosurgery for vestibular schwannomas: evaluation of 440 patients more than10 years after treatment with Gamma Knife surgery. J Neurosurg. 2013;118:557–65. doi: 10.3171/2012.10.JNS12523. [DOI] [PubMed] [Google Scholar]
- 49.Carlson ML, Jacob JT, Pollock BE, et al. Long-term hearing outcomes following stereotactic radiosurgery for vestibular schwannoma: patterns of hearing loss and variables influencing audiometric decline. J Neurosurg. 2013;118:579–87. doi: 10.3171/2012.9.JNS12919. [DOI] [PubMed] [Google Scholar]
- 50.Mousavi SH, Niranjan A, Akpinar B, et al. Hearing subclassification may predict long-term auditory outcomes after radiosurgery for vestibular schwannoma patients with good hearing. J Neurosurg. 2016;125(4):845–52. doi: 10.3171/2015.8.JNS151624. [DOI] [PubMed] [Google Scholar]
- 51.Jacob JT, Carlson ML, Schiefer TK, et al. Significance of cochlear dose in the radiosurgical treatment of vestibular schwannoma: controversies and unanswered questions. Neurosurgery. 2014;74(5):466–74. doi: 10.1227/NEU.0000000000000299. [DOI] [PubMed] [Google Scholar]
- 52.Akpinar B, Mousavi SH, McDowell MM, et al. Early Radiosurgery Improves Hearing Preservation in Vestibular Schwannoma Patients With Normal Hearing at the Time of Diagnosis. Int J Radiat Oncol Biol Phys. 2016;95(2):729. doi: 10.1016/j.ijrobp.2016.01.019. [DOI] [PubMed] [Google Scholar]
- 53.Matthies C, Samii M, Krebs S. Management of Vestibular Schwannomas: Radiological Features in 202 Cases. Neurosurg. 1997;40(3):469–482. doi: 10.1097/00006123-199703000-00009. [DOI] [PubMed] [Google Scholar]
- 54.Piccirillo E, Wiet MR, Flanagan S, et al. Cystic vestibular schwannoma: classification, management and facial nerve outcomes. Otol Neurotol. 2009;30(6):826–34. doi: 10.1097/MAO.0b013e3181b04e18. [DOI] [PubMed] [Google Scholar]
- 55.Pendl G, Ganz JC, Kitz K, et al. Acoustic neurinomas with macrocysts treated with gamma knife radiosurgery. Stereotact Funct Neurosurg. 1996;66:103–11. doi: 10.1159/000099775. [DOI] [PubMed] [Google Scholar]
- 56.Benech F, Perez R, Fontanella MM, et al. Cystic versus solid vestibular schwannomas: a series of 80 grade IIIYIVpatients. Neurosurg Rev. 2005;28:209–13. doi: 10.1007/s10143-005-0380-y. [DOI] [PubMed] [Google Scholar]
- 57.Delsanti C, Regis J. Cystic vestibular schwannomas. Neurochirurgie. 2004;50:401–6. [PubMed] [Google Scholar]
- 58.Goddard J, Schwartz M, Friedman R. Fundal fluid as a predictor of hearing preservation in the middle cranial fossa approach for vestibular schwannoma. Otol Neurotol. 2010;31(7):1128–1134. doi: 10.1097/MAO.0b013e3181e8fc3f. [DOI] [PubMed] [Google Scholar]
- 59.Somers T, Casselman J, deCeulaer G, et al. Prognostic value of magnetic resonance imaging findings in hearing preservation surgery for vestibular schwannoma. Otol Neurotol. 2001;22(1):87–94. doi: 10.1097/00129492-200101000-00017. [DOI] [PubMed] [Google Scholar]
- 60.Abele TA, Besachio DA, Quigley EP, et al. Diagnostic accuracy of screening MR imaging using unenhanced axial CISS and coronal T2WI for detection of small internal auditory canal lesions. AJNR. 2014 doi: 10.3174/ajnr.A4041. Published online before print July 17, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bhadelia RA, Tedesco KL, Hwang S, et al. Increased cochlear Fluid-Attenuation Inversion Recovery Signal in patients with vestibular schwannomas. AJNR. 2008;29:720–23. doi: 10.3174/ajnr.A0968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee IH, Kim HJ, Chung WH, et al. Signal intensity change of the labyrinth in patients with surgically confirmed or radiologically diagnosed vestibular schwannoma on isotropic 3D fluid-attenuated inversion recovery MR imaging at 3 T. Eur Radiol. 2010;20:949–57. doi: 10.1007/s00330-009-1626-9. [DOI] [PubMed] [Google Scholar]
- 63.Yoshida T, Sugiura M, Naganawa S, et al. Three-dimensional fluid attenuated inversion recovery magnetic resonance imaging findings and prognosis in sudden sensorineural hearing loss. Laryngoscope. 2008;118:1433–37. doi: 10.1097/MLG.0b013e318172ef85. [DOI] [PubMed] [Google Scholar]
- 64.O’Connor AF, France MW, Morrison AW. Perilymph total protein levels associated with cerebellopontine angle lesions. Am J Otol. 1981;2:193–95. [PubMed] [Google Scholar]
- 65.Kim DY, Lee JH, Sung YS, et al. Clinical significance of an increased cochlear 3D fluid-attenuated inversion recovery signal intensity on an MR imaging examination in patients with acoustic neuroma. AJNR. 2014;35:1825–1829. doi: 10.3174/ajnr.A3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamazaki M, Naganawa S, Kawai H, et al. Increased signal intensity of the cochlea on pre- and post-contrast enhanced 3D-FLAIR in patients with vestibular schwannoma. Neuroradiology. 2009;51:855–63. doi: 10.1007/s00234-009-0588-6. [DOI] [PubMed] [Google Scholar]
- 67.Lee IH, Kim HJ, Chung WH, et al. Signal intensity change of the labyrinth in patients with surgically confirmed or radiologically diagnosed vestibular schwannoma on isotropic 3D fluid-attenuated inversion recovery MR imaging at 3T. Eur Radiol. 2010;20:949–957. doi: 10.1007/s00330-009-1626-9. [DOI] [PubMed] [Google Scholar]
- 68.Committee on Hearing and Equilibrium. Committee on Hearing and Equilibrium guidelines for the evaluation of hearing preservation in acoustic neuroma. Otol Head Neck Surg. 1995;113:179–180. doi: 10.1016/S0194-5998(95)70101-X. [DOI] [PubMed] [Google Scholar]
- 69.Kanzaki J, Tos M, Sanna M, et al. New and modified reporting systems from the consensus meeting on systems for reporting results in vestibular schwannomas. Otol & Neuotol. 2003;24:642–649. doi: 10.1097/00129492-200307000-00019. [DOI] [PubMed] [Google Scholar]
- 70.Walsh RM, Bath AP, Bance ML, et al. Comparison of two radiologic methods for measuring the size and growth rate of extracanalicular vestibular schwannomas. Am J Otol. 2000;21:716–721. [PubMed] [Google Scholar]
- 71.Nagano O, Higuchi Y, Serizawa T, et al. Transient expansion of vestibular schwannoma following stereotactic radiosurgery. J Neurosurg. 2008;109:811–6. doi: 10.3171/JNS/2008/109/11/0811. [DOI] [PubMed] [Google Scholar]
- 72.Delsanti C, Roche PH, Thomassin JM, et al. Morphological changes of vestibular schwannomas after radiosurgical treatment: pitfalls and diagnosis of failure. Prog Neurol Surg. 2008;21:93–7. doi: 10.1159/000156712. [DOI] [PubMed] [Google Scholar]
- 73.Park CK, Kim DC, Park SH, et al. Microhemorrhage, a possible mechanism for cyst formation in vestibular schwannomas. J Neurosurg. 2006;105:576–80. doi: 10.3171/jns.2006.105.4.576. [DOI] [PubMed] [Google Scholar]
- 74.Warren FM, Kaylie DM, Aulino JM, et al. Magnetic resonance appearance of the inner ear after hearing-preservation surgery. Otol Neurotol. 2006;27(3):393–397. doi: 10.1097/00129492-200604000-00016. [DOI] [PubMed] [Google Scholar]