Abstract
Purpose
To determine if adrenal calcifications seen at computed tomography (CT) are associated with familial cerebral cavernous malformations (fCCMs) in carriers of the CCM1 Common Hispanic Mutation.
Materials and Methods
This study was approved by the institutional review board. The authors retrospectively reviewed abdominal CT scans in 38 patients with fCCM, 38 unaffected age- and sex-matched control subjects, and 13 patients with sporadic, nonfamilial cerebral cavernous malformation (CCM). The size, number, and laterality of calcifications and the morphologic characteristics of the adrenal gland were recorded. Brain lesion count was recorded from brain magnetic resonance (MR) imaging in patients with fCCM. The prevalence of adrenal calcifications in patients with fCCM was compared with that in unaffected control subjects and those with sporadic CCM by using the Fisher exact test. Additional analyses were performed to determine whether age and brain lesion count were associated with adrenal findings in patients with fCCM.
Results
Small focal calcifications (SFCs) (≤5 mm) were seen in one or both adrenal glands in 19 of the 38 patients with fCCM (50%), compared with 0 of the 38 unaffected control subjects (P < .001) and 0 of the 13 subjects with sporadic CCM (P = .001). Adrenal calcifications in patients with fCCM were more frequently left sided, with 17 of 19 patients having more SFCs in the left adrenal gland than the right adrenal gland and 50 of the 61 observed SFCs (82%) found in the left adrenal gland. No subjects had SFCs on the right side only. In patients with fCCM, the presence of SFCs showed a positive correlation with age (P < .001) and number of brain lesions (P < .001).
Conclusion
Adrenal calcifications identified on CT scans are common in patients with fCCM and may be a clinically silent manifestation of disease.
Cerebral cavernous malformations (CCMs) are low-flow vascular lesions representing 5%–13% of vascular anomalies in the central nervous system (1,2). When symptomatic, CCMs may cause epilepsy, intracerebral hemorrhage, and focal neurologic deficits. Histologically, they are composed of sinusoidal, endothelial-lined spaces without other vascular wall elements or intervening brain parenchyma (2,3). CCMs are readily detected at T2-weighted gradient-echo (GRE) and susceptibility-weighted brain magnetic resonance (MR) imaging owing to the presence of blood breakdown products in various stages, such as methemoglobin and hemosiderin, located within the lesions (4,5).
CCMs develop sporadically or in the setting of a hereditary disorder. Sporadic CCMs are typically solitary lesions, whereas familial CCMs (fCCMs) exhibit multiplicity, numbering in the hundreds in some patients (4,5). Mutations at three genetic loci are implicated in fCCM: CCM1 (also known as KRIT1), CCM2, and CCM3, all displaying autosomal dominant inheritance with incomplete penetrance and variable expression (6,7). Familial CCM type 1 (CCM1) is especially prevalent in Hispanic Americans of Mexican and Spanish ancestry owing to a founder mutation in the CCM1 gene known as the Common Hispanic Mutation (CHM) or CCM1-CHM (8,9). Clinical presentation is highly variable, even among those who share the CCM1-CHM haplotype. At least 30%–50% of heterozygotes remain asymptomatic into adulthood, whereas the remainder experience clinical effects, most commonly seizures (5,10,11). The biologic basis for this clinical variability is unknown. CCM lesions are known to accumulate with age (5,11,12), but the number of brain lesions does not correlate well with disease expression (5,10–13).
There is growing evidence that fCCM has manifestations beyond the central nervous system. The frequency of cutaneous vascular lesions and retinal hemangiomas is increased in all hereditary forms of CCM (14–16). An association with vertebral hemangiomas has also been described (17,18). Liver hemangiomas have previously been reported in two small CCM1 kindreds (18–20), but an association has not been studied in larger case series. We have observed several patients with fCCM with small focal calcifications (SFCs) of the adrenal glands. The purpose of this study was to determine if adrenal calcifications seen at computed tomography (CT) are associated with fCCM in carriers of the CCM1-CHM.
Materials and Methods
Study Population
The local institutional review board approved this Health Insurance Portability and Accountability Act–compliant, retrospective case-control study. All patients were identified by means of a thorough electronic search of records in the hospital database or were enrolled in the Brain Vascular Malformation Consortium study for patients with fCCM (21). Participants in the Brain Vascular Malformation Consortium study gave written informed consent to undergo prospective brain MR imaging, medical record review, and targeted CCM1-CHM genetic testing. The requirement to obtain informed consent was waived by the institutional review board for local subjects identified by means of medical record review.
Patients were included if they had CT scans of the abdomen or chest that completely showed the adrenal glands and if fCCM was diagnosed by means of DNA testing for CCM1-CHM or by the presence of at least three CCM lesions on brain MR images. Of 286 participants in the Brain Vascular Malformation Consortium study enrolled between June 2010 and February 2016, 26 underwent CT through the adrenal glands. An additional 12 patients with fCCM identified by means of a records search met the inclusion criteria, for a total of 38 patients with fCCM. Of the 38 patients, 24 have previously been reported on (22,23). Previous publications dealt with neuroimaging and genetic aspects of fCCM, whereas herein we report abdominal findings in these patients. Clinical records from patients with fCCM were reviewed for initial clinical presentation leading to the diagnosis of fCCM. Records were also reviewed for a history of granulomatous disease or adrenal hemorrhage as potential alternate causes of adrenal calcification.
Unaffected control subjects were matched to patients with fCCM with regard to age (within 1 year for children and 2 years for adults) and sex. Control subjects were included if they had previously undergone brain MR imaging that showed the absence of CCM lesions or, if they were a first-degree relative of a patient known to have CCM1-CHM, a negative finding at CCM1-CHM DNA testing. As an additional control group, patients with a clinical diagnosis of nonfamilial (sporadic) CCM were identified. Patients were considered to have nonfamilial CCM if they had a solitary CCM lesion on brain MR images, no family history of CCM, and no history of cranial radiation. A records search identified 35 patients with sporadic CCM, 13 of whom had previously undergone adrenal CT.
Adrenal CT
CT scans of the chest, abdomen, or entire torso were obtained in all 89 subjects between August 2005 and March 2016. Except for one study obtained at an outside institution, CT scans were obtained at our institution with use of 16– or 64–detector row scanners (Somatom Sensation 16 or Somatom Definition, Siemens), with 3–5-mm-thick axial sections. Forty-two examinations were performed with intravenous contrast material in a single phase, 34 were performed without contrast material, and 13 were performed both with and without contrast material.
Adrenal calcification was assessed in both the left and right adrenal glands for each subject and graded as definitely absent, probably absent, equivocal, probably present, and definitely present. Grades were determined by means of consensus between two readers (S.C.E. and C.D.S., with 18 and 4 years of experience, respectively). Radiologists were aware when patients had a clinical diagnosis of fCCM but did not review neurologic images concurrently. Adrenal calcification was considered present in subjects in whom calcification was determined to be probably present or definitely present in at least one gland. Clinical indication for and age at adrenal CT, the number of focal adrenal calcifications, and the size of each calcification on the basis of greatest measurement in the transaxial plane were recorded. Adrenal gland size and contour and the presence of any adrenal nodules were also noted.
Brain MR Imaging
Brain MR imaging was performed between November 2007 and February 2016. Except for two unaffected control subjects, who underwent imaging at outside institutions, all subjects underwent imaging at our institution. Participants in the Brain Vascular Malformation Consortium study underwent prospective brain MR imaging with a 3.0-T unit (Trio; Siemens Healthcare, Erlangen, Germany) by using a sagittal T1-weighted magnetization-prepared rapid acquisition GRE sequence, an axial turbo spin-echo T2-weighted sequence, an axial fluid-attenuated inversion-recovery sequence, an axial T2-weighted GRE sequence, and a susceptibility-weighted imaging sequence without gadolinium contrast material. The other 12 patients with fCCM underwent brain MR imaging at either 1.5 T (Siemens Symphony, nine subjects) or 3.0 T (Siemens Trio, two subjects; Philips Ingenia [Philips Healthcare, Best, the Netherlands], one subject). These studies all included a T2-weighted GRE sequence; in addition, four included a susceptibility-weighted imaging sequence. Ten of the patients underwent brain MR imaging without gadolinium contrast material and two underwent brain MR imaging without and with gadolinium contrast material.
Unaffected control subjects (excluding two with negative DNA test results who did not undergo neurologic imaging) underwent brain MR imaging at either 1.5 T (Siemens Symphony, 26 subjects; two subjects underwent imaging at an outside institution) or 3.0 T (Siemens Trio, eight subjects). All 36 subjects underwent echo-planar imaging, 29 underwent T2-weighted GRE imaging, and none underwent susceptibility-weighted imaging. For the patients with sporadic CCM, brain MR imaging was performed at 1.5 T (Siemens Symphony) in eight subjects and at 3.0 T (Siemens Trio) in five. All but one subject underwent a T2-weighted GRE sequence, and susceptibility-weighted imaging was used in two subjects.
Brain lesion count in patients with fCCM was obtained from T2-weighted GRE and, when available, susceptibility-weighted images read concurrently. Absence of CCM lesions in control subjects was determined by review of all available images. Lesion counting and review of all brain MR studies were performed by a neuroradiologist (B.L.H., with 24 years of experience) who was blinded to the results of adrenal imaging.
Statistical Analysis
The prevalence of adrenal calcification in patients with fCCM was compared with that in unaffected control subjects and patients with sporadic CCM by using the Fisher exact test. We tested whether subjects had a disproportionate number of adrenal calcifications in the left or right adrenal gland by using a Wilcoxon signed rank test. Two-sample t tests were used to determine whether age, brain lesion count, or the residuals of brain lesion count regressed on age were associated with the presence of adrenal calcifications. Brain lesion counts were log transformed to accommodate for outliers. Exponentiated differences of log-lesion and residual log-lesion counts, and corresponding 95% confidence intervals, are interpreted as the proportional increase in brain lesion counts between those with SFC and those without. The Fisher exact test was used to determine whether sex was associated with the presence of adrenal calcifications. Statistical analyses were performed by using software (Stata 13.1; StataCorp, College Station, Tex). P < .05 was considered indicative of a statistically significant difference.
Results
Demographic Characteristics and Indications for Imaging
The Table summarizes demographic characteristics and clinical indications for adrenal imaging among patients with fCCM and the two control groups. Most subjects were adults; six patients younger than 18 years were included in the fCCM group and in the unaffected control group. Mean ages were 43.6 years in the fCCM group, 43.5 years in the unaffected control group, and 51.2 years in the sporadic CCM group. Male subjects represented a slight majority in all three groups; 18 of the 38 subjects in the fCCM and unaffected control groups (47%) and six of the 13 subjects in the sporadic CCM group (46%) were female. Thirty-seven of 38 subjects in the fCCM group (97%) and all subjects in the unaffected control group were of self-reported Hispanic ethnicity. One patient with fCCM reported White and/or Anglo Non-Hispanic ethnicity. Among the 13 subjects in the sporadic CCM group, self-reported ethnicity was Hispanic in nine subjects (69%), White and/or Anglo Non-Hispanic in one subject, and American Indian and/or Alaska Native in one subject. Ethnicity was not reported by two of the 13 subjects.
Table 1.
Demographic and Adrenal Imaging Characteristics according to Group
| Characteristic | fCCM (n = 38) | Unaffected Control Subjects (n = 38) | Sporadic CCM (n = 13) |
|---|---|---|---|
| Female sex | 18 (47) | 18 (47) | 6 (46) |
| Age at CT (y)* | 43.6 ± 18.1 | 43.5 ± 17.9 | 51.2 ± 12.7 |
| Hispanic ethnicity | 37 (97) | 38 (100) | 9 (69) |
| Indication for CT | |||
| Abdominal pain | 12 (32) | 14 (37) | 1 (7) |
| Cancer staging | 7 (18) | 8 (21) | 3 (23) |
| Trauma | 7 (18) | 3 (8) | 2 (15) |
| Other | 12 (32) | 13 (34) | 7 (54) |
| Time between CT and MR imaging (y)† | 1.3 (0–6.5) | 0.6 (0–5.8) | 0.7 (0–4.7) |
| Presence of adrenal calcifications | 19 (50) | 0 (0) | 0 (0) |
Note.—Except where indicated, data are numbers of patients, with percentages in parentheses.
Data are means ± standard deviations.
Data are medians, with ranges in parentheses.
Abdominal pain was the most common indication for adrenal imaging, followed by cancer staging and trauma (Table). Other indications for adrenal imaging included hematuria, characterization of a liver or renal lesion, suspected pulmonary embolism, infection, planning for gastrostomy tube placement, aortic aneurysm or dissection, and lung nodule surveillance. Adrenal imaging was unrelated to CCM diagnosis except in two patients with fCCM who had undergone CT for gastrostomy planning secondary to underlying neurologic impairment. The median time between adrenal CT and brain MR imaging for all groups was less than 2 years (range, 0.0–6.5 years).
Seizures were the most common symptom leading to the diagnosis of fCCM, occurring in 19 of the 38 patients with fCCM (50%). Eight of the 38 patients (21%) presented with symptomatic intracerebral or intraspinal hemorrhage, two (5%) had chronic headaches, and nine (24%) had no history of CCM-related symptoms.
Presence of Adrenal Calcification
Adrenal calcifications were present in 19 of the 38 patients with fCCM (50%) compared with 0 of the 38 unaffected control subjects (P < .001) and 0 of the 13 patients with sporadic CCM (P = .001). When present, adrenal SFCs were 1–5 mm in size (mean, 3.1 mm; standard deviation, 1.16 mm). Fifteen of the 19 patients with SFCs (79%) had multiple calcifications, with as many as seven distinct calcific foci present in a single subject and six in any one gland (Figs 1–5). In sum, 61 SFCs were observed across all subjects.
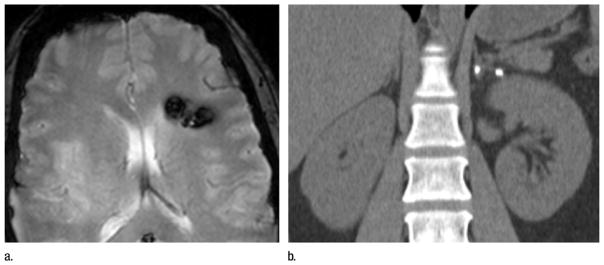
Figure 1.
Images in 36-year-old woman with CCM1-CHM genotype who presented with symptomatic intracerebral hemorrhage. (a) Axial T2-weighted GRE MR image shows left frontal cavernous malformation. Patient had 10 additional brain lesions (not pictured). (b) Coronal reconstruction image from unenhanced abdominal CT performed for renal colic 3 years earlier reveals focal calcifications without associated mass in left adrenal gland.
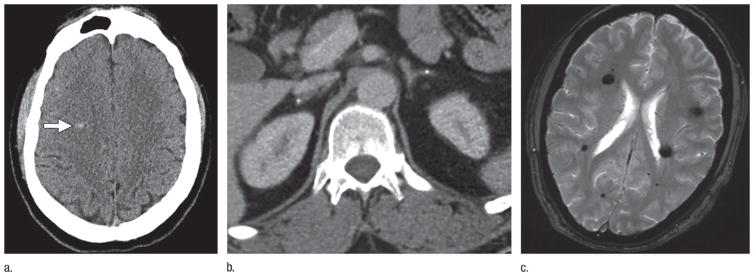
Figure 5.
Images in 55-year-old man without prior neurologic symptoms who underwent imaging after fall from a horse. (a) Unenhanced axial head CT scan shows high-attenuation focus in right frontal lobe (arrow), which was interpreted as acute hemorrhage versus pre-existing cavernous malformation. (b) Contrast-enhanced axial CT scan of abdomen shows bilateral adrenal calcifications, which increased suspicion for fCCM. (c) Axial T2-weighted GRE brain MR image obtained at 1-month follow-up reveals multiple cavernous malformations, which confirms diagnosis of fCCM.
Morphologic Characteristics
Adrenal glands had normal size and contour in 82 of the 89 subjects (92%). Solitary adrenal nodules measuring 0.8–2.2 cm were identified in three patients with fCCM, all of which were in the left adrenal gland. One of these nodules could be characterized as likely adenoma (on the basis of an un-enhanced CT attenuation value of 8.7 HU), and two were indeterminate. Ipsilateral SFCs were present in the patients with indeterminate nodules but were separate from the nodules. One matched control subject had an indeterminate 1.1-cm left adrenal nodule. Two patients with sporadic CCM and one matched control subject had thickening of one adrenal gland without a measurable nodule.
Laterality
SFCs were more common in the left adrenal gland than in the right adrenal gland. Of the 19 patients with SFC, 17 patients (89%) had more left-sided SFCs, one patient (5%) had an equal number on both sides, and one patient (5%) had more on the right side (P < .001). Ten patients had SFCs on the left side only, nine had bilateral SFCs, and none had SFC on the right side only. Fifty of the 61 SFCs (82%) were in the left adrenal gland.
Association of SFC with Age, Sex, and Brain Lesion Count
SFCs were seen in 0 of six patients with fCCM younger than 20 years of age (0%), in three of nine patients aged 20–39 years (33%), and in 16 of 23 subjects aged 40 years and older (70%). The average age of patients with fCCM with SFCs was significantly higher than that of those without SFCs (54 vs 33 years, respectively; P < .001). No association was observed between SFC and sex (P = .999).
Subjects in the fCCM group had as few as two and as many as 305 brain lesions (mean, 59 lesions; median, 13 lesions). Brain lesion count was not assessed in one subject, a 43-year-old woman with more than 200 lesions who had a history of radiation therapy for head and neck malignancy in addition to fCCM. Cranial radiation is known to increase the risk of sporadic CCM development (24), likely because of a “second-hit” mechanism in patients with fCCM mutations (23). Subjects with SFCs had significantly higher brain lesion counts than those without SFCs (proportional increase: 5.7; 95% confidence interval: 2.6, 12.4; P < .001) and also tended to have more lesions than expected for their age (proportional increase: 1.6; 95% confidence interval: 0.9, 3.1; P = .131).
Discussion
We found that SFCs in the adrenal gland were common (50%) in patients with fCCM and absent in age- and sex-matched control subjects and patients with sporadic CCM. The nature of the SFCs is speculative; none of our subjects’ adrenal glands underwent histologic assessment, and SFCs were incidental and asymptomatic. We could not find a reference estimating the frequency of adrenal calcifications seen at CT in the general population, but our experience is that these calcifications are very infrequent, perhaps on the order of one instance in 300–500 CT scans. When seen on CT scans, adrenal calcifications are most commonly attributed to previous hemorrhage, granulomatous disease, and calcified masses (25–27). Hemangiomas occur rarely in the adrenal gland as a known vascular mass and can calcify (26,28). Other rare systemic causes of adrenal calcification include lysosomal acid lipase deficiency (Wolman disease) (29) and disseminated Pneumocystis jiroveci infection (30). None of our subjects had these diagnoses, had masses associated with calcifications, or had a clinical history of granulomatous disease. Except for two indeterminate nodules that did not contain calcification, adrenal glands harboring calcification in our study were morphologically normal. We suspect that these were adenomas, given that this diagnosis accounts for 75% of adrenal masses detected incidentally at CT (31).
Because our study population is known to have cerebral CCMs, we propose that the adrenal calcifications reflect microscopic adrenal vascular lesions, possibly cavernous angiomas, leading to hemorrhage and subsequent calcification. In support of this, a case report of a large multigenerational family with CCM describes an obligate carrier in whom a cavernous angioma was found in the left adrenal gland at autopsy after a fatal motor vehicle accident (32). The hypothesized vascular lesions in our patient population are apparently clinically silent, because no subject had a documented history of symptomatic retroperitoneal hemorrhage or adrenal insufficiency. In addition, these conditions have not been reported in larger clinical series (6,10). Whether our patients with adrenal calcification would have biochemical adrenal abnormality if tested is unknown and could be a topic of further investigation.
The left-sided predominance of adrenal calcifications observed in our patients is an interesting phenomenon that, to our knowledge, has not been described in other adrenal abnormalities. Neonatal adrenal hemorrhage is reported more frequently on the right side (33), and the right adrenal gland is more prone to hemorrhage from blunt trauma (34,35). Different vascular anatomy on the right and left sides may explain the right-sided predilection observed in these settings. Venous drainage from the right adrenal gland occurs via a short right adrenal vein directly connected to the inferior vena cava, whereas the longer left adrenal vein drains into the left renal vein. This anatomic difference may expose the right and left adrenal glands to different local venous pressure shifts (34). Theoretically, the same mechanism could influence the rate of de novo lesion development or propensity of lesions to hemorrhage in adrenal glands of patients with fCCM. Further understanding of the histologic basis of CCM adrenal calcifications and the left-sided predilection we observed might provide insight into cerebral lesion development and causes of intra-cerebral hemorrhage in familial CCM.
Our study had several limitations, including the retrospective design and small number of patients with CCM with available abdominal CT scans. The fact that CT scans were interpreted by two readers in consensus with knowledge of the clinical diagnosis of fCCM represents a potential source of bias. The fCCM patient population at our institution is composed predominantly of CCM1-CHM carriers; thus, it is unknown if our findings are applicable to fCCM disease caused by mutations in the CCM2 or CCM3 genes or different mutations in the CCM1 gene.
Our finding that adrenal calcifications were significantly associated with fCCM adds to existing evidence that this is a multisystem disorder with effects beyond the central nervous system. Further investigation is needed to establish the histologic basis of adrenal calcifications in our population and to determine whether the same association exists for other CCM gene mutations. When encountered incidentally on CT scans, adrenal SFCs could lead to the detection of unrecognized fCCM. Timely diagnosis of fCCM has important implications for clinical and genetic counseling and the management of neurologic complications. At our institution, detection of adrenal SFCs has already improved confidence in an fCCM diagnosis questioned after initial neurologic imaging. The presence of adrenal calcifications may also help differentiate familial from sporadic CCM in patients with a solitary cerebral lesion or negative family history, especially when combined with defining lesions of other extraneural tissues such as retina and skin. Our results suggest that fCCM should be considered in the differential diagnosis when patients have CT scans that show small focal adrenal calcifications without an associated mass.
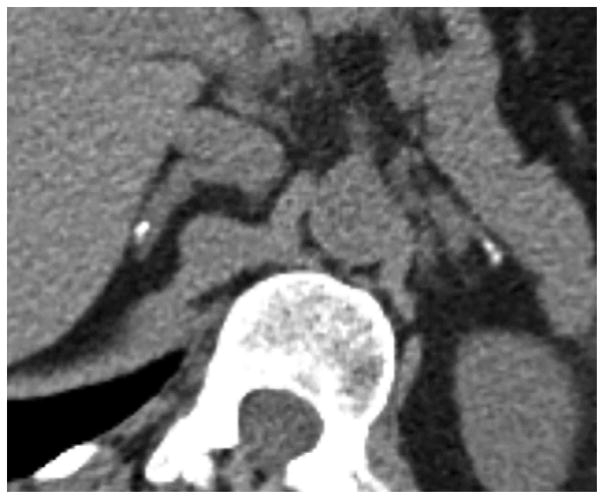
Figure 2.
Unenhanced axial CT scan in 58-year-old asymptomatic man with CCM1-CHM genotype who underwent multiphase abdominal CT to characterize liver lesion. Image shows bilateral adrenal calcifications with left side predominance.
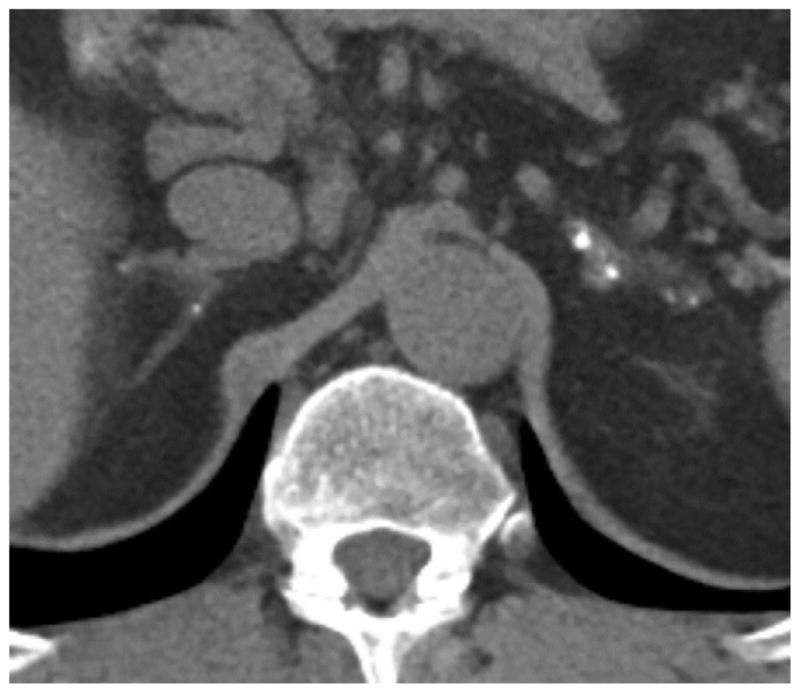
Figure 3.
Unenhanced axial CT scan in 54-year-old woman with multiple cerebral cavernous malformations, genotype unknown. Patient underwent CT of abdomen for suspected urinary calculi. Image depicts bilateral adrenal calcifications.
Figure 4.
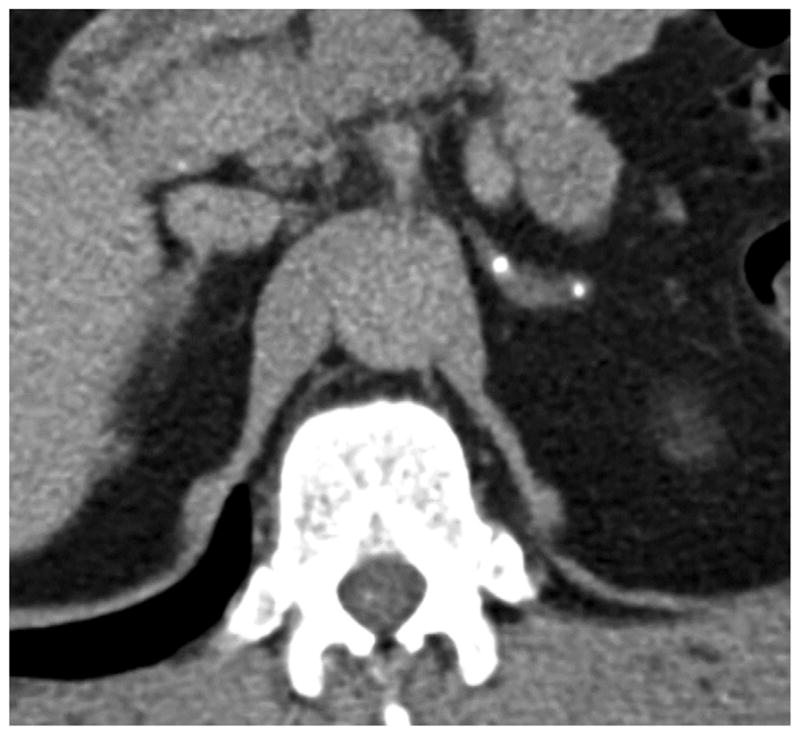
Image in 56-year-old man with history of seizures and documented CCM1-CHM genotype. Delayed phase axial image from multiphase CT of abdomen performed because of hematuria shows focal calcifications in left adrenal gland. Right adrenal calcification was also present (not pictured).
Advances in Knowledge.
Small focal calcifications (SFCs) in the adrenal gland are common in patients with familial cerebral cavernous malformations (fCCMs) and were found in 19 of the 38 patients (50%) in our series.
SFCs showed a positive correlation with patient age, occurring in 0 of six subjects with fCCM younger than 20 years (0%), three of nine subjects aged 20–39 years (33%), and 16 of 23 subjects aged 40 years and older (70%).
Adrenal calcifications in patients with fCCM were more frequent on the left side, with 17 of 19 patients having more SFCs in the left adrenal gland than the right and 50 of the 61 observed SFCs (82%) found in the left adrenal gland.
Subjects with SFCs had significantly higher brain lesion counts than did those without SFCs (proportional increase, 5.7; 95% confidence interval: 2.6, 12.4; P < .001) and also tended to have more brain lesions than expected for their age (proportional increase, 1.6; 95% confidence interval: 0.9, 3.1; P = .131).
Implications for Patient Care.
fCCM should be considered in the differential diagnosis of SFCs in the adrenal gland without mass because recognition of fCCM may allow for appropriate clinical and genetic counseling and management of neurologic complications.
SFCs in the adrenal gland add diagnostic confidence when patients with equivocal neuroimaging findings and negative family history are suspected of having fCCM.
Acknowledgments
Supported by National Center for Advancing Translational Sciences (grants U54 NS065705, UL1 TR001449) and the National Institute of Neurological Disorders and Stroke (grant U54 NS065705).
We thank Beth Baca, MSW, and Kathryn Epstein, MD, of the University of New Mexico for study coordination and data management and Li Luo, PhD, of the University of New Mexico for statistical assistance.
Abbreviations
- CCM
cerebral cavernous malformation
- CCM1
CCM type 1
- CHM
Common Hispanic Mutation
- fCCM
familial CCM
- GRE
gradient echo
- SFC
small focal calcification
Footnotes
Author contributions:
Guarantors of integrity of entire study, C.D.S., L.A.M., B.L.H.; study concepts/study design or data acquisition or data analysis/interpretation, all authors; manuscript drafting or manuscript revision for important intellectual content, all authors; manuscript final version approval, all authors; agrees to ensure any questions related to the work are appropriately resolved, all authors; literature research, C.D.S., S.C.E., L.A.M., B.L.H.; clinical studies, C.D.S., S.C.E., H.K., L.A.M., B.L.H.; statistical analysis, M.R.B., J.N., H.K., L.A.M., B.L.H.; and manuscript editing, C.D.S., S.C.E., M.R.B., H.K., L.A.M., B.L.H.
Disclosures of Conflicts of Interest: C.D.S. disclosed no relevant relationships. S.C.E. disclosed no relevant relationships. M.R.B. disclosed no relevant relationships. J.N. disclosed no relevant relationships. H.K. disclosed no relevant relationships. L.A.M. disclosed no relevant relationships. B.L.H. disclosed no relevant relationships.
References
- 1.McCormick WF, Hardman JM, Boulter TR. Vascular malformations (“angiomas”) of the brain, with special reference to those occurring in the posterior fossa. J Neurosurg. 1968;28(3):241–251. doi: 10.3171/jns.1968.28.3.0241. [DOI] [PubMed] [Google Scholar]
- 2.Giombini S, Morello G. Cavernous angiomas of the brain: account of fourteen personal cases and review of the literature. Acta Neurochir (Wien) 1978;40(1–2):61–82. doi: 10.1007/BF01773116. [DOI] [PubMed] [Google Scholar]
- 3.Simard JM, Garcia-Bengochea F, Ballinger WE, Jr, Mickle JP, Quisling RG. Cavernous angioma: a review of 126 collected and 12 new clinical cases. Neurosurgery. 1986;18(2):162–172. doi: 10.1227/00006123-198602000-00008. [DOI] [PubMed] [Google Scholar]
- 4.de Souza JM, Domingues RC, Cruz LC, Jr, Domingues FS, Iasbeck T, Gasparetto EL. Susceptibility-weighted imaging for the evaluation of patients with familial cerebral cavernous malformations: a comparison with T2-weighted fast spin-echo and gradient-echo sequences. AJNR Am J Neuroradiol. 2008;29(1):154–158. doi: 10.3174/ajnr.A0748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunereau L, Labauge P, Tournier-Lasserve E, Laberge S, Levy C, Houtteville JP. Familial form of intracranial cavernous angioma: MR imaging findings in 51 families. French Society of Neurosurgery Radiology. 2000;214(1):209–216. doi: 10.1148/radiology.214.1.r00ja19209. [DOI] [PubMed] [Google Scholar]
- 6.Requena I, Arias M, López-Ibor L, et al. Cavernomas of the central nervous system: clinical and neuroimaging manifestations in 47 patients. J Neurol Neurosurg Psychiatry. 1991;54(7):590–594. doi: 10.1136/jnnp.54.7.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavalcanti DD, Kalani MY, Martirosyan NL, Eales J, Spetzler RF, Preul MC. Cerebral cavernous malformations: from genes to proteins to disease. J Neurosurg. 2012;116(1):122–132. doi: 10.3171/2011.8.JNS101241. [DOI] [PubMed] [Google Scholar]
- 8.Gunel M, Awad IA, Finberg K, et al. A founder mutation as a cause of cerebral cavernous malformation in Hispanic Americans. N Engl J Med. 1996;334(15):946–951. doi: 10.1056/NEJM199604113341503. [DOI] [PubMed] [Google Scholar]
- 9.Sahoo T, Johnson EW, Thomas JW, et al. Mutations in the gene encoding KRIT1, a Krev-1/rap1a binding protein, cause cerebral cavernous malformations (CCM1) Hum Mol Genet. 1999;8(12):2325–2333. doi: 10.1093/hmg/8.12.2325. [DOI] [PubMed] [Google Scholar]
- 10.Denier C, Labauge P, Brunereau L, et al. Clinical features of cerebral cavernous malformations patients with KRIT1 mutations. Ann Neurol. 2004;55(2):213–220. doi: 10.1002/ana.10804. [DOI] [PubMed] [Google Scholar]
- 11.Zabramski JM, Wascher TM, Spetzler RF, et al. The natural history of familial cavernous malformations: results of an ongoing study. J Neurosurg. 1994;80(3):422–432. doi: 10.3171/jns.1994.80.3.0422. [DOI] [PubMed] [Google Scholar]
- 12.Horowitz M, Kondziolka D. Multiple familial cavernous malformations evaluated over three generations with MR. AJNR Am J Neuroradiol. 1995;16(6):1353–1355. [PMC free article] [PubMed] [Google Scholar]
- 13.Labauge P, Brunereau L, Laberge S, Houtteville JP. Prospective follow-up of 33 asymptomatic patients with familial cerebral cavernous malformations. Neurology. 2001;57(10):1825–1828. doi: 10.1212/wnl.57.10.1825. [DOI] [PubMed] [Google Scholar]
- 14.Labauge P, Enjolras O, Bonerandi JJ, et al. An association between autosomal dominant cerebral cavernomas and a distinctive hyper-keratotic cutaneous vascular malformation in 4 families. Ann Neurol. 1999;45(2):250–254. doi: 10.1002/1531-8249(199902)45:2<250::aid-ana17>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 15.Labauge P, Krivosic V, Denier C, Tournier-Lasserve E, Gaudric A. Frequency of retinal cavernomas in 60 patients with familial cerebral cavernomas: a clinical and genetic study. Arch Ophthalmol. 2006;124(6):885–886. doi: 10.1001/archopht.124.6.885. [DOI] [PubMed] [Google Scholar]
- 16.Toll A, Parera E, Giménez-Arnau AM, et al. Cutaneous venous malformations in familial cerebral cavernomatosis caused by KRIT1 gene mutations. Dermatology. 2009;218(4):307–313. doi: 10.1159/000199461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toldo I, Drigo P, Mammi I, Marini V, Carollo C. Vertebral and spinal cavernous angiomas associated with familial cerebral cavernous malformation. Surg Neurol. 2009;71(2):167–171. doi: 10.1016/j.surneu.2007.07.067. [DOI] [PubMed] [Google Scholar]
- 18.Lanfranconi S, Ronchi D, Ahmed N, et al. A novel CCM1 mutation associated with multiple cerebral and vertebral cavernous malformations. BMC Neurol. 2014;14:158. doi: 10.1186/s12883-014-0158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drigo P, Mammi I, Battistella PA, Ricchieri G, Carollo C. Familial cerebral, hepatic, and retinal cavernous angiomas: a new syndrome. Childs Nerv Syst. 1994;10(4):205–209. doi: 10.1007/BF00301155. [DOI] [PubMed] [Google Scholar]
- 20.Davenport WJ, Siegel AM, Dichgans J, et al. CCM1 gene mutations in families segregating cerebral cavernous malformations. Neurology. 2001;56(4):540–543. doi: 10.1212/wnl.56.4.540. [DOI] [PubMed] [Google Scholar]
- 21.Akers AL, Ball KL, Clancy M, et al. Brain Vascular Malformation Consortium: overview, progress and future directions. J Rare Disord. 2013;1(1):5. [PMC free article] [PubMed] [Google Scholar]
- 22.Choquet H, Trapani E, Goitre L, et al. Cytochrome P450 and matrix metalloproteinase genetic modifiers of disease severity in cerebral cavernous malformation type 1. Free Radic Biol Med. 2016;92:100–109. doi: 10.1016/j.freeradbiomed.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Golden M, Saeidi S, Liem B, Marchand E, Morrison L, Hart B. Sensitivity of patients with familial cerebral cavernous malformations to therapeutic radiation. J Med Imaging Radiat Oncol. 2015;59(1):134–136. doi: 10.1111/1754-9485.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain R, Robertson PL, Gandhi D, Gujar SK, Muraszko KM, Gebarski S. Radiation-induced cavernomas of the brain. AJNR Am J Neuroradiol. 2005;26(5):1158–1162. [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson DA, Muchmore HG, Tisdal RG, Fahmy A, Pitha JV. Histoplasmosis of the adrenal glands studied by CT. Radiology. 1984;150(3):779–783. doi: 10.1148/radiology.150.3.6695079. [DOI] [PubMed] [Google Scholar]
- 26.Hindman N, Israel GM. Adrenal gland and adrenal mass calcification. Eur Radiol. 2005;15(6):1163–1167. doi: 10.1007/s00330-004-2509-8. [DOI] [PubMed] [Google Scholar]
- 27.Ma ES, Yang ZG, Li Y, Guo YK, Deng YP, Zhang XC. Tuberculous Addison’s disease: morphological and quantitative evaluation with multidetector-row CT. Eur J Radiol. 2007;62(3):352–358. doi: 10.1016/j.ejrad.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Otal P, Escourrou G, Mazerolles C, et al. Imaging features of uncommon adrenal masses with histopathologic correlation. RadioGraphics. 1999;19(3):569–581. doi: 10.1148/radiographics.19.3.g99ma07569. [DOI] [PubMed] [Google Scholar]
- 29.Ozmen MN, Aygün N, Kiliç I, Kuran L, Yalçin B, Besim A. Wolman’s disease: ultrasono-graphic and computed tomographic findings. Pediatr Radiol. 1992;22(7):541–542. doi: 10.1007/BF02013008. [DOI] [PubMed] [Google Scholar]
- 30.Radin DR, Baker EL, Klatt EC, et al. Visceral and nodal calcification in patients with AIDS-related Pneumocystis carinii infection. AJR Am J Roentgenol. 1990;154(1):27–31. doi: 10.2214/ajr.154.1.2104720. [DOI] [PubMed] [Google Scholar]
- 31.Song JH, Chaudhry FS, Mayo-Smith WW. The incidental adrenal mass on CT: prevalence of adrenal disease in 1,049 consecutive adrenal masses in patients with no known malignancy. AJR Am J Roentgenol. 2008;190(5):1163–1168. doi: 10.2214/AJR.07.2799. [DOI] [PubMed] [Google Scholar]
- 32.Gil-Nagel A, Wilcox KJ, Stewart JM, Anderson VE, Leppik IE, Rich SS. Familial cerebral cavernous angioma: clinical analysis of a family and phenotypic classification. Epilepsy Res. 1995;21(1):27–36. doi: 10.1016/0920-1211(95)00005-u. [DOI] [PubMed] [Google Scholar]
- 33.Mutlu M, Karagüzel G, Aslan Y, Cansu A, Okten A. Adrenal hemorrhage in newborns: a retrospective study. World J Pediatr. 2011;7(4):355–357. doi: 10.1007/s12519-011-0259-7. [DOI] [PubMed] [Google Scholar]
- 34.Rana AI, Kenney PJ, Lockhart ME, et al. Adrenal gland hematomas in trauma patients. Radiology. 2004;230(3):669–675. doi: 10.1148/radiol.2303021345. [DOI] [PubMed] [Google Scholar]
- 35.Lee YS, Jeong JJ, Nam KH, Chung WY, Chang HS, Park CS. Adrenal injury following blunt abdominal trauma. World J Surg. 2010;34(8):1971–1974. doi: 10.1007/s00268-010-0537-x. [DOI] [PubMed] [Google Scholar]