Abstract
Background
Cerebral arteriovenous malformations (AVM) are common in patients with hereditary hemorrhagic telangiectasia (HHT). However, due to rarity of HHT, and little published evidence of outcomes from management of brain AVMs in this disease, current International HHT Guidelines recommend an individualized approach. Specifically, the outcomes for surgical versus non-surgical management of these lesions have not been reported to date.
Objective
We report long-term outcomes of surgical resection of brain AVMs in HHT patients compared to outcomes in non-surgically treated patients.
Methods
From the database of Brain Vascular Malformation Consortium HHT project, 19 patients with 20 resected AVMs (group 1), and 22 patients with 33 AVMs who received non-surgical treatment (group 2) were studied. The groups were retrospectively reviewed for changes in functional status (modified Rankin Scale score) during the follow-up period.
Results
During the follow-up period, 9% of patients in group 1 suffered from worsening of functional status whereas this figure was 16% for group 2 (P> 0.05). Functional outcomes were not statistically different between the two groups at the latest the follow-up (P > 0.05).
Conclusion
HHT patients treated surgically for brain AVMs appear to have long-term functional outcomes comparable to non-surgical (including observational) therapy with fewer unfavorable outcomes. It is therefore reasonable to consider surgical resection as a management option, in the multidisciplinary team’s individualized treatment strategy for HHT patients with brain AVMs.
Keywords: Arteriovenous malformation, Hereditary Hemorrhagic Telangiectasia, Rendu-Osler-Weber disease, Embolization, Radiosurgery, AVM grading, Microsurgical Resection, Brain Vascular Malformation Consortium
Introduction
Hereditary hemorrhagic telangiectasia (HHT) is a rare familial disorder with autosomal dominant inheritance.1 It is characterized by multiple mucocutaneous telangiectasias, and visceral vascular malformations. HHT has a reported overall prevalence of 1–2 in 10,000, although geographical location has great impact on this figure.2–4 Neurological complications affect about 8–27% of HHT patients.5–8 The majority of neurologic complications in HHT patients are associated with pulmonary AVMs, causing stroke or brain abscess.9–11 However, cerebral vascular malformations (VM) account for one third of neurological manifestations in HHT patients.10, 12 VMs of the brain are found in 5–23% of HHT patients.12–15 Generally, three types of cerebral VMs are described in HHT patients: (1) arteriovenous malformations (AVMs); (2) non-shunting, small, superficially located collections of enhancing vessels with no enlarged feeding artery or draining vein, named “capillary VMs”; and (3) arteriovenous fistulas (AVFs).16–18 AVMs, with evidence of shunting and the presence of a nidus, comprise 15.8–83.3% of all cerebral VMs in HHT patients.12, 15–20 Most of these brain AVMs are superficial and small (< 3cm); and, have a single feeder and a single draining vein (Figure 1).9, 17, 18, 20–22
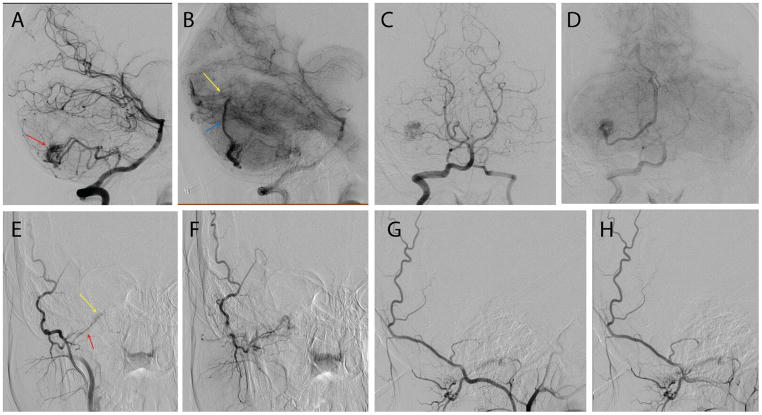
Figure 1.
Cerebral digital subtraction angiograms of a 19-year-old female presenting with intractable headaches and syncopal episodes demonstrate a characteristic AVM in an HHT patient. Lateral (A & B) and antero-posterior (C &D) projections of vertebral artery injection in arterial and capillary phases showing a right superficial cerebellar 1.2×1.3 cm arteriovenous malformation (red arrow in A) supplied by posterior inferior cerebellar artery with superficial hemispheric drainage (blue arrow in B) to the straight sinus near the torcula (yellow arrow in B). Although there are no flow-related or intra-nidal aneurysms there is a high-grade stenosis of the venous outflow at the junction between the cortical vein and the straight sinus. Antero-posterior (E & F) and lateral (G & H) projections of selective right occipital artery injection in the same patient also show a hypoglossal canal arteriovenous fistula (yellow arrow in E) fed by the hypoglossal branch of the right occipital artery (red arrow in E) and draining into condylar veins.
The annual risk of an AVM rupture in HHT patients has been estimated to be 0.36–1.02% per year.15, 23, 24 In the largest series to date, however, the confidence intervals were large, with intracranial hemorrhage rates ranging from 0.42–2.44% per year, and higher rates reported in those with initial hemorrhagic presentation.23 Most brain AVMs in these patients were discovered by magnetic resonance (MR) screening, as routinely performed for HHT patients in north American HHT centers.13, 15, 16, 24 Although multiple radiological and clinical descriptions of cerebral VMs in HHT patients exist, few studies have focused on the treatment of these lesions.13, 21, 25, 26 Due to the paucity of the literature in this regard, there is no accepted standard treatment paradigm for brain AVMs in HHT. Current International HHT Guidelines recommend an individualized approach conducted by a multidisciplinary team with neurovascular expertise.27 Presently, there are various available treatment techniques (microsurgical resection, radiosurgery, and embolization therapy), as well as combined modalities, and finally the ‘wait-and-see’ approach.
The development of treatment guidelines for HHT patients with brain AVMs is difficult for many reasons. First, HHT-related brain AVMs are rare lesions and single institution experiences are small; second, the anatomical characteristics of these lesions differ from sporadic brain AVMs, which may prevent experiences and guidelines derived from sporadic AVMs therapy from being applied to HHT AVMs; and third, some studies have suggested a dynamic clinical course for HHT-related brain AVMs with clear growth and regression25, 28, 29, which may modify their response to standard AVM therapies. Nonetheless, guidelines are important for these patients because their lesions are increasingly detected by screening studies, rather than by hemorrhagic presentation, and the diagnosis of an unruptured brain AVM creates a management dilemma, particularly in the aftermath of the ARUBA results. Therefore, we formed a multicenter consortium of academic institutions with dedicated expertise in the management of HHT patients - the Brain Vascular Malformations Consortium (BVMC) - with the intention of studying HHT-related AVMs and their treatments. The BVMC, which is funded by the National Institute of Health and part of the Rare Diseases Clinical Research Network, facilitated the assembly of the largest cohort of operated HHT-brain AVM patients to date for analysis of surgical therapy and outcomes, and comparing these results to those from non-surgically treated brain AVMs in HHT patients.
Patients Methods
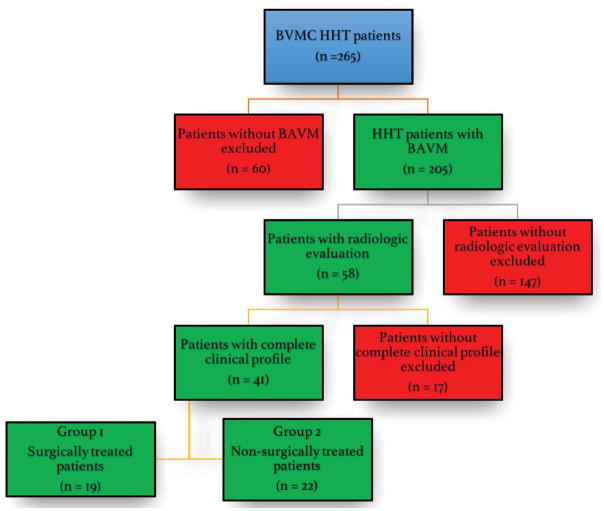
This retrospective study was approved by the institutional review board and performed in compliance with Health Insurance Portability and Accountability Act regulations. HHT patients enrolled in the Brain Vascular Malformation Consortium (BVMC) HHT project between April 2010 and November 2015 were studied (n = 265). After obtaining informed consent, patients were recruited to the BVMC as previously described.18 All patients had a genetic or clinical diagnosis of HHT (definite HHT with ≥3 criteria of the 4 Curaçao criteria).30 We included all patients recruited with known history of brain AVMs and who also had available diagnostic imaging for review (n=58). Of these, we excluded 17 patients with incomplete clinical profile. Only 2 of these 17 patients underwent surgery and none of the operated patients showed worsening of functional status at long term follow-up, as the mRS scores for these 2 patients were 2, both before surgery and at the latest follow-up visit. Patients with brain AVMs had their brain imaging reviewed by our senior radiologist (T.K.). These patients were allocated to either of two groups: (1) patients with at least one of the brain AVMs resected (n =19), and (2) patients without a history of surgery for brain AVMs (n = 22) (Figure 2). Clinical information retrieved from the BVMC dataset included (1) age at the time of diagnosis (and surgery, if performed), (2) symptoms at clinical presentation (including cerebral hemorrhage from AVM rupture), (3) the functional status (i.e., modified Rankin [mRS] score) at the time of diagnosis (for non-surgically treated patients) or at the time of surgery (for the surgically treated patients), and (4) the latest follow-up mRS score. For patients who underwent surgery, the early post-operative mRS score (at 6 weeks) was also recorded.
Figure 2.
Selection process of the patients in the present study. BAVM, brain arteriovenous malformation; BVMC, brain vascular malformation consortium; HHT, hereditary hemorrhagic telangiectasia.
Radiological evaluation included recording type, count, and location of all cerebral VMs (including AVMs, AVFs and capillary VMs), as well as criteria for determining Spetzler-Martin (SM), Lawton-Young (LY), and Supplemented Spetzler-Martin (Supp-SM) scores31 for brain AVMs. Lesion obliteration was confirmed by post-operative imaging reviewed by BVMC radiologists.
Statistical analysis
Statistical analysis included Mann-Whitney U test to compare median mRS scores between the two groups, student’s t-test to compare parametric means, and the two-sample z-test to compare proportions of various parameters (e.g., functional status, radiological grades) between the two groups. Spearmen’s Rho was calculated to evaluate correlation between the radiological lesion grade and the surgical outcome.
Results
Group 1: Surgically Treated
Of the 14 centers enrolled in BVMC, the 19 surgically treated patients were recruited at 8 centers. Average total number of brain AVM cases enrolled in each site was 15 patients (range, 9–24). Considering all patients diagnosed with brain AVM in these 8 centers regardless of the completeness of clinical profile or radiological evaluation (n = 118), average surgery rate for the BAVM was 16% (range: 8–25%, SD 6%).
There were 12 female and 7 male patients with 20 brain AVMs resected, and a total number of 27 lesions including 21 AVMs and 6 capillary VMs (mean 1.4 lesions per patient, range 1–5). No cerebral AVF was present in any of the surgically treated patients. Median age at surgery was 28 years (range, 0.5–69 years) whereas median age at diagnosis was 27 years (range, 0.1–55 years). One patient (5%) presented with clinical manifestations of AVM rupture (i.e. intracranial hemorrhage). Six patients (32%) were diagnosed through screening studies or while evaluating for other cerebral lesions. The most common presenting symptoms were headache (52%) and focal neurological deficit (32%) (Table 1).
Table 1.
Clinical data for surgically treated and non-surgically treated HHT patients with brain arteriovenous malformation in current series.
| Surgically Treated (n = 19) | Non-surgically Treated (n = 22) | |
|---|---|---|
| Age at diagnosis, median (range) | 27 (0.1–55) | 42 (0.6–72.5) |
| Female, n (%) | 12 (63%) | 12 (54%) |
| Presentation, n (% lesions) | ||
| Asymptomatic | 6 (32%) | 18 (82%) |
| Headache | 10 (52%) | 3 (14%) |
| Seizure | 3 (16%) | 3 (14%) |
| Focal neurological deficit | 6 (32%) | 2 (9%) |
| Hemorrhage | 1 (5%) | 3 (14%) |
AVM, arteriovenous malformation
Lesions were most commonly located in frontal (45%) and parietal (20%) lobes (Table 2). Eight lesions were in eloquent areas (40%). Average size of the lesions was 18.6mm (range, 8–36mm; SD, 8). Most lesions were superficially located with superficial venous drainage (90%); two lesions had deep venous drainage. Lesion grade was SM grade 1 or 2 in 90% (Table 2). Most of the parietal lobe (3/4, 75%) and frontal lobe (8/9, 89%) lesions were symptomatic in this group (Table 3).
Table 2.
Radiological characteristics and lesion grades of 20 surgically resected arteriovenous malformations and 33 lesions managed non-surgically.
| Surgery | No surgery | |
|---|---|---|
| Characteristic | n (%) | |
| Deep Venous Drainage | 2 (10) | 2 (6) |
| Eloquent | 8 (40) | 10 (30) |
| Lobe | ||
| Frontal | 9 (45) | 18 (55) |
| Parietal | 4 (20) | 2 (6) |
| Occipital | 2 (10) | 7 (21) |
| Cerebellum | 2 (10) | 3 (9) |
| Temporal | 2 (10) | 2 (6) |
| Deep | 1* (5) | 1# (3) |
| Spetzler-Martin Grade | ||
| 1 | 9 (45) | 22 (66) |
| 2 | 9 (45) | 9 (27) |
| 3 | 2 (10) | 1 (3.5) |
| 4 | 0 | 1 (3.5) |
| 5 | 0 | 0 |
| Lawton-Young grade | ||
| 1 | 1 (5) | 0 |
| 2 | 7 (35) | 8 (24) |
| 3 | 3 (15) | 12 (37) |
| 4 | 9 (45) | 11 (33) |
| 5 | 0 | 2 (6) |
| Supplemented Spetzler-Martin Grade† | ||
| 2 | 0 | 0 |
| 3 | 3 (15) | 5 (15) |
| 4 | 6 (30) | 9 (27.5) |
| 5 | 7 (35) | 12 (36.5) |
| 6 | 3 (15) | 7 (21) |
| 7 | 1 (5) | 0 |
| 8 | 0 | 0 |
| 9 | 0 | 0 |
| 10 | 0 | 0 |
brainstem
thalamus
Supplemented Spetzler-Martin Grade is the add-up of Spetzler-Martin and Lawton-Young grades.
Table 3.
Clinical and radiological features of individual patients in group 1 (surgically treated) and their initial, early post-op, and late post-op functional status.
| Patient No. | Age at surgery (years), Sex | Symptoms Prior to surgery | Lesion Location | Lesion Grade | Pre-op mRS score | Early post-op mRS score | Latest post-op mRS score | Complications | Adjuvant therapy | |
|---|---|---|---|---|---|---|---|---|---|---|
| SM | LY | |||||||||
| 1 | 10, F | Headache, Seizure, FND | R Frontal | 1 | 2 | 2 | 0 | 2 | None | E |
| 2 | 29, F | Asymptomatic | R Temporal | 1 | 2 | 0 | 1 | 2 | None | - |
| 3 | 24, F | Headache | R Parietal | 2 | 3 | 2 | 2 | 2 | None | - |
| 4 | 50, M | Headache | R Frontal | 1 | 4 | 2 | 2 | 1 | None | - |
| R Parietal | 1 | 4 | ||||||||
| 5 | 45, F | Headache | R Frontal | 2 | 4 | 1 | 1 | 0 | None | - |
| 6 | 41, M | Congenital facial weakness due to encephalomalacia | R Frontal | 2 | 4 | 1 | 1 | 1 | None | - |
| 7 | 15, F | Asymptomatic | L Frontal | 2 | 2 | 1 | 1 | 1 | None | E |
| 8 | 11, F | Headache, Somatosensory partial Seizures | L Frontal | 2 | 2 | 1 | 1 | 1 | seizures, sensorimotor deficit resolved in 30 days | - |
| 9 | 69, M | Gait instability | Brainstem | 3 | 4 | 0 | 0 | 2 | None | E |
| 10 | 19, F | Headache, Fainting | Cerebellar | 1 | 2 | 1 | 0 | 1 | mild dysmetria resolved within 1 day after surgery | - |
| 11 | 0.5, M | Hemorrhage | L Parietal | 3 | 1 | 0 | 0 | 1 | R hemiparesis resolved 10 days after surgery | - |
| 12 | 39, M | Asymptomatic | Cerebellar | 1 | 4 | 0 | 0 | 0 | None | E,RS |
| 13 | 9, M | Asymptomatic | Cerebellar | 2 | 2 | 0 | 0 | 0 | None | - |
| 14 | 26, F | Headache | L Frontal | 1 | 3 | 0 | 0 | 0 | None | E |
| 15 | 40, F | Asymptomatic* | L Parietal | 2 | 3 | 2 | 2 | 1 | Aneurysm rupture hemiparesis | E |
| 16 | 27, M | Headache# | L Frontal | 1 | 4 | 0 | 4 | 0 | None | - |
| 17 | 6, F | Headache | R Frontal | 2 | 2 | 0 | 0 | 0 | None | - |
| 18 | 55, F | Asymptomatic | L Temporal | 2 | 4 | 0 | 0 | 1 | None | E |
| 19 | 65, F | Partial Seizure | L Occipital | 1 | 4 | 1 | 0 | 2 | None | - |
underwent angiography for decreased vision which revealed an ophthalmic artery aneurysm and the asymptomatic arteriovenous malformation
aneurysm rupture on operating table
M, male; F, female; FND, focal neurological deficit; R, right; L, left; E, embolization; RS, radiosurgery
Complications and Outcomes
All lesions were eradicated surgically as confirmed by post-operative imaging. Median preoperative, early post-operative, and late follow-up mRS scores were 1. Three patients suffered from early postoperative neurological complications that resolved within 1 month after surgery. These included transient sensorimotor deficits in 1 frontal (SM grade 2) and 1 parietal AVM (SM grade 3), and transient dysmetria in a cerebellar AVM (SM grade 1). One patient with a left frontal AVM (SM grade 2) had an associated aneurysm that ruptured while the patient was on the operating table and caused hemiparesis and dysphasia. This patient’s neurological deficits rapidly improved over the following months and he was completely symptom-free in the first long-term follow-up visit. Table 3 summarizes the clinical picture of the individual patients in this group.
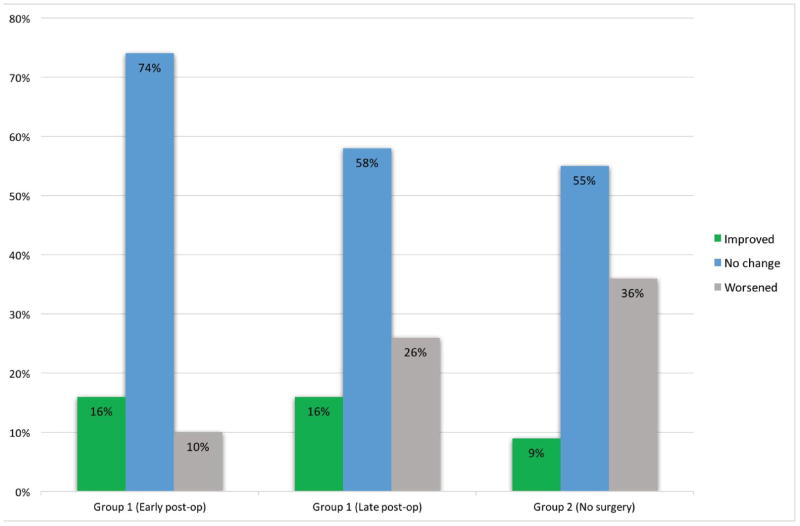
Median mRS score at the time of diagnosis was 1. Overall, the early post-operative functional status (6-weeks after surgery) was unchanged in 14 (74%), improved in 3 (16%), and worsened in 2 (10%) patients. Patient follow-up was available for an average of 9.6 years (median 6.3 years; 1.6–33.9 years). Overall, the functional status of the patients was improved in 3 (16%), worsened in 5 (26%), and not changed in 11 (58%) during the entire period of follow-up. At the latest follow-up, 14 patients (74%) had good functional status (mRS 0 & 1) and 5 had an mRS score of 2 (Figure 3). Calculation of Spearman’s Rho did not reveal any significant correlation between pre-operative SM or SSM grades with early and late post-operative mRS scores (P > 0.05).
Figure 3.
Diagram showing the relative frequencies of different pattern of change (improved, no change, and worsened) in the functional status of the patients in groups 1 (early and late postoperative & 2 (non-surgically treated).
Twelve patients (63%) did not receive any adjuvant therapy including radiosurgery or embolization. One patient had received stereotactic radiosurgery one year before surgery without any evidence of shrinkage of the AVM. A total of 10 embolization sessions had been performed pre-operatively in 7 patients, with the goal of facilitating surgery (5 patients), and with a goal of definitive treatment in two patients. Attempts at curative embolization in these two patients were unsuccessful.
Group 2: Non-surgically treated
A total of 22 patients (12 females and 10 males) were included (Table 1). In this group of patients, 33 AVMs and 9 micro-AVMs were present (1.9 lesions per patient). No cerebral AVF was present in these patients. Median age of this group was 42 years at diagnosis. Three patients/lesions were diagnosed after initial presentation with ICH. Overall, five patients/lesions were diagnosed after they became symptomatic (headache, seizure, focal deficit, and/or hemorrhage), while the rest of the lesions were diagnosed during screening studies or incidentally during brain angiography performed for the symptomatic lesion.
Most common locations for the lesions were the frontal (55%) and occipital (21%) lobes. (Table 2). Ten lesions were in eloquent locations (30%). Mean size of the lesions in the non-surgically treated group was 14.7mm (range 7–35mm, SD 6.9). Lesions were superficially located with superficial drainage (94%) except one thalamic lesion and 1 temporal AVM with deep venous drainage. The majority of lesions (93%) were of SM grade 1 or 2 (Table 4). In this group, all the occipital and parietal lesions, and the majority of frontal lobe lesions (15/18) were asymptomatic.
Table 4.
Clinical and radiological features of individual patients in group 2 (non-surgically treated) patients and their initial and long-term functional status.
| Patient No. | Age at diagnosis (years), Sex | Symptoms | Lesion Location | Lesion Grade | mRS score at Diagnosis | Latest follow-up mRS score | Adjuvant therapy | |
|---|---|---|---|---|---|---|---|---|
| SM | LY | |||||||
| 1 | 43, M | Asymptomatic | R Frontal | 1 | 4 | 0 | 1 | - |
| 2 | 38, M | Asymptomatic | R Occipital | 1 | 3 | 2 | 1 | RS |
| 3 | 64, F | Asymptomatic | R Occipital | 2 | 4 | 1 | 1 | - |
| 4 | 72, M | Asymptomatic | L Frontal | 1 | 4 | 0 | 2 | - |
| 5 | 0.6, M | ICH, Seizure | Thalamus | 4 | 2 | 0* | 2 | E |
| 6 | 53, M | Asymptomatic | L Occipital | 2 | 4 | 0* | 1 | - |
| 7 | 45, F | Asymptomatic | L Frontal | 1 | 4 | 1 | 2 | - |
| 8 | 23, F | Asymptomatic | R Parietal | 1 | 3 | 1 | 3 | - |
| 9 | 40, F | Asymptomatic | R Temporal | 1 | 4 | 0 | 0 | RS |
| 10 | 52, M | Asymptomatic | L Frontal | 1 | 4 | 0 | 0 | RS |
| 11 | 50, F | Headache, Hemiparesis | L Frontal | 2 | 3 | 0 | 1 | RS |
| 12 | 55, F | Asymptomatic | L Frontal | 1 | 4 | 0 | 0 | - |
| 13 | 9, M | Asymptomatic | L Frontal | 1 | 2 | 1 | 1 | RS,E |
| 14 | 7, M | Asymptomatic | R Temporal | 2 | 2 | 2 | 2 | E |
| 15 | 48, F | Asymptomatic | L Frontal | 2 | 4 | 2 | 2 | RS |
| 16 | 21, F | Asymptomatic | L Frontal | 3 | 3 | 0 | 1 | RS |
| 17 | 18, M | Asymptomatic | L Frontal | 1 | 3 | 1 | 1 | - |
| 18 | 39, F | Asymptomatic | L Parietal | 1 | 3 | 1 | 1 | - |
| 19 | 52, M | Hemiparesis | L Occipital | 1 | 5 | 1 | 1 | - |
| R Occipital | 1 | 5 | - | |||||
| 20 | 57, F | Headache, Seizure | R Frontal | 1 | 4 | 1 | 2 | - |
| R Occipital | 1 | 4 | - | |||||
| 21 | 23, F | Headache, Seizure | R Frontal | 2 | 3 | 2 | 1 | - |
| R Frontal | 2 | 3 | - | |||||
| L Frontal | 1 | 3 | - | |||||
| L Frontal | 1 | 3 | - | |||||
| Cerebellum | 1 | 3 | - | |||||
| L Occipital | 2 | 3 | - | |||||
| 22 | 14, F | Asymptomatic | R Frontal | 2 | 2 | 1 | 1 | - |
| R Frontal | 1 | 2 | - | |||||
| R Frontal | 1 | 2 | - | |||||
| Cerebellum | 1 | 2 | - | |||||
| Cerebellum | 1 | 2 | - | |||||
M, male; F, female; R, right; L, left; E, embolization; RS, radiosurgery
Complications and Outcomes
Median mRS score at the time of diagnosis was 1. Follow-up was available for an average of 4.6 years. During the entire follow-up period, 8 (36%) worsened functionally, while 12 (55%) did not change and 2 (9%) improved. At the latest follow-up, median mRS score was 1, and fifteen patients (68%) had good functional status (mRS 0 & 1). (Figure 3).
Thirteen patients (59%) were followed-up without any therapeutic intervention. Six patients (27%) were treated only with radiosurgery, 2 patients (9%) received only embolization and 1 (5%) patient received both.
Summary of clinical and radiological data of the lesions is depicted in tables 1 and 2. Tables 3 and 4 show the individual patients’ clinico-radiological characteristics and functional status data in the surgically treated and non-surgically treated groups, respectively.
Comparison of the two groups
There was no statistically significant difference between the surgically treated and non-surgically treated groups in terms of number of AVMs, lesion size, venous drainage, and mRS scores at the time of diagnosis and latest follow-up. Although the median ages at the time of diagnosis differed (27 years for group 1 versus 42 years for group 2), student’s t-test failed to show a statistically significant difference in the mean age of patients at the time of diagnosis between the two groups. The percentages of low-radiological grade lesions (i.e., SM grade ≤2 and SSM grade ≤6) were not statistically different between groups (p > 0.05). When the latest follow-up mRS of the surgically treated group was compared with the patients who received ‘no form of treatment’ in the non-surgically treated group (13 patients), no significant difference was observed (Mann-Whitney U test, p > 0.05). Two-sample z-test did not reveal any statistically significant difference regarding the proportions of various functional status changes (i.e., not changed, improved, and worsened) between two groups (p > 0.05).
Discussion
Operative Risk
Previous reports on surgery for brain AVMs in HHT patients are scant with limited information on post-operative outcomes (Table 5).24, 25 We report the outcomes for the largest surgical cohort of HHT patients (n=19) with cerebral AVMs (not including capillary VMs or AVFs) to date, and compared them to outcomes of a similar group of HHT patients who did not receive surgical treatment for their brain AVM(s). This comparison shows that, when carefully selected, operative resection of brain AVMs can be considered a safe treatment option in HHT patients (Figure 4). The multi-center nature of our study generalizes this point of view, as different centers with variable levels of surgical expertise were involved in treating these patients. Surgical patients had a relatively long follow-up time (mean 9.6 years, minimum 1.6 years), and the majority of the patients had good functional status (median mRS = 1) at early and late follow-up (Figure 3). Small lesion size (average 18.6mm), superficial location, low frequency of deep drainage (10%), and low SM and Supp-SM grades overall, as typically seen in HHT patients18, 24, may have contributed to the favorable early and late post-operative outcomes, even though 40% of the operated lesions were in eloquent brain areas. We found no correlation between the pre-operative lesion grade and post-operative mRS scores, suggesting that SM (or Supp-SM) grading may not be the best pre-operative tool for surgical decision making in HHT. This finding might also be due to the small sample size, absence of high-grade lesions in our sample, and a high percentage of favorable post-operative functional outcomes.
Table 5.
Previous reports of surgical treatment of brain arteriovenous malformations in HHT patients
| Study (year) | Patient | Lesion location | presentation | Early | Late |
|---|---|---|---|---|---|
| Kikuchi et al (2004) | 2-year-old girl | Left parietal AVM | ICH | Uneventful | Not reported |
| Du et al (2007) | 26-day-old | Left parasagittal parietal AVM | ICH | Immediate hemiparesis | Completely resolved after 4 months |
| Yang et al (2015) | 19-year-old male | Right Temporal | None | No residual neurological deficit | n/s |
| n/s | Left frontal | n/s | No residual neurological deficit | n/s |
AVM, arteriovenous malformation; ICH, intracranial hemorrhage; n/s, not specified
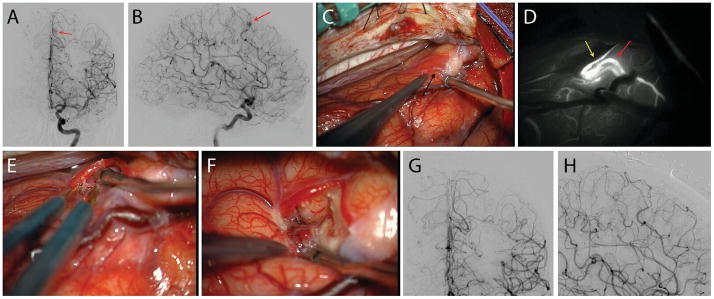
Figure 4.
Case illustration. This 49-year-old lady was evaluated for chronic headaches and a history of recurrent nose bleeds and multiple cutaneous telangiectasias. She had a family history of bleeding lesions in her first and second degree relatives. Antero-posterior (A) and lateral (B) cerebral angiograms with left internal carotid artery injection showed a small left medial frontal arteriovenous malformation (Spetzler-Martin grade 1, Lawton-Young grade 3, Supplemented Spetzler-Martin grade 4) (red arrows). The lesion was exposed through a left interhemispheric approach (C, black arrow). Intra-operative indocyanine green video angiography showed the feeder artery (yellow arrow) and the single arterialized draining vein (red arrow) leading from the nidus (D). The feeding artery was skeletonized, coagulating the small branches supplying the nidus and preserving distal flow in the parent artery. (E) The nidus was circumferentially dissected and removed. Post-operative antero-posterior (G) and lateral (H) angiograms confirmed complete resection of the lesion. The patient was neurologically intact postoperatively.
Comparison of surgically treated and non-surgically treated groups
1. Mode of Presentation
There is controversy about the hemorrhage risk of brain AVMs in HHT patients.15, 23, 24 However, it is generally accepted that the hemorrhage risk of brain AVMs in HHT patients does not exceed that of sporadic AVMs.15, 23, 24, 32 In both groups, hemorrhage was not a common mode of presentation in the patients. However, most lesions were symptomatic in group 1 (headache and/or focal neurological deficit). Although headaches are common in patients with AVMs, it is difficult to attribute solitary headaches to non-ruptured AVMs. 15, 19, 20, 33–35 This can be more problematic when an HHT patient with multiple AVMs presents with headache. Nevertheless, as one of the common presentations of cerebral AVMs in undiagnosed HHT patients, headaches may prompt a neurological work-up and lead to diagnosis of AVMs.
2. Radiological Features
The 2 groups were similar regarding the radiological grades of the lesions. The frontal lobe harbored most AVMs in both groups. However, parietal lobe lesions were more frequently encountered in the surgically treated group whereas occipital lobe lesions were more commonly found in the non-surgically treated group. The higher frequency of symptomatic lesions in frontal and parietal lobes of group 1 patients might have lowered the threshold for surgical resection. On the other hand, higher frequency of lesions of the occipital lobe as well as lack of symptoms in most frontal lobe lesions (83%) of group 2 patients might have increased the threshold of decision to operate in the non-surgically treated group.
3. Clinical Outcome
Long-term clinical outcomes between the surgical and non-surgical groups were not significantly different. Although both groups had a favorable functional status at long-term follow-up (median mRS score 1), the non-surgical group suffered from worsening functional status at a rate of almost 1.5× the surgically treated group (Figure 3). This finding is emphasized by the longer duration of follow-up in group 1 (see below). This is not in concordance with the results of the ARUBA trial, which showed a relatively unfavorable outcome for surgically treated sporadic AVMs compared to observation. However, several subsequent studies showed a benefit from operating sporadic brain AVMs,36–39 and our study now shows a benefit from operating HHT brain AVMs. Therefore, surgery remains a viable option for brain AVMs in HHT patient, protecting them from the risk of future hemorrhage with minimal associated surgical morbidity.
We acknowledge the selection bias in the current series, with symptomatic lesions easily selected for surgical resection, and observation more readily selected for asymptomatic AVMs (the patient groups are heterogeneous). It is also important to note that different follow-up periods might have affected our results. As mentioned, the follow-up duration for the surgically treated group was almost twice the non-surgically treated group. Since the cumulative risk of hemorrhage in non-ruptured AVMs is lower with a shorter follow-up, the probability of failing to detect a significant difference between long-term outcomes of the two groups should not be underestimated. In other words, if the non-surgically treated group was followed for a longer period, declining outcomes might have been detected. This fact favors our suggestion for the surgical treatment of these lesions. Another important bias to consider is the survivor bias. While some centers may be reluctant to recruit deceased HHT patients, which might influence results, BVMC centers are encouraged to recruit all of their patients (current and previous) with brain VM history, even if deceased. Therefore, such bias may have had little effect on our results.
The Dilemma of Decision to Operate
The management strategy of brain AVMs in HHT patients is controversial. Due to the rarity of HHT, landmark previous studies such as the ARUBA trial did not include HHT patients. 37–41 This fact, along with the unknown natural history of the disease, further adds to the complexity of developing a treatment algorithm of brain AVMs in HHT patients. Several factors need to be considered when deciding to treat a brain AVM in these patients. First, the spontaneous hemorrhage risk of these lesions (on a per-lesion basis) seems to be lower, on average, than sporadic AVMs.15, 23, 24, 33–35 Second, the outcome of hemorrhage from HHT-related AVMs is reported to be relatively favorable compared to sporadic AVMs.19, 24 Third, multiplicity is very common in brain vascular malformations of HHT patients, which lowers the likelihood of achieving curative resection or obliteration of all lesions with surgery. Fourth, there have been rare reports of spontaneous regression of brain VMs in HHT.25, 28, 29 Also, a recent study has shown that bleeding in HHT-related brain AVMs is associated with an increased risk of future hemorrhage.23 These factors make patient management more challenging than with sporadic AVM, with regards to the decision to intervene, and with which treatment modality. These factors lead some clinicians to favor conservative observation for these HHT-brain AVMs instead of active intervention treatment. In fact, the same strategy seems to prevail in BVMC centers as the average surgery rate for HHT-related brain AVMs was low (16%).
On the other hand, risk of hemorrhage in critical regions of the brain along with the low-complication profile of treatment modalities favor aggressive treatment for these lesions. In addition, our consortium has previously demonstrated higher risk for intracranial hemorrhage amongst HHT patients with previous AVM rupture.23 Although radiosurgery is a promising method for treatment, especially for small lesions that are multiple and far apart (as occurs frequently in HHT patients), the latency period after radiosurgery and lower obliteration rates than surgical extirpation favor a surgical strategy when curative resection of lesions is possible.42–46 Embolization therapy is usually used as an adjunct for definitive surgical treatment of an AVM. Although the obliteration rate for brain AVMs after embolization treatment is low with high rate of recurrence, small lesions such as those seen in HHT may appear favorable for endovascular treatment.47, 48 However, small feeding artery size and superficial location of many HHT-related AVMs can also make embolization more challenging than surgery or radiosurgery for such lesions. In the current series, embolization was primarily used as a pre-operative adjunct to facilitate surgical resection (5 of 7 patients undergoing embolization). In the remaining two patients, embolization elected as the definitive treatment failed to obliterate the lesion and completion surgery was undertaken. This is consistent with the results of embolization in sporadic AVMs48 and supports multimodality treatment of AVMs using a multidisciplinary team approach.49, 50
The results of our study show a low risk and favorable long-term outcome for surgical resection of brain AVMs in HHT patients. Compared to observation, surgery remains a viable option for selected brain AVMs in HHT patients, protecting them from the risk of future hemorrhage with minimal associated surgical morbidity. Previous reports of surgical results for AVMs (although in very limited numbers) in HHT patients are also favorable (Table 5).13, 24, 25 Compared to radiosurgery and embolization therapy, surgery confers immediate and durable cure without a latency period, and thus provides the most definitive extirpation of the lesion. Studies show that when possible, surgical resection should be considered in low-grade AVMs.40, 41, 51 This recommendation may be applied to HHT-related AVMs that frequently have low SM and Supp-SM grades (i.e., they are small, superficial, compact, and have a single feeder), which may translate to a less challenging surgical resection. However, it is important to note that even though only 2 patients were worse as a result of treatment (early post-operative functional status), not all low SM/Supp-SM grade lesions had a favorable long-term post-operative outcome in our study (90% low SM grade lesions vs. 74% favorable outcome). It is also important to note that the small AVMs seen in HHT patients can be more difficult to identify than sporadic AVMs seen in other patients, although intraoperative navigation with frameless stereotactic guidance and intraoperative videoangiography with fluorescent dyes like indocyanine green dye (Figure 4) can help localize the AVM, as can anatomical clues like an arterialized cortical vein. Therefore, our conclusion on the relative safety of surgical intervention needs to be cautiously interpreted, and an individualized treatment strategy is essential for every patient. Further delineation of lesion characteristics and predictors of surgical outcome is necessary to help guide decisions on surgical treatment.
The decision to proceed with surgical resection of a symptomatic AVM causing neurological deficit (e.g. seizure, hemiparesis) may be more straightforward. However, in asymptomatic cases or cases in which the only presenting symptom is headache, it is even more difficult to decide for surgery or other interventions. Again, with the current level of evidence, no definitive treatment paradigm can be suggested for these lesions and the best individualized treatment must be proposed to patients by a multidisciplinary team that considers all aspects of the disease and its neurological impact, as well as the particular expertise and experience of the team.
Preoperative considerations
It is important to note several points before proceeding with surgical treatment of an HHT-related brain AVM. Screening for pulmonary AVMs is recommended in all HHT patients, followed by preventative embolization of significant AVMs and life-long pulmonary AVM precautions.27 This screening and treatment should be performed prior to cranial surgery, when feasible, to reduce perioperative risks (intrapulmonary hemorrhage, stroke, brain abscess) from pulmonary AVMs. In urgent cases, with unknown pulmonary AVM status, pulmonary AVMs should be presumed present, and pulmonary AVM precautions should be followed, including antibiotic prophylaxis and the use of IV air filters, until screening can be completed post-operatively.
Patients with brain AVM may have an unrecognized HHT diagnosis, as the disease remains under-diagnosed. Multiplicity of brain AVMs is a strong predictor for the diagnosis of HHT.9, 52 In addition, a personal and/or family history of epistaxis and the presence of mucocutaneous telangiectasias should be sought as clues towards the diagnosis of HHT. If the diagnosis of HHT is suspected, family members need to be screened to rule out HHT.
Study limitations
The major limitation of the current study is the small sample size. Although this consortium study involved multiple centers, the total number of HHT patients is a small fraction of those with sporadic brain AVMs. The BVMC continues to record all deaths including those caused by surgery or its complications, and poor outcomes. Although we did not see any surgical mortality in the current reported series, exclusion of 17 patients with incomplete clinical data is a possible source of selection bias. However, only 2 of these 17 patient underwent surgery and they did not show any functional decline in long-term follow-up. On the other hand, the heterogeneous nature of the two groups of patients causes their comparison to be less reliable. Presentation mode did not follow a similar pattern between groups. Some of the patients in this series received radiosurgery and/or embolization before surgery, making the patient population nonhomogeneous. Pre-operative embolization and/or radiosurgical treatment of a brain AVM may affect the difficulty of its surgical resection. Embolization might reduce the intra-operative bleeding and improve surgical outcomes, while radiosurgical treatment may shrink the AVM and facilitate resection. Lack of a sizable control group who did not undergo any treatment modality limits the generalizability of our results (our series had only 13 patients with these features). Without such a control group, it is difficult to reach the statistical power to generate firm guidelines.
The current study also has the weaknesses of any retrospective review. However, it is the largest series of its kind and the good outcomes found in group 1 support the operative management of HHT-related brain AVMs. Future studies with larger patient populations, including larger numbers of observed patients, will be possible as the BVMC increases its enrollment of patients, which may better delineate the role of different treatment modalities in HHT-related brain AVMs.
Conclusion
HHT-related brain AVMs are rare lesions with a genetic basis, a, different radiological profile from sporadic AVMs, and a more benign natural history. A comprehensive treatment paradigm for brain AVMs in HHT patients has not been established. This multi-center study shows that HHT patients with brain AVMs treated surgically appear to have good long-term functional outcomes. This surgical cohort, although small, is the largest reported to date and it is reasonable to consider surgical resection as a therapeutic option in the context of an individualized, multidisciplinary team approach for HHT patients with brain AVMs. The decisions regarding management of AVMs in HHT patients presently parallel those for sporadic AVMs, but future research should identify determinants of outcomes in HHT-related brain AVMs.
Footnotes
Disclosure
A.T.M. was supported by a fellowship training grant from the Brain Vascular Malformations Consortium (BVMC). The BVMC is part of the Rare Diseases Clinical Research Network (RDCRN) and is supported through collaboration between the National Institutes of Health Office of Rare Diseases Research at the National Center for Advancing Translational Science, and the National Institute of Neurological Disorders and Stroke (NIH/NINDS grant: U54 NS065705).
References
- 1.Guttmacher AE, Marchuk DA, White RI. Hereditary hemorrhagic telangiectasia. N Engl J Med. 1995 Oct;333(14):918–924. doi: 10.1056/NEJM199510053331407. [DOI] [PubMed] [Google Scholar]
- 2.Dakeishi M, Shioya T, Wada Y, et al. Genetic epidemiology of hereditary hemorrhagic telangiectasia in a local community in the northern part of Japan. Hum Mutat. 2002 Feb;19(2):140–148. doi: 10.1002/humu.10026. [DOI] [PubMed] [Google Scholar]
- 3.Kjeldsen AD, Vase P, Green A. Hereditary haemorrhagic telangiectasia: a population-based study of prevalence and mortality in Danish patients. J Intern Med. 1999 Jan;245(1):31–39. doi: 10.1046/j.1365-2796.1999.00398.x. [DOI] [PubMed] [Google Scholar]
- 4.Bideau A, Brunet G, Heyer E, Plauchu H, Robert JM. An abnormal concentration of cases of Rendu-Osler disease in the Valserine valley of the French Jura: a genealogical and demographic study. Ann Hum Biol. 1992 May-Jun;19(3):233–247. doi: 10.1080/03014469200002112. [DOI] [PubMed] [Google Scholar]
- 5.Fuchizaki U, Miyamori H, Kitagawa S, Kaneko S, Kobayashi K. Hereditary haemorrhagic telangiectasia (Rendu-Osler-Weber disease) Lancet. 2003 Nov;362(9394):1490–1494. doi: 10.1016/S0140-6736(03)14696-X. [DOI] [PubMed] [Google Scholar]
- 6.Salaria M, Taylor J, Bogwitz M, Winship I. Hereditary haemorrhagic telangiectasia, an Australian cohort: clinical and investigative features. Intern Med J. 2014 Jul;44(7):639–644. doi: 10.1111/imj.12457. [DOI] [PubMed] [Google Scholar]
- 7.HODGSON CH, BURCHELL HB, GOOD CA, CLAGETT OT. Hereditary hemorrhagic telangiectasia and pulmonary arteriovenous fistula: survey of a large family. N Engl J Med. 1959 Sep;261:625–636. doi: 10.1056/NEJM195909242611301. [DOI] [PubMed] [Google Scholar]
- 8.BOCZKO ML. NEUROLOGICAL IMPLICATIONS OF HEREDITARY HEMORRHAGIC TELANGIECTASIS. J Nerv Ment Dis. 1964 Dec;139:525–536. doi: 10.1097/00005053-196412000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Putman CM, Chaloupka JC, Fulbright RK, Awad IA, White RI, Fayad PB. Exceptional multiplicity of cerebral arteriovenous malformations associated with hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu syndrome) AJNR Am J Neuroradiol. 1996 Oct;17(9):1733–1742. [PMC free article] [PubMed] [Google Scholar]
- 10.Román G, Fisher M, Perl DP, Poser CM. Neurological manifestations of hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber disease): report of 2 cases and review of the literature. Ann Neurol. 1978 Aug;4(2):130–144. doi: 10.1002/ana.410040207. [DOI] [PubMed] [Google Scholar]
- 11.White RI, Lynch-Nyhan A, Terry P, et al. Pulmonary arteriovenous malformations: techniques and long-term outcome of embolotherapy. Radiology. 1988 Dec;169(3):663–669. doi: 10.1148/radiology.169.3.3186989. [DOI] [PubMed] [Google Scholar]
- 12.Fulbright RK, Chaloupka JC, Putman CM, et al. MR of hereditary hemorrhagic telangiectasia: prevalence and spectrum of cerebrovascular malformations. AJNR Am J Neuroradiol. 1998 Mar;19(3):477–484. [PMC free article] [PubMed] [Google Scholar]
- 13.Kikuchi K, Kowada M, Sasajima H. Vascular malformations of the brain in hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber disease) Surg Neurol. 1994 May;41(5):374–380. doi: 10.1016/0090-3019(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 14.Haitjema T, Disch F, Overtoom TT, Westermann CJ, Lammers JW. Screening family members of patients with hereditary hemorrhagic telangiectasia. Am J Med. 1995 Nov;99(5):519–524. doi: 10.1016/s0002-9343(99)80229-0. [DOI] [PubMed] [Google Scholar]
- 15.Willemse RB, Mager JJ, Westermann CJ, Overtoom TT, Mauser H, Wolbers JG. Bleeding risk of cerebrovascular malformations in hereditary hemorrhagic telangiectasia. J Neurosurg. 2000 May;92(5):779–784. doi: 10.3171/jns.2000.92.5.0779. [DOI] [PubMed] [Google Scholar]
- 16.Matsubara S, Mandzia JL, ter Brugge K, Willinsky RA, Faughnan ME, Manzia JL. Angiographic and clinical characteristics of patients with cerebral arteriovenous malformations associated with hereditary hemorrhagic telangiectasia. AJNR Am J Neuroradiol. 2000 Jun-Jul;21(6):1016–1020. [PMC free article] [PubMed] [Google Scholar]
- 17.Krings T, Ozanne A, Chng SM, Alvarez H, Rodesch G, Lasjaunias PL. Neurovascular phenotypes in hereditary haemorrhagic telangiectasia patients according to age. Review of 50 consecutive patients aged 1 day-60 years. Neuroradiology. 2005 Oct;47(10):711–720. doi: 10.1007/s00234-005-1390-8. [DOI] [PubMed] [Google Scholar]
- 18.Krings T, Kim H, Power S, et al. Neurovascular manifestations in hereditary hemorrhagic telangiectasia: imaging features and genotype-phenotype correlations. AJNR Am J Neuroradiol. 2015 May;36(5):863–870. doi: 10.3174/ajnr.A4210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maher CO, Piepgras DG, Brown RD, Friedman JA, Pollock BE. Cerebrovascular manifestations in 321 cases of hereditary hemorrhagic telangiectasia. Stroke. 2001 Apr;32(4):877–882. doi: 10.1161/01.str.32.4.877. [DOI] [PubMed] [Google Scholar]
- 20.Woodall MN, McGettigan M, Figueroa R, Gossage JR, Alleyne CH. Cerebral vascular malformations in hereditary hemorrhagic telangiectasia. J Neurosurg. 2014 Jan;120(1):87–92. doi: 10.3171/2013.10.JNS122402. [DOI] [PubMed] [Google Scholar]
- 21.Maarouf M, Runge M, Kocher M, Zähringer M, Treuer H, Sturm V. Radiosurgery for cerebral arteriovenous malformations in hereditary hemorrhagic telangiectasia. Neurology. 2004 Jul;63(2):367–369. doi: 10.1212/01.wnl.0000130197.31844.16. [DOI] [PubMed] [Google Scholar]
- 22.Willinsky RA, Lasjaunias P, Terbrugge K, Burrows P. Multiple cerebral arteriovenous malformations (AVMs). Review of our experience from 203 patients with cerebral vascular lesions. Neuroradiology. 1990;32(3):207–210. doi: 10.1007/BF00589113. [DOI] [PubMed] [Google Scholar]
- 23.Kim H, Nelson J, Krings T, et al. Hemorrhage rates from brain arteriovenous malformation in patients with hereditary hemorrhagic telangiectasia. Stroke. 2015 May;46(5):1362–1364. doi: 10.1161/STROKEAHA.114.007367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang W, Liu A, Hung A, et al. Lower Risk of Intracranial Arteriovenous Malformation Hemorrhage in Patients With Hereditary Hemorrhagic Telangiectasia. Neurosurgery. 2015 Nov; doi: 10.1227/NEU.0000000000001103. [DOI] [PubMed] [Google Scholar]
- 25.Du R, Hashimoto T, Tihan T, Young WL, Perry V, Lawton MT. Growth and regression of arteriovenous malformations in a patient with hereditary hemorrhagic telangiectasia. Case report. J Neurosurg. 2007 Mar;106(3):470–477. doi: 10.3171/jns.2007.106.3.470. [DOI] [PubMed] [Google Scholar]
- 26.Kuo YH, Santoreneos S, Roos D, Brophy BP. Treatment of multiple arteriovenous malformations in pediatric patients with hereditary hemorrhagic telangiectasia and spontaneous hemorrhage. Report of two cases. J Neurosurg. 2007 Dec;107(6 Suppl):489–494. doi: 10.3171/PED-07/12/489. [DOI] [PubMed] [Google Scholar]
- 27.Faughnan ME, Palda VA, Garcia-Tsao G, et al. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J Med Genet. 2011 Feb;48(2):73–87. doi: 10.1136/jmg.2009.069013. [DOI] [PubMed] [Google Scholar]
- 28.Leung KM, Agid R, terBrugge K. Spontaneous regression of a cerebral arteriovenous malformation in a child with hereditary hemorrhagic telangiectasia. Case report. J Neurosurg. 2006 Nov;105(5 Suppl):428–431. doi: 10.3171/ped.2006.105.5.428. [DOI] [PubMed] [Google Scholar]
- 29.Cloft HJ. Spontaneous regression of cerebral arteriovenous malformation in hereditary hemorrhagic telangiectasia. AJNR Am J Neuroradiol. 2002 Jun-Jul;23(6):1049–1050. [PMC free article] [PubMed] [Google Scholar]
- 30.Shovlin CL, Guttmacher AE, Buscarini E, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome) Am J Med Genet. 2000 Mar;91(1):66–67. doi: 10.1002/(sici)1096-8628(20000306)91:1<66::aid-ajmg12>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 31.Kim H, Abla AA, Nelson J, et al. Validation of the supplemented Spetzler-Martin grading system for brain arteriovenous malformations in a multicenter cohort of 1009 surgical patients. Neurosurgery. 2015 Jan;76(1):25–31. doi: 10.1227/NEU.0000000000000556. discussion 31–22; quiz 32–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Easey AJ, Wallace GM, Hughes JM, Jackson JE, Taylor WJ, Shovlin CL. Should asymptomatic patients with hereditary haemorrhagic telangiectasia (HHT) be screened for cerebral vascular malformations? Data from 22,061 years of HHT patient life. J Neurol Neurosurg Psychiatry. 2003 Jun;74(6):743–748. doi: 10.1136/jnnp.74.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hernesniemi JA, Dashti R, Juvela S, Väärt K, Niemelä M, Laakso A. Natural history of brain arteriovenous malformations: a long-term follow-up study of risk of hemorrhage in 238 patients. Neurosurgery. 2008 Nov;63(5):823–829. doi: 10.1227/01.NEU.0000330401.82582.5E. discussion 829–831. [DOI] [PubMed] [Google Scholar]
- 34.Mast H, Young WL, Koennecke HC, et al. Risk of spontaneous haemorrhage after diagnosis of cerebral arteriovenous malformation. Lancet. 1997 Oct;350(9084):1065–1068. doi: 10.1016/s0140-6736(97)05390-7. [DOI] [PubMed] [Google Scholar]
- 35.Abecassis IJ, Xu DS, Batjer HH, Bendok BR. Natural history of brain arteriovenous malformations: a systematic review. Neurosurg Focus. 2014 Sep;37(3):E7. doi: 10.3171/2014.6.FOCUS14250. [DOI] [PubMed] [Google Scholar]
- 36.Bervini D, Morgan MK, Ritson EA, Heller G. Surgery for unruptured arteriovenous malformations of the brain is better than conservative management for selected cases: a prospective cohort study. J Neurosurg. 2014 Oct;121(4):878–890. doi: 10.3171/2014.7.JNS132691. [DOI] [PubMed] [Google Scholar]
- 37.Abla AA, Nelson J, Kim H, Hess CP, Tihan T, Lawton MT. Silent arteriovenous malformation hemorrhage and the recognition of “unruptured” arteriovenous malformation patients who benefit from surgical intervention. Neurosurgery. 2015 May;76(5):592–600. doi: 10.1227/NEU.0000000000000686. discussion 600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rutledge WC, Abla AA, Nelson J, Halbach VV, Kim H, Lawton MT. Treatment and outcomes of ARUBA-eligible patients with unruptured brain arteriovenous malformations at a single institution. Neurosurg Focus. 2014 Sep;37(3):E8. doi: 10.3171/2014.7.FOCUS14242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohr JP, Parides MK, Stapf C, et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet. 2014 Feb;383(9917):614–621. doi: 10.1016/S0140-6736(13)62302-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nerva JD, Mantovani A, Barber J, et al. Treatment outcomes of unruptured arteriovenous malformations with a subgroup analysis of ARUBA (A Randomized Trial of Unruptured Brain Arteriovenous Malformations)-eligible patients. Neurosurgery. 2015 May;76(5):563–570. doi: 10.1227/NEU.0000000000000663. discussion570; quiz 570. [DOI] [PubMed] [Google Scholar]
- 41.Potts MB, Lau D, Abla AA, et al. Current surgical results with low-grade brain arteriovenous malformations. J Neurosurg. 2015 Apr;122(4):912–920. doi: 10.3171/2014.12.JNS14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Beijnum J, van der Worp HB, Buis DR, et al. Treatment of brain arteriovenous malformations: a systematic review and meta-analysis. JAMA. 2011 Nov;306(18):2011–2019. doi: 10.1001/jama.2011.1632. [DOI] [PubMed] [Google Scholar]
- 43.Ding D, Yen CP, Starke RM, Xu Z, Sheehan JP. Radiosurgery for ruptured intracranial arteriovenous malformations. J Neurosurg. 2014 Aug;121(2):470–481. doi: 10.3171/2014.2.JNS131605. [DOI] [PubMed] [Google Scholar]
- 44.Pollock BE, Lunsford LD, Kondziolka D, Maitz A, Flickinger JC. Patient outcomes after stereotactic radiosurgery for “operable” arteriovenous malformations. Neurosurgery. 1994 Jul;35(1):1–7. doi: 10.1227/00006123-199407000-00001. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 45.Wang YC, Huang YC, Chen HC, et al. Linear accelerator stereotactic radiosurgery in the management of intracranial arteriovenous malformations: long-term outcome. Cerebrovasc Dis. 2014;37(5):342–349. doi: 10.1159/000360756. [DOI] [PubMed] [Google Scholar]
- 46.Kano H, Lunsford LD, Flickinger JC, et al. Stereotactic radiosurgery for arteriovenous malformations, Part 1: management of Spetzler-Martin Grade I and II arteriovenous malformations. J Neurosurg. 2012 Jan;116(1):11–20. doi: 10.3171/2011.9.JNS101740. [DOI] [PubMed] [Google Scholar]
- 47.Yu SC, Chan MS, Lam JM, Tam PH, Poon WS. Complete obliteration of intracranial arteriovenous malformation with endovascular cyanoacrylate embolization: initial success and rate of permanent cure. AJNR Am J Neuroradiol. 2004 Aug;25(7):1139–1143. [PMC free article] [PubMed] [Google Scholar]
- 48.Reig AS, Rajaram R, Simon S, Mericle RA. Complete angiographic obliteration of intracranial AVMs with endovascular embolization: incomplete embolic nidal opacification is associated with AVM recurrence. J Neurointerv Surg. 2010 Sep;2(3):202–207. doi: 10.1136/jnis.2009.001636. [DOI] [PubMed] [Google Scholar]
- 49.Pandey P, Marks MP, Harraher CD, et al. Multimodality management of Spetzler-Martin Grade III arteriovenous malformations. J Neurosurg. 2012 Jun;116(6):1279–1288. doi: 10.3171/2012.3.JNS111575. [DOI] [PubMed] [Google Scholar]
- 50.Nataraj A, Mohamed MB, Gholkar A, et al. Multimodality treatment of cerebral arteriovenous malformations. World Neurosurg. 2014 Jul-Aug;82(1–2):149–159. doi: 10.1016/j.wneu.2013.02.064. [DOI] [PubMed] [Google Scholar]
- 51.Alén JF, Lagares A, Paredes I, et al. Cerebral microarteriovenous malformations: a series of 28 cases. J Neurosurg. 2013 Sep;119(3):594–602. doi: 10.3171/2013.4.JNS121740. [DOI] [PubMed] [Google Scholar]
- 52.Bharatha A, Faughnan ME, Kim H, et al. Brain arteriovenous malformation multiplicity predicts the diagnosis of hereditary hemorrhagic telangiectasia: quantitative assessment. Stroke. 2012 Jan;43(1):72–78. doi: 10.1161/STROKEAHA.111.629865. [DOI] [PMC free article] [PubMed] [Google Scholar]