Abstract
Background
While national human papillomavirus (HPV) vaccination estimates exist by sex, little is known about HPV vaccination rates by gender identity.
Methods
We conducted a self-administered, anonymous online cross-sectional survey, with recruitment through Facebook ads, of lesbian, gay, bisexual, and transgender individuals in rural areas of the US. We compared HPV vaccine recommendation and uptake by self-reported sex assigned at birth and current gender identity.
Results
Six hundred sixty respondents were age eligible for HPV vaccination: 84% reported gender identity aligned with their sex assigned at birth, while 10% reported gender identity the differed from their sex assigned at birth; an additional 6% reported non-binary gender identity. Only 14% of male sex assigned at birth and 44% of female sex assigned at birth received HPV vaccine, similar to estimates by current gender identity. Transgender respondents’ HPV vaccination experience mirrored that of cisgender respondents with regard to sex assigned at birth.
Conclusions
Providers may base HPV vaccine recommendations on individuals’ sex assigned at birth, which may impact transgender individuals’ vaccine coverage. Future HPV vaccine uptake studies should account for gender identity. With sex-specific catch-up HPV vaccination recommendations, the role of gender identity on provider recommendation and reimbursement needs to be addressed.
Keywords: human papillomavirus, vaccine, transgender, gender identity
1. Introduction
Despite being recommended since 2006,[1] human papillomavirus (HPV) vaccines are sub-optimally used, particularly when compared to other routinely recommended adolescent vaccines.[2] HPV vaccination was initially recommended only for females;[3] routine recommendation for HPV vaccination of males came five years later.[4] While HPV vaccination is recommended at 11–12 years of age, catch-up vaccination is recommended to age 26 years for women as well as for men who have sex with men and up to 21 years for men who have sex with women.[1] These recommendations are based solely on sex assigned at birth. With the time difference between female and male recommendations, there are lingering disparities in sex-specific vaccination coverage. In 2014, only 40% of female and 22% of male adolescents eligible for HPV vaccine were fully vaccinated against HPV.[2]
National HPV vaccination estimates for lesbian and bisexual women (45%)[5] and gay and bisexual men (13%)[6] aged 18–26 years are available. While these estimates exist by sex assigned at birth, they do not currently exist by gender identity, defined by the Institute of Medicine as “a person’s basic sense of being a man or boy, a woman or girl, or another gender (e.g., transgender, bigender, or genderqueer—a rejection of the traditional binary classification of gender)”. [7] This may impact our ability to assess HPV vaccine uptake differences among transgender individuals (those whose gender identity differs from the sex they were assigned at birth). With HPV vaccination catch-up recommendations differing by sex[1] it is possible that healthcare providers may base vaccination recommendations on sex assigned at birth, with a preferential focus on individuals assigned female at birth. Not accounting for gender identity may contribute to inequitable vaccination recommendation and delivery.
It is known that LGBT adults are less likely than non-LGBT adults to have a primary care provider.[8] Fears of experiencing stigma or actual experience of stigma based on sexual identity may be underlying the lower rates of primary health care utilization among LGBT populations. Given this knowledge, it would follow that LGBT individuals may also experience reduced uptake of HPV vaccinations. As part of a larger study of outness and medical care-related stigma among LGBT individuals residing in rural areas of the United States,[9] we examined HPV vaccination among an online sample of LGBT adults residing in rural areas. We assessed vaccination coverage by gender identity as a preliminary comparison between cisgender (those whose current gender identity is the same as their sex assigned at birth) and transgender individuals (those whose current gender identity is different to their sex assigned at birth).
2. Methods
2.1. Data collection
The survey methodology has been described previously.[9] Briefly, data were collected using a self-administered, electronic survey distributed via a social networking site (Facebook) during 10 days in August 2014, with recruitment targeted to site users aged 18 and older, with LGBT-related interests, who lived in rural zip codes. Individuals who consented to participate by providing electronic consent were taken to the electronic survey instrument. No incentive was offered for participation, and the survey took approximately 20 minutes to complete. In addition to sex assigned at birth and gender identity (described in detail below), we collected other demographic (e.g., age, race, ethnicity, and education status) and self-reported HPV vaccination data. Race and ethnicity data were collected because of their known associations with healthcare and vaccination disparities. The study was approved by Institutional Review Board at the lead author’s university.
2.2. Measures
To evaluate our exposure of interest, we used self-reported sex assigned at birth (SAAB) and current gender identity independently to create a composite categorization containing five categories: cis-male (male SAAB/male gender identity); cis-female (female SAAB female gender identity); transman (female SAAB/male gender identity); transwoman (male SAAB/female gender identity); and non-binary gender identity. The main outcomes assessed were self-reported receipt of a health care providers’ recommendation to receive HPV vaccine (“has a health care worker ever recommended that you should get the HPV vaccine?”) and self-reported receipt of at least one dose of HPV vaccine (“have you received any does of the HPV vaccine?”); among HPV vaccine recipients we assessed age at first HPV vaccination and number of vaccine doses received.
2.2. Statistical Analysis
Counts and proportions of all outcomes were compared by SAAB, gender identity, and our composite gender identity-related categorization. We also compared HPV vaccine receipt by history of a health care provider’s recommendation, across all SAAB/gender identity categories. Survey data were analyzed with SAS v9.3 (The SAS Institute, Cary NC). Analysis was restricted to individuals aged 18 to 34 at the time of the survey, corresponding to being within the recommended ages for HPV vaccination since it was first recommended in 2006. Because we evaluated both SAAB and gender identity, we did not make sex-related distinctions based on the timing of the initial male HPV vaccine recommendation.
3. Results
Facebook users were shown the banner advertisements 220,053 times, yielding 5,317 click-throughs to the survey website, resulting in 972 completed surveys by eligible individuals. Additionally, 24 completed surveys were obtained after posting the survey to a Tumblr site, 18 completed surveys came from referrals from study participants, and four completed surveys were obtained after posting the survey to a listserv of transgender individuals residing primarily in the southeastern US. These 1,018 complete surveys from eligible individuals came from an initial 1,909 completed surveys, of which 891 were disqualified for not meeting eligibility criteria (531 did not complete the survey, 323 did not live in a rural area, 37 were younger than 18 years, 10 were both cisgender and heterosexual; 8 individuals were disqualified for more than one of these reasons, yielding a sum of individual disqualification factors (N = 901) that is greater than the number of individuals disqualified). For this analysis, 4 responses from intersex participants were dropped due to small sample size; and 354 were dropped because the individuals were not age-eligible for HPV vaccination (not aged 18 to 34 years). This yielded 660 eligible respondents for this analysis.
Slightly more respondents reported male SAAB (51%) than female SAAB (49%). With regard to gender identity, 54% reported male identity (47% cis-male, 7% transman) and 41% reported female identity (37% cis-female, 4% transwoman); an additional 6% reported non-binary gender identity (Table 1). No significant demographic differences were observed across our gender identity categorization (Table 2). Overall, approximately one-third of respondents were completely out about their sexual orientation to their primary care provider, though age at which they came out to their provider was not assessed (data not shown).
Table 1.
Sex assigned at birth and gender identity of rural LGBT recruited online in August 2014 who were age-eligible for HPV vaccination.
| Sex/gender measure | Category | N | % |
|---|---|---|---|
| Sex assigned at birth | Male | 338 | 51.2 |
| Female | 322 | 48.8 | |
| Gender identity | Male | 354 | 53.6 |
| Female | 269 | 40.8 | |
| Non-binary | 37 | 5.6 | |
| Sex assigned at birth/gender identity | Cismale | 306 | 46.6 |
| Cisfemale | 245 | 37.3 | |
| Transman | 46 | 7.0 | |
| Transwoman | 23 | 3.5 | |
| Non-binary, assigned male at birth | 7 | 1.1 | |
| Non-binary, assigned female at birth | 30 | 4.6 |
Table 2.
Demographic characteristics rural LGBT recruited online in August 2014 who were age-eligible for HPV vaccination, stratified by current gender identity relative to sex assigned at birth.
| Cismale (N = 306) | Cisfemale (N = 245) | Transwoman (N = 23) | Transman (N = 46) | Non-binary (N = 40) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 18 to 21 | 124 | 40.5 | 77 | 31.4 | 8 | 34.8 | 20 | 43.5 | 22 | 55.0 |
| 22 to 26 | 101 | 33.0 | 86 | 35.1 | 8 | 34.8 | 13 | 28.3 | 14 | 35.0 | |
| 27 to 34 | 81 | 26.5 | 82 | 33.5 | 7 | 30.4 | 13 | 28.3 | 4 | 10.0 | |
| Race | White | 269 | 88.2 | 211 | 86.5 | 20 | 90.9 | 41 | 89.1 | 35 | 89.7 |
| Black | 5 | 1.6 | 12 | 4.9 | 0 | 0.0 | 2 | 4.4 | 1 | 2.6 | |
| Other | 31 | 10.2 | 21 | 8.6 | 2 | 9.1 | 3 | 6.5 | 3 | 7.7 | |
| Missing | 1 | 1 | 1 | 0 | 1 | ||||||
| Ethnicity | Hispanic | 24 | 7.9 | 21 | 8.7 | 1 | 4.3 | 6 | 13.0 | 2 | 5.3 |
| Non-Hispanic | 280 | 92.1 | 221 | 91.3 | 22 | 95.7 | 40 | 87.0 | 36 | 94.7 | |
| Missing | 2 | 3 | 0 | 0 | 2 | ||||||
| Education | HS or less | 84 | 27.5 | 55 | 22.7 | 6 | 26.1 | 13 | 28.3 | 7 | 17.5 |
| Some college | 159 | 52.0 | 133 | 55.0 | 12 | 52.1 | 25 | 54.3 | 23 | 57.5 | |
| College graduate | 63 | 20.6 | 54 | 22.3 | 5 | 21.7 | 5 | 17.4 | 10 | 25.0 | |
| Missing | 0 | 3 | 0 | 0 | 0 | ||||||
| Has health insurance | Yes | 242 | 80.4 | 196 | 82.7 | 14 | 60.9 | 24 | 85.7 | 29 | 85.3 |
| No | 59 | 19.6 | 41 | 17.3 | 9 | 39.1 | 4 | 14.3 | 5 | 14.7 | |
| Missing | 5 | 8 | 0 | 2 | 6 | ||||||
Note: No demographic characteristics were significantly associated with respondents gender identity and sex assigned at birth, at the alpha = 0.05 level.
Receipt of HPV vaccination recommendation and at least one HPV vaccine dose was higher for female SAAB (47% and 44%, respectively) compared to male SAAB (17% and 14%, respectively), as well as female or transmale gender identity compared to male or transfemale gender identity. HPV vaccine recommendations and receipt were highest among those with female SAAB (cis-female recommendation 46.2%, receipt 43.1%; transmen recommendation 41.9%, receipt 36.6%) than those with male SAAB (cis-male recommendation 17.5%, receipt 14.2%, transwomen recommendation 5.3%, receipt 5.3%) With regard to SAAB, non-binary gender respondents indicated HPV vaccine recommendation and receipt similar to other respondents (Table 3).
Table 3.
Receipt of (a) HPV vaccine recommendation or (b) at least one dose of HPV vaccine among rural LGBT recruited online in August 2014 who were age-eligible for HPV vaccination, stratified by current gender identity relative to sex assigned at birth.
| Received HPV vaccine recommendation | Received 1+ dose of HPV vaccine | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Sex/gender measure | Category | Total N (non-missing) | N | % | Total N (non-missing) | N | % |
| Sex assigned at birth | Male | 312 | 53 | 17.0 | 292 | 40 | 13.7 |
| Female | 309 | 146 | 47.2 | 296 | 130 | 43.9 | |
| Missing | 39 | 72 | |||||
| Gender identity | Male | 294 | 51 | 17.3 | 275 | 39 | 14.2 |
| Female | 241 | 109 | 45.2 | 230 | 97 | 42.2 | |
| Transman | 36 | 17 | 47.2 | 34 | 14 | 41.2 | |
| Transwoman | 15 | 1 | 6.7 | 15 | 1 | 6.7 | |
| Non-binary | 14 | 6 | 42.9 | 14 | 7 | 50.0 | |
| Missing | 60 | 92 | |||||
| Sex assigned at birth/Gender identity | Cismale | 286 | 50 | 17.5 | 267 | 38 | 14.2 |
| Cisfemale | 236 | 109 | 46.2 | 225 | 97 | 43.1 | |
| Transman | 43 | 18 | 41.9 | 41 | 15 | 36.6 | |
| Transwoman | 19 | 1 | 5.3 | 19 | 1 | 5.3 | |
| Non-binary, assigned male at birth | 6 | 2 | 33.3 | 5 | 1 | 20.0 | |
| Non-binary, assigned female at birth | 29 | 19 | 41.9 | 29 | 18 | 62.1 | |
| Missing | 31 | 74 | |||||
Among individuals who received a health care provider’s recommendation for HPV vaccination, vaccine uptake was consistently high (at least 70% of those who received a recommendation received at least one HPV vaccine dose) across all gender identity categories (Figure 1). Approximately half of vaccinated respondents reported receiving HPV vaccine between 13 and 17 years of age (Table 2). Regardless of SAAB/gender identity, most individuals who initiated the HPV vaccine series reported receiving all three doses (Table 4).
Figure 1.
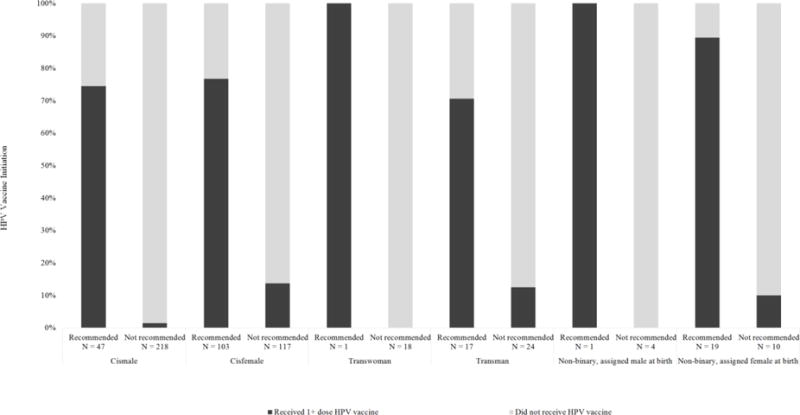
HPV vaccination status among individuals who either received or did not receive a healthcare provider recommendation for HPV vaccination, among rural LGBT recruited online in August 2014 who were age-eligible for HPV vaccination, stratified by current gender identity relative to sex assigned at birth.
Table 4.
Age at receipt of first HPV vaccine and number of doses of HPV vaccine received, among rural LGBT recruited online in August 2014who received HPV vaccine, stratified by current gender identity relative to sex assigned at birth.
| Cismale (N = 38) | Cisfemale (N = 97) | Transwoman (N = 1) | Transman (N = 15) | Non-binary, male assigned at birth (N = 1) | Non-binary, female assigned at birth (N = 18) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age at HPV vaccination | N | % | N | % | N | % | N | % | N | % | N | % |
| Less than 11 years | 1 | 2.6% | 2 | 2.1% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 1 | 5.6% |
| 11–12 years | 1 | 2.6% | 12 | 12.4% | 0 | 0.0% | 1 | 6.7% | 0 | 0.0% | 4 | 22.2% |
| 13–17 years | 15 | 39.5% | 60 | 61.9% | 1 | 100.0% | 7 | 46.7% | 1 | 100.0% | 9 | 50.0% |
| 18–26 years | 18 | 47.4% | 23 | 23.7% | 0 | 0.0% | 7 | 46.7 | 0 | 0.0% | 4 | 22.2% |
| 27 years and older | 1 | 2.6% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% |
| Missing | 2 | 0 | 0 | 0 | 0 | 0 | ||||||
| HPV vaccine doses received | ||||||||||||
| 1 | 11 | 28.9% | 13 | 13.4% | 1 | 100.0% | 1 | 6.7% | 0 | 0.0% | 1 | 5.6% |
| 2 | 7 | 18.4% | 13 | 13.4% | 0 | 0.0% | 6 | 40.0% | 0 | 0.0% | 1 | 5.6% |
| 3 | 20 | 52.6% | 71 | 73.2% | 0 | 0.0% | 8 | 53.3% | 1 | 100.0% | 16 | 88.8% |
4. Discussion
While national-level HPV vaccination rates among gay and bisexual men[6] and lesbian and bisexual women[5] are available, these studies may not have fully accounted for differences by gender identity. We evaluated HPV vaccination recommendations and uptake across SAAB and gender identity, finding that HPV vaccination recommendation and uptake closely aligns with SAAB.
On the basis of sex assigned at birth, HPV vaccine initiation among our sample of rural LGBT young adults (42% for female and 14% for male) was similar to that published for national-level HPV vaccine initiation among lesbian and bisexual women (47%)[5] and gay and bisexual men (13%)[6]. Lack of provider recommendation is a key barrier to HPV vaccination.[10] Healthcare providers may cue their vaccination recommendations on their perceptions of an individual’s sex based on presentation in the clinical setting, in which case individuals with female SAAB may be preferentially recommended vaccination. Importantly, within gender identity categories, HPV vaccine recommendation and uptake was more similar with regard to SAAB. For example, transmen had HPV vaccine recommendation and uptake levels similar to cisfemales, when considering SAAB. However, we found that HPV vaccine receipt was extremely high among those who received a provider’s recommendation for vaccination, across measures of SAAB and gender identity. This highlights the need for healthcare providers to recommend HPV vaccine to all patients in the recommended age range, without making recommendations based on providers’ perceptions of gender identity or sexual activity or sexual orientation.[11]
Another recent study of vaccination coverage in LGBT populations was conducted in Kentucky in the two years prior to our data collection. While this study reported the proportion of their sample who were transgender (4.6% of total population), they reported uptake of at least one dose of HPV vaccine in aggregate for females (15.4%) and males (10.3%).[12] While estimates of HPV vaccination among males are similarly low between this study and our results, we did find higher coverage among females. These differences may be due to a number of factors, including time (e.g. adolescent HPV vaccine coverage increased by nearly 7 percentage points for females and 20 percentage points for males between 2012 and 2014[2, 13]) and geography (one state versus a national sample). However, the limited reporting of vaccine coverage among LGBT populations highlights the need for continued surveillance and analysis of vaccination in these populations.
HPV vaccination is most effective when given prior to exposure to this common virus, with the recommendation for vaccination at 11–12 years of age.[1] While there have been recent gains in the proportion of vaccinated adolescent females who received HPV vaccine at 11–12 years (from 12% to 56% of those vaccinated),[14] only approximately 30% of adolescent girls are getting HPV vaccine at 11–12 years of age. Nearly 90% of HPV vaccinated individuals in this study reported receiving their first HPV vaccine dose at age 13 or older. Given differences in catch-up vaccination recommendations for females, heterosexual males, and men who have sex with men,[1] healthcare providers specializing in care of transgender patients need to be aware of the recommendation differences. This is important for assessing and understanding HPV vaccine coverage in their patients as well as addressing potential issues during catch-up vaccination (e.g. for insurance reimbursement for vaccinations, is SAAB or gender identity to be considered when considering the differential catch-up recommendations?). In this population, approximately 81% reported having health insurance, with no differences in health insurance coverage by gender identity. This coverage level is similar to national estimates of health insurance coverage among 18–24 (85.6%) and 25–34 (82.1%) year-olds in the US.[15]
This study has some limitations. First, recruitment was conducted through targeted advertisements on a social networking site directed to rural-residing individuals, and the representativeness of the sample cannot be determined. However, this is a preliminary descriptive study to begin understanding HPV vaccine as it relates to gender identity, to facilitate future studies. Therefore, the potential lack of national representativeness due to online recruitment (which may attract more young and technology-savvy individuals) may help provide insights into younger populations, for whom HPV vaccination is recommended, and into methodologic concerns for these future studies (e.g. appropriate survey design and response options to accurately assess transgender individuals’ vaccination). Second, there may be selection bias related to the length of the survey with no participation incentive. Future studies on this topic should consider ways to minimize this bias. Third, though the HPV vaccine was first routinely recommended for males five years after it was recommended for females,[1] we did not distinguish by sex when identifying vaccine-eligible individuals, because of our focus on gender identity as opposed to SAAB This may have artificially depressed HPV vaccine uptake in males due to inclusion of non-eligible individuals. Fourth, we did not assess age when respondents came out, with regard to sexual identity, to their healthcare providers or transitioned, in the case of transgender individuals, precluding assessment of that age relative to age at HPV vaccination. Future studies assessing the role of gender identity on vaccination recommendation and uptake among LGBT populations should include collection of both outness with regard to gender identity or sexual orientation, or age at transition. Finally, in this anonymous survey, we relied on self-reported HPV vaccination status, with no review of medical records, which may lead to potential recall bias in vaccination history. However, in a recent study comparing self-reported and medical record verified adult vaccination, the positive and negative predicted values for HPV vaccine recall (80% and 93%, respectively) were among the highest of all vaccines.[16] Future targeted surveys should include sufficient recruitment for more detailed assessment of gender identity, including ages at which they came out with regard to sexual orientation or nonconforming gender identity, as well as mechanisms for verification of vaccination status.
Conclusions
This is the first study to address HPV vaccination with regard to gender identity. Our finding of high HPV vaccine uptake among individuals whose healthcare provided recommended the vaccine, regardless of SAAB or gender identity, stands in contrast to the differential provision of recommendations to this population. As recommended by the Advisory Committee on Immunization Practices,[17, 18] HPV vaccination should be recommended and provided to all age-eligible adolescents and young adults. Our findings that there are differences in recommendation and vaccine provision among LGBT young adults highlight the need for providers to make HPV vaccine recommendation a universal recommendation. However there is evidence that providers across a range of disciplines lack the training needed to provide culturally competent care to LGBT individuals.[19–22] Future HPV vaccination studies should make concerted efforts to collect gender identity data and enroll transgender individuals to understand the dynamics of HPV vaccination across gender identity. Transgender patient’s healthcare providers should understand the potential issues related to sex-based differences in HPV vaccine recommendations, particularly regarding administration of and reimbursement for catch-up vaccination.
Acknowledgments
This project was supported in part by NIH grant K01AI106961. The funder had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this research have previously been presented at the National Foundation for Infectious Diseases 18th Annual Conference on Vaccine Research, Bethesda MD, April 2015, and the 2016 American Society for Colposcopy and Cervical Pathology Annual Scientific Meeting, New Orleans LA, April 2016.
The authors declare that no competing financial interests exist.
References
- 1.Markowitz LE, Dunne EF, Saraiya M, Chesson HW, Curtis CR, Gee J, et al. Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2014;63:1–30. [PubMed] [Google Scholar]
- 2.Reagan-Steiner S, Yankey D, Jeyarajah J, Elam-Evans LD, Singleton JA, Curtis CR, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years—United States, 2014. MMWR Morb Mortal Wkly Rep. 2015;64:784–92. doi: 10.15585/mmwr.mm6429a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent Human Papillomavirus Vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56:1–24. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1705–8. [PubMed] [Google Scholar]
- 5.McRee AL, Katz ML, Paskett ED, Reiter PL. HPV vaccination among lesbian and bisexual women: Findings from a national survey of young adults. Vaccine. 2014;32:4736–42. doi: 10.1016/j.vaccine.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reiter PL, McRee AL, Katz ML, Paskett ED. Human Papillomavirus Vaccination Among Young Adult Gay and Bisexual Men in the United States. Am J Public Health. 2014:e1–e7. doi: 10.2105/AJPH.2014.302095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Institute of Medicine. The National Academies Collection: Reports funded by National Institutes of Health. The Health of Lesbian, Gay, Bisexual, and Transgender People: Building a Foundation for Better Understanding. Washington (DC): National Academies Press (US), National Academy of Sciences; 2011. [PubMed] [Google Scholar]
- 8.Ward B, Dahlhamer J, Galinsky A, Joestl S. Sexual orientation and health among US adults: National Health Interview Survey, 2013. National Center for Health Statistics; 2014. [PubMed] [Google Scholar]
- 9.Whitehead J, Shaver J, Stephenson R. Outness, Stigma, and Primary Health Care Utilization among Rural LGBT Populations. PloS one. 2016;11:e0146139. doi: 10.1371/journal.pone.0146139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168:76–82. doi: 10.1001/jamapediatrics.2013.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stokley S, Freed G, Curtis R, Gordon L, Humiston S, Parnell T, et al. Adolescent vaccination: recommendations from the National Vaccine Advisory Committee. Am J Prev Med. 2009;36:278–9 e6. doi: 10.1016/j.amepre.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Jones J, Poole A, Lasley-Bibbs V, Johnson M. LGBT health and vaccinations: Findings from a community health survey of Lexington-Fayette County, Kentucky, USA. Vaccine. 2016;34:1909–14. doi: 10.1016/j.vaccine.2016.02.054. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13–17 years—United States, 2012. MMWR Morb Mortal Wkly Rep. 2013;62:685–93. [PMC free article] [PubMed] [Google Scholar]
- 14.Rahman M, McGrath CJ, Hirth JM, Berenson AB. Age at HPV vaccine initiation and completion among US adolescent girls: Trend from 2008 to 2012. Vaccine. 2015;33:585–7. doi: 10.1016/j.vaccine.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward B, Clarke T, Nugent C, Schiller J. Early release of selected estimates based on data from the 2015 National Health Interview Survey. National Center for Health Statistics; 2016. [Google Scholar]
- 16.Rolnick SJ, Parker ED, Nordin JD, Hedblom BD, Wei F, Kerby T, et al. Self-report compared to electronic medical record across eight adult vaccines: Do results vary by demographic factors? Vaccine. 2013;31:3928–35. doi: 10.1016/j.vaccine.2013.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meites E, Kempe A, Markowitz LE. Use of a 2-Dose Schedule for Human Papillomavirus Vaccination — Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65:1405–8. doi: 10.15585/mmwr.mm6549a5. [DOI] [PubMed] [Google Scholar]
- 18.Petrosky E, Bocchini JA, Jr, Hariri S, Chesson H, Curtis CR, Saraiya M, et al. Use of 9-Valent Human Papillomavirus (HPV) Vaccine: Updated HPV Vaccination Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2015;64:300–4. [PMC free article] [PubMed] [Google Scholar]
- 19.Amato P, Morton D. Original Research: Lesbian Health Education: A Survey of Obstetrics and Gynecology Residency Training Programs. Journal of the Gay and Lesbian Medical Association. 2002;6:47–51. [Google Scholar]
- 20.Corliss HL, Shankle MD, Moyer MB. Research, curricula, and resources related to lesbian, gay, bisexual, and transgender health in US schools of public health. Am J Public Health. 2007;97:1023–7. doi: 10.2105/AJPH.2006.086157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNair R. Outing lesbian health in medical education. Women & health. 2003;37:89–103. doi: 10.1300/J013v37n04_07. [DOI] [PubMed] [Google Scholar]
- 22.Tesar CM, Rovi SL. Survey of curriculum on homosexuality/bisexuality in departments of family medicine. Family medicine. 1998;30:283–7. [PubMed] [Google Scholar]


