Abstract
Neural correlates of face processing were examined in 12-month-olds at high-risk for autism spectrum disorder (ASD), including 21 siblings of children with ASD (ASIBs) and 15 infants with fragile X syndrome (FXS), as well as 21 low-risk (LR) controls. Event-related potentials were recorded to familiar and novel face and toy stimuli. All infants demonstrated greater N290 amplitude to faces than toys. At the Nc component, LR infants showed greater amplitude to novel stimuli than to their mother’s face and own toy, whereas infants with FXS showed the opposite pattern of responses and ASIBs did not differentiate based on familiarity. These results reflect developing face specialization across high- and low-risk infants and reveal neural patterns that distinguish between groups at high-risk for ASD.
Atypical processing of faces is one of the most commonly documented areas of abnormal visual attention in individuals diagnosed with autism spectrum disorders (ASD; e.g., Grelotti, Gauthier, & Schultz, 2002; Hubl et al., 2003; McPartland, Dawson, Webb, Panagiotides, & Carver, 2004). Recent research indicates that these differences emerge early in development, with infants at an increased risk of ASD displaying different electrophysiological responses from low-risk infants within the first year of life (e.g., Key et al., 2014; Key & Stone, 2012; McCleery, Akshoomoff, Dobkins, & Carver, 2009). Existing studies of high-risk infants have primarily focused on infant siblings of children with ASD, and it is unclear whether similar abnormalities are present in other high-risk groups. In the current study, we measured event-related potentials during a face processing task in two samples of infants at high risk of ASD – infant siblings of children diagnosed with ASD (ASIBs) and infants diagnosed with fragile X syndrome (FXS), as well as low-risk (LR) controls. We expected to observe distinct electrophysiological responses that would differentiate the high-risk ASD groups from each other and from the low-risk group.
Event-related potentials (ERPs) provide a discrete timeline of neural activation associated with face processing and reveal distinct differences in neural responses to faces in adults with ASD compared with typical adults (e.g., McPartland et al., 2004). The N170 ERP component has been most strongly associated with face detection and processing in adults and is characterized by a negative peak occurring approximately 170 ms after stimulus onset at lateral posterior scalp regions. Both typical adults and adults with ASD show greater amplitude N170 to faces compared with objects (e.g., Bentin, Allison, Puce, Perez, & McCarthy, 1996; McPartland et al., 2004; Neuhaus, Kresse, Faja, Bernier, & Webb, 2015; Rossion et al., 2000; Webb et al., 2010, 2012). However, adults with ASD have shown a longer latency to peak N170 than typical controls (McPartland et al., 2004, 2011). Additionally, adults with ASD do not show a right hemisphere advantage for faces, which is typically seen in adults and reflected by greater amplitude N170 at right than left lateral electrodes (McPartland et al., 2004), nor do they exhibit greater amplitude N170 responses to inverted compared with upright faces, which is exhibited in typical adults (McPartland et al., 2011; Webb et al., 2012). Thus, although adults with ASD exhibit typical increases in N170 amplitude toward faces versus objects, the temporal and spatial characteristics of the N170 response remain atypical.
Because ASD is not typically diagnosed until toddlerhood or later, recent studies have examined electrophysiological responses to faces in infants at high-risk of ASD as a means to understanding early developmental sequences of risk. This area of research has focused primarily on infant siblings of children with ASD (ASIBs), as ASD diagnosis is 18 to 20 times more prevalent in ASIBs than in the general population (Ozonoff et al., 2011). In addition to exhibiting increased rates of ASD, first-degree relatives of individuals with ASD have been shown to exhibit higher rates of subclinical ASD symptoms than the general population, a phenomenon known as the broader autism phenotype (BAP; Dawson et al., 2002; Losh, Childress, Lam, & Piven, 2008; Ozonoff et al., 2014). Subclinical symptoms associated with the BAP may manifest through atypical cognitive and social function (Landa, Gross, Stuart, & Bauman, 2012; Messinger et al., 2013). Thus, investigating early patterns of face processing among ASIBs can inform both early markers of ASD risk, as well as broader endophenotypes of ASD that manifest in clinically unaffected individuals.
Event-related potentials have been used to study the development of face processing in infant ASIBs (Elsabbagh et al., 2009; Key et al., 2014; Key & Stone, 2012; Luyster, Powell, Tager-Flusberg, & Nelson, 2014; Luyster et al., 2011; McCleery et al., 2009). Of primary interest to these studies are two infant ERP components strongly associated with face processing, the N290 and P400. It has been hypothesized that the N290 and P400 together function as the precursor to the adult N170 (e.g., Luyster et al., 2014). However, recent research has indicated that the N290 is more strongly linked to face processing, as cortical source analysis has localized the component to temporal and occipital brain regions including the middle fusiform gyrus (Guy, Zieber, & Richards, 2016).
Like the N170, the N290 is a negative ERP component that occurs over lateral posterior electrodes and is characterized by greater amplitude to faces than other classes of stimuli (Halit, Csibra, Volein, & Johnson, 2004; Guy et al., 2016). The results of a recent study examining N290 responses in conjunction with infant heart rate-defined phases of attention indicate that sustained attention may further contribute to the differentiation of face and toy stimuli in 4.5- to 7.5-month-old infants (Guy et al., 2016). This typical pattern of greater amplitude responses to faces than objects has also been observed in ASIBs (McCleery et al., 2009). However, ASIBs showed a shorter latency N290 response to objects than faces, whereas LR infants showed a trend of responses in the opposite direction (McCleery et al., 2009). Additionally, research conducted with LR and ASIB infants has indicated that N290 amplitude may be sensitive to stimulus exposure (Key & Stone, 2012; Luyster et al., 2014), although several studies of 4.5- through 12-month-old LR infants (de Haan & Nelson, 1997, 1999; Guy et al., 2016; Luyster et al., 2011; McCleery et al., 2009) and ASIB infants (Luyster et al., 2014; McCleery et al., 2009) have failed to find a significant effect of stimulus familiarity on N290 amplitude. Although the N290 is relevant to ASD due to its association with face processing, the limited ERP studies in ASIBs to date have failed to identify consistent group-specific differences related to ASD risk on N290 amplitude, latency, or topography.
The P400 is a positive component that is seen over occipital electrodes (de Haan, Johnson, & Halit, 2003). Research conducted with low-risk infants has reported shorter latency P400 responses to face stimuli than other classes of stimuli (de Haan & Nelson, 1999; Halit et al., 2004; McCleery et al., 2009). McCleery and colleagues (2009) found that 10-month-old ASIBs did not demonstrate this face processing advantage and exhibited slower P400 latencies to faces than LR infants. Differences in P400 amplitude and latency have been reported based on stimulus exposure, such that LR and ASIB infants showed greater P400 amplitude to a stranger’s face than their mother’s face, but only LR infants showed longer P400 latency to the stranger’s face than their mother’s face (Key & Stone, 2012). These studies indicate that the investigation of the P400 may be informative to the examination of atypical face processing in ASIBs during infancy.
Additionally, the Negative central (“Nc;” Courchesne, Ganz, & Norcia, 1981) is an ERP component that is relevant to the examination of infant face processing and that has been examined in studies of ASIBs. The Nc is a negative ERP component seen from 350 to 750 ms after stimulus onset at frontal and central midline electrodes. The Nc is evident in response to a variety of visual stimuli. It is greater in amplitude in response to salient or novel stimuli and during heart rate defined stages of attention (de Haan & Nelson, 1997, 1999; Guy, Reynolds, & Zhang, 2013; Guy et al., 2016; Reynolds, Courage, & Richards, 2010; Richards, 2003; Webb, Long, & Nelson, 2005). In several studies of face processing in the first year of life, Nc amplitude has been reported to be greater in response to an infant’s mother’s face than a stranger’s face (e.g., de Haan & Nelson, 1997, 1999; Luyster et al., 2014; Webb et al., 2005). However, additional research including ASIBs and LR infants suggest variable patterns in response to these stimuli across age and risk status. Key and Stone (2012) found that 9-month-old LR and ASIB infants showed greater amplitude Nc responses to a stranger’s face than their mothers’ faces. This effect was replicated in a separate cohort of 12-month-old infants and was stronger in LR infants, as a larger proportion of LR infants demonstrated greater Nc amplitude to a stranger’s face than their mother’s face (Luyster et al., 2011). However, a recent longitudinal examination of LR and ASIB infants from 6 to 36 months of age found that LR infants consistently showed greater Nc amplitude to their mother’s face than a stranger’s face, but ASIBs did not respond differentially across ages (Luyster et al., 2014). Overall, these studies suggest that the Nc may be sensitive to emerging differences in attention to salient and novel faces among ASIBs.
Notably, these extant electrophysiological studies of face processing in high-risk infants have exclusively focused on ASIBs, and no studies to date have contrasted patterns of face processing with other high-risk groups such as infants with ASD-associated genetic syndromes. Fragile X syndrome (FXS), a single gene trinucleotide (CGG) repeat disorder located on the FMR1 gene (Xq27.3), affects approximately 1 in 3,700–8,900 males (Coffee et al., 2009; Crawford, Acuña, & Sherman, 2001; Hunter et al., 2014). FXS is the most common known genetic cause of ASD, accounting for approximately 5% of cases (Hagerman, Rivera, & Hagerman, 2008), with co-morbidity of ASD with FXS associated with deleterious phenotypic effects (Bailey, Raspa, Olmsted, & Holiday, 2008; Hatton et al., 2006; Loesch et al., 2007). The relation between FXS and ASD is well established, with 60–74% of FXS cases meeting criteria for ASD (Clifford et al., 2007; Harris et al., 2008; Kaufman et al., 2004; Philofsky, et al., 2004). There is considerable interest in studying the association of FXS and ASD due to both the clinical consequences of their co-occurrence and potential to increase understanding of ASD (Budimirovic & Kaufmann, 2011). Controversy exists, however, regarding the shared phenomenology across these two etiologically distinct disorders, as some studies indicate a high degree of concordance (Bailey, Hatton, & Skinner, 1998; Dissanayake, et al., 2009; Rogers, Wehner, & Hagerman, 2001), yet others report distinct neurobiological pathways and behavioral presentations. For instance, Rogers and colleagues (2001) compared the development of toddlers diagnosed with FXS, ASD, and developmental delay and found that half of the toddlers with FXS were nearly identical in ASD related behavior and symptoms to the toddlers with ASD, while the other half of the FXS sample scored very similarly to the toddlers with developmental delay. Given the complex, overlapping symptom profiles associated with FXS and ASD, examining the early emergence of ASD-associated features in FXS may inform early risk factors specific to FXS, as well as broader heterogeneous pathways of ASD emergence (McCary & Roberts, 2013).
Relative to ASIBs and those diagnosed with ASD, few studies have examined the development of face processing in FXS. Farzin, Rivera, and Hessl (2009) found that adults with FXS made fewer fixations to faces than control adults during a passive face viewing task, but fixation patterns were not significantly correlated with symptoms of ASD. In a neuroimaging study, adults with FXS or ASD and control participants were asked to determine whether photographs of faces were emotional or neutral (Dalton, Holsen, Abbeduto, & Davidson, 2008). FXS and ASD groups exhibited similar emotional recognition accuracy, and demonstrated decreased activation of the fusiform gyrus relative to controls. Decreased fusiform gyrus activation in individuals with FXS has been reported elsewhere (e.g., Garrett, Menon, MacKenzie, & Reiss, 2004), and could indicate that face processing is less automatic in FXS compared with typical adolescents and adults. Although face processing has not been examined in infants with FXS, previous research has shown that during a toy play task, 12-month-olds with FXS show longer look durations, take longer to disengage, and experience more shallow heart rate decelerations during periods of attention than control infants, all indicative of a muted attentional response (Roberts et al., 2012). These results suggest that face processing deficits are present in FXS, although the emergence of these behaviors in early childhood is unclear.
The aim of the current study was to examine electrophysiological correlates of specialized face processing in two etiologically distinct groups of 12-month-old infants at high-risk for ASD based on a diagnosis of FXS or familial history of ASD (i.e., an older sibling diagnosed with ASD; “ASIBs”) contrasted to LR controls. Differences in amplitude and latency of face-sensitive ERP components (N290, P400) and the Nc ERP component were measured in response to each participant’s mother’s face, a stranger’s face, their own toy, and a novel toy. Past research has indicated that younger infants demonstrate greatest differentiation of face and object processing during heart-rate defined periods of attention (Guy et al., 2016), and we measured electrocardiogram (ECG) to examine the effect of heart rate-defined attention versus inattention on ERP responses in the current study. Consistent with previous studies, we expected ASIBs to exhibit (1) typical patterns of greater N290 amplitude to faces than toys, (2) slower latency P400 responses to faces relative to LR controls, and (3) greater Nc amplitude toward the mother’s face compared with the stranger’s face, while LR infants were expected to demonstrate greater Nc amplitude to the stranger’s face than the mother’s face. We expected that infants with FXS would display a similar pattern of responses to ASIBs due to shared risk for ASD, although we also predicted that the FXS group would exhibit developmentally immature responses, reflecting developmental delay. Specifically, we predicted that infants with FXS would demonstrate less differentiation of faces and toys at the N290 relative to control infants, or they would only differentiate these stimuli during HR-defined phases of attention, similar to results recently reported in younger infants (Guy et al., 2016). Additionally, infants with FXS were expected to demonstrate a greater amplitude Nc response to the mother’s face than the stranger’s face.
Method
Participants
Fifty-seven 12-month-old infants were recruited for the current study. Demographic information is included in Table 1. Participants included 21 LR infants, 21 ASIBs, and 15 infants with FXS. An additional three infants were tested but not included in the final sample due to technical errors (n = 1), insufficient ERP data (n = 1), and diagnosis with a neurological disorder (n = 1). Low-risk infants had no known developmental anomalies, no family history of ASD, and were recruited from the Columbia, SC area. ASIBs were recruited through the South Carolina Department of Disabilities and Special Needs. Letters were mailed to families in South Carolina with a child with an ASD diagnosis describing the current study and inviting participation from families with an infant sibling. Infants with FXS were identified through collaborations with researchers across the United States in addition to emails and postings through social media (e.g., LISTSERVs, Facebook). All infants were full term (at least 38 weeks gestation, birth weight at least 2,500 g). Participants were primarily Caucasian and of middle socioeconomic status. All infants participated with the informed, signed consent of their parents.
Table 1.
Participant demographics
| ASIB | FXS | TD | |
|---|---|---|---|
| n | 21 | 15 | 21 |
| n male (%) | 18 (86%) | 8 (53%) | 16 (76%) |
| Age | 12.68 (0.80) | 12.29 (0.38) | 12.28 (0.37) |
| Race | |||
| Caucasian | 18 | 11 | 20 |
| Hispanic | 1 | 1 | 0 |
| Black | 2 | 2 | 1 |
| Asian | 0 | 1 | 0 |
| * AOSI Total Score | 5.62 (3.26) | 8.60 (6.25) | 1.14 (3.45) |
| * AOSI Number of Markers | 3.86 (1.98) | 5.00 (3.32) | 0.67 (1.74) |
| * MSEL Early Learning Composite | 99.50 (14.03) | 83.47 (20.29) | 99.84 (11.11) |
| * MSEL Age Equivalent | 12.44 (1.60) | 10.27 (2.88) | 12.20 (1.25) |
Data were not available from three participants (2 TD, 1 ASIB)
MSEL = Mullen Scales of Early Learning
Apparatus
The experiment took place in a darkened room, where participants were seated on a parent’s lap, approximately 55 cm away from a 29″ LCD monitor (NEC Multisync XM29). The participants’ looking behavior was recorded by a video camera positioned above the monitor. An experimenter judged infant fixation online and controlled stimulus presentation using Electrical Geodesics Inc. (EGI) Net Station software and an E-Prime experiment program from an adjacent room.
Stimuli
Stimuli included photographs of female faces and infant toys. Sesame Street characters were used as attractors. All stimuli were presented on colorful, variegated backgrounds. Faces and toys: The face and toy stimuli were created from photographs taken upon participant arrival at the lab. Photographs were taken from a straight-on view of the mother’s face and the infant’s toy. The images of the participant’s mother and toy were paired with photographs of the mother and toy from the previous participant. The images measured approximately 17° visual angle. Sesame Street Characters: Dynamic videos of 15 Sesame Street characters were used as attractor stimuli. Video segments of individual Sesame Street characters were taken from the movie, “Sesame Street’s 25th Birthday: A Musical Celebration!” Attractor stimuli were presented in a 2° × 3° area in the center of the screen. Backgrounds: Five static backgrounds containing simple patterns, such as water, sand, and grass, were presented on the entire monitor.
Procedure
The experiment began when the participant became fixated on the center of the monitor. A button press by the experimenter initiated a sequence of brief image presentations and paired comparison (PC) trials. Each brief stimulus presentation began with a blank screen for a period of 100 ms, followed by a 500 ms stimulus presentation, and a variable inter-trial interval of 500–1500 ms. This ITI was selected to facilitate infant attention and the collection of a greater number of trials during the ERP task (Xie & Richards, 2016). The PC trials consisted of side-by-side presentations of the two faces (mother and stranger) or the two toys (familiar toy and novel toy). Each PC presentation lasted until 4 s of looking time was accumulated. The PC and brief trials were presented in random order in a 10-trial block, including two brief presentations of each face and toy stimulus, one face PC, and one toy PC. Sample presentations from this experimental procedure are presented in the Supplemental Information of Guy et al. (2016). At the beginning of the experiment or if the infant looked away from the screen, a dynamic Sesame Street video clip was presented to attract fixation. After completion of the experiment, a digital video recording was viewed offline to confirm infant looking during the brief stimulus presentations and to measure visual preferences during the PC presentations. The purpose of the PC trials was to generate preference measures for familiar and unfamiliar faces and toys (as well as to keep the infant interested), but these trials were not included in the ERP analyses. The experiment continued for as long as the infant was not fussy in order to collect as much data as possible.
ECG Recording and Heart-Rate Defined Attention
Two Ag-AgCl electrodes were placed on the chest to measure the ECG. They were digitized with the EGI 128-channel EEG recording system concurrently with the EEG data. The ECG data was analyzed offline to assess changes in HR used to define periods of attention and inattention in a continuous presentation method (Guy et al., 2016; Mallin & Richards, 2012; Pempek et al., 2010; Reynolds et al., 2010). Periods of attention included periods when the infant was looking toward the screen and showed a deceleration of HR below the prestimulus level (five beats with inter-beat intervals [IBIs] > median prestimulus IBIs) and sustained lowered HR (IBIs > prestimulus median). The periods of inattention included periods when the infant looked toward the screen before the HR deceleration occurred or continued looking toward the screen after the lowered HR returned to prestimulus levels. If the infant looked away the trial was not included in the analyses, the attention phase was undefined, and the sequence began again when the infant looked back toward the screen.
EEG Recording and Segmentation
The EEG was recorded using the EGI 128-channel EEG recording system (Johnson et al., 2001; Tucker, 1993). Participants’ were fitted with a “hydrocel geodesic sensor net” (HGSN), chosen based on the infant’s head circumference. Net application took 5–10 minutes, during which a second experimenter entertained the infant with various toys. EEG was measured from 124 channels in the electrode net and two Ag-AgCl electrodes measured electrooculogram (EOG). The EEG signal was referenced to the vertex, recorded with 20K amplification at a 250 Hz sampling rate with bandpass filters set from 0.1–100 Hz and 100 kΩ impedance. The vertex-referenced EEG was algebraically recomputed to an average reference. Recorded EEG was inspected for artifacts (ΔEEG > 100 μV), poor recordings, and blinks using the ERPLAB toolbox in Matlab and visual inspection of the EOG data. Individual channels or locations within trials were eliminated from the analyses if these occurred and if more than 10 channels within a trial were affected, that trial was rejected from further analysis.
ERP Data Analysis
The EEG was filtered with a 0.5 Hz high-pass bandpass filter and the ERP trials were segmented from 50 ms before stimulus onset through 1 s following onset. The ERP data processing procedure was completed using the EEGLAB and ERPLAB toolboxes (Delorme & Makeig, 2004; Lopez-Calderon & Luck, 2014) within MATLAB (MATLAB R2014a, the Mathworks, Inc.). Clusters of virtual “10–10” electrodes were created from the mean of EGI electrodes surrounding the traditional 10–10 electrode locations (see Guy et al., 2016, Supplemental Information). The N290 was examined at lateral posterior-inferior scalp areas including Parietal Occipital (PO7: 59, 65, 66; PO8: 84, 90, 91; PO9: 64, 65, 68, 69; PO10: 89, 90, 94, 95), Parietal (P7: 51, 58, 59; P8: 91, 96, 97; P9: 57, 58, 63, 64; P10: 95, 96, 99, 100), and Temporal Parietal electrodes (TP7: 46, 50, 51; TP8: 97, 101, 102, TP9: 50, 56, 57; TP10: 100, 101, 107). The P400 was examined at medial posterior-inferior scalp areas including Parietal Occipital (PO7–10), Occipital (Oz: 71, 75, 76; O1: 66, 70, 71; O2: 76, 83, 84), and Inion electrodes (Iz: 74, 75, 81, 82; I1: 69, 70, 73, 74; I2: 82, 83, 88, 89). The Nc was analyzed at frontal and central midline virtual electrodes (Fz: 5, 10, 11, 12, 16, 18; FCz: 5, 6, 7, 12, 106; Cz: 7, 31, 55, 80, 106).
Mixed-design ANOVAs were calculated to determine the effects of participant group, stimulus type, stimulus familiarity, attention phase, and electrode hemisphere on N290, P400, and Nc peak amplitude and N290 and P400 peak latency. The N290 peak amplitude was calculated from the positive peak proceeding the N290 to the negative trough of the N290. Due to the unequal distribution of the number of trials in the cells of the factorial design, the ANOVAs were completed using the “Proc GLM” of SAS with a general linear models approach using nonorthogonal design (see Searle, 1987). The statistical tests used error terms derived from the related interval effect analyses and Scheffe-type methods to control for inflation of test wise error rate. Simple effects were examined through the calculation of least squares means with Bonferroni corrections for multiple comparisons. All significant tests are reported at p < .05 and effect sizes (Cohen’s d) are reported to describe comparisons within significant effects.
Results
On average each participant contributed over 60 total trials to the ERP analysis (M = 67.26, SD = 27.00). LR infants contributed an average of 58.33 trials (SD = 22.31), while ASIBs contributed 66.95 trials on average (SD = 28.91), and infants with FXS contributed an average of 80.20 trials (SD = 26.70). The number of trials contributed to the analyses did not vary across faces (M = 33.35) and toys (M = 33.91) for any of the participant groups (LR: faces M = 29.00, toys M = 29.33; ASIB: faces M = 33.24, toys M = 33.71; FXS: faces M = 39.60, toys M = 40.60). Overall, participants contributed more good trials during periods of attention (M = 39.68) than inattention (M = 27.58). This trend was also observed in all participant groups (LR: attention M = 36.67, inattention M = 21.67; ASIB: attention M = 36.86, inattention M = 30.10; FXS: attention M = 47.87, inattention M = 32.33). An average of 15.51 trials were rejected from each participant’s analysis due to looks away or excessive ERP artifact. The number of rejected trials was very similar across groups (LR: M = 16.05; ASIB: M = 14.23; FXS: M = 16.53).
N290 Amplitude
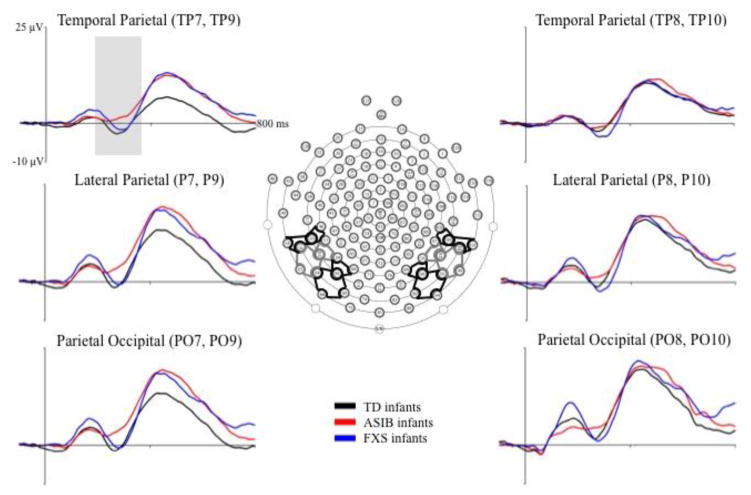
Figure 1 presents grand average N290 responses by group across left and right electrode clusters included in the analyses. The N290 is evident as a negative deflection in the ERP occurring approximately 300 ms after stimulus onset. Table 2 shows the mean N290 peak amplitude for the data in Figure 1, separately by electrode cluster, electrode hemisphere, and the three participant groups. The N290 amplitude was largest in the Parietal and Parietal Occipital clusters, and appears to be largest for the infants with FXS, followed by the LR infants, and was smallest in the ASIBs. This interaction was not tested in the current design, as ERP components were averaged across electrode clusters of interest to focus analyses on the effects of the factors: participant group, stimulus type, stimulus familiarity, HR-defined attention phase, and electrode hemisphere.
Figure 1.
Grand average N290 responses by group at Parietal Occipital, Lateral Parietal, and Temporal Parietal electrode clusters. The grand average ERP activity is shown from 100 ms preceding stimulus onset through 800 ms post stimulus onset for the three participant groups at electrode clusters included in the N290 analysis.
Table 2.
Mean N290 peak amplitude (μV) and corresponding standard errors by group across left and right Parietal Occipital, Parietal, and Temporal Parietal electrode clusters
| Left | Right | |||||
|---|---|---|---|---|---|---|
| Group | Parietal Occipital | Parietal | Temporal Parietal | Parietal Occipital | Parietal | Temporal Parietal |
| TD | −10.93 (0.726) | −10.90 (0.802) | −9.09 (0.646) | −9.75 (0.779) | −10.50 (0.794) | −8.85 (0.725) |
| ASIB | −7.70 (0.694) | −7.47 (0.832) | −6.80 (0.595) | −7.83 (0.720) | −9.06 (0.853) | −8.81 (0.612) |
| FXS | −13.31 (0.915) | −11.78 (1.068) | −9.32 (0.798) | −14.78 (0.928) | −13.17 (1.035) | −9.01 (0.752) |
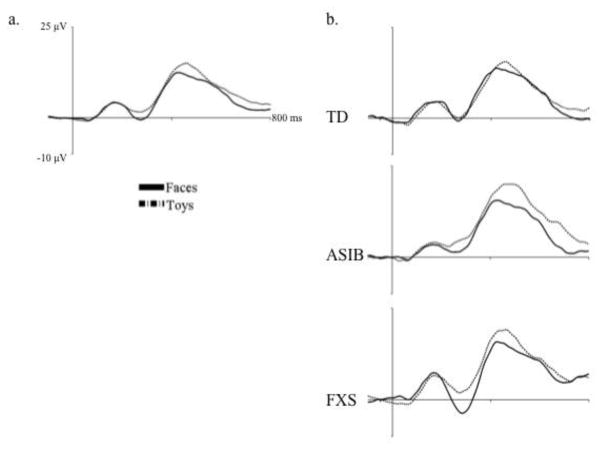
The effects of stimulus type and stimulus familiarity on N290 amplitude were examined, as well as their interaction with participant group and electrode hemisphere. An ANOVA was carried out that included group (3: LR, ASIB, FXS), electrode hemisphere (2: left, right), stimulus type (2: faces, toys), and stimulus familiarity (2: familiar, novel) as factors. Results included a significant main effect of stimulus type, F (1, 54) = 11.92, p = .0011. Figure 2a shows N290 amplitude as a function of stimulus type averaged across all participant groups. It can be seen that responses were significantly greater to faces, M = −11.11 μV, than toys, M = −8.39 μV, p < .0001, d = .201. Figure 2b shows N290 amplitude for faces and toys separately for each participant group. Although the stimulus type by participant group interaction was not significant, differences between N290 responses to faces and toys were greatest in infants with FXS. There was a marginally significant interaction of stimulus familiarity and participant group, F (2, 54) = 3.01, p = .0578. This interaction was due to greater N290 amplitude in response to familiar stimuli in infants with FXS, M = −14.50 μV, than LR infants, M = −9.88 μV, d = .347, and ASIBs, M = −7.70 μV, d = .497, all ps < .0001. The FXS group’s responses to familiar stimuli were also greater in amplitude than all groups’ responses to novel stimuli: LR, M = −10.13 μV, d = .318, ASIB, M = −8.20 μV, d = .477, FXS, M = −9.29 μV, d = .373, all ps < .0001. LR and ASIB infants did not differentiate familiar from novel stimuli based on N290 amplitude. These results indicate that all groups demonstrated a greater N290 response to faces than toys, but only infants with FXS responded differentially based stimulus familiarity.
Figure 2.
The N290 in response to faces and toys, (a) across all participant groups and (b) separately for each participant group. In the left figure, the N290 is shown in response to face and toy stimuli averaged across participant groups. The right figure panel presents N290 responses to faces and toys separately for each participant group.
To examine the effect of stimulus type and attention on N290 amplitude an ANOVA was conducted, including group (3: LR, ASIB, FXS), electrode hemisphere (2: left, right), stimulus type (2: faces, toys), and HR-defined attention phase (2: attention, inattention). The main effect of stimulus type was replicated, F (1, 54) = 11.94, p = .0011. However, there were no significant effects or interactions of attention on N290 amplitude.
N290 Latency
Latency to the N290 peak was examined across participant group, electrode hemisphere, stimulus type, stimulus familiarity, and HR-defined attention phase. An ANOVA was calculated including the factors participant group (3: LR, ASIB, FXS), electrode hemisphere (2: left, right), stimulus type (2: faces, toys), and stimulus familiarity (2: familiar, novel). There was a significant interaction of participant group and electrode hemisphere, F (2, 54) = 4.84, p = .0117. Least squares means revealed that the latency to the N290 peak was significantly shorter in ASIBs at left electrodes, M = 277.07 ms, than LR infants at left electrodes, M = 289.63 ms, p = .0022, d = .247, and infants with FXS at right electrodes, M = 291.39 ms, p = .0014, d = .292. There were no additional significant differences in latency to peak N290 based on group and electrode hemisphere (LR infants at right electrodes, M = 282.57 ms; ASIBs at right electrodes, M = 284.92 ms; infants with FXS at left electrodes, M = 285.89 ms). Additionally, there was a significant interaction of stimulus type and electrode hemisphere on N290 latency, F (1, 54) = 4.43, p = .0400. However, follow-up examination of least squares means did not reveal significant differences in latency to peak N290 for faces and toys across left and right hemispheres: faces in the right hemisphere, M = 285.29 ms, faces in the left hemisphere, M = 287.17 ms, toys in the right hemisphere, M = 287.16, and toys in the left hemisphere, M = 281.51 ms.
A second ANOVA was conducted examining the effect of participant group (3: LR, ASIB, FXS), electrode hemisphere (2: left, right), stimulus type (2: faces, toys), and attention phase (2: attention, inattention) on N290 latency. The interactions of participant group and electrode hemisphere, F (2, 54) = 4.94, p = .0107, and stimulus type and electrode hemisphere, F (1, 54) = 5.07, p = .0284, were replicated. There were no significant effects or interactions of HR-defined attention phase on N290 latency.
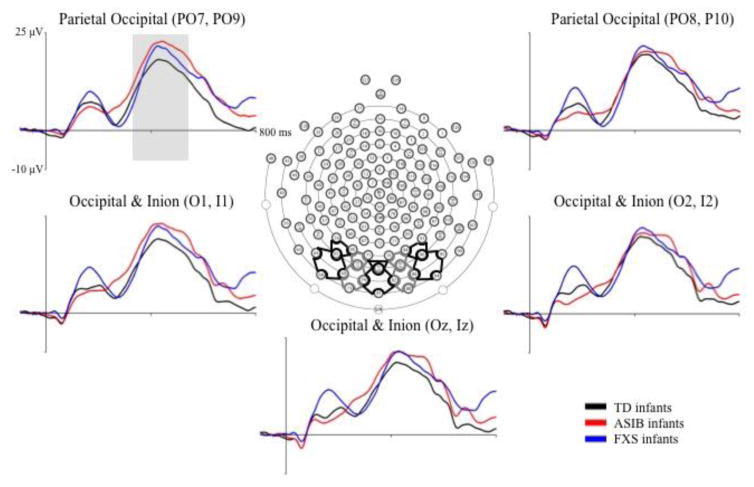
P400 Amplitude
Grand average P400 amplitude responses are presented in Figure 3 by group across posterior electrode clusters included in the analyses. The P400 is evident as a positive ERP component occurring approximately 400 ms after stimulus onset. An analysis of P400 amplitude was conducted to identify differences across group, electrode hemisphere, stimulus type, stimulus familiarity, and HR-defined attention phase. The first ANOVA conducted included participant group (3: LR, ASIB, FXS), electrode hemisphere (3: left, midline, right), stimulus type (2: faces, toys), and stimulus familiarity (2: familiar, novel). A second ANOVA was conducted, including participant group (3: LR, ASIB, FXS), electrode hemisphere (3: left, midline, right), stimulus type (2: faces, toys), and HR-defined attention phase (2: attention, inattention). Both ANOVAs revealed no significant effects of these factors on P400 amplitude.
Figure 3.
Grand average P400 amplitude and latency by group at Occipital Inion and Parietal Occipital electrode clusters. The grand average ERP activity is shown from 100 ms preceding stimulus onset through 800 ms post stimulus onset for the three participant groups at electrode clusters included in the P400 analysis.
P400 Latency
The effects of participant group, electrode hemisphere, stimulus type, stimulus familiarity, and HR-defined attention phase on P400 latency were examined in two ANOVAs. The first ANOVA included the factors participant group (3: LR, ASIB, FXS), electrode hemisphere (3: left, midline, right), stimulus type (2: faces, toys), and stimulus familiarity (2: familiar, novel). There was a main effect of electrode hemisphere, F (2, 104) = 4.59, p = .0123. The latency to P400 peak amplitude was significantly shorter at left electrodes, M = 487.38 ms, than right electrodes, M = 495.03 ms, d = .078, and midline electrodes, M = 497.55 ms, d = .102, p = .0006. This effect was replicated in an ANOVA including participant group (3: LR, ASIB, FXS), electrode hemisphere (3: left, midline, right), stimulus type (2: faces, toys), and HR-defined attention phase (2: attention, inattention).
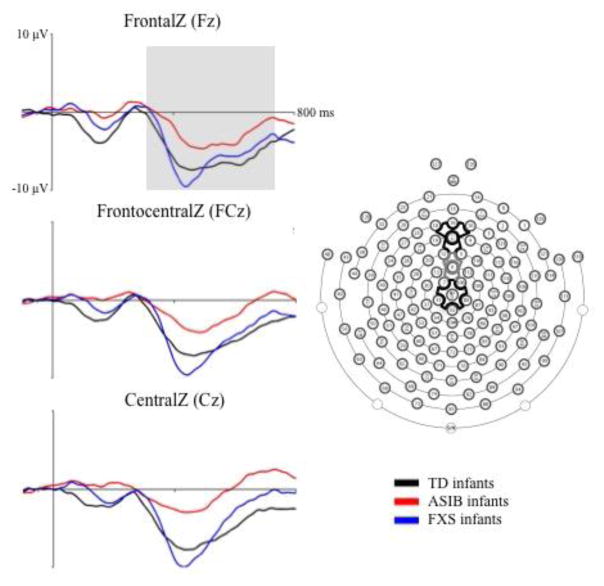
Nc Amplitude
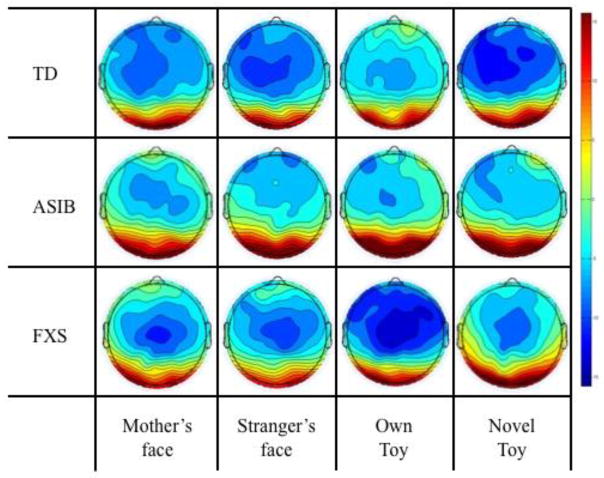
Figure 4 presents grand average Nc responses by group across midline frontal and central electrode clusters that were included in the analyses. The Nc can be seen as a negative component that occurs from approximately 350–750 ms after stimulus onset. Analyses were conducted to examine the effect of participant group, stimulus type and familiarity, and attention phase on Nc amplitude. Differences between participant groups were investigated in an analysis of grand average Nc amplitude. An ANOVA was conducted including group (3: LR, ASIB, FXS), stimulus type (2: faces, toys), and stimulus familiarity (2: familiar, novel). There was a marginally significant main effect of Nc amplitude across groups, F (2, 52) = 3.00, p = .0586. As shown in Figure 4, Nc amplitude was significantly greater in LR infants, M = −10.91 μV, and infants with FXS, M = −11.87 μV, than ASIBs, M = −6.09 μV, both ps < .0001, d = .369 and d = .501, respectively. There was a significant interaction of group and stimulus familiarity, F (2, 48) = 3.88, p = .0274. Figure 5 presents topographical plots of infants’ Nc responses as a function of stimulus familiarity separately for each participant group. As shown in Figure 5, LR infants showed a greater amplitude response to novel stimuli, M = −15.54 μV, than familiar stimuli, M = −12.21 μV, d = .232, while ASIBs showed a very similar response to both stimulus types, familiar M = −10.41 μV and novel M = −10.23 μV, d = .014, and infants with FXS showed a greater Nc response to familiar stimuli, M = −13.50 μV, than novel stimuli, M = −11.89 μV, d = .140.
Figure 4.
Grand average Nc amplitude by group at FrontalZ, FrontalCentralZ, and CentralZ virtual 10–10 electrodes. The grand average ERP activity is shown from 100 ms preceding stimulus onset through 800 ms post stimulus onset for the three participant groups at virtual 10–10 electrodes included in the Nc analysis.
Figure 5.
Nc amplitude by group across all electrode clusters in response to the mother’s face, stranger’s face, familiar toy, and novel toy. Mean Nc amplitude is presented in topographical plots in response to the mother’s face, stranger’s face, familiar toy, and novel toy, separately for each group.
An additional analysis included group, electrode cluster, stimulus type, and HR-defined attention phase to examine the effects of stimulus type and attention on Nc amplitude. There were no significant effects or interactions of stimulus type or attention on Nc amplitude.
Discussion
The primary goal of the current study was to examine neural correlates of face and object processing in two etiologically distinct groups of 12-month-old infants at high-risk for ASD (infants with FXS versus ASIBs) relative to a low-risk control group. The N290, P400, and Nc ERP components were investigated across participant groups in response to infants’ mothers’ faces, strangers’ faces, infants’ own toys, and novel toys. We predicted that both LR and ASIB infants would demonstrate greater N290 amplitude to faces than toys. Infants with FXS were expected to respond in a similar manner, but we hypothesized that they may exhibit less differentiation of faces and toys, similar to younger infants. The results revealed greater amplitude N290 in response to faces compared with toys across all groups. Contradictory to our hypothesis, the greatest N290 amplitude responses and greatest differentiation of faces and toys were observed in infants with FXS. Additionally, although the analyzed results did not include a significant effect of participant group on N290 amplitude, the ASIBs showed the smallest amplitude N290 of all three groups. We hypothesized that latency to the P400 peak would be greater in high-risk than control infants, but found no differences in P400 latency across participant groups. Additionally, participant group did not interact with stimulus type, stimulus familiarity, HR-defined attention, or electrode hemisphere to influence P400 amplitude or latency. The Nc response was significantly greater in LR and FXS groups than ASIBs. Analysis of the Nc also revealed an interaction of participant group and stimulus familiarity. As hypothesized, LR infants showed greater amplitude Nc responses to novel than familiar stimuli. We expected that ASIBs would demonstrate greater Nc amplitude to familiar than novel stimuli, but found no differences based on stimulus familiarity. However, infants with FXS did show greater amplitude to familiar than novel stimuli.
Analysis of the N290 revealed significant effects of participant group, stimulus type, and stimulus familiarity. Our finding of greater amplitude N290 responses to faces than toys replicates previous findings from LR and ASIB infants (e.g., Guy et al., 2016; Halit et al., 2004; McCleery et al., 2009) and supports the role of the N290 in the development of specialized face processing across diverse groups of infants. The lack of an interaction with participant group may indicate that this early component reflects automatic face processing or recognition and that it is not strongly influenced by risk factors. However, visual review of the results (see Figure 1 and 2 and Table 2) indicates that there was a trend of greatest N290 amplitude in infants with FXS, followed by LR infants, and ASIBs. This may reflect a greater orienting response toward face stimuli in infants FXS than is typically observed, and a more muted orienting response in ASIBs. This trend does indicate that heterogeneous groups of infants at high risk of ASD process social stimuli differently from one another. This hypothesis was also supported by a marginally significant interaction of participant group and stimulus familiarity. Infants with FXS showed significantly greater N290 responses to familiar stimuli than to novel stimuli. Their responses to familiar stimuli were also significantly greater than LR and ASIB infants’ N290 responses to familiar and novel stimuli. This interaction indicates that the N290 is sensitive to stimulus familiarity, at least under some circumstances or in some participant groups, although previous research has often suggested otherwise (de Haan & Nelson, 1997, 1999; Guy et al., 2016; Luyster et al., 2011; McCleery et al., 2009). Two previous studies that found significant differences in N290 amplitude based on stimulus familiarity in LR and ASIB infants (Key & Stone, 2012; Luyster et al., 2014) only utilized familiar and novel face stimuli. It is possible that the more frequent presentation of these stimuli in those studies led to greater differentiation between familiar and novel at the level of the N290.
The specific role of the P400 in face processing is not as well established as that of the N290, and the current study did not provide evidence that it is a face-sensitive ERP component, as P400 amplitude and latency did not differ across face and toy stimuli. Additionally, our results revealed no significant effects of participant group on P400 amplitude or latency. Past studies have reported shorter P400 latency to faces than objects (de Haan & Nelson, 1999; Halit et al., 2004; McCleery et al., 2009) and to the mother’s face than a stranger’s face in LR infants (Key & Stone, 2012), but not ASIBs (Key & Stone, 2012; McCleery et al., 2009). Greater P400 amplitude to novel compared with familiar face stimuli has been reported in LR infants and ASIBs (Key & Stone, 2012), but was not observed in the current study. The current results did not provide any additional insight into the functional significance of the P400 in response to social and nonsocial stimuli or in comparison of infants at increased risk of ASD and LR infants.
The Nc component was included in our analyses because it reflects attentional engagement (e.g., Reynolds et al., 2010) and we sought to examine the effect of stimulus salience or novelty across participant groups. Results of the current study showed that Nc amplitude differed significantly across participant group. Amplitude of the Nc was similar across LR and FXS groups and was greater than in ASIBs. A greater Nc response in LR infants compared with ASIBs has not been previously reported (e.g., Key et al., 2014; Key & Stone, 2012; Luyster et al., 2011), but may reflect decreased attentional engagement in the task for ASIBs relative to LR and FXS groups. Stimulus familiarity also influenced infants’ Nc responses, which differed across participant group. LR infants showed a greater Nc to novel than familiar stimuli. This replicates previous findings from research comparing responses to novel stimuli with familiar, yet meaningful, stimuli in infants at 12 months of age (Carver et al., 2003; Luyster et al., 2011). Alternatively, ASIBs did not differentiate their mother’s face and own toy from a stranger’s face and novel toy based on Nc amplitude. Both Key and Stone (2012) and Luyster and colleagues (2011) found that like LR infants, ASIBs demonstrated a greater amplitude Nc response to the novel face than their mother’s face. However consistent with the results of the current study, Luyster and colleages (2014) did not find differences in Nc amplitude based on stimulus familiarity in their longitudinal study of face processing in LR and ASIB infants. Infants with FXS showed a greater amplitude response to familiar than novel stimuli, a finding most commonly reported in LR infants under 12 months (de Haan & Nelson, 1997, 1999; Luyster et al., 2014; Webb et al., 2005). Results from the examination of stimulus familiarity and Nc amplitude indicate an enhanced novelty response in LR infants, an enhanced familiarity response in infants with FXS, and a null preference in ASIBs. This pattern of results may indicate that ASIBs were less responsive to the stimuli than the other groups and that infants with FXS may show immature stimulus processing, similar to that of younger infants.
Differences observed in the ERP responses of infants with FXS relative to ASIB infants, despite shared risk status and behavioral phenotypes, may reflect differences in the developmental trajectories of each group. Previous research contrasting the structural brain development of toddlers with FXS and toddlers with idiopathic ASD has indicated that shared behavioral ASD characteristics across these groups may actually have roots in distinct neural mechanisms (Hazlett et al., 2009; Hoeft et al., 2011). For example, amygdala volume was found to be enlarged in toddlers with idiopathic ASD and decreased in volume in toddlers with FXS relative to control toddlers (Hazlett et al., 2009). It was hypothesized that both patterns of brain development may contribute to the similar atypicalities in social behavior. Differences in level of intellectual maturity may also contribute to the differences observed in the ERP responses of infants with FXS and ASIBs. As shown in Table 1, infants with FXS had lower scores on the Mullen Scales of Early Learning than ASIBs and LR infants, reflecting a younger mental age. This effect was strongest in males with FXS, however, due to small sample sizes, it was not feasible to examine sex effects on the current ERP results. Behavioral characteristics of the FXS group indicate that including females in the sample may have diluted our results, however, significant group effects were observed in the results of our N290 and Nc analyses. Future research should further investigate the impact of participant sex on ERP responses in infants with FXS.
The unique pattern of ERP responses observed in infants with FXS may also reflect high rates of anxiety observed in FXS, with approximately 86% of males meeting DSM-IV criteria for one or more anxiety disorders (Cordeiro, Ballinger, Hagerman & Hessl, 2011), 60% of males meeting criteria for social anxiety (Cordeiro et al., 2011), and 70% seeking anxiety-related treatment (Bailey et al., 2008). Given the commonality and intensity of anxiety in FXS, it is possible that the enhanced amplitude of N290 and Nc responses may reflect a hyper-responsiveness to social stimuli in infants with FXS relative to ASIBs and LR infants. Although differences in N290 amplitudes have not been previously reported in studies of ASIBs relative to LR infants, the presence of enhanced N290 amplitude in FXS may reflect an especially strong orientation to face stimuli than object stimuli early in the processing stream. Similarly, the enhanced Nc responses in FXS infants may support greater arousal or interest in the experimental stimuli compared with ASIBs. These hypotheses are consistent with a number of studies characterizing behavioral and biological predictors of anxiety in young children with FXS as early as infancy. For example, infants and toddlers with FXS exhibit abnormal behavioral and physiological responses to anxiety, as measured during an experimental stranger approach paradigm (Tonnsen, Shinkareva et al., 2013). In addition, accelerations in parent reported temperamental approach, characterized as engagement in novel situations, have been shown to predict later anxiety symptoms among infants and toddlers with FXS (Tonnsen, Malone, Hatton & Roberts, 2013). Given these behavioral and biological prodromal anxiety features are detectable in early childhood within FXS, it is possible that atypical neural patterns – particularly enhanced amplitude of N290 and Nc responses – may reflect emergent anxiety risk in this population.
As the first study to examine and detect cross-syndrome neural differences in infant groups at risk for ASD, we suggest a number of future directions. First, although the prospective examination of group differences in ERP responses among ASIBs is a standard method for studying the effects of the broader autism phenotype (Elsabbagh et al., 2009; Key et al., 2014; Key & Stone, 2012; Luyster et al., 2011; McCleery et al., 2009), it will be important to examine developmental outcomes associated with infant risks. These include comparisons across groups based on diagnosis of ASD and intellectual disability. Monitoring change in neural responses over time will also be essential to determining whether the effects observed in the current study increase in intensity over time, thus informing points of greatest risk and timing for interventions.
Results of the current study indicate that infants at increased risk of ASD already display unique patterns of neural responses to familiar and novel, face and toy stimuli at 12 months of age. Although previous studies have examined ASIBs’ ERP responses to face and object stimuli (e.g., Luyster et al., 2011, 2014; McCleery et al., 2009; Key & Stone, 2012; Key et al., 2014), this was the first study to incorporate an additional at-risk group of infants with FXS. Both ASIBs and infants with FXS differed from LR infants in their ERP responses, but patterns of responses also differed across the at-risk groups. Infants with FXS showed enhanced N290 and Nc amplitude responses, whereas ASIBs demonstrated a more muted response, reflected in decreased N290 and Nc amplitude relative to controls. Additionally, at the N290 and Nc ERP components, infants with FXS showed sensitivity to stimulus familiarity, while ASIBs’ responses did not differ across familiar and novel stimuli. These group-specific differences suggest that despite shared risk for ASD outcomes, infants with FXS and ASIBs exhibit distinct neural patterns of attention and face processing within the first year of life, potentially suggesting syndrome-specific pathways to similar behavioral outcomes.
Acknowledgments
This research was supported by grants R37 HD18942 to J. E. Richards, NIMH-1RO1MH090194-01A1 to J. E. Roberts, and 1F31MH095318 to B. L. Tonnsen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailey DB, Jr, Hatton DD, Skinner M. Early developmental trajectories of males with fragile X syndrome. American Journal on Mental Retardation. 1998;103:29–39. doi: 10.1352/0895-8017(1998)103<0029:EDTOMW>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Raspa M, Olmsted M, Holiday DB. Co-occurring conditions associated with FMR1 gene variations: Findings from a national parent survey. American journal of medical genetics part A. 2008;146:2060–2069. doi: 10.1002/ajmg.a.32439. [DOI] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budimirovic DB, Kaufmann WE. What can we learn about autism from studying fragile X syndrome? Developmental Neuroscience. 2011;33:379–394. doi: 10.1159/000330213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver LJ, Dawson G, Panagiotides H, Meltzoff AN, McPartland J, Gray J, Munson J. Age-related differences in neural correlates of face recognition during the toddler and preschool years. Developmental Psychobiology. 2003;42:148–159. doi: 10.1002/dev.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford SM, Dissanayake C, Bui QM, Huggins R, Taylor AK, Loesch DZ. Autism spectrum phenotype in males and females with fragile X full mutation and premutation. Journal of Autism and Developmental Disorders. 2007;37:738–747. doi: 10.1007/s10803-006-0205-z. [DOI] [PubMed] [Google Scholar]
- Coffee B, Keith K, Albizua I, Malone T, Mowrey J, … Warren ST. Incidence of fragile X syndrome by newborn screening for methylated FMR1. DNA. 2009;85:503–514. doi: 10.1016/j.ajhg.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordeiro L, Ballinger E, Hagerman R, Hessl D. Clinical assessment of DSM-IV anxiety disorders in fragile X syndrome: prevalence and characterization. Journal of Neurodevelopmental Disorders. 2011;3(1):57–67. doi: 10.1007/s11689-010-9067-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Ganz L, Norcia AM. Event-related potentials to human faces in infants. Child Development. 1981;52:804–811. [PubMed] [Google Scholar]
- Crawford DC, Acuña JM, Sherman SL. FMR1 and the fragile X syndrome: Human genome epidemiology review. Genetics in Medicine. 2001;3:359–371. doi: 10.1097/00125817-200109000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton KM, Holsen L, Abbeduto L, Davidson RJ. Brain function and gaze-fixation during facial emotion processing in fragile-X and autism. Autism Research. 2008;1:231–239. doi: 10.1002/aur.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Webb S, Schellenberg GD, Dager S, Friedman S, Aylward E, Richards T. Defining the broader phenotype of autism: Genetic, brain, and behavioral perspectives. Development and Psychopathology. 2002;14:581–611. doi: 10.1017/s0954579402003103. [DOI] [PubMed] [Google Scholar]
- de Haan M, Johnson MH, Halit H. Development of face-sensitive event-related potentials during infancy: a review. International Journal of Psychophysiology. 2003;51:45–58. doi: 10.1016/s0167-8760(03)00152-1. [DOI] [PubMed] [Google Scholar]
- de Haan M, Nelson CA. Recognition of the mother’s face by six-month-old infants: A neurobehavioral study. Child Development. 1997;68:187–210. [PubMed] [Google Scholar]
- de Haan M, Nelson CA. Brain activity differentiates face and object processing in 6-month-old infants. Developmental Psychology. 1999;35:1113–1121. doi: 10.1037//0012-1649.35.4.1113. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dissanayake C, Bui Q, Bulhak-Paterson D, Huggins R, Loesch DZ. Behavioural and cognitive phenotypes in idiopathic autism versus autism associated with fragile X syndrome. Journal of Child Psychology and Psychiatry. 2009;50:290–299. doi: 10.1111/j.1469-7610.2008.01988.x. [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Volein A, Csibra G, Holmboe K, Garwood H, … Johnson MH. Neural correlates of eye gaze processing in the infant broader autism phenotype. Biological Psychiatry. 2009;65:31–38. doi: 10.1016/j.biopsych.2008.09.034. [DOI] [PubMed] [Google Scholar]
- Farzin F, Rivera SM, Hessl D. Visual processing of faces in individuals with fragile X syndrome: An eye tracking study. Journal of Autism and Developmental Disorders. 2009;39:946–952. doi: 10.1007/s10803-009-0744-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett AS, Menon V, MacKenzie K, Reiss AL. Here’s looking at you, kid: Neural systems underlying face and gaze processing in fragile X syndrome. Archives of General Psychiatry. 2004;61:281–288. doi: 10.1001/archpsyc.61.3.281. [DOI] [PubMed] [Google Scholar]
- Grelotti DJ, Gauthier I, Schultz RT. Social interest and the development of cortical face specialization: What autism teaches us about face processing. Developmental Psychobiology. 2002;40:213–225. doi: 10.1002/dev.10028. [DOI] [PubMed] [Google Scholar]
- Guy MW, Reynolds GD, Zhang D. Visual attention to global and local stimulus properties in six-month-old infants: Individual differences and event-related potentials. Child Development. 2013;84:1392–1406. doi: 10.1111/cdev.12053. [DOI] [PubMed] [Google Scholar]
- Guy MW, Zieber N, Richards JE. The cortical development of specialized face processing in infancy. Child Development. 2016 doi: 10.1111/cdev.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman RJ, Rivera SM, Hagerman PJ. The fragile X family of disorders: a model for autism and targeted treatments. Current Pediatric Reviews. 2008;4:40–52. [Google Scholar]
- Halit H, Csibra G, Volein Á, Johnson MH. Face-sensitive cortical processing in early infancy. Journal of Child Psychology and Psychiatry. 2004;45:1228–1234. doi: 10.1111/j.1469-7610.2004.00321.x. [DOI] [PubMed] [Google Scholar]
- Harris SW, Hessl D, Goodlin-Jones B, Ferranti J, Bacalman S, Barbato I, … Hagerman RJ. Autism profiles of males with fragile X syndrome. American Journal of Mental Retardation. 2008;113(6) doi: 10.1352/2008.113:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey DB, Jr, … Mirrett P. Autistic behavior in chidlren with fragile X syndrome: prevalence, stability, and the impact of FMRP. American Journal of Medical Genetics, Part A. 2006;140:1804–13. doi: 10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Lightbody AA, Gerig G, MacFall JR, … Piven J. Teasing apart the heterogeneity of autism: Same behavior, different brains in toddlers with fragile X syndrome and autism. Journal of Neurodevelopmental Disorders. 2009;1:81–90. doi: 10.1007/s11689-009-9009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Walter E, Lightbody AA, Hazlett HC, Chang C, Piven J, Reiss AL. Neuroanatomical differences in toddler boys with fragile X syndrome and idiopathic autism. Archives of General Psychiatry. 2011;68:295–305. doi: 10.1001/archgenpsychiatry.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubl D, Bölte S, Feineis-Matthews S, Lanfermann H, Federspiel A, Strik W, … Dierks T. Functional imbalance of visual pathways indicates alternative face processing strategies in autism. Neurology. 2003;61:1232–1237. doi: 10.1212/01.wnl.0000091862.22033.1a. [DOI] [PubMed] [Google Scholar]
- Hunter J, Rivero-Arias O, Angelov A, Kim E, Fotheringham I, Leal J. Epidemiology of fragile X syndrome: A systematic review and meta-analysis. American Journal of Medical Genetics Part A. 2014;164:1648–1658. doi: 10.1002/ajmg.a.36511. [DOI] [PubMed] [Google Scholar]
- Johnson MH, de Haan M, Oliver A, Smith W, Hatzakis H, Tucker LA, Csibra G. Recording and analyzing high-density event-related potentials with infants using the Geodesic sensor net. Developmental Neuropsychology. 2001;19:295–323. doi: 10.1207/S15326942DN1903_4. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Cortell R, Kau AS, Bukelis I, Tierney E, Gray RM, … Stanard P. Autism spectrum disorder in fragile X syndrome: communication, social interaction, and specific behaviors. American Journal of Medical Genetics Part A. 2004;129:225–234. doi: 10.1002/ajmg.a.30229. [DOI] [PubMed] [Google Scholar]
- Key AP, Ibanez LV, Henderson HA, Warren Z, Messinger DS, Stone WL. Positive affect processing and joint attention in infants at high risk for autism: An exploratory study. Journal of Autism and Developmental Disorders. 2014 doi: 10.1007/s10803-014-2191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key APF, Stone WL. Processing of novel and familiar faces in infants at average and high risk for autism. Developmental Cognitive Neuroscience. 2012 doi: 10.1016/j.dcn.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa RJ, Gross AL, Stuart EA, Bauman M. Latent class analysis of early developmental trajectory in baby siblings of children with autism. Journal of Child Psychology and Psychiatry. 2012;53:986–996. doi: 10.1111/j.1469-7610.2012.02558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesch DZ, Bui QM, Dissanayake C, Clifford S, Gould E, Bulhak-Paterson D, … Huggins RM. Molecular and cognitive predictors of the continuum of autistic behaviours in fragile X. Neuroscience & Biobehavioral Reviews. 2007;31:315–326. doi: 10.1016/j.neubiorev.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Calderon J, Luck SJ. ERPLAB: an open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience. 2014;8 doi: 10.3389/fnhum.2014.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losh M, Childress D, Lam K, Piven J. Defining key features of the broad autism phenotype: A comparison across parents of multiple- and single-incidence autism families. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2008;147B:424–433. doi: 10.1002/ajmg.b.30612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyster RJ, Powell C, Tager-Flusberg H, Nelson CA. Neural measures of social attention across the first years of life: Characterizing typical development and markers of autism risk. Developmental Cognitive Neuroscience. 2014;8:131–143. doi: 10.1016/j.dcn.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyster RJ, Wagner JB, Vogel-Farley V, Tager-Flusberg H, Nelson CA. Neural correlates of familiar and unfamilar face processing in infants at risk for autism spectrum disorders. Brain Topography. 2011 doi: 10.1007/s10548-011-0176-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallin BM, Richards JE. Peripheral stimulus localization by infants of moving stimuli on complex backgrounds. Infancy. 2012;17:692–714. doi: 10.1111/j.1532-7078.2011.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCary L, Roberts J. Early identification of autism in fragile X syndrome: a review. Journal of Intellectual Disability Research. 2013;57:803–814. doi: 10.1111/j.1365-2788.2012.01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleery JP, Akshoomoff N, Dobkins KR, Carver LJ. Atypical face versus object processing and hemispheric asymmetries in 10-month-old infants at risk for autism. Biological Psychiatry. 2009;66:950–957. doi: 10.1016/j.biopsych.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPartland J, Dawson G, Webb SJ, Panagiotides H, Carver LJ. Event-related brain potentials reveal anomalies in temporal processing of faces in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2004;45:1235–1245. doi: 10.1111/j.1469-7610.2004.00318.x. [DOI] [PubMed] [Google Scholar]
- McPartland JC, Wu J, Bailey CA, Mayes LC, Schultz RT, Klin A. Atypical neural specialization for social percepts in autism spectrum disorder. Social Neuroscience of Psychiatric Disorders. 2011;6 doi: 10.1080/17470919.2011.586880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger D, Young G, Ozonoff S, Dobkins K, Carter A, Zwaigenbaum L, … Sigman M. Beyond autism: A baby siblings research consortium study of high-risk children at three years of age. Journal of the American Academy of Child and Adolescent Psychiatry. 2013;52:300–308. doi: 10.1016/j.jaac.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus E, Kresse A, Faja S, Bernier RA, Webb SJ. Face processing among twins with and without autism: Social correlates and twin concordance. Social Cognitive and Affective Neuroscience. 2015 doi: 10.1093/scan/nsv085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Carter A, Messinger D, Yirmiya N, Zwaigenbaum L, … Stone WL. Recurrence risk for autism spectrum disorders: A Baby Siblings Research Consortium study. Pediatrics. 2011;128:e488–e495. doi: 10.1542/peds.2010-2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young G, Belding A, Hill M, Hill A, Hutman T, … Iosif A. The broader autism phenotype in infancy: When does it emerge? Journal of the American Academy of Child and Adolescent Psychiatry. 2014;53:398–407. doi: 10.1016/j.jaac.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pempek TA, Kirkorian HL, Richards JE, Anderson DR, Lund AF, Stevens M. Video comprehensibility and attention in very young children. Developmental Psychology. 2010;46:1283–1293. doi: 10.1037/a0020614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philofsky A, Hepburn SL, Hayes A, Hagerman R, Rogers SJ. Linguistic and Cognitive Functioning and Autism Symptoms in Young Children with Fragile X syndrome. American Journal on Mental Retardation. 2004;109:208–218. doi: 10.1352/0895-8017(2004)109<208:LACFAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Reynolds GD, Courage ML, Richards JE. Infant attention and visual preferences: Converging evidence from behavior, event-related potentials, and cortical source localization. Developmental Psychology. 2010;46:886–904. doi: 10.1037/a0019670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE. Attention affects the recognition of briefly presented visual stimuli in infants: an ERP study. Developmental Science. 2003;6:312–328. doi: 10.1111/1467-7687.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Hatton DD, Long ACJ, Anello V, Colombo J. Visual attention and autistic behavior in infants with fragile X syndrome. Journal of Autism and Developmental Disorders. 2012;42:937–946. doi: 10.1007/s10803-011-1316-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Wehner EA, Hagerman R. The behavioral phenotype in fragile X: Symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. Developmental and Behavioral Pediatrics. 2001;22:409–417. doi: 10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Rossion B, Gauthier I, Tarr MJ, Despland P, Bruyer R, Linotte S, Crommelinck M. The N170 occipito-temporal component is delayed and enhanced to inverted faces but not to inverted objects: an electrophysiological account of face-specific processes in the human brain. NeuroReport. 2000;11:69–74. doi: 10.1097/00001756-200001170-00014. [DOI] [PubMed] [Google Scholar]
- Searle SR. Linear models for unbalanced data. New York: Wiley; 1987. [Google Scholar]
- Tonnsen BL, Malone PS, Hatton DD, Roberts JE. Early negative affect predicts anxiety, not autism, in preschool boys with fragile X syndrome. Journal of Abnormal Child Psychology. 2013;41(2):267–80. doi: 10.1007/s10802-012-9671-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnsen BL, Shinkareva SV, Deal SC, Hatton DD, Roberts JE. Biobehavioral indicators of social fear in young children with fragile x syndrome. American Journal on Intellectual and Developmental Disabilities. 2013;118(6):447–59. doi: 10.1352/1944-7558-118.6.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker DM. Spatial sampling of head electrical fields - the Geodesic Sensor Net. Electroencephalography and Clinical Neurophysiology. 1993;87:154–163. doi: 10.1016/0013-4694(93)90121-b. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Jones EJH, Merkle K, Murias M, Greenson J, Richards T, Aylward E, Dawson G. Response to familiar faces, newly familiar faces, and novel faces as assessed by ERPs is intact in adults with autism spectrum disorders. International Journal of Psychophysiology. 2010;77:106–117. doi: 10.1016/j.ijpsycho.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Long JD, Nelson CA. A longitudinal investigation of visual event-related potentials in the first year of life. Developmental Science. 2005;8:605–616. doi: 10.1111/j.1467-7687.2005.00452.x. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Merkle K, Murias M, Richards T, Aylward E, Dawson G. ERP responses differentiate inverted but not upright face processing in adults with ASD. SCAN. 2012;7:578–587. doi: 10.1093/scan/nsp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Richards JE. Effects of interstimulus intervals on behavioral, heart rate, and event-related potential indices of infant engagement and sustained attention. Psychophysiology. 2016 doi: 10.1111/psyp.12670. [DOI] [PMC free article] [PubMed] [Google Scholar]