Abstract
Several studies have shown that young children who have experienced early caregiving adversity (e.g. previously institutionalization (PI)) exhibit flattened diurnal cortisol slopes; however, less is known about how these patterns might differ between children and adolescents, since the transition between childhood and adolescence is a time of purported plasticity in the hypothalamic-pituitary-adrenal (HPA) axis. PI youth experience a massive improvement in caregiving environment once adopted into families; therefore we anticipated that a developmental increase in HPA axis plasticity during adolescence might additionally allow for an enhanced enrichment effect by the adoptive family. In a cross-sectional sample of 197 youths (PI and Comparison; 4–15 years old) we observed age-related group differences in diurnal slope. First replicating previous findings, PI children exhibited flattened diurnal slope. This group difference, however, was not observed in adolescents. Moderation analyses showed that pubertal development, increased time with family, and early adoption contributed to the steeper diurnal cortisol slope in PI adolescents. These findings add support to existing theories positing that the transition between middle childhood and adolescence may mark an additional sensitive period for diurnal cortisol patterning, allowing PI youth to benefit from the enriched environment provided by adoptive parents during this period of development.
Keywords: Childhood, Adolescence, Stress, Enrichment, Early Adversity
1 Introduction
Early rearing environments play an important role in the development of the hypothalamic-pituitary-adrenal (HPA) axis, a central system in the body’s ability to appropriately respond and adapt to stress (Gunnar & Quevedo, 2007; Lupien, McEwen, Gunnar, & Heim, 2009). Across several species, early adverse caregiving is followed by alterations to diurnal cortisol activity, such as flattened diurnal slopes with blunted morning and elevated evening levels (Gunnar & Vazquez, 2001; Sánchez et al., 2005). These early alterations in cortisol activity have been correlated with long-term future physiological and psychological outcomes, such as inflammation and increased rates of psychiatric disorders (e.g. anxiety and depression; Loman, Gunnar, & Experience, 2010). Children with a history of early parental deprivation as a result of institutional (i.e., “orphanage”) caregiving are at significantly elevated risk for long-term alterations to stress physiology and socio-emotional functioning (Loman et al., 2010). Nonetheless, many previously-institutionalized (PI) children that have been adopted, exhibit tremendous rebound in a number of developmental domains (Loman et al., 2010; Nelson et al., 2007; Tottenham, 2012), raising the possibility that the HPA axis may also exhibit change in the post-adoption home. Though much research has demonstrated flattened diurnal cortisol profiles for younger PI children, some work has shown that there is also dynamic change in the HPA axis observed into early adolescence (Gunnar, Morison, Chisholm, & Schuder, 2012; Kertes, Gunnar, Madsen, & Long, 2008; Quevedo, Johnson, Loman, LaFavor, & Gunnar, 2012), which is suggested to be another sensitive period for HPA axis programming (Hostinar & Gunnar, 2013).
1.1 International adoption as end of early life adversity
Examining the sequelae of early adversity is particularly difficult in humans because adverse caregiving contexts often do not have a discrete end point. Moreover, children who experience early adversity may continue to be living under conditions of adversity at the time of testing. It is therefore difficult to disentangle the effects of adversity during an early sensitive period of development from the chronic effects of adversity. Individuals with a history of previous institutional care and subsequent adoption by families, however, provide a rare chance to examine the sequelae of a discrete and early exposure to adverse caregiving, which ends upon adoption by families. Institutional rearing, even under the best conditions is a species atypical experience (Tottenham, 2012). However, the majority of parents who adopt internationally tend to have a higher income, increased education, and two-parent homes (Hellerstedt et al., 2008), and importantly they tend to exhibit an extraordinary degree of parental investment (Hamilton, Cheng, & Powell, 2007) and involvement (Levy-Shiff, Naomi Zoran, and Shmuel, 1997; McGuinness, McGuinness, & Dyer, 2000); therefore, PI children and adolescents are likely to be adopted into highly enriched family environments (Hamilton et al., 2007). This transition into adoptive homes provides the rare opportunity to examine how HPA axis activity can change with an enriched home environment.
1.2 Adolescence as another sensitive period in HPA programming
Strong evidence suggests that early postnatal development is a sensitive period in the HPA axis. However, mounting evidence from both rodent and human models suggests that the transition from childhood to adolescence may represent a second period of heightened HPA axis plasticity (Gunnar, Wewerka, Frenn, Long, & Griggs, 2009; Romeo, 2010), when the HPA axis can be “reorganized” by positive social environments (Francis, Diorio, Plotsky, & Meaney, 2002; Morley-fletcher et al., 2003). This period of potential reorganization could provide another opportunity to influence the development of the HPA axis, which is particularly important for individuals with a history of early adversity. For example, evidence from rodent studies demonstrates that HPA axis dysregulation from early maternal deprivation can be reversed by enriched environments in the juvenile-to-adolescent period (Francis et al., 2002; Morley-fletcher et al., 2003).
There are many changes occurring around this transition into adolescence that could increase the malleability of the HPA axis (Lupien et al., 2009). One proposed mechanism of this increase in HPA axis activity is pubertal maturation that typically begins in late childhood/early adolescence and extends into late adolescence. Although pubertal effects are often difficult to separate from age effects (Gunnar, Wewerka, Frenn, Long, & Griggs, 2009), Quevedo et al., 2012 demonstrated that puberty is a time of HPA axis recalibration. Specifically, between ages 12–14 years old, PI adolescents who were in pre/early pubertal development displayed blunted morning cortisol levels (cortisol awakening response, specifically), which was not observed in PI adolescents in post/late pubertal development. This “pubertal recalibration” hypothesis would suggest that puberty instantiates another sensitive period of HPA axis programming, providing an opportunity for PI youth to take advantage of their post-adoptive home environments in late childhood/early adolescence. Here, we extended these findings to assess diurnal cortisol production across the day (i.e., diurnal slope) as it relates to puberty and time with adoptive family across a broader age range.
1.3 Current study
The current study aimed to characterize age-related patterns of diurnal cortisol slope in a cross-sectional sample of both PI and never-institutionalized (Comparison) youth (4–15 years old) and to further examine the moderating effects of adoption-related variables and pubertal development on age-related changes in diurnal cortisol slope. First, we hypothesized that at younger ages (i.e., childhood), the PI group would exhibit a flattened diurnal cortisol slope, characterized by lower morning values (i.e., shallower slope). Secondly, we hypothesized that group differences would be less apparent between PI and Comparisons groups as age increased (i.e., adolescence), resulting from steeper age-related change in the PI group relative to the Comparison group. Lastly, we hypothesized that these age-related changes in the PI group’s diurnal cortisol slope would be associated with pubertal development and moderated by time with adoptive family, such that increased pubertal development and time with adoptive family would be associated with a steeper diurnal slope.
Methods and Materials
2.1 Participants
A total of 197 youths (79 PI and 118 Comparison) participated in the study. A wide age range (4–15 years old) was studied to examine cross-sectional age-related differences in diurnal cortisol. Participants were recruited as part of a larger longitudinal study examining the neural correlates of emotional development. The larger study included participants, ages 3–17 years old; however, due to the relatively smaller diurnal cortisol sample size for the PI group at either tail of the study (mean < 3 participants per year), we restricted the range to 4–15 years old (see Table 1). PI participants (defined as previously institutionalized in an “orphanage” abroad and subsequently adopted to the United States) were recruited via local international adoption agencies, adoption family networks, posted flyers, and friend referral. Healthy comparison participants (defined as raised by biological parents in the United States and never adopted) were recruited via birth records, posted flyers, and friend referral. Comparison participants were prescreened for prior diagnoses of any behavioral/psychological concerns or learning disabilities, and confirmed by in-lab assessments (e.g., CBCL internalizing problems mean(SD) = 46.90(10.82); CBCL externalizing problems mean(SD) = 44.99(9.60)). The protocol was approved by the Institutional Review Board at the University of California, Los Angeles. Participants and their parents provided both informed assent and consent, respectively.
Table 1.
Demographics Between Groups. PI= previously institutionalized. Comparison= never institutionalized. Peterson’s Pubertal Development Scale (PPDS; 1 (not begun) - 4 (fully developed)). Age and time variables all represented in years. Income ranges were between 1–10. Income range of 7= $100,001–150,000; income range of 6= $70,001–85,000 *above the US national average(~$52,250)40. Parent education ranges were between 1–10. Parent education range of 6= Some Graduate; parent education range of 5= 4 Year Degree.
| PI mean(SD) | Comparison mean(SD) | Significance | |
|---|---|---|---|
| Sex | M:26 F:50 | M:59 F:59 | χ2= 4.73, p=.04 |
| Puberty | 2.13 (0.77) | 2.14 (0.91) | t(87)=0.04, p=.97 |
| Age | 9.50 (3.11) | 8.79 (3.53) | t(192)=−1.42, p=.16 |
| Estimated IQ | 103.21 (15.01) | 111.61 (17.52) | t(152)=3.14, p<.003 |
| Parent Income | 7.59*(2.08) | 6.29*(2.94) | t(185)=−3.29, p<.002 |
| Parent Education | 6.25(1.43) | 5.44(1.91) | t(188)=−3.11, p<.003 |
2.1.2 Inclusion criteria
Early life adversity is associated with a number of health and behavioral outcomes, including increased allergies, and psychotropic medication use for mental health related problems (Kozyrskyj, Bahreinian, & Azad, 2011; Tottenham, 2012). Therefore, we did not exclude based on psychotropic (PI: 13, C: 0) or steroid (PI: 14, C: 3) medication. However, to properly control for the associations of these medications on cortisol values, both psychotropic and steroid medication usage were controlled for in all of the statistical models as a covariate of no interest. Sensitivity analyses were also performed to assess the contributing role of PI youth taking psychotropic and/or steroid medication. Overly influential outlying data points were calculated a priori using criteria of 3 standard deviations above or below the mean for the time of day, calculated separately in each group. Outlier removal did not result in the exclusion of an entire participant’s data.
Although there is no possible “control” group for the PI group, to better match the PI and Comparison samples, participants were removed with an estimated IQ less than 70 (based on standard intellectual impairment cutoffs; PI: 3, C: 0). Because it is possible that lower IQ in PI individuals is a result of early adversity, sensitivity analyses were performed with the inclusion of these three participants. Independent sample t-tests results indicated that there were significant overall group differences between PI and Comparison youth in estimated IQ (see Table 1), although both groups had estimated IQ means that were average or above. Although comparison families had lower mean parental education and income relative to the adoptive families, mean parental education and income in the Comparison group were well above national averages, representing middle-to-upper class range (Ryan & Bauman, 2016; DeNavas-Walt, Proctor, & Smith, 2015; see Table 1).
2.2 Measures
Adoption Descriptives
Institutional care measures were collected from PI participants’ adoptive parents. These questionnaires provided indexes of quality and quantity of institutional care, such as time in institutional care, age of placement and age of adoption. These measures (see Table 2) were used to assess the role of pre-adoptive conditions on age-related cortisol effects. To assess the associations of early versus late adoption and cortisol patterning, early adoption was defined as adopted before 1 year of age, and late adoption was defined as adopted at 1 year of age or older. Time with family was calculated by subtracting age of adoption from age at test.
Table 2.
PI Adoption-Related Demographics. PI= previously institutionalized. Age and time variables all represented in years. Descriptives of institution were rated on a 10-point scale (10 = greater quality). Eastern Europe: Russia 26, Ukraine 4, Belarus 1, Hungary 1; East Asia: China 26, South Korea 2, Vietnam 1; Central Asia: Kazakhstan 10; West Asia: Azerbaijan 2; Central America: Guatemala 2.
| Mean(SD) | Range | |
|---|---|---|
| Age Adopted | 2.25(2.10) | 0–10 |
| Age Orphaned | .82 (1.31) | 0–6 |
| Time Institutionalized | 1.71(1.43) | 0–8 |
| Time with Family | 7.33 (3.64) | 1–15 |
| Percent of Life w/Family | .75(.22) | 0–1 |
| Quantity Caregivers | 5.81(2.87) | 1–10 |
| Quality Caregivers | 6.37(2.64) | 3–10 |
| Clean Facilities | 7.54(1.95) | 1–10 |
| Quality Buildings | 6.16(2.54) | 1–10 |
| Quality Heath at Adoption | 6.59(2.56) | 1–10 |
| City Size | 7.02(3.02) | 1–10 |
| Origin | n | % |
| Eastern Europe | 32 | 42% |
| East Asia | 29 | 38% |
| Central Asia | 10 | 13% |
| West Asia | 2 | .03% |
| Central America | 2 | .03% |
| Missing | 1 | .01% |
Pubertal Development
The 5-item Peterson Pubertal Development Scale (PPDS25) was used to assess pubertal development for children 10 years of age and older. Sample items included: body hair growth, height growth, breast growth (female), and voice change (males). Responses were given on a 4-point scale, ranging from 1 (not begun) to 4 (fully developed). Pre/early pubertal development was defined as a PPDS score below 3, and late/post pubertal development was defined as a PPDS score of 3 to 4.
Health Diary
Take-home saliva diaries were included for each day of cortisol sampling. Parents were asked to report the following: date, bedtime, illness, medication use, and/or unusual levels of activity. Daily diaries were used for analysis covariates of psychotropic and steroid medication use.
Diurnal Salivary Cortisol
Families were instructed to collect cortisol samples over 2 days at target time points of: wake up, 45 minutes after wake up, 5pm, and 8pm. They were instructed to collect samples before eating or drinking, or at least 15 minutes after eating or drinking, and not to collect samples on days they felt ill. Following Salimetrics protocol, children under 6 years old used two sorbettes under their tongue for 1 minute per sample (Salimetrics, State College, PA). Children 6 years old and older were instructed to chew on a piece of Trident original sugarless gum, shown to not interfere with cortisol collection, to stimulate saliva flow28. Participants were then instructed to place a sorbette underneath their tongue for 60–90 seconds before placing the sample back in the salivette (Salimetrics, State College, PA). Samples were stored in a locked freezer at −20C until they were mailed over dried ice to Dr. Clemens Kirschbaum’s Biological Psychology Laboratory at the Technical University Dresden. Salivary cortisol concentrations were measured in singlet, using commercially available chemiluminescence-immunoassays with high sensitivity. The inter-assay coefficient for cortisol was below 8%(Kirschbaum & Hellhammer, 2000). Though we do not have an intra-assay coefficient for the data because the data were assayed in singlet, individual’s cortisol values between the days (e.g., samples collected at the same time across two days) were highly correlated (all p’s < .001).
2.4 Data Analysis
Statistical model
We employed multilevel modeling (Singer & Willett, 2003) to examine age-related differences in diurnal cortisol across the day between PI and Comparison groups. Multilevel modeling was implemented to handle non-independence in the data, as well as missing data. Statistical analyses were conducted using Statistical Analysis Software (SAS) 9.3, utilizing the PROC MIXED model to create a multilevel model with repeated measures to nest the four cortisol samples per day within two days of sample collection for each individual (see SAS Proc Mixed documentation for further description of Proc Mixed models; support.sas.com). Multilevel modeling assesses change by estimating two types of parameters that characterize a trajectory, initial status (model intercept) and slope, and we examined differences between PI and Comparison youths for both of these parameters. Satterthwaite approximation degrees of freedom were used and the covariance matrix was unstructured. In the main model, the following variables were treated as categorical: sample day (1,2), sample number (1,2,3,4), group (0,1), psychotropic medication (0,1), steroid medication (0,1), sex (0,1), and the following variables were treated as numeric: time of day (participant reported sample collection time), and age (continuous). All smaller order interactions were included for the three way interaction of Age x Time of Day x Group. To understand the significant Age x Time of Day x Group interaction, simple effects tests were performed using a SAS equivalent procedure to the Johnson-Neyman procedure in other programs (i.e., ESTIMATE in SAS). This allowed us to maintain the model structure and the entire dataset to describe the Age x Time of Day x Group interaction. Based on prior work that assessed the role of morning cortisol as a measure of healthy diurnal cortisol functioning (e.g., Quevedo et al., 2012), simple effects for Age X Time of Day X Group interaction were tested for both wake up cortisol values and 45 minutes after wake up.
Multilevel model assumptions were verified. Covariance structure testing indicated an unstructured covariance provided a better model fit than a compound symmetry covariance structure according to lower akaike’s information criterion (AIC; 9053.7 vs. 9409.4) and lower bayesian information criterion (BIC; 9203.6 vs. 9448.5). Test for skew demonstrated that all but one of the cortisol values (8pm) were normally distributed, and a log transformation caused non-normality in several cortisol variables. Therefore raw (non-transformed) values were used for the multilevel model, which multilevel modeling is robust to non-normality at the first level (Maas & Hox, 2004). To better account for multicollinearity in the model, numeric values were centered (e.g., age, time of day, time with family).
Covariates
Variables of potential influence on cortisol values were independently tested in the main multilevel model as a covariate of no interest: parental education, income, child estimated IQ, sex, psychotropic, and steroid medications. There were no significant simple effects of income, education, or estimated IQ on cortisol values (all p>.05) and the three-way interaction remained significant when the covariates were separately tested (all p<.05); therefore these variables were not included in the final model. Due to the group difference in measures of socioeconomic status, we tested the combination of education and income covariates in the model: the simple effects remained non-significant (p>.05) and the Age x Time of Day x Group interaction remained significant (p<.05). Psychotropic medications (PI only), steroid medication, and sex were significant predictors in the model (all p<.02) and were therefore included in all further analyses as covariates.
Follow-up analyses
The original multilevel model with repeated measures was maintained for all post hoc analyses. Covariates (i.e., steroid medications, psychotropic medications, and sex) and age (centered) were included in all post hoc analyses. For pubertal analyses conducted between ages 10–15, age was re-centered within the restricted age-range. To examine the contribution of pre/early or late/post pubertal development on PI youth’s diurnal cortisol slope, PDDS scores lower than 3 were coded as “pre/early” and 3 and higher as “late/post”. For sensitivity analyses, we also assessed pubertal development continuously. For post-hoc analyses the additional variables were treated as categorical: early/late pubertal development (1,2), early/late adoption (1,2) and the additional variables were treated as numeric: time with family (centered), puberty (continuous). Last, to assess the role of a cortisol awakening response between wake up and 45 minutes after wake up in the data set, we ran a constrained version of the multilevel model using only morning samples across the two days, with participant-reported time of sample (morning time).
3 Results
3.1 Group Differences in Age-Related Change in Diurnal Cortisol
A multilevel model with repeated measures was used to test the effects of Group (PI, Comparison), Age (in continuous years) and Time of Day (wake-up, 45 minutes after wake-up, 5PM and 8PM), on cortisol levels. Simple effects showed cortisol values were positively associated with Age (F(1,178)=10.73, p<.002), and negatively associated with Time of Day (F(1,180)=76.57, p<.0001). Overall, the PI group (versus Comparison) had significantly lower cortisol values (F(1,180)=5.27, p=.02). Cortisol slope across the day declined (i.e., steeper slope) with age (Age X Time of Day; F(1,179)=9.84, p<.003), and differed by group (Time of Day X Group; F(1,180)=6.77, p=.01) Importantly, these simple effects and lower-order interactions were qualified by a significant three-way interaction of Age X Time of Day X Group (F(1,179)=4.91, p=.03). Simple effects showed with increasing age, the PI group exhibited a significantly steeper slope across the day (PI: Age X Time of Day, (F(1,66.1)=8.82, p<.005; Comparison: Age X Time of Day, F(1,116)=.97, p=.33; see Figure 1). Further simple effect analyses were performed to describe the pattern of change observed in the main model. The model empirically derived that the Age X Time of Day x Group interaction was the result of group differences at younger ages and no group differences at older ages. Follow-up simple effect tests at childhood age-points, for example at 4 or at 8 years old, showed that the PI group had a significantly flatter slope across the day (versus the Comparison group); in contrast, tests at adolescent age-points, for example at 11 or at 15 years old, showed that PI and Comparison groups were not significantly different (see Table 3). The model empirically derived that the Age X Group X Time of Day effect was also the result of group differences in morning values across age; specifically, simple effects tests showed that a model-assumed wakeup time of 7am and 45 minutes later resulted in lower cortisol values for PI participants (relative to Comparisons) between early to mid-childhood (e.g., ages 4 and 8 years old), whereas there was no such group difference in morning values in adolescents (e.g., ages 11 and 15 years old; see Table 4). Follow-up tests to compare wake-up values to values 45 minutes after wake up indicated no interaction with time (Morning Time x Age x Group; F(1,180)=.36, p=.55). Testing only within the PI group showed a similar pattern: morning cortisol values were not significantly different across age between time of sampling for wake up and 45 minutes after wake up (Morning Time x Age; F(1,66.1)=.01, p=.94).
Fig. 1.
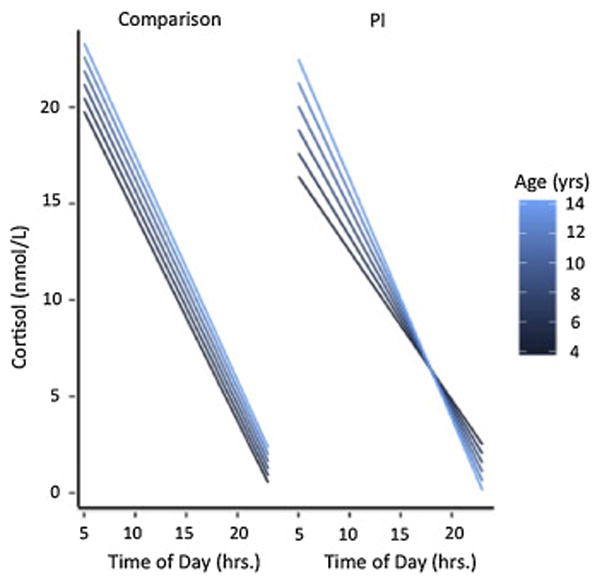
Group Differences in Diurnal Cortisol Slope Across Age. PI children (versus Comparison) exhibited significantly flatter cortisol slopes in childhood, and significantly steeper cortisol as age increased, resulting in no group difference in cortisol slope by adolescence. PI= previously institutionalized. Comparison =never institutionalized.
Table 3.
Simple Effects of Diurnal Cortisol Slope Across Age. Multilevel model interaction descriptives indicated PI children show a significantly flatter slope than comparison children at younger ages. PI= previously institutionalized. Comp= Comparison; never institutionalized. Age is in years.
| Age | Comp mean | PI mean | t | df | p |
|---|---|---|---|---|---|
| 4 | −1.06 | −0.75 | 2.70 | 181 | <.008 |
| 8 | −1.09 | −0.95 | 2.24 | 182 | 0.03 |
| 11 | −1.12 | −1.10 | 0.25 | 179 | 0.80 |
| 15 | −1.15 | −1.3 | −1.20 | 178 | 0.23 |
Table 4.
Simple Effects of Morning Cortisol Across Age. Multilevel model interaction descriptives indicated PI children show a significantly blunted morning cortisol values than comparison children at younger ages. PI= previously institutionalized. Comp= Comparison; never institutionalized. Age is in years.
| Wake up | Comp Mean | PI Mean | t | df | p |
|---|---|---|---|---|---|
| 4 | 18.31 | 14.80 | −2.29 | 181 | 0.02 |
| 8 | 19.20 | 17.08 | −2.21 | 188 | 0.01 |
| 11 | 19.86 | 18.80 | −1.20 | 187 | 0.25 |
| 15 | 20.75 | 21.08 | 0.14 | 180 | 0.71 |
| Wake up +45 minutes | |||||
| 4 | 17.51 | 14.23 | −2.29 | 180 | 0.02 |
| 8 | 18.38 | 16.37 | −2.48 | 189 | 0.01 |
| 11 | 19.02 | 17.97 | −1.20 | 188 | 0.23 |
| 15 | 19.89 | 20.11 | 0.14 | 181 | 0.89 |
3.2 Moderating Effects of Adoption-Related Variables in Diurnal Cortisol
Across age, we assessed the moderating role of time in adoptive family for PI youth’s diurnal cortisol slope using the same multilevel model employed above using repeated measures, and retaining the same covariates (i.e., sex, medications, and age). Due to the high correlation between age of adoption and time with family (p<.001), age of adoption and time with family were both included in analyses. Age and time with family were both centered and early/late adoption was treated as categorical. Controlling for age and age of adoption, increased time with family was associated with a steeper slope across the day (Time of Day X Time with Family; F(1,67.2)=10.04, p<.003; see Figure 2a). Similarly, controlling for age and time with family, earlier adoption (i.e, adoption before one year old) was associated with a steeper slope across the day (Time of Day X Age Adoption; F(1,72.3)=13.61, p<.0005; see Figure 2b). There were no significant interactions with measures of parent-reported quality of pre-adoptive environment and cortisol slope across the day (all p’s >.05).
Fig. 2.
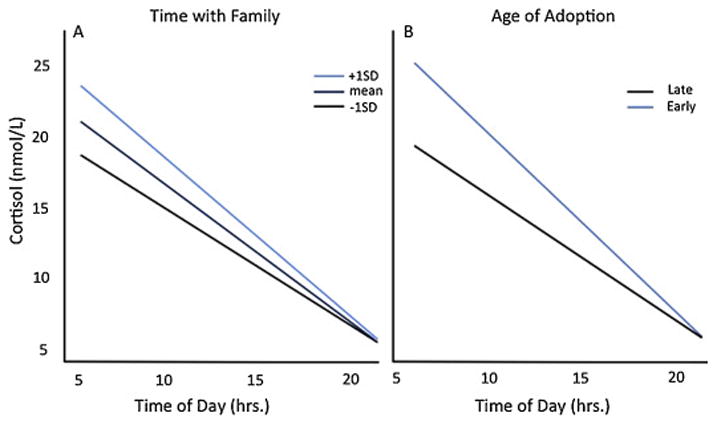
Adoption Related Variables on Diurnal Cortisol Slope Across Age. Fig. 2A. Time with Family on Diurnal Cortisol Slope Across Age. Controlling for age, PI participants that spent more time with family had a steeper diurnal cortisol slope. Fig. 2B. Age of Adoption on Diurnal Cortisol Slope Across Age. Controlling for age, PI participants adopted before one year old showed a significantly steeper diurnal cortisol slope than PI participants adopted later.
3.3 Moderating Effects of Pubertal Development in PI Adolescent Diurnal Cortisol
Multilevel models with repeated measures were used to assess the moderating role of pubertal development on the observed age-related change in the PI group’s cortisol slope (10–15 years old). Controlling for sex, age and puberty were highly correlated (age with puberty continuously, r=.73, p<.001; age with puberty categorically, r=.63, p<.001); therefore, all covariates were retained (sex, medications, and age centered within adolescence). Controlling for age and sex, more advanced pubertal development was associated with a steeper diurnal cortisol slope (Time of Day X Puberty Development; F(1,29.6)=11.55, p<.003, see Figure 3). This interaction remained significant when controlling for both early/late adoption and time with family (Time of Day X Puberty Development; F(1,29.8)=13.97, p<.001). When puberty was examined as a continuous measure, results were consistent: as pubertal development increased, the PI group’s diurnal slope steepened (F(1,28.7)=12.57, p<.002). There was no significant interaction between puberty, adoption variables, and time of day on cortisol values (all p’s>.05).
Fig. 3.
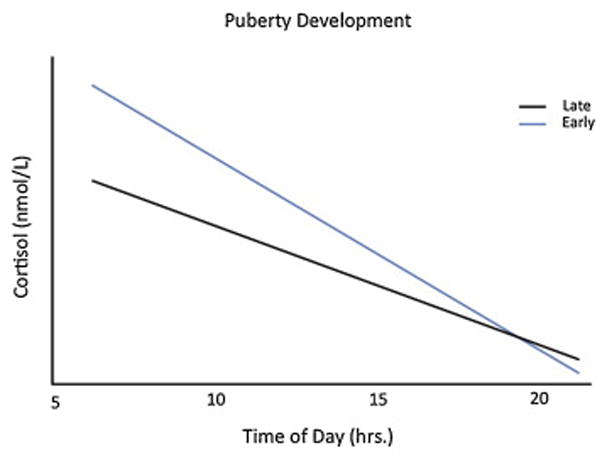
PI Pubertal Development on Diurnal Cortisol Slope. PI adolescents in late pubertal development display a significantly steeper slope than PI adolescents in early pubertal development. Early pubertal development =PPDS score < 3; Late pubertal development =PPDS score 3 and higher.
3.4 Sensitivity Analyses
We examined the potential influence of medication use in the PI group on cortisol production throughout the day using the following separate multilevel models. In the PI group, there was a main effect of psychotropic medication use (F(1,67.4)=12.77, p<.008) and steroid medications (F(1,64.6)=6.33, p=.01) on increased overall cortisol levels, but the Age X Time of Day interaction remained significant (F(1,68.3)=9.06, p<.004). Importantly, the original three-way interaction (Age X Time of Day X Group) was robust to sensitivity analyses that excluded PI participants taking psychotropic and/or steroid medication (p<.05). Next we assessed the role of medication use within the PI sample across age and within the restricted pubertal age range. Both the age-related and puberty-related differences in cortisol (steeper) slope were robust to sensitivity analyses performed with the exclusion of PI youth taking psychotropic and/or steroid medications (p<.05). Lastly, the Age X Time of Day X Group interaction remained significant (p<.05) with the inclusion of PI participants with an estimated IQ less than 70.
4 Discussion
The present study builds on a large body of animal and human literature indicating that HPA axis activity is highly influenced by early experiences, but that it also remains dynamic over the course of development (Lupien et al., 2009). Here, we showed an age-related change in group differences between PI and comparison youth’s diurnal cortisol slope. The Age X Time of Day X Group interaction showed that in early and middle childhood, the PI group exhibited a flatter slope, as a result of blunted morning cortisol levels (versus Comparisons). This finding is highly consistent with several published reports across different species (Gunnar & Vazquez, 2001; Sánchez et al., 2005). However, this group difference was not apparent in adolescence. The lack of group differences in the adolescent group was the result of the PI group showing a large age-related change in diurnal slope, so that PI adolescents were not different from the comparison adolescents. Simple effects indicated this age-related change in the PI group was the result of increasing cortisol morning levels (both wake up and 45 minutes after wake up) levels as age increased. These age-related changes in this cross-sectional sample are consistent with the hypothesis that the transition between middle childhood and adolescence may represent a time of increased plasticity of the HPA axis (Lupien et al., 2009; Quevedo et al., 2009).
4.1 Pubertal Development and Diurnal Cortisol in the PI sample
Steeper diurnal slope and higher morning cortisol values are typically associated with a healthy diurnal cortisol profile (Lupien et al., 2009), and although the current sample was cross-sectional, the age related change suggests an improvement in HPA axis function during adolescence in the PI group. Furthermore, there was a significant difference in diurnal slope across the day depending if PI youth were at earlier or later points in pubertal development. These data add to the findings of Quevedo et al., 2012, providing support that the pubertal transitions between childhood and adolescence may increase hormonal plasticity and provide a mechanism for improved diurnal cortisol for youth with a history of early institutional rearing.
4.2 Moderating Roles of Adoption Timing Variables on Diurnal Cortisol
Consistent with prior findings showing increased time spent in an institutional rearing environment is associated with flatter cortisol slopes throughout the day (Gunnar, Morison, Chisholm, & Schuder, 2001; McLaughlin et al., 2015; Quevedo et al., 2012), we observed a dose-response association such that older age at adoption (>1 year in institution) was associated with flatter cortisol slopes in the PI group. We observed no significant effect of parent-reported quality/quantity of institutional caregiving (e.g., quality of facility and quantity of caregivers) on diurnal cortisol slope, consistent with the notion that even under the best conditions, institutional care still represents a severely suboptimal care experience for human infants (e.g., Tottenham, 2012). However, we also observed that time with adopted family contributed independently to a steeper cortisol production slope (even when controlling for age adopted and age), suggesting that these two variables, age of adoption and time with family, have independent effects on HPA axis function. These data speak to the power of enriching environments in impacting the functioning of stress-responsive systems.
4.3 Implications
These data suggest several noteworthy implications. The increasing similarity in Comparison and PI youths’ diurnal slopes as age increased may indicate that PI adolescents are able to take advantage of their improved home environment (i.e., adoptive home). The associations with pubertal development additionally indicate that pubertal transitions facilitate this environmental enrichment, and pubertal development may provide another sensitive period for HPA axis re-calibration. We did not observe an interaction between adoption-related variables and pubertal development however, suggesting that these variables independently describe some of the individual variation in the age-related changes in the PI group’s diurnal cortisol slope.
Later age of adoption (e.g., after the child’s first birthday in the current study) was associated with a flatter diurnal cortisol slope, consistent with previous publications. For example, Gunnar et al., 2001 showed that children who spent more than 8 months in an institutional care showed significantly higher overall daytime cortisol production, and significantly lower cortisol awakening response in pre/early pubertal development than non-adopted youth (Quevedo et al., 2012). In another study, children randomly assigned to foster care after two years of age did not show an intervention effect in cortisol reactivity (McLaughlin et al., 2015).
It is important to note that, despite the potential rescue of the HPA axis observed in the current study, other work has shown that brain regions sensitive to cortisol (e.g., amygdala, prefrontal cortex, and ventral striatum) may not normalize for PI adolescents (Nelson, Bos, Gunnar, & Sonuga-Barke, 2011; Gee et al., 2013; Goff & Tottenham, 2015). This discrepancy is consistent with several previous findings that cortisol exerts its largest effects on brain structure and function in the early organizational states of these neural regions (Raineki et al., 2012 & reviewed in Gunnar & Quevedo, 2007). However, more normative diurnal cortisol functioning may play a future protective role in long-term immune health, such as inflammation and aging, by decreasing wear and tear on the system (Mcewen & Gianaros, 2010).
4.4 Limitations and Future Directions
There are limitations to consider regarding our sample and generalizability of our findings. Due to the nature of the sample, random assignment is impossible. Furthermore, although we tested the influence of the pre-adoptive variables on the models, we had minimal knowledge of pre-adoptive life (other than timing) and therefore we were only able to model aspects of this period provided to us by adoptive families. Secondly, due to the nature of sampling, compliance with diurnal cortisol sampling was self-reported and therefore should be interpreted with some caution until future work with more objective measures (e.g., timestamping) can confirm these initial findings. This study used a cross-sectional design, so we do not know if PI adolescents previously exhibited bunted diurnal cortisol patterning or if PI children will exhibit increased cortisol slope with increased age; however, we observed no significant differences in the pre-adoptive environment between child and adolescent PI participants, providing some confidence that these are not merely cohort effects (see Supplementary Table 1). Lastly, pubertal development was only assessed using a self-report measure in participants 10 year and older. Some studies have shown that early adversity, such as harsh parenting, can be associated with accelerated pubertal development (Belsky et al., 2010); therefore, it is possible our results are underselling the influence of pubertal development on diurnal cortisol patterning in younger PI participants. Future studies should seek to validate these findings using several measures of pubertal development, including hormone levels, as well as measures of pubertal development across childhood. In addition, oversampling within this childhood to adolescent timeframe would allow future studies a greater ability to parse apart pubertal effects from age (Quevedo et al., 2012)
4.5 Conclusion
These data suggest that early-life plasticity may be followed by additional moments of plasticity during the transition between childhood and adolescence, allowing PI adolescents to benefit from the enriched environments provided by adoptive families. These data also provide supportive preliminary evidence that pubertal development may be part of the mechanism by which plasticity of the HPA axis occurs.
Acknowledgments
This work was supported by NIMH R01MH091864 (to NT), the Dana Foundation (toNT), the National Science Foundation Conference Grant conference grant BCS-1439258 (N. Tottenham, co-I), and the National Science Foundation Graduate Research Fellowship 2015172132 to Jessica Flannery. We would also like to thank UCLA statistical consulting for assistance building the SAS PROC MIXED and PROC PLM models and John Flournoy at the University of Oregon for graphical assistance in R Studio for our Figure 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belsky J, Steinberg L, Houts RM, Halpern-Felsher BL. The development of reproductive strategy in females: Early maternal harshness→ earlier menarche→ increased sexual risk taking. Developmental psychology. 2010;46(1):120. doi: 10.1037/a0015549. [DOI] [PubMed] [Google Scholar]
- DeNavas-Walt C, Proctor BD, Smith JC. Income and poverty in the United States: 2014. United States Census Bureau; 2015. p. 22. [Google Scholar]
- Francis DD, Diorio J, Plotsky PM, Meaney MJ. Environmental enrichment reverses the effects of maternal separation on stress reactivity. Journal of Neuroscience. 2002;22(18):7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, … Tottenham N. Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff B, Tottenham N. Early-life adversity and adolescent depression: mechanisms involving the ventral striatum. CNS spectrums. 2015;20(04):337–345. doi: 10.1017/S1092852914000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–73. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm KIM, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Development and psychopathology. 2001;13(03):611–628. doi: 10.1017/S095457940100311X. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Development and psychopathology. 2001;13(03):515–538. doi: 10.1017/S0954579401003066. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Stress Neurobiology and Developmental Psychopathology. Development and Psychopathology. 2006;2:533–577. [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus–pituitary–adrenal activity over the transition to adolescence: normative changes and associations with puberty. Development and psychopathology. 2009;21(01):69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton L, Cheng S, Powell B. Adoptive parents, adaptive parents: Evaluating the importance of biological ties for parental investment. American Sociological Review. 2007;72(1):95–116. doi: 10.1177/000312240707200610. [DOI] [Google Scholar]
- Hellerstedt WL, Madsen NJ, Gunnar MR, Grotevant HD, Lee RM, Johnson DE. The International Adoption Project: Population-based surveillance of Minnesota parents who adopted children internationally. Maternal and child health journal. 2008;12(2):162–171. doi: 10.1007/s10995-007-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Gunnar MR. Future directions in the study of social relationships as regulators of the HPA axis across development. Journal of Clinical Child & Adolescent Psychology. 2013;42(4):564–575. doi: 10.1080/15374416.2013.804387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertes DA, Gunnar MR, Madsen NJ, Long JD. Early deprivation and home basal cortisol levels: A study of internationally adopted children. Development and psychopathology. 2008;20(02):473–491. doi: 10.1017/S0954579408000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer DH. Salivary cortisol. Encyclopedia of stress. 2000;3:379–383. doi: 10.1097/ACI.0b013e328349b166. [DOI] [Google Scholar]
- Levy-Shiff R, Zoran N, Shulman S. International and domestic adoption: Child, parents, and family adjustment. International Journal of Behavioral Development. 1997;20(1):109–129. doi: 10.1080/016502597385478. [DOI] [Google Scholar]
- Loman MM, Gunnar MR. Early experience and the development of stress reactivity and regulation in children. Neuroscience & Biobehavioral Reviews. 2010;34(6):867–876. doi: 10.1016/j.neubiorev.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Maas CJ, Hox JJ. Robustness issues in multilevel regression analysis. Statistica Neerlandica. 2004;58(2):127–137. doi: 10.1046/j.0039-0402.2003.00252.x. [DOI] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences. 2010;1186(1):190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinness TM, McGuinness JP, Dyer JG. Risk and protective factors in children adopted from the former Soviet Union. Journal of Pediatric Health Care. 2000;14(3):109–116. [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Tibu F, Fox NA, Zeanah CH, Nelson CA. Causal effects of the early caregiving environment on development of stress response systems in children. Proceedings of the National Academy of Sciences. 2015;112(18):5637–5642. doi: 10.1073/pnas.1423363112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley-Fletcher S, Rea M, Maccari S, Laviola G. Environmental enrichment during adolescence reverses the effects of prenatal stress on play behaviour and HPA axis reactivity in rats. European Journal of Neuroscience. 2003;18(12):3367–3374. doi: 10.1046/j.1460-9568.2003.03070.x. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Bos K, Gunnar MR, Sonuga-Barke EJ. V. The neurobiological toll of early human deprivation. Monographs of the Society for Research in Child Development. 2011;76(4):127–146. doi: 10.1111/j.1540-5834.2011.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: The Bucharest Early Intervention Project. Science. 2007;318(5858):1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- Quevedo K, Johnson AE, Loman ML, LaFavor TL, Gunnar M. The confluence of adverse early experience and puberty on the cortisol awakening response. International journal of behavioral development. 2012;36(1):19–28. doi: 10.1177/0165025411406860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Cortés MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: Infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. Journal of Neuroscience. 2012;32(22):7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD. Pubertal maturation and programming of hypothalamic–pituitary–adrenal reactivity. Frontiers in neuroendocrinology. 2010;31(2):232–240. doi: 10.1016/j.yfrne.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Ryan CL, Bauman K. Educational attainment in the United States: 2015. March Current Population Report 2016 [Google Scholar]
- Sánchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, Winslow JT. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biological psychiatry. 2005;57(4):373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford university press; 2003. [Google Scholar]
- Tottenham N. Risk and developmental heterogeneity in previously institutionalized children. Journal of Adolescent Health. 2012;51(2):S29–S33. doi: 10.1016/j.jadohealth.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. The developing human brain. 2009:204. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


