Abstract
Kynurenine pathway (KP) is the primary path of tryptophan (Trp) catabolism in most mammalian cells. The KP generates several bioactive catabolites, such as kynurenine (Kyn), kynurenic acid (KA), 3-hydroxykynurenine (3-HK), xanthurenic acid (XA), and 3-hydroxyanthranilic acid (3-HAA). Increased catabolite concentrations in serum are associated with several cardiovascular diseases (CVD), including heart disease, atherosclerosis, and endothelial dysfunction, as well as their risk factors, including hypertension, diabetes, obesity, and aging. The first catabolic step in KP is primarily controlled by indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO). Following this first step, the KP has two major branches, one branch is mediated by kynurenine 3-monooxygenase (KMO) and kynureninase (KYNU) and is responsible for the formation of 3-HK, 3-HAA, and quinolinic acid (QA); and another branch is controlled by kynurenine aminotransferase (KAT), which generates KA. Uncontrolled Trp catabolism has been demonstrated in distinct CVD, thus, understanding the underlying mechanisms by which regulates KP enzyme expression and activity is paramount. This review highlights the recent advances on the effect of KP enzyme expression and activity in different tissues on the pathological mechanisms of specific CVD, KP is an inflammatory sensor and modulator in the cardiovascular system, and KP catabolites act as the potential biomarkers for CVD initiation and progression. Moreover, the biochemical features of critical KP enzymes and principles of enzyme inhibitor development are briefly summarized, as well as the therapeutic potential of KP-enzyme inhibitors against CVD is briefly discussed.
Keywords: Tryptophan, kynurenine pathway, aortic aneurysm, atherosclerosis, aging, obesity, diabetes
Introduction
The essential amino acid tryptophan (Trp) is either used for protein synthesis or metabolized to a variety of bioactive molecules via the serotonin pathway or the kynurenine pathway (KP) which accounts for the metabolism of more than 95% of dietary Trp [1], that involves several vitamin B6- or B2-dependent enzymes (Fig. 1). KP is the major pathway for Trp catabolism in human [2, 3], animals [4, 5], and some fungi and bacteria [6, 7]. Generation of KP catabolites is triggered by the enzymes tryptophan 2,3-dioxygenase (TDO) [8], which is mainly expressed in liver and brain with high substrate specificity for L-tryptophan [9], or indoleamine-2,3-dioxygenase (IDO), the first and rate-limiting enzyme catabolizing both D- and L-tryptophan [10]. Generally, TDO mediates basal KP, whereas IDO may control KP under disease conditions [11]. IDO has two isoforms IDO1 and IDO2 [12, 13], and IDO1 is widely expressed at low levels under normal conditions and the major isoform contributing to Trp degradation [14]. IDO2 is primarily expressed in mouse liver and kidney [12], as well as human liver, spleen, placenta, kidney, and brain, but not in the heart [15]. IDO1 is inducible while IDO2 appears to be basally expressed [5]. IDO catalyzes the conversion of Trp to N-formylkynurenine, which is then catabolized to the pivotal KP intermediate kynurenine (Kyn). From Kyn, one branch of the KP leads to the formation of kynurenic acid (KA) or xanthurenic acid (XA) catalyzed by kynurenine amino-transferases (KATs, KAT I~IV), one family of the key enzymes in KP (Fig. 1); and another branch results in the biosynthesis of oxidized nicotinamide adenine dinucleotide (NAD+) via 3-hydroxykynurenine (3-HK) and quinolinic acid (QA) [16]. Within this branch, another key enzyme kynureninase (KYNU) directly catalyzes the hydrolysis of Kyn or 3-hydroxykynurenine (3-HK) to form the anthranilic acid (AA) or 3-hydroxyanthranilic acid (3-HAA), respectively [17, 18] (Fig. 1). Human KYNU presents a 20-fold higher affinity for 3-HK than for Kyn, favoring the generation of 3-HAA over AA [19]. KP is well controlled under physiological conditions but altered as part of the activated immune response. Dysregulated KP has been linked to various diseases, including neurodegenerative disorders (such as Huntington, Alzheimer, and Parkinson disease) [8], multiple sclerosis [20], depression [21, 22], schizophrenia [23, 24], diabetes [25], cancer [26, 27], and inflammatory bowel disease [28]. Emerging evidence indicates that catabolites in KP of Trp catabolism play critical roles in cardiovascular physiology and pathology [29–31], in addition to regulating immune system functions [2, 32] and inflammation [7, 33]. Here we briefly review the recent research progress on the role of disregulated KP in cardiovascular diseases (CVD) development and the regulation of CVD risk factors on KP, highlight KP functions as the inflammatory sensor and modulator in the cardiovascular system, as well as discuss some controversies and raise questions in this field.
Fig. 1.
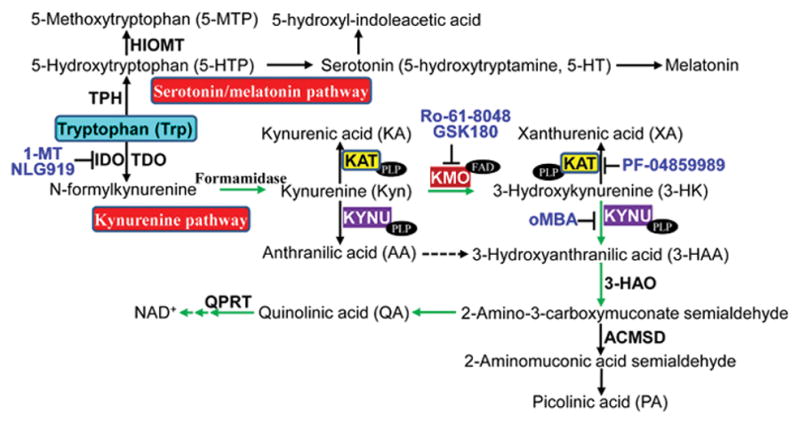
Major enzymes, catabolites, and inhibitors in tryptophan metabolism. Tryptophan is metabolized via two major pathways, kynurenine pathway and serotonin/melatonin pathway. 3-HAO, 3-hydroxyanthranilate 3,4-dioxygenase; ACMSD, 2-amino-3-carboxymuconate semialdehyde decarboxylase; FAD, flavin adenine dinucleotide; HIOMT, hydroxyindole O-methyltransferase; oMBA, o-Methoxybenzoyalanine; QPRT, quinolinate phosphoribosyltransferase; TPH, tryptophan hydroxylase. Refer to the text for the expanded form of abbreviations.
1 Role of dysregulated KP in CVD
CVD is the most common cause of death in the world. Evidence indicates that KP catabolites play important roles in cardiac pathophysiology. The expression of IDO1, the first and rate-limiting enzyme in KP, is tightly controlled and confined to certain cell types, including endothelial cells (EC) [30, 34, 35] vascular smooth muscle cells (VSMC), macrophages [36], leucocytes [37], and dendritic cells (DCs) [38], all of which are present in the artery wall. Proinflammatory modulators, such as lipopolysaccharide (LPS), tumor necrosis factor (TNF), interleukin 1 (IL-1), and IL-2, induce IDO1 expression in many cell types [39]. Notably, interferon-gamma (IFN-γ) released from activated CD4+ T cells robustly induces IDO1 expression contributing to Trp catabolism [39, 40], where Trp is converted to immunomodulating compounds, collectively termed kynurenines [41]. Epidemiologically, tryptophan catabolites, Kyn, AA, 3-HK, and XA are reported to associate with CVD mortality [42]. 3-HAA was identified as a precursor of cinnabarinic acid, a novel endogenous ligand of the transcription factor aryl hydrocarbon receptor (AHR) [43], which may contribute to cardiotoxicity, vascular inflammation, and endothelial dysfunction [44]. Here, we will discuss the effects of major catabolites in KP on the main CVD (Table 1).
Table 1.
Function and CVD-association of key catabolites in kynurenine pathway
| Catabolites | Function | Associated disease | References |
|---|---|---|---|
| 3-HAA | Activator of aryl hydrocarbon receptor (AHR), induction of Tregs from naïve CD4+ T cells and T cell death | Vascular inflammation, atherogenesis | [43–46] |
| 3-HK | ROS generator, cell apoptosis | EC dysfunction | [30, 47–49] |
| AA | Inhibits proinsulin synthesis | T1D | [50] |
| Kyn | Vasodilation, neurotoxicity and brain degeneration, endogenous ligand of AHR | Hypotension, heart failure, insulin resistance | [29, 51] [52, 53] |
| KA | Antagonist of NMDA receptor and α7 nicotinic acetylcholine receptor, antioxidant, AHR agonist, ligand for GPR35 | Ischemic post stroke, hypertension, preeclampsia, myocardial infarction, maternal hypertension, unstable atherosclerotic plaque, T2D, schizophrenia | [54–57] |
| QA | Agonist of the NMDA receptor, elevates Ca2+, ROS generator, neurotoxicity | Aging, T1D | [53] |
| XA | Induces apoptosis, inhibits BH4 synthesis | Diabetes, insulin resistance | [58, 59] |
NMDA, N-methyl-D-aspartate; ROS, reactive oxygen species. For definitions of other abbreviations, please see the main text.
1.1 KP and atherosclerosis
Atherosclerotic cardiovascular diseases including coronary heart disease and cerebrovascular disease are the most common forms of CVD. There are several reports about abnormal KP and atherogenesis. In the Tampere Vascular Study, elevated IDO expression was observed in the macrophage-rich cores of human advanced atherosclerotic plaques [36]. IDO activity in blood has a significant positive correlation with more advanced atherosclerosis in both sexes (Table 2) [60]. These data suggest that activated KP may play an important role in atherogenesis. However, treatment with KP catabolite 3-HAA for 8 weeks significantly decreases plasma cholesterol and triglyceride levels, inhibits the uptake of oxidized low-density lipoproteins (oxLDL) by macrophages, and the resultant inhibition of atherogenesis in hypercholesterolaemic low-density lipoprotein receptor-deficient (LDLr−/−) mice [61]. Furthermore, systemic IDO inhibition by 1-methyl tryptophan (1-MT) enhances vascular inflammation with upregulation of vascular cell adhesion molecule-1 (VCAM-1) and monocyte chemoattractant protein-1 (MCP-1/CCL2), and increasing CD68+ macrophage infiltration in vascular area, which leads to a significant increase in atherosclerotic lesions in the aortic arch and root of high-fat diet (HFD)-fed apolipoprotein E knockout (ApoE−/−) mice [45]. Interestingly, the increased VCAM-1 expression is not limited to the plaque but also found in VSMC of the tunica media. Moreover, IDO inhibition-induced acceleration of atherosclerosis and vascular inflammation can be reversed by exogenous administration of the Trp catabolite 3-HAA [45]. All these data strongly suggest that IDO-mediated KP catabolite 3-HAA blocks atherogenesis (Fig. 2). It will be important to investigate 3-HAA levels in plasma and local atherosclerotic areas of mice and to identify if endogenous 3-HAA can be used as a biomarker for atherosclerosis initiation and progression. Additionally, 3-HK level correlates with intima media thickness of the carotid artery [62].
Table 2.
Cardiovascular diseases and their risk factors associate with alternations of KP catabolites and enzymes.
| CVD and risk factors | Alternation of KP catabolites | Samples | KP enzymes | Samples | References |
|---|---|---|---|---|---|
| Atherosclerosis | AA↓, 3-HAA↓, KA↑ | Serum | IDO↑, IDO activity↑ | Plaque, serum | [36, 67] |
| Myocardial infarction | KA↑, 3-HK↑, AA↑, 3-HAA↑ | Plasma | IDO↑ | Plasma | [68, 69] |
| Coronary artery disease | Kyn↑, 3-HK↑ | Urine | IDO activity↑ | Urine | [70] |
| Stroke | KA↑ | Serum | Active IDO1 | Serum | [71] |
| Diabetes | KA↑, AA↑, XA↑, 3-HAA↑, Kyn↑, PLP↓ | Plasma, urine | IDO↑, KAT activity↑ | Plasma | [25, 50, 72–74] |
| Hypertension | Kyn↑, KA↓ | Rat brain | Mutation in KAT I, KYNU mRNA↑, KYNU activity↓ | Brainstem | [75–77] |
| Obesity | Kyn↑, KA↑, QA↑ | Serum | IDO1↑, KYNU↑, KMO↑, KAT III↑, | Omental adipose tissue | [78–81] |
| Aging | Kyn↑, KA↑, 3-HK↑, AA↑, QA↑ | Rat brain | IDO1↑, TDO↓ | Brain striatum, blood | [82–86] |
| Smoking (nicotine) | KA↑, PLP↓ | Brain | N/A | [87] |
Refers to the main text for the expanded form of abbreviations.
Fig. 2.
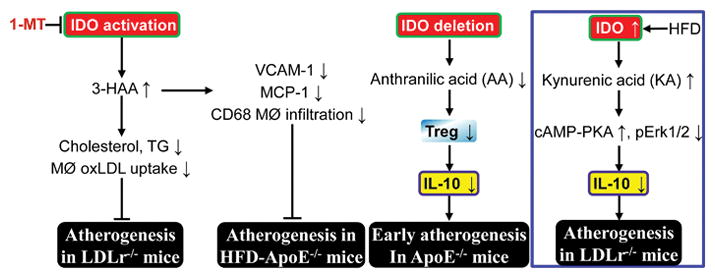
Abnormal kynurenine pathway and atherogenesis. Elevation of 3-hydroxyanthranilic acid (3-HAA) or anthranilic acid (AA) by IDO activation inhibits vascular inflammation and subsequent atherosclerosis in LDLr−/− or ApoE−/− mice. However, kynurenic acid (KA) elevation by IDO upregulation enhances vascular inflammation and exacerbates atherogenesis in LDLr−/−mice (blue box). MØ, macrophage; PKA, protein kinase A; Treg, regulatory T cell. For definitions of other abbreviations, please see the main text. ⊥, inhibits.
On the one hand, IDO exerts a protective effect against atherogenesis. It was reported that IDO1 deficiency accelerates early (at 15 weeks of age) atherosclerotic lesion formation in the aortic root of ApoE−/−mice fed with a regular chow diet, through downregulation of the anti-inflammatory cytokine IL-10 in peripheral blood, spleen, and lymph node B cells [63]. IDO1 deletion does not significantly alter serum cholesterol levels in ApoE−/−mice. However, IDO1 deletion for 15 weeks increases infiltration of CD68+ macrophages and CD4+ T cells in atherosclerotic plaque [63]. Moreover, IL-10 production in cultured splenic B cells is stimulated by 3,4,-dimethoxycinnamoyl anthranilic acid (3,4-DAA), an orally active synthetic derivative of the tryptophan catabolite AA [64], but not Kyn. Finally, 3,4-DAA treatment inhibits inflammation and lesion formation after perivascular collar-mediated arterial injury in ApoE−/−mice, and decreases cytokine and chemokine production in ex vivo human atheroma cell cultures. These data imply that compensated upregulation of IDO may play protective function from atherogenesis at an early stage via AA generation (Fig. 2). Importantly, IDO1-expressing aortic plasmacytoid dendritic cells (pDCs) protect against atherosclerosis through induction of regulatory T cells [38]. Also, IDO1 may play a critical role in maintaining plaque stability [63].
On the other hand, HFD dramatically increases IDO activity in macrophages and VSMC of aortic sinus, as well as circulating levels of KA and QA in atherosclerosis-prone LDLr−/−mice compared with chow diet. Furthermore, levels of Kyn, KA, and QA are higher in spleen extracts of LDLr−/−mice fed with HFD [65]. Interestingly, it was demonstrated that IDO1 deficiency inhibits atherogenesis in aortic root of LDLr−/−mice fed with HFD compared with LDLr−/−IDO1+/+ littermates via IL-10 upregulation in macrophages and FMS-related tyrosine kinase 3 (Flt3) ligand-induced bone-marrow-derived DCs [65]. Moreover, KP catabolite KA is responsible for the IL-10 reduction in macrophages via activation of the cAMP-dependent pathway and inhibition of Erk1/2 phosphorylation [65] (Fig. 2). However, deletion of both IDO1 and IDO2 does not alter IL-10 levels in macrophages in response to LPS + IFNγ compared to WT [66]. Additionally, high concentrations of KA in atheromatous plaques are associated with an unstable plaque phenotype in human atherosclerotic lesions, whereas there is no detection or low levels of KA in stable fibrous plaques [65]. It is of interest to test whether endogenous KA can work as the biomarker for atherosclerosis development and plaque instability.
The reason for the discrepancy of IDO1 function in atherogenesis may be due to the different animal genetic background, different cell types involved, and specific KP downstream catabolites. Nevertheless, this contradictory evidence highlights the importance of clarifying the function and the underlying mechanisms by which IDO1 expression and activation in atherogenesis. Further investigation with tissue-specific gene knockout/knockin mouse models is needed to understand the role of these particular catabolites in different cells in atherogenesis under different disease condition.
1.2 Abnormal KP and EC dysfunction
Endothelial dysfunction is featured by increased endothelium apoptosis, impaired endothelial-dependent vasomotion, and dysregulated endothelial cell activation [88, 89]. Endothelial dysfunction accounts for a significant portion of all CVD [90]. Abnormal KP is highly associated with EC dysfunction. For example, IDO-deleted pregnant mice with C57BL/6J background display preeclampsia featured by aortic endothelial dysfunction, renal structural damage (glomerular endotheliosis) and dysfunction (proteinuria) [91]. However, the catabolites of KP responsible for preeclampsia are unknown. In contrast, our group demonstrated that KP catabolite 3-HK mediates angiotensin II (Ang II)-induced EC apoptosis and subsequent endothelial dysfunction via NAD(P)H oxidase-derived superoxide anions in vivo. Whereas, IDO1 deletion blocks Ang II action on EC [30]. KP catabolite KA elicits the adhesion of neutrophils and leukocytes to vascular endothelium via G protein-coupled receptor 35 (GPR35) in an in vitro vascular flow model [92]. Thus KA may lead to abnormal EC activation and inflammation. 3-HAA inhibits the expression and activity of inducible nitric oxide synthase (iNOS) in macrophages [93]. Also, KP catabolite XA is a potent inhibitor of sepiapterin reductase (SPR) [58], the last enzyme in the de novo synthesis of tetrahydrobiopterin (BH4) which is an enzyme cofactor essential for the activation of endothelial nitric oxide synthase (eNOS). These results suggest that elevated XA arising out of up-regulated KP could attenuate BH4 biosynthesis and consequent EC dysfunction. On the other hand, dysregulated eNOS would increase O2.− production, which reacts with nitric oxide (NO) to generate peroxynitrite. Peroxynitrite then nitrates IDO at Tyr15, Tyr345, and Tyr353, and inactivates IDO. The levels of Tyr nitration and peroxynitrite inhibition on IDO activity are significantly reduced in the Tyr15-to-Phe mutant [94]. These results indicate that IDO can be nitrated and inactivated by peroxynitrite, and that nitration of IDO-Tyr15 is the most important factor for IDO inactivation. Also, another Trp metabolite, 5-methoxytryptophan (5-MTP) (not from KP) (Fig. 1) derived from endothelium inhibits LPS-induced endothelial leakage and suppresses the overexpression of proinflammatory mediators (cyclooxgenase-2 and interleukin-6) regulated by LPS- or cecal ligation and puncture [95]. Furthermore, 5-MTP treatment protects against ligation injury-induced endothelial loss and detachment, decreases endothelial expression of intercellular adhesion molecular-1 (ICAM-1) and VCAM-1, suggesting attenuation of endothelial dysfunction [96].
1.3 KP and heart diseases
Epidemiologically, elevated levels of plasma kynurenines, including KA, 3-HK, AA, and 3-HAA, can predict the increased risk of acute myocardial infarction in patients with suspected stable angina pectoris [68]. Indeed, active IDO1 and higher concentrations of several Kyn metabolites including KA have been observed in poststroke [71], where they have been associated with increased mortality and the development of poststroke cognitive impairment [97]. Recently, urine Kyn:Trp ratio (KTR), an indicator of IDO activity, was identified as a particularly strong predictor of coronary events and mortality after elective coronary angiography [70]. The human heart itself has KAT I, its catalytic features are different from human brain KAT I or KAT II, can synthesize KA from low concentrations of L-Kyn [98]. Kyn fluctuation markedly influences KA and its effect on cardiac activity. Blood KA levels can be employed to predict death and recurrent myocardial infarction in patients with coronary artery disease. Patients with coronary heart disease and depression have activated KP [69]. Additionally, cardiac arrest patients with an initial non-shockable rhythm have higher plasma levels of KP metabolites, including Kyn, KA, and 3-HAA, than those with a shockable rhythm at intensive care unit (ICU) admission. Patients with higher plasma levels of Kyn, KA, 3-HAA, and IDO activity have lower 24-h systolic arterial pressure. Patients died in the ICU have higher plasma KP metabolites (Kyn, 3-HAA, and KA) than those who survived. All these results indicate that early activation of KP after cardiac arrest is an independent predictor of survival [99]. Patients with heart failure have higher plasma Kyn level [100]. High incidence of heart calcification on the endometrium proximal to the right ventricle was reported in female IDO-deficient BALB/c mice [101]. It is warranted to identify the causative function of specific KP catabolites for the particular heart diseases and to elucidate the effects of KP catabolites on the progression of different heart diseases.
1.4 KP and abdominal aortic aneurysm (AAA)
Abdominal aortic aneurysm (AAA) is a continual expansion of the abdominal aorta, a unique vascular disease, is found in up to 9% of men aged >65 years [102], yet usually remains asymptomatic until it ruptures. Disruption of an AAA and its associated catastrophic physiological insult carries overall mortality more than 80%, and 2% of all deaths are AAA-associated [103]. Currently, there are no therapeutic strategies proven to block AAA progression and rupture, making endovascular or open surgical repair as the solely available approach [103]. Pathologically, AAA is highly associated with inflammation [104–107], smooth muscle cell apoptosis [108], and matrix degradation [109]. However, there is a paucity of knowledge on the mechanisms and factors controlling AAA growth at early and middle–stage [110]. Currently, there is no direct evidence to demonstrate that KP exerts its role in AAA development. Ang II, a principal mediator for the initiation and progression of AAA [111], strongly induces IDO expression in mouse aorta in vivo [30], suggesting that activated KP may play an important role in AAA development. Importantly, IFN-γ, a T cell-derived cytokine, which is elevated in Ang II-treated mice or AAA patients [112], induces IDO1 expression in human VSMC with allogeneic T cell inhibition in vitro and in vivo [40]. IFN-γ-induced IDO1 expression and activity in cultured human VSMC is substantially stronger than in endothelial cells (EC) or T cells [40]. Inhibition of IDO by 1-MT enhances medial vessel infiltration of allogeneic T cells and VSMC loss [40], which may associate with AAA development. Furthermore, aging, a key risk factor for AAA formation in humans [113] and animal model, increases IDO1 mRNA and activity in brain striatum of YAC128 mouse model [82]. It is well known that obesity is an independent risk factor for AAA formation in human [114] and animal model [115]. Obese adults have strikingly increased Kyn serum levels and dramatically enhanced Kyn/Trp ratio (IDO1 activity), suggesting obesity activates IDO1 [78]. All these AAA risk factors explicitly activate IDO1, suggesting that activated KP may play a critical role in AAA initiation and development. Clinically, the level of plasma C-reactive protein (CRP), a critical biomarker for AAA incidence [116–118], is strongly correlated positively with Kyn, 3-HK, and 3-HAA, while negatively corrected with XA [42]. Lastly, matrix metalloproteinases (MMPs) exert critical roles in AAA initiation and progression [119, 120]. Kyn increases MMP-1 and MMP-3 expression in dermal fibroblasts via MEK-Erk1/2 signaling pathway [121] or AHR activation [122]. Genetic and pharmacological approaches implicate the Erk1/2 pathway as a critical regulator of CCN3-dependent AAA development [123]. These results imply that catabolites from the activated KP may play a causative role in AAA formation. However, there is a missing link between the central enzymes of KP, dysregulated KP catabolites, and aortic aneurysm formation.
2. Abnormal KP and risk factors for CVD
2.1 KP metabolites and blood pressure
Hypertension is a critical risk factor for CVD. Both Trp and Kyn have been shown to dilate pre-constricted porcine coronary arteries and mouse or rabbit aorta in a dose-dependent manner [29]. The dilating effect of Trp requires the presence of active IDO and an intact endothelium, and the effect of Kyn is endothelium-independent. Kyn-induced arterial relaxation is mediated by activation of the adenylate cyclase and soluble guanylate cyclase pathways. Intravenous administration of Kyn transiently and dose-dependently decreases the mean blood pressure in spontaneously hypertensive rats in vivo [29]. Also, IDO1 is expressed in EC of resistance vessels and is a potential novel contributor to hypotension in human sepsis [51]. Thus, IDO-mediated Trp catabolites might be implicated in the regulation of the vascular tone [29]. Pharmacological inhibition of IDO by 1-MT increases blood pressure in mice but not in mice deficient in IDO1 [29]. IDO deletion deteriorates hypoxia-induced pulmonary hypertension (PH) in mice [35]. In contrast, overexpression of IDO in endothelium protects against hypoxia-induced PH in mice and monocrotaline-induced PH in rats, as well as right ventricular hypertrophy and vascular remodeling [35]. Preeclampsia, a pregnancy-specific disease of maternal hypertension, is associated with decreased IDO activity and mRNA level in placentas [124]. Moreover, elevated maternal plasma KA levels in early pregnancy (at gestational week 18) are associated with a substantially increased risk of preeclampsia in obese women [56]. However, KP is less active in preeclampsia. Clinically, median Kyn levels are higher in hypertensive than in normotensive patients (Table 2) [68]. Also, renovascular hypertensive Wistar male rats induced by surgical clips on the left renal artery have higher plasma levels of Kyn, KA, 3-HK, and AA, as well as higher TDO activity in liver than sham rats, suggesting renovascular hypertension is associated with KP activation [125].
The levels of a key catabolite KA in different regions of the brain of spontaneously hypertensive rats are dramatically lower than those of normotensive rats [75]. Injection of KA, an antagonist of the α7 nicotinic receptor in the central nervous system, decreases blood pressure in spontaneously hypertensive rats [76]. Moreover, a missense mutation in KAT I, an enzyme that metabolizes Kyn to KA, has been identified in spontaneously hypertensive rats [77], suggesting that the mutation might inhibit KAT I activity. Additionally, KYNU catabolizing Kyn to AA or 3-HAA also controls KA level (Fig. 1). Interestingly, elevated KYNU mRNA levels were detected in the brainstem of spontaneously hypertensive rats compared with normotensive controls [126]. Furthermore, KYNU Arg188Gln mutation is linked to lower KYNU activity and essential hypertension (higher systolic and diastolic blood pressure) in the Han Chinese population [127]. All these data indicate that KA may have an anti-hypertension function.
2.2 Obesity and KP
Obesity is recognized as an independent predictor for CVD [128]. However, experimental data on potential effects of kynurenines on obesity are limited. Clinically, obese adults have dramatically elevated Kyn serum levels and significantly increased Kyn/Trp ratio (IDO activity), suggesting obesity activates IDO [78, 129]. Furthermore, IDO activity is positively associated with body mass index (BMI), fat mass, visceral or subcutaneous adipose tissue area, and subcutaneous adipocyte size [129]. Circulating Kyn levels are positively related to BMI and a higher HOMA2-IR insulin resistance index in the D.E.S.I.R. cohort (data from an Epidemiological Study on the Insulin Resistance Syndrome). The serum levels of Kyn, KA, and QA are associated with higher BMI. The mRNA expression of several KP enzyme genes, including IDO1, KYNU, KMO, and KAT III (CCBL2) is increased in the omental adipose tissue of obese women in ABOS (Biological Atlas of Severe Obesity, ClinicalGov NCT01129297) cohort compared to lean [81] (Table 2). The mRNA expression of IDO1, KYNU, and KAT III in human primary adipocytes is induced by proinflammatory cytokines. However, KMO is not expressed in human primary adipocytes. Moreover, the mRNA level of IDO1, KYNU, KMO, and KAT III is higher in proinflammatory than in anti-inflammatory macrophages [81]. A correlation of Kyn, KA, and QA with BMI and HOMA-IR is presented in another cohort [80]. Several KP enzymes, including IDO1, but neither IDO2 nor TDO2 is known to be more expressed in the omental and subcutaneous adipose tissues and liver of women with severe obesity compared to lean individuals [79]. This increase is due to the presence of pro-inflammatory T-cells in the adipose tissue and also comes from the adipocyte response to inflammatory stimuli. Furthermore, the ratio of Kyn to Trp (IDO1 activity) in obese patients is higher than that in lean subjects [79]. Also, it was observed that the serum levels of Kyn and Kyn over Trp ratio are higher in women with higher BMI and they both decrease one year after body weight loss induced by bariatric surgery [130]. Importantly, mice deficient in IDO1 are resistant to Western diet-induced obesity [131]. Mechanistically, KP catabolite Kyn, but not KA activates AHR-mediated luciferase expression in mouse hepatocyte cell, suggesting that IDO1-produced Kyn leads to obesity via AHR pathway [131].
3-HAA treatment to LDLr−/− mice markedly reduces plasma cholesterol and triglyceride concentration, as well as inhibits the oxLDL uptake by macrophages. The reduction in total cholesterol may be due to the signaling modulation by peroxisome proliferator-activated receptors [61]. In another experiment, LDLr−/−/IDO−/− mice demonstrated a remarkable induction of serum lipids, particularly triglycerides [101]. However, the underlying mechanism by which KP catabolites regulate lipid metabolism remains elusive.
Upregulated IDO1 in both adipose tissues and liver is inversely correlated with arterial blood pressure, suggesting IDO1 upregulation may represent a local compensatory mechanism to inhibit obesity-induced hypertension [79]. Further research is needed to investigate whether IDO1 play a causative role in obesity-related hypertension. Interestingly, periaortic fat from rat thoracic aorta has strong staining for IDO and the brown fat marker uncoupling protein-1 (UCP1), while the white fat around rat mesenteric artery or abdominal aorta has weak staining for IDO and UCP1 [132]. Moreover, rat perithoracic aortic fat has higher IDO activity than visceral and mesenteric artery fat. Periadventitial fat inhibits Ang II-induced rat thoracic aortic and mesenteric artery maximal contraction. However, periadventitial fat does not blunt agonist (phenylephrine)-induced contraction of the abdominal aorta. The IDO inhibitor 1-MT blocks the fat-induced inhibition of Ang II-mediated contraction in the thoracic aorta but not in the mesenteric artery. The IDO product Kyn relaxes the rat thoracic aorta at high concentrations (9 mM), whereas the downstream catabolite QA relaxes the contracted rat thoracic aorta (approximately 80%) at a relatively lower concentration (1 mM). All these data imply that IDO is active primarily in the brown fat surrounding the thoracic aorta and inhibits aortic contractility [132]. It is interesting to identify catabolites from KP in the perivascular adipose tissue which control blood vessel contraction and relaxation. Nevertheless, a causal function has yet to be demonstrated in any of these conditions.
2.3 KP and Diabetes
Diabetes mellitus is a major risk factor for CVD which is the principal cause of death in more than 70% of type 2 diabetic patients [133]. Despite advances in our understanding of some early markers of CVD, the mechanisms underlying the increased risk of CVD in diabetes remain incompletely delineated. Recently, there are several reports on upregulated KP in type 1 (T1D) or type 2 diabetes (T2D), as well as the diabetic cardiovascular complication. Clinically, T1D, but not T2D has higher concentrations of plasma AA (2.3 fold induction) compared with non-diabetic subjects [50]. However, the cause and consequence of AA elevation in T1D are unknown. The underlying mechanism may be the individual kynurenines inhibit proinsuline synthesis from pancreatic islets in rat models [64] or generate complexes with insulin decreasing its biological activity [134]. Elevated plasma KA and XA are observed in both T1D and T2D compared with non-diabetic persons [25]. TDO inactive mutation attenuates high sucrose diet-induced insulin resistance (IR) in Drosophila, suggesting upregulated KP mediates high sucrose diet-induced IR [135]. Levels of kynurenine-modified serum proteins (KMSP) are significantly higher in diabetic patients and particularly in type 2 diabetic patients with CVD. Moreover, KMSP level is only associated with the onset of diabetes and might participate in CVD development [136]. However, the role of KMSP in diabetes and diabetic cardiovascular complication remains elusive.
Some catabolites of the KP have been proposed to be risk factors for the IR development. Surplus dietary Trp induces IR in pigs [137]. Diabetic retinopathy patients have elevated serum Kyn [72]. mRNA expression of KAT I and KAT II is modestly reduced in skeletal muscle from type 2 diabetic patients compared with that in normal glucose tolerant humans [73]. Exercise enhances KAT activity [73] may through upregulation of skeletal muscle KATs mediated by PGC-1α1 [22]. As expected, bariatric surgery is associated with the improvement and even the remission of T2D. It was shown that higher levels of KA and QA one year after the bariatric surgery are associated respectively with T2D mitigation and better glucose homeostasis and that lower levels of XA are associated with better glucose homeostasis [130]. Recently, it was demonstrated that serum Kyn concentration in chronic hepatitis C virus (HCV) patients is highly associated with HOMA-IR and HOMA-beta scores [136], suggesting that Kyn may contribute to higher incidence of IR in HCV patients [53].
2.4 Aging and abnormal KP
Aging is a critical risk factor for CVD. Importantly, aging increases IDO1 mRNA and activity in brain striatum of YAC128 mouse model [82]. Upregulation of KP in aging may be due to the activation of IDO by age-related chronic inflammation [138]. Depletion of TDO-2 (homology to human TDO) in Caenorhabditis elegans suppresses the toxicity of age-related aggregation-prone proteins, including α-synuclein, β-amyloid, and polyglutamine proteins, as well as extends life span by increasing Trp levels [139]. Kyn increases with age apparently [83]. Kyn levels and IDO1 activity are highly associated with aging [84]. Moreover, KA, 3-HK, and AA are associated positively with age [68]. XA treatment induces mitochondria damage, cytochrome C release in VSMC, consequent activation of caspase-3 and -9, and resultant cellular apoptosis [59]. Further investigations are warranted to clarify the causal or resultant roles of KP catabolites on age-related CVD.
2.5 Smoking and KP
16% higher XA serum concentrations are presented in heavy drinkers than never or occasional drinkers [140]. There is no substantial change in Kyn metabolites was observed among smokers [140], although smoking is associated with considerably lower levels of PLP, biologically active form of vitamin B6 [141], a cofactor for KATs and KYNU. KA, an endogenous antagonist for NMDA and α7 nicotinic acetylcholine receptor, is dramatically increased in rat hippocampus, striatum, and frontal cortex, but not in the serum by prolonged (for 10 days) subcutaneous injections or osmotic minipumps of nicotine. Nicotine has no effect on Kyn in brain or blood [87]. There is no report about the effect of smoking on KP and its function in CVD.
3 Therapeutic implications for abnormal KP-associated CVD
Dysregulation of KP of tryptophan catabolism has been correlated with several cardiovascular diseases. Investigation quantifying KP catabolites in local tissue, plasma, and urine samples from patients diagnosed with CVD or its risk factors has presented associations between kynurenines concentrations and pathology (Table 2). Inflammatory condition usually upregulates IDO1, KMO, and KYNU. Notably, 3,4-DAA, a synthetic derivative of the tryptophan catabolite AA, as well as 3-HAA play an anti-inflammatory function in the vascular system and protect from atherosclerosis [45, 63]. However, catabolite KA exerts a pro-inflammatory role [92] and enhances atherogenesis [65]. Taken together, KP activation might be implicated in inflammation-related CVD, such as atherosclerosis, AAA, and endothelial dysfunction. Indeed, the inflammation-responsive and stress-reactive feature of KP enzymes has promoted biomarkers development and brought into focusing several promising therapeutic targets of relevance across a broad range of CVD and cancer [9]. Animal studies have demonstrated that KP enzyme inhibition, especially involving IDO1, KMO, KYNU, and KAT II, can serve as the viable drug targets for treating cardiovascular diseases. Pharmacological manipulation of the KP enzymes employing structure-based drug design has, therefore, become an attractive area of drug development. Here, we briefly summarize the biochemical features of key KP enzymes and principles of inhibitors development.
3.1 IDO regulation
IDO is expressed unremarkably in most tissue but highly active in placenta required for the maternal immune suppression of T cells to prevent rejection of fetus [142]. Usually, IDO is silent under physiological condition, but strongly induced by inflammatory mediators under disease state. IDO1 induction in macrophages and VSMC by HFD contributes to the exacerbated atherosclerosis [65]. However, deletion of IDO1-positive pDCs enhances atherosclerosis in LDLr−/−mice, while aortic IDO1-positive pDCs prevents atherosclerosis [38]. Also, IDO1 deletion accelerates early atherogenesis in aortic root and promotes plaque destabilization of ApoE−/−mice [63]. IDO inhibition by 1-MT promotes atherosclerosis in ApoE−/−mice [45]. Thus, IDO demonstrates the greatest potential as the druggable target for CVD treatment. Three major kinds of small-molecule IDO inhibitors have been used for cancer treatment in clinical trials (Table 3). For example, 1-MT (referred to as Indoximod) is the first and widely used competitive inhibitor of IDO1 [143], 1-MT is being used as cancer immunotherapy combined with Docetaxel or Paclitaxel chemotherapy in phase II clinical trials for metastatic breast cancer [144]. There is another phase I/II trial using Indoximod (overcoming tumor-induced immune suppression) in combination with Tergenpumatucel-L (activating human immune system) immunotherapy and Docetaxel to treat subjects with advanced non-small cell lung cancer (NSCLC) [145]. 1-MT was also used for atherosclerotic research in mouse [45]. Another IDO1 inhibitor, 4-amino-1,2,5-oxadizaole-3-carboximidamide (INCB024360, also named Epacadostat) was in phase II clinical trial for patients with myelodysplastic syndromes [146]. NLG919 (an imidazoleisoindole derivative), a potent direct IDO1 inhibitor (Ki = 7 nM) [147], is currently in phase I clinical trials for adult patients with recurrent advanced solid tumors [148]. In addition, peptide-based IDO1-targeting vaccines were employed in phase I trial for stage III-IV non-small-cell lung cancer patients [149]. However, there is no clinical trial with IDO inhibitors for CVD treatment.
Table 3.
Inhibitors of targeted KP enzymes
| Targeted enzymes | Inhibitors | Ki | IC50 | Testing systems | Outcomes | Ref. |
|---|---|---|---|---|---|---|
| IDO1 | 1-Methyl D-tryptophan | 34 μM | 120 μM (HeLa) | Rabbit small intestinal IDO | Anti-tumor | [143, 150] |
| INCB024360 | 10 nM | 72 nM (IDO1), 7.1 nM (HeLa) | Tumor cells | Anti-tumor | [150, 151] | |
| NLG919 | 7 nM | 75 nM | Effector T cells | Immune activation and tumor regression | [152, 153] | |
| KMO | GSK180 | N/A | 6 nM | Mouse model of acute pancreatitis | Anti-inflammation | [154] |
| Ro-61-8048 | 4.88 nM | 37 nM | Rat neuropathic pain model | Decrease neuropathic pain | [155] | |
| UPF-648 | 56.7 nM | 20 nM | Human KMO | Neuroprotection | [156] | |
| KYNU | o-Methoxybenzoyalanine | 4 μM | N/A | Rat | Sedation | [157] |
| 2-Amino-4-[3′-hydroxyphenyl]-4-hydroxybutanoic acid | 100 nM | N/A | Recombinant Human KYNU | N/A | [158] | |
| S-phenyl-L-cysteine sulfoxide | 140 μM | N/A | Recombinant KYNU of P. aeruginosa | Potential antivirulence | [159] | |
| KAT II | PF-04859989 | N/A | 23 nM | Rat brain | Anti-schizophrenia | [160] |
| BFF 122 | N/A | 1 μM | Rat brain | Cognitive enhancement | [161, 162] |
Ki, inhibition constant; N/A, not available. For definitions of the abbreviations, please see the main text.
The novel and potent IDO1 (EC 1.13.11.52) inhibitors are being developed intensively [163]. IDO1 is a monomeric, redox-sensitive, heme-containing dioxygenase enzyme, making that possible to develop competitive inhibitor binding to the heme active site [164]. As crystal structures of IDO1 [165], IDO1 bound with an inhibitor [147, 166], and IDO1 bound with substrate [167] were revealed, it is indicated that occupation of both hydrophobic pockets A and B, as well as heme ion-atom in IDO1, is critical for IDO1 inhibition [147]. Furthermore, the JK loop (a 20-amino acid protein segment) in human IDO1 connecting the J and K helices undergoes structural rearrangement upon substrate binding. JK loop N-terminal (JK loopN) may function as a target for post-translational modifications such as phosphorylation or a mediator for protein-protein interactions. The residue Thr379 in highly conversed “GTGG” motif of JK loop C-terminal (JK loopC) is crucial for hydrogen bonding interactions between substrate Trp and IDO enzyme, highlighting the importance of JK loop conformation in regulating the enzyme activity. Interestingly, Tyr353 nearby helix J can be nitrated upon exposure to peroxynitrite, resulting in IDO inhibition [168]. The structure-activity relationships (SAR) analysis by molecular docking technologies and pharmacophore models indicates that 1H-indazole scaffold is a novel key pharmacophore and necessary for potent IDO1 inhibition [169]. Recently, rational drug design demonstrates that O-alkylhydroxylamines function as IDO1 inhibitors by binding heme iron with nanomolar-level cell-based potency and limited toxicity [170]. Finally, IDO2 is clearly different from IDO1 regarding substrate specificity, affinity, kinetic parameters, and inhibition profiles [171, 172]. IDO2, but not IDO1, generates a distinct signal for activation of transcription factor liver-enriched inhibitory protein (LIP) that is Trp-restoration independent [15]. All the results suggest it is necessary to develop specific inhibitors of these enzymes. For example, D-1MT but not L-1MT strongly inhibits IDO2 but not IDO1 in overexpression T-REx cells system [15]. However, we are far from understanding the profile of IDO expression and activation during CVD initiation and development. A better understanding of IDO biology is needed to comprehend the effect of IDO inhibitors and to provide a rationale for their therapeutic application in CVD. As a note, no IDO1 inhibitor is currently being used in clinical trial for CVD treatment.
3.2 Kynurenine monooxygenase (KMO) inhibition
KMO (EC 1.14.13.9, also is referred to as kynurenine 3-hydroxylase), a pivotal enzyme in KP [4], is a flavin adenine dinucleotide (FAD)-dependent monooxygenase locating in the outer mitochondrial membrane where it catalyzes Kyn to 3-HK [156]. KMO is expressed in kidney, liver, and brain, as well as macrophages and monocytes. A crystal structure of truncated yeast KMO has been reported [156]. KMO inhibitors are being researched as potential treatments for neurodegenerative diseases, such as Alzheimer’s, Huntington’s, and Parkinson’s diseases [162, 173, 174]. There have been no reports or detailed studies on KMO inhibition for CVD in animal or clinical trial. Reported KMO inhibitors contain an aryl ring appropriately spaced from an acidic functionally or bioisostere [175]. The most well-known and potent KMO inhibitor is Ro-61-8048, which decreases neuropathic pain intensity induced by chronic constriction injury and potentiates the analgesic properties of morphine [176]. In addition, KMO is central to the pathogenesis of acute-pancreatitis-multiple organ dysfunction syndrome (MODS). KMO inhibition by genetic deletion or a potent and specific inhibitor, oxazolidinone GSK180 (3-(5,6-dichloro-2-oxobenzo[d]oxazol-3(2H)-yl)propanoic acid), prevents extrapancreatic tissue injury to the lung, kidney and liver in mouse model of acute-pancreatitis (Table 3), may through upregulation of Kyn, KA, and AA, or downregulation of 3-HK and 3-HAA [154]. Therefore, KMO inhibition may prevent inflammation-related CVD including atherosclerosis and AAA. It is warranted to test the currently available KMO inhibitors for CVD treatment in animal and clinical trial.
3.3 Kynureninase (KYNU) inhibition
Eukaryotic kynureninase (KYNU, EC 3.7.1.3) preferentially catalyzes the conversion of 3-hydroxy-L-kynurenine rather than L-kynurenine to 3-hydroxyanthranilic acid than anthranilic acid, as well as L-alanine [177]. KYNU is a cytoplasmic enzyme with 465 amino acids and 52 kDa, one member of the aminotransferase superfamily, contains PLP as the cofactor [178]. A conserved lysine residue Lys276 was identified to be the critical residue for cofactor covalent-binding within the pyridoxal-p-binding site consensus sequence. The reaction specificity of KYNU is determined in part by active site residues, Trp64, Gly281, and Thr282 in P. fluorescens, and the homologous His102, Ser332, and Asn333 in human KYNU. Asn333 can form a hydrogen bond to the 3-OH of 3-HK in the human enzyme [19, 178]. These results may help to develop the selective inhibitor for KYNU. A structural analogue of the reduced form of substrate 3-HK, 2-amino-4-[3’-hydroxyphenyl]-4-hydroxybutanoic acid, is the most potent and reversible inhibitor of KYNU from both rat and human, with a Ki value of 100 nM [158]. Interestingly, removal of the aryl amino group coupled with a reduction of the carbonyl group at position 7 of the alanine side chain greatly enhances the potency of the inhibitor [158]. Importantly, rats treated with KYNU inhibitor, o-methoxybenzoylalanine (oMBA) (50–400 mg/kg i.p.) have increased Kyn and KA levels in the brain, blood, liver, and kidney, associating with a decreased locomotor activity [157]. Recently, another structural analogue of Kyn, S-phenyl-L-cysteine sulfoxide, was shown to competitively inhibit P. aeruginosa KYNU activity in vitro, suggesting a potential antivirulence strategy [159]. Since KYNU directly controls 3-HAA formation, which regulates cardiovascular cells inflammation and apoptosis, it would be interesting to exam the effect of KYNU inhibition on CVD initiation and development.
3.4 Kynurenine aminotransferases (KATs) inhibition
KATs are homodimeric PLP-dependent enzymes that mediate the irreversible and permanent transamination of Kyn to KA, a known neuroactive agent, as well as 3-HK to XA [179] (Fig. 1). Evidence suggests that abnormal levels of KA are implicated in many neurodegenerative diseases such as Parkinson’s disease, Huntington’s disease, Alzheimer’s disease and schizophrenia [179, 180]. Four isoforms (KAT I to IV) of this enzyme have been identified [9]. Human KAT I and KAT III have the highest sequence identity (51.7%) [179]. Human KAT I is located in the heart, it more efficiently catalyzes the transamination of hydrophobic amino acids, such as Trp. It is not inhibited by glutamine [98]. KAT II seems to be the major isoform contributing to KA formation (75%) in human brain [179]. However, KAT II is undetectable in murine heart and skeletal muscle [22]. Thus, KAT II inhibitors have been proposed as new therapeutic approaches for psychiatric and cognitive disorders [181]. Human KAT III shares 30.1% similarity of amino acids to that of KAT II and has higher mRNA expression levels in heart, liver, and kidney [182]. KAT IV is identical with glutamic-oxaloacetic transaminase 2 or mitochondrial aspartate aminotransferase [183]. It plays a major role in KA formation in mouse brain [184]. KAT (I, II, and III) protein levels in mouse gastrocnemius muscle can be induced by exercise, but not chronic mild stress [22]. Importantly, exercise training increase KATs (I, II, III, and IV) mRNA and protein levels in human vastus lateralis skeletal muscle [22, 185]. KATs are insensitive to minerals changes, but sensitive to dietary vitamin B6, which is sequestered by an intricate hydrogen bond networks and covalently binds to the conserved catalytic Lys263 to form a reactive Schiff base [186]. Thus, Lys263 in the active site of KAT II is very important for KAT II activity. Recently, the three-dimensional (3D) crystal structure of the PLP form of hKAT II at 1.83 Å resolution was reported [180, 187]. Several KAT II inhibitors have been designed to interact with Kyn-PLP imine adduct and function as irreversible inhibitors [9]. For example, Pfizer developed cyclic hydroxamic acid PF-04859989 (KAT II IC50 =23 nM) which forms an irreversible covalent bond with the enzyme cofactor PLP in the active site, leading to a highly potent complex [188]. The hydroxyl and keto moieties in the quinolone core of PF-04859989 make two critical hydrogen bonds with Asn202 and Arg399 in hKAT II. PF-04859989 is a brain penetrant and irreversible inhibitor of human and rat KAT II, and it is highly selective for KAT II versus KAT I, III, and IV isoforms [160]. KATs inhibition may be a novel therapeutic strategy for treating preeclampsia and other CVD. However, the expression profile and pathophysiological function of KATs in cardiovascular system remain unknown. There are not many in vivo studies presented for hAKT II inhibitor. There is no KAT III or KAT IV inhibitor was reported to date.
4 Conclusions and perspectives
It is still unclear whether endogenous kynurenines directly contribute to the initiation or promotion of CVD development, although emerging evidence suggests that Trp metabolism has diverse and complex effects on the cardiovascular outcomes in human. Gaining a better understanding of cell type specificity and the mechanisms of regulating KP enzyme expression/activities is extremely important because they generate and determine the balance of different metabolites. It is critical to eemploy pharmacological or genetic strategy with a gain of function and loss of function to clarify the causal or consequent roles of different KP catabolites in identical cells-associated typical CVD. As mentioned above, the manipulation of KP metabolism through the administration of enzyme inhibitors can serve as a novel therapeutic strategy for several CVD and their risk factors and the development of specific KP enzyme inhibitors is desirable. However, this manipulation should be controlled precisely by monitoring kynurenines levels to identify new therapeutic targets and biomarkers and assess the normalization of KP imbalances. Numerous medicinal chemistry studies are currently aimed at the design of novel, potent, and selective inhibitors for each of these aforementioned enzymes. Taken together, to target the pathway effectively, it is essential to better understand: 1, the effects of KP metabolites on initiation and progression of CVD in vivo; 2, controlling of particular KP enzymes on the levels of the bioactive metabolites in vivo; 3, unique features and regulation of KP enzymes under physiological and CVD pathological conditions; 4, structure-activity relationship to develop the specific and potent inhibitors or activators for KP enzymes, as well as metabolites analogue to mimic or antagonize KP intermediates’ function in vitro and in vivo. All deep investigation on KP would accelerate our understanding of KP in the prevention, early detection (biomarkers developing), treatment, and cure of CVD, and benefit the personalized and precision cardiovascular medicine.
Acknowledgments
This study was supported by funding from the following agencies: National Institutes of Health RO1 (HL132500, HL128014, HL110488, HL080499, HL089920, AG047776, and CA213022). This work is in part supported by the Georgia Research Alliance. Dr. Zou is a Georgia Research Alliance Eminent Scholar in Molecular Medicine.
Abbreviations
- 1-MT
1-methyl tryptophan
- 3-HAA
3-hydroxyanthranilic acid
- 3-HK
3-hydroxykynurenine
- AA
anthranilic acid
- AAA
abdominal aortic aneurysm
- AHR
aryl hydrocarbon receptor
- Ang II
angiotensin II
- DCs
dendritic cells
- HFD
high-fat diet
- IDO1
indoleamine-2,3-dioxygenase 1
- IFN-γ
interferon-gamma
- IR
insulin resistance
- KA
kynurenic acid
- KAT
kynurenine amino-transferase
- KMO
kynurenine-3-monooxygenase
- KYNU
kynureninase
- KP
kynurenine pathway
- Kyn
Kynurenine
- MMPs
matrix metalloproteinases
- oxLDL
oxidized low density lipoprotein
- pDCs
plasmacytoid dendritic cells
- PLP
pyridoxal 5′-phosphate
- QA
quinolinic acid
- TDO
tryptophan 2,3-dioxygenase
- Trp
tryptophan
- VCAM-1
vascular cell adhesion molecule-1
- VSMC
vascular smooth muscle cell
- XA
xanthurenic acid
Footnotes
Disclosures
The authors declare that there are no conflicts of interest.
References
- 1.Polyzos KA, Ketelhuth DF. The role of the kynurenine pathway of tryptophan metabolism in cardiovascular disease. An emerging field. Hamostaseologie. 2015;35:128–136. doi: 10.5482/HAMO-14-10-0052. [DOI] [PubMed] [Google Scholar]
- 2.Jones SP, Franco NF, Varney B, Sundaram G, Brown DA, de Bie J, Lim CK, Guillemin GJ, Brew BJ. Expression of the Kynurenine Pathway in Human Peripheral Blood Mononuclear Cells: Implications for Inflammatory and Neurodegenerative Disease. PLoS One. 2015;10:e0131389. doi: 10.1371/journal.pone.0131389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guillemin GJ, Cullen KM, Lim CK, Smythe GA, Garner B, Kapoor V, Takikawa O, Brew BJ. Characterization of the kynurenine pathway in human neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:12884–12892. doi: 10.1523/JNEUROSCI.4101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giorgini F, Huang SY, Sathyasaikumar KV, Notarangelo FM, Thomas MA, Tararina M, Wu HQ, Schwarcz R, Muchowski PJ. Targeted deletion of kynurenine 3-monooxygenase in mice: a new tool for studying kynurenine pathway metabolism in periphery and brain. J Biol Chem. 2013;288:36554–36566. doi: 10.1074/jbc.M113.503813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prendergast GC, Metz R, Muller AJ, Merlo LM, Mandik-Nayak L. IDO2 in Immunomodulation and Autoimmune Disease. Front Immunol. 2014;5:585. doi: 10.3389/fimmu.2014.00585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Phillips RS. Structure, mechanism, and substrate specificity of kynureninase. Biochim Biophys Acta. 2011;1814:1481–1488. doi: 10.1016/j.bbapap.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell BM, Charych E, Lee AW, Moller T. Kynurenines in CNS disease: regulation by inflammatory cytokines. Front Neurosci. 2014;8:12. doi: 10.3389/fnins.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breda C, Sathyasaikumar KV, Sograte Idrissi S, Notarangelo FM, Estranero JG, Moore GG, Green EW, Kyriacou CP, Schwarcz R, Giorgini F. Tryptophan-2,3-dioxygenase (TDO) inhibition ameliorates neurodegeneration by modulation of kynurenine pathway metabolites. Proc Natl Acad Sci U S A. 2016;113:5435–5440. doi: 10.1073/pnas.1604453113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dounay AB, Tuttle JB, Verhoest PR. Challenges and Opportunities in the Discovery of New Therapeutics Targeting the Kynurenine Pathway. Journal of medicinal chemistry. 2015;58:8762–8782. doi: 10.1021/acs.jmedchem.5b00461. [DOI] [PubMed] [Google Scholar]
- 10.Platten M, Wick W, Van den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer Res. 2012;72:5435–5440. doi: 10.1158/0008-5472.CAN-12-0569. [DOI] [PubMed] [Google Scholar]
- 11.Larkin PB, Sathyasaikumar KV, Notarangelo FM, Funakoshi H, Nakamura T, Schwarcz R, Muchowski PJ. Tryptophan 2,3-dioxygenase and indoleamine 2,3-dioxygenase 1 make separate, tissue-specific contributions to basal and inflammation-induced kynurenine pathway metabolism in mice. Biochim Biophys Acta. 2016;1860:2345–2354. doi: 10.1016/j.bbagen.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ball HJ, Yuasa HJ, Austin CJ, Weiser S, Hunt NH. Indoleamine 2,3-dioxygenase-2; a new enzyme in the kynurenine pathway. The international journal of biochemistry & cell biology. 2009;41:467–471. doi: 10.1016/j.biocel.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Fukunaga M, Yamamoto Y, Kawasoe M, Arioka Y, Murakami Y, Hoshi M, Saito K. Studies on tissue and cellular distribution of indoleamine 2,3-dioxygenase 2: the absence of IDO1 upregulates IDO2 expression in the epididymis. J Histochem Cytochem. 2012;60:854–860. doi: 10.1369/0022155412458926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lob S, Konigsrainer A, Zieker D, Brucher BL, Rammensee HG, Opelz G, Terness P. IDO1 and IDO2 are expressed in human tumors: levo- but not dextro-1-methyl tryptophan inhibits tryptophan catabolism. Cancer immunology, immunotherapy : CII. 2009;58:153–157. doi: 10.1007/s00262-008-0513-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Metz R, Duhadaway JB, Kamasani U, Laury-Kleintop L, Muller AJ, Prendergast GC. Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan. Cancer Res. 2007;67:7082–7087. doi: 10.1158/0008-5472.CAN-07-1872. [DOI] [PubMed] [Google Scholar]
- 16.Fujigaki H, Yamamoto Y, Saito K. L-Tryptophan-kynurenine pathway enzymes are therapeutic target for neuropsychiatric diseases: Focus on cell type differences. Neuropharmacology. 2017;112:264–274. doi: 10.1016/j.neuropharm.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matino D, Gargaro M, Santagostino E, Di Minno MN, Castaman G, Morfini M, Rocino A, Mancuso ME, Di Minno G, Coppola A, Talesa VN, Volpi C, Vacca C, Orabona C, Iannitti R, Mazzucconi MG, Santoro C, Tosti A, Chiappalupi S, Sorci G, Tagariello G, Belvini D, Radossi P, Landolfi R, Fuchs D, Boon L, Pirro M, Marchesini E, Grohmann U, Puccetti P, Iorio A, Fallarino F. IDO1 suppresses inhibitor development in hemophilia A treated with factor VIII. J Clin Invest. 2015;125:3766–3781. doi: 10.1172/JCI81859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lima S, Kumar S, Gawandi V, Momany C, Phillips RS. Crystal structure of the Homo sapiens kynureninase-3-hydroxyhippuric acid inhibitor complex: insights into the molecular basis of kynureninase substrate specificity. Journal of medicinal chemistry. 2009;52:389–396. doi: 10.1021/jm8010806. [DOI] [PubMed] [Google Scholar]
- 20.Lovelace MD, Varney B, Sundaram G, Franco NF, Ng ML, Pai S, Lim CK, Guillemin GJ, Brew BJ. Current Evidence for a Role of the Kynurenine Pathway of Tryptophan Metabolism in Multiple Sclerosis. Front Immunol. 2016;7:246. doi: 10.3389/fimmu.2016.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adibfar A, Saleem M, Lanctot KL, Herrmann N. Potential Biomarkers for Depression Associated with Coronary Artery Disease: A Critical Review. Curr Mol Med. 2016;16:137–164. doi: 10.2174/1566524016666160126144143. [DOI] [PubMed] [Google Scholar]
- 22.Agudelo LZ, Femenia T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, Correia JC, Izadi M, Bhat M, Schuppe-Koistinen I, Pettersson AT, Ferreira DM, Krook A, Barres R, Zierath JR, Erhardt S, Lindskog M, Ruas JL. Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159:33–45. doi: 10.1016/j.cell.2014.07.051. [DOI] [PubMed] [Google Scholar]
- 23.Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett. 2001;313:96–98. doi: 10.1016/s0304-3940(01)02242-x. [DOI] [PubMed] [Google Scholar]
- 24.Wonodi I, Schwarcz R. Cortical kynurenine pathway metabolism: a novel target for cognitive enhancement in Schizophrenia. Schizophr Bull. 2010;36:211–218. doi: 10.1093/schbul/sbq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oxenkrug GF. Increased Plasma Levels of Xanthurenic and Kynurenic Acids in Type 2 Diabetes. Mol Neurobiol. 2015;52:805–810. doi: 10.1007/s12035-015-9232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wainwright DA, Dey M, Chang A, Lesniak MS. Targeting Tregs in Malignant Brain Cancer: Overcoming IDO. Front Immunol. 2013;4:116. doi: 10.3389/fimmu.2013.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Munn DH, Mellor AL. IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance. Trends Immunol. 2016;37:193–207. doi: 10.1016/j.it.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf AM, Wolf D, Rumpold H, Moschen AR, Kaser A, Obrist P, Fuchs D, Brandacher G, Winkler C, Geboes K, Rutgeerts P, Tilg H. Overexpression of indoleamine 2,3-dioxygenase in human inflammatory bowel disease. Clin Immunol. 2004;113:47–55. doi: 10.1016/j.clim.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Liu H, McKenzie G, Witting PK, Stasch JP, Hahn M, Changsirivathanathamrong D, Wu BJ, Ball HJ, Thomas SR, Kapoor V, Celermajer DS, Mellor AL, Keaney JF, Jr, Hunt NH, Stocker R. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat Med. 2010;16:279–285. doi: 10.1038/nm.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Q, Zhang M, Ding Y, Wang Q, Zhang W, Song P, Zou MH. Activation of NAD(P)H Oxidase by Tryptophan-Derived 3-Hydroxykynurenine Accelerates Endothelial Apoptosis and Dysfunction In Vivo. Circ Res. 2014;114:480–492. doi: 10.1161/CIRCRESAHA.114.302113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mangge H, Stelzer I, Reininghaus EZ, Weghuber D, Postolache TT, Fuchs D. Disturbed tryptophan metabolism in cardiovascular disease. Curr Med Chem. 2014;21:1931–1937. doi: 10.2174/0929867321666140304105526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Platten M, von Knebel Doeberitz N, Oezen I, Wick W, Ochs K. Cancer Immunotherapy by Targeting IDO1/TDO and Their Downstream Effectors. Front Immunol. 2014;5:673. doi: 10.3389/fimmu.2014.00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Q, Liu D, Song P, Zou MH. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Frontiers in bioscience (Landmark edition) 2015;20:1116–1143. doi: 10.2741/4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen AM, Driussi C, Turner V, Takikawa O, Hunt NH. Tissue distribution of indoleamine 2,3-dioxygenase in normal and malaria-infected tissue. Redox Rep. 2000;5:112–115. doi: 10.1179/135100000101535384. [DOI] [PubMed] [Google Scholar]
- 35.Xiao Y, Christou H, Liu L, Visner G, Mitsialis SA, Kourembanas S, Liu H. Endothelial indoleamine 2,3-dioxygenase protects against development of pulmonary hypertension. Am J Respir Crit Care Med. 2013;188:482–491. doi: 10.1164/rccm.201304-0700OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niinisalo P, Oksala N, Levula M, Pelto-Huikko M, Jarvinen O, Salenius JP, Kytomaki L, Soini JT, Kahonen M, Laaksonen R, Hurme M, Lehtimaki T. Activation of indoleamine 2,3-dioxygenase-induced tryptophan degradation in advanced atherosclerotic plaques: Tampere vascular study. Ann Med. 2010;42:55–63. doi: 10.3109/07853890903321559. [DOI] [PubMed] [Google Scholar]
- 37.Sakash JB, Byrne GI, Lichtman A, Libby P. Cytokines induce indoleamine 2,3-dioxygenase expression in human atheroma-asociated cells: implications for persistent Chlamydophila pneumoniae infection. Infect Immun. 2002;70:3959–3961. doi: 10.1128/IAI.70.7.3959-3961.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yun TJ, Lee JS, Machmach K, Shim D, Choi J, Wi YJ, Jang HS, Jung IH, Kim K, Yoon WK, Miah MA, Li B, Chang J, Bego MG, Pham TN, Loschko J, Fritz JH, Krug AB, Lee SP, Keler T, Guimond JV, Haddad E, Cohen EA, Sirois MG, El-Hamamsy I, Colonna M, Oh GT, Choi JH, Cheong C. Indoleamine 2,3-Dioxygenase-Expressing Aortic Plasmacytoid Dendritic Cells Protect against Atherosclerosis by Induction of Regulatory T Cells. Cell Metab. 2016;23:852–866. doi: 10.1016/j.cmet.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol. 2013;34:137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuffy MC, Silverio AM, Qin L, Wang Y, Eid R, Brandacher G, Lakkis FG, Fuchs D, Pober JS, Tellides G. Induction of indoleamine 2,3-dioxygenase in vascular smooth muscle cells by interferon-gamma contributes to medial immunoprivilege. J Immunol. 2007;179:5246–5254. doi: 10.4049/jimmunol.179.8.5246. [DOI] [PubMed] [Google Scholar]
- 41.Vecsei L, Szalardy L, Fulop F, Toldi J. Kynurenines in the CNS: recent advances and new questions. Nat Rev Drug Discov. 2013;12:64–82. doi: 10.1038/nrd3793. [DOI] [PubMed] [Google Scholar]
- 42.Zuo H, Ueland PM, Ulvik A, Eussen SJ, Vollset SE, Nygard O, Midttun O, Theofylaktopoulou D, Meyer K, Tell GS. Plasma Biomarkers of Inflammation, the Kynurenine Pathway, and Risks of All-Cause, Cancer, and Cardiovascular Disease Mortality: The Hordaland Health Study. American journal of epidemiology. 2016;183:249–258. doi: 10.1093/aje/kwv242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lowe MM, Mold JE, Kanwar B, Huang Y, Louie A, Pollastri MP, Wang C, Patel G, Franks DG, Schlezinger J, Sherr DH, Silverstone AE, Hahn ME, McCune JM. Identification of cinnabarinic acid as a novel endogenous aryl hydrocarbon receptor ligand that drives IL-22 production. PLoS One. 2014;9:e87877. doi: 10.1371/journal.pone.0087877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sallee M, Dou L, Cerini C, Poitevin S, Brunet P, Burtey S. The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: a new concept to understand cardiovascular complications of chronic kidney disease. Toxins (Basel) 2014;6:934–949. doi: 10.3390/toxins6030934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polyzos KA, Ovchinnikova O, Berg M, Baumgartner R, Agardh H, Pirault J, Gistera A, Assinger A, Laguna-Fernandez A, Back M, Hansson GK, Ketelhuth DF. Inhibition of indoleamine 2,3-dioxygenase promotes vascular inflammation and increases atherosclerosis in Apoe−/− mice. Cardiovasc Res. 2015;106:295–302. doi: 10.1093/cvr/cvv100. [DOI] [PubMed] [Google Scholar]
- 46.Hayashi T, Mo JH, Gong X, Rossetto C, Jang A, Beck L, Elliott GI, Kufareva I, Abagyan R, Broide DH, Lee J, Raz E. 3-Hydroxyanthranilic acid inhibits PDK1 activation and suppresses experimental asthma by inducing T cell apoptosis. Proc Natl Acad Sci U S A. 2007;104:18619–18624. doi: 10.1073/pnas.0709261104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson K, Auer M, Binnie M, Zheng X, Pham NT, Iredale JP, Webster SP, Mole DJ. Overexpression of human kynurenine-3-monooxygenase protects against 3-hydroxykynurenine-mediated apoptosis through bidirectional nonlinear feedback. Cell death & disease. 2016;7:e2197. doi: 10.1038/cddis.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okuda S, Nishiyama N, Saito H, Katsuki H. Hydrogen peroxide-mediated neuronal cell death induced by an endogenous neurotoxin, 3-hydroxykynurenine. Proc Natl Acad Sci U S A. 1996;93:12553–12558. doi: 10.1073/pnas.93.22.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eastman CL, Guilarte TR. The role of hydrogen peroxide in the in vitro cytotoxicity of 3-hydroxykynurenine. Neurochem Res. 1990;15:1101–1107. doi: 10.1007/BF01101711. [DOI] [PubMed] [Google Scholar]
- 50.Oxenkrug G, van der Hart M, Summergrad P. Elevated anthranilic acid plasma concentrations in type 1 but not type 2 diabetes mellitus. Integr Mol Med. 2015;2:365–368. doi: 10.15761/IMM.1000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Changsirivathanathamrong D, Wang Y, Rajbhandari D, Maghzal GJ, Mak WM, Woolfe C, Duflou J, Gebski V, dos Remedios CG, Celermajer DS, Stocker R. Tryptophan metabolism to kynurenine is a potential novel contributor to hypotension in human sepsis. Crit Care Med. 2011;39:2678–2683. doi: 10.1097/CCM.0b013e31822827f2. [DOI] [PubMed] [Google Scholar]
- 52.Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M, Jugold M, Guillemin GJ, Miller CL, Lutz C, Radlwimmer B, Lehmann I, von Deimling A, Wick W, Platten M. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature. 2011;478:197–203. doi: 10.1038/nature10491. [DOI] [PubMed] [Google Scholar]
- 53.Oxenkrug GF, Turski WA, Zgrajka W, Weinstock JV, Summergrad P. Tryptophan-kynurenine metabolism and insulin resistance in hepatitis C patients. Hepat Res Treat. 2013;2013:149247. doi: 10.1155/2013/149247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.DiNatale BC, Murray IA, Schroeder JC, Flaveny CA, Lahoti TS, Laurenzana EM, Omiecinski CJ, Perdew GH. Kynurenic acid is a potent endogenous aryl hydrocarbon receptor ligand that synergistically induces interleukin-6 in the presence of inflammatory signaling. Toxicol Sci. 2010;115:89–97. doi: 10.1093/toxsci/kfq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thomsen MS, Hansen HH, Timmerman DB, Mikkelsen JD. Cognitive improvement by activation of alpha7 nicotinic acetylcholine receptors: from animal models to human pathophysiology. Current pharmaceutical design. 2010;16:323–343. doi: 10.2174/138161210790170094. [DOI] [PubMed] [Google Scholar]
- 56.Nilsen RM, Bjorke-Monsen AL, Midttun O, Nygard O, Pedersen ER, Ulvik A, Magnus P, Gjessing HK, Vollset SE, Ueland PM. Maternal tryptophan and kynurenine pathway metabolites and risk of preeclampsia. Obstet Gynecol. 2012;119:1243–1250. doi: 10.1097/AOG.0b013e318255004e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J, Simonavicius N, Wu X, Swaminath G, Reagan J, Tian H, Ling L. Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35. J Biol Chem. 2006;281:22021–22028. doi: 10.1074/jbc.M603503200. [DOI] [PubMed] [Google Scholar]
- 58.Haruki H, Hovius R, Pedersen MG, Johnsson K. Tetrahydrobiopterin Biosynthesis as a Potential Target of the Kynurenine Pathway Metabolite Xanthurenic Acid. J Biol Chem. 2016;291:652–657. doi: 10.1074/jbc.C115.680488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Malina HZ, Richter C, Mehl M, Hess OM. Pathological apoptosis by xanthurenic acid, a tryptophan metabolite: activation of cell caspases but not cytoskeleton breakdown. BMC Physiol. 2001;1:7. doi: 10.1186/1472-6793-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Niinisalo P, Raitala A, Pertovaara M, Oja SS, Lehtimaki T, Kahonen M, Reunanen A, Jula A, Moilanen L, Kesaniemi YA, Nieminen MS, Hurme M. Indoleamine 2,3-dioxygenase activity associates with cardiovascular risk factors: the Health 2000 study. Scand J Clin Lab Invest. 2008;68:767–770. doi: 10.1080/00365510802245685. [DOI] [PubMed] [Google Scholar]
- 61.Zhang L, Ovchinnikova O, Jonsson A, Lundberg AM, Berg M, Hansson GK, Ketelhuth DF. The tryptophan metabolite 3-hydroxyanthranilic acid lowers plasma lipids and decreases atherosclerosis in hypercholesterolaemic mice. Eur Heart J. 2012;33:2025–2034. doi: 10.1093/eurheartj/ehs175. [DOI] [PubMed] [Google Scholar]
- 62.Pawlak K, Mysliwiec M, Pawlak D. Kynurenine pathway - a new link between endothelial dysfunction and carotid atherosclerosis in chronic kidney disease patients. Adv Med Sci. 2010;55:196–203. doi: 10.2478/v10039-010-0015-6. [DOI] [PubMed] [Google Scholar]
- 63.Cole JE, Astola N, Cribbs AP, Goddard ME, Park I, Green P, Davies AH, Williams RO, Feldmann M, Monaco C. Indoleamine 2,3-dioxygenase-1 is protective in atherosclerosis and its metabolites provide new opportunities for drug development. Proc Natl Acad Sci U S A. 2015;112:13033–13038. doi: 10.1073/pnas.1517820112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:609–620. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- 65.Metghalchi S, Ponnuswamy P, Simon T, Haddad Y, Laurans L, Clement M, Dalloz M, Romain M, Esposito B, Koropoulis V, Lamas B, Paul JL, Cottin Y, Kotti S, Bruneval P, Callebert J, den Ruijter H, Launay JM, Danchin N, Sokol H, Tedgui A, Taleb S, Mallat Z. Indoleamine 2,3-Dioxygenase Fine-Tunes Immune Homeostasis in Atherosclerosis and Colitis through Repression of Interleukin-10 Production. Cell Metab. 2015;22:460–471. doi: 10.1016/j.cmet.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 66.Van de Velde LA, Gingras S, Pelletier S, Murray PJ. Issues with the Specificity of Immunological Reagents for Murine IDO1. Cell Metab. 2016;23:389–390. doi: 10.1016/j.cmet.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 67.Kato A, Suzuki Y, Suda T, Suzuki M, Fujie M, Takita T, Furuhashi M, Maruyama Y, Chida K, Hishida A. Relationship between an increased serum kynurenine/tryptophan ratio and atherosclerotic parameters in hemodialysis patients. Hemodial Int. 2010;14:418–424. doi: 10.1111/j.1542-4758.2010.00464.x. [DOI] [PubMed] [Google Scholar]
- 68.Pedersen ER, Tuseth N, Eussen SJ, Ueland PM, Strand E, Svingen GF, Midttun O, Meyer K, Mellgren G, Ulvik A, Nordrehaug JE, Nilsen DW, Nygard O. Associations of plasma kynurenines with risk of acute myocardial infarction in patients with stable angina pectoris. Arterioscler Thromb Vasc Biol. 2015;35:455–462. doi: 10.1161/ATVBAHA.114.304674. [DOI] [PubMed] [Google Scholar]
- 69.Nikkheslat N, Zunszain PA, Horowitz MA, Barbosa IG, Parker JA, Myint AM, Schwarz MJ, Tylee AT, Carvalho LA, Pariante CM. Insufficient glucocorticoid signaling and elevated inflammation in coronary heart disease patients with comorbid depression. Brain Behav Immun. 2015;48:8–18. doi: 10.1016/j.bbi.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 70.Pedersen ER, Svingen GF, Schartum-Hansen H, Ueland PM, Ebbing M, Nordrehaug JE, Igland J, Seifert R, Nilsen RM, Nygard O. Urinary excretion of kynurenine and tryptophan, cardiovascular events, and mortality after elective coronary angiography. Eur Heart J. 2013;34:2689–2696. doi: 10.1093/eurheartj/eht264. [DOI] [PubMed] [Google Scholar]
- 71.Ormstad H, Verkerk R, Amthor KF, Sandvik L. Activation of the kynurenine pathway in the acute phase of stroke and its role in fatigue and depression following stroke. J Mol Neurosci. 2014;54:181–187. doi: 10.1007/s12031-014-0272-0. [DOI] [PubMed] [Google Scholar]
- 72.Munipally PK, Agraharm SG, Valavala VK, Gundae S, Turlapati NR. Evaluation of indoleamine 2,3-dioxygenase expression and kynurenine pathway metabolites levels in serum samples of diabetic retinopathy patients. Arch Physiol Biochem. 2011;117:254–258. doi: 10.3109/13813455.2011.623705. [DOI] [PubMed] [Google Scholar]
- 73.Mudry JM, Alm PS, Erhardt S, Goiny M, Fritz T, Caidahl K, Zierath JR, Krook A, Wallberg-Henriksson H. Direct effects of exercise on kynurenine metabolism in people with normal glucose tolerance or type 2 diabetes. Diabetes Metab Res Rev. 2016;32:754–761. doi: 10.1002/dmrr.2798. [DOI] [PubMed] [Google Scholar]
- 74.Hattori M, Kotake Y, Kotake Y. Studies on the urinary excretion of xanthurenic acid in diabetics. Acta Vitaminol Enzymol. 1984;6:221–228. [PubMed] [Google Scholar]
- 75.Kapoor V, Kapoor R, Chalmers J. Kynurenic acid, an endogenous glutamate antagonist, in SHR and WKY rats: possible role in central blood pressure regulation. Clin Exp Pharmacol Physiol. 1994;21:891–896. doi: 10.1111/j.1440-1681.1994.tb02460.x. [DOI] [PubMed] [Google Scholar]
- 76.Ito S, Komatsu K, Tsukamoto K, Sved AF. Excitatory amino acids in the rostral ventrolateral medulla support blood pressure in spontaneously hypertensive rats. Hypertension. 2000;35:413–417. doi: 10.1161/01.hyp.35.1.413. [DOI] [PubMed] [Google Scholar]
- 77.Kwok JB, Kapoor R, Gotoda T, Iwamoto Y, Iizuka Y, Yamada N, Isaacs KE, Kushwaha VV, Church WB, Schofield PR, Kapoor V. A missense mutation in kynurenine aminotransferase-1 in spontaneously hypertensive rats. J Biol Chem. 2002;277:35779–35782. doi: 10.1074/jbc.C200303200. [DOI] [PubMed] [Google Scholar]
- 78.Mangge H, Summers KL, Meinitzer A, Zelzer S, Almer G, Prassl R, Schnedl WJ, Reininghaus E, Paulmichl K, Weghuber D, Fuchs D. Obesity-related dysregulation of the tryptophan-kynurenine metabolism: role of age and parameters of the metabolic syndrome. Obesity (Silver Spring) 2014;22:195–201. doi: 10.1002/oby.20491. [DOI] [PubMed] [Google Scholar]
- 79.Wolowczuk I, Hennart B, Leloire A, Bessede A, Soichot M, Taront S, Caiazzo R, Raverdy V, Pigeyre M, Guillemin GJ, Allorge D, Pattou F, Froguel P, Poulain-Godefroy O Consortium A. Tryptophan metabolism activation by indoleamine 2,3-dioxygenase in adipose tissue of obese women: an attempt to maintain immune homeostasis and vascular tone. Am J Physiol Regul Integr Comp Physiol. 2012;303:R135–143. doi: 10.1152/ajpregu.00373.2011. [DOI] [PubMed] [Google Scholar]
- 80.Ho JE, Larson MG, Ghorbani A, Cheng S, Chen MH, Keyes M, Rhee EP, Clish CB, Vasan RS, Gerszten RE, Wang TJ. Metabolomic Profiles of Body Mass Index in the Framingham Heart Study Reveal Distinct Cardiometabolic Phenotypes. PLoS One. 2016;11:e0148361. doi: 10.1371/journal.pone.0148361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Favennec M, Hennart B, Caiazzo R, Leloire A, Yengo L, Verbanck M, Arredouani A, Marre M, Pigeyre M, Bessede A, Guillemin GJ, Chinetti G, Staels B, Pattou F, Balkau B, Allorge D, Froguel P, Poulain-Godefroy O. The kynurenine pathway is activated in human obesity and shifted toward kynurenine monooxygenase activation. Obesity (Silver Spring) 2015;23:2066–2074. doi: 10.1002/oby.21199. [DOI] [PubMed] [Google Scholar]
- 82.Mazarei G, Budac DP, Lu G, Adomat H, Tomlinson Guns ES, Moller T, Leavitt BR. Age-dependent alterations of the kynurenine pathway in the YAC128 mouse model of Huntington disease. J Neurochem. 2013;127:852–867. doi: 10.1111/jnc.12350. [DOI] [PubMed] [Google Scholar]
- 83.Theofylaktopoulou D, Midttun O, Ulvik A, Ueland PM, Tell GS, Vollset SE, Nygard O, Eussen SJ. A community-based study on determinants of circulating markers of cellular immune activation and kynurenines: the Hordaland Health Study. Clin Exp Immunol. 2013;173:121–130. doi: 10.1111/cei.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Capuron L, Schroecksnadel S, Feart C, Aubert A, Higueret D, Barberger-Gateau P, Laye S, Fuchs D. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry. 2011;70:175–182. doi: 10.1016/j.biopsych.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 85.Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Grant R. Changes in kynurenine pathway metabolism in the brain, liver and kidney of aged female Wistar rats. FEBS J. 2011;278:4425–4434. doi: 10.1111/j.1742-4658.2011.08366.x. [DOI] [PubMed] [Google Scholar]
- 86.Pertovaara M, Raitala A, Juonala M, Lehtimaki T, Huhtala H, Oja SS, Jokinen E, Viikari JS, Raitakari OT, Hurme M. Indoleamine 2,3-dioxygenase enzyme activity correlates with risk factors for atherosclerosis: the Cardiovascular Risk in Young Finns Study. Clin Exp Immunol. 2007;148:106–111. doi: 10.1111/j.1365-2249.2007.03325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rassoulpour A, Wu HQ, Albuquerque EX, Schwarcz R. Prolonged nicotine administration results in biphasic, brain-specific changes in kynurenate levels in the rat. Neuropsychopharmacology. 2005;30:697–704. doi: 10.1038/sj.npp.1300583. [DOI] [PubMed] [Google Scholar]
- 88.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 89.Song P, Zou MH. Redox regulation of endothelial cell fate. Cell Mol Life Sci. 2014;71:3219–3239. doi: 10.1007/s00018-014-1598-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 91.Santillan MK, Pelham CJ, Ketsawatsomkron P, Santillan DA, Davis DR, Devor EJ, Gibson-Corley KN, Scroggins SM, Grobe JL, Yang B, Hunter SK, Sigmund CD. Pregnant mice lacking indoleamine 2,3-dioxygenase exhibit preeclampsia phenotypes. Physiol Rep. 2015;3:e12257. doi: 10.14814/phy2.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Barth MC, Ahluwalia N, Anderson TJ, Hardy GJ, Sinha S, Alvarez-Cardona JA, Pruitt IE, Rhee EP, Colvin RA, Gerszten RE. Kynurenic acid triggers firm arrest of leukocytes to vascular endothelium under flow conditions. J Biol Chem. 2009;284:19189–19195. doi: 10.1074/jbc.M109.024042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sekkai D, Guittet O, Lemaire G, Tenu JP, Lepoivre M. Inhibition of nitric oxide synthase expression and activity in macrophages by 3-hydroxyanthranilic acid, a tryptophan metabolite. Arch Biochem Biophys. 1997;340:117–123. doi: 10.1006/abbi.1997.9913. [DOI] [PubMed] [Google Scholar]
- 94.Fujigaki H, Saito K, Lin F, Fujigaki S, Takahashi K, Martin BM, Chen CY, Masuda J, Kowalak J, Takikawa O, Seishima M, Markey SP. Nitration and inactivation of IDO by peroxynitrite. J Immunol. 2006;176:372–379. doi: 10.4049/jimmunol.176.1.372. [DOI] [PubMed] [Google Scholar]
- 95.Wang YF, Hsu YJ, Wu HF, Lee GL, Yang YS, Wu JY, Yet SF, Wu KK, Kuo CC. Endothelium-Derived 5-Methoxytryptophan Is a Circulating Anti-Inflammatory Molecule That Blocks Systemic Inflammation. Circ Res. 2016;119:222–236. doi: 10.1161/CIRCRESAHA.116.308559. [DOI] [PubMed] [Google Scholar]
- 96.Ho YC, Wu ML, Su CH, Chen CH, Ho HH, Lee GL, Lin WS, Lin WY, Hsu YJ, Kuo CC, Wu KK, Yet SF. A Novel Protective Function of 5-Methoxytryptophan in Vascular Injury. Sci Rep. 2016;6:25374. doi: 10.1038/srep25374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gold AB, Herrmann N, Swardfager W, Black SE, Aviv RI, Tennen G, Kiss A, Lanctot KL. The relationship between indoleamine 2,3-dioxygenase activity and post-stroke cognitive impairment. J Neuroinflammation. 2011;8:17. doi: 10.1186/1742-2094-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baran H, Amann G, Lubec B, Lubec G. Kynurenic acid and kynurenine aminotransferase in heart. Pediatr Res. 1997;41:404–410. doi: 10.1203/00006450-199703000-00017. [DOI] [PubMed] [Google Scholar]
- 99.Ristagno G, Latini R, Vaahersalo J, Masson S, Kurola J, Varpula T, Lucchetti J, Fracasso C, Guiso G, Montanelli A, Barlera S, Gobbi M, Tiainen M, Pettila V, Skrifvars MB Investigators F. Early activation of the kynurenine pathway predicts early death and long-term outcome in patients resuscitated from out-of-hospital cardiac arrest. Journal of the American Heart Association. 2014;3:e001094. doi: 10.1161/JAHA.114.001094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Konishi M, Ebner N, Springer J, Schefold JC, Doehner W, Dschietzig TB, Anker SD, von Haehling S. Impact of Plasma Kynurenine Level on Functional Capacity and Outcome in Heart Failure. - Results From Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF) Circulation Journal. 2017;81:52. doi: 10.1253/circj.CJ-16-0791. [DOI] [PubMed] [Google Scholar]
- 101.Chang MY, Smith C, DuHadaway JB, Pyle JR, Boulden J, Soler AP, Muller AJ, Laury-Kleintop LD, Prendergast GC. Cardiac and gastrointestinal liabilities caused by deficiency in the immune modulatory enzyme indoleamine 2,3-dioxygenase. Cancer biology & therapy. 2011;12:1050–1058. doi: 10.4161/cbt.12.12.18142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Silverstein MD, Pitts SR, Chaikof EL, Ballard DJ. Abdominal aortic aneurysm (AAA): cost-effectiveness of screening, surveillance of intermediate-sized AAA, and management of symptomatic AAA. Proc (Bayl Univ Med Cent) 2005;18:345–367. doi: 10.1080/08998280.2005.11928095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nordon IM, Hinchliffe RJ, Loftus IM, Thompson MM. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nature reviews Cardiology. 2011;8:92–102. doi: 10.1038/nrcardio.2010.180. [DOI] [PubMed] [Google Scholar]
- 104.Xiong W, MacTaggart J, Knispel R, Worth J, Persidsky Y, Baxter BT. Blocking TNF-alpha attenuates aneurysm formation in a murine model. J Immunol. 2009;183:2741–2746. doi: 10.4049/jimmunol.0803164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Golledge AL, Walker P, Norman PE, Golledge J. A systematic review of studies examining inflammation associated cytokines in human abdominal aortic aneurysm samples. Dis Markers. 2009;26:181–188. doi: 10.3233/DMA-2009-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hellenthal FA, Buurman WA, Wodzig WK, Schurink GW. Biomarkers of abdominal aortic aneurysm progression. Part 2: inflammation. Nature reviews Cardiology. 2009;6:543–552. doi: 10.1038/nrcardio.2009.102. [DOI] [PubMed] [Google Scholar]
- 107.Bruemmer D, Collins AR, Noh G, Wang W, Territo M, Arias-Magallona S, Fishbein MC, Blaschke F, Kintscher U, Graf K, Law RE, Hsueh WA. Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J Clin Invest. 2003;112:1318–1331. doi: 10.1172/JCI18141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Henderson EL, Geng YJ, Sukhova GK, Whittemore AD, Knox J, Libby P. Death of smooth muscle cells and expression of mediators of apoptosis by T lymphocytes in human abdominal aortic aneurysms. Circulation. 1999;99:96–104. doi: 10.1161/01.cir.99.1.96. [DOI] [PubMed] [Google Scholar]
- 109.Abdul-Hussien H, Soekhoe RG, Weber E, von der Thusen JH, Kleemann R, Mulder A, van Bockel JH, Hanemaaijer R, Lindeman JH. Collagen degradation in the abdominal aneurysm: a conspiracy of matrix metalloproteinase and cysteine collagenases. Am J Pathol. 2007;170:809–817. doi: 10.2353/ajpath.2007.060522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Davis FM, Rateri DL, Daugherty A. Mechanisms of aortic aneurysm formation: translating preclinical studies into clinical therapies. Heart. 2014;100:1498–1505. doi: 10.1136/heartjnl-2014-305648. [DOI] [PubMed] [Google Scholar]
- 111.Krettek A, Sukhova GK, Libby P. Elastogenesis in human arterial disease: a role for macrophages in disordered elastin synthesis. Arterioscler Thromb Vasc Biol. 2003;23:582–587. doi: 10.1161/01.ATV.0000064372.78561.A5. [DOI] [PubMed] [Google Scholar]
- 112.Juvonen J, Surcel HM, Satta J, Teppo AM, Bloigu A, Syrjala H, Airaksinen J, Leinonen M, Saikku P, Juvonen T. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1997;17:2843–2847. doi: 10.1161/01.atv.17.11.2843. [DOI] [PubMed] [Google Scholar]
- 113.Singh K, Bonaa KH, Jacobsen BK, Bjork L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study : The Tromso Study. American journal of epidemiology. 2001;154:236–244. doi: 10.1093/aje/154.3.236. [DOI] [PubMed] [Google Scholar]
- 114.Stackelberg O, Bjorck M, Sadr-Azodi O, Larsson SC, Orsini N, Wolk A. Obesity and abdominal aortic aneurysm. Br J Surg. 2013;100:360–366. doi: 10.1002/bjs.8983. [DOI] [PubMed] [Google Scholar]
- 115.Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2009;29:1458–1464. doi: 10.1161/ATVBAHA.109.192658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Folsom AR, Yao L, Alonso A, Lutsey PL, Missov E, Lederle FA, Ballantyne CM, Tang W. Circulating Biomarkers and Abdominal Aortic Aneurysm Incidence: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2015;132:578–585. doi: 10.1161/CIRCULATIONAHA.115.016537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.De Haro J, Bleda S, Acin F. C-reactive protein predicts aortic aneurysmal disease progression after endovascular repair. Int J Cardiol. 2016;202:701–706. doi: 10.1016/j.ijcard.2015.09.122. [DOI] [PubMed] [Google Scholar]
- 118.De Haro J, Acin F, Bleda S, Varela C, Medina FJ, Esparza L. Prediction of asymptomatic abdominal aortic aneurysm expansion by means of rate of variation of C-reactive protein plasma levels. J Vasc Surg. 2012;56:45–52. doi: 10.1016/j.jvs.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 119.Freestone T, Turner RJ, Coady A, Higman DJ, Greenhalgh RM, Powell JT. Inflammation and matrix metalloproteinases in the enlarging abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1995;15:1145–1151. doi: 10.1161/01.atv.15.8.1145. [DOI] [PubMed] [Google Scholar]
- 120.Thompson RW, Parks WC. Role of matrix metalloproteinases in abdominal aortic aneurysms. Ann N Y Acad Sci. 1996;800:157–174. doi: 10.1111/j.1749-6632.1996.tb33307.x. [DOI] [PubMed] [Google Scholar]
- 121.Li Y, Kilani RT, Rahmani-Neishaboor E, Jalili RB, Ghahary A. Kynurenine increases matrix metalloproteinase-1 and -3 expression in cultured dermal fibroblasts and improves scarring in vivo. J Invest Dermatol. 2014;134:643–650. doi: 10.1038/jid.2013.303. [DOI] [PubMed] [Google Scholar]
- 122.Poormasjedi-Meibod MS, Salimi Elizei S, Leung V, Baradar Jalili R, Ko F, Ghahary A. Kynurenine Modulates MMP-1 and Type-I Collagen Expression Via Aryl Hydrocarbon Receptor Activation in Dermal Fibroblasts. Journal of cellular physiology. 2016;231:2749–2760. doi: 10.1002/jcp.25383. [DOI] [PubMed] [Google Scholar]
- 123.Zhang C, van der Voort D, Shi H, Zhang R, Qing Y, Hiraoka S, Takemoto M, Yokote K, Moxon JV, Norman P, Rittie L, Kuivaniemi H, Atkins GB, Gerson SL, Shi GP, Golledge J, Dong N, Perbal B, Prosdocimo DA, Lin Z. Matricellular protein CCN3 mitigates abdominal aortic aneurysm. J Clin Invest. 2016;126:1282–1299. doi: 10.1172/JCI82337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kudo Y, Boyd CA, Sargent IL, Redman CW. Decreased tryptophan catabolism by placental indoleamine 2,3-dioxygenase in preeclampsia. Am J Obstet Gynecol. 2003;188:719–726. doi: 10.1067/mob.2003.156. [DOI] [PubMed] [Google Scholar]
- 125.Bartosiewicz J, Kaminski T, Pawlak K, Karbowska M, Tankiewicz-Kwedlo A, Pawlak D. The activation of the kynurenine pathway in a rat model with renovascular hypertension. Experimental Biology and Medicine. 2017:242. doi: 10.1177/1535370217693114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mizutani K, Sugimoto K, Okuda T, Katsuya T, Miyata T, Tanabe T, Higaki J, Ogihara T, Yamori Y, Tsujita Y, Tago N, Iwai N. Kynureninase is a novel candidate gene for hypertension in spontaneously hypertensive rats. Hypertens Res. 2002;25:135–140. doi: 10.1291/hypres.25.135. [DOI] [PubMed] [Google Scholar]
- 127.Zhang Y, Shen J, He X, Zhang K, Wu S, Xiao B, Zhou X, Phillips RS, Gao P, Jeunemaitre X, Zhu D. A rare variant at the KYNU gene is associated with kynureninase activity and essential hypertension in the Han Chinese population. Circulation Cardiovascular genetics. 2011;4:687–694. doi: 10.1161/CIRCGENETICS.110.959064. [DOI] [PubMed] [Google Scholar]
- 128.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH, American Heart A Obesity Committee of the Council on Nutrition PA, Metabolism. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 129.Boulet MM, Chevrier G, Grenier-Larouche T, Pelletier M, Nadeau M, Scarpa J, Prehn C, Marette A, Adamski J, Tchernof A. Alterations of plasma metabolite profiles related to adipose tissue distribution and cardiometabolic risk. Am J Physiol Endocrinol Metab. 2015;309:E736–746. doi: 10.1152/ajpendo.00231.2015. [DOI] [PubMed] [Google Scholar]
- 130.Favennec M, Hennart B, Verbanck M, Pigeyre M, Caiazzo R, Raverdy V, Verkindt H, Leloire A, Guillemin GJ, Yengo L, Allorge D, Froguel P, Pattou F, Poulain-Godefroy O. Post-Bariatric Surgery Changes in Quinolinic and Xanthurenic Acid Concentrations Are Associated with Glucose Homeostasis. PLoS One. 2016;11:e0158051. doi: 10.1371/journal.pone.0158051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moyer BJ, Rojas IY, Kerley-Hamilton JS, Hazlett HF, Nemani KV, Trask HW, West RJ, Lupien LE, Collins AJ, Ringelberg CS, Gimi B, Kinlaw WB, 3rd, Tomlinson CR. Inhibition of the aryl hydrocarbon receptor prevents Western diet-induced obesity. Model for AHR activation by kynurenine via oxidized-LDL, TLR2/4, TGFbeta, and IDO1. Toxicol Appl Pharmacol. 2016;300:13–24. doi: 10.1016/j.taap.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Watts SW, Shaw S, Burnett R, Dorrance AM. Indoleamine 2,3-diooxygenase in periaortic fat: mechanisms of inhibition of contraction. Am J Physiol Heart Circ Physiol. 2011;301:H1236–1247. doi: 10.1152/ajpheart.00384.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Laakso M. Cardiovascular disease in type 2 diabetes from population to man to mechanisms: the Kelly West Award Lecture 2008. Diabetes Care. 2010;33:442–449. doi: 10.2337/dc09-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Oxenkrug G. Insulin resistance and dysregulation of tryptophan-kynurenine and kynurenine-nicotinamide adenine dinucleotide metabolic pathways. Mol Neurobiol. 2013;48:294–301. doi: 10.1007/s12035-013-8497-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Navrotskaya V, Oxenkrug G, Vorobyova L, Summergrad P. Attenuation of high sucrose diet-induced insulin resistance in tryptophan 2,3-dioxygenase deficient Drosophila melanogaster vermilion mutants. Integr Obes Diabetes. 2015;1:93–95. doi: 10.15761/iod.1000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ahmadou MA, JOHA MS. kynurenine-modified serum proteins are a new marker of cardiovascular discorders developing in type 2 diabetes. International J Academic Scientific Research. 2015;3:101–105. [Google Scholar]
- 137.Koopmans SJ, Ruis M, Dekker R, Korte M. Surplus dietary tryptophan inhibits stress hormone kinetics and induces insulin resistance in pigs. Physiol Behav. 2009;98:402–410. doi: 10.1016/j.physbeh.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 138.Oxenkrug G. Interferon-gamma - Inducible Inflammation: Contribution to Aging and Aging-Associated Psychiatric Disorders. Aging Dis. 2011;2:474–486. [PMC free article] [PubMed] [Google Scholar]
- 139.van der Goot AT, Zhu W, Vazquez-Manrique RP, Seinstra RI, Dettmer K, Michels H, Farina F, Krijnen J, Melki R, Buijsman RC, Ruiz Silva M, Thijssen KL, Kema IP, Neri C, Oefner PJ, Nollen EA. Delaying aging and the aging-associated decline in protein homeostasis by inhibition of tryptophan degradation. Proc Natl Acad Sci U S A. 2012;109:14912–14917. doi: 10.1073/pnas.1203083109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Deac OM, Mills JL, Shane B, Midttun O, Ueland PM, Brosnan JT, Brosnan ME, Laird E, Gibney ER, Fan R, Wang Y, Brody LC, Molloy AM. Tryptophan catabolism and vitamin B-6 status are affected by gender and lifestyle factors in healthy young adults. The Journal of nutrition. 2015;145:701–707. doi: 10.3945/jn.114.203091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ulvik A, Theofylaktopoulou D, Midttun O, Nygard O, Eussen SJ, Ueland PM. Substrate product ratios of enzymes in the kynurenine pathway measured in plasma as indicators of functional vitamin B-6 status. Am J Clin Nutr. 2013;98:934–940. doi: 10.3945/ajcn.113.064998. [DOI] [PubMed] [Google Scholar]
- 142.Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 143.Cady SG, Sono M. 1-Methyl-DL-tryptophan, beta-(3-benzofuranyl)-DL-alanine (the oxygen analog of tryptophan), and beta-[3-benzo(b)thienyl]-DL-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Arch Biochem Biophys. 1991;291:326–333. doi: 10.1016/0003-9861(91)90142-6. [DOI] [PubMed] [Google Scholar]
- 144. [Accessed 24 October 2016];Study of chemotherapy in combination with IDO inhibitor in metastatic breast cancer. https://clinicaltrialsgov/show/NCT01792050.
- 145. [Accessed 24 October 2016];Immunotherapy combination study in advanced previously treated non-small cell lung cancer. https://clinicaltrialsgov/show/NCT02460367.
- 146. [Accessed 24 October 2016];Phase II INCB024360 study for patients with myelodysplastic syndromes (MDS) https://clinicaltrialsgov/show/NCT01822691.
- 147.Peng YH, Ueng SH, Tseng CT, Hung MS, Song JS, Wu JS, Liao FY, Fan YS, Wu MH, Hsiao WC, Hsueh CC, Lin SY, Cheng CY, Tu CH, Lee LC, Cheng MF, Shia KS, Shih C, Wu SY. Important Hydrogen Bond Networks in Indoleamine 2,3-Dioxygenase 1 (IDO1) Inhibitor Design Revealed by Crystal Structures of Imidazoleisoindole Derivatives with IDO1. Journal of medicinal chemistry. 2016;59:282–293. doi: 10.1021/acs.jmedchem.5b01390. [DOI] [PubMed] [Google Scholar]
- 148. [Accessed 24 October 2016];Indoleamine 2,3-Dioxygenase (IDO) inhibitor in advanced solid tumors. https://clinicaltrialsgov/show/NCT02048709.
- 149. [Accessed 24 October 2016];IDO peptid vaccination for stage III–IV non small-cell lung cancer patients. (IDOvaccine) https://clinicaltrialsgov/show/NCT01219348.
- 150.Liu X, Shin N, Koblish HK, Yang G, Wang Q, Wang K, Leffet L, Hansbury MJ, Thomas B, Rupar M, Waeltz P, Bowman KJ, Polam P, Sparks RB, Yue EW, Li Y, Wynn R, Fridman JS, Burn TC, Combs AP, Newton RC, Scherle PA. Selective inhibition of IDO1 effectively regulates mediators of antitumor immunity. Blood. 2010;115:3520–3530. doi: 10.1182/blood-2009-09-246124. [DOI] [PubMed] [Google Scholar]
- 151.Koblish HK, Hansbury MJ, Bowman KJ, Yang G, Neilan CL, Haley PJ, Burn TC, Waeltz P, Sparks RB, Yue EW, Combs AP, Scherle PA, Vaddi K, Fridman JS. Hydroxyamidine inhibitors of indoleamine-2,3-dioxygenase potently suppress systemic tryptophan catabolism and the growth of IDO-expressing tumors. Mol Cancer Ther. 2010;9:489–498. doi: 10.1158/1535-7163.MCT-09-0628. [DOI] [PubMed] [Google Scholar]
- 152.Kumar S, Jaller D, Patel B, LaLonde JM, DuHadaway JB, Malachowski WP, Prendergast GC, Muller AJ. Structure based development of phenylimidazole-derived inhibitors of indoleamine 2,3-dioxygenase. Journal of medicinal chemistry. 2008;51:4968–4977. doi: 10.1021/jm800512z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Li M, Bolduc AR, Hoda MN, Gamble DN, Dolisca SB, Bolduc AK, Hoang K, Ashley C, McCall D, Rojiani AM, Maria BL, Rixe O, MacDonald TJ, Heeger PS, Mellor AL, Munn DH, Johnson TS. The indoleamine 2,3-dioxygenase pathway controls complement-dependent enhancement of chemo-radiation therapy against murine glioblastoma. J Immunother Cancer. 2014;2:21. doi: 10.1186/2051-1426-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mole DJ, Webster SP, Uings I, Zheng X, Binnie M, Wilson K, Hutchinson JP, Mirguet O, Walker A, Beaufils B, Ancellin N, Trottet L, Beneton V, Mowat CG, Wilkinson M, Rowland P, Haslam C, McBride A, Homer NZ, Baily JE, Sharp MG, Garden OJ, Hughes J, Howie SE, Holmes DS, Liddle J, Iredale JP. Kynurenine-3-monooxygenase inhibition prevents multiple organ failure in rodent models of acute pancreatitis. Nat Med. 2016;22:202–209. doi: 10.1038/nm.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rover S, Cesura AM, Huguenin P, Kettler R, Szente A. Synthesis and biochemical evaluation of N-(4-phenylthiazol-2-yl)benzenesulfonamides as high-affinity inhibitors of kynurenine 3-hydroxylase. Journal of medicinal chemistry. 1997;40:4378–4385. doi: 10.1021/jm970467t. [DOI] [PubMed] [Google Scholar]
- 156.Amaral M, Levy C, Heyes DJ, Lafite P, Outeiro TF, Giorgini F, Leys D, Scrutton NS. Structural basis of kynurenine 3-monooxygenase inhibition. Nature. 2013;496:382–385. doi: 10.1038/nature12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chiarugi A, Carpenedo R, Molina MT, Mattoli L, Pellicciari R, Moroni F. Comparison of the neurochemical and behavioral effects resulting from the inhibition of kynurenine hydroxylase and/or kynureninase. J Neurochem. 1995;65:1176–1183. doi: 10.1046/j.1471-4159.1995.65031176.x. [DOI] [PubMed] [Google Scholar]
- 158.Walsh HA, Leslie PL, O’Shea KC, Botting NP. 2-Amino-4-[3′-hydroxyphenyl]-4-hydroxybutanoic acid; a potent inhibitor of rat and recombinant human kynureninase. Bioorganic & medicinal chemistry letters. 2002;12:361–363. doi: 10.1016/s0960-894x(01)00758-2. [DOI] [PubMed] [Google Scholar]
- 159.Kasper SH, Bonocora RP, Wade JT, Musah RA, Cady NC. Chemical Inhibition of Kynureninase Reduces Pseudomonas aeruginosa Quorum Sensing and Virulence Factor Expression. ACS Chem Biol. 2016;11:1106–1117. doi: 10.1021/acschembio.5b01082. [DOI] [PubMed] [Google Scholar]
- 160.Dounay AB, Anderson M, Bechle BM, Campbell BM, Claffey MM, Evdokimov A, Evrard E, Fonseca KR, Gan X, Ghosh S, Hayward MM, Horner W, Kim JY, McAllister LA, Pandit J, Paradis V, Parikh VD, Reese MR, Rong S, Salafia MA, Schuyten K, Strick CA, Tuttle JB, Valentine J, Wang H, Zawadzke LE, Verhoest PR. Discovery of Brain-Penetrant, Irreversible Kynurenine Aminotransferase II Inhibitors for Schizophrenia. ACS Med Chem Lett. 2012;3:187–192. doi: 10.1021/ml200204m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rossi F, Valentina C, Garavaglia S, Sathyasaikumar KV, Schwarcz R, Kojima S, Okuwaki K, Ono S, Kajii Y, Rizzi M. Crystal structure-based selective targeting of the pyridoxal 5′-phosphate dependent enzyme kynurenine aminotransferase II for cognitive enhancement. Journal of medicinal chemistry. 2010;53:5684–5689. doi: 10.1021/jm100464k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Amori L, Guidetti P, Pellicciari R, Kajii Y, Schwarcz R. On the relationship between the two branches of the kynurenine pathway in the rat brain in vivo. J Neurochem. 2009;109:316–325. doi: 10.1111/j.1471-4159.2009.05893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rohrig UF, Majjigapu SR, Vogel P, Zoete V, Michielin O. Challenges in the Discovery of Indoleamine 2,3-Dioxygenase 1 (IDO1) Inhibitors. Journal of medicinal chemistry. 2015;58:9421–9437. doi: 10.1021/acs.jmedchem.5b00326. [DOI] [PubMed] [Google Scholar]
- 164.Yue EW, Douty B, Wayland B, Bower M, Liu X, Leffet L, Wang Q, Bowman KJ, Hansbury MJ, Liu C, Wei M, Li Y, Wynn R, Burn TC, Koblish HK, Fridman JS, Metcalf B, Scherle PA, Combs AP. Discovery of potent competitive inhibitors of indoleamine 2,3-dioxygenase with in vivo pharmacodynamic activity and efficacy in a mouse melanoma model. Journal of medicinal chemistry. 2009;52:7364–7367. doi: 10.1021/jm900518f. [DOI] [PubMed] [Google Scholar]
- 165.Sugimoto H, Oda S, Otsuki T, Hino T, Yoshida T, Shiro Y. Crystal structure of human indoleamine 2,3-dioxygenase: catalytic mechanism of O2 incorporation by a heme-containing dioxygenase. Proc Natl Acad Sci U S A. 2006;103:2611–2616. doi: 10.1073/pnas.0508996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tojo S, Kohno T, Tanaka T, Kamioka S, Ota Y, Ishii T, Kamimoto K, Asano S, Isobe Y. Crystal Structures and Structure-Activity Relationships of Imidazothiazole Derivatives as IDO1 Inhibitors. ACS Med Chem Lett. 2014;5:1119–1123. doi: 10.1021/ml500247w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Alvarez L, Lewis-Ballester A, Roitberg A, Estrin DA, Yeh SR, Marti MA, Capece L. Structural Study of a Flexible Active Site Loop in Human Indoleamine 2,3-Dioxygenase and Its Functional Implications. Biochemistry. 2016;55:2785–2793. doi: 10.1021/acs.biochem.6b00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fujigaki H, Seishima M, Saito K. Posttranslational modification of indoleamine 2,3-dioxygenase. Anal Bioanal Chem. 2012;403:1777–1782. doi: 10.1007/s00216-012-5946-2. [DOI] [PubMed] [Google Scholar]
- 169.Qian S, He T, Wang W, He Y, Zhang M, Yang L, Li G, Wang Z. Discovery and preliminary structure-activity relationship of 1H-indazoles with promising indoleamine-2,3-dioxygenase 1 (IDO1) inhibition properties. Bioorg Med Chem. 2016;24:6194–6205. doi: 10.1016/j.bmc.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 170.Malachowski WP, Winters M, DuHadaway JB, Lewis-Ballester A, Badir S, Wai J, Rahman M, Sheikh E, LaLonde JM, Yeh SR, Prendergast GC, Muller AJ. O-alkylhydroxylamines as rationally-designed mechanism-based inhibitors of indoleamine 2,3-dioxygenase-1. Eur J Med Chem. 2016;108:564–576. doi: 10.1016/j.ejmech.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pantouris G, Serys M, Yuasa HJ, Ball HJ, Mowat CG. Human indoleamine 2,3-dioxygenase-2 has substrate specificity and inhibition characteristics distinct from those of indoleamine 2,3-dioxygenase-1. Amino Acids. 2014;46:2155–2163. doi: 10.1007/s00726-014-1766-3. [DOI] [PubMed] [Google Scholar]
- 172.Meininger D, Zalameda L, Liu Y, Stepan LP, Borges L, McCarter JD, Sutherland CL. Purification and kinetic characterization of human indoleamine 2,3-dioxygenases 1 and 2 (IDO1 and IDO2) and discovery of selective IDO1 inhibitors. Biochim Biophys Acta. 2011;1814:1947–1954. doi: 10.1016/j.bbapap.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 173.Smith JR, Jamie JF, Guillemin GJ. Kynurenine-3-monooxygenase: a review of structure, mechanism, and inhibitors. Drug Discov Today. 2016;21:315–324. doi: 10.1016/j.drudis.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 174.Zwilling D, Huang SY, Sathyasaikumar KV, Notarangelo FM, Guidetti P, Wu HQ, Lee J, Truong J, Andrews-Zwilling Y, Hsieh EW, Louie JY, Wu T, Scearce-Levie K, Patrick C, Adame A, Giorgini F, Moussaoui S, Laue G, Rassoulpour A, Flik G, Huang Y, Muchowski JM, Masliah E, Schwarcz R, Muchowski PJ. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell. 2011;145:863–874. doi: 10.1016/j.cell.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Stone TW, Forrest CM, Darlington LG. Kynurenine pathway inhibition as a therapeutic strategy for neuroprotection. FEBS J. 2012;279:1386–1397. doi: 10.1111/j.1742-4658.2012.08487.x. [DOI] [PubMed] [Google Scholar]
- 176.Rojewska E, Piotrowska A, Makuch W, Przewlocka B, Mika J. Pharmacological kynurenine 3-monooxygenase enzyme inhibition significantly reduces neuropathic pain in a rat model. Neuropharmacology. 2016;102:80–91. doi: 10.1016/j.neuropharm.2015.10.040. [DOI] [PubMed] [Google Scholar]
- 177.Fitzgerald DH, Muirhead KM, Botting NP. A comparative study on the inhibition of human and bacterial kynureninase by novel bicyclic kynurenine analogues. Bioorg Med Chem. 2001;9:983–989. doi: 10.1016/s0968-0896(00)00318-7. [DOI] [PubMed] [Google Scholar]
- 178.Phillips RS. Structure and mechanism of kynureninase. Arch Biochem Biophys. 2014;544:69–74. doi: 10.1016/j.abb.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 179.Han Q, Cai T, Tagle DA, Li J. Structure, expression, and function of kynurenine aminotransferases in human and rodent brains. Cell Mol Life Sci. 2010;67:353–368. doi: 10.1007/s00018-009-0166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sun G, Nematollahi A, Nadvi NA, Kwan AH, Jeffries CM, Church WB. Expression, purification and crystallization of human kynurenine aminotransferase 2 exploiting a highly optimized codon set. Protein Expr Purif. 2016;121:41–45. doi: 10.1016/j.pep.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 181.Koola MM. Kynurenine pathway and cognitive impairments in schizophrenia: Pharmacogenetics of galantamine and memantine. Schizophr Res Cogn. 2016;4:4–9. doi: 10.1016/j.scog.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yu P, Li Z, Zhang L, Tagle DA, Cai T. Characterization of kynurenine aminotransferase III, a novel member of a phylogenetically conserved KAT family. Gene. 2006;365:111–118. doi: 10.1016/j.gene.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 183.Han Q, Robinson H, Cai T, Tagle DA, Li J. Biochemical and structural characterization of mouse mitochondrial aspartate aminotransferase, a newly identified kynurenine aminotransferase-IV. Bioscience reports. 2011;31:323–332. doi: 10.1042/BSR20100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Guidetti P, Amori L, Sapko MT, Okuno E, Schwarcz R. Mitochondrial aspartate aminotransferase: a third kynurenate-producing enzyme in the mammalian brain. J Neurochem. 2007;102:103–111. doi: 10.1111/j.1471-4159.2007.04556.x. [DOI] [PubMed] [Google Scholar]
- 185.Schlittler M, Goiny M, Agudelo LZ, Venckunas T, Brazaitis M, Skurvydas A, Kamandulis S, Ruas JL, Erhardt S, Westerblad H, Andersson DC. Endurance exercise increases skeletal muscle kynurenine aminotransferases and plasma kynurenic acid in humans. American journal of physiology Cell physiology. 2016;310:C836–840. doi: 10.1152/ajpcell.00053.2016. [DOI] [PubMed] [Google Scholar]
- 186.Han Q, Robinson H, Li J. Crystal structure of human kynurenine aminotransferase II. J Biol Chem. 2008;283:3567–3573. doi: 10.1074/jbc.M708358200. [DOI] [PubMed] [Google Scholar]
- 187.Nematollahi A, Sun G, Harrop SJ, Hanrahan JR, Church WB. Structure of the PLP-form of the human kynurenine aminotransferase II in a novel spacegroup at 1.83 A resolution. International journal of molecular sciences. 2016;17:446. doi: 10.3390/ijms17040446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Nematollahi A, Sun G, Jayawickrama GS, Church WB. Kynurenine aminotransferase isozyme inhibitors: a review. International journal of molecular sciences. 2016;17:946. doi: 10.3390/ijms17060946. [DOI] [PMC free article] [PubMed] [Google Scholar]


