Abstract
The astrocyte-neuronal lactate shuttle hypothesis (ANLSH) posits that lactate released from astrocytes into the extracellular space is metabolized by neurons. The lactate released should alter extracellular pH (pHe), and changes in pH in central chemosensory regions of the brainstem stimulate ventilation. Therefore, we assessed the impact of disrupting the lactate shuttle by administering 100 microM α-cyano-4-hydroxy-cinnamate (4-CIN), a dose that blocks the neuronal monocarboxylate transporter (MCT2), but not the astrocytic MCTs (MCT1 and MCT4). Administration of 4-CIN focally in the retrotrapezoid nucleus (RTN), a medullary central chemosensory nucleus, increased ventilation and decreased pHe in intact animals. In medullary brain slices, 4-CIN reduced astrocytic intracellular pH (pHi) slightly, but alkalinized neuronal pHi. Nonetheless, pHi fell significantly in both cell types when they were treated with exogenous lactate, although 100 microM 4-CIN significantly reduced the magnitude of the acidosis in neurons, but not astrocytes. Finally, 4-CIN treatment increased the uptake of a fluorescent 2-deoxy-d-glucose analogue in neurons, but did not alter the uptake rate of this 2-deoxy-d-glucose analogue in astrocytes. These data confirm the existence of an astrocyte to neuron lactate shuttle in intact animals in the RTN, and lactate derived from astrocytes forms part of the central chemosensory stimulus for ventilation in this nucleus. When the lactate shuttle was disrupted by treatment with 4-CIN, neurons increased the uptake of glucose. Thus, neurons seem to metabolize a combination of glucose and lactate (and other substances such as pyruvate) depending, in part, on the availability of each of these particular substrates.
Keywords: astrocytes, neurons, lactate shuttle, metabolism, ventilation, pH
Introduction
Magistretti and Pellerin (1999) proposed that an astrocytic-neuronal lactate shuttle exists in which glutamate, released from neurons during synaptic activity, is taken up by astrocytes in a sodium-coupled process and the ensuing sodium-dependent activation of the Na-K-ATPase triggers glucose uptake, which results in the rapid formation and release of lactate from astrocytes via MCT1 and MCT4. The lactate released by astrocytes into the extracellular space is, according to this hypothesis, transported into neurons by MCT2 and used to fuel activity-dependent energy demands. A family of monocarboxylate transporters (MCTs) transport lactate, pyruvate and ketone bodies across biological membranes (Halestrap and Price, 1999), and MCT1, MCT2 and MCT4 have been identified in the central nervous system. MCT1 and MCT4 are expressed in astrocytes, and MCT2 is expressed in neurons (Bröer et al., 1997; Gerhart et al., 1997; Pierre et al., 2002; Debernardi et al., 2003). Since MCT2 has a lower Km for lactate (~ 0.7 mM) than the Km for either MCT1 or MCT4 (~ 5-7 mM and 28-35 mM, respectively; (Bröer et al., 1997; Poole and Halestrap, 1997; Bröer et al., 1999; Halestrap and Price, 1999; Manning Fox et al., 2000), the cell specific distribution of these transporters between astrocytes and neurons seems designed to move lactate from astrocytes to neurons.
One consequence of shuttling lactate between astrocytes and neurons is the effect of lactate on extracellular and intracellular pH. The buffering power of the extracellular space is low (Pappenheimer et al., 1965), and changes in either lactate efflux or influx in astrocytes or neurons can result in significant reciprocal fluctuations in pH. Blood and brain tissue pH are maintained at constant levels by central respiratory chemoreceptors located primarily in the medulla. These chemosensors detect pH and adjust ventilatory drive to alter blood and tissue levels of CO2 and thereby regulate pH. The retrotrapezoid nucleus (RTN) in the ventral medulla contains chemosensory neurons that provide an excitatory pH-dependent drive to breathing. RTN neurons, many of which are glutamatergic (Mulkey et al., 2004), increase their activity as the CO2 rises and the pH falls (Ritucci et al., 2005). In the current study, we used the respiratory effects of changes in extracellular pH (pHe) and intracellular pH (pHi) to test aspects of the ANLSH. Much of the support for the lactate shuttle hypothesis comes from cultured cells (Kimmelberg, 2004), and we were, therefore, interested in testing the lactate shuttle hypothesis in vivo or in situ in brain slices. To that end, we first we examined the ventilatory effects of focally inhibiting MCT2 in the RTN in vivo, and we measured pHe to determine whether inhibiting MCT2 altered pHe within the RTN. Second, we tested the hypothesis that inhibition of MCT2 and impaired lactate transport would lead to accumulation of lactate in astrocytes and a fall in intracellular pH (pHi), but prevent uptake of lactate in neurons and alkalinize neuronal pHi. Finally, we tested the hypothesis that inhibition of neuronal lactate uptake would be associated with a reciprocal increase in neuronal glucose uptake.
Methods
The St. Lawrence University Animal Review Committee approved this study, and all procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals as put forth by the Public Health Service, National Institutes of Health.
In vivo studies of MCT inhibition
Adult Sprague-Dawley rats of either sex aged 7-11 weeks weighing between 250-350 grams were used for this study. Animals were kept on a 12:12 hour light/dark cycle and provided food and water ad libitum. Each rat was prepared for the study by placing a push-pull cannula (Plastics One, Roanoke, VA) unilaterally in the RTN. To do this, each rat was anesthetized with xylazine (6 mg/kg I.P.) followed by ketamine•HCl (70 mg/kg I.M.) and secured in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). A midline incision was made; a hole was drilled in the skull 5 mm caudal to lamda and 2.2 mm from the midline (left side); and a push-pull guide cannula (I.D.= 0.29 mm, O.D. = 0.56 mm) was inserted through the burr hole. Two anchor screws were placed adjacent to the cannula, and the guide cannula and the anchor screws were attached to the skull with cranioplast (Plastics One). The scalp was sutured together up to the border of the guide cannula. A dummy obturator was screwed into the guide cannula to maintain patency of the lumen during the recovery period and between experiments. The obturator extended 0.3 mm beyond the end of the guide cannula. Approximately one week following surgery, ventilatory measurements were made in each animal. In previous work we found that ventilation was unaffected by the surgery or by perfusion of the RTN with aCSF alone.
Cerebrospinal fluid solutions and solution delivery
Artificial CSF (aCSF) contained the following salts (in mM): NaCl 124, KCl 3, CaCl2 2.4, MgSO4, 1.3, KH2PO4 1.24, NaHCO3 26 and glucose 10. Prior to delivery, aCSF was equilibrated with 95% O2 and 5% CO2, warmed to 37°C and loaded into 3 ml plastic syringes. The pH of all solutions was 7.48. Solutions were delivered to the RTN using a push-pull syringe pump (World Precision Instruments, Sarasota, FL). Flow was directed through polyethylene tubing that was connected to an inner delivery cannula (Plastics One, Roanoke, VA; I.D. = 0.1 mm, O.D. = 0.2 mm). Prior to perfusate delivery, the line and inner cannula were flushed with aCSF or aCSF plus the drug to be tested, and the solution was perfused throughout each experiment. After the dead space of the inner cannula was filled, the cannula was screwed into the guide cannula, and the return vacuum line was connected. Like the dummy cannula, the inner delivery cannula extended 0.3 mm beyond the end of the guide cannula. The flow through the cannula was 0.12 ml/hr. Push-pull cannulae resemble microdialysis in that the amount of tissue exposed to the agent is restricted, and the concentration of the test agent remains constant at the tissue adjacent to the probe throughout the perfusion period.
Ventilatory responses to carbon dioxide
Body weight and barometric pressure were recorded before each experiment. Each animal was placed in the plethysmograph and allowed to accommodate to the chamber for 30 minutes. Perfusion of the push-pull cannula began at the start of the 30 min accommodation period in the plethysmograph and was continued throughout each experiment. During the experiment, each animal was exposed serially to increasing levels of humidified CO2 in the plethysmograph beginning with room air and progressing in 2% increments to a final fractional inspired CO2 (FICO2) of 8%. Animals were maintained at each level of CO2 for approximately 10 minutes following the stabilization of CO2 levels within the plethysmograph chamber. The chamber had a volume of ~1 L, and the rate of airflow through the box was 1.25 L*min-1. Stable CO2 values within the chamber were achieved in 2-3 minutes. The chamber carbon dioxide concentration (Applied Electrochemistry, CD3A) and pressure (Grass Instruments, PT5) were measured continuously, and the signals were low pass filtered at 10 Hz, digitized at 20 Hz, smoothed using a moving-average transformation and stored on a personal computer (World Precision Instruments, Sarasota, FL, Biopac). Both the chamber and rat body temperature were measured at the end of each step-change in CO2 concentration. Body temperature was measured using an infrared sensor (Linear Laboratories, C1600). For tidal volume calculations, the measured body temperature was corrected to rectal temperature based on previous experiments in which these body temperatures were determined by infrared emissivity and rectal temperature measured simultaneously. The average pressure change of each breath, which reflects the magnitude of the tidal volume, was determined from 100 breaths during the last two minutes of each test period (i.e. room air or CO2). The frequency of breathing was measured during the last minute of each period. Tidal volume was calculated using the method described by Pappenheimer (1977).
Inhibition of lactate transport
MCT transport was inhibited using α-cyano-4-hydroxy-cinnamate (4-CIN; stock solution dissolved in 0.1% DMSO and used at a final concentration of 100 or 500 μM; Sigma, St. Louis, MO) dissolved in aCSF. 4-CIN is a competitive, nontransportable inhibitor of MCTs. The IC50 (the concentration of drug required to reduce lactate transport by one-half) is 425 μM for MCT1, 900 μM for MCT4 and 24 μM for MCT2 (Bröer et al., 1999). A 500 μM concentration of 4-CIN would be expected to inhibit MCT1 and MCT4 transport by no more than 50%, whereas transport by MCT2 would be virtually eliminated (500 μM is ~21 time the IC50). A 100 μM concentration of 4-CIN should inhibit monocarboxylate transport by MCT1 and MCT4 by less than 13% while eliminating monocarboxylate transport by MCT2 (100 μM 4-CIN is ~4 times the IC50 MCT2). In the in vivo experiments, we focally perfused the RTN unilaterally with normal aCSF (pH 7.48) or aCSF plus 100 μM 4-CIN to determine if inhibition of MCT2 increased ventilation at each level of CO2 tested (Hewitt et al., 2004; Parisian et al., 2004). Each perfusate was tested on a separate day, and at least one day separated each perfusate trial. The order in which the perfusates were delivered was randomized within each animal.
In vivo tissue pH measurements
Tissue pH measurements were made using a custom fabricated electrode (Beetrode; WPI) placed in the RTN through a steel guide cannula located just dorsal to the retrotrapezoid nucleus. After measuring pHe in the control state while the animal breathed room air, MCT function was impaired by injecting 200-300 nL of 100 uM 4-CIN unilaterally into the RTN through the guide tube (a larger volume of 4-CIN was administered since we could not continuously perfuse and measure pHe simultaneously). The pH electrode was placed in the RTN, and the pHe adjacent to the electrode was recorded in mV for ten minutes in intact awake rats. In separate experiments, the pH electrode was placed into the RTN via the guide cannula and the FICO2 of the animal was increased to 5% while the pHe was measured for 10 minutes in intact awake rats. The mV readings occasionally drifted for no apparent reason, and measurements in which the control pHe values before and after each treatment differed by more than 2 mV (0.034 pH units) were excluded. Stable pHe measurements were recorded as mV with pencil and paper during in each condition and converted to pH using a calibration factor measured in vitro. The pH electrodes were calibrated in vitro with standard pH buffers (pH 4, 7 and 10) at 38 °C, and the conversion factor was 58.0 ± 1.8 mV/pH unit (Li and Nattie, 2002).
Histological analysis of cannula locations
After completing all experimental conditions, Fast Green was added to the aCSF and perfused into the RTN for approximately 1 hour (a time similar to the duration of a typical experiment) to evaluate the location of each cannula and the distribution of perfusate within the medulla. After the dye was perfused, the rat was deeply anesthetized and perfused transcardially through the left ventricle with a tissue fixative (Streck S.T.F. pH 7.4, Streck Laboratories). After fixation, the brain was removed, flash frozen (Fisher Scientific, Histofreeze) and sectioned (40 μm thickness) with a cryostat maintained at -20°C (Reichert, Histostat). Tissue sections were mounted on ultrastick glass slides (Becton Dickinson, Gold Seal), stained with neutral red or cresyl violet and serial sections were photographed using a digital camera (Hamamatsu, Orca-ER). The images were compared to photographic plates in a stereotaxic atlas to identify the location of the cannula in each section (Paxinos and Watson, 1998). Each cannula made a small hole in the tissue at the end of the cannula track, and the placement of the cannula was determined by identifying the section with the largest lucent cross-sectional area. The tissue stained by Fast Green was located directly adjacent to the tip of the guide cannula (~600 μm in diameter), as in our previous studies in the RTN (Holleran et al., 2001; Hewitt et al., 2004; Parisian et al., 2004).
In vitro experiments: Slice preparation for pHi measurements
Brain tissue slices were prepared from adult transgenic mice of either sex that express enhanced green fluorescent protein (EGFP) in astrocytes (FVB/N × Tg GFAPGFP; Jackson Laboratories; see Fig. 1A). The mice were 3.6 ± 0.6 months of age at the time of study. Each animal was killed by rapid decapitation, and the brain was removed from the skull and submerged in chilled (4°C) choline-substituted slicing solutions (described below) for 2-3 minutes. The caudal cerebellum was removed, and a tissue block extending from the obex caudally to the rostral portion of the pons was prepared. Transverse medullary slices (400 μm thick) were sectioned in chilled, oxygenated choline-substituted slicing solution using a vibratome (Pelco, Series 1000, Redding, CA). Following sectioning, slices were transferred to a holding chamber containing oxygenated aCSF (described below) at room temperature.
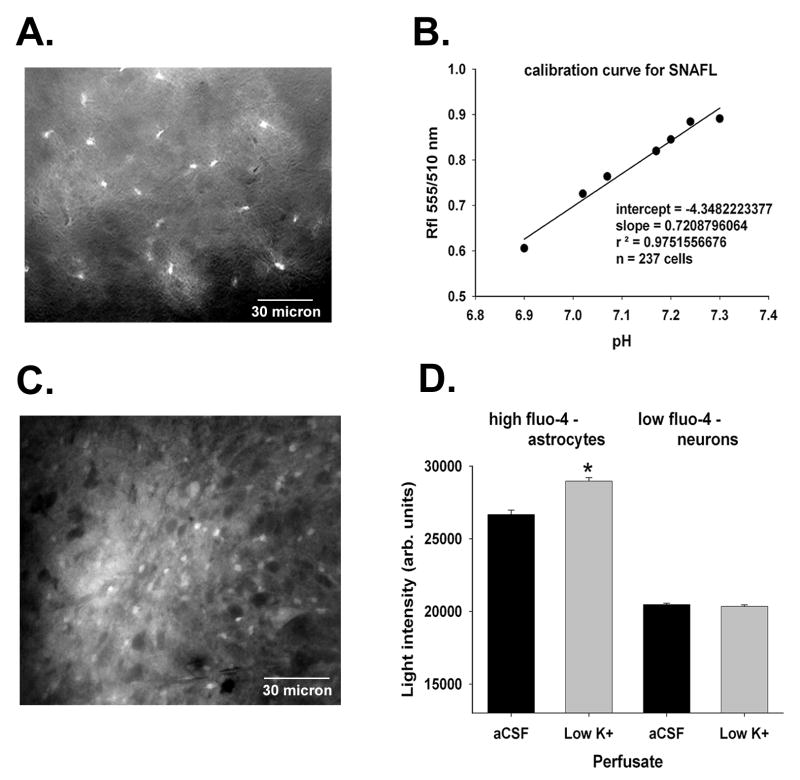
Figure 1.
Expression of EGFP in astrocytes in brain slices prepared from transgenic mice is shown in panel A (scale bar = 30 micron). In panel B, the calibration curve for SNAFL is shown. Cells were exposed to solutions containing nigericin at known pH values ranging from 6.9-7.3 and the ratio of fluorescence at two excitation wave lengths (550 and 510 nm) was determined. The calibration data were fit using a linear regression equation because we used only a small pH range close to the pKa of SNAFL. Fluo-4 loading is shown in panel C (scale bar = 30 micron); the cells that stained more intensely for Fluo-4 were astrocytes. Fluo-4 is a Ca++ sensitive dye, and when the population of Fluo-4 loaded cells was divided into two groups on the bases of the intensity of Fluo-4 fluorescence in aCSF (panel D), only those cells with more intense uptake demonstrated increased intracellular Ca++ levels after exposure to low potassium solutions (Dallwig and Deitmer, 2002). “*” indicates P < 0.05.
Solutions for brain slice studies
The control aCSF contains (in mM): NaCl - 124, KCl -3, CaCl2 - 2.4, MgSO4- 1.3, KH2PO4 - 1.24, NaHCO3 - 26, and glucose - 10. In solutions containing high concentrations of K+, Na+ was reduced equally to keep the solution isosomotic. Except for the dose-response experiments, 4-CIN was used at a final concentration of 100 μM in the in vitro experiments. Prior to delivery to the tissue, all aCSF solutions were loaded into 60 ml syringes, equilibrated with 95% O2 - 5% CO2 to maintain a pH of 7.48 at 37°C, and heated to 37°C using a servo-controlled syringe heater block, in-line perfusion heater and servo-controlled stage heater (Model SW-707; SH-27B; TC-344B; Warner Instruments, New Haven, CT).
We used a modified aCSF solution to reduce cell death reducing intracellular sodium and calcium and glutamatergic excitation. Therefore, the slicing solution contained no sodium (choline was substituted for sodium), reduced calcium, elevated magnesium, kynurenic acid (a glutamate receptor antagonist) and ascorbic acid as an antioxidant. Thus, the choline-substituted slicing solution contained (in mM): choline chloride - 135, KCl - 1, CaCl2 - 0.5, MgCl2 - 20, NaH2PO4 - 1.4, choline bicarbonate - 24, kynurenic acid - 20, ascorbic acid - 5, and glucose - 10. The choline substituted slicing solution is equilibrated with 95% O2 - 5% CO2 and chilled to 4 °C. The pH of the choline substituted slicing solution was ~6.9.
Measurement of pHi in brain slices
Since a proton is co-transported with the lactate anion across the cell membrane, pHi may be used as a surrogate measure of lactate transport (Schneider et al., 1993; Bröer et al., 1999). We used a pH-sensitive, red-shifted fluorescent probe, 5-(and-6)-carboxy SNAFL®-1 (SNAFL, Molecular Probes, Eugene, OR), for studies pHi. The emission spectra of SNAFL do not overlap the emission spectrum of EGFP in the mice we studied; slices were loaded with 20 μM SNAFL in control saline bubbled with 95% O2/ 5% CO2 for 45 minutes at room temperature. Following loading with SNAFL, the slices were returned a holding chamber which contained aCSF equilibrated with 95% O2/ 5% CO2 at room temperature for 30 minutes prior to experimentation. Intracellular pH was measured in single, SNAFL-loaded slices placed in a chamber that was continuously superfused with a CSF and mounted on the stage of a Nikon Eclipse TE200S inverted microscope (Nikon Inc, Melville, NY). The pHi of individual cells was measured within discrete areas (~ 0.042 mm2) of the medulla by alternately exciting the area with light from a 75W xenon lamp (Chiu Industries, Kings Park, NY) at 510 ± 10 nm and 555 ± 10 nm wavelengths for 0.3-0.8 seconds. The excitation filters were switched by a computer controlled filter wheel (Lambda 10-2, Sutter Instruments Co, Novato, CA). The emitted fluorescence (620 nm) from the intracellular SNAFL was filtered using a 585 nm dichroic mirror (Chroma Technology, Bennington, VT), intensified and measured with a cooled CCD camera (Hamamatsu Orca ER, Japan). The digital images were acquired and processed with Compix software (C Imaging Systems, Cranberry Township, PA).
The ratiometric values of pHi based on emitted SNAFL fluorescence were calibrated using the nigericin method (Boyarsky et al., 1988). The nigericin calibration solution contained (in mM): KCl - 104, CaCl2 - 2.4, MgSO4 - 1.3, KH2PO4 - 1.24, glucose - 10, NMDG-HEPES - 25, K-HEPES - 25, and nigericin 1.6 ×10-2. A calibration curve was constructed by measuring the 555/510 nm ratio fluorescence (RFL) of cells (n= 237) exposed to nigericin containing calibration solutions of known pH ranging from 6.9-7.3 pH units at 37 °C. These ratio measurements were fit by linear regression and changes in pHi were calculated from the following calibration equation: pHi = RFL+ 4.34/0.721 (r2 = 0.98; Figure 1B). After each calibration, the entire perfusion apparatus was soaked and washed three times in 70% ethanol in water and subsequently rinsed repeatedly in distilled water to remove any retained nigericin.
Measurement of glucose uptake
We used EGFP(-) mice to study glucose uptake in brain slices. Brain slices containing the RTN were prepared from the medulla of adult mice as described above. Astrocytes were identified using a protocol in which brain slices were incubated in Fluo-4-AM (4 μM; Molecular Probes; Eugene, OR) in aCSF and the extracellular K+ was elevated to 5 mM for 45 minutes at room temperature (Dallwig and Deitmer, 2002). Astrocytes loaded using this method demonstrate Ca++ oscillations when exposed to K+ solutions containing nominal potassium (Figure 1C) and co-label with glial fibrillary acidic protein (Dallwig and Deitmer, 2002). Neurons take up Fluo-4 as well. However, the staining was less intense compared to glia, and there were no intracellular Ca++ oscillations when neurons were exposed to low potassium solutions (Fig. 1D). Fluo-4 loaded cells were visualized by exciting the fluorophore at 480 ± 10 nm and filtering the emitted light (535±10 nm) through a 505 nm long pass dichroic filter (Chroma Technology, Bennington, VT). Cells were defined as glia if they were Fluo-4 positive and if the emitted light intensity after loading was above the mean light intensity level for the entire group of cells measured. The Fluo-4 negative group (putative neurons) was comprised of cells in which the intensity of emitted fluorescent light was below the mean intensity for the entire group of cells measured. That this Fluo-4 method accurately identified astrocytes and neurons in our studies was confirmed subsequently when we obtained mice expressing EGFP exclusively in astrocytes. The correspondence between high intensity Fluo-4 fluorescence and the presence of EGFP in brain cells in these animals was virtually 1:1 (Erlichman, J.S., unpublished observations).
We used 2-[N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxyglucose (2-NBDG) a fluorescent glucose tracer to measure glucose uptake (Yamada et al., 2007). Regions of interest were defined by the somatic distribution of Fluo-4, and 2-NBDG uptake was subsequently measured within these regions. To measure 2-NBDG uptake, 2-NBDG (15 μM) was added to aCSF, and this solution was used to perfuse the slice for one minute after which time the solution was switched back to control aCSF and washed for five minutes. The five minute wash was necessary to remove free 2-NBDG from the extracellular space. This procedure was repeated four times during each experiment and resulted in progressively greater loading with each 2-NBDG pulse. Between each loading/wash period the fluorescent intensity of 2-NBDG was measured in each cell each minute for three minutes after the wash was completed. We determined in preliminary experiments that a one minute loading period with the tracer was a) sufficient to generate an incremental increase in light intensity that yielded a satisfactory signal above baseline Fluo-4 fluorescence, and b) would not saturate our camera following four periods of loading. Once the tissue was washed, the light intensity collected from subsequent images declined slowly, which likely reflected the degradation of phosphorylated 2-NBDG to a non-fluorescent metabolite (Yamada et al., 2000; Yamada et al., 2007). The effect of inhibition of MCT2 with 100 μM 4-CIN was assessed by comparing 2-NBDG uptake before 4-CIN treatment to 2-NBDG uptake after 4-CIN treatment in astrocytes and neurons. The 4-CIN was added to the perfusate between the second and third 2-NBDG treatment periods. In control studies, 2-NBDG uptake in astrocytes and neurons was measured after all four 2-NBDG treatment periods and no 4-CIN treatment was given.
Data analysis and statistics
In the studies of intact animals, the type of perfusate delivered (aCSF or aCSF plus 4-CIN) and the FICO2 (0-8%) were used in a two-way repeated measures ANOVA (Systat 9.0, SPSS Science, Chicago, IL) to analyze the whole animal ventilatory responses. In the studies of pHe in intact animals, and the studies of pHi in brain slices, paired t-tests were used to compare steady-state pH values before and after treatment with 4-CIN. The analysis of the intensity of 2-NBDG uptake was conducted using a three-way ANOVA in which time of exposure (treatment 1, 2, 3, or 4) was a repeated within subjects factors and drug treatment (4-CIN versus no 4-CIN exposure) and cell type (astrocytes versus neurons) were between subjects factor. These data were also analyzed as a percent of control 2-NBDG uptake intensity using a two-way ANOVA in which time of exposure and drug treatment with or without 4-CIN were the two factors. When any of the analyses of variance indicated that significant differences existed among treatments, specific pre-planned comparisons were made using P-values adjusted by the Bonferroni method. Values reported are the mean ± the standard error of the mean (SEM). P-values ≤ 0.05 were considered statistically significant.
Results
Ventilatory effects of unilateral perfusion of RTN with 4-CIN
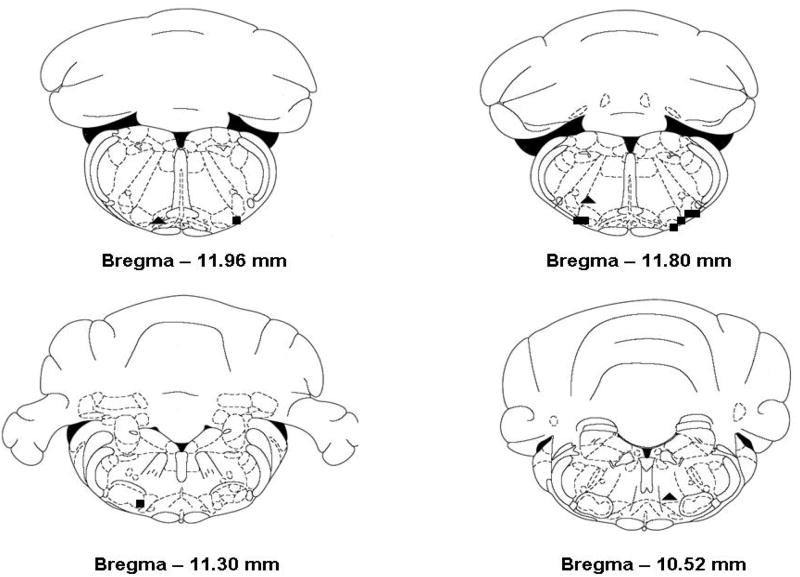
We studied hypercapnic ventilatory responses during focal unilateral perfusion of the RTN with aCSF or aCSF plus 100 μM 4-CIN in nine animals. The slope of the response of minute ventilation, tidal volume and respiratory frequency was not changed by perfusion of the RTN with aCSF containing 4-CIN (no CO2 by drug interaction in the ANOVA; Fig. 2, panels A, B, and C, left side). However, minute ventilation, tidal volume and respiratory frequency were increased significantly for all three variables at every value of inspired CO2 during focal perfusion of aCSF plus 4-CIN compared to aCSF alone (P < 0.01 for all three variables). Moreover, ventilation, tidal volume and respiratory frequency all increased significantly as the inspired FICO2 increased from 0 to 8% (P < 0.001 for all variables). Thus, perfusion with 4-CIN did not change the apparent CO2 sensitivity, but did shift the ventilatory response curves up and to the left significantly for all three variables. The locations of all the cannulae in these nine animals fell within or closely adjacent to the RTN (Fig. 3; squares, ■), which extends from the rostral end of the facial nucleus caudally to the rostral end of the nucleus ambiguus and medio-laterally between the pyramidal tract and the spinotrigeminal tract and is deep to the ventral surface 100-300 μm (Pearce et al., 1989).
Figure 2.
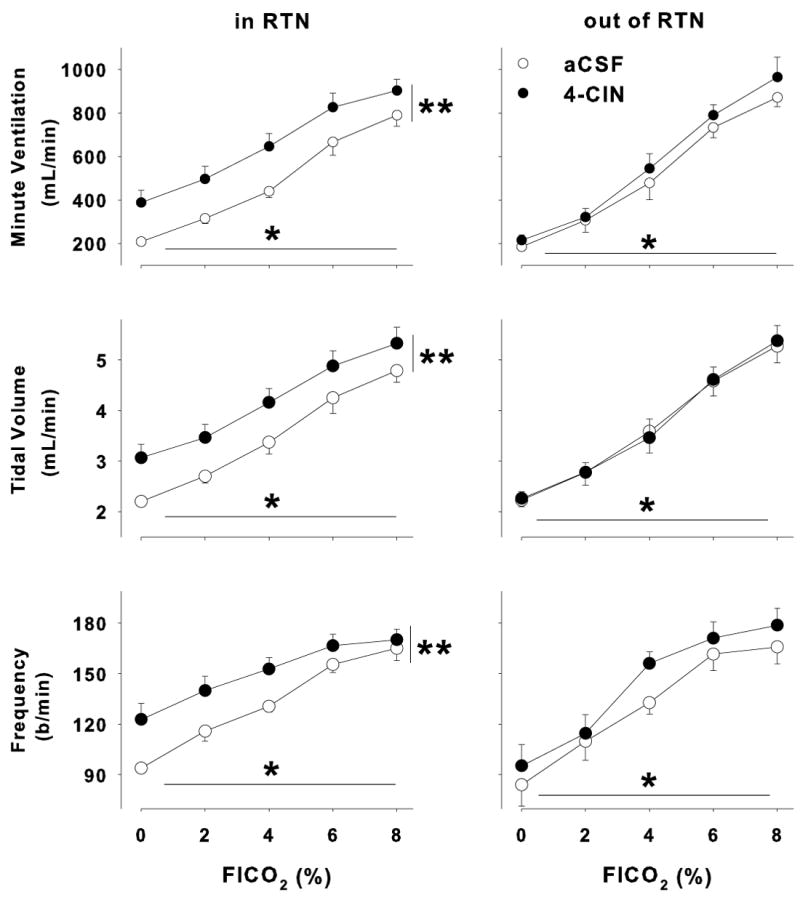
Ventilatory effects of 4-CIN perfusion unilaterally in the RTN. (A) Minute ventilation, (B) tidal volume and (C) frequency increased significantly as the fractional inspired CO2 increased (P <0.01 for all three variables). In addition, focal unilateral perfusion of 4-CIN into the RTN increased minute ventilation, tidal volume and frequency at all levels of FICO2 (P < 0.01 for all three variables; left panels; n = 9). On the other hand, 4-CIN perfused focally in the ventral medulla outside the area of the RTN did not change minute ventilation, tidal volume or frequency (n = 3), although increasing the FICO2 increased these variables just as it had in the RTN treatment group.
Figure 3.
Histological placement of cannulae for ventilatory studies within the RTN (■; n = 9) and in adjacent sites in the medulla (▲; n = 3).
To evaluate the specificity of the ventilatory effects of 4-CIN, three additional animals were cannulated in regions outside the RTN, and the ventilatory effects of 4-CIN were examined. In contrast to our findings in the RTN, unilateral perfusion of 4-CIN in regions adjacent to the RTN had no significant effect on any of the measured ventilatory variables (Fig. 2: A, B and C - right panels). Hypercapnia still resulted in a significant increase in ventilation, but there was no effect of drug treatment on the ventilatory response to CO2 (p > 0.05 all three variables). The locations of the probes in these three animals are also shown in Fig. 3 (triangles, ▼). Focal administration of 100 μM 4-CIN unilaterally within the RTN increased minute ventilation at all levels of inspired CO2, and focal administration of 4-CIN outside the RTN did not increase minute ventilation. Thus, the effect of blocking neuronal lactate uptake on ventilation was specific to the RTN.
Effects of 4-CIN on pHe in the RTN
Microinjection of aCSF plus 4-CIN into the RTN in awake rats resulted in a rapid and significant acid shift in pHe of approximately 0.08 ± 0.02 pH units (p < 0.05). To compare the level of extracellular acidification associated with 4-CIN to the effect of inhaled CO2, we performed a separate set of experiments in which the FICO2 was increased to 5% while simultaneously measuring pHe in the RTN. The elevated inspired CO2 reduced the pHe in the RTN by 0.07 ± 0.1 pH units (P < 0.05), but the pHe change was similar to that observed with the 4-CIN injection alone. These results suggest that both treatments result in a similar stimulus to the acid sensitive cells in the RTN, an area that is known to increase ventilation when acidified in similar studies in adult rats (Akilesh et al., 1997; Erlichman et al., 1998; Li et al., 1999; Li and Nattie, 2002). Administration of 4-CIN may have decreased pHe outside the RTN (we did not make these measurements), but these regions do not contain a density of chemosensory neurons sufficient to stimulate ventilation (Akilesh et al., 1997; Li et al., 1999) or the density of astrocytes may be too low to generate sufficient lactate (Erlichman et al., 2004).
Effects of 4-CIN on pHi in the RTN
We studied the effects of 4-CIN on pHi in EGFP(-) cells, which we defined as neurons, and in EGFP(+) cells, which we defined as astrocytes, in the region of the RTN in brain slices. Intracellular pH was measured using the ratiometric fluorescent dye, SNAFL. Under control conditions, astrocytic pHi was 7.34 ± 0.01 in glia (n = 80). The addition of 4-CIN to the perfusate resulted in small, rapid, and dose-dependent decrease in pHi compared to control conditions (Figure 4A; p < 0.05; n = 92). The peak acid shift in astrocytic pHi following 10 minutes exposure to 100 μM 4-CIN was small, 0.005 ± 0.004 pH units, but significant (P < 0.05; Fig. 4C). The reduction in pHi was significantly greater after exposure to 500 μM 4-CIN (0.02 ± 0.01 pH units) although the absolute decline in pHi was still modest.
Figure 4.
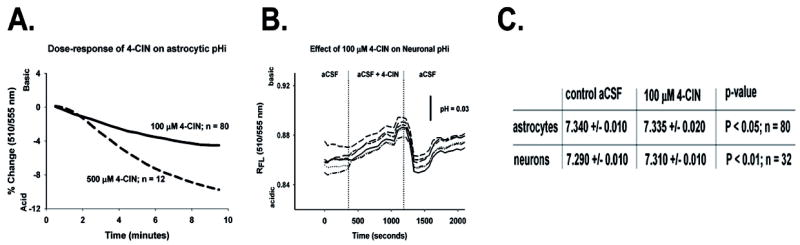
Effects of 4-CIN on pHi in astrocytes and neurons. Astrocytes (eGFP(+) cells; panel A) showed a dose-dependent acidification in pHi during 4-CIN exposure, expressed as the percent change in pHi. Neurons (eGFP(+) cells; panel B), on the other hand, were alkalinized slightly, but significantly after treatment with 4-CIN, expressed as the fluorescence ratio of SNAFL at two excitation wave lengths. The control and peak pHi changes before and after 100 μM 4-CIN are shown for each cell type in panel C along with the p-values for comparisons between the control and test conditions.
In neurons, the baseline control pHi was 7.27 ± 0.01 (n = 32), and this values was significantly lower than the pHi in astrocytes (p< 0.01), just as we found previously (Nottingham et al., 2001). The neuronal pHi was, however, slightly more alkaline than our previous estimate of neuronal pHi (Ritucci et al., 2005). In contrast to astrocytes, which were very slightly acidified after exposure to 4-CIN, neurons were consistently alkalinized when exposed to 100 μM 4CIN (peak pHi = 7.31 ± 0.01; n = 32; P < 0.01; Fig. 4B). The intracellular alkalosis was reversed when 4-CIN was removed from the perfusate. During the washout of 4-CIN, the neurons demonstrated a transient, acidifying undershoot (nadir of pHi = 7.26 ± 0.009) before returning to control values. The acidifying undershoot indicates that the cells had activated pH regulatory mechanisms (likely the Cl-/HCO3- exchanger) during the alkalinizing phase of 4-CIN treatment in an attempt to regulate pHi toward the initial control value. The pHi values measured during exposure to 4-CIN as well as the washout period were significantly different from control (p<0.001 for both time points). Measurements of pHi alone cannot identify the form of the monocarboxylate being transported (i.e. lactate, pyruvate or ketones), but it is likely that lactate was the predominant monocarboylate transported in our studies. The baseline extracellular lactate concentration in the CNS is ~ 1-3 mM during resting conditions in vivo, and although the Km of MCT2 for pyruvate is 5 fold less than the Km for lactate (Bröer et al., 1999), the pyruvate concentration in the cortex in vivo is approximately 20 fold less than the concentration of lactate (Homola et al., 2006). Lactate was likely the major monocarboxylate transported between cellular compartments, and therefore changes in lactate levels were the cause of the pHi changes that we measured. Mitochondrial monocarboxylate transporters in neurons can be inhibited by 4-CIN. Had this happened, we might have expected an increase in glycolysis and a decrease in neuronal pHi. In fact, 4-CIN treatment was associated with a slight alkaline shift in neuronal pHi, and it seems unlikely that 100 μM 4-CIN inhibited mitochondrial MCTs.
Effects of 5 mM lactate pulse on pHi in the RTN
To assess the cellular specificity of lactate transport and the selectivity of the 4-CIN dose that we studied, we examined the effect of exogenous lactate (5 mM) on pHi in astrocytes and neurons in the presence or absence of 100 μM 4-CIN. The addition of sodium lactate to the aCSF solution created an inwardly direct lactate gradient and resulted in a decrease in pHi in all cells tested (Fig. 5A and B; n = 31; p < 0.001). The lactate pulse decreased pHi in astrocytes by 0.08 ± 0.01 pH units, and decreased neuronal pHi by 0.11 ± 0.01 pH units. These changes in pHi after exposure to equal concentrations of extracellular lactate were significantly difference between the cell types (p < 0.01). The addition of 100 μM 4-CIN to the aCSF/lactate solution had little effect on the magnitude of the lactic acidosis in astrocytes (Figure 5C; Δ pHi 0.08 versus 0.08; n = 13; p = 0.83), whereas, the addition of 100 μM 4-CIN decreased the size of the shift in neuronal pHi by approximately 20%; the pHi difference between lactate alone and lactate plus 100 μM 4-CIN was statistically significant (Fig. 5C; Δ pHi before 4-CIN = 0.11 vs 0.08 pH units after 4-CIN; n = 18; p < 0.001). Thus, 4-CIN inhibited the movement of lactate into neurons, but not into astrocytes, which is consistent with the hypothesis that lactate moves from astrocytes to neurons.
Figure 5.
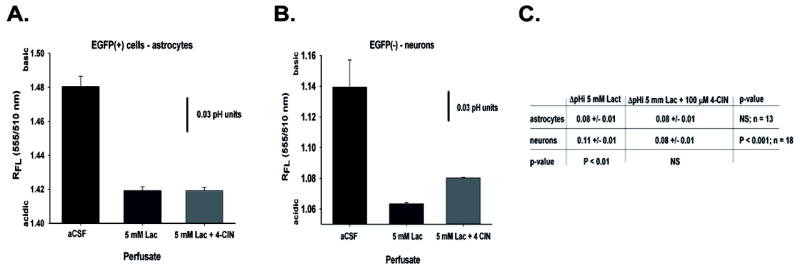
Effects of 5 mM sodium lactate and 100 μM 4-CIN on pHi, in astrocytes is shown in panel A. The peak change in pHi from control before and after 100 μM 4-CIN treatment are shown for each cell type in panel B along with the p-values for comparisons between the control and test conditions and between cell types within each condition. ‘NS’- not statistically significant.
Loading of the glucose analogue, 2-NBDG, in the RTN
If lactate contributes significantly to the metabolism of neurons or astrocytes, then blocking lactate uptake using 4-CIN should result in a compensatory increase in glucose uptake. To assess this possibility, we measured the uptake of a fluorescent analogue of glucose, 2-NBDG, before and after exposure to 100 μM 4-CIN. Both cell types demonstrated a significant increase in the accumulation of the fluorescent probe with repeated loading (n = 426; p < 0.001). The uptake of 2-NBDG was similar in neurons and astrocytes, but within astrocytes, the initial loading with 2-NBDG was a bit variable. Therefore, the intensity of 2-NBDG uptake in subsequent test periods was expressed as a percentage of the control values to facilitate comparisons of 2-NBDG uptake overtime within each cell type (Fig. 6). The ANOVA of 2-NBDG uptake in astrocytes indicated that uptake increased significantly over time in all astrocytes tested (P < 0.001). However, the rate of uptake of 2-NBDG in astrocytes was unaffected by the addition of 4-CIN to the perfusate between the second and third 2-NBDG exposure periods. The uptake of 2-NBDG by neurons also increased significantly over time, but the relative increase in 2-NBDG uptake was significantly greater in neurons that were treated with 4-CIN compared to neurons during the same 2-NBDG exposure periods that were not treated with 4-CIN (P< 0.05 for both the third and fourth 2-NBDG exposure periods). Thus, 4-CIN treatment significantly increased 2-NBDG uptake in neurons, but not in astrocytes.
Figure 6.
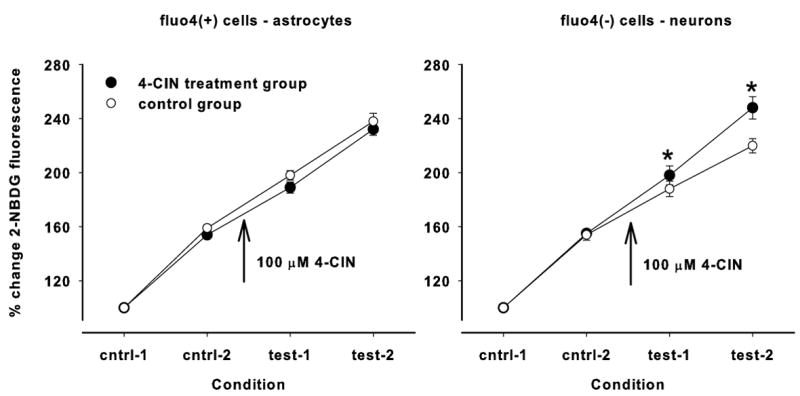
Uptake of 2-NBDG, expressed as a percent of the first exposure period, increased in each subsequent loading period (P < 0.001). The comparison between control conditions (no 4-CIN treatment; open circles) and test conditions (100 μM 4-CIN added between the second control period and the first test period; closed circles) are shown for astrocytes on the left and neurons on the right. Astrocytic loading of 2-NBDG was unaffected by 4-CIN treatment, but neuronal loading of 2-NBDG was significantly increased (P < 0.05; indicated by ‘*’) in each test condition.
Discussion
Effects of MCT inhibition
The ANLSH, as originally described (Pellerin and Magistretti, 1994; Magistretti and Pellerin, 1999), has been criticized: glutamate uptake by astrocytes is not uniquely coupled to anaerobic glycolysis, except possibly in cultured cells; it is not clear that there was net flux of lactate from astrocytes to neurons; and it has not be established that astrocytes are the source of lactate production or that neurons are the sink for lactate consumption, both cell types possess the enzymatic machinery for anaerobic and oxidative glycolysis (Chih et al., 2001; Hertz, 2004). Moreover, support for the hypothesis comes largely from cultured cells rather than in vivo measurements (Kimmelberg, 2004). Subsequently, the ANLSH was modified to include just two ideas: glutamate uptake by astrocytes depends on anaerobic glycolysis, and neurons oxidize lactate produced by astrocytes (Pellerin and Magistretti, 2003).
Others have tested the first of these hypotheses in intact animals (Lam et al., 2005), but we concerned ourselves with the second part of the ANLSH and tried to demonstrate the existence of a flux of lactate from astrocytes to neurons in vivo. Stimulation of central chemosensory neurons within the RTN increases minute ventilation (Li and Nattie, 2002), and we reasoned that interruption of the lactate flux between astrocytes and neurons by selective inhibition of MCT2 using a low dose of 4-CIN should reduce pHe in the RTN as lactate accumulates in the extracellular space, which should stimulate ventilation. Thus, we used the minute ventilation of intact animals to probe the effect of disrupting the lactate shuttle, and we measured pHe in the RTN before and after 4-CIN treatment to prove that 4-CIN stimulated ventilation by interrupting the flux of lactate. Focal administration of 4-CIN in the RTN did increase ventilation, and pHe decreased ~0.8 pH units, which indicates the presence of a lactate flux dependent on monocarboxylate transport in the RTN and provides support for the ANLSH. We cannot exclude the possibility of a non-specific effect of 4-CIN on blood flow or some other aspect of metabolism or cell function in the RTN, which might result in extracellular acidosis, but we know of no such non-specific effect of 4-CIN.
Nothing in the ventilatory and pHe measurements revealed which cellular elements were the source of lactate and which were the sink for lactate. Since lactate is transported 1:1 with a proton, an indirect means to investigate the direction of lactate transport is to follow the time course of changes in pHi using a fluorescent probe (Carpenter and Halestrap, 1994; Bröer et al., 1997; Bröer et al., 1999). Exposure of brain slices to 4-CIN led to a dose-dependent acidosis in astrocytes and alkalosis in neurons. This is consistent with the hypothesis that 4-CIN blocked the uptake of lactate by neurons, and they were alkalinized slightly. On the other hand, lactate accumulated in the extracellular space, decreased the concentration gradient driving lactate out of astrocytes, lactate accumulated in astrocytes and the pHi of astrocytes fell. We conclude, therefore, that lactate originates in astrocytes. Moreover, the greater acidification in neurons compared to astrocytes following the lactate pulse is consistent with a larger lactate concentration gradient in neurons, which favors movement of lactate into neurons where the lactate is consumed; a conclusion that supports the ANLSH (Pellerin and Magistretti, 2004).
Glucose uptake in astrocytes and neurons in the RTN
Astrocytes and neurons accumulated 2-NBDG at similar rates in the absence of 4-CIN (Wittendorp-Rechenmann et al., 2002; Nehlig et al., 2004). These investigators found that the amount of radiolabeled 2-deoxyglucose accumulated in neurons and astrocytes was roughly equivalent in brain slice studies. Similar uptake rates of 2-NBDG in astrocytes and neurons, nonetheless, represents a disproportionate astrocytic uptake of glucose since the metabolic rate of astrocytes is less than the metabolic rate of neurons. Presumably much of the glucose taken up by astrocytes is shuttled to neurons in the form of lactate and contributes only modestly to energy consumption in the astrocytes (Rothman et al., 2003; Nehlig et al., 2004).
The uptake of 2-NBDG was increased in neurons in our study when monocarboxylate transport was impaired. This effect of 4-CIN on 2-NBDG uptake by neurons is similar, in general, to previous studies using radiolabeled 2-fluoro-2-deoxy-d-glucose to assess cell specific metabolic rates in brain slices (Tanaka et al., 2004; Ogawa et al., 2005). These investigators found that 4-CIN treatment increased glucose uptake, but only in some regions of the brain or only after periods of neuronal activation. We detected an increase in neuronal 2-NBDG uptake after 4-CIN treatment in unstimulated brain slices, indicating that neurons in the RTN metabolize some lactate even during resting conditions. Ventilation is a surrogate measure of pHe and a surrogate measure of changes in lactate levels in our study, and the slope of the ventilatory response to CO2 was not affected by 4-CIN treatment. This suggests that even during hypercapnic activation of the RTN when neuronal activity increases (Mulkey et al., 2004), the relative dependence of neurons on lactate was unchanged. Had the use of lactate increased in neurons as neuronal activity increased, we would have expected a steeper ventilatory response to CO2 since as the level of neuronal activity and astrocytic lactate release increased, the relative fall in pHe should have been greater and greater – but we did not see this. These results and those of others (Tanaka et al., 2004; Ogawa et al., 2005) raise two issues. First, dependence on lactate seems to vary from area to area within the brain. Presumably this reflects the relative importance of glutamate as a neurotransmitter in each particular brain region, but must also reflect the relative distribution of astrocytes within various areas of the brain. Second, lactate may be important in basal metabolism in some areas, but may participate more importantly in activated states in others, which may also reflect the relative rates of basal and activated glutamate release. Our findings support the ANLSH in general, but metabolic dependence on the ANLS may be heterogeneous throughout the brain, and we were not able to demonstrate any dependence of the magnitude of lactate shuttling on the magnitude of neuronal activation, at least in so far as we may infer that from measurements of ventilation.
Implications for ventilatory central chemosensitivity
There has been a long debate about the exact stimulus of central CO2 chemoreceptors (Winterstein, 1910; Jacobs, 1920; Putnam et al., 2004). Studies of arterial pH, pHi, pHe and neuronal activity indicate that changes in pHi are a sufficient stimulus of central chemosensory neurons (Filosa et al., 2002; Wang et al., 2002; Putnam et al., 2004), but not a necessary stimulus. In addition, many of the putative ion channels (Putnam et al., 2004; Chernov et al., 2007) and neurotransmitter receptors (Loeschcke, 1982) thought to mediate chemosensitivity are sensitive to changes in pHe, rather than pHi. Our measurements strongly suggest that pHe is also a sufficient stimulus of central chemosensors since ventilation was increased when pHe decreased even though it seems likely that 4-CIN treatment alkalinized neuronal pHi. An alternative interpretation is that the increase in pHi in RTN neurons during MCT inhibition may have decreased activity of neurons alkalinized by 4-CIN treatment, but the extracellular acidosis extended beyond the direct effects of 4-CIN. Where this the case, we might have expected ventilatory stimulation from this extended volume of extracellular acidosis following administration of 4-CIN just outside the RTN, but we did not see this in our control studies (Figures 2 and 3). Thus, pHe may also be a sufficient stimulus, but like pHi, not a necessary stimulus since chemosensory neurons may be activated when pHe is held constant (Filosa et al., 2002; Wang et al., 2002; Ritucci et al., 2005). Central chemoreceptors probably have no necessary pHi or pHe stimulus, but respond to the particular combination of pHi and pHe present in central chemosensory nuclei.
Students of the biology of astrocytes are fond of stating that astrocytes participate in information processing (Araque et al., 2001) although the evidence supporting such a statement in intact animals is almost non-existent. The impact of inhibiting monocarboxylate transport on ventilation and pH in the RTN indicates that astrocytes actually determine, at least in part, the local pH and therefore the stimulus of central chemoreceptors in the RTN in intact animals. The distribution of astrocytes is heterogeneous in the CNS: astrocytes are densely distributed throughout the RTN, but the nucleus tractus solitarius (NTS), which also contains central chemosensory cells (Putnam et al., 2004), has relatively few astrocytes (Erlichman et al., 2004). It seems likely that the ANLS is a significant factor setting pHe for chemosensory cells in the RTN, but not in the NTS. In this way, lactate transport and other astrocytic processes may make a significant, but anatomically variable contribution to the ‘information’ processing that constitutes the central chemosensory stimuli (Erlichman et al., 2004). These ideas are consistent with focal measurements of pHe in the brainstem, which indicated that significant regional variation exists in pHe in the brainstem even when the CO2 stimulus is constant (Arita et al., 1989).
Acknowledgments
This work was supported by grants NIH HL-71001 and NSF IOB-0517698.
References
- Akilesh MR, Kamper M, Li A, Nattie EE. Effects of unilateral lesion of retrotrapezoid nucleus on breathing in awake rats. J Appl Physiol. 1997;82:469–479. doi: 10.1152/jappl.1997.82.2.469. [DOI] [PubMed] [Google Scholar]
- Araque A, Carmingoto G, Haydon PG. Dynamic signaling between astrocytes and neurons. Ann Rev Physiol. 2001;63:795–813. doi: 10.1146/annurev.physiol.63.1.795. [DOI] [PubMed] [Google Scholar]
- Arita H, Ichikawa K, Kuwana S, Kogo N. Possible locations of pH-dependent central chemoreceptors: intramedullary regions with acidic shift of extracellular fluid pH during hypercapnia. Brain Res. 1989;485:285–293. doi: 10.1016/0006-8993(89)90572-6. [DOI] [PubMed] [Google Scholar]
- Boyarsky G, Ganz MB, Sterzel RB, Boron WF. pH regulation in single glomerular mesangial cells. I. Acid extrusion in the absence and presence of HCO3- Am J Physiol. 1988;255:C844–C856. doi: 10.1152/ajpcell.1988.255.6.C844. [DOI] [PubMed] [Google Scholar]
- Bröer S, Bröer A, Schneider H-P, Stegen C, Halestrap AP, Deitmer JW. Characterization of the high-affinity monocarboxylate transporter MCT2 in Xenopus laevis oocytes. Biochem J. 1999;341:529–535. doi: 10.1042/0264-6021:3410529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröer S, Rahman B, Pellegri G, Pellerin L, Martin J-L, Verleysdonk S, Hamprecht B, Magistretti PJ. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT1) expressing Xenopus laevis oocytes. J Biol Chem. 1997;272:3009–30102. doi: 10.1074/jbc.272.48.30096. [DOI] [PubMed] [Google Scholar]
- Carpenter L, Halestrap AP. The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumor cells studied with the intracellular oH indicator BCECF. Biochem J. 1994;304:751–760. doi: 10.1042/bj3040751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernov MM, Leiter JC, Putnam RW. A computer model of mammalian central CO2 chemoreception. Adv Exp Med Biol. 2007 doi: 10.1007/978-0-387-73693-8_52. in press. [DOI] [PubMed] [Google Scholar]
- Chih C-P, Lipton P, Roberts EL., Jr Do active cerebral neurons really use lactate rather than glucose? TINS. 2001;24:573–578. doi: 10.1016/s0166-2236(00)01920-2. [DOI] [PubMed] [Google Scholar]
- Dallwig R, Deitmer JW. Cell-type specific calcium responses in acute rat hippocampal slices. J Neurosci Meth. 2002;116:77–87. doi: 10.1016/s0165-0270(02)00030-4. [DOI] [PubMed] [Google Scholar]
- Debernardi R, Pierre K, Lengacher S, Magistretti PJ, Pellerin L. Cell-specific expression pattern of monocarboxylate transporters in astrocytes and neurons observed in different mouse brain cortical cell cultures. J Neurosci Res. 2003;73:141–155. doi: 10.1002/jnr.10660. [DOI] [PubMed] [Google Scholar]
- Erlichman JS, Li A, Nattie EE. Ventilatory effects of glial dysfunction in a rat brain stem chemoreceptor region. J Appl Physiol. 1998;85:1599–1604. doi: 10.1152/jappl.1998.85.5.1599. [DOI] [PubMed] [Google Scholar]
- Erlichman JS, Cook A, Schwab MC, Budd TW, Leiter JC. Heterogeneous patterns of pH regulation in glial cells in the dorsal and ventral medulla. Am J Physiol. 2004;286:R289–R302. doi: 10.1152/ajpregu.00245.2003. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Dean JB, Putnam RW. Role of intracellular and extracellular pH in the chemosensitive response of rat locus coeruleus neurones. J Physiol (Lond) 2002;541(2):493–509. doi: 10.1113/jphysiol.2001.014142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart EZ, Enerson BE, Zhdankina OY, Leino RL, Drewes LR. Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. Am J Physiol. 1997;273:E207–E213. doi: 10.1152/ajpendo.1997.273.1.E207. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Price NP. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999;343:281–299. [PMC free article] [PubMed] [Google Scholar]
- Hertz L. The astrocyte-neuron lactate shuttle: A challenge of a challenge. J Cerebral Blood Flow Metab. 2004;24:1241–1248. doi: 10.1097/00004647-200411000-00008. [DOI] [PubMed] [Google Scholar]
- Hewitt A, Barrie R, Graham M, Bogus K, Leiter JC, Erlichman JS. Ventilatory effects of gap junctions in the RTN in awake rats. Am J Physiol. 2004;287:R1407–R1418. doi: 10.1152/ajpregu.00404.2004. [DOI] [PubMed] [Google Scholar]
- Holleran J, Babbie M, Erlichman JS. The ventilatory effects of impaired glial function in a rat brainstem chemoreceptor region in the conscious rat. J Appl Physiol. 2001;90:1539–1547. doi: 10.1152/jappl.2001.90.4.1539. [DOI] [PubMed] [Google Scholar]
- Homola A, Zoremba N, Šlais K, Kuhlen R, Syková E. Changes in diffusion parameters, energy-related metabolites and glutamate in the rat cortex after transient hypoxia/ischemia. Neurosci Lett. 2006;404:137–142. doi: 10.1016/j.neulet.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Jacobs MH. The production of intracellular acidity by neutral and alkaline solutions containing carbon dioxide. Am J Physiol. 1920;53:457–463. [Google Scholar]
- Kimmelberg HK. The role of hypotheses in current research, illustrated by hypotheses on the possible role of astrocytes in energy metabolism and cerebral blood flow: From Newton to now. J Cerebral Blood Flow Metab. 2004;24:1235–1239. doi: 10.1097/01.WCB.0000138668.10058.8C. [DOI] [PubMed] [Google Scholar]
- Lam TKT, Gutierrez-Juarez R, Pocai A, Rossetti L. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science. 2005;309:943–947. doi: 10.1126/science.1112085. [DOI] [PubMed] [Google Scholar]
- Li A, Nattie EE. CO2 dialysis in one chemoreceptor site, the RTN: stimulus intensity and sensitivity in the awake rat. Respir Physiol Neurobiol. 2002;133:11–22. doi: 10.1016/s1569-9048(02)00134-9. [DOI] [PubMed] [Google Scholar]
- Li A, Randall M, Nattie EE. CO2 microdialysis in retrotrapezoid nucleus of the rat increase breathing in wakefulness but not sleep. J Appl Physiol. 1999;87:910–919. doi: 10.1152/jappl.1999.87.3.910. [DOI] [PubMed] [Google Scholar]
- Loeschcke HH. Central chemosensitivity and the reaction theory. J Physiol (Lond) 1982;332:1–24. doi: 10.1113/jphysiol.1982.sp014397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ, Pellerin L. Astrocytes couple synaptic activity to glucose utilization in the brain. NIPS. 1999;14:177–182. doi: 10.1152/physiologyonline.1999.14.5.177. [DOI] [PubMed] [Google Scholar]
- Manning Fox JE, Meredith D, Halestrap AP. Characterisation of human monocarboxylate transporter 4 substantiates its role in lactic acid efflux from skeletal muscle. J Physiol (Lond) 2000;529(2):285–293. doi: 10.1111/j.1469-7793.2000.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG. Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci. 2004;7:1360–1369. doi: 10.1038/nn1357. [DOI] [PubMed] [Google Scholar]
- Nehlig A, Wittendorp-Rechenmann E, Lam CD. Selective uptake of [14C]2-deoxyglucose by neurons and astrocytes: High resolution microautoradiopraphic imaging by cellular 14C-trajectory combined with immunohistochemistry. J Cerebral Blood Flow Metab. 2004;24:1004–1014. doi: 10.1097/01.WCB.0000128533.84196.D8. [DOI] [PubMed] [Google Scholar]
- Nottingham S, Leiter JC, Wages P, Buhay S, Erlichman JS. Developmental changes in intracellular pH regulation in medullary neurons of the rat. Am J Physiol. 2001;281:R1940–R1951. doi: 10.1152/ajpregu.2001.281.6.R1940. [DOI] [PubMed] [Google Scholar]
- Ogawa M, Watabe H, Teramoto N, Miyake Y, Hayashi T, Iida H, Murata T, Magata Y. Understanding of cerebral energy metabolism by dynamic living brain slice imaging system with [18F]FDG. Neurosci Res. 2005;52:357–361. doi: 10.1016/j.neures.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Pappenheimer J. Sleep and respiration of rats during hypoxia. J Physiol (London) 1977;266:191–207. doi: 10.1113/jphysiol.1977.sp011763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappenheimer JR, Fencl V, Heisey SR, Held D. Role of cerebral fluids in control of respiration as studied in unanesthetized goats. Am J Physiol. 1965;208:436–450. doi: 10.1152/ajplegacy.1965.208.3.436. [DOI] [PubMed] [Google Scholar]
- Parisian K, Wages P, Hewitt A, Leiter JC, Erlichman JS. Ventilatory effects of gap junction blockade in the NTS in awake rats. Respir Physiol Neurobiol. 2004;142:127–143. doi: 10.1016/j.resp.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Second Edition. Sydney: Academic Press; 1998. [Google Scholar]
- Pearce R, Stornetta RL, Guyenet P. Retrotrapezoid nucleus in the rat. Neurosci Lett. 1989;101:138–142. doi: 10.1016/0304-3940(89)90520-x. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Food for thought: Challenging the dogmas. J Cerebral Blood Flow Metab. 2003;23:1282–1286. doi: 10.1097/01.WCB.0000096064.12129.3D. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Empiricism and rationalism: Two paths toward the same goal. J Cerebral Blood Flow Metab. 2004;24:1240–1241. doi: 10.1097/00004647-200411000-00007. [DOI] [PubMed] [Google Scholar]
- Pierre K, Magistretti PJ, Pellerin L. MCT2 is a major neuronal monocarboxylate transporter in the adult mouse brain. J Cerebral Blood Flow Metab. 2002;22:586–595. doi: 10.1097/00004647-200205000-00010. [DOI] [PubMed] [Google Scholar]
- Poole RC, Halestrap AP. Interaction of the erythrocyte lactate transporter (monocarboxylate transporter 1) with an integral 70-kDa membrane glycoprotein of the immunoglobulin superfamily. J Biol Chem. 1997;272:14624–14628. doi: 10.1074/jbc.272.23.14624. [DOI] [PubMed] [Google Scholar]
- Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid sensing in chemosensitive neurons. Am J Physiol. 2004;287:C1493–C1526. doi: 10.1152/ajpcell.00282.2004. [DOI] [PubMed] [Google Scholar]
- Ritucci NA, Erlichman JS, Leiter JC, Putnam RW. Response of membrane potential (Vm) and intracellular pH (pHi) to hypercapnia in neurons and astrocytes from rat retrotrapezoid nucleus (RTN) Am J Phsyiol. 2005;289:R851–R861. doi: 10.1152/ajpregu.00132.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman DL, Behar KL, Hyder F, Shulman RG. In vivo NMR studies of the glutamate neurotransmitter flux and neuroenergetics: Implications for brain function. Annu Rev Physiol. 2003;65:401–427. doi: 10.1146/annurev.physiol.65.092101.142131. [DOI] [PubMed] [Google Scholar]
- Schneider U, Poole RC, Halestrap AP, Grafe P. Lactate-proton co-transport and its contribution to interstitial acidification during hypoxia in isolated rat spinal roots. Neurosci. 1993;53:1153–1162. doi: 10.1016/0306-4522(93)90497-4. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Nakamura F, Mizokawa S, Matsumura A, Matsumura K, Murata T, Shigematsu M, Kageyama K, Ochi H, Watanabe Y. Role of lactate in the brain energy metabolism: revaled by bioradiography. Neurosci Res. 2004;48:13–20. doi: 10.1016/j.neures.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Wang W, Bradley SR, Richerson GB. Quantification of the response of rat medullary raphe neurones to independent changes in pHo and PCO2. J Physiol (Lond) 2002;540(3):951–970. doi: 10.1113/jphysiol.2001.013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterstein H. Das Überleben neugeborener Säugetiere bei künstlicher Durchspülung. Wien med Wochenschr. 1910;60:2274. [Google Scholar]
- Wittendorp-Rechenmann E, Lam CD, Steibel F, Lasbennes F, Nehlig A. High resolution tracer targeting combining microautoradiographic imaging by cellular 14C-trajectory with immunohistochemistry: a novel protocol to demonstrate metabolism of [14C]2-deoxyglucose by neurons and astrocytes. J Trace Microprobe Tech. 2002;20:505–515. [Google Scholar]
- Yamada KA, Saito M, Matsuoka H, Inagaki N. A real-time method of imaging glucose uptake in single, living mammalian cells. Nature Protocols. 2007;2:753–762. doi: 10.1038/nprot.2007.76. [DOI] [PubMed] [Google Scholar]
- Yamada KA, Nakata M, Horimoto N, Saito M, Matsuoka H, Inagaki N. Measurement of glucose uptake and intracellular calcium concentration in single, living, pancreatic β–cells. J Biol Chem. 2000;275:22278–22283. doi: 10.1074/jbc.M908048199. [DOI] [PubMed] [Google Scholar]