Abstract
Ca-stimulated adenylyl cyclases (AC) transduce neuronal stimulation-evoked increase in calcium to the production of cyclic AMP (cAMP), which impinges on the regulation of many aspects of neuronal function. Type 1 and type 8 AC (AC1 and AC8) are the only ACs that are directly stimulated by Ca. Although AC1 function was implicated in regulating reference spatial memory, the function of AC8 in memory formation is not known. Due to the different biochemical properties of AC1 and AC8, these two enzymes may have distinct functions. For example, AC1 activity is regulated by both Ca and G proteins. In contrast, AC8 is a pure Ca sensor. It is neither stimulated by Gs, nor inhibited by Gi. Recent studies also suggested that AC1 and AC8 were differentially concentrated at different sub-cellular domains, implicating that Ca-stimulated signaling might be compartmentalized. In this study, we used AC8 knockout (KO) mice and found behavioral deficits in memory retention for temporal dissociative passive avoidance and object recognition memory. When examined by Morris water maze, AC8 KO mice showed normal reference memory. However, the acquisition of newer spatial information was defective in AC8 KO mice. Furthermore, AC8 KO mice were severely impaired in hippocampus-dependent episodic-like memory when examined by the delayed matching-to-place task. Because AC8 is preferentially localized at the presynaptic active zone, our results suggest a novel role of presynaptic cAMP signaling in memory acquisition and retention, as well as distinct mechanisms underlying reference and working/episodic-like memory.
Keywords: Adenylyl cyclase, cAMP, synaptic plasticity, learning, memory, knockout mice
Introduction
The adaptation of animal behavior to the changing environment requires activity-dependent modification of neuronal functions. Cellular and molecular studies have suggested the role of cAMP-and cAMP dependent protein kinase (PKA)-mediated signal transduction in regulating many forms of neuroplasticity, including long-term potentiation (LTP), long-term depression (LTD), and long-term memory formation (LTM) (Nguyen and Woo, 2003; Wang and Storm, 2003).
Ca-stimulated adenylyl cyclases (AC) couple the activity-evoked Ca rise to the production of cAMP, which may lead to further activation of PKA and extracellular signal-regulated kinase (ERK) signaling (Impey et al., 1998). Among all cloned ACs, type 1 and type 8 AC (AC1 and AC8) are stimulated by calcium and calmodulin. In deed, biochemical and genetic evidence indicated that AC1 and AC8 are the only ACs that are directly stimulated by Ca in the central nervous system (Wong et al., 1999). Membrane preparation from the brains of AC1/AC8 double knockout (DKO) mice showed no Ca stimulation in AC activity. Interestingly, the late phase of LTP in the CA1 region of the hippocampus was significantly impaired in DKO mice, indicating an essential role of Ca-stimulated AC in synaptic plasticity (Wong et al., 1999). Although DKO mice showed normal short-term memory for passive avoidance (Stubley-Weatherly et al., 1996) and contextual memory (Kim and Fanselow, 1992; Logue et al., 1997), their LTM for these two hippocampus-dependent tasks was defective (Wong et al., 1999).
Although AC1 and AC8 are both stimulated by Ca/calmodulin, they show different biochemical properties. AC1 is regulated by both G protein-coupled receptors and Ca (Choi et al., 1992). It is stimulated by Gs (Wayman et al., 1994) and inhibited by Gi (Nielsen et al., 1996). In contrast, AC8 is a pure Ca sensor, and not regulated by either Gs or Gi (Nielsen et al., 1996). Interestingly, AC1 and AC8 may be differentially targeted to distinct sub-cellular domains, suggesting possible compartmentalization of Ca-stimulated signaling. When the epitope-tagged AC1 and AC8 were over-expressed in hippocampal neurons, we found that AC8, but not AC1, showed concentrated expression pattern at the excitatory synapses (Wang et al., 2003). Moreover, we found that the endogenous AC1 was mainly localized at the post-synaptic density (PSD). The endogenous AC8 was preferentially concentrated at the pre-synaptic active zone (PAZ) (Conti et al., 2007). These findings suggest that AC1 and AC8 may play distinct roles in regulating activity-dependent plasticity.
When examined by hippocampus-dependent learning paradigms, AC1 KO mice showed impaired reference memory in Morris water maze test (Wu et al., 1995), and normal performance in passive avoidance and contextual fear conditioning. However, the function of AC8 in memory formation is largely unknown. Interestingly, earlier investigation on AC8 KO mice demonstrated that AC8 is not required for hippocampus-dependent associative memories, such as passive avoidance and contextual fear conditioning (Wong et al., 1999). In this study, we identified un-discovered roles of AC8, the pure Ca sensor, in memory retention, acquisition of newer spatial information, and working/episodic-like memory.
Materials and Methods
Animals
The mice mutants for AC8 were generated by gene-specific recombination as described (Schaefer et al., 2000). The mice were bred into C57BL6 background for at least 10 generations. Animals were housed in the university lab animal research facility, and all the manipulations were in compliance with the guidelines of Institutional Animal Care and Use Committee (IACUC) at Michigan State University. The mice have free access to water and food, and housed under 12h dark/12h light cycles.
Behavioral analysis
Open-field analysis was used to measure the activity of wild type (WT) and AC8 KO mice in a novel environment. Parameters, including total movement time, moving distance, and velocity, were determined by the TruScan Photo Beam Activity System (Coulbourn Instruments).
Passive avoidance
During training, mouse was introduced to the lit half of the training chamber (Coulbourn Instruments), and allowed to explore the lit chamber for 1min before opening the trap door. The trap door was closed, and a mild foot-shock (0.7mA for 2sec) was delivered immediately after the mouse entered the darkened half. The trained mouse was retained in the dark chamber for 20sec after the shock, and then returned to its home cage. When tested, the trained mouse was re-introduced to the lit chamber. The time spent in the lit half before entering the darkened half was scored as crossover latency, and used as index for memory formation. We chose 300 sec as the cut-off value for crossover latency. Mice were removed manually from the lit chamber when the cut-off value was reached.
Temporal dissociative passive avoidance
The behavioral protocol was similar to that of passive avoidance except for that the shock was delivered 10min after the mouse entered the dark chamber. The animals were trained by 1 trial/day for 5 days. The animals were tested on day 6 without shock. During each training trial, the crossover latency was recorded. If the mice reached the cutoff value (300sec) before the 5th trial, they would not be subjected to further training and the value of their crossover latency was used for the later sessions. To test long-term memory retention, the trained mouse, which reached the cut-off value (300 sec), was tested again 8 days later. For example, if a trained mouse showed 300sec crossover latency on day 3, no more training trial was performed on this particular mouse. The value of 300 sec was used for day 3, 4, 5, and 6. The mouse was tested for long-term memory retention 8 days later, which is day 11.
Object recognition memory
First, the mouse was habituated for 12h in the training/testing chamber (46.5cm L, 25cm W, 15cm H). During training, two objects with different shapes were presented to the mouse for 10min. One hour after training, another set of objects (one old object and one novel object) was presented to the trained mouse. The interaction of mouse with each object, including approaches and sniffing, was scored. If the mouse had memory retention for the old object, it would show preference to the novel object during testing. The percentage of preference is defined as “number of interaction for a specific object” divided by the “total number of interaction for both objects”.
Morris water maze was used for testing hippocampus-dependent spatial memory (Morris et al., 1982). Animal activity was measured by a video-based tracking system (WaterMaze, Coulbourn Instruments). The pool was filled with opaque water (by adding washable white paint), and surrounded by extra-maze cues. The escape platform (10cm in diameter) was placed in the center of a designated quadrant with its top positioned 1cm below the water surface. During the visible platform training, the platform was marked by a flag. Mice were trained by 6 trials/day for 2 days. The 6 trials were divided into 2 blocks with an interval of 1hr (interblock interval). There were 3 trials for each block with 10min interval between the trials (intertrial interval, or ITI). Mice were allowed to navigate in the circular pool for up to 60 sec till they found the platform. Mice were allowed to stay on the platform for 30sec. If mice failed to find and land on the platform within 60 sec, they were manually guided to the platform. The visible platform was randomly placed in different locations for each trial. The time each mouse spent to land on the platform was scored as escape latency.
After the visible platform training, the mice were further trained by the hidden platform paradigm, during which the platform was placed 1cm beneath the opaque water. Mice were trained by 4 trials (with 1h ITI)/day for 5 days. For each trial, mice were dropped into the pool randomly from 4 different designated start points.
Probe trials were carried out 1 day after the hidden platform training. With the escape platform removed, the mice were allowed to swim in the pool for 60 sec. The time spent in each quadrant, number of crossing for the location of the hidden platform, and swimming speed were recorded.
During the reversal platform training, the hidden platform was moved to the opposite quadrant. The mice were trained by 4 trials/day (with ITI of 1h) for 4 days. A probe trial was carried out 1 day after the last training session.
Delayed matching-to-place (DMP) task
The hippocampus-dependent DMP task was performed, as described (Chen et al., 2000; Zeng et al., 2001), to examine the working/episodic-like memory. Naïve WT and AC8 KO mice were first pre-trained by the visible platform paradigm (4 trials/day for 2 days with an ITI of 30 min). After the pre-training, these mice were trained to find the 6 new different locations of the hidden platform. For each trial, a maximal duration of 90 sec was allowed for the mice to find the hidden platform. For each platform location, the mice were trained for a maximum of 8 trials/day for 2 days (with an ITI of 10min). If the mice find the platform within 20 sec for 3 consecutive trials, they will be trained for the next new platform location. The least number of trials was 5 for each platform location even if a mouse reached the criterion in fewer than 5 trials.
Hippocampal lesion
Bilateral hippocampal lesions were performed as described (de Hoz et al., 2005; Martel et al., 2007). Briefly, mice were anesthetized with pentobarbital sodium (100 mg/kg, i.p.), and mounted on a stereotaxic frame (David Kopf, Model 963-LS) with Bregma and Lambda on the same horizontal plane. The injection cannulas were placed to the dorsal hippocampus with coordinates of 2.0 mm posterior, 1.4 mm lateral and 1.6 mm ventral. Ibotenic acid (0.3ul with a concentration of 10mg/ml in PBS) was delivered at a flow rate of 0.05ul/min by a WPI syringe pump. After injection, the infusion cannula was kept in place for 2 min, and then pulled out slowly. Sham lesions were performed the same way, but injected with 0.3ul PBS. At the end of behavioral test, animals were perfused with ice-cold PBS. The brains were removed, fixed in 6% paraformaldehyde/PBS, and cryo-protected with 30% sucrose/PBS. Coronal sections (30-micron thick) were subjected to histological examination by cresyl violet staining.
Detection of endogenous AC1 and AC8
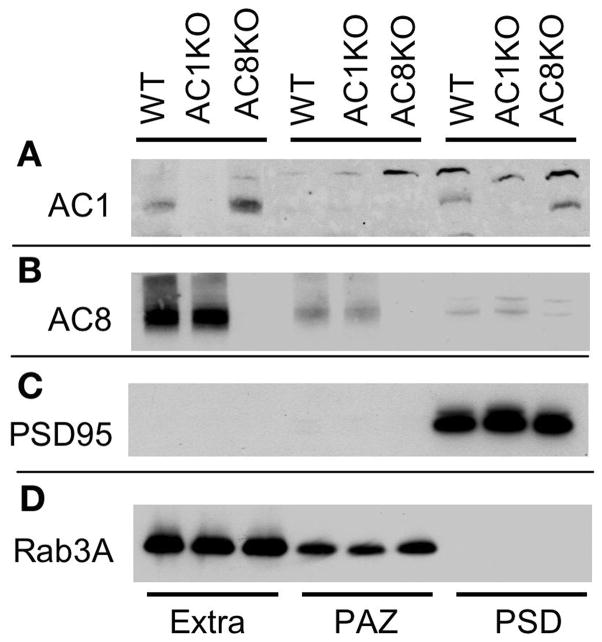
The forebrain tissues including hippocampus were freshly dissected from WT, AC1 KO, and AC8 KO mice. After homogenization, the synaptosomes were isolated by sucrose gradient centrifugation (100,000×g for 1.5 h) as described (Phillips et al., 2001; Conti et al., 2007). The purified synaptosomes were further separated into extrasynaptic, PSD, and PAZ fractions as described (Conti et al., 2007), and subjected to 4–12% SDS-PAGE. AC1 and AC8 were detected by Western blot analysis with the ECL methods (SuperSignal WestDura; Pierce Biotechnology). Antibodies against AC1 (1:500; rabbit polyclonal; developed in house), AC8 (1:500; rabbit polyclonal; Santa Cruz Biotechnology), PSD 95 (1:4000; monoclonal; Affinity Bioreagents), and Rab3A (1:1000, rabbit polyclonal, Affinity Bioreagents) were used.
Data analysis
Two-way repeated measures ANOVA was performed for water maze and TDPA data (genotype and time/trial as between-within subject factor). Three-way repeated measures ANOVA was used for delayed matching-to-pace task. Student’s t-test was used to assess significance for data between two groups. Data were expressed as the mean ± SEM. Differences with p-values less than 0.05 were considered significant.
Results
AC8 KO mice showed normal locomotor activity, passive avoidance memory and shock sensitivity
We first examined the basal locomotor activity of AC8 KO mice in a novel environment by the open field analysis. WT mice and AC8KO mice showed comparable movement time, similar travel distance, and ambulatory velocity (see Fig 1 of supplementary data). These data indicated normal movement of AC8 KO mice.
It was reported that AC1/AC8 double KO mice (DKO) were significantly impaired in two hippocampus-dependent tasks, passive avoidance memory and contextual memory (Wong et al., 1999). However, single KO mice for AC8 showed normal memory retention in both tasks (Wong et al., 1999). These results implicated that AC8 activity is not necessary to support certain forms of associative memory. Here, we confirmed that the memory retention is normal with AC8 KO mice for passive avoidance (Fig. 2 of supplementary data). Both WT and AC8 KO mice showed significant increase in crossover latency when tested 24 hours after training (F[1, 14]=548, p<0.001, two-way ANOVA), and there was no significant difference between the genotypes (F[1, 14]=650, p=0.4). We also found no difference in shock sensitivity between WT mice and AC8 KO mice. WT mice and AC8 KO mice showed similar reaction during the delivery of the mild electric foot shock (0.7mA for 2 sec). They showed similar increase in ambulatory velocity during the shock and an after-shock reduction in movement (supplementary Fig. 3).
AC8 KO mice are impaired for temporal dissociative passive avoidance
Although AC8 KO mice were normal for contextual and passive avoidance memory, we reasoned that it might be due to the intrinsic features of these two paradigms. These two paradigms are very strong and less demanding training protocols, and may not be sensitive enough to detect behavioral phenotypes in AC8 KO mice. We used a modified passive avoidance protocol, temporal dissociative passive avoidance (TDPA), in which the delivery of an aversive unconditioned stimulus (the mild electric foot shock) was delayed. In the standard passive avoidance, the mild electric foot shock was delivered immediately after the mouse entered the darkened half of the training chamber. In the TDPA, the foot shock was delivered 10min after mouse entering the darkened half, so that the association of the conditioned stimuli with the unconditioned stimuli was weaker. Normally a single passive avoidance training may lead to very strong memory formation, as indicated by reaching the cutoff value for crossover latency during testing (supplementary Fig. 2). When trained by the TDPA, multiple training sessions are needed for mice to reach the cutoff value (Fig. 1A).
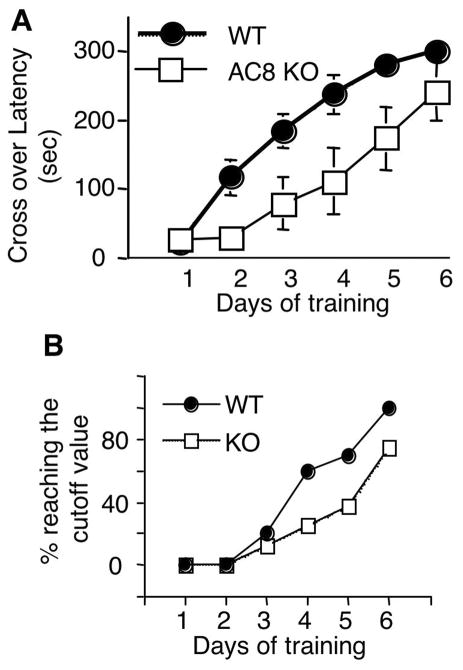
Figure 1.
AC8 KO mice show deficits in TDPA. WT (n=10) and AC8 KO (n=8) mice, at the age of 2 to 3 months, were trained by TDPA everyday (from day 1 to 5). The trained mice were tested 24hr after each training (from day 2 to 6). The crossover latency was recorded. A. AC8 KO mice showed slower learning than WT mice, as indicated by the lower crossover latency on testing days 2, 3, 4, and 5. All values are average +/− SEM. B. The percentage of mice reaching the cutoff value was lower for AC8 KO mice.
Compared to WT mice, AC8 KO mice showed significantly slower increase in crossover latency when trained by for the TDPA. Although the two-way ANOVA analysis demonstrated significant learning and memory formation for both WT and AC8 KO mice (F[5, 80]=42, p<0.001), significant difference in crossover latency was revealed between WT and AC8 KO mice (F[1, 16]=13, p=0.002). The trial X genotype interaction also revealed significant difference (F[5, 80]=2.7, p=0.03). These data demonstrated a slower learning curve with AC8 KO mice in TDPA. In addition, WT mice required less training sessions to reach the cutoff value (300sec of crossover latency). The percentage of animals with 300sec crossover latency was higher for WT mice in all testing sessions (Fig 1B). After 5 consecutive daily trainings, all WT mice reached 300sec crossover latency when tested on day 6. In contrast, 75% of AC8 KO mice (6 out of 8) reached the cutoff value on day 6.
To test long-term memory retention, the mice reached the 300 sec cutoff value after TDPA training were tested again 8 days later. Because not all AC8 KO mice showed cutoff value on day 6, we only chose the individuals (6 out of 8) with 300sec crossover latency for the 8-day memory retention test. We observed significant loss of TDPA memory for AC8 KO mice. The crossover latency of WT was 275 +/−13 sec when tested 8 days later. In contrast, the value for AC8 KO mice was 157 +/− 52 sec (p=0.05, student t test). The difference between WT and AC8 KO mice during training and testing was not due to different shock sensitivity (see supplementary Fig. 3).
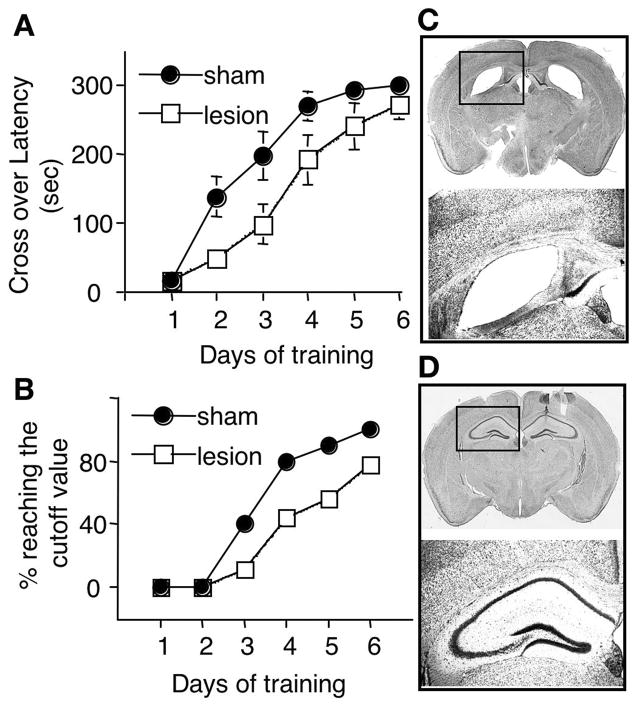
To determine whether TDPA depends on the function of hippocampus, we did bilateral hippocampal lesions. After lesion/surgery, mice were recovered for 9 days, and tested for TDPA. As shown in Fig 2, both groups significantly increased their crossing-over latency during training (F[5, 85] = 54.4, P < 0.0001). However, the lesioned mice (injected with ibotenic acid) displayed significant slower learning than the sham-operated mice (injected with vehicle PBS) (F[1, 17] = 10.7, P < 0.01, repeated measures two-way ANOVA). Post-hoc comparison showed that there was significant difference between the lesioned and the sham group on day 2 (P = 0.01) and day 3 (P = 0.04), and a tendency to significant difference on day 4 (P = 0.06). Furthermore, the percentage of animals with 300sec crossover latency (the cut-off value) was lower for the lesioned mice in all testing sessions (Fig 2B). After 5 consecutive daily trainings, all sham-operated mice reached 300sec crossover latency when tested on day 6. In contrast, 78% of the lesioned mice (7 out of 9) reached the cutoff value on day 6. Histology assessment revealed that significant hippocampal lesions occurred in ibotenic acid-injected mice (Fig. 2C). No apparent hippocampal damages were observed in the sham-operated mice (Fig. 2D). These results implicated that TDPA depends on intact hippocampus.
Figure 2.
Memory formation for TDPA depends on hippocampus. Mice with hippocampal lesion (injected with ibotenic acid, n=9) and sham-operated mice (injected with PBS, n=10) were trained by TDPA as described in Figure 1. A. Lesioned mice displayed poor TDPA performance, as indicated by shorter crossover latency during testing. B. The percentage of animals reaching the cutoff value (300sec) was lower for lesioned mice. C and D. The hippocampal morphology was examined by cresyl violet staining. Hippocampal lesion occurred in ibotenic acid-injected (C), but not in PBS-injected mice (D). The boxed regions in the upper panels were enlarged, and shown in the lower panels.
AC8 KO mice are impaired for object recognition memory
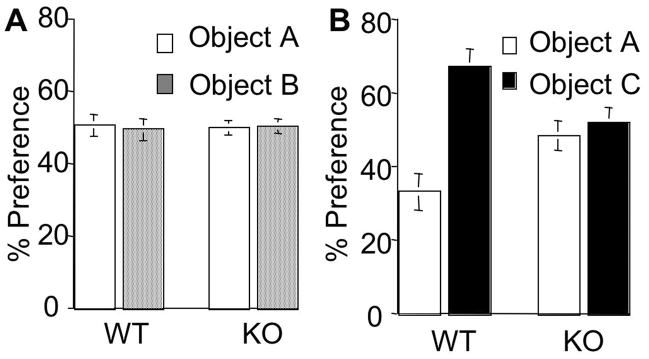
We further examined AC8 KO mice by another sensitive hippocampus-dependent paradigm, object recognition memory (Myhrer, 1988; Reed and Squire, 1997). Because there is no reinforcement (such as an aversive US) during the object recognition training, a single training trial usually results in weaker memory formation, which may last for less than 1 day. During the 10min training session, WT and AC8 KO mice showed similar interaction with the objects (38 +/− 3 for WT mice, 37 +/− 3 for AC8 KO mice, p>0.05, student t test), indicating normal motivation and exploratory activity. WT and AC8 KO mice also displayed equal preference to the two objects during training (Fig. 3A). During testing, one conditioned old object was replaced by a novel object. If mice retained memory for the old objects, they would show preference to the novel object. When tested 1h after training, only WT mice, but not AC8 KO mice, showed significant preference to the novel object (67 +/− 2% for WT mice, 52 +/− 2% for AC8 KO mice, p<0.001, student t test) (Fig. 3B). These data indicated that AC8 is required for the formation of recognition memory.
Figure 3.
AC8 KO mice show deficits in object recognition memory. WT (n=9) and AC8 KO (n=8) mice were trained for object recognition memory. A. WT and AC8 KO mice showed equal preference to the two objects during training. B. WT, but not AC8 KO mice, showed significant preference to the novel object during testing. The values are average +/− SEM.
The function of AC8 in spatial reference memory
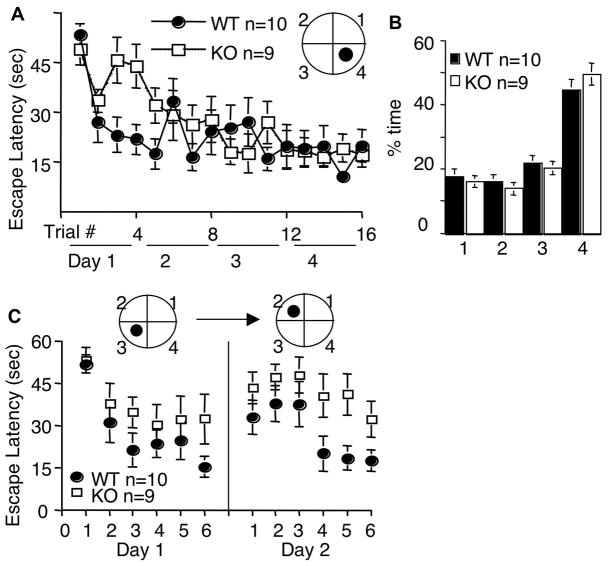
We examined hippocampus-dependent spatial memory by Morris water maze (Morris et al., 1982). We first trained mice with the visible platform paradigm, in which the animals learn to find the escape platform with an attached visual cue. Although WT showed better improvement for the first 4 trials on day 1, there was no significant difference in escape latency after 12 trials (p=0.267, two-way ANOVA) (Fig. 4A). Both genotypes showed significant improvement in escape latency (F[11, 187]=19.5, p<0.001, two-way ANOVA). Animals of both group showed similar swimming speed during visible platform training (1.8 +/− 0.1 arbitrary units for both genotypes, p=0.317, t test). These data indicated that AC8 KO mice were normal in motor activity, vision, and motivation to escape from the water.
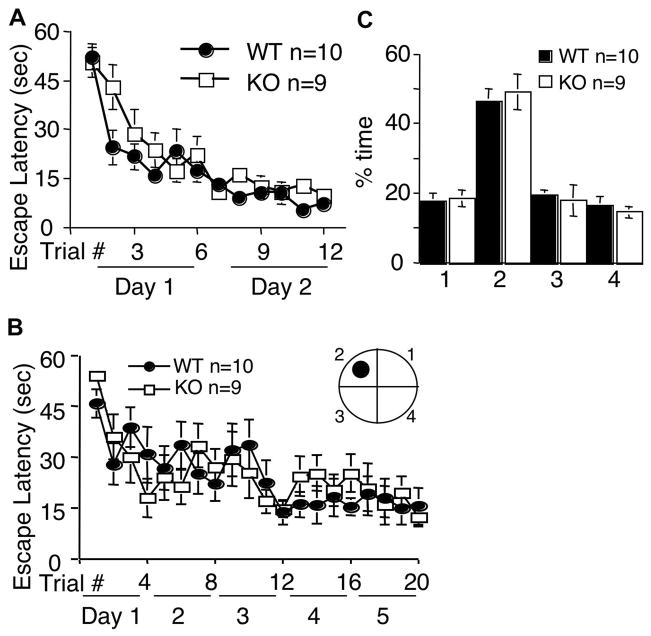
Figure 4.
AC8 KO mice show normal reference memory for Morris water maze. WT and AC8 KO mice were first trained by the visible platform paradigm, and showed comparable improvement in escape latency (A). The same set of mice were further trained by the hidden platform paradigm, and showed comparable improvement in escape latency (B). During training, the hidden platform was positioned in the center of a designated quadrant (arbitrarily quadrant 2 as indicated in the inset diagram). During the probe trial, both WT and AC8 KO mice spent significant more time in the target quadrant (quadrant 2) (C). All values are average +/− SEM.
We further examined AC8 KO mice with the hidden platform paradigm, in which animals learned to find the escape platform by using the extra-maze cues. AC8 KO mice showed similar performance to that of WT mice. Both group displayed significant training-related decrease in escape latency, correlating well with the number of trials (Fig. 4B) (F[19, 323]=5, p<0.001, two-way ANOVA), and there was no significant difference between WT and AC8 KO mice (F[1, 17]=201, p=0.919, two-way ANOVA). During the probe test, both WT and AC8 KO mice spent significant more time in the target quadrant (46 +/− 4% for WT mice, and 49 +/− 5% for AC8 KO mice; p=0.674, student t test) (Fig. 4C). The number of crossing the platform location was also comparable between the two groups (WT and AC8 KO mice) (3.4 +/− 0.5 for WT mice, 2.9 +/− 1 for AC8 KO mice, p=0.63, student t test). During the probe test, AC8 KO mice also displayed similar swim speed to that of WT mice (data not shown). These data indicated that AC8 is not required for reference spatial memory.
The function of AC8 in working/episodic-like memory during the delayed matching-to-place task
We next tested whether AC8 KO mice could re-learn new platform formation. During the reversal trials, the platform was moved to the opposite quadrant from the previously trained position. After 4 days of training, both WT and AC8 KO mice significantly improved in escape latency (F[15, 255]=5.4, p<0.001, two-way ANOVA) (Fig. 5A). There was no statistical difference in escape latency between WT and AC8 KO mice for the whole training session (F[1, 17]=1.4, p=0.253, two-way ANOVA). Furthermore, AC8 KO and WT mice showed comparable percentage of time in the target quadrant (Fig. 5B; 45 +/− 3% for WT mice, 49 +/− 4% for AC8 KO mice, p=0.317, t test), number of crossing for the location of the escape platform (3.1 +/−0.5 for WT mice, 4.0 +/− 0.7 for AC8 KO mice, p=0.205, t test), and swim speed (1.8 +/− 0.1 for WT mice, 1.8 +/− 0.1 for AC8 KO mice, p=0.465, t test) during the probe trial after the last reversal training.
Figure 5.
AC8 KO mice show impaired acquisition of newer spatial information. After the hidden platform trials, WT and AC8 KO mice were trained by the reversal test, during which the platform was moved from quadrant 2 to 4 (as indicated by the inset diagram) (A). After 4 days of training, both groups (WT and AC8 KO mice) learned the reversed platform position, as indicated by the improvement in escape latency (A). A probe trial was carried out after the reversal protocol. Both WT and AC8 KO mice spent significant more time in the new target quadrant (quadrant 4) (B). After the reversal paradigm, the mice were further trained to find 2 new platform positions (placed in quadrant 3 and 2) on 2 consecutive days (C). All values are expressed as average +/− SEM.
Although there was no significant difference between WT and AC8 KO mice in escape latency, the p value for the interaction between genotype and trial was less than 0.05 (F[15, 255]=2.2, p=0.008, two-way ANOVA). During the first day of reversal training, we noticed that the reduction of escape latency was significantly less for AC8 KO mice in the 3rd and 4th trials (Fig. 5A). The post-hoc comparison detected difference for trial 3 and 4 (F[1, 17]=7, p=0.018 for trial 3; F[1, 17]=8, p=0.013 for trial 4), and suggested that AC8 KO mice might be impaired for acquiring newer spatial information. Therefore, we further examined memory acquisition by moving the escape platform to another location. The platform was moved clockwise to 2 new locations (as indicated in Fig. 5C) on two consecutive days. AC8 KO mice showed significant impairments in finding the new platform location on day 2 (F[1,17]=7, p=0.017, two-way ANOVA) (Fig. 5C).
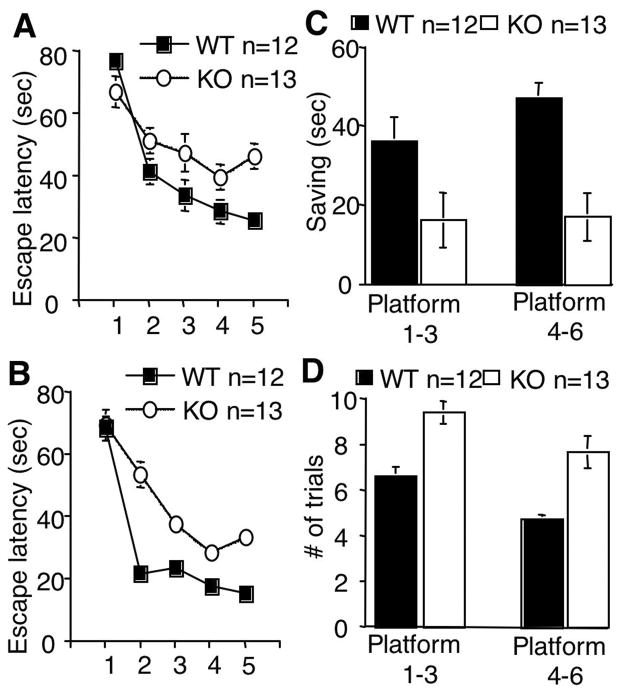
The data from Fig 5 suggested that AC8 might be required for working/episodic-like memory. Therefore, we examined naïve cohorts of WT and AC8 KO mice by delayed matching-to-place (DMP) task in Morris water maze (Chen et al., 2000; Zeng et al., 2001). During the DMP task, which requires dynamic acquisition of on-going events and measures hippocampus-dependent working/episodic-like memory, mice were trained to learn the 6 changing locations of the platform. For each platform location, the mice ware trained by a maximum of 8 trials/day for up to 2 days. If the mice were able to find the platform within 20 sec for 3 consecutive trials, they would be trained for the next platform position. However, a minimum of 5 trials was performed for each platform location. Again, WT and AC8 KO mice showed comparable performance during the visible platform pre-training (data not shown). They also displayed similar swimming speed during all pre-training and DMP training sessions (data not shown). Although both WT and AC8 KO mice showed learning and improvement in escape latency for all platform locations (F[5,55]=6.9, p<0.0001, for WT mice; F[5, 60]=6.6, p<0.0001 for AC8 KO mice; two-way ANOVA), WT mice showed significantly faster learning of the new platform location than AC8 KO mice (Fig. 6A and B) (F[1, 23]=33.7, p<0.0001, three-way repeated measures ANOVA). The difference in trial x genotype interaction was also significant (F[4, 92]=7.6, p<0.0001). To determine the function of AC8 in rapid learning after a single trial for the new platform, we calculated the “saving time”, which was the reduction of escape latency between the first and second trial. AC8 KO mice showed much smaller saving value than WT mice (Fig. 6C). The average saving for the first 3 platform positions was 36 +/− 6 sec for WT mice, and 16 +/− 7 sec for AC8 KO mice (Fig. 6C, p<0.05, t test). The average saving for the last 3 platform locations was 47 +/− 4 sec for WT mice, and 17 +/− 6 sec for AC8 KO mice (Fig. 6C, p<0.001, t test). We also noticed that AC8 KO mice needed more trials to reach the criterion for each platform location (i.e. less than 20 sec in escape latency) (Fig. 6D). For the first 3 platform locations, the average number of trials was 6.6 +/− 0.4 for WT mice, and 9.4 +/− 0.5 for AC8 KO mice (Fig. 6D, p<0.01, t test). For the last 3 locations, it was 4.8 +/− 0.2 for WT mice, and 7.6 +/−0.7 for AC8 KO mice (Fig. 6D, p<0.005, t test). These data strongly suggested that AC8 is required for the hippocampus-dependent working/episodic-like memory. When data were analyzed for the last 2 platform locations, AC8 KO mice displayed more dramatic impairments for the improvement in escape latency (supplementary Fig. 4A), saving time (supplementary Fig. 4B), and trail numbers needed to reach the escape latency criterion of 20sec (supplementary Fig. 4C). These data demonstrated that AC8 is required for working/episodic-like memory.
Figure 6.
AC8 KO mice show impairments in delayed matching-to-place (DMP) task. WT and AC8 KO mice were trained in the Morris water maze by the DMP task. The values were averaged for the first 3 platform locations (platform 1, 2, and 3) and the last 3 platform locations (platform 4, 5, and 6). A) Cross over latency for the first five trials when averaged for the first 3 platform locations. B) Cross over latency for the first five trials when averaged for the last 3 platform locations. C) The reduction of escape latency between the first and second trials was calculated and expressed as saving. D) The number of trials required for WT or AC8 KO mice to reach the arbitrary criterion (20 sec escape latency) are plotted. The values are averaged +/− SEM. In C and D, the values are presented for the first 3 (platform 1–3) and last 3 platform (platform 4–6) locations, as indicated/
AC8 is preferentially localized in the presynaptic active zone and co-purifies with Rab3A
As described earlier, mice mutants with AC1, another Ca-stimulated AC, showed impairments in spatial reference memory (Wu et al., 1995). In contrast, AC8 KO mice displayed normal reference memory in Morris water maze, as indicated by their normal performance in the probe tests (Fig. 4C and 5B). Possibly, the different phenotypes could be due to the distinct biochemical properties and differential sub-cellular localization of these two Ca-stimulated enzymes. Here, we re-confirmed that AC1 is concentrated in the PSD fraction and AC8 is concentrated in the PAZ fraction. AC1 was co-purified with PSD95 but not with Rab3A (Fig. 7A, C, and D). Rab3A is a small GTPase, whose function is demonstrated in regulating vesicle fusion and neurotransmitter release (Schluter et al., 2002; Sudhof, 2004). Interestingly, Rab3A mutant mice showed similar phenotypes to that of AC8 KO mice. They are both impaired for mossy fiber LTP (Castillo et al., 1997; Wang et al., 2003) and acquisition of newer spatial information [(D’Adamo et al., 2004), and this study]. Here, we show that AC8 was also co-purified with Rab3A, which was present in the PAZ but not in PSD preparations (Fig. 7B, C, and D). Although the antibodies against AC1 and AC8 detected a few non-specific bands (Fig. 7A, and B), they appeared to be highly specific for AC1 and AC8. The specific immuno-signal was lost in AC1 KO mice when AC1 antibody was used (Conti et al., 2007, and Fig. 7A). Similarly, AC8 antibody failed to detect AC8 in AC8 KO mice (Conti et al., 2007, and Fig. 7B). We consider the doublets (detected by AC8 antibody) in the PSD fraction non-specific signals, because they were also present in AC8 KO samples (Fig. 7B). These molecules might be extensively enriched in PSD, and picked up by non-specific antibody binding. They were not present in PAZ preparations from AC8 KO mice (Fig. 7B).
Figure 7.
AC8 is co-purified with Rab3A in the presynaptic active zone (PAZ). Brain homogenates from WT, AC1 KO, and AC8 KO mice were separated into extrasynaptic, PAZ and PSD fractions. The presence of AC1 (A), AC8 (B), PSD-95 (C), and Rab3A (D) was detected by Western blot analysis.
Discussion
Ca/calmodulin-stimulated ACs couple the two major second messengers, Ca and cAMP, in the central nervous system. Supported by biochemistry, molecular and genetic studies, it was concluded that AC1 and AC8 are the only ACs that are directly activated by Ca. The activity-dependent up-regulation of the enzymatic activity of AC1 and AC8 may be pivotal for the activation of many signaling molecules, such as PKA, ERK and cAMP-responsive element binding protein (CREB), which play essential roles in regulating synaptic plasticity (Nguyen and Woo, 2003).
The function of Ca-stimulated ACs in regulating neuroplasticity was addressed by using gene knockout strategies. Double knockout mice (DKO) for both AC1 and AC8 showed no late phase LTP in the CA1 region of the hippocampus (Wong et al., 1999). When examined by passive avoidance and contextual fear conditioning, the DKO mice showed no long-term memory (LTM) retention. These data demonstrated that Ca-stimulated ACs are required for many aspects of plasticity. Recently, it was shown that the activity-dependent activation of ERK and CREB was lost in DKO neurons (Sindreu et al., 2007).
The contribution of AC1 and AC8 to Ca-stimulated cyclase activity was determined by the use of DKO mice and single KO mice of AC1 and AC8. The Ca-stimulated AC activity was significantly reduced in the hippocampus of AC1 KO and AC8 KO, and totally lost in DKO mice (Wong et al., 1999). Despite the significant reduction in Ca-stimulated AC activity, AC8 KO mice showed normal transcription-dependent late phase LTP (L-LTP) at the Schaffer collateral/CA1 synapses (Wong et al., 1999). Although the level of CA1 LTP was lower during the first hour after induction (Wu et al., 1995), L-LTP was normal in AC1 KO mice (Wong et al., 1999). Furthermore, AC1 KO and AC8 KO mice both showed normal LTM for passive avoidance and contextual fear conditioning (Wong et al., 1999). These data implicated that AC1 or AC8 alone might be sufficient to support certain forms of synaptic plasticity.
Are AC1 and AC8 redundant? Although passive avoidance and contextual memory do not depend on AC1 activity, AC1 KO mice showed significant deficits in spatial reference memory formation. In the Morris water maze paradigm, AC1 KO mice showed normal acquisition in the hidden platform training, but were impaired in the probe test (Wu et al., 1995). As described earlier, the regulatory properties of AC1 and AC8 are different. Compared to AC1, whose activity is regulated by both G proteins and Ca, AC8 is a pure Ca sensor. Recently, we found that the endogenous AC1 is preferentially localized in the postsynaptic density, whereas AC8 is detected mainly in the presynaptic active zone [(Conti et al., 2007) and Fig. 7]. Therefore, they may play distinct roles in synaptic plasticity. Although AC8 KO mice showed normal CA1 LTP, they were impaired in CA1 LTD (Schaefer et al., 2000). Interestingly, AC8 is required for mossy fiber LTP (Wang et al., 2003), which is mechanistically different from CA1 LTP, and does not depend on the activation of NMDA receptors (Zalutsky and Nicoll, 1990; Johnston et al., 1992). However, the function of AC8 in memory formation is basically unknown. In this study, we found that AC8 is required for a more sensitive form of passive avoidance memory, TDPA. We assume that TDPA and standard PA are essentially similar. They both involve the same US and CS, except that the coupling of US and CS is delayed in TDPA. With this kind of perturbation, more training sessions are required for animals to form strong memory. The same TDPA protocol was successfully used to detect impairments with mice mutants for an APP (beta-amyloid precursor protein) interacting protein FE65 (Wang et al., 2004a). By the hippocampal lesion experiments, we, for the first time, demonstrated that this sensitive paradigm of PA is indeed hippocampus-dependent. Compared to WT mice, AC8 KO mice showed weaker memory formation, as well as weaker memory retention in TDPA. Although AC8 KO mice showed normal passive avoidance and contextual memory, they were defective in object recognition memory, which also depends on the function of hippocampus.
We found that the function of AC8 and AC1 is different in regulating spatial memory. While the reference memory is significantly impaired in AC1 KO mice (Wu et al., 1995), AC8 KO mice showed normal performance in acquisition and the probe test for the hidden platform test. However, the ability to acquire newer platform location was lost in AC8 KO mice (Fig. 5C). Such impairment suggests a role of AC8 in working/episodic-like memory, which involves dynamic acquisition of on-going information. Therefore, we further examined AC8 KO mice with DMP task using Morris water maze. Compared to WT mice, AC8 KO mice showed slower learning for the 6 changing platform locations, as indicated by slower improvement in latency and smaller saving values.
The postsynaptic mechanisms for learning and memory formation were intensively investigated. Recently, emerging evidence started to suggest the role of presynaptic function in certain forms of hippocampus-dependent learning (Powell, 2006). Specifically, the functional relevance of LTP at the mossy fiber/CA3 synapses (mfLTP) in memory formation was investigated in genetically engineered mice. As described earlier, the deletion of Rab3A, a molecule involved in neurotransmitter release, resulted in severe impairments of mfLTP (Castillo et al., 1997). However, the Rab3A KO mice showed normal reference memory in the hidden platform test of Morris water maze. Their contextual memory formation was also comparable to that of WT mice (Powell et al., 2004). Although a recent report suggested a role of Rab3A in the regulation of emotion (Yang et al., 2007), the phenotypes of Rab3A KO mice in cued fear conditioning were controversial (Powell et al., 2004; Yang et al., 2007). The lack of behavioral phenotypes was also demonstrated in mice mutant for PKA. Although genetic deletion of the Cβ1 or the RIβ isoform of PKA caused significant deficits in mfLTP, these KO mice displayed normal hidden platform performance and contextual memory (Huang et al., 1995). While the functional role of mfLTP in learning was challenged, D’Adamo and colleagues reported interesting defective phenotypes in reversal and episodic-like memory with Rab3A KO mice (D’Adamo et al., 2004). Our study revealed a striking similarity between Rab3A KO and AC8 KO mice. They are both defective in mfLTP, show normal contextual memory (Wong et al., 1999; Powell et al., 2004) and hidden platform performance, but display impairments in reversal and DMP test. Although our data do not prove the causal effects of mfLTP, we suggest that this form of presynaptic plasticity may be required for more sensitive and demanding learning paradigms. This possibility was demonstrated by the phenotypes of AC8 KO mice in passive avoidance and TDPA. We showed that AC8 KO mice were normal for the standard passive avoidance, but impaired for the more sensitive TDPA test. It would be interesting to examine Rab3A and PKA mutant mice with TDPA and recognition memory, in which AC8 KO mice were also defective.
Although the essential role of Ca-stimulated ACs in memory was well accepted, the function of pre-and post-synaptic cAMP signaling is unknown. Due to the different sub-cellular localization, we suggest that cAMP-regulated memory is mainly mediated by AC1 at the postsynaptic site and by AC8 at the presynaptic site. It is important to mention that AC1 KO mice are also defective for mfLTP (Villacres et al., 1998). Although plasticity at the mossy fiber synapses is considered to be mainly presynaptic (Zalutsky and Nicoll, 1990; Weisskopf and Nicoll, 1995), the role of postsynaptic Ca and cAMP was demonstrated (Yeckel et al., 1999; Wang et al., 2004b). Because mild deficits in CA1 LTP were observed with AC1 KO mice, it is pre-mature to postulate how regulation of mfLTP by cAMP at pre-and post-synaptic sites affects reference and working memory. Nevertheless, the distinct properties of AC1 and AC8 are reflected by the different phenotypes in AC1 KO and AC8 KO mice.
In summary, we identified novel roles of AC8 in regulating memory retention. Furthermore, We found that AC8 and AC1 have distinct function in spatial memory. While AC1 is required for reference memory, AC8 is required for the acquisition of newer spatial information and working/episodic-like memory. Our results also suggest that compartmentalized Ca-stimulated cAMP signaling with distinct biochemical properties may differentially regulate neuronal function and behavior.
Supplementary Material
Acknowledgments
This study was supported by National Institute of Health Grants MH076906 (H.W.), NS20498 (D.R.S.), MH073601 (D.R.S.), and AG18876 (L.J.M).
References
- Castillo PE, Janz R, Sudhof TC, Tzounopoulos T, Malenka RC, Nicoll RA. Rab3A is essential for mossy fibre long-term potentiation in the hippocampus. Nature. 1997;388:590–593. doi: 10.1038/41574. [DOI] [PubMed] [Google Scholar]
- Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, Justice A, McConlogue L, Games D, Freedman SB, Morris RG. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer’s disease. Nature. 2000;408:975–979. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- Choi EJ, Wong ST, Hinds TR, Storm DR. Calcium and muscarinic agonist stimulation of type I adenylylcyclase in whole cells. J Biol Chem. 1992;267:12440–12442. [PubMed] [Google Scholar]
- Conti AC, Maas JW, Jr, Muglia LM, Dave BA, Vogt SK, Tran TT, Rayhel EJ, Muglia LJ. Distinct regional and subcellular localization of adenylyl cyclases type 1 and 8 in mouse brain. Neuroscience. 2007;146:713–729. doi: 10.1016/j.neuroscience.2007.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Adamo P, Wolfer DP, Kopp C, Tobler I, Toniolo D, Lipp HP. Mice deficient for the synaptic vesicle protein Rab3a show impaired spatial reversal learning and increased explorative activity but none of the behavioral changes shown by mice deficient for the Rab3a regulator Gdi1. Eur J Neurosci. 2004;19:1895–1905. doi: 10.1111/j.1460-9568.2004.03270.x. [DOI] [PubMed] [Google Scholar]
- de Hoz L, Moser EI, Morris RG. Spatial learning with unilateral and bilateral hippocampal networks. Eur J Neurosci. 2005;22:745–754. doi: 10.1111/j.1460-9568.2005.04255.x. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER, Varshavsky L, Brandon EP, Qi M, Idzerda RL, McKnight GS, Bourtchouladze R. A genetic test of the effects of mutations in PKA on mossy fiber LTP and its relation to spatial and contextual learning. Cell. 1995;83:1211–1222. doi: 10.1016/0092-8674(95)90146-9. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB- dependent transcription and ERK nuclear translocation. Neuron. 1998;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Johnston D, Williams S, Jaffe D, Gray R. NMDA-receptor-independent long-term potentiation. Annu Rev Physiol. 1992;54:489–505. doi: 10.1146/annurev.ph.54.030192.002421. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Logue SF, Paylor R, Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci. 1997;111:104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- Martel G, Blanchard J, Mons N, Gastambide F, Micheau J, Guillou JL. Dynamic interplays between memory systems depend on practice: the hippocampus is not always the first to provide solution. Neuroscience. 2007;150:743–753. doi: 10.1016/j.neuroscience.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Myhrer T. Exploratory behavior and reaction to novelty in rats with hippocampal perforant path systems disrupted. Behav Neurosci. 1988;102:356–362. doi: 10.1037//0735-7044.102.3.356. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Woo NH. Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases. Prog Neurobiol. 2003;71:401–437. doi: 10.1016/j.pneurobio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Nielsen MD, Chan GC, Poser SW, Storm DR. Differential regulation of type I and type VIII Ca2+-stimulated adenylyl cyclases by Gi-coupled receptors in vivo. J Biol Chem. 1996;271:33308–33316. doi: 10.1074/jbc.271.52.33308. [DOI] [PubMed] [Google Scholar]
- Phillips GR, Huang JK, Wang Y, Tanaka H, Shapiro L, Zhang W, Shan WS, Arndt K, Frank M, Gordon RE, Gawinowicz MA, Zhao Y, Colman DR. The presynaptic particle web: ultrastructure, composition, dissolution, and reconstitution. Neuron. 2001;32:63–77. doi: 10.1016/s0896-6273(01)00450-0. [DOI] [PubMed] [Google Scholar]
- Powell CM. Gene targeting of presynaptic proteins in synaptic plasticity and memory: across the great divide. Neurobiol Learn Mem. 2006;85:2–15. doi: 10.1016/j.nlm.2005.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell CM, Schoch S, Monteggia L, Barrot M, Matos MF, Feldmann N, Sudhof TC, Nestler EJ. The presynaptic active zone protein RIM1alpha is critical for normal learning and memory. Neuron. 2004;42:143–153. doi: 10.1016/s0896-6273(04)00146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JM, Squire LR. Impaired recognition memory in patients with lesions limited to the hippocampal formation. Behav Neurosci. 1997;111:667–675. doi: 10.1037//0735-7044.111.4.667. [DOI] [PubMed] [Google Scholar]
- Schaefer ML, Wong ST, Wozniak DF, Muglia LM, Liauw JA, Zhuo M, Nardi A, Hartman RE, Vogt SK, Luedke CE, Storm DR, Muglia LJ. Altered stress-induced anxiety in adenylyl cyclase type VIII-deficient mice. J Neurosci. 2000;20:4809–4820. doi: 10.1523/JNEUROSCI.20-13-04809.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter OM, Khvotchev M, Jahn R, Sudhof TC. Localization versus function of Rab3 proteins. Evidence for a common regulatory role in controlling fusion. J Biol Chem. 2002;277:40919–40929. doi: 10.1074/jbc.M203704200. [DOI] [PubMed] [Google Scholar]
- Sindreu CB, Scheiner ZS, Storm DR. Ca2+-stimulated adenylyl cyclases regulate ERK-dependent activation of MSK1 during fear conditioning. Neuron. 2007;53:79–89. doi: 10.1016/j.neuron.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubley-Weatherly L, Harding JW, Wright JW. Effects of discrete kainic acid-induced hippocampal lesions on spatial and contextual learning and memory in rats. Brain Res. 1996;716:29–38. doi: 10.1016/0006-8993(95)01589-2. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Villacres EC, Wong ST, Chavkin C, Storm DR. Type I adenylyl cyclase mutant mice have impaired mossy fiber long-term potentiation. J Neurosci. 1998;18:3186–3194. doi: 10.1523/JNEUROSCI.18-09-03186.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Hu Q, Hearn MG, Shimizu K, Ware CB, Liggitt DH, Jin LW, Cool BH, Storm DR, Martin GM. Isoform-specific knockout of FE65 leads to impaired learning and memory. J Neurosci Res. 2004a;75:12–24. doi: 10.1002/jnr.10834. [DOI] [PubMed] [Google Scholar]
- Wang H, Storm DR. Calmodulin-regulated adenylyl cyclases: cross-talk and plasticity in the central nervous system. Mol Pharmacol. 2003;63:463–468. doi: 10.1124/mol.63.3.463. [DOI] [PubMed] [Google Scholar]
- Wang H, Pineda VV, Chan GC, Wong ST, Muglia LJ, Storm DR. Type 8 adenylyl cyclase is targeted to excitatory synapses and required for mossy fiber long-term potentiation. J Neurosci. 2003;23:9710–9718. doi: 10.1523/JNEUROSCI.23-30-09710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Yeckel MF, Johnston D, Zucker RS. Photolysis of postsynaptic caged Ca2+ can potentiate and depress mossy fiber synaptic responses in rat hippocampal CA3 pyramidal neurons. J Neurophysiol. 2004b;91:1596–1607. doi: 10.1152/jn.01073.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Wu Z, Kindsvogel W, Prichard L, Storm DR. Synergistic activation of the type I adenylyl cyclase by Ca2+ and Gs-coupled receptors in vivo. J Biol Chem. 1994;269:25400–25405. [PubMed] [Google Scholar]
- Weisskopf MG, Nicoll RA. Presynaptic changes during mossy fibre LTP revealed by NMDA receptor-mediated synaptic responses. Nature. 1995;376:256–259. doi: 10.1038/376256a0. [DOI] [PubMed] [Google Scholar]
- Wong ST, Athos J, Figueroa XA, Pineda VV, Schaefer ML, Chavkin CC, Muglia LJ, Storm DR. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase LTP. Neuron. 1999;23:787–798. doi: 10.1016/s0896-6273(01)80036-2. [DOI] [PubMed] [Google Scholar]
- Wu ZL, Thomas SA, Villacres EC, Xia Z, Simmons ML, Chavkin C, Palmiter RD, Storm DR. Altered behavior and long-term potentiation in type I adenylyl cyclase mutant mice. Proc Natl Acad Sci U S A. 1995;92:220–224. doi: 10.1073/pnas.92.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Farias M, Kapfhamer D, Tobias J, Grant G, Abel T, Bucan M. Biochemical, molecular and behavioral phenotypes of Rab3a mutations in the mouse. Genes, Brain and Behvior. 2007;6:77–96. doi: 10.1111/j.1601-183X.2006.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeckel MF, Kapur A, Johnston D. Multiple forms of LTP in hippocampal CA3 neurons use a common postsynaptic mechanism. Nat Neurosci. 1999;2:625–633. doi: 10.1038/10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalutsky RA, Nicoll RA. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]
- Zeng H, Chattarji S, Barbarosie M, Rondi-Reig L, Philpot BD, Miyakawa T, Bear MF, Tonegawa S. Forebrain-specific calcineurin knockout selectively impairs bidirectional synaptic plasticity and working/episodic-like memory. Cell. 2001;107:617–629. doi: 10.1016/s0092-8674(01)00585-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.