Abstract
Objectives
To determine whether menopausal hormone therapy (HT) affects regional brain volumes, including hippocampal and frontal regions.
Methods
Brain MRI scans were obtained in a subset of 1403 women ages 71–89 years who participated in the Women’s Health Initiative Memory Study (WHIMS). WHIMS was an ancillary study to the Women’s Health Initiative, which consisted of two randomized, placebo-controlled trials: 0.625 mg of conjugated equine estrogens (CEE) with or without 2.5 mg medroxyprogesterone acetate (MPA) in one daily tablet. Scans were performed, on average, 3.0 years post-trial for the CEE+MPA trial and 1.4 years post-trial for the CEE-Alone trial; average on-trial exposures were 4.0 years for CEE+MPA and 5.6 years for CEE-Alone. Total brain, ventricular, hippocampal and frontal lobe volumes, adjusted for age, clinic site, estimated intracranial volume, and dementia risk factors, were the main outcome variables.
Results
Compared to placebo, covariate-adjusted mean frontal lobe volume was 2.37 cc lower among women assigned to HT (p=0.004), mean hippocampal volume was slightly (0.10 cc) lower (p=0.05), and differences in total brain volume approached significance (p=0.07). Results were similar for CEE+MPA and CEE-Alone therapy. HT-associated reductions in hippocampal volumes were greatest in women with the lowest baseline Modified Mini-Mental State (3MS) scores (3MS ≤ 90).
Conclusions
CEE+MPA and CEE-Alone are associated with greater brain atrophy among women aged 65 years and older; however, the adverse effects are most evident in women experiencing cognitive deficits before initiating HT.
INTRODUCTION
The Women’s Health Initiative Memory Study (WHIMS) trials1–4 showed that conjugated equine estrogens (CEE) alone or combined with medroxyprogesterone acetate (MPA) increase dementia risk and adversely affect global cognition in women aged 65 years or older. In view of these results and findings that hormone therapy (HT) increases the risk of clinical stroke in older women5, 6, we examined potential mechanisms through magnetic resonance imaging (MRI) scans of former WHIMS participants.
HT may influence clinical outcomes through vascular changes or effects on regional brain volumes, including neuronal architecture and synaptic density. Increases in gray matter7, 8 and hippocampal volumes7, 9, 10, hippocampal blood flow11, and temporal glucose metabolism12, 13 have been reported in observational studies of estrogen users. Effects of HT on frontal function also were reported.14, 15 These generally small studies were conducted in cohorts with average ages less than 70 years. However, the increased risk of stroke and thromboembolic disease associated with HT in older women6, 16 may offset potential neurocognitive benefits, resulting in a net increase in dementia risk.
We investigated whether global and regional brain volumes differ post-trial between older women who had been randomly assigned to HT or placebo during the Women’s Health Initiative (WHI) HT trials. We focused on whether total brain, hippocampal, and frontal lobe volumes, measured by MRI, differed by treatment assignment. A companion manuscript17 reports findings on lesion volume, the primary outcome of the WHIMS-MRI study.
Analysis of global cognitive function in the WHIMS trials uncovered only one factor moderating the adverse HT effects: baseline cognitive function at WHI enrollment. Women with lower baseline scores on the modified Mini-Mental State (3MS) exam18 had significantly greater on-trial HT-associated declines in cognitive function than women with higher scores.4 Thus, a second goal is to determine whether a low 3MS score at baseline is associated with a greater HT effect on global and regional brain volumes. Finally, we tested whether HT benefits women with the lowest vascular lesion burden, as suggested by animal models19, by comparing HT effects on brain volumes in women with the lowest ischemic lesion volume to the remaining women.
Methods
WHIMS was an ancillary study to WHI, which consisted of parallel placebo-controlled randomized clinical trials of 0.625 mg/day CEE therapy alone in women post-hysterectomy and in combination with 2.5 mg/day MPA in women with a uterus. WHIMS design, eligibility criteria, and recruitment procedures have been described.20 Participants were recruited from 39 of the 40 clinical centers participating in the WHI CEE-Alone or CEE+MPA clinical trials. To be eligible for WHIMS, women were 65 to 79 years of age at enrollment, and free of dementia.20 Written informed consent was obtained; Institutional Review Boards for participating institutions and the NIH approved the protocols and consent forms.
The WHIMS CEE+MPA trial terminated earlier than planned (July, 2002)1, 3 due to an adverse risk-to-benefit profile in the main WHI trial. Subsequently, the WHI, and ancillary WHIMS, CEE-Alone trial also terminated early (February, 2004).2, 4
WHIMS-MRI was designed to contrast MRI outcomes post-trial among WHIMS participants who had been assigned to active treatment versus placebo. It was conducted in 14 of the 39 WHIMS clinical centers, selected based on interest, experience with multi-center MRI studies, participation in the Women’s Health Initiative Study of Cognitive Aging, and availability of necessary equipment. Participants in these centers were eligible for recruitment to WHIMS-MRI, regardless of prior adherence to the WHI study protocol, on-trial use of study medications, on-study measures of cognitive function, or willingness to continue post-trial follow-up.21 Scans were performed, on average, 3.0 years post-trial for the CEE+MPA trial and 1.4 years post-trial for the CEE-Alone trial; average on-trial exposures were 4.0 years for CEE+MPA and 5.6 years for CEE-Alone. Exclusion criteria included the presence of pacemakers and other implants or foreign bodies contra-indicated for MRI.
Baseline demographic, lifestyle, and clinical factors were collected via self-report and standardized assessments. We included body mass index, as lower values may signal underlying brain pathologies in older individuals,22 and education, as higher education may identify individuals whose cognitive function and participation is less responsive to atrophy.23 The 3MS 18 was administered by a centrally trained and certified technician. It measures temporal and spatial orientation, immediate and delayed recall, executive function, naming, verbal fluency, abstract reasoning, praxis, writing, and visuo-constructional abilities. Scores range from 0 to 100 (higher score reflecting better cognitive functioning).
MRI protocol
MRI scans were conducted using a standardized protocol, developed by investigators at the MRI Quality Control Center (MRIQCC) in the Department of Radiology, University of Pennsylvania, Philadelphia. Additional detail and quality control procedures are provided in Coker et al.17 MRI series were acquired with field of view = 22 and matrix = 256×256. They included oblique axial spin density/T2-weighted spin echo (3200/0/30,120/3), FLAIR T2- weighted spin echo (8000/2000/100/3), and oblique axial 3D T1-weighted gradient echo (flip angle 30; 21/0/8/1.5) images from the vertex to skull base parallel to the anterior commissure-posterior commissure (AC-PC) plane.
To quantify regional brain volumes, the T1-weighted volumetric MRI scans were first pre-processed according to a standardized protocol24: 1) alignment to the AC-PC orientation; 2) removal of extra-cranial material; 3) segmentation of brain parenchyma into gray matter (GM), white matter (WM) and cerebrospinal fluid (CSF). Regional volumetric measurements of GM, WM and CSF were subsequently obtained via a validated, automated computer-based template warping method.25 This technique is based on a digital atlas labeled for brain lobes and individual structures, including the hippocampus. Atlas definitions were transferred to MRI scans via an image warping algorithm performing pattern matching of anatomically corresponding brain regions. The volumes of GM, WM and CSF of each labeled brain region were obtained by summing the number of respective voxels within each region. Volumes of brain lesions and peri-ventricular abnormal WM were also measured separately via the same procedure, using the three sets of images; total lesion volume was measured, as described in the accompanying paper.17 Volumes of GM and WM reported in this paper refer to normal brain tissue only. Intracranial volume (ICV) was estimated as the total cerebral hemispheric volumes, including ventricular CSF and the CSF within the sulcal spaces.
Statistical methods
Characteristics of participants at the time of WHI enrollment were described and differences among treatment groups were compared using chi-square tests. Differences in volumes of the total brain, ventricles, hippocampus, and frontal lobe (pre-specified as secondary outcomes) were contrasted among women grouped by WHI treatment assignment, both separately within each trial and pooled across trials, using analyses of covariance that adjusted for age at WHI enrollment, time between enrollment and scanning, intracranial volume, clinical center site (the WHIMS stratification factor), and other baseline dementia risk factors (education level, ethnicity, smoking status, body mass index, hypertension status, prior cardiovascular disease, diabetes, prior HT, and baseline 3MS score). Dementia risk factors were included to account for the possibility that balance among the groups originally developed by randomization had been diminished by attrition, non-consent, and MRI-related eligibility. Each volume measure was analyzed separately. Because WHIMS-MRI was primarily designed to provide mechanistic support for the findings of the WHIMS trials, no adjustment for comparisons of its multiple endpoints was specified in its protocol. Associations between MRI outcomes and dementia risk factors were assessed with analyses of covariance. To test the hypothesis that the effect of HT on MRI volumes varied by baseline 3MS, we fitted an interaction term between treatment effect and baseline 3MS scores as a continuous variable and presented fitted means for women grouped by baseline scores. We also grouped women according to total ischemic lesion volume, which includes infarcts and white matter signal abnormalities17, using the cutpoint of < 2 cm3 (lowest quartile) versus ≥ 2 cm3 (upper three quartiles). Analyses of covariance for total brain, ventricular, hippocampal and frontal volumes were repeated using this grouping as a stratification factor to test the hypothesis that women with the lowest ischemic volume and the healthiest brains might show a benefit of HT on regional volumes.
RESULTS
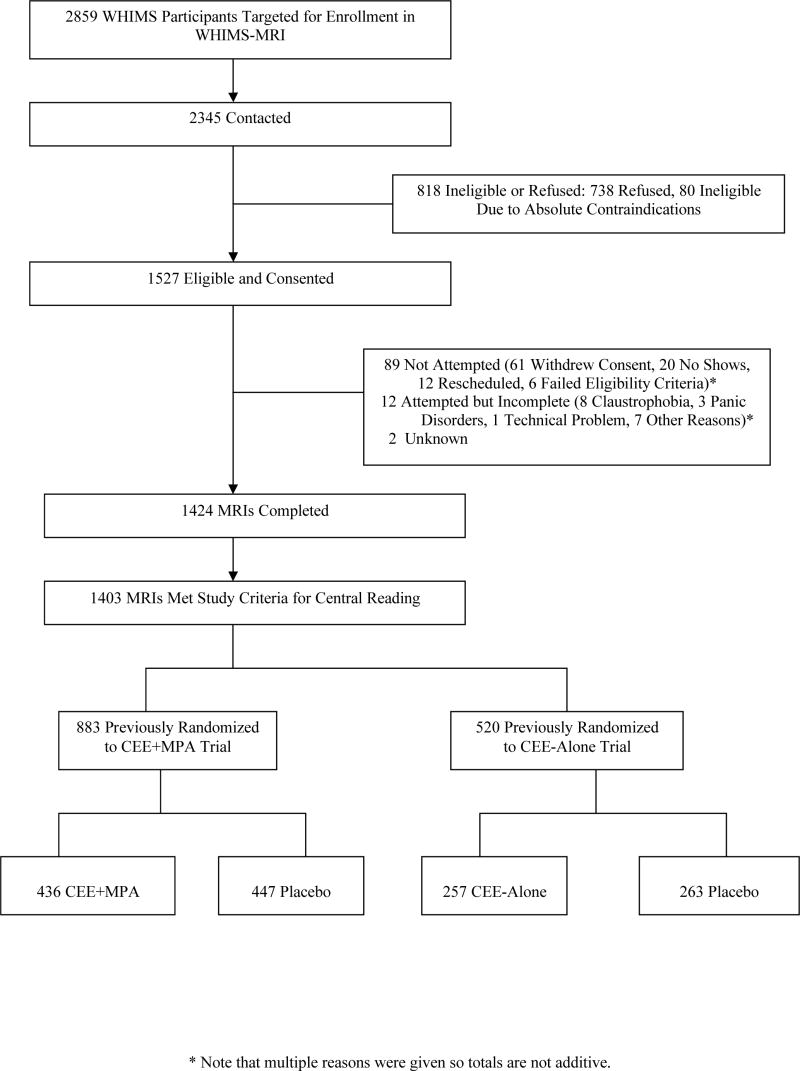
WHIMS-MRI contacted 2,345 WHIMS participants, of which 1,527 (65.1%) provided consent. Of these, 1,424 (93.3%) received brain MRI scans, of which 1,403 (98.5%) met central reading criteria for analysis: 883 women in the CEE+MPA trial and 520 women in the CEE-Alone trial. The study flow diagram is shown in Figure 1. Compared to the 1610 WHIMS participants at the 14 WHIMS-MRI sites who did not join the MRI study, WHIMS-MRI women were younger (mean ages 77.5 vs. 78.3 years; p<0.001), had higher baseline 3MS scores (mean 96.1 vs. 95.1; p<0.01), and were fewer years post menopausal (mean 28.7 vs. 30.5 years; p<0.001). However, participation rates did not differ among treatment assignments (p=0.10), race (p=0.36), education (p=0.10), or body mass index (p=0.15).
Figure 1.
Diagram describing enrollment and follow-up of WHIMS-MRI participants.
Table 1 presents dementia risk factors within the WHIMS-MRI cohort by WHI treatment assignment at the time of WHI enrollment. While there were differences with respect to many risk factors between women enrolled in the CEE+MPA versus CEE-Alone trials, there were no marked differences between women who had been randomly assigned to HT versus placebo. The mean (SD) age at the time of the MRI was 78.5 (3.7) years, which occurred an average of 8.0 years after WHI enrollment. The overall deficit in 3MS performance in association with HT observed on-trial was apparent in WHIMS-MRI women at their annual evaluation preceding the MRI scan, treatment effect of 0.43 (0.21) units.
Table 1.
Demographic, SES, and lifestyle characteristics at the time of WHI enrollment by treatment assignment: frequency and (percent).
| Variable | WHIMS-MRI CEE+MPA |
WHIMS-MRI CEE-Alone |
HT vs No HT (p-value) | ||
|---|---|---|---|---|---|
| CEE+MPA N=436 | Placebo N=447 | CEE-Alone N=257 | Placebo N=263 | ||
| Age --yrs, No. (%) | |||||
| 65–69 | 229 (52.5) | 231 (51.7) | 124 (48.2) | 131 (49.8) | |
| 70–74 | 153 (35.1) | 153 (34.2) | 94 (36.6) | 92 (35.0) | 0.81 |
| 75+ | 54 (12.4) | 63 (14.1) | 39 (15.2) | 40 (15.2) | |
|
| |||||
| Education, No. (%) | |||||
| < High school | 16 (3.7) | 22 (4.9) | 16 (6.2) | 9 (3.4) | |
| High school/GED | 88 (20.0) | 98 (22.0 | 70 (27.2) | 69 (26.2) | 0.97 |
| > High school < 4 yr college | 179 (41.2) | 165 (37.1) | 95 (37.0) | 118 (44.9) | |
| > 4 yr college | 152 (35.0) | 160 (36.0) | 76 (29.6) | 67 (25.5) | |
|
| |||||
| Ethnicity, No. (%) | |||||
| Amer. Indian/Alaskan Native | 1 (0.2) | 0 (0.0) | 3 (1.2) | 0 (0.0) | |
| Asian/Pacific Islander | 4 (0.9) | 14 (3.1) | 3 (1.2) | 2 (0.8) | |
| Black/African-American | 18 (4.1) | 16 (3.6) | 13 (5.1) | 17 (6.5) | 0.88* |
| Hispanic/Latino | 7 (1.6) | 5 (1.1) | 7 (2.8) | 2 (0.8) | |
| White, non-Hispanic | 405 (92.9) | 409 (91.7) | 224 (87.8) | 238 (90.7) | |
| Other | 1 (0.2) | 2 (0.4) | 5 (2.0) | 3 (1.2) | |
|
| |||||
| Smoking Status, No. (%) | |||||
| Never | 257 (59.2) | 252 (57.1) | 149 (58.7) | 148 (56.5) | |
| Former | 159 (36.6) | 172 (39.0) | 96 (37.8) | 99 (37.8) | 0.67 |
| Current | 18 (4.2) | 17 (3.8) | 9 (3.5) | 15 (5.7) | |
|
| |||||
| Body Mass Index—kg/m2, No. (%) | |||||
| < 25 | 138 (31.7) | 156 (35.0) | 60 (23.5) | 64 (24.4) | |
| 25–29 | 165 (37.9) | 155 (34.8) | 98 (38.4) | 108 (41.2) | 0.81 |
| 30–34 | 90 (20.7) | 90 (20.2) | 62 (24.3) | 58 (22.1) | |
| ≥35 | 42 (9.7) | 45 (10.1) | 35 (10.1) | 32 (12.2) | |
|
| |||||
| Hypertension Status, No. (%) | |||||
| None | 234 (53.7) | 240 (53.7) | 122 (47.5) | 139 (52.8) | 0.75 |
| Current/Controlled1 | 55 (12.6) | 49 (11.0) | 51 (19.8) | 54 (20.5) | |
| Current/Uncontrolled | 147 (33.7) | 158 (35.4) | 84 (32.7) | 70 (26.6) | |
|
| |||||
| Prior CVD, No. (%) | |||||
| No | 413 (94.7) | 435 (95.1) | 236 (91.8) | 239 (90.9) | |
| History of stroke | 2 (0.5) | 5 (1.1) | 3 (1.2) | 4 (1.5) | 0.56 |
| History of other CVD2 | 21 (4.8) | 17 (3.8) | 18 (7.0) | 20 (7.6) | |
|
| |||||
| Diabetes, No. (%) | |||||
| No | 420 (96.3) | 425 (95.1) | 241 (93.8) | 242 (92.0) | 0.23 |
| Yes | 16 (3.7) | 22 (4.9) | 16 (6.2) | 21 (8.0) | |
|
| |||||
| Prior Hormone Therapy, No. (%) | |||||
| No | 340 (78.0) | 346 (77.4) | 131 (51.0) | 127 (48.3) | 0.59 |
| Yes | 96 (22.0) | 101 (22.6) | 126 (49.0) | 136 (51.7) | |
|
| |||||
| 3MS Exam Score | |||||
| <90 | 22 (5.10 | 19 (4.3) | 20 (7.8) | 19 (7.3) | |
| 90–94 | 66 (15.2) | 80 (18.1) | 50 (19.6) | 58 (22.2) | 0.36 |
| 95–100 | 347 (79.8) | 342 (77.6) | 185 (72.6) | 184 (70.5) | |
Measured to be less than 140/90 mmHg
Other CVD defined as MI, angina, PCTA, or CABG
Based on collapsing to three categories (African-American, White, and other
Mean (SE) ICV, an estimate of cranial size, was similar between HT and placebo groups: 1095.9 (5.06) cm3 vs 1087.1 cm3 for the CEE+MPA trial (p=0.19) and 1088.0 (5.96) cm3 vs 1086.4 (6.66) cm3 for the CEE-Alone trial (p=0.86). Table 2 presents mean volumes for total brain (gray plus white matter), ventricles, hippocampus, and frontal lobe, after adjustment for age at WHI enrollment, time between enrollment and scan, ICV, clinic site, and dementia risk factors listed in Table 1. Mean hippocampal (p=0.05) and frontal lobe (p=0.004) volumes were lower in HT-treated women, and mean overall brain volumes were slightly lower among women who had been assigned to HT compared with placebo (p=0.07). These differences were consistent between the CEE+MPA and CEE-Alone trials. Mean ventricular volumes were unaffected by prior HT assignment.
Table 2.
Mean (SE) volumes by treatment assignment after adjustment for age, time since enrollment, intracranial volume, clinic site, and other potential confounders listed in Table 1.
| Total Brain Volume Mean (SE) | Ventricle Volume Mean (SE) | Hippocampal Volume Mean (SE) | Frontal Lobe Volume Mean (SE) | |
|---|---|---|---|---|
| Pooled trials | ||||
| HT | 798.37 (1.30) | 37.62 (0.55) | 5.69 (0.04) | 282.72 (0.57) |
| Placebo | 801.69 (1.29) | 37.15 (0.55) | 5.79 (0.04) | 285.09 (0.57) |
| Difference | −3.32 (1.84) | 0.47 (0.78) | −0.10 (0.05) | −2.37 (0.81) |
| p-value | 0.07 | 0.55 | 0.05 | 0.004 |
|
| ||||
| E+P Trial | ||||
| CEE+MPA | 800.92 (1.63) | 37.84 (0.68) | 5.72 (0.04) | 283.61 (0.72) |
| Placebo | 803.11 (1.63) | 36.53 (0.68) | 5.83 (0.04) | 285.46 (0.72) |
| Difference | −2.19 (2.32) | 1.31 (0.97) | −0.11 (0.06) | −1.85 (1.03) |
| p-value | 0.35 | 0.18 | 0.09 | 0.07 |
|
| ||||
| E-Alone Trial | ||||
| CEE-Alone | 794.53 (2.21) | 37.53 (0.95) | 5.63 (0.06) | 281.47 (0.95) |
| Placebo | 799.03 (2.16) | 37.85 (0.94) | 5.75 (0.06) | 284.25 (0.94) |
| Difference | −4.50 (3.13) | −0.33 (1.36) | −0.12 (0.09) | −2.78 (1.36) |
| p-value | 0.15 | 0.81 | 0.18 | 0.04 |
Consistency of treatment effects of CEE+MPA vs CEE-Alone
Total brain volume: p=0.36
Ventricle volume: p=0.20
Hippocampal volume: p=0.99
Frontal lobe volume: p=0.45
Tissue volumes include gray and white matter but not CSF.
Associations that volumes had with dementia risk factors are described in (E-Table 1). Consistent with expectation, mean adjusted brain volumes were lower among women with higher age, lower BMI, uncontrolled hypertension, prior cardiovascular disease, or diabetes (all p≤0.05). Higher educational level also was associated with lower brain volumes. Older women had larger mean ventricular volumes and smaller mean hippocampal and frontal lobe volumes. Lower BMI was associated with smaller hippocampal and frontal lobe volumes.
Table 3 presents mean differences in volumes between women assigned to HT versus placebo therapy who are grouped according to 3MS score at WHI enrollment, with adjustment for age, ICV, and clinic site, and additionally for all other dementia risk factors in Table 1. Decrements in hippocampal volumes associated with HT therapy were greatest in women with the lowest pre-treatment 3MS scores. Parallel analyses found that the association of HT assignment with the brain volume measures did not appear to depend on age.
Table 3.
Fitted mean difference in volumes for women assigned to HT versus placebo, after adjustment for age, time since enrollment, intracranial volume, clinic site, and other potential confounders listed in Table 1.
| Region | Baseline 3MS |
p-value* | ||
|---|---|---|---|---|
| <90 Mean (SE) | 90–94 Mean (SE) | 95–100 Mean (SE) | ||
| Total brain | −16.93 (7.71) | −7.40 (4.34) | −1.41 (2.10) | 0.07 |
| Ventricles | 3.19 (3.29) | −0.69 (1.85) | 0.52 (0.90) | 0.77 |
| Hippocampus | −0.53 (0.21) | −0.21 (0.12) | −0.04 (0.06) | 0.02 |
| Frontal | −7.62 (3.40) | −2.59 (1.92) | −1.96 (0.93) | 0.43 |
p-values are based on interaction terms between treatment effect and baseline 3MS score as a continuous variable.
Women whose total ischemic lesion volume was below the approximate 25% percentile (2 cm3) were selected to represent those with relatively little evidence of vascular disease: 359 women, 26.1% of HT group and 25.1% of placebo group (p=0.65). Table 4 contrasts mean HT-related differences in total brain, ventricular, hippocampal and frontal volumes among women with lesion volumes lower than 2 cm3 with those with lesion volumes ≥ 2 cm3, with adjustment for all covariates. The small differences between treatment groups were not significant among women with with the lowest ischemic lesion volumes. However, for women with ischemic lesion volumes ≥ 2 cm3, mean total brain (p < 0.05), hippocampal (p < 0.01) and frontal (p < 0.01) volumes were lower among women who had been assigned to HT.
Table 4.
Mean (SE) volumes by treatment assignment for women grouped according to total abnormal tissue volumes: <2 cm3 or ≥2 cm3, after adjustment for age, time since enrollment, intracranial volume, clinic site, and other potential confounders listed in Table 1
| Total Brain Volume Mean (SE) | Ventricle Volume Mean (SE) | Hippocampal Volume* Mean (SE) | Frontal Lobe Volume Mean (SE) | |
|---|---|---|---|---|
| Lesion Volume < 2 cm3 | ||||
| HT | 788.12 (2.41) | 30.90 (0.93) | 6.08 (0.07) | 278.15 (1.05) |
| Placebo | 785.51 (2.43) | 31.71 (0.94) | 5.93 (0.07) | 278.66 (1.06) |
| Difference | 2.62 (3.52) | −0.81 (1.36) | 0.15 (0.10) | −0.51 (1.54) |
| p-value | p=0.41 | p=0.55 | p=0.13 | p=0.74 |
| Lesion Volume ≥ 2 cm3 | ||||
| HT | 802.27 (1.55) | 39.78 (0.67) | 5.57 (0.04) | 284.29 (0.69) |
| Placebo | 806.93 (1.54) | 39.13 (0.66) | 5.73 (0.04) | 287.31 (0.68) |
| Difference | −4.67 (2.20) | 0.66 (0.95) | −0.16 (0.06) | −3.01 (0.98) |
| p-value | p=0.03 | p=0.49 | p=0.005 | p=0.002 |
Significant HT
lesion volume interaction, p=0.01
DISCUSSION
Through post-trial MRI scans of WHIMS participants, we found that randomization to CEE, with or without MPA, was associated with small but significant mean decrements in frontal (2.37±0.81 cc) and hippocampal (0.10±0.05 cc) volumes. Women randomized to HT continued to express a persistent treatment-related deficit in 3MS test scores through the time of the MRI assessment. Analysis of brain volume measures as a function of 3MS scores at WHIMS baseline showed that HT-associated reductions in hippocampal volume were greatest in women with the lowest cognitive function at WHI enrollment. These associations were similar for CEE+MPA and CEE-Alone trials. In addition, HT-associated reductions in total brain hippocampal, and frontal volumes were apparent in women with vascular lesion burden volumes of 2 cm3 or larger, but not lower than 2 cm3.
In contrast to several earlier reports of increased volumes of the hippocampus and other brain regions in HT users7–10, we found no evidence of increased frontal, hippocampal, or total brain volumes in women randomized to CEE+MPA or CEE-Alone compared with placebo. Our findings are based on the largest sample of postmenopausal women studied to date. However, our sample differs from most prior reports in that we studied older women, mean age 77.5 years at the time of MRI assessment, who initiated HT at age 65 and older within the framework of the WHI clinical trial, and who had discontinued study medications an average of 3.0 (CEE+MPA trial) and 1.4 (CEE-Alone trial) years before the MRI. In contrast, studies reporting increased volumes of the hippocampus and other gray matter regions in HT users7–10 were based on younger women who were long-term users of HT, generally initiated close to menopause, but not all studies have reported increased brain volumes in association with HT in younger women and long-term HT users.26, 27 Moreover, hormone use prior to WHI enrollment was not associated with differences in regional brain volumes in WHIMS-MRI.
The relationships between HT and hippocampal volumes varied significantly with baseline cognitive function, with a trend to similar effects for total brain volume. HT-associated reductions in hippocampal volume were greater in women with low cognitive function (3MS score < 90) at WHIMS baseline prior to WHI HT randomization. Reductions in total brain, hippocampal and frontal volumes in women randomized to HT also were observed in the 75% of women with vascular lesion burdens greater than 2 cm3, but not in women with lesion volumes less than 2 cm3. These findings parallel the earlier WHIMS report that the degree to which HT adversely affected cognitive function was greatest in women with the lowest baseline 3MS scores (p<0.001).4 It also is consistent with the short timeframe in which HT increased risk of dementia (4 to 5 years on average)1, 2, which seems to be too rapid to be linked to the primary initiation of a protracted disease process.
Greater vulnerability of postmenopausal women with low baseline cognitive function and higher lesion volumes to reduced brain volumes in association with HT is consistent with other evidence of the greater vulnerability of an already compromised brain28 and the potential that estrogen may adversely affect cognition among women with existing pathology.19 These findings also point to the growing body of evidence that vascular lesions and Alzheimer-type pathology act additively to influence the risk for clinical dementia.29 As hippocampal volume loss is a well-documented risk factor for dementia30 and may be a biomarker for AD-type neuropathology31, our findings suggest a possible contributory mechanism to HT-associated increase in dementia risk in women with low baseline cognitive function or existing neuropathology. Further research is required to elucidate whether the contribution of HT to lower total and regional brain volumes results from acceleration of Alzheimer-type pathology, from vascular disease, or some other mechanism.
The mechanism underlying this possible neurotoxicity is unclear. Results from the companion paper17 suggest that the effect is not conveyed primarily through an increase in ischemic lesions. It may be that there is an optimal level of estrogen exposure beyond which HT is neurotoxic.32 The optimum level may vary as a function of age or time since menopause as estrogen receptors may lose sensitivity in the absence of hormone exposure.33 CEE contains many equine estrogens that are not normally found in human blood and that have varying affinities to estradiol binding sites and a range of biologic activities.34, 35 While many constituents appear to have neuroprotective properties36, the role of others remains unclear.
While ours is the largest study conducted to date of possible HT effects on brain structure, a number of issues limit the generality of our findings. We investigated the effects of particular CEE-based hormone regimens in older postmenopausal women, aged 65 and older at initiation of treatment, and do not address possible effects in younger postmenopausal women. However, adverse effects of CEE+MPA on verbal memory (word list recall) were similar in older WHI participants37 and younger menopausal women with cognitive complaints.38 Another limitation is that MRI scans were conducted post-trial, on average 3.0 and 1.4 years post-trial for CEE+MPA and CEE-Alone. As pre-treatment MRI scans were not obtained, we have no information on brain volumes at baseline. However, the HT and placebo groups were well-balanced with respect to many dementia risk factors. We repeated analyses in Table 2 using propensity scores adjustment to account for potential differential enrollment39 which resulted in essentially identical results.
The automated approach to image processing may be prone to image registration errors, especially in some small regions. However, previous validation studies of this methodology40 have confirmed its accuracy in measuring hippocampal and lobar volumes. Moreover, total and regional brain volumes showed the predicted relationships with age and medical co-morbidities such as uncontrolled hypertension and diabetes, providing an internal validation of our approach. More refined analyses of smaller regions, including voxel-based analysis, may identify other regions of vulnerability to HT which potentially cannot be resolved via the current methodology. Finally, our study is cross-sectional and longitudinal volumetric studies may yield greater sensitivity to HT effects on brain.
Our findings emphasize the need for continued investigation of the joint effects of brain volume changes and vascular changes to further understanding of HT effects on cognitive and brain aging.
Supplementary Material
Acknowledgments
WHIMS-MRI Clinical Centers: Albert Einstein College of Medicine, Bronx, NY: Sylvia Wassertheil-Smoller, Mimi Goodwin, Richard DeNise, Michael Lipton, James Hannigan; Medical College of Wisconsin, Milwaukee: Jane Morley Kotchen, Diana Kerwin, John Ulmer, Steve Censky; Stanford Center for Research in Disease Prevention, Stanford University, CA: Marcia L. Stefanick, Sue Swope, Anne Marie Sawyer-Glover; The Ohio State University, Columbus: Rebecca Jackson, Rose Hallarn, Bonnie Kennedy; University of California at Davis, Sacramento: John Robbins, Sophia Zaragoza, Cameron Carter, John Ryan; University of California at Los Angeles: Lauren Nathan, Barbara Voigt, Pablo Villablanca, Glen Nyborg; University of Florida, Gainesville/Jacksonville: Marian Limacher, Sheila Anderson, Mary Ellen Toombs, Jeffrey Bennett, Kevin Jones, Sandy Brum, Shane Chatfield; University of Iowa, Davenport: Jennifer Robinson, Candy Wilson, Kevin Koch, Suzette Hart; University of Massachusetts, Worcester: Judith Ockene, Linda Churchill, Douglas Fellows, Anthony Serio; University of Minnesota, Minneapolis: Karen Margolis, Cindy Bjerk, Chip Truwitt, Margaret Peitso; University of Nevada, Reno: Robert Brunner, Ross Golding, Leslie Pansky; University of North Carolina, Chapel Hill: Carol Murphy, Maggie Morgan, Mauricio Castillo, Thomas Beckman; University of Pittsburgh, PA: Lewis Kuller, Pat McHugh, Carolyn Meltzer, Denise Davis.
WHIMS-MRI Clinical Coordinating Center: Wake Forest University Health Sciences, Winston-Salem, NC: Sally Shumaker, Mark Espeland, Laura Coker, Jeff Williamson, Debbie Felton, LeeAnn Andrews, Steve Rapp, Claudine Legault, Maggie Dailey, Julia Robertson, Patricia Hogan, Sarah Jaramillo, Pam Nance, Cheryl Summerville, Josh Tan.
WHIMS-MRI Quality Control Center: University of Pennsylvania, Philadelphia: Nick Bryan, Christos Davatzikos, Lisa Desiderio.
WHIMS-MRI Working Group: Wake Forest University Health Sciences, Winston-Salem, NC: LeeAnn Andrews; University of Pennsylvania, Philadelphia: Nick Bryan; Wake Forest University Health Sciences, Winston-Salem, NC: Laura Coker; Wake Forest University Health Sciences, Winston-Salem, NC: Mark Espeland; Wake Forest University Health Sciences, Winston-Salem, NC: Debbie Felton; University of Pittsburgh, PA: Lew Kuller; University of Minnesota, Minneapolis: Karen Margolis; University of Minnesota, Minneapolis: Anne Murray; Gerontology Research Center, National Institute on Aging, Baltimore, MD: Susan Resnick; Wake Forest University Health Sciences, Winston-Salem, NC: Sally Shumaker; Wake Forest University Health Sciences, Winston-Salem, NC: Jeff Williamson.
U.S. National Institutes of Health: National Institute on Aging, Bethesda, MD: Neil Buckholtz, Susan Molchan, Susan Resnick; National Heart, Lung, and Blood Institute, Bethesda, MD, Jacques Rossouw, Linda Pottern.
The Women’s Health Initiative and Women’s Health Initiative Memory Study (WHIMS)-MRI Study are funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health, U.S. Department of Health and Human Services. WHIMS was funded in part by Wyeth Pharmaceuticals, Inc, St. Davids, PA. Dr. Resnick is supported by the Intramural Research Program, NIA, NIH. Drs. Resnick and Espeland had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Disclosures: Dr. Espeland received salary support from Wyeth Pharmaceuticals from 1995–2003 as a WHIMS investigator. He also was compensated by Wyeth Pharmaceuticals from 2002–5 for serving on a monitoring board for an unrelated clinical trial. The remaining authors have nothing to disclose.
Footnotes
ClinicalTrials.gov Identifier: NCT00000611
Literature Cited
- 1.Shumaker SA, Legault C, Thal L, et al. Estrogen Plus Progestin and the Incidence of Dementia and Mild Cognitive Impairment in Postmenopausal Women: The Women’s Health Initiative Memory Study: A Randomized Controlled Trial. JAMA. 2003;289:2651–2662. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 2.Shumaker SA, Legault C, Kuller L, et al. Conjugated equine estrogens and incidence of probable dementia and mild cognitive impairment in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2947–2958. doi: 10.1001/jama.291.24.2947. [DOI] [PubMed] [Google Scholar]
- 3.Rapp SR, Espeland MA, Shumaker SA, et al. Effect of Estrogen Plus Progestin on Global Cognitive Function in Postmenopausal Women: The Women’s Health Initiative Memory Study: A Randomized Controlled Trial. JAMA. 2003;289:2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- 4.Espeland MA, Rapp SR, Shumaker SA, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women’s Health Initiative Memory Study. JAMA. 2004;291:2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- 5.Wassertheil-Smoller S, Hendrix SL, Limacher M, et al. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289:2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 6.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 7.Boccardi M, Ghidoni R, Govoni S, et al. Effects of hormone therapy on brain morphology of healthy postmenopausal women: a Voxel-based morphometry study. Menopause. 2006;13:584–591. doi: 10.1097/01.gme.0000196811.88505.10. [DOI] [PubMed] [Google Scholar]
- 8.Erickson KI, Colcombe SJ, Raz N, et al. Selective sparing of brain tissue in postmenopausal women receiving hormone replacement therapy. Neurobiol Aging. 2005;26:1205–1213. doi: 10.1016/j.neurobiolaging.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Eberling JL, Wu C, Haan MN, Mungas D, Buonocore M, Jagust WJ. Preliminary evidence that estrogen protects against age-related hippocampal atrophy. Neurobiol Aging. 2003;24:725–732. doi: 10.1016/s0197-4580(02)00056-8. [DOI] [PubMed] [Google Scholar]
- 10.Lord C, Buss C, Lupien SJ, Pruessner JC. Hippocampal volumes are larger in postmenopausal women using estrogen therapy compared to past users, never users and men: A possible window of opportunity effect. Neurobiol Aging. 2008;29:95–101. doi: 10.1016/j.neurobiolaging.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Resnick SM, Maki PM, Golski S, Kraut MA, Zonderman AB. Estrogen effects on PET cerebral blood flow and neuropsychological performance. Hormones and Behavior. 1998;34:171–182. doi: 10.1006/hbeh.1998.1476. [DOI] [PubMed] [Google Scholar]
- 12.Rasgon NL, Small GW, Siddarth P, et al. Estrogen use and brain metabolic change in older adults. A preliminary report. Psychiatry Res. 2001;107:11–18. doi: 10.1016/s0925-4927(01)00084-1. [DOI] [PubMed] [Google Scholar]
- 13.Eberling JL, Reed BR, Coleman JE, Jagust WJ. Effect of estrogen on cerebral glucose metabolism in postmenopausal women [In Process Citation] Neurology. 2000;55:875–877. doi: 10.1212/wnl.55.6.875. [DOI] [PubMed] [Google Scholar]
- 14.Eberling JL, Wu C, Tong-Turnbeaugh R, Jagust WJ. Estrogen- and tamoxifen-associated effects on brain structure and function. Neuroimage. 2004;21:364–371. doi: 10.1016/j.neuroimage.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 15.Shaywitz SE, Shaywitz BA, Pugh KR, et al. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA. 1999;281:1197–1202. doi: 10.1001/jama.281.13.1197. [DOI] [PubMed] [Google Scholar]
- 16.Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 17.Coker LH, Hogan PS, Bryan NR, et al. Postmenopausal hormone therapy and subclinical cerebrovascular disease: the WHIMS-MRI study. 2008 doi: 10.1212/01.wnl.0000339036.88842.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teng EL, Chui H. The Modified Mini-Mental State (3MS) Exam. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 19.Wise PM, Dubal DB, Rau SW, Brown CM, Suzuki S. Are estrogens protective or risk factors in brain injury and neurodegeneration? Reevaluation after the Women’s health initiative. Endocr Rev. 2005;26:308–312. doi: 10.1210/er.2004-0014. [DOI] [PubMed] [Google Scholar]
- 20.Shumaker SA, Reboussin BA, Espeland MA, et al. The Women’s Health Initiative Memory Study (WHIMS): a trial of the effect of estrogen therapy in preventing and slowing the progression of dementia. Control Clin Trials. 1998;19:604–621. doi: 10.1016/s0197-2456(98)00038-5. [DOI] [PubMed] [Google Scholar]
- 21.Jaramillo SA, Felton D, Andrews L, et al. Enrollment in a brain magnetic resonance study: results from the Women’s Health Initiative Memory Study Magnetic Resonance Imaging Study (WHIMS-MRI) Acad Radiol. 2007;14:603–612. doi: 10.1016/j.acra.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gustafson D. A life course of adiposity and dementia. Eur J Pharmacol. 2008;585:163–175. doi: 10.1016/j.ejphar.2008.01.052. [DOI] [PubMed] [Google Scholar]
- 23.Coffey CE, Saxton JA, Ratcliff G, Bryan R, Lucke JF. Relation of education to brain size in normal aging: implications for the reserve hypothesis. Neurology. 1999;53:189–196. doi: 10.1212/wnl.53.1.189. [DOI] [PubMed] [Google Scholar]
- 24.Goldszal AF, Davatzikos C, Pham DL, Yan MX, Bryan RN, Resnick SM. An image-processing system for qualitative and quantitative volumetric analysis of brain images. J Comput Assist Tomogr. 1998;22:827–837. doi: 10.1097/00004728-199809000-00030. [DOI] [PubMed] [Google Scholar]
- 25.Shen D, Davatzikos C. HAMMER: hierarchical attribute matching mechanism for elastic registration. IEEE Trans Med Imaging. 2002;21:1421–1439. doi: 10.1109/TMI.2002.803111. [DOI] [PubMed] [Google Scholar]
- 26.Low LF, Anstey KJ, Maller J, et al. Hormone replacement therapy, brain volumes and white matter in postmenopausal women aged 60–64 years. Neuroreport. 2006;17:101–104. doi: 10.1097/01.wnr.0000194385.10622.8e. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg DL, Payne ME, MacFall JR, Provenzale JM, Steffens DC, Krishnan RR. Differences in brain volumes among males and female hormone-therapy users and nonusers. Psychiatry Res. 2006;147:127–134. doi: 10.1016/j.pscychresns.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 28.Gamaldo A, Moghekar A, Kilada S, Resnick SM, Zonderman AB, O’Brien R. Effect of a clinical stroke on the risk of dementia in a prospective cohort. Neurology. 2006;67:1363–1369. doi: 10.1212/01.wnl.0000240285.89067.3f. [DOI] [PubMed] [Google Scholar]
- 29.Schneider JA, Wilson RS, Cochran EJ, et al. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003;60:1082–1088. doi: 10.1212/01.wnl.0000055863.87435.b2. [DOI] [PubMed] [Google Scholar]
- 30.Jack CR, Jr, Shiung MM, Weigand SD, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology. 2005;65:1227–1231. doi: 10.1212/01.wnl.0000180958.22678.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bobinski M, de Leon MJ, Wegiel J, et al. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer’s disease. Neuroscience. 2000;95:721–725. doi: 10.1016/s0306-4522(99)00476-5. [DOI] [PubMed] [Google Scholar]
- 32.Chen S, Nilsen J, Brinton RD. Dose and temporal pattern of estrogen exposure determines neuroprotective outcome in hippocampal neurons: therapeutic implications. Endocrinology. 2006;147:5303–5313. doi: 10.1210/en.2006-0495. [DOI] [PubMed] [Google Scholar]
- 33.Toran-Allerand CD. Estrogen as a treatment for Alzheimer disease [letter; comment] JAMA. 2000;284:307–308. [PubMed] [Google Scholar]
- 34.de Lignieres B, Silberstein S. Pharmacodynamics of oestrogens and progestogens. Cephalalgia. 2000;20:200–207. doi: 10.1046/j.1468-2982.2000.00042.x. [DOI] [PubMed] [Google Scholar]
- 35.Turgeon JL, Carr MC, Maki PM, Mendelsohn ME, Wise PM. Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: Insights from basic science and clinical studies. Endocr Rev. 2006;27:575–605. doi: 10.1210/er.2005-0020. [DOI] [PubMed] [Google Scholar]
- 36.Zhao L, Brinton RD. Select estrogens within the complex formulation of conjugated equine estrogens (Premarin) are protective against neurodegenerative insults: implications for a composition of estrogen therapy to promote neuronal function and prevent Alzheimer’s disease. BMC Neurosci. 2006;7:24. doi: 10.1186/1471-2202-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Resnick SM, Maki PM, Rapp SR, et al. Effects of combination estrogen plus progestin hormone treatment on cognition and affect. J Clin Endocrinol Metab. 2006;91:1802–1810. doi: 10.1210/jc.2005-2097. [DOI] [PubMed] [Google Scholar]
- 38.Maki PM, Gast MJ, Vieweg AJ, Burriss SW, Yaffe K. Hormone therapy in menopausal women with cognitive complaints: a randomized, double-blind trial. Neurology. 2007;69:1322–1330. doi: 10.1212/01.wnl.0000277275.42504.93. [DOI] [PubMed] [Google Scholar]
- 39.Rubin DB. Using multivariate matched sampling and regression adjustment to control bias in observational studies. Journal of the American Statistical Association. 1979;74:318–324. [Google Scholar]
- 40.Shen D, Moffat S, Resnick SM, Davatzikos C. Measuring size and shape of the hippocampus in MR images using a deformable shape model. Neuroimage. 2002;15:422–434. doi: 10.1006/nimg.2001.0987. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.