Abstract
Objective
To determine the effectiveness of the Metropolitan Atlanta Community Adolescent Rapid Testing Initiative (MACARTI) intervention relative to standard of care (SOC), in achieving early diagnosis, linkage and retention among HIV-infected youth ages 18-24 years.
Design
MACARTI was a pilot single-center, prospective, non-randomized study
Methods
MACARTI combined non-traditional venue HIV testing, motivational interviewing and case management. We collected demographic, clinical variables and calculated linkage and appointment adherence rates. We obtained SOC data from an adolescent HIV clinic. Longitudinal data were analyzed using inverse propensity-treatment weighted linear growth models; medians, interquartile ranges (IQR), means and 95% confidence intervals (CI) are provided.
Results
MACARTI screened 435 participants and identified 49 (11.3%) HIV infections. The SOC arm enrolled 49 new HIV-infected individuals. The 98 participants, (49 in each arm) were: 85% male; 91% Black; mean age=21 years (SD:1.8). Overall, 63% were linked within three months of diagnosis; linkage was higher for MACARTI compared with SOC (96% vs. 57%, p<0.001). Median linkage time for MACARTI participants compared to SOC was 0.39 (IQR:0.20-0.72) vs. 1.77 (IQR:1.12-12.65) months (p<0.001). MACARTI appointment adherence was higher than SOC (86.1% vs. 77.2%, p=0.018). In weight-adjusted models, mean CD4+T-cell counts increased and mean HIV-1 RNA levels decreased in both arms over 12 months, but the differences were more pronounced in the MACARTI arm.
Conclusions
MACARTI successfully identified and linked HIV-infected youth in Atlanta. MACARTI may serve as an effective linkage and care model for clinics serving HIV-infected youth.
Introduction
Georgia (GA) had the fifth-highest rate of new HIV diagnoses (12.9/100,000) in the United States (U.S.) in 2015; 66% of HIV-infected individuals lived in and 69% of new diagnoses were reported from Atlanta Metropolitan Statistical Area (Atlanta) in 2011.[1, 2] Gaps along the HIV care continuum among youth in Atlanta are evidenced by low rates of testing, linkage to care, and viral suppression. Among people living with diagnosed HIV in GA, only 68% of youth are linked to care within 30 days, and just 52% and 38% of 13-19 and 20-24 year olds, respectively, achieved viral suppression at last measurement.[2] Additionally, Atlanta youth are likely to be diagnosed at more advanced stages of illness with more 13-24 year olds progressing to Stage 2 HIV (CD4 count 200-499) at diagnosis compared to any other age group.[3, 4]
Youth living with HIV have a higher prevalence of psychosocial stressors contributing to unfavorable clinical outcomes and broader gaps along the HIV continuum of care compared with HIV-infected adults.[5-9] Singer's syndemic theory suggests that healthcare is affected by multiple epidemics that should be addressed simultaneously.[10] Adverse social structures such as poverty, discrimination, stigma, and psychiatric co-morbidities (including depressive disorder, substance use) increase risk for HIV acquisition and adversely impact adherence to HIV treatment. [11-13]
Effective interventions tailored to HIV-infected youth are urgently needed. Hall et al. showed that only 62% of youth between 13-24 years were linked and 44% retained in care nationally.[6] The Reaching for Excellence in Adolescent Care and Health study documented that only 32.5% of youth achieved viral suppression,[14] while a Pediatric AIDS Clinical Trials Group study demonstrated a 6-month retention rate of 58%.[15] These studies underscore deficiencies in traditional approaches of HIV diagnosis and management for youth.[16, 17] Newer, comprehensive approaches tailored for youth that address HIV diagnosis and management including of psychiatric co-morbidities and psychosocial stressors may improve HIV outcomes.
Despite recommendations for routine HIV testing in health-care settings,[16] implementation gaps remain; especially in racial/ethnic minority youth.[17] Venue-based HIV testing can improve testing rates among youth. A study with young men who have sex with men (MSM), showed that factors associated with no previous HIV test included young age (13–24 years) and identifying as non-Hispanic black or Hispanic.[18] Alternate testing strategies have shown high positivity rates among those tested in non-traditional settings.[19]
Motivational interviewing (MI) has been successful in the treatment of chronic diseases,;[20, 21] however, data on MI-based interventions in HIV-infected youth are limited.[22] A randomized trial in youth assessing the effectiveness of MI delivered either by paraprofessional or professional staff showed improved retention in both arms with no differences between staff members delivering the intervention. However, pre-intervention data were incomplete for the majority of participants.[23] Another study used MI and financial incentives with 11 perinatally-infected youth with advanced immunosuppression; five achieved viral suppression at one year with a median CD4 count recovery of 140 cells/μl. This study was limited by small sample size and the potential confounding of financial incentives. [24]
Case management (CM) has improved linkage/retention in care of HIV-infected individuals. The Antiretroviral Treatment Access Study (ARTAS) randomized recently diagnosed HIV-infected participants to a brief strength-based model of CM (SBCM) and care planning versus usual care; 64% in the intervention arm were linked-to and retained-in care compared with 49% in the control arm [RR(adj) 1.41; p=0.006]. ARTAS is recommended as an effective intervention by the Centers for Disease Control and Prevention (CDC) [25-27]. However, 90% of ARTAS participants were over 26 years of age and youth was underrepresented [28].
Building on these studies, we developed the Metropolitan Atlanta Community Adolescent Rapid Testing Initiative (MACARTI) a multi-pronged intervention combining non-traditional venue HIV testing, MI and CM support to improve diagnosis, linkage and retention in care of youth ages 18-24 years. The intervention started with a formative phase of focus groups with HIV-infected and uninfected youth to inform a youth-friendly strategy.[29, 30] MI/CM approach was implemented through the first year post-diagnosis using a developmentally informed approach.
Our goals were to: 1) increase opportunities for HIV testing and diagnosis for youth at places where they routinely gather, and 2) strengthen HIV treatment and care for those living with HIV using the MACARTI intervention.
Methods
Study Design
MACARTI was a pilot single-center, prospective non-randomized interventional study of HIV-infected youth. Enrollment occurred from December 2012 through January 2015, with follow-up through February 2016. The MACARTI trial flow (Figure 1) is described briefly below. The Emory Institutional Review Board, the Grady Research Oversight Committee, and the CDC's National Center for HIV, Viral Hepatitis, STD, and TB Prevention approved this study. All participants provided written informed consent.
Figure 1. The MACARTI Trial Flow Diagram.
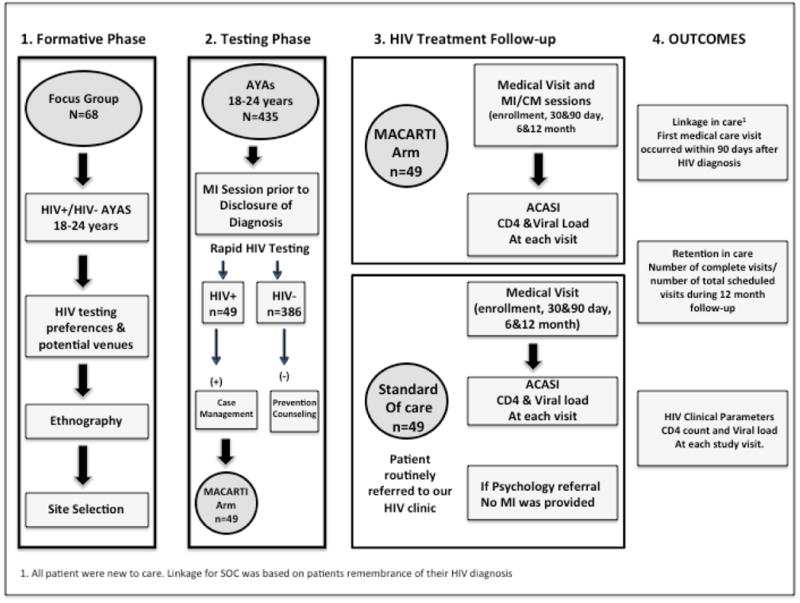
Formative Phase and Venue Testing Selection
We conducted focus groups with 68 HIV-infected and uninfected youth to understand testing preferences and potential venues for testing. Seventeen focus groups (11 with HIV-infected, two with HIV-uninfected and four with a mixed group of HIV-infected and uninfected youth) were conducted (four participants per group). Their responses were used to develop a youth-friendly testing strategy, to select testing sites and to better characterize post-diagnosis support. Following the focus groups, we conducted ethnographic observations of prospective venues to inform site selection.[30] Venues were selected only if they provided a private space for testing.
Testing Phase
Study Team
The MACARTI study staff implementing the intervention included: a physician (Study PI-AFCG), a psychology fellow (KF), one case manager, and three recruiters/testers. Community partners (AID Atlanta, AIDS Healthcare Foundation and Positive Impact) also provided personnel during testing events as needed. Study personnel had no previous MI experience and received training by the study psychologist and/or fellow (KF or CG), utilizing an MI group facilitator manual with MI information, MI techniques, and motivational activities. Study staff learned theoretical and practical applications of MI including how to apply reflectively listening and ask open-ended questions, assess levels of motivation and confidence, and elicit barriers to adherence, confidence and commitment language.[31]
Participants
Participants in the testing phase included youth ages 18-24 years. The intervention arm (MACARTI) included youth diagnosed with HIV at non-traditional venues by either the study team or a community partner. The standard of care (SOC) arm included participants ages 18-24 years referred to the Ponce Family and Youth Clinic (PFYC) of Grady Health Systems for HIV care. Participants from both arms were selected only if they had a previously negative or unknown HIV test.
MACARTI arm enrollment procedures
Members of the study team (at least one recruiter and one tester) conducted testing in venues selected during the formative phase. For participants who agreed to be tested, testing was performed using a sixty-second INSTI™ HIV-1/HIV-2 antibody test (BioLytical Laboratories, Inc; sensitivity and specificity of 99.8% and 99.5% respectively).[32] The tester conducted an MI/CM session prior to disclosure of diagnosis. MI/CM was used prior to disclosure to address potential ambivalence towards seeking HIV-related care and to generate a plan of action to either improve clinical outcomes (if test was positive) or to establish HIV prevention strategies (if test was negative). HIV-infected patients were given instructions on how to get to the PFYC and provided information about documents needed for enrollment into medical care. After the diagnosis was made, study personnel maintained contact and assisted with clinic enrollment. At the initial medical visit, each participant had his or her blood drawn for HIV-1 RNA (VL) and CD4+T cell (CD4) count. Participants had an MI session with the psychology fellow to continue addressing potential ambivalence towards follow-up care with follow-up visits at 1, 3, 6 and 12 months. All participants received reminder calls the day prior to their study visits and for rescheduling purposes if the visit was missed. If participants stopped attending visits or answering phone calls from the study team, they were considered lost to follow-up, triggering a referral to the health department for tracking purposes. Participants also had the option to enroll in another HIV clinic (this was requested by two participants); we then helped these participants arrange appropriate follow-up. Participants enrolled at a non-PFYC site were asked if they wished to continue in the study. If they did, a signed release of medical records was required and the same number of visits occurred with study personnel travelling to their preferred clinic to deliver the intervention. No significant differences were noted with the delivery of the intervention at the non-PFYC sites.
Standard of Care Participants
SOC participants were newly HIV-diagnosed youth referred for care to the PFYC through conventional referrals from other agencies, hospitals or medical providers. The PFYC policy is to try to link patients within 72 hours after referral; therefore, confounding from the referral process/scheduling was not a concern. SOC participants received standard support services upon request including psychological and CM support. SOC psychological support met practice standards and CM support was limited to providing referrals for housing, food stamps and transportation as needed. SOC participants also received reminder calls prior to each appointment for rescheduling purposes from PFYC personnel.
Data Collection
Once consented, participants from both arms completed baseline Audio Computer-Assisted Self-Interview (ACASI) questionnaires. Data collected included demographic information, employment, education, drug use and sexual history. Clinical information was obtained from the medical records and included baseline and follow-up CD4 count, VL, any antiretroviral therapy (ART) prescriptions, any AIDS-defining diagnoses, and condom use at last sexual encounter. Baseline and follow-up questionnaires were obtained at screening, enrollment, at 30 and 90 days, and at six and 12 months.
MACARTI Intervention Components
Motivational Interviewing
A detailed description of the MI component of the intervention is presented in Appendix I. Briefly, MI is an evidence-based therapeutic approach. Treatment fidelity depends upon the provider's adherence to the “spirit” of the approach (namely, partnership, acceptance, compassion and evocation), which can be reliably measured (see quality and fidelity section below) as opposed to adherence to specific guidelines.[20, 31, 33-35] MI focuses on strengths and self-efficacy, while emphasizing collaboration, empowerment, respect for choice, and understanding of the participant's perspective.[33] MACARTI participants received MI sessions at the venue before disclosure of HIV diagnosis, and at all study visits.
Strength-Based Model of Case Management
An SBCM was employed to empower the client, build self-esteem and enable the participants' utilization of available resources. This model provides care that is beyond accessing services; it empowers participants to identify their own needs in utilizing available resources and services. CM was provided at each study visit for the MACARTI arm participants. Problem-solving, goal-planning, and guidance counseling were used to help participants with concerns identified by CM. An average meeting for CM lasted approximately 45-60 minutes.
Quality and Fidelity
A standard operating procedure manual was developed and available to study staff, ensuring quality and fidelity to study procedures. To evaluate fidelity to the MI protocol, the MI trainer assessed 20% of the sessions for consistent use of MI techniques and re-trained staff if deviations were noted.
Definitions
Linkage to Care
The first medical care visit occurred within 90 days after HIV diagnosis.
Retention in care
Number of completed visits divided by the number of total scheduled visits during the 12 month follow-up,[36] among participants who attended at least one medical care visit. Individuals who never linked (4/98; 4%) were not counted in retention in care calculations.
Viral Suppression
The number of participants who had VL <40 copies/ml at the one-year visit among participants who completed the study.
Statistical Methods
Statistical analyses were performed using SAS v9.4 (Cary, NC) and CRAN R v.3.3 (Vienna, Austria), and significance was evaluated two-sided at the 0.05 level. Demographic, drug use, sexual history, and clinical characteristics were summarized overall and by SOC and MACARTI arms using means and standard deviations, medians and interquartile ranges (IQR), or frequencies and percentages as appropriate. Two-sample testing, including both parametric (t-tests and Chi-square tests) and non-parametric (Wilcoxon and Fisher's) approaches were used to gauge dissimilarities across the study groups at baseline. Differences in visit attendance, retention and linkage between SOC and MACARTI arms were similarly considered. Due to noted baseline covariate differences across SOC and MACARTI arms, an inverse propensity-treatment weighted (IPTW) score was calculated using binary logistic regression and added as an observation weight characteristic to the sample, to control for baseline study arm disparities.
Linear mixed-effects growth models were used to evaluate statistical differences over study visit follow-up in CD4 count and VL between the SOC and MACARTI arms. The fixed effect for each model was treatment arm (2 levels), and the random effects were participant-specific intercepts and study visit slopes. Interactions between treatment arm and study visit were included, and due to curve-linear associations in the raw data, quadratic terms were added to each model for study visit. For CD4 count, a square-root transformation was applied to the outcome; for VL, both the outcome values and study visit were natural-log transformed. All observations in the mixed-effects regression models were evaluated unweighted and weighted using the IPTW score. All presented results have been back-transformed to their original units, and results are given as least-squares mean estimates with associated 95% confidence intervals (CI). Further details for the propensity and linear growth models are provided in Appendix II.
Results
We tested 435 participants and identified 49 as HIV-infected, for a positivity rate of 11.3%. Multiple sites were used for testing, however the highest positivity rate was seen in nightclubs (30%) and street testing in areas identified as high risk by ethnographic studies (18%) (Table 1). The SOC arm screened 62 participants to enroll 49 HIV-infected individuals new to HIV care; thirteen were excluded because they were not new to HIV care. Ninety-eight participants, 49 in each arm, were enrolled; 85% male; 91% Black; mean age was 21 years (SD: 1.8 years); 78% identified as homosexual/bisexual or queer; 62% had high school education or less; 23% percent reported currently using drugs (marijuana, cocaine, heroin, methamphetamines, ecstasy, inhalant or other); 14% reported a history of abuse (Table 2). After IPTW-adjustment, all differences were balanced between MACARTI arm and SOC participants (Table 2), per a weighted standardized difference cutoff of 0.25.
Table 1. Testing Venues and Positivity Rate The MACARTI Trial, Atlanta, GA 2012-2016.
| Venue Type | Number Tested | Identified Positives | Positivity Rate |
|---|---|---|---|
| Night Clubs | 122 | 37 | 30% |
| College Campus | 98 | 5 | 5% |
| Street Testing1 | 38 | 7 | 18% |
| Private Parties | 19 | 0 | 0% |
| Pride Events | 38 | 0 | 0% |
| Malls and surroundings | 6 | 0 | 0% |
| Fairs | 19 | 0 | 0% |
| Shelters | 95 | 0 | 0% |
| Total | 435 | 49 |
Previously determined high risk areas by ethnographic studies
Table 2. Baseline characteristics for standard of care and intervention participants, The MACARTI Trial, Atlanta, GA 2012-2016.
| Characteristic, N (%) | Overall N = 98 |
SOC N = 49 |
MACARTI N = 49 |
P-Value | Unweighted Std. Diff |
Weighted Std. Diff2 |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Male | 83 (84.7%) | 36 (73.5%) | 47 (95.9%) | 0.004 | 0.656 | 0.097 |
| Female | 15 (15.3%) | 13 (26.5%) | 2 (4.1%) | |||
| Race | ||||||
| Black | 89 (90.8%) | 47 (95.9%) | 42 (85.7%) | 0.159 | 0.359 | 0.230 |
| Other (White, Hispanic, Other) | 9 (9.2%) | 2 (4.1%) | 7 (14.3%) | |||
| Age (yr), Mean ± SD | 21.5 ± 1.8 | 21.3 ± 1.8 | 21.7 ± 1.7 | 0.175 | 0.276 | 0.083 |
| Work Status | ||||||
| Employed/In School | 74 (75.5%) | 32 (65.3%) | 42 (85.7%) | 0.019 | 0.489 | 0.139 |
| Neither | 24 (24.5%) | 17 (34.7%) | 7 (14.3%) | |||
| Education, N = 97 | ||||||
| High school or Less | 60 (61.9%) | 35 (72.9%) | 25 (51%) | 0.026 | 0.463 | 0.154 |
| College or More | 37 (38.1%) | 13 (27.1%) | 24 (49%) | |||
| Ever Abused Alcohol | 15 (15.3%) | 3 (6.1%) | 12 (24.5%) | 0.022 | 0.528 | 0.083 |
| Currently Using Drugs | 22 (22.5%) | 9 (18.4%) | 13 (26.5%) | 0.333 | 0.197 | 0.008 |
| Abused Type | ||||||
| No Abuse | 84 (85.7%) | 42 (85.7%) | 42 (85.7%) | 1.000 | <0.001 | <0.001 |
| Abused | 14 (14.3%) | 7 (14.3%) | 7 (14.3%) | |||
| Sexual Orientation | ||||||
| Straight | 22 (22.5%) | 19 (38.8%) | 3 (6.1%) | <0.001 | 0.850 | 0.198 |
| Gay/Bisexual/Queer | 76 (77.5%) | 30 (61.2%) | 46 (93.9%) | |||
| Condom Usage | ||||||
| Always/Usually | 71 (72.5%) | 33 (67.4%) | 38 (77.6%) | 0.258 | 0.230 | 0.249 |
| Sometimes/Never | 27 (27.5%) | 16 (32.6%) | 11 (22.4%) | |||
| Ever had STI1 – Patient Report, N = 97 | 47 (48.5%) | 28 (57.1%) | 19 (39.6%) | 0.084 | 0.357 | 0.071 |
| Any AIDS defining conditions, N = 94 | 34 (36.2%) | 25 (51%) | 9 (20%) | 0.002 | 0.685 | 0.112 |
Sexually Transmitted infection
Baseline propensity balancing results are presented in the supplemental materials; a cutoff of <0.25 was utilized to indicate covariate balance
Baseline HIV characteristics
Compared with SOC, MACARTI arm participants reported fewer AIDS-defining conditions (20% vs 51%, p=0.002) and a higher mean CD4 count (317 (IQR: 218-512) vs. 196.5 (IQR: 61-377.5) cells/mm3, p=0.007).
Linkage to Care
Overall, 63% of participants were linked to care within 90 days of diagnosis; however, linkage was higher for the MACARTI arm compared with SOC (88% vs. 39%, p<0.001). Weighted, MACARTI linkage remained higher than SOC (96% vs 57%, p<0.001). Weighted median linkage time for MACARTI participants compared to SOC was 0.39 (IQR: 0.20-0.72) vs. 1.77 (IQR: 1.12-12.65) months (p<0.001). An IPTW-adjusted multivariable logistic model showed that MACARTI participants had significantly higher odds of linking within 90 days than those in SOC arm (aOR= 18.17, 95% CI: 3.27-100.90).
Retention in Care
MACARTI arm participants had better appointment adherence compared to SOC participants (86.1% vs. 77.2%, p=0.018). MACARTI participants also had better adherence throughout each of the follow-up study visits, albeit only significant at 90 days (Table 3). We also looked at the percentage of participants who attended 80 and 100% of clinical visits scheduled. Although there was no statistical difference at 80% of scheduled visits, 50% of MACARTI participants attended 100% of the visits compared to 26% in the SOC arm (p=0.017).
Table 3. Proportion of appointment adherence stratified by study arm, The MACARTI Trial, Atlanta, GA 2012-2016.
| Visit, n/N (%) | Standard Arm Appointment Adherence |
MACARTI Arm Appointment Adherence |
P-Value |
|---|---|---|---|
| Unweighted | |||
| 30 Days | 35/49 (71.4%) | 38/45 (84.4%) | 0.130 |
| 90 Days | 37/49 (75.5%) | 43/45 (95.6%) | 0.008 |
| 6 Months | 30/49 (61.2%) | 34/45 (75.6%) | 0.137 |
| 12 Months | 30/49 (61.2%) | 33/45 (73.3%) | 0.212 |
| Overall | 181/245 (73.9%) | 197/229 (86%) | 0.001 |
| Weighted | |||
| 30 Days | 42.6/52.7 (80.8%) | 30/37.8 (79.3%) | 0.864 |
| 90 Days | 36.8/52.7 (69.9%) | 36.1/37.8 (95.6%) | 0.002 |
| 6 Months | 32.4/52.7 (61.5%) | 29.1/37.8 (77%) | 0.119 |
| 12 Months | 39/52.7 (74%) | 29.8/37.8 (78.8%) | 0.603 |
| Overall | 203.6/263.6 (77.2%) | 162.7/188.9 (86.1%) | 0.018 |
CD4 Count and HIV-1 RNA Levels
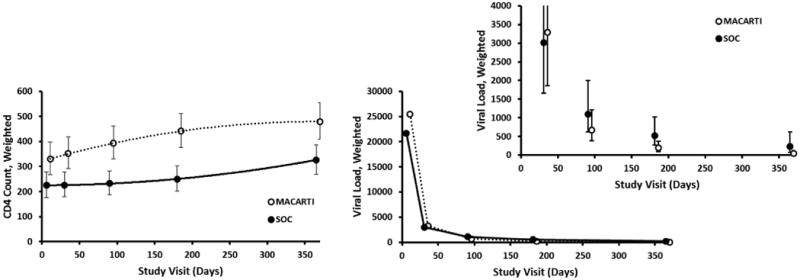
CD4 counts increased significantly within both arms. Growth model estimates indicated MACARTI and SOC participants gained 149 cell/mm3 and 101 cells/mm3, respectively, at 12 months. Additionally, CD4 counts in the MACARTI arm were significantly higher at all study visits relative to the SOC arm ( Appendix II-Table 3b). The growth trajectory in CD4 count over participant follow-up was significantly higher in the MACARTI arm relative to the SOC (p=0.004) (Figure 2; Appendix II-Table 3a). Growth model estimates for VL indicated significant decreases in both arms, and although the overall growth trajectories were not significantly different between the two arms (p=0.1) (Figure 2; Appendix II-Table 4a), MACARTI arm participants had significantly lower VL at six months (p=0.031) and one year (p=0.008), respectively ( Appendix II-Table 4b). At one year, the weighted percentage of participants in the MACARTI arm who had an undetectable VL was 83% compared to 41% in SOC arm (p<0.001); concurrently, the odds of having an undetectable VL at one year was significantly higher in MACARTI compared to the SOC arm (aOR= 6.80, 95% CI: 2.09-22.15, p=0.002).
Figure 2. Model-based change in CD4 count and viral load overtime by treatment arm – Mean estimates and 95% confidence intervals, The MACARTI Trial, Atlanta, GA 2012-2016.
Discussion
The MACARTI intervention successfully identified HIV-infected youth in the community, linking them to HIV care within 90 days of diagnosis and achieving high retention rates consistent with National HIV/AIDS Strategy goals.[37] Factors such as psychological distress, fear, lack of information, traumatic experiences, and lack of food, transport and housing, create syndemics of risk and add complexity to the care of HIV-infected youth.[38] MACARTI utilized MI and CM to address behavioral, motivational, and socioeconomic factors that affect HIV care. In MACARTI, MI started in the venue prior to disclosure of the diagnosis to build rapport, prepare participants emotionally in the event of a positive HIV test, and to enable participants to develop a plan of action proactively, regardless of the test result. After linkage, MI promoted achievement of: 1) attending medical visits, 2) initiating and adhering to ART, and 3) achieving viral suppression.
MACARTI identified high-risk youth, validating our formative work and targeted testing strategy. Strategies designed without youth input may not be able to access this hard-to-reach population, underscoring the importance of developing youth-oriented, culturally-competent interventions. MACARTI also enabled diagnosing youth at earlier stages of HIV disease compared to participants in the SOC arm. Early diagnosis and treatment of HIV has significant individual and public health advantages, including increased survival and decreased secondary transmission.[39, 40] Interventions incorporating enhanced testing, linkage, and retention components can reduce HIV incidence by 54% and mortality rate by 64%; these outcomes are cost-effective compared to no intervention.[41]
Although MACARTI was not powered to look at differences in HIV clinical parameters, we noted decreases in VL and increases in CD4 count in both arms. The CD4 count trend over time was significantly better for the MACARTI than the SOC arm participants. VL was lower at all time points for MACARTI arm participants, however, statistical significance was reached during the latter part of the follow-up period suggesting that youth-informed interventions, such as MACARTI, provide additional support time-points beyond the first few months post-diagnosis. This type of intervention may seem more labor intensive and challenging for broader implementation purposes; however, the psychosocial needs of youth may require such interventions in order to achieve the desired HIV continuum of care goals in this population.
This study has several limitations. First, the study population reflected a convenience sample that was not identified randomly, and the study was conducted in a single site. Although results may not be generalizable, the HIV epidemiology in GA reflects the current US epidemic. [1] Additionally, since the PFYC is the only adolescent HIV clinic in GA, we potentially accessed the majority of HIV-infected youth in Atlanta. Second, several differences in baseline characteristics were noted between groups. Some of these differences may be related to the venue selection process (not all potential venues where chosen as we required specific standards for testing confidentially and privacy), which may have shifted the MACARTI population towards a more employed/educated population that could afford entrance to specific sites. Additionally, since the intervention included targeted testing based on our formative phase results and positivity rates obtained in the different venues, we could have inadvertently oversampled the gay/bisexual population. However, the use of IPTW balanced both groups, which allowed us to control for differences in baseline characteristics during the analysis. Third, for the linear growth models, missing data were handled under a mixed-model framework, allowing for incomplete observations in the analysis. For all other analyses, complete case data were used, and missing observations were removed. Concurrent with the mixed-model framework, missing data were assumed to be at random after visual evaluation of the participation logs for patterns in attrition, as well as quantitative analyses considering univariate differences in the baseline covariates between those that attended their study visits versus those that did not. While we feel missing at random is an appropriate assumption for our data, we acknowledge that some missing data may not be random. Fourth, although we found significant differences in CD4 count and VL trends, which suggests improved immunologic recovery and viral control in the MACARTI arm, our sample sizes were small; larger studies are warranted to confirm this finding.
In conclusion, despite the need of a larger randomized control study to further test this intervention, the results of the MACARTI trial are very promising and suggest that the combination of non-traditional venue testing, MI and CM has the potential to effectively decrease gaps for youth along the HIV care continuum.
Supplementary Material
Acknowledgments
The authors will like to acknowledge our community partners AIDS Healthcare Foundation, AID Atlanta and Positive Impact, the Fulton County Department of Health and Wellness and the LGTBQ unit of the Atlanta Police Department.
Funding: This work was supported by the Centers for Disease Control and Prevention, grant number 5U01PS003322, the National Center for Advancing Translational Sciences of the National Institutes of Health under Award number UL1TR000454 and by the Center for AIDS Research At Emory University (P30AI050409)
Footnotes
These data were presented in part at the National HIV Prevention Conference, Atlanta, GA, December 2015 and at the Conference of Retroviruses and Opportunistic Infections, Seattle Washington, February 2017
Disclaimer: The findings and conclusions in the paper are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Author Contribution:
- Andres F. Camacho-Gonzalez: Conception and design of the work; data acquisition, analysis, and interpretation, manuscript preparation, revision and submission of final manuscript.
- Scott E. Gillespie: Statistical analysis and interpretation, manuscript preparation and revision.
- LaTeshia Thomas-Seaton: Data acquisition, and interpretation, manuscript revision.
- Krystal Frieson: Data acquisition, analysis, and interpretation, manuscript revision.
- Sophia A. Hussen: Analysis, and interpretation of data, manuscript revision.
- Ashley Murray: Analysis, and interpretation of data, manuscript revision.
- Zaneta Gaul: Analysis, and interpretation of data, manuscript revision.
- Traci Leong: Design of the work, analysis and interpretation of data, manuscript revision.
- Chanda Graves: Design of the work, analysis and interpretation of data, manuscript revision
- Madeline Y. Sutton: Design of the work, analysis and interpretation of data, manuscript revision
- Rana Chakraborty: Conception and design of the work; manuscript preparation, manuscript revision
- All authors reviewed the final submitted manuscript and gave their approval. All authors are in agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Financial disclosure: Andres F. Camacho-Gonzalez has received research support from Gilead and Janssen Pharmaceuticals. Rana Chakraborty has received research support from Gilead.
References
- 1.The Georgia Department of Public Health. HIV Surveillance Fact Sheet. 2014 Accessed at https://dph.georgia.gov/sites/dph.georgia.gov/files/HIV_EPI_Fact%20Sheet_Surveillance_2014.pdf.
- 2.The Georgia Department of Public Health. HIV Care Continuum Report. Georgia: 2014. Accessed at https://dph.georgia.gov/sites/dph.georgia.gov/files/HIV%20Care%20Continuum%20Georgia%202014_07%2007%2016_final.pdf. [Google Scholar]
- 3.HIV Surveillance Update. Online: Georgia Department of Public Health Epidemiology. 2013 Jul 22; [Google Scholar]
- 4.Centers for Disease C, Prevention. HIV incidence among young men who have sex with men--seven U.S. cities, 1994-2000. MMWR Morb Mortal Wkly Rep. 2001;50(21):440–444. [PubMed] [Google Scholar]
- 5.Philbin MM, Tanner AE, DuVal A, Ellen JM, Xu J, Kapogiannis B, et al. Factors affecting linkage to care and engagement in care for newly diagnosed HIV-positive adolescents within fifteen adolescent medicine clinics in the United States. AIDS Behav. 2014;18(8):1501–1510. doi: 10.1007/s10461-013-0650-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall HI, Gray KM, Tang T, Li J, Shouse L, Mermin J. Retention in care of adults and adolescents living with HIV in 13 U.S.areas. J Acquir Immune Defic Syndr. 2012;60(1):77–82. doi: 10.1097/QAI.0b013e318249fe90. [DOI] [PubMed] [Google Scholar]
- 7.Minniear TD, Gaur AH, Thridandapani A, Sinnock C, Tolley EA, Flynn PM. Delayed entry into and failure to remain in HIV care among HIV-infected adolescents. AIDS Res Hum Retroviruses. 2013;29(1):99–104. doi: 10.1089/AID.2012.0267. [DOI] [PubMed] [Google Scholar]
- 8.Ryscavage P, Anderson EJ, Sutton SH, Reddy S, Taiwo B. Clinical outcomes of adolescents and young adults in adult HIV care. J Acquir Immune Defic Syndr. 2011;58(2):193–197. doi: 10.1097/QAI.0b013e31822d7564. [DOI] [PubMed] [Google Scholar]
- 9.Kuhns LM, Hotton AL, Garofalo R, Muldoon AL, Jaffe K, Bouris A, et al. An Index of Multiple Psychosocial, Syndemic Conditions Is Associated with Antiretroviral Medication Adherence Among HIV-Positive Youth. AIDS Patient Care STDS. 2016;30(4):185–192. doi: 10.1089/apc.2015.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer MC, Erickson PI, Badiane L, Diaz R, Ortiz D, Abraham T, et al. Syndemics, sex and the city: understanding sexually transmitted diseases in social and cultural context. Soc Sci Med. 2006;63(8):2010–2021. doi: 10.1016/j.socscimed.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oldenburg CE, Perez-Brumer AG, Reisner SL. Poverty matters: contextualizing the syndemic condition of psychological factors and newly diagnosed HIV infection in the United States. AIDS. 2014;28(18):2763–2769. doi: 10.1097/QAD.0000000000000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer M, Clair S. Syndemics and public health: reconceptualizing disease in bio-social context. Med Anthropol Q. 2003;17(4):423–441. doi: 10.1525/maq.2003.17.4.423. [DOI] [PubMed] [Google Scholar]
- 13.Luo X, Liu G, Frush K, Hey LA. Children's health insurance status and emergency department utilization in the United States. Pediatrics. 2003;112(2):314–319. doi: 10.1542/peds.112.2.314. [DOI] [PubMed] [Google Scholar]
- 14.Ding H, Wilson CM, Modjarrad K, McGwin G, Jr, Tang J, Vermund SH. Predictors of suboptimal virologic response to highly active antiretroviral therapy among human immunodeficiency virus-infected adolescents: analyses of the reaching for excellence in adolescent care and health (REACH) project. Arch Pediatr Adolesc Med. 2009;163(12):1100–1105. doi: 10.1001/archpediatrics.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudy BJ, Lindsey JC, Flynn PM, Bosch RJ, Wilson CM, Hughes ME, et al. Immune reconstitution and predictors of virologic failure in adolescents infected through risk behaviors and initiating HAART: week 60 results from the PACTG 381 cohort. AIDS Res Hum Retroviruses. 2006;22(3):213–221. doi: 10.1089/aid.2006.22.213. [DOI] [PubMed] [Google Scholar]
- 16.Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. quiz CE11-14. [PubMed] [Google Scholar]
- 17.Millett GA, Ding H, Marks G, Jeffries WLt, Bingham T, Lauby J, et al. Mistaken assumptions and missed opportunities: correlates of undiagnosed HIV infection among black and Latino men who have sex with men. J Acquir Immune Defic Syndr. 2011;58(1):64–71. doi: 10.1097/QAI.0b013e31822542ad. [DOI] [PubMed] [Google Scholar]
- 18.Mdodo R, Thomas PE, Walker A, Chavez P, Ethridge S, Oraka E, et al. Rapid HIV testing at gay pride events to reach previously untested MSM: U.S., 2009-2010. Public Health Rep. 2014;129(4):328–334. doi: 10.1177/003335491412900407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellen JM, McCree DH, Muvva R, Chung SE, Miazad RM, Arrington-Sanders R, et al. Recruitment approaches to identifying newly diagnosed HIV infection among African American men who have sex with men. Int J STD AIDS. 2013;24(5):335–339. doi: 10.1177/0956462412472459. [DOI] [PubMed] [Google Scholar]
- 20.Levensky ER, Forcehimes A, O'Donohue WT, Beitz K. Motivational interviewing: an evidence-based approach to counseling helps patients follow treatment recommendations. The American journal of nursing. 2007;107(10):50–58. doi: 10.1097/01.NAJ.0000292202.06571.24. quiz 58-59. [DOI] [PubMed] [Google Scholar]
- 21.Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55(513):305–312. [PMC free article] [PubMed] [Google Scholar]
- 22.Mbuagbaw L, Ye C, Thabane L. Motivational interviewing for improving outcomes in youth living with HIV. Cochrane Database Syst Rev. 2012;(9) doi: 10.1002/14651858.CD009748.pub2. CD009748. [DOI] [PubMed] [Google Scholar]
- 23.Naar-King S, Outlaw A, Green-Jones M, Wright K, Parsons JT. Motivational interviewing by peer outreach workers: a pilot randomized clinical trial to retain adolescents and young adults in HIV care. AIDS Care. 2009;21(7):868–873. doi: 10.1080/09540120802612824. [DOI] [PubMed] [Google Scholar]
- 24.Foster C, McDonald S, Frize G, Ayers S, Fidler S. “Payment by Results”--financial incentives and motivational interviewing, adherence interventions in young adults with perinatally acquired HIV-1 infection: a pilot program. AIDS Patient Care STDS. 2014;28(1):28–32. doi: 10.1089/apc.2013.0262. [DOI] [PubMed] [Google Scholar]
- 25.Craw JA, Gardner LI, Marks G, Rapp RC, Bosshart J, Duffus WA, et al. Brief strengths-based case management promotes entry into HIV medical care: results of the antiretroviral treatment access study-II. Journal of Acquired Immune Deficiency Syndromes: JAIDS. 2008;47(5):597–606. doi: 10.1097/QAI.0b013e3181684c51. [DOI] [PubMed] [Google Scholar]
- 26.Stoll P, Duncan T, Marr O. CDC National HIV Prevention Conference. Atlanta GA: 2011. The Diffusion of ARTAS: What CDC Plans to Do. [Google Scholar]
- 27.Centers for Disease Control and Prevention. Effective interventions, HIV Prevention that works. 2016 Dec; accessed at https://effectiveinterventions.cdc.gov/en/highimpactprevention/PublicHealthStrategies.aspx.
- 28.Gardner LI, Metsch LR, Anderson-Mahoney P, Loughlin AM, del Rio C, Strathdee S, et al. Anti-Retroviral Treatment and Access Study Group Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;19(4):423–431. doi: 10.1097/01.aids.0000161772.51900.eb. [DOI] [PubMed] [Google Scholar]
- 29.Camacho-Gonzalez AF, Wallins A, T Lauren, Murray Ashley, Gaul Zaneta, Sutton Madeline, Gillespie Scott, Leong Traci, Graves Chanda, Chakraborty Rana. Key Elements for Venue-based HIV-testing and Linkage to care for Adolescent and Young Adults in Metropolitan Atlanta. Abstract Presented at the 20th International AIDS Conference; Melbourne Australia. 2014. [Google Scholar]
- 30.Camacho-Gonzalez AF, Wallins A, Toledo L, Murray A, Gaul Z, Sutton MY, et al. Risk Factors for HIV Transmission and Barriers to HIV Disclosure: Metropolitan Atlanta Youth Perspectives. AIDS Patient Care STDS. 2016;30(1):18–24. doi: 10.1089/apc.2015.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller WR, Stephen . Motivational Interviewing- Helping People Change. Third. United States of America: The Guilford Press; 2013. [Google Scholar]
- 32.Biolytical Laboratories. Accesed at http://www.biolytical.com/products/instiHIV.
- 33.Dominguez Rosales R, Albar Marin MJ, Tena Garcia B, Ruiz Perez MT, Garzon Real MJ, Rosado Poveda MA, et al. Effectiveness of the application of therapeutic touch on weight, complications, and length of hospital stay in preterm newborns attended in a neonatal unit. Enferm Clin. 2009;19(1):11–15. doi: 10.1016/j.enfcli.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Maissi E, Ridge K, Treasure J, Chalder T, Roche S, Bartlett J, et al. Nurse-led psychological interventions to improve diabetes control: Assessing competencies. Patient Educ Couns. 2010 doi: 10.1016/j.pec.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 35.Madson MB, Campbell TC. Measures of fidelity in motivational enhancement: a systematic review. J Subst Abuse Treat. 2006;31(1):67–73. doi: 10.1016/j.jsat.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 36.Mugavero MJ, Davila JA, Nevin CR, Giordano TP. From access to engagement: measuring retention in outpatient HIV clinical care. AIDS Patient Care STDS. 2010;24(10):607–613. doi: 10.1089/apc.2010.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National HIV/AIDS Strategy for the United States. The White House. 2015 Updated to 2020. accessed at https://www.aids.gov/federal-resources/national-hiv-aids-strategy/nhas-update.pdf.
- 38.Sprague C, Simon SE. Understanding HIV care delays in the US South and the role of the social-level in HIV care engagement/retention: a qualitative study. Int J Equity Health. 2014;13:28. doi: 10.1186/1475-9276-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral Therapy for the Prevention of HIV-1 Transmission. N Engl J Med. 2016;375(9):830–839. doi: 10.1056/NEJMoa1600693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah M, Risher K, Berry SA, Dowdy DW. The Epidemiologic and Economic Impact of Improving HIV Testing, Linkage, and Retention in Care in the United States. Clin Infect Dis. 2016;62(2):220–229. doi: 10.1093/cid/civ801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


