Abstract
The vascular endothelium is essential to normal vascular homeostasis. Its dysfunction participates in various cardiovascular disorders. The mouse is an important model for cardiovascular disease research. This study demonstrates a simple method to isolate and culture endothelial cells from the mouse aorta without any special equipment. To isolate endothelial cells, the thoracic aorta is quickly removed from the mouse body, and the attached adipose tissue and connective tissue are removed from the aorta. The aorta is cut into 1 mm rings. Each aortic ring is opened and seeded onto a growth factor reduced matrix with the endothelium facing down. The segments are cultured in endothelial cell growth medium for about 4 days. The endothelial sprouting starts as early as day 2. The segments are then removed and the cells are cultured continually until they reach confluence. The endothelial cells are harvested using neutral proteinase and cultured in endothelial cell growth medium for another two passages before being used for experiments. Immunofluorescence staining indicated that after the second passage the majority of cells were double positive for Dil-ac-LDL uptake, Lectin binding, and CD31 staining, the typical characteristics of endothelial cells. It is suggested that cells at the second to third passages are suitable for in vitro and in vivo experiments to study the endothelial biology. Our protocol provides an effective means of identifying specific cellular and molecular mechanisms in endothelial cell physiopathology.
Keywords: Cellular Biology, Issue 118, Primary culture, Mouse, Aorta, Segment attachment, Endothelial cells, Endothelial sprouting
Introduction
The vascular endothelium is not only a barrier layer that separates blood and tissue, it is considered a vast endocrine gland that stretches over the entire vascular tree with a surface area of 400 square meters1. The well-being of the endothelium is essential to vascular homeostasis. The dysfunctional endothelium participates in various cardiovascular disorders, including atherosclerosis, vasculitis and ischemia/reperfusion injuries, etc. 2–4. To date, the specific cellular and molecular mechanisms involved in these disease settings are not well understood due to the diffused anatomic nature of endothelium.
The mouse is an important model for research because genetic manipulation techniques are more developed in mice than in any other mammalian species. However, the isolation of primary murine aortic endothelial cells is considered particularly difficult because the small size of the aorta makes enzymatic digestion of endothelium impractical. Some reported procedures to isolate and purify ECs require 5–7.
The goal of this protocol is to use a simple method to isolate and expand endothelial cells from the mouse aorta without using any special equipment. In this protocol, the freshly isolated aorta is cut into small segments and seeded onto a matrix with the endothelium facing down to allow for endothelial sprouting. After segments are removed, endothelial cells are expanded in endothelium-favored medium and are ready for experiments after two or three passages. The advantages of the described method are that: 1) considerably high numbers of endothelial cells are harvested from a single aorta; 2) cell viability is well preserved; and 3) no special equipment or technique is needed. It provides an effective means of identifying specific cellular and molecular mechanisms in endothelial cell pathophysiology. For those who are interested in studying primary cultured endothelial cells from either gene knock-out mice, gene knock-in mice, or a murine disease model, this protocol is very useful and easy to practice.
Protocol
1. Isolation of Aorta from Mice
All the procedures described here were approved by the Institutional Animal Care and Use Committee of Wayne State University.
Put the mouse into the induction chamber of the anesthesia machine. Set the isoflurane flow to 4% in conjunction with 25% fresh air and 75% O2. Anesthetize the mouse by inhalation of isoflurane (4% for induction, ± 1.0% for maintenance) in conjunction with 25% fresh air and 75% O2 via induction chamber followed by a dedicated nose cone. NOTE: Anesthesia with isoflurane can be delivered in 75% oxygen/25% air or in 100% oxygen.
Check the mouse every 5 min until the appropriate level of anesthesia is reached (lack of withdrawal to toe pinch).
Take the mouse out of the induction chamber and continue isoflurane inhalation though the dedicated nose cone that is connected to the anesthesia machine. Switch the isoflurane flow to 1% to maintain anesthesia.
While it is under anesthesia, put the mouse onto a surgery pad, positioned on its back. Use laboratory tape to secure the limbs.
Use a heating lamp to keep the mouse warm. Keep the lamp in an appropriate distance from the mouse so that the mouse will not over-heat.
Spray the chest with 70% ethanol.
Use dissection scissors to open the abdomen from the midline, and expose the abdominal aorta. Open the chest cavity and expose the heart and lungs.
Cut the abdominal aorta at the middle with dissection scissors to release the blood.
Fill a 1 mL syringe (with 25 G needle) with 1 mL of PBS containing 1,000 U/mL of heparin. Inject PBS containing 1,000 U/mL of heparin to the left ventricle and perfuse the aorta. Push the heart and the lung with forceps at the great arteries to the right side of the mice to expose the thoracic aorta.
Quickly remove the thoracic aorta using micro-dissection forceps and put it in ice-cold 1x PBS (sterile), then transport the container into a laminar airflow hood.
Insert a 1 mL syringe fitted with 25 G needle into one end of the aorta, and gently flush the aorta with ice-cold PBS to remove the blood.
Use micro-dissection forceps to remove as much of the attached adipose tissue and small lateral vessels as possible.
Immediately transfer the aorta to endothelial growth medium.
Cut the aorta into 1 mm rings using a sterile scalpel blade. Harvest about 8 – 10 rings per aorta.
Open each aortic ring using a pair of micro-dissection scissors.
2. Seed the Aortic Segments on Matrix
When not in use, keep the growth factor-reduced matrix in −20 °C to prevent solidification. Put the growth factor-reduced matrix in 4 °C at least overnight to allow a complete thaw.
Precool a 6-well plate and pipet tips to −20 °C for at least 10 min. Put the 6-well plate on ice and coat one well of the plate with 1 mL of matrix without introducing any air bubbles. Place the plate in a 37 °C incubator for 20 min to allow the matrix to solidify.
Implant the aortic pieces onto the solidified matrix using sterile micro-dissection forceps. Place the pieces lumen-side-down on the matrix without touching the endothelium. Place 3 – 4 aortic segments close to each other on the matrix.
Add just enough endothelial cell growth medium to keep segments wet (~ 200 μL).
Incubate the plate at 37 °C under 5% CO2 for 4 to 6 h. At the end of the day, add just enough medium to cover the aortic segments.
Observe the aortic segment sprouting periodically under a phase-contrast microscope. Check medium level and cell growth each day. Add medium if necessary to keep aortic segments covered.
On the 4th day, gently remove the medium and remove the aortic segments from the matrix using a sterile needle without interrupting the growing endothelial cells.
Add 2 mL of new endothelial cell growth medium and allow the endothelial cells continue to proliferate on matrix for 2 – 3 days.
3. Initial Passaging of the Mouse Aortic Endothelial Cells
Coat a new T12.5 flask with gelatin (0.1%), incubate at 37 °C for 30 min.
Wash the matrix plates carefully with sterile 37 °C PBS.
Add 2 mL of neutral proteinase (50 U/mL) to the matrix plates and incubate at room temperature on platform rocker with occasional shaking. Check the cells under a phase-contrast microscope to make sure the majority of cells are detached.
Add 2 mL of D-Val to inactivate the neutral proteinase.
Collect the supernatant carefully in a 15 mL centrifuge tube. Wash the plate with 2 mL D-Val and collect the supernatant.
Centrifuge the cell suspension at 900 × g for 5 min at room temperature.
Re-suspend the cell pellet in 4 mL of endothelial cell growth medium and plate in the T12.5 flask coated with gelatin. Incubate the cells at 37°C, 5% CO2 for 2 h.
Replace the medium. Incubate the cells at 37 °C, 5% CO2 until they are 85% – 90% confluent.
4. Passaging of the Mouse Aortic Endothelial Cells
Coat two new T12.5 flasks with gelatin (0.1%), incubate at 37 °C for 30 min. Pre-heat Trypsin-EDTA (0.25% Trypsin, 0.02 EDTA in PBS) and sterile PBS at 37 °C for about 15 min.
Wash the cells carefully with sterile 37 °C PBS.
Add 0.5 mL of Trypsin-EDTA (0.25% Trypsin, 0.02 EDTA in PBS) and incubate at 37 °C for ~ 1 min or until the majority of the cells turn round.
Add 2 mL of endothelial cell growth medium to stop the digestion. Pipette the cell suspension up and down within the flask several times. If necessary, use a cell scraper to collect the cells.
Collect the cell suspension in a 15 mL centrifuge tube. Centrifuge the cell suspension at 900 × g for 5 min at room temperature.
Re-suspend the cell pellet in 4 mL of endothelial cell growth medium and plate in 2 T12.5 flasks coated with gelatin. Incubate the cells at 37°C, 5% CO2 for 2 h.
Replace the medium. Incubate the cells at 37 °C, 5% CO2 until they are 85% – 90% confluent.
Use the mouse aortic endothelial cells after 2 – 3 passages.
5. Characterization of the Mouse Aortic Endothelial Cells
-
Re-plate the cells.
Coat a 6-well plate with gelatin (0.1%), incubate at 37 °C for 30 min. Rinse the plate with PBS.
Pre-heat the Trypsin-EDTA and sterile PBS to 37 °C for about 15 min.
Wash the cells with sterile PBS 3 times. Add 0.5 mL of Trypsin/EDTA to the cells, incubate for ~1 min at 37 °C or until the majority of the cells turn round.
Add 2 mL of endothelial cell growth medium to stop the digestion. Pipette the cell suspension up and down within the flask several times. If necessary, use a cell scraper to collect the cells.
Collect the cell suspension in a 15 mL centrifuge tube. Centrifuge the cell suspension at 900 × g for 5 min at room temperature.
Discard the supernatant, re-suspend the cells in endothelial cell growth medium. Seed the cells in 6-well plate coated with gelatin at the density of 3 × 105/well.
Incubate the cells overnight at 37 °C, 5% CO2 to allow the cells to attach to the culture surface.
-
Dil-ac-LDL uptaken and Ulex-lectin binding.
Store the 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine perchlorate-labeled acetylated LDL (Dil-ac-LDL) at −20 °C. Before staining, thaw Dil-ac-LDL on ice. The stock Dil-ac-LDL solution is 1 mg/mL. Store the FITC-labeled Ulex europaeus agglutinin (Ulex-Lectin, 1 μg/mL) at 4 °C. The stock Ulex-Lectin solution is 1 mg/mL. Store Hoechst 33342 at 4 °C. The stock Hoechst 33342 solution is 100 μg/mL.
Add 10 μL of Dil-ac-LDL stock solution to 1 mL of culture medium to make a final concentration of 10 μg/mL.
Prior to staining, remove the old medium and wash the cells in each well with new medium.
Remove the new medium and add the Dil-ac-LDL staining medium.
Incubate the cells at 37 °C, 5% CO2 for 1 h.
Wash the cells with sterile 1x PBS 3 times.
Add 1 mL of 10% formalin to fix the cells. Put the plate in room temperature for about 30 min.
Wash the cells with sterile 1x PBS 3 times. Avoid light.
Add 1 mL sterile 1x PBS that contains 10 μL of Ulex-Lectin.
Incubate the cells at room temperature in the dark for 1 h.
Remove the Ulex-Lectin by washing the cells with sterile 1x PBS 3 times.
Add Hoechst 33342 to final concentration of 1 μg/mL, incubate 10 min in the dark.
View the fluorescence of Dil-ac-LDL (red, excitation wavelength of 576 nm), Ulex-Lectin (green, excitation wavelength of 519 nm) and Hoechst (blue, excitation wavelength of 361 nm) with an inverted fluorescent microscope.
-
Fluorescent staining for CD31, VEGFR2, eNOS, VE-Cadherin and Calponin.
Store FITC-conjugated anti-mouse CD31 antibody, eNOS antibody, VE-Cadherin antibody, FITC-conjugated anti-rabbit IgG at 4 °C in the dark. Store VEGFR2 antibody in −20 °C.
Wash the cells with sterile 1x PBS 3 times.
Add 1 mL of 10% formalin to fix the cells. Put the plate in room temperature for about 30 min.
Wash the cells with sterile 1x PBS 3 times.
To stain CD31, add 1 mL of sterile 1x PBS that contains 10 μL of CD31-FITC antibody. To stain either VEGFR2, eNOS, VE-Cadherin or calponin, add 1 mL of sterile 1x PBS that contains 4 μL of VEGFR2, eNOS, VE-Cadherin or calponin primary antibody, respectively.
Incubate the cells on ice for 1 h in the dark.
Remove the antibodies by washing the cells with sterile PBS 3 times.
For the staining of VEGFR2, eNOS, VE-Cadherin or calponin, add 1 mL of sterile 1x PBS that contains 10 μL of FITC-conjugated anti-rabbit IgG. Incubate the cells on ice for 1 h in the dark. Remove the IgG by washing the cells with sterile PBS 3 times.
Add Hoechst 33342 to final concentration of 1 μg/mL, incubate 10 min in the dark.
View the fluorescence of CD31, VEGFR2, eNOS, VE-Cadherin, calponin (green, excitation wavelength of 518–535 nm) and Hoechst (blue, excitation wavelength of 361 nm) with an inverted fluorescent microscope.
Representative Results
Endothelial Cell Sprouting
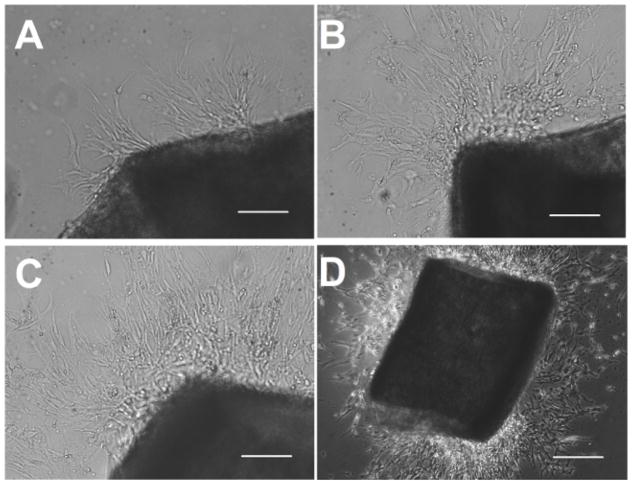
Spontaneous endothelial cell sprouting started from a mouse aorta segment. The mouse aorta segment was allowed to grow on a growth factor-reduced matrix then in endothelial cell growth medium for 4 days. The endothelial cell sprouting usually appears in 2 – 4 days. Photomicrographs were taken on day 4 (Figure 1). As shown in the pictures, numerous endothelial cells migrate away from the segment. The newly formed sprouts continue to extend from the segment and the branch.
Figure 1. Endothelial Cell Sprouting.
Mouse aortic segment was seeded onto growth factor-reduced matrix and cultured in endothelial cell growth medium. Endothelial cell sprouting started as early as on day 2 (A, bar = 100 μm) and increased in the following 2 – 3 days (B, C, bar = 100 μm). The segment was removed on day 4 (D, bar = 500 μm).
Endothelial Cell Phenotypes
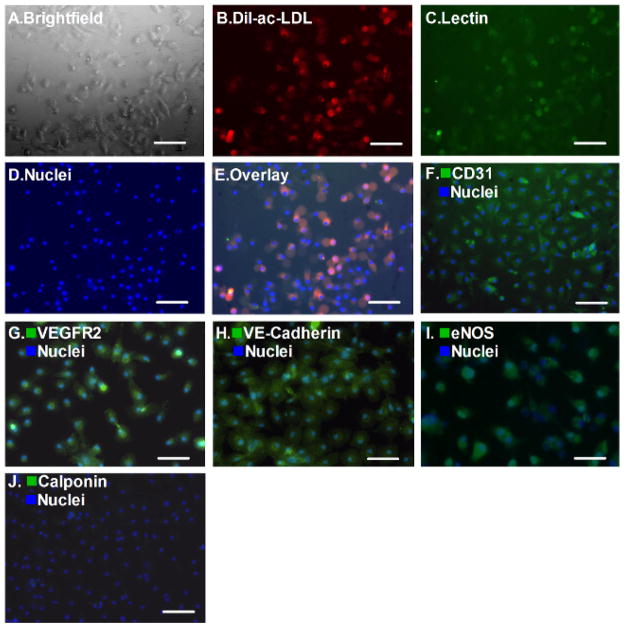
These cells demonstrated spindle-shaped and cobblestone-like appearances after initial passage (Figure 2A). The attached cells were labeled with Dil-ac-LDL and Ulex-Lectin for one hour. As shown in Figures 2B–2E, most of the cells were double-positive for Dil-ac-LDL uptake (red) and Ulex-Lectin binding (green). Meanwhile, after the second passage, more than 95% of the cells were positive for platelet endothelial cell adhesion molecule 1 (CD31, PECAM-1, Figure 2F), VEGFR2 (Figure 2G), VE-Cadherin (Figure 2H), eNOS (Figure 2I) but negative for Calponin (Figure 2J) which is a smooth muscle cell marker.
Figure 2. Endothelial Cell Phenotypes.
The attached cells display spindle-shaped and cobblestone-like appearances (A, bar = 100 μm), double-positive fluorescence of Dil-ac-LDL and Ulex-Lectin (B–E, bar = 100 μm). Meanwhile, platelet endothelial cell adhesion molecule 1 (CD31, PECAM-1), VEGFR2, VE-Cadherin, eNOS were positive in >95% of the cells after the second passage (F–I, bar = 100 μm) Most of the cells were negative for Calponin staining, which is a smooth muscle cell marker (J, bar = 100 μm).
Discussion
This study demonstrates a simple method to isolate and culture endothelial cells from a mouse aorta without any special equipment. The immunofluorescence staining indicated that the majority of cells were endothelial cells after the second passage. It is suggested that cells at second to third passage are suitable for in vitro and in vivo experiments to study endothelial biology.
The Key Notes from the Present Protocol
There are five critical points in the procedure. First, the vascular lumen is flushed with PBS containing heparin to minimize endothelial cell activation and clot formation. Second, the time between cardiac arrest and the seeding of aortic segment onto matrix is critical to endothelium viability. While clearing the peri-arterial adipose tissue and connective tissue, avoid stretching the aorta and limit the time spent on this step to 10 – 15 min. Third, pour just enough media to cover the segments after they are seeded. Too much media will cause the aortic segments to float. Fourth, the culture medium contains a high concentration of endothelial cell growth supplement, making endothelial cells grow much faster than when cultured in endothelial growth medium-2. The outgrowth of endothelial cells will suppress other cell types such as smooth muscle cells and fibroblasts. Fifth, the timing to remove aortic segment from matrix is also critical to the purity of endothelial cells. This step prevents contamination by fibroblasts and smooth muscle cells. The segment should be removed before the development of the tube network. Delayed removal of aortic segment will result in contamination of other cell types such as fibroblasts or smooth muscle cells. Please note that this approach is also used to study angiogenesis in vitro and the formation of capillary like structures indicates angiogenesis capacity of the aorta segment. Based on our experience, if the segments are removed after the capillary-like structure fully develops, the incidence of smooth muscle cells contamination increases dramatically. Therefore, we feel that the best time point to remove the segment is when the network starts to be visible but is not fully developed. In this way, we are able to harvest as many endothelial cells as possible while keeping contamination by smooth muscle cells to a minimum.
The Limitations of the Present Protocol
There are some limitations of the described methods. First, there are chances of contamination of fibroblasts and smooth muscle cells, if the aortic segments are not removed before tube networks develop. The fibroblasts or smooth muscle cells may start to attach to the matrix and proliferate 3 to 5 days after seeding. Therefore, always remove the aortic segments in a timely manner. As a secondary precaution, changing the medium 2 hours after re-plating the cells onto a new flask also helps to eliminate the possible contamination of fibroblasts and smooth muscle cells. Second, these cells are cultured in vitro for 7 – 10 days after isolation. It is possible that their phenotypes may be different from freshly isolated endothelial cells. For example, if the endothelial cells from a mouse model of hyperlipidemia are cultured in endothelial cell growth medium without a high concentration of lipids, they are not exposed to the hyperlipidemic environment as they were in animals. The cultured endothelial cells may behave differently from freshly isolated endothelial cells8. This problem exists in all in vitro cultured primary cells and cell lines. Adding the pathological stimuli into the culture medium to mimic the in vivo environment may be a solution.
Endothelial Cells Demonstrate Different Phenotypes in Different Vascular Branches
The vascular system is a hierarchical structure composed of arteries, veins, and capillaries. The heterogeneity of endothelial cells that reside in the specific ‘zones’ of vasculature plays a large part in creating functional diversity in the vascular system9,10. Even in a single vascular bed, such as the aorta, endothelial heterogeneity exists. Laminar blood flow with high shear stress in the straight part of the aorta induces endothelial nitric oxide synthase and thrombomodulin11. Therefore, endothelial cells that resides in the straight part of the aorta demonstrate anti-coagulant, anti-adhesive, and anti-inflammatory properties12,13. In contrast, turbulent blood flow at areas where arteries branch or turn sharply (aortic arch) reduces endothelial nitric oxide synthase expression and induces atherogenic genes in endothelial cells, i.e., the monocyte chemotactic protein-1 (MCP-1), and platelet-derived growth factors (PDGFs), resulting in a pro-inflammatory and atherosclerotic endothelial phenotype6. In addition, endothelial cells in the vessels of each organ undergo anatomic and functional adaptive changes that are specific to that organ’s functions14,15. Nonetheless, there is a consensus that several cell surface markers, functional genes and cell activities can be used to characterize endothelial cell lineage. These cell surface markers include Platelet endothelial cell adhesion molecule (PCAM-1, CD31), von Willebrand factor (vWF), Vascular Endothelial-Cadherin (VE-Cadherin, CD144). The most frequently used functional genes include endothelial nitric oxide synthase (eNOS) and Vascular Endothelial Growth Factor Receptor (VEGFR). The cell activities that are usually seen in endothelial cell lineage include uptake of Dil-ad-LDL and binding Lectin, which forms cord-like structures on the matrix.
The Significance and Future Applications of Primary Cultured Mouse Aortic Endothelial Cells
The method described here is used to study macrovascular endothelial cells. The significance of this protocol is that it provides a great opportunity to study the endothelial-specific activities of targeted molecules and can be done in knockout and transgenic mouse models, making it very useful in cardiovascular research. The ability to grow high numbers of mouse aortic endothelial cells under defined conditions makes this ideal technique to better define the phenotypes and functions of endothelium. This technique improves the practicality of testing the prospective potential of endothelial cell-based therapy in murine models, through either intravenous injection or engraftment of endothelial cells.
Acknowledgments
This study is supported by American Heart Association Scientist Development Grant 13SDG16930098 and the National Science Foundation of China Youth Award 81300240 (PI: Wang). We thank Roberto Mendez from Wayne State University for assisting in the preparation of the manuscript.
Footnotes
Video Link
The video component of this article can be found at http://www.jove.com/video/52965/
Disclosures
The authors have nothing to disclose.
References
- 1.Simionescu M. Implications of early structural-functional changes in the endothelium for vascular disease. Arteriosclerosis, thrombosis, and vascular biology. 2007;27:266–274. doi: 10.1161/01.ATV.0000253884.13901.e4. [DOI] [PubMed] [Google Scholar]
- 2.Cid MC, Segarra M, Garcia-Martinez A, Hernandez-Rodriguez J. Endothelial cells, antineutrophil cytoplasmic antibodies, and cytokines in the pathogenesis of systemic vasculitis. Current rheumatology reports. 2004;6:184–194. doi: 10.1007/s11926-004-0067-3. [DOI] [PubMed] [Google Scholar]
- 3.Wang JM, et al. C-Reactive protein-induced endothelial microparticle generation in HUVECs is related to BH4-dependent NO formation. Journal of vascular research. 2007;44:241–248. doi: 10.1159/000100558. [DOI] [PubMed] [Google Scholar]
- 4.Wang JM, et al. Increased circulating CD31+/CD42− microparticles are associated with impaired systemic artery elasticity in healthy subjects. American journal of hypertension. 2007;20:957–964. doi: 10.1016/j.amjhyper.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Mackay LS, et al. Isolation and characterisation of human pulmonary microvascular endothelial cells from patients with severe emphysema. Respiratory research. 2013;14:23. doi: 10.1186/1465-9921-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marelli-Berg FM, Peek E, Lidington EA, Stauss HJ, Lechler RI. Isolation of endothelial cells from murine tissue. Journal of immunological methods. 2000;244:205–215. doi: 10.1016/s0022-1759(00)00258-1. [DOI] [PubMed] [Google Scholar]
- 7.Bowden RA, et al. Role of alpha4 integrin and VCAM-1 in CD18-independent neutrophil migration across mouse cardiac endothelium. Circulation research. 2002;90:562–569. doi: 10.1161/01.res.0000013835.53611.97. [DOI] [PubMed] [Google Scholar]
- 8.Lu Y, et al. Palmitate induces apoptosis in mouse aortic endothelial cells and endothelial dysfunction in mice fed high-calorie and high-cholesterol diets. Life sciences. 2013;92:1165–1173. doi: 10.1016/j.lfs.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Atkins GB, Jain MK, Hamik A. Endothelial differentiation: molecular mechanisms of specification and heterogeneity. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:1476–1484. doi: 10.1161/ATVBAHA.111.228999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aitsebaomo J, Portbury AL, Schisler JC, Patterson C. Brothers and sisters: molecular insights into arterial-venous heterogeneity. Circulation research. 2008;103:929–939. doi: 10.1161/CIRCRESAHA.108.184937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimamoto T. Drugs and foods on contraction of endothelial cells as a key mechanism in atherogenesis and treatment of atherosclerosis with endothelial-cell relaxants (cyclic AMP phosphodiesterase inhibitors) Advances in experimental medicine and biology. 1975;60:77–105. doi: 10.1007/978-1-4615-9029-3_6. [DOI] [PubMed] [Google Scholar]
- 12.Chiu JJ, Chien S. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiological reviews. 2011;91:327–387. doi: 10.1152/physrev.00047.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gimbrone MA, Jr, Topper JN, Nagel T, Anderson KR, Garcia-Cardena G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Annals of the New York Academy of Sciences. 2000;902:230–239. doi: 10.1111/j.1749-6632.2000.tb06318.x. discussion 239–240. [DOI] [PubMed] [Google Scholar]
- 14.Rostgaard J, Qvortrup K. Electron microscopic demonstrations of filamentous molecular sieve plugs in capillary fenestrae. Microvascular research. 1997;53:1–13. doi: 10.1006/mvre.1996.1987. [DOI] [PubMed] [Google Scholar]
- 15.Brutsaert DL. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiological reviews. 2003;83:59–115. doi: 10.1152/physrev.00017.2002. [DOI] [PubMed] [Google Scholar]