Abstract
The Psychiatric Genomics Consortium-Posttraumatic Stress Disorder group (PGC-PTSD) combined genome-wide case-control molecular genetic data across eleven multi-ethnic studies to quantify PTSD heritability, to examine potential shared genetic risk with schizophrenia, bipolar disorder, and major depressive disorder, and to identify risk loci for PTSD. Examining 20 730 individuals, we report a molecular genetics-based heritability estimate (h2SNP) for European American females of 29%, which is similar to h2SNP for schizophrenia and is substantially higher than h2SNP in European American males (estimate not distinguishable from zero). We found strong evidence of overlapping genetic risk between PTSD and schizophrenia along with more modest evidence of overlap with bipolar and major depressive disorder. No SNPs exceeded genome-wide significance in the trans-ethnic (overall) meta-analysis and we do not replicate previously reported associations. Still, SNP-level summary statistics made available here afford the best-available molecular genetic index of PTSD – for both European and African American individuals – and can be used in polygenic risk prediction and genetic correlation studies of diverse phenotypes. Publication of summary statistics for approximately 10 000 African Americans contributes to the broader goal of increased ancestral diversity in genomic data resources. In sum, the results demonstrate genetic influences on the development of PTSD, identify shared genetic risk between PTSD and other psychiatric disorders, and highlight the importance of multi-ethnic/racial samples. As has been the case with schizophrenia and other complex genetic disorders, larger sample sizes are needed to identify specific risk loci.
Introduction
Posttraumatic Stress Disorder (PTSD) is a common and debilitating mental disorder that occurs in some persons following a traumatic event. It includes symptoms such as re-experiencing the event, avoidance of event-related stimuli, and chronic hyperarousal. In the U.S., one in nine women and one in twenty men will meet criteria for the diagnosis at some point in their lives.1 The societal impact of PTSD is large, with increased rates of suicide, hospitalization, and substance use.2 Regarding etiology, the role of nature versus nurture in response to traumatic events has been debated for over a century.3 Psychiatrists who treated “shell shock” in soldiers returning from World War I argued over whether soldiers who succumbed to the stressors of war were “moral invalids” or whether such breakdowns could occur in any man who was “buried in a trench or saw his friend’s brains scattered before him”.4 This historical controversy has been reignited as the age of genomic medicine has reached PTSD. Genomic research is given the highest priority in the U.S. National Research Action Plan on PTSD that was established in response to an executive order by President Obama aimed at improving mental health care and services for veterans, military service members, and their families.5 However, fundamental questions remain as to the role of genetic factors in PTSD etiology.
Twin study estimates of PTSD heritability range from 24–72% following trauma,6–9 with female heritability two to three times higher than that in males.8 Twin studies have also documented that genetic influences on PTSD are shared with other mental disorders, particularly major depression.10–12 Such studies have made important contributions beyond heritability estimation, e.g. the discordant twin study by Gilbertson et al. showed that smaller hippocampal volume is likely a risk factor for PTSD rather than a consequence of the disorder.13 Despite this excellent work, the importance of genetic influences for PTSD etiology is not universally accepted amongst mental health clinicians or researchers. This may be in part because the latent modeling of genetic variation in twin studies is not easily interpretable to those not immersed in the methodology of behavioral genetics.
To more definitively establish the contribution of genetic variants to PTSD risk, to examine the genetic overlap between PTSD and other mental disorders, and to identify risk loci, the PGC-PTSD group employed the consortium science approach of aggregating genomic (GWAS) data from multiple contributing groups. In recent years, the consortium approach has delivered groundbreaking results for many complex genetic phenotypes – including height,14 diabetes,15 and schizophrenia16 – and here we report initial findings on the genetic architecture of PTSD.
Materials and Methods
Overview
Eleven groups contributed data for analysis; nine contributed individual level data and two groups could only share SNP-level summary statistics. These two groups implemented the quality control (QC) and GWAS analyses outlined below, prior to sharing summary statistics. Individual level genotypes are necessary for genome-wide complex trait analysis (GCTA) and polygenic risk scoring (PRS), so these two datasets are not included in the polygenic analyses. For all datasets, the first analytical step was ancestry assignment. As described below, this yielded nineteen ancestry-assigned datasets for analysis across the eleven contributing studies. Next QC procedures and imputation were performed and then single SNP (GWA and meta-analyses) and polygenic analyses (GCTA, PRS, LD score regression = LDSC) were completed.
Participants
All participants were adults. Contributing studies provided individual-level genotype data or summary statistics consistent with their IRB approved protocols. Descriptions of each of the 11 contributing studies are provided in the Supplementary Text. In many cohorts included in the consortium, all the controls were trauma exposed (e.g. Nurses Health Study II PTSD sub-study). Thus, in the combined analysis, the vast majority of controls were trauma exposed (N controls = 15,548, N trauma exposed controls = 13,638, 87.7% of controls were trauma exposed). Case and control numbers along with other dataset information is provided in Supplementary Table S1, and Figure 1 illustrates the study and analytical design.
Figure 1. Study design for Phase 1 PGC-PTSD: A) single variant GWAS and meta-analyses B) polygenic analyses.
A) Each of the 19 gray boxes represents one ancestry-assigned dataset/GWAS. Within-ancestry meta-analyses were followed by the trans-ethnic meta-analysis. B) Blue boxes denote polygenic analyses. LDSC could not be applied to the AA subsample. Latino/Hispanic (LA) and South African samples were deemed too small for polygenic analyses.
Ancestry assignment
SNPweights software17 was used to assign ancestry, applied to uncleaned data because the use of Hardy-Weinberg equilibrium (HWE) filters can remove ancestry informative SNPs in samples with mixed ancestry background, and is therefore not compatible with this protocol. Ancestry assignment is separate from controlling for ancestry analytically, described below. Briefly, SNPweights uses external genomic reference panels to derive ancestry informative weights for individual SNPs, hence SNPweights. We used the four-group, continental ancestry panel to quantify individual participants’ proportions of: African (YRI), European (CEU), Asian (ASI), and Native American (NAT)17 ancestry. The specific cutoffs implemented in this report for assigning ancestry – after inspection of PC plots and adjusting cutoffs – were as follows: European American (EA) was defined as having ≥ 90% European ancestry. African American (AA) was defined as ≥ 90% for the combination of African and European ancestry, and also < 3% Asian and Native American ancestry. Individuals that self-identify as Latino or Hispanic (LA) in the US may have predominately European ancestry and may have relatively recent admixture with Native American ancestry or both Native American and African ancestry. In this report we focused on Latino/Hispanic individuals with two-way admixture; cutoffs used were >= 85% for the combination of European and Native American ancestry, < 10% African ancestry, and <3% Asian Ancestry. With exception of the South African data (see below), individuals not falling into the EA, AA, and LA categories were excluded from analysis (on the basis of low case and/or control numbers).
Data contributed from South Africa were handled separately because the African ancestry panel used in SNPweights (YRI) is a West African sample. For the South African sample, the typical GWAS method of conducting principal components analysis, followed by visual inspection of data, was used. In doing so, we identified two populations, i.e., the two South African datasets in this report. For comparison of PGC-PTSD ancestry to previously published meta-analyses of psychiatric phenotypes see Figure 2A. Recent admixture is evident for AA and Latino/Hispanic participants in this study (Figure 2B).
Figure 2. Ancestral composition for Phase 1 of PGC-PTSD and principal components plot of individuals’ data.
A) PGC-PTSD (left), compared to the largest psychiatric meta-analyses (center), and estimated world ancestry (right). B) Plot of first two principal components and assigned ancestry according to the protocol described in the text. Each dot is one person.
QC methods, relatedness testing, imputation
QC procedures were performed sequentially on each of the 19 data subsets as follows: monomorphic SNPs and SNPs with missingness > 0.05 removed, individuals with missingness > 0.02 removed. Individuals with FHET > |0.2| and individuals failing sex checks removed. SNPs with missingness >0.02 removed (a more stringent SNP missingness filter was applied after individual level filters). SNPs with differential missingness between cases and controls >0.02 removed. SNPs failing HWE: controls (p<1×10−6) and cases (p<1×10−10) removed. All analyses were performed using second generation PLINK.18
Principal components analysis (PCA) was performed within each dataset and then across all datasets using FastPCA.19 PCA was conducted on high quality SNPs with low linkage disequilibrium (LD) passing filters: SNP directly genotyped in all datasets; minor allele frequency (MAF) > 0.05; HWE p >1×10−4; not strand ambiguous (AT or GC SNPs); not in high LD region (MHC chr6:25–35Mb, chr8 inversion chr8:7–13Mb); and r2 between SNPs <0.2 (i.e., the PLINK option: ‘--indep-pairwise 200 100 0.2’, applied twice). Within each dataset, scatterplots of principal components were visually examined and outliers removed. This process was repeated until cases and controls appeared evenly interspersed across all PC pairs.
Imputation to the 1000 Genomes20 phase 1 reference was performed within the PGC pipeline16 using SHAPEIT for phasing21 and IMPUTE2 for imputation.22 Imputation was performed with a chunk size of 3 Mb with default parameters on the full set of 2,186 phased haplotypes (August 2012, 30,069,288 variants, release “v3.macGT1”). Samples were then combined (within ancestry groups) for relatedness testing and calculation of principal component covariates. The same filters as above were employed and we removed one individual from each pair of related or duplicate individuals (pi-hat value >0.2), preferentially retaining cases.
Single variant analyses, gene, and pathway analyses
Single variant analysis (GWAS within each of the 19 data subsets) was performed using an additive model in PLINK, with the first ten principal components as covariates, on dosage data. Fixed-effects meta-analysis was accomplished using METAL23, 24 with inverse variance weighting. Plotting was performed in R.25 Analyses were completed with both study specific PCs and with PCs computed within each ancestry group (‘generic’ PCs for AA, EA, LA and separately the two S. African datasets), with similar results. Final results in Manhattan, QQ plot, top hits tables, and online supplements use study-specific PCs.
Gene and pathway analyses were completed using MAGMA (Multi-marker Analysis of GenoMic Annotation)26 and default parameters as in the manual (version 1.06). The main gene and pathway analyses (as we defined them) were conducted on the 16 datasets with cleaned genotype data (versus SNP-level p-values) so that we could control for ancestry using PCs. This was particularly important for the AA, LA, and SA samples given poorer external sequence data resources, which are needed for the option that uses summary statistics. Thus, we performed gene-based analyses on each of the 16 datasets with genotype data and PCs, followed by pathway analyses on the gene level results (as per MAGMA procedures). For completeness we also then used the summary statistic method on the three remaining datasets for which raw data were not available, and meta-analyzed (at the gene level, per MAGMA procedures) with the other 16 datasets. Like de Leeuw et al (2015), we used the MSigDB Canonical Pathways because this list contains a wide variety of gene sets, drawn from different gene-set databases, thus providing results that are not overly dependent on the choice of a narrow set of gene sets.
SNP-chip heritability estimation with Genome-wide Complex Trait Analysis (GCTA)
GCTA27–29 was used to estimate SNP-chip heritability (h2SNP) in the EA and AA subsamples (separately) as follows: Following QC, datasets were combined using PLINK18, 30 (i.e. the 7 EA bed/bim/fam file sets were combined into one EA7 bed/bim/fam file set). Genetic relationship matrices (GRMs) were made (one chromosome at a time for computational efficiency) using‘--chr n’ for chromosomes 1–22, ‘--maf 0.01’ to restrict to SNPs with MAF > 1%, ‘--make-grm-bin’ to make the GRMs, and then combined with ‘--mgrm' command. Heritability estimation with ‘--reml-no-constrain’ command, specifying a GRM with ‘--grm-bin’, a phenotype file with ‘--pheno’, prevalence with ‘--prevalence’ quantitative covariates (here, PCs 1–10) with ‘--qcovar' and binary covariates (here, sex and study indicator covariates) with ‘--covar'. Prevalence specified as 11% (females), 5% (males), and 8% (combined).
Polygenic risk scoring (PRS)
PRS was conducted using PLINK18, 30 for the three major adult psychiatric disorders (SCZ16, BIP31, MDD32) for which GWAS results from large studies are publicly available. SNP level summary statistics from each of the three ‘discovery’ disorders were used to create the ‘score’ files. Each individual in this study was scored for genetic risk by weighting risk alleles according to the natural log of the odds ratio from each of the discovery disorder meta-analyses. The following commands were used to specify the bed/bim/fam fileset from this study to be scored (--bfile), specify the file with logORs (--score), stop the default behavior of mean imputation (no-mean-imputation), specify the file with p-values from the discovery disorder (--q-score-file) and provide specified ranges of p-values to be scored (--q-score-range). See Supplementary Table S2 for 38 p-value bins (twelve bins per disorder plus one additional bin for genome-wide significant loci for both SCZ and BIP). After scoring, the significance and magnitude of polygenic PTSD prediction was calculated for each of the 38 p-value bins. Two logistic regressions were run. The first regressed PTSD on polygenic risk score, ten principal components, and study indicator covariates. The second regression was the same as the first, but with the polygenic risk score term removed. Nagelkerke’s r2 was calculated for both models, and the difference was the r2 for the polygenic risk score term.
SNP-chip heritability and genetic correlation estimation with Linkage Disequilibrium Score regression (LDSC)
LDSC33, 34 was used on the SNP level summary statistics from the 7 EA datasets for which raw genotype data were available. With raw data we could rule out population stratification and the presence of related individuals, and consequently the constrained version of LDSC could be used, affording greater power for heritability estimation. By constraining the LDSC regression intercept to be 1 (i.e. the expected chi-square for a single SNP with LD score equal to zero and with no influence from population stratification), there is one less parameter to estimate in the LDSC regression and standard error of the heritability estimate is reduced. For genetic correlation, we report both constrained and unconstrained results. As with GCTA, we use population prevalence estimates of 11% (female), 5% (male), and 8% (combined). Separate male and female heritability estimates were calculated using sex specific subsamples of the data. General instructions for LDSC are provided here: https://github.com/bulik/ldsc
Results
Single variant (GWAS and meta-analyses), gene, and pathway analyses
No variants achieved genome-wide significance in either the trans-ethnic or EA meta-analyses. For Manhattan plots, QQ plots, and top hits tables for each of the three meta-analyses, see Supplementary Figures S1–S2 and Supplementary Tables S3–S5. Though not currently informative about individual risk loci, summary statistics are useful for polygenic predictions and cross-disorder analyses, and are available for download: www.med.unc.edu/pgc.
In the AA meta-analysis, one variant on chromosome 13 exceeded genome-wide significance, (rs139558732, OR=2.19, p=3.33×10−8). This SNP and nearby variants were not present for analysis in the EA and Latino/Hispanic studies due to low frequency (MAF <=1%) in those populations. The variant was present in the two South African datasets, and was no longer genome-wide significant in the trans-ethnic meta-analysis (OR=2.05, p=1.31×10−07, only AA and S. African datasets contributing data). To further investigate the possibility that this chromosome 13 locus, in the KLHL1 gene, (see Supplementary Figure S3 for regional plot in AA meta-analysis) was associated with PTSD in AA individuals, we requested data from the Army STARRS consortium, and meta-analyzed results from their data and ours.36 Doing so also resulted in loss of genome wide significance for rs139558732 (OR=1.90, p=1.0×10−06). Thus, despite nominally achieving genome-wide significance in the AA meta-analyses, we do not report rs139558732 as a PTSD risk variant.
We also conducted meta-analyses of our data with the two SNPs reported as genome wide significant by the Army STARRS consortium.36 STARRS reported one locus for AA (rs159572) and one for EA (rs11085374).36 Results post meta-analysis for the reported SNPs were not genome-wide significant (rs159572, p=0.2744; rs11085374, p=1.74×10−05), perhaps unsurprisingly given lack of consistent association within the Army STARRS report itself.36
Gene-based and pathway analyses using MAGMA26 yielded no significant results after correction for multiple testing, consistent with observation from other GWAS analyses in which gene and pathway methods did not yield significant findings until the primary GWAS was well powered enough to identify specific risk loci. Nevertheless, we present the top gene and pathway results in Supplementary Tables S7–S8. Top results for genes did not include PTSD candidate genes, though GRINA, a glutamate receptor, was among the top twenty results. The top pathway was the neurotrophic factor-mediated Trk receptor signaling pathway37, which includes BNDF (brain-derived neurotrophic factor), NGF (nerve growth factor), and other neurotrophin-related genes, which collectively regulate synaptic strength and plasticity in the nervous system of mammals38. Given the primacy of learning in the PTSD phenotype, this is an intriguing pathway result, which awaits follow-up in a better-powered PTSD analyses.
Heritability estimation from molecular genetic (SNP) data, h2SNP
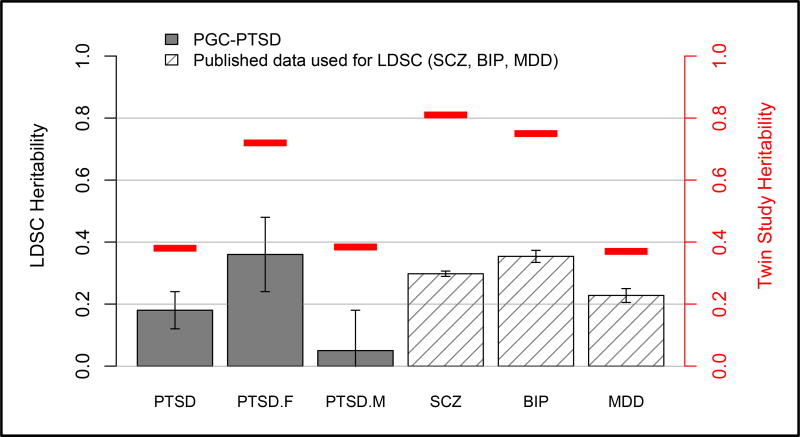
LDSC and GCTA were used to estimate SNP-chip heritability (h2SNP). Using both methods, female heritability estimates (29% average of LDSC=0.36 SE=0.12, p=0.003 and GCTA=0.21, SE=0.09, p=0.019) were comparable to those for other psychiatric disorders (see Figure 3). In contrast, in males, the point estimate was not significantly different from zero and was lower than previously reported estimates for major psychiatric disorders39 (7% average of LDSC=0.05, SE=0.13, p=0.69 and GCTA=0.08, SE=0.10, p=0.43). Analyzing males and females together - as has been done in published reports - the point estimate was 15% (average of LDSC=0.18, SE=0.06, p=0.003 and GCTA=0.12, SE=0.05, p=0.016). Partitioned heritability estimation35 will be conducted on future releases of PGC-PTSD data when the z-score for overall heritability is higher. The current z-score is 3.0, and a z-score of 7 was deemed adequate for partitioned heritability analysis in the primary publication.35
Figure 3. PTSD SNP-chip heritability (h2SNP) overall and for males and females separately and comparison to other psychiatric disorders.
Gray bars denote PTSD heritability estimates. Slashed bars reflect SCZ, BIP, and MDD heritability estimates calculated using LDSC and published data.16, 31, 32 Red lines denote twin study heritability estimates, see discussion. EA samples only per description in text; error bars reflect standard error (SE).
PGC=psychiatric genomics consortium, PTSD=posttraumatic stress disorder, LDSC=Linkage Disequilibrium Score Regression, SCZ=schizophrenia, BIP=bipolar disorder, MDD=major depressive disorder
In our sample, the point estimate for heritability among AAs was much lower and not statistically different from zero (GCTA= −0.005, se=0.04, p=0.45). Unlike our EA samples, we could not compare this estimate to one from LDSC because LDSC is currently not suitable for use in populations with recent admixture (e.g., AA, LA).
Cross disorder genetic effects between PTSD and SCZ, BIP, and MDD
Due to the relatively lower power for heritability estimation for PTSD with our current samples, we decided to limit the number of genetic correlations tested to focus on three major adult psychiatric disorders (SCZ16, BIP31, MDD32) for which GWAS results from large studies are publicly available. As shown in the top (EA) portion of Table 1, PRS suggested overlap with both SCZ and BIP even after overly stringent (due to correlated tests) Bonferroni correction. Moreover, 58% (22/38) of PRS tests among EA individuals were nominally significant. For variance explained by discovery disorder p-value bins and associated statistics, see Supplementary Table S6. In contrast, PRS revealed no evidence of overlap with MDD, but this could be due to low power in both the MDD40 and the present PTSD analysis.41 This possibility is consistent with evidence of PTSD-MDD genetic overlap found with the more powerful (constrained) version of LDSC. Constrained LDSC results further supported the PRS finding of shared PTSD-SCZ genetic effects.
Table 1. Cross disorder effects between PTSD and the three adult psychiatric disorders.
LDSC cannot be applied to data from individuals with more recent admixture (e.g., AA), hence ‘NA’.
| PRS | LDSC unconstrained RG(SE), p |
LDSC constrained RG(SE), p |
||
|---|---|---|---|---|
| EA | ||||
| SCZ | Yes min p = 3.9×10−4 | No 0.10(0.06) p=0.13 | Yes 0.33(0.08) p=1.3×10−5 | |
| BIP | Yes min p = 7.0×10−5 | No −0.02(0.13) p=0.85 | No 0.16(0.10) p=0.11 | |
| MDD | No min p = 0.15 | No 0.21(0.17) p=0.22 | Yes 0.34(0.12) p=0.006 | |
|
| ||||
| AA | ||||
| SCZ | No min p = 0.14 | NA | NA | |
| BIP | No min p = 0.05 | NA | NA | |
| MDD | No min p = 0.35 | NA | NA | |
RG=genetic correlation from LDSC, SE=standard error, SCZ=schizophrenia, BIP=bipolar disorder, MDD=major depressive disorder, PRS=polygenic risk scoring, LDSC=Linkage disequilibrium score regression. Bonferroni multiple testing correction for PRS is 0.05/38=1.3×10−3.
Discussion
The present report provides the first molecular genetic evidence of PTSD heritability and extends previous findings about shared genetic effects between PTSD and other disorders.8, 10–12 These results portend future success in identifying specific PTSD risk loci when viewed in the context of the trajectories of genetic discoveries for other complex genetic disorders,16, 42 which yielded significant polygenic results prior to identification of robust single variant associations. Specifically, examining 20 730 individuals, we found a molecular genetics-based heritability estimate for European American females of 29%, which is similar to h2SNP for schizophrenia and is substantially higher than h2SNP in European American males (estimate not distinguishable from zero). We found strong evidence of overlapping genetic risk between PTSD and schizophrenia along with more modest evidence of overlap with bipolar and major depressive disorder. The combination of informative polygenic results and lack of robust single variant analyses (in our data and in the evaluation of previously reported PTSD loci36, 43–46) strongly suggests the need for better powered analyses.
Regarding polygenic results, a distinction must be drawn between the EA and AA components of this study. For reasons discussed below, the present report was far more informative for PTSD genetics in EA individuals than AA individuals, despite comparable sample sizes (both approximately 10,000 with 25% cases). Among EA individuals, these molecular genetic results parallel twin study results,6–9 which showed moderate heritability for PTSD overall, and higher heritability in females than males.
There are several possible reasons why female heritability may be higher than male heritability, including differences in trauma exposure47, 48, which itself is heritable49, 50, sex-based biology, and sex-based symptom differences. Given that heritability estimates convey the relative importance of genetic and environmental influences, sex-based differences in either would impact heritability. We can assume comparable genetic variation in female and male subjects because we only used genetic variants that conformed to HWE expectations (thus implying no selective removal of particular alleles). This leaves sex-based differences in relevant environmental influences as one explanation, and indeed, rates of exposure to various types of trauma are known to vary by sex51. As well, particular trauma types vary in the degree to which they are associated with PTSD52. Greater variability in the profile of PTSD-inducing environmental influences in males, as compared to females, would lead to greater relative importance of environmental factors in the development of PTSD in males, and this could explain observed lower heritability estimates in males. This possibility could be tested via detailed study of trauma and environmental histories of males and females, combined with mathematical quantification of PTSD risk based on the frequency and magnitude of PTSD-inducing effects of relevant variables.
Second, female and male sex may be viewed as environmental variables, in that genetic variation is expressed in the context of a particular individual, and there are biological differences in female and male biology (e.g. sex hormone levels). To the extent that the female biological ‘environment’ is more conducive the expression of genetic variation, and the male biological ‘environment’ tends to dampen genetically-influenced variability in PTSD liability, sex itself may account for different heritability estimates in females vs. males. Biological differences have been reported between sexes regarding responses to trauma51 and responses to environmental variables thought to mediate the later development of PTSD53. Thus, there may be sex-based biological differences in trauma liability. In order for such effects to explain the observed greater heritability in females compared to males, the specified biological processes would need to lead to greater expression of genetically influenced liability to PTSD in females compared to males.
The most pedestrian explanation for sex-based heritability differences for PTSD is that reliability and/or validity of PTSD diagnosis differs by sex. Lower male heritability could be a result of lower reliability and validity of PTSD diagnosis in males, because heritability estimates are always capped by reliability and validity of measurement. Regarding this possibility, it would be worthwhile to examine reasons why particular populations might systematically over or under-report PTSD (thereby decreasing reliability and validity). Perhaps cultural factors that are more permissive for accurate reporting of PTSD symptoms in females contribute to more precise measurement of PTSD in females, and this would permit higher heritability estimates. The same issue of reliability and validity applies if diagnostic nosology is more appropriate for females than males. If the current PTSD diagnosis ‘carves nature at it’s joints’ better for females than males, there will be a lower bound on male heritability estimates compared to females.
In sum, greater measurement precision of phenotypic and environmental variables relevant to PTSD, as well as PTSD diagnosis, will aid accurate estimation of heritability. It will also increase power in primary GWAS analyses. PGC-PTSD is pursuing these strategies for continued GWAS efforts, in addition to increasing sample size.
A common misconception about SNP-chip heritability estimates calculated with GCTA and LDSC is that they should be similar to twin study estimates, when in reality twin studies have the advantage of capturing all genetic effects – common, rare, and those not genotyped by available methods. Thus, the assumption should be that h2SNP < h2TWIN when using GCTA and LDSC, and this is what we observe for PTSD, as has been observed for many other phenotypes.58 Though somewhat limited by power in the present study, strong evidence of shared genetic effects between PTSD and SCZ, and more modest evidence of shared effects with both MDD and BIP, are consistent with recent reports of partially shared genetic effects across nearly all psychiatric disorders34, 39 and with twin study evidence of shared genetic influences on MDD and PTSD.10–12 No evidence of overlap was found with ADHD and autism. However, this could be due to low power and should be reexamined when PGC PTSD is substantially expanded.
In contrast, there were no significant findings for heritability or shared genetic effects in AA individuals. This should not be taken as evidence that genetic effects in AA individuals differ from those in EA individuals. Rather, this report highlights an interpretational disparity between EA and AA individuals. There are no twin studies conducted in primarily non-EA populations, so there is no prior information indicating whether heritability might differ across ancestry groups (which could occur for a number of reasons). Second, polygenic methods like PRS and LDSC rely on external data resources, which are far less common generally, and nonexistent for many non-EA populations. Third, newer methods, such as LDSC, have not been adapted for recently admixed genomes, thus they can’t be applied to AA and many LA individuals. Other factors responsible for lower power in the AA PTSD analysis are Eurocentric bias on genotyping arrays and the inherently greater genetic variation in the African portions of AA individuals’ chromosomes, which necessitates more markers to achieve the same proportion of genomic coverage. Given these factors, publication of this relatively large sample of AA individuals is particularly important.
Our findings suggest that a sample of approximately 10,000 individuals (with 25% cases) is not sufficient – in EAs or in AAs – to identify robust risk loci. A larger sample size and greater genotyping coverage (particularly in AA individuals), will afford greater power in future single variant association analyses. As well, it is possible that highly standardized phenotyping and/or ascertainment of more specific populations may increase power by decreasing phenotypic heterogeneity, as is arguably the explanation for success of the CONVERGE consortium.59 Future work needs to more carefully consider trauma exposure, a necessary though not sufficient condition for the development of PTSD. Although the majority of controls (87.7%) in the current analysis were trauma exposed, the inclusion of non-trauma exposed controls may have reduced power to detect PTSD loci. Moreover, sex differences in both type and quantity of trauma exposure are well documented54 and will be important to consider in future research examining sex differences in heritability. In the interim, the successful polygenic analyses discussed above (i.e., significant heritability estimates and genetic correlations) mean that the summary statistics made available with this report will be informative for PTSD studies when used with appropriate polygenic methods (e.g., PRS and LDSC).
In summary, we find that PTSD – a disorder that by definition requires an environmental exposure, trauma – is also partly genetic in origin. This result comes as a foregone conclusion for researchers steeped in the behavioral genetics literature, but for those unfamiliar with twin studies or skeptical of their results, heritability based on molecular genetic data is compelling. Reassuringly, these molecular genetic heritability results are consistent with twin studies. All available evidence suggests that PTSD heritability among females is higher than males. Overall, PTSD heritability is comparable to that of MDD, but female heritability is close to that of SCZ and BIP, two of the most genetically-influenced psychiatric disorders. Male PTSD SNP-chip heritability, in contrast, was lower than any recorded for a major psychiatric disorder and not distinguishable from zero with current sample sizes. While large in comparison to previous reports, the current sample size of over 20 000 was still a limiting factor in single variant analyses. The strong evidence of shared genetic effects between PTSD and SCZ is worth further investigation and consistent with data from birth cohort studies, which have found early childhood factors such as low IQ60, 61 and psychotic symptoms62 to be risk factors for both syndromes, but not for MDD or BIP. More variable evidence suggests that larger samples are needed to precisely resolve genetic overlap for BIP and MDD. Finally, the distribution of individuals of different ancestries in this analysis illuminated analytical and interpretational disparities for individuals of all ancestries as compared to EA individuals, and highlights the need for large genetic studies in non-European populations. Data from the largest genetic examination of PTSD to date demonstrates significant heritability for the development of PTSD, suggests at least partial genetic mediation of comorbidities, and outlines important areas for future progress in understanding the genomic architecture of PTSD.
Supplementary Material
Acknowledgments
We thank study participants and research groups contributing to PGC-PTSD for sharing their data and their time to make this work possible. We thank One Mind, Cohen Veterans Bioscience, and the Stanley Center for Psychiatric Research for their financial support. Finally, we acknowledge Steve Hyman of the Stanley Center for Psychiatric Research and Harvard University for comments on various versions of this manuscript. For study specific acknowledgments, see Supplementary text.
Footnotes
Conflict of interest
See Supplementary text for conflict of interest statements.
Contributor Information
Laramie E Duncan, Department of Psychiatry, Stanford University, Stanford, CA 94305, Broad Institute of MIT and Harvard, Stanley Center for Psychiatric Research, Boston, MA 02113, The Analytic and Translational Genetics Unit, Massachusetts General Hospital, Boston, MA, 02114.
Andrew Ratanatharathorn, Department of Epidemiology, Columbia University, New York, NY 10032.
Allison E Aiello, Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina, Chapel Hill, NC 27599.
Lynn M Almli, Department of Psychiatry and Behavioral Sciences, Emory University, Atlanta, GA 30322.
Ananda B Amstadter, Department of Psychiatry, Virginia Commonwealth University, Richmond, VA 23298.
Allison E Ashley-Koch, Department of Medicine, Duke Molecular Physiology Institute, Duke University Medical Center, Durham, NC 27701.
Dewleen G Baker, Veterans Affairs San Diego Healthcare System and Veterans Affairs Center of Excellence for Stress and Mental Health, San Diego, CA, 92093, Department of Psychiatry and Department of Family Medicine and Public Health, University of California San Diego, San Diego, CA, 92093.
Jean C Beckham, Veterans Affairs Durham Healthcare System, Durham, NC 27705, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Durham NC 27705.
Laura J Bierut, Department of Psychiatry, Washington University School of Medicine, St. Louis, MO 63110.
Jonathan Bisson, Division of Psychological Medicine and Clinical Neurosciences, Cardiff University, Cardiff, UK.
Bekh Bradley, Atlanta VA Medical Center, Atlanta, GA 30322, Department of Psychiatry, Emory University, Atlanta, GA 30322.
Chia-Yen Chen, Psychiatric and Neurodevelopmental Genetics Unit, Center for Human Genetic Research, and Department of Psychiatry, Massachusetts General Hospital, Boston, MA 02114, The Analytic and Translational Genetics Unit, Massachusetts General Hospital, Boston, MA, 02114, Department of Psychiatry, Harvard University, Cambridge, MA 02138.
Shareefa Dalvie, Division of Human Genetics, University of Cape Town, Cape Town, South Africa 7935.
Lindsay A Farrer, Biomedical Genetics, Boston University School of Medicine, Boston, MA 02118.
Sandro Galea, Boston Unviersity School of Public Health, Boston, MA 02118.
Melanie E Garrett, Department of Medicine, Duke Molecular Physiology Institute, Duke University Medical Center, Durham, NC 27701.
Joel E Gelernter, Department of Psychiatry, Yale University School of Medicine, New Haven, CT 06510, VA CT Healthcare system.
Guia Guffanti, Department of Psychiatry, McLean Hospital, Belmont, MA 02478, Department of Psychiatry, Harvard University, Cambridge, MA 02138.
Michael A Hauser, Department of Medicine, Duke Molecular Physiology Institute, Duke University Medical Center, Durham, NC 27701.
Eric O Johnson, RTI International, Research Triangle Park, NC 27709.
Ronald C Kessler, Department of Health Care Policy, Harvard Medical School, Boston, MA 02138.
Nathan A Kimbrel, Veterans Affairs Durham Healthcare System, Durham, NC 27705, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Durham NC 27705.
Anthony King, Department of Psychiatry, University of Michigan, Ann Arbor, MI 48105.
Nastassja Koen, Department of Psychiatry and Mental Health, University of Cape Town, Cape Town, South Africa 7935, MRC Unit on Anxiety & Stress Disorders, Groote Schuur Hospital, Cape Town, South Africa 7935.
Henry R Kranzler, Department of Psychiatry, University of Pennsylvania Perelman School of Medicine and VISN 4 MIRECC, Crescenz VAMC, Philadelphia, PA 19104.
Mark W Logue, VA Boston Healthcare System, Jamaica Plain, MA 02130, Department of Medicine, Boston University School of Medicine, Boston, MA 02118.
Adam X Maihofer, Veterans Affairs San Diego Healthcare System and Veterans Affairs Center of Excellence for Stress and Mental Health, San Diego, CA, 92093, Department of Psychiatry and Department of Family Medicine and Public Health, University of California San Diego, San Diego, CA, 92093.
Alicia R Martin, The Analytic and Translational Genetics Unit, Massachusetts General Hospital, Boston, MA, 02114, Broad Institute of MIT and Harvard, Stanley Center for Psychiatric Research, Boston, MA 02113.
Mark W Miller, VA Boston Healthcare System, Jamaica Plain, MA 02130, Department of Psychiatry, Boston University School of Medicine, Boston, MA 02118.
Rajendra A Morey, Department of Psychiatry and Behavioral Sciences, Duke University Medical Center, Durham NC 27705, Durham VA Medical Center, Durham, NC 27710.
Nicole R Nugent, Division of Behavioral Genetics, Department of Psychiatry, Rhode Island Hospital, Providence, RI 02903, Department of Psychiatry and Human Behavior, Alpert Medical School of Brown University, Providence, RI 02903.
John P Rice, Department of Psychiatry, Washington University, St. Louis, MO 63110.
Stephan Ripke, The Analytic and Translational Genetics Unit, Massachusetts General Hospital, Boston, MA, 02114, Broad Institute of MIT and Harvard, Stanley Center for Psychiatric Research, Boston, MA 02113, Department of Psychiatry and Psychotherapy, Charit, Campus Mitte, 10117 Berlin, Germany.
Andrea L Roberts, Department of Social and Behavioral Sciences, Harvard T. H. Chan School of Public Health Cambridge, MA 02138.
Nancy L Saccone, Department of Genetics, Washington University, St. Louis, MO 63110.
Jordan W Smoller, Psychiatric and Neurodevelopmental Genetics Unit, Center for Human Genetic Research, and Department of Psychiatry, Massachusetts General Hospital, Boston, MA 02114, Broad Institute of MIT and Harvard, Stanley Center for Psychiatric Research, Boston, MA 02113.
Dan J Stein, Department of Psychiatry and Mental Health, University of Cape Town, Cape Town, South Africa 7935, MRC Unit on Anxiety & Stress Disorders, Groote Schuur Hospital, Cape Town, South Africa 7935.
Murray B Stein, Department of Psychiatry, University of California, San Diego, La Jolla, CA 92093.
Jennifer A Sumner, Center for Cardiovascular Behavioral Health, Columbia University Medical Center, New York, NY 10032.
Monica Uddin, Department of Psychology and Carl R. Woese Institute for Genomic Biology, University of Illinois, Champaign, IL 61820.
Robert J Ursano, Center for the Study of Traumatic Stress, Department of Psychiatry, Uniformed Services University of the Health Sciences, Bethesda, MD 20814.
Derek E Wildman, Carl R. Woese Institute for Genomic Biology and Department of Molecular & Integrative Physiology, University of Illinois, Champaign, IL 61820.
Rachel Yehuda, James J. Peters Bronx Veterans Affairs and Department of Psychiatry, Department of Neuroscience, Icahn School of Medicine at Mount Sinai, Bronx, NY 10468.
Hongyu Zhao, Department of Biostatistics, Yale University, New Haven, CT 06510.
Mark J Daly, The Analytic and Translational Genetics Unit, Massachusetts General Hospital, Boston, MA, 02114, PhD, Broad Institute of MIT and Harvard, Stanley Center for Psychiatric Research, Boston, MA 02113.
Israel Liberzon, VA Ann Arbor Health System, Ann Arbor, MI, 28105, Department of Psychiatry, University of Michigan, Ann Arbor, MI 48105.
Kerry J Ressler, Department of Psychiatry, McLean Hospital, Belmont, MA 02478, Department of Psychiatry, Harvard University, Cambridge, MA 02138.
Caroline M Nievergelt, Veterans Affairs San Diego Healthcare System and Veterans Affairs Center of Excellence for Stress and Mental Health, San Diego, CA, 92093, Department of Psychiatry and Department of Family Medicine and Public Health, University of California San Diego, San Diego, CA, 92093.
Karestan C Koenen, Department of Epidemiology, Harvard University, Cambridge, MA 02138, Psychiatric and Neurodevelopmental Genetics Unit, Center for Human Genetic Research, and Department of Psychiatry, Massachusetts General Hospital, Boston, MA 02114, Broad Institute of MIT and Harvard, Stanley Center for Psychiatric Research, Boston, MA 02113.
References
- 1.Kessler RC, et al. Prevalence and Treatment of Mental Disorders, 1990 to 2003. N. Engl. J. Med. 2005;352:2515–2523. doi: 10.1056/NEJMsa043266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davidson JR. Trauma: the impact of post-traumatic stress disorder. J. Psychopharmacol. Oxf. Engl. 2000;14:S5–12. doi: 10.1177/02698811000142S102. [DOI] [PubMed] [Google Scholar]
- 3.Herman J. Trauma and Recovery: The Aftermath of Violence--from Domestic Abuse to Political Terror. Basic Books; 1997. [Google Scholar]
- 4.Myers C. A Final Contribution to the Study of Shell Shock - Being a Consideration of Unsettled Points Needing Investigation. The Lancet. 1915;185:316–330. [Google Scholar]
- 5.White House. National Research Action Plan Responding to the Executive Order Improving Access to Mental Health Services for Veterans, Service Members, and Military Families (August 31, 2012) 2013 [Google Scholar]
- 6.True WR, et al. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch. Gen. Psychiatry. 1993;50:257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- 7.Stein MB, Jang KL, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder symptoms: a twin study. Am. J. Psychiatry. 2002;159:1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- 8.Sartor CE, et al. Common genetic and environmental contributions to post-traumatic stress disorder and alcohol dependence in young women. Psychol. Med. 2011;41:1497–1505. doi: 10.1017/S0033291710002072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolf EJ, Mitchell KS, Koenen KC, Miller MW. Combat exposure severity as a moderator of genetic and environmental liability to post-traumatic stress disorder. Psychol. Med. 2014;44:1499–1509. doi: 10.1017/S0033291713002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolf EJ, et al. Posttraumatic stress disorder and the genetic structure of comorbidity. J. Abnorm. Psychol. 2010;119:320–330. doi: 10.1037/a0019035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koenen KC, et al. Common Genetic Liability to Major Depression and Posttraumatic Stress Disorder in Men. J. Affect. Disord. 2008;105:109–115. doi: 10.1016/j.jad.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sartor CE, et al. Common Heritable Contributions to Low-Risk Trauma, High-Risk Trauma, Posttraumatic Stress Disorder, and Major Depression. Arch. Gen. Psychiatry. 2012;69:293–299. doi: 10.1001/archgenpsychiatry.2011.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbertson MW, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat. Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lango Allen H, et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010;467:832–838. doi: 10.1038/nature09410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Voight BF, et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat. Genet. 2010;42:579–589. doi: 10.1038/ng.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C-Y, et al. Improved ancestry inference using weights from external reference panels. Bioinforma. Oxf. Engl. 2013;29:1399–1406. doi: 10.1093/bioinformatics/btt144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang CC, et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galinsky KJ, et al. Fast principal components analysis reveals independent evolution of ADH1B gene in Europe and East Asia. bioRxiv. 2015:018143. doi: 10.1101/018143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abecasis GR, et al. A map of human genome variation from population-scale sequencing. Nature. 2010;467:1061–1073. doi: 10.1038/nature09534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delaneau O, Marchini J, Zagury J-F. A linear complexity phasing method for thousands of genomes. Nat. Methods. 2012;9:179–181. doi: 10.1038/nmeth.1785. [DOI] [PubMed] [Google Scholar]
- 22.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanna S, et al. Common variants in the GDF5-UQCC region are associated with variation in human height. Nat. Genet. 2008;40:198–203. doi: 10.1038/ng.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willer CJ, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat. Genet. 2008;40:161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ihaka R, Gentleman R. R: A Language for Data Analysis and Graphics. J. Comput. Graph. Stat. 1996;5:299–314. [Google Scholar]
- 26.de Leeuw CA, Mooij JM, Heskes T, Posthuma D. MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput. Biol. 2015;11:e1004219. doi: 10.1371/journal.pcbi.1004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SH, Wray NR, Goddard ME, Visscher PM. Estimating missing heritability for disease from genome-wide association studies. Am. J. Hum. Genet. 2011;88:294–305. doi: 10.1016/j.ajhg.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am. J. Hum. Genet. 2011;88:76–82. doi: 10.1016/j.ajhg.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang J, et al. Common SNPs explain a large proportion of the heritability for human height. Nat. Genet. 2010;42:565–569. doi: 10.1038/ng.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Purcell S, et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Psychiatric GWAS Consortium - Bipolar Disorder. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat. Genet. 2011;43:977–983. doi: 10.1038/ng.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium et al. A mega-analysis of genome-wide association studies for major depressive disorder. Mol. Psychiatry. 2013;18:497–511. doi: 10.1038/mp.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bulik-Sullivan BK, et al. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 2015;47:291–295. doi: 10.1038/ng.3211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bulik-Sullivan B, et al. An atlas of genetic correlations across human diseases and traits. Nat. Genet. 2015 doi: 10.1038/ng.3406. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finucane HK, et al. Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat. Genet. 2015;47:1228–1235. doi: 10.1038/ng.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stein MB, Chen C, Ursano RJ, et al. GEnome-wide association studies of posttraumatic stress disorder in 2 cohorts of us army soldiers. JAMA Psychiatry. 2016 doi: 10.1001/jamapsychiatry.2016.0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liberzon A, et al. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–425. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nestler E, Hyman S, Malenka R. Molecular Neuropharmacology: A Foundation for Clinical Neuroscience, Second Edition. McGraw-Hill Professional; 2008. [Google Scholar]
- 39.Cross-Disorder Group of the Psychiatric Genomics Consortium et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat. Genet. 2013;45:984–994. doi: 10.1038/ng.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium et al. A mega-analysis of genome-wide association studies for major depressive disorder. Mol. Psychiatry. 2013;18:497–511. doi: 10.1038/mp.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dudbridge F. Power and Predictive Accuracy of Polygenic Risk Scores. PLoS Genet. 2013;9:e1003348. doi: 10.1371/journal.pgen.1003348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Purcell SM, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nievergelt CM, et al. Genomic predictors of combat stress vulnerability and resilience in U.S. Marines: A genome-wide association study across multiple ancestries implicates PRTFDC1 as a potential PTSD gene. Psychoneuroendocrinology. 2015;51:459–471. doi: 10.1016/j.psyneuen.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 44.Guffanti G, et al. Genome-wide association study implicates a novel RNA gene, the lincRNA AC068718.1, as a risk factor for post-traumatic stress disorder in women. Psychoneuroendocrinology. 2013;38:3029–3038. doi: 10.1016/j.psyneuen.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie P, et al. Genome-wide Association Study Identifies New Susceptibility Loci for Posttraumatic Stress Disorder. Biol. Psychiatry. 2013;74:656–663. doi: 10.1016/j.biopsych.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Logue MW, et al. A genome-wide association study of post-traumatic stress disorder identifies the retinoid-related orphan receptor alpha (RORA) gene as a significant risk locus. Mol. Psychiatry. 2012 doi: 10.1038/mp.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benjet C, et al. The epidemiology of traumatic event exposure worldwide: results from the World Mental Health Survey Consortium. Psychol. Med. 2015:1–17. doi: 10.1017/S0033291715001981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tolin DF, Foa EB. Sex differences in trauma and posttraumatic stress disorder: a quantitative review of 25 years of research. Psychol. Bull. 2006;132:959–992. doi: 10.1037/0033-2909.132.6.959. [DOI] [PubMed] [Google Scholar]
- 49.Kremen WS, Koenen KC, Afari N, Lyons MJ. Twin studies of posttraumatic stress disorder: differentiating vulnerability factors from sequelae. Neuropharmacology. 2012;62:647–653. doi: 10.1016/j.neuropharm.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Afifi TO, Asmundson GJG, Taylor S, Jang KL. The role of genes and environment on trauma exposure and posttraumatic stress disorder symptoms: a review of twin studies. Clin. Psychol. Rev. 2010;30:101–112. doi: 10.1016/j.cpr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Olff M, Langeland W, Draijer N, Gersons BPR. Gender differences in posttraumatic stress disorder. Psychol. Bull. 2007;133:183–204. doi: 10.1037/0033-2909.133.2.183. [DOI] [PubMed] [Google Scholar]
- 52.Breslau N, Chilcoat HD, Kessler RC, Peterson EL, Lucia VC. Vulnerability to assaultive violence: further specification of the sex difference in post-traumatic stress disorder. Psychol. Med. 1999;29:813–821. doi: 10.1017/s0033291799008612. [DOI] [PubMed] [Google Scholar]
- 53.Francis DD, Young LJ, Meaney MJ, Insel TR. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: gender differences. J. Neuroendocrinol. 2002;14:349–353. doi: 10.1046/j.0007-1331.2002.00776.x. [DOI] [PubMed] [Google Scholar]
- 54.Benjet C, et al. The epidemiology of traumatic event exposure worldwide: results from the World Mental Health Survey Consortium. Psychol. Med. 2016;46:327–343. doi: 10.1017/S0033291715001981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kremen WS, Koenen KC, Afari N, Lyons MJ. Twin studies of posttraumatic stress disorder: differentiating vulnerability factors from sequelae. Neuropharmacology. 2012;62:647–653. doi: 10.1016/j.neuropharm.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Afifi TO, Asmundson GJG, Taylor S, Jang KL. The role of genes and environment on trauma exposure and posttraumatic stress disorder symptoms: a review of twin studies. Clin. Psychol. Rev. 2010;30:101–112. doi: 10.1016/j.cpr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 57.Inslicht SS, et al. Sex differences in fear conditioning in posttraumatic stress disorder. J. Psychiatr. Res. 2013;47:64–71. doi: 10.1016/j.jpsychires.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. Am. J. Hum. Genet. 2012;90:7–24. doi: 10.1016/j.ajhg.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.CONVERGE consortium. Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature. 2015;523:588–591. doi: 10.1038/nature14659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koenen KC, et al. Childhood IQ and Adult Mental Disorders: A Test of the Cognitive Reserve Hypothesis. Am. J. Psychiatry. 2009;166:50–57. doi: 10.1176/appi.ajp.2008.08030343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koenen KC, Moffitt TE, Poulton R, Martin J, Caspi A. Early childhood factors associated with the development of post-traumatic stress disorder: results from a longitudinal birth cohort. Psychol. Med. 2007;37:181–192. doi: 10.1017/S0033291706009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fisher HL, et al. Specificity of childhood psychotic symptoms for predicting schizophrenia by 38 years of age: a birth cohort study. Psychol. Med. 2013;43:2077–2086. doi: 10.1017/S0033291712003091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.