Abstract
Objectives
The potential growth-promoting effects of antibiotics are not well understood among undernourished children in environments with high pathogen exposure. We aimed to assess whether early antibiotic exposure duration and class were associated with growth to two years of age across 8 low-resource sites in the MAL-ED birth cohort study.
Methods
We followed 1,954 children twice per week from birth to two years to record maternally-reported antibiotic exposures and measure anthropometry monthly. We estimated the associations between antibiotic exposure before 6 months of age and weight-for-age (WAZ) and length-for-age (LAZ) z-scores to two years. We assessed the impact of class-specific exposures and duration, and compared these results to effects of antibiotic exposures after 6 months of age.
Results
Antibiotic use before 6 months of age was associated with increased weight from 6 months to 2 years, while associations with length were less consistent across sites and antibiotic classes. Compared to unexposed children, two or more courses of metronidazole, macrolides, and cephalosporins were associated with adjusted increases in WAZ of 0.24 (95% confidence interval (CI): 0.04, 0.43), 0.23 (95% CI: 0.05, 0.42), and 0.19 (95% CI: 0.04, 0.35) from 6 months to 2 years, respectively.
Conclusions
Antibiotic use in low-resource settings was most associated with the ponderal growth of children who had multiple exposures to antibiotics with broad spectrum and anaerobic activity in early infancy. Opportunities for rational and targeted antibiotic therapy in low resource settings may also promote short-term weight gain in children, though longer-term physical growth and metabolic impacts are unknown.
Introduction
After decades of antibiotic use to promote growth of livestock in the agricultural industry, researchers have begun to explore the potential role of antibiotic use in promoting growth in humans as well (1). Evidence of the potential for antibiotics to cause long-lasting perturbations of the gut microbiota (2,3) and the role of the microbiota in metabolism (4-6) has suggested that this exposure could be an important research target as a modifiable factor that may be contributing to the obesity epidemic in high-income countries. Antibiotic exposure early in life, during the maturation process of the gut microbiota and enteric immune system, has been hypothesized to have the largest potential impact on growth (6,7). Epidemiologic studies investigating the role of early life antibiotic exposures in high-income settings of Finland (8), United Kingdom (9,10), United States (11,12), The Netherlands (13), and Denmark (14) have shown associations between early antibiotic exposure and increased weight gain in children from 2-10 years of age, though some found mixed or negative results (14,15).
In low-income settings, obesity among young children is less common, and poor growth and undernutrition is often a more pressing public health concern. In these settings, antibiotics are recommended by the WHO as part of the treatment for severe acute malnutrition (16), despite the mixed evidence for the impact of antibiotic treatment on recovery and mortality (17,18). Outside of clinical settings, it is not clear whether antibiotic use promotes growth in children who are not acutely malnourished but remain below the average growth curve and reside in environments with high pathogen exposure. In these settings, antibiotics may impact growth through clearance of infections or through modifications of the microbiota, the hypothesized mechanism in high-income settings (19,20). Unique to low-income settings, there is additional confounding in observational studies by the indications for treatment, since some of the most common illnesses resulting in antibiotic treatment (e.g. diarrhea) can have long-term negative impacts on growth. An international cross-sectional study that found a positive association between antibiotic treatment during infancy and BMI among boys did not include these factors and was also limited by self-reported heights and weights (21).
To appropriately account for these factors, we leveraged the high-resolution illness and treatment data from The Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project (MAL-ED), a birth cohort study performed at eight sites in South America, sub-Saharan Africa, and south Asia. Antibiotic use was highly prevalent in almost all sites (22). We aimed to assess whether early antibiotic use in the first 6 months of life was associated with physical growth from 6 months to two years of age and determine the impact of antibiotic class and duration of use.
Methods
The MAL-ED study was conducted between November 2009 and February 2014 at eight sites in Dhaka, Bangladesh, Fortaleza, Brazil, Vellore, India, Bhaktapur, Nepal, Naushahro Feroze, Pakistan, Loreto, Peru, Venda, South Africa, and Haydom, Tanzania. Study design and methods have been previously described (23). Children were enrolled within 17 days of birth if their enrolelment weight was ≥1500 g, they were not hospitalized, and they did not have severe or chronic conditions. Follow-up was conducted twice per week at home visits until two years of age to document illnesses, breastfeeding practices, and antibiotic use. Caregivers reported all oral or injected antibiotics given to their child. Medication packaging and prescriber documentation were used to confirm antibiotic use and class. Non-diarrheal surveillance stool samples were collected monthly and tested for 40 enteropathogens (24). Weight and length were measured monthly, and weight-for-age (WAZ) and length-for-age (LAZ) z-scores were calculated using the 2006 WHO child growth standards (25). Length measurements from Pakistan were excluded due to poor data quality. Socioeconomic status was assessed biannually and summarized using the child’s average WAMI (Water, Assets, Maternal education, Income) score, which is based on monthly household income, maternal education, wealth measured by eight assets, and access to improved water and sanitation (26). All sites received ethical approval from their respective governmental, local institutional, and collaborating institutional ethical review boards. Written, informed consent was obtained from the caregiver of each child.
Data and definitions
Assessment of antibiotic use practices in the MAL-ED cohort has been previously described (22). A new antibiotic course was defined after two antibiotic-free days, assuming antibiotics were not received on the 2% of days with missing surveillance information. Diarrhea was defined as maternal report of three or more loose stools in 24 hours or at least one loose stool with visible blood (27). Respiratory illness was defined as cough or shortness of breath, and was considered an acute lower respiratory infection (ALRI) if accompanied by fieldworker-determined rapid respiratory rate (27). Fever and vomiting were caregiver reported.
Analysis
We used multivariable linear regression to estimate the association between antibiotic use in the first 6 months of life and monthly WAZ and LAZ from 6-24 months of age. Antibiotic exposure was modelled as a continuous measure of duration in days from 0-5 months of age and as a categorical variable by number of courses received to assess the potential for a non-linear dose-response. We also stratified antibiotic effects by sex and site. Generalized estimating equations (GEE) with robust variance were used to account for correlation between anthropometric measurements within children across time points. Confounding variables for adjustment included baseline characteristics and indications for treatment, and were selected by causal diagram (28) based on expert opinion as well as a previous analysis of factors associated with antibiotic use in MAL-ED (22). All analyses were adjusted for site, child sex, enrollment WAZ, WAMI score, crowding (people/room in household), maternal height, maternal education, and characteristics of the child’s first 6 months of life: percent days exclusively breastfed, number of diarrhea episodes, days with fever, vomiting, and respiratory illness, and presence of ALRI, bloody stools, and hospitalization. Length models also included enrollment LAZ.
We further explored the effects of class-specific antibiotic exposure use by modeling class-specific exposure as dichotomous (exposed to a specific class on at least one day versus not) and as a categorical variable by number of class-specific courses received, using the models above and additionally adjusting for other antibiotic class exposures to isolate class-specific effects. We also explored effect measure modification by malnutrition (stunted and underweight) at 6 months and pathogen burden from 0-5 months (presence of Campylobacter, enteroaggregative Escherichia coli (EAEC), and Giardia, and average number of bacterial pathogens detected (24)) by including interaction terms between the exposures and these variables and estimating subgroup-specific effects.
To assess the period during which the effects of early antibiotic use (before 6 months of age) were manifested, we estimated these effects on anthropometry at different age periods, from 0-5, 6-11, 12-17, and 17-24 months, adjusting for the child’s anthropometric z-scores at the beginning of the age period.
To compare early life exposures with later exposures, we used linear regression to estimate the effects of exposures from 6-24 months on cross-sectional WAZ and LAZ at 2 years. A child’s measurement closest to 24 months between 23 and 25 months was considered their anthropometry at two years. Adjustment variables included the same baseline characteristics as above, including enrollment WAZ, enrollment LAZ (for length models only), illness burden as characterized above over the whole two years of follow-up, and antibiotic use in the first 6 months of life. Using these models with cross-sectional outcomes of WAZ and LAZ at 2 years, we demonstrated that the early life effects were insensitive to further statistical adjustment for illnesses and antibiotic use after 6 months of age.
Results
Across eight sites in the MAL-ED cohort, 1,954 children were followed until at least 6 months of age and had at least one subsequent anthropometric measurement. The majority of these children (n=1736, 88.3%) remained under surveillance and had anthropometric measurements at two years. Baseline characteristics, early antibiotic exposure, and growth outcomes differed across sites (Table 1), with the highest antibiotic use in the South Asian sites.(22) Mean enrollment weight and length within 17 days of birth across sites were near one standard deviation below the WHO standard. All sites except Brazil showed reductions in average WAZ and LAZ over the two years of follow-up with overall means at two years of -1.06 and -1.71, respectively.
Table 1.
Baseline characteristics and antibiotic use by site among 1,954 children in the MAL-ED cohort who were followed until at least 6 months of age with subsequent anthropometry.
| Dhaka, Bangladesh No. (%) | Fortaleza, Brazil No. (%) | Vellore, India No. (%) | Bhaktapur, Nepal No. (%) | Loreto, Peru No. (%) | Naushahro Feroze, Pakistan No. (%) | Venda, South Africa No. (%) | Haydom, Tanzania No. (%) | Overall No. (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Children followed until 6 mo. With subsequent anthropometry | 240 | 207 | 234 | 235 | 269 | 265 | 259 | 245 | 1954 |
| Children with anthropometry at 2 years | 212 (87.6) | 168 (80.4) | 227 (96.2) | 228 (96.6) | 200 (74.1) | 249 (94.0) | 238 (91.2) | 214 (86.3) | 1736 (88.3) |
| Female gender | 122 (50.8) | 101 (48.8) | 128 (54.7) | 109 (46.4) | 123 (45.7) | 136 (51.3) | 129 (49.8) | 124 (50.6) | 972 (49.7) |
| Crowding in the home (2+ people per room) | 225 (93.8) | 29 (14.0) | 185 (79.0) | 93 (39.6) | 100 (37.2) | 229 (86.4) | 35 (13.5) | 107 (43.7) | 1003 (51.3) |
| Maternal education <6 years | 152 (63.3) | 27 (13.0) | 82 (35.3) | 60 (25.5) | 60 (22.3) | 218 (82.3) | 6 (2.3) | 94 (38.4) | 699 (35.8) |
| Monthly income <150 USD | 157 (65.4) | 7 (3.4) | 216 (92.3) | 114 (48.5) | 188 (69.9) | 145 (54.7) | 51 (19.7) | 242 (98.8) | 1120 (57.3) |
| Median months of exclusive breastfeeding (IQR) | 5.0 (3.8, 5.7) | 2.6 (1.3, 4.3) | 3.5 (2.5, 4.6) | 3.0 (1.5, 4.4) | 2.7 (1.0, 4.3) | 0.5 (0.3, 0.7) | 1.0 (0.6, 1.7) | 1.8 (1.1, 2.7) | 2.2 (0.9, 4) |
| Courses of antibiotics before 6 mo. | |||||||||
| 0 | 4 (1.7) | 182 (87.9) | 87 (37.2) | 106 (45.1) | 58 (21.6) | 4 (1.5) | 179 (69.1) | 50 (20.4) | 670 (34.3) |
| 1 | 18 (7.5) | 25 (12.1) | 64 (27.4) | 90 (38.3) | 71 (26.4) | 14 (5.3) | 55 (21.2) | 76 (31.0) | 413 (21.1) |
| 2 | 36 (15.0) | 0 (0) | 36 (15.4) | 26 (11.1) | 71 (26.4) | 25 (9.4) | 21 (8.1) | 54 (22.0) | 269 (13.8) |
| 3+ | 182 (75.8) | 0 (0) | 47 (20.1) | 13 (5.5) | 69 (25.7) | 222 (83.8) | 4 (1.5) | 65 (26.5) | 602 (30.8) |
| Median days of antibiotics before 6 mo. (IQR) | 26 (15, 38) | 0 (0, 0) | 4 (0, 9) | 3 (0, 7) | 7 (3, 13) | 31 (16, 48) | 0 (0, 2) | 8 (3, 16) | 6 (0,18) |
| At least one day of class-specific antibiotic exposure before 6 mo. | |||||||||
| Penicillins | 202 (84.2) | 18 (8.7) | 93 (39.7) | 61 (26.0) | 173 (64.3) | 151 (57.0) | 59 (22.8) | 150 (61.2) | 907 (46.4) |
| Cephalosporins | 158 (65.8) | 5 (2.4) | 62 (26.5) | 27 (11.5) | 33 (12.3) | 205 (77.4) | 1 (0.4) | 2 (0.8) | 493 (25.2) |
| Macrolides | 143 (59.6) | 1 (0.5) | 21 (9) | 28 (11.9) | 86 (32) | 79 (29.8) | 7 (2.7) | 6 (2.5) | 371 (19.0) |
| Metronidazole | 8 (3.3) | 0 (0) | 11 (4.7) | 37 (15.7) | 2 (0.7) | 156 (58.9) | 3 (1.2) | 59 (24.1) | 276 (14.1) |
| Sulfonamides | 3 (1.3) | 1 (0.5) | 22 (9.4) | 19 (8.1) | 45 (16.7) | 73 (27.6) | 10 (3.9) | 55 (22.5) | 228 (11.7) |
| Fluoroquinolones | 34 (14.2) | 0 (0) | 16 (6.8) | 4 (1.7) | 1 (0.4) | 9 (3.4) | 0 (0) | 2 (0.8) | 66 (3.4) |
| Campylobacter detection before 6 mo. | 128 (53.3) | 27 (13.0) | 99 (42.3) | 90 (38.3) | 103 (38.3) | 175 (66.0) | 105 (40.5) | 147 (60.0) | 874 (44.7) |
| EAEC detection before 6 mo. | 166 (69.2) | 150 (72.5) | 178 (76.1) | 147 (62.6) | 107 (39.8) | 219 (82.6) | 161 (62.2) | 223 (91.0) | 1351 (69.1) |
| Giardia detection before 6 mo. | 3 (1.3) | 6 (2.9) | 15 (6.4) | 7 (3.0) | 19 (7.1) | 99 (37.4) | 3 (1.2) | 20 (8.2) | 172 (8.8) |
| Mean enrollment WAZ* | -1.28 | -0.16 | -1.30 | -0.91 | -0.62 | -1.42 | -0.38 | -0.13 | -0.78 |
| Mean enrollment LAZ* | -1.03 | -0.78 | -1.03 | -0.70 | -0.96 | -0.71 | -1.01 | -0.89 | |
| Mean WAZ at 2 years | -1.61 | 0.37 | -1.65 | -0.93 | -0.82 | -1.65 | -0.51 | -1.33 | -1.06 |
| Mean LAZ at 2 years | -2.03 | -0.07 | -1.92 | -1.35 | -1.89 | -1.71 | -2.66 | -1.71 |
Within 17 days of birth
USD, United States Dollars. IQR, interquartile range. EAEC, enteroaggregative E. coli. WAZ, weight-for-age z-score. LAZ, length-for-age z-score.
A 7-day increase in duration of antibiotic exposure in the first 6 months of life was associated with an adjusted 0.03 (95% confidence interval (CI): 0.00, 0.05) higher WAZ from 6 months to 2 years of age compared to unexposed children. There was no difference between boys and girls, and this association was largely consistent across sites, except in Brazil and South Africa, where antibiotic use was least common and the estimates are least precise (Figure 1A). The largest differences in WAZ were associated with children who received more than 3 courses in the first 6 months (Figure, Supplemental Digital Content 2A). Children who received more than 3 courses of antibiotics in the first 6 months of life had an average 0.18 (95% CI: 0.06, 0.30) higher WAZ from 6 months to 2 years compared to children receiving 3 courses or less. This effect was largest in Pakistan (WAZ difference: 0.46, 95% CI: 0.25, 0.67) and Tanzania (WAZ difference: 0.21, 95% CI: -0.01, 0.43), but these estimates translate to relatively small differences in weight (600 g and 280 g at 2 years, respectively).
Figure 1.
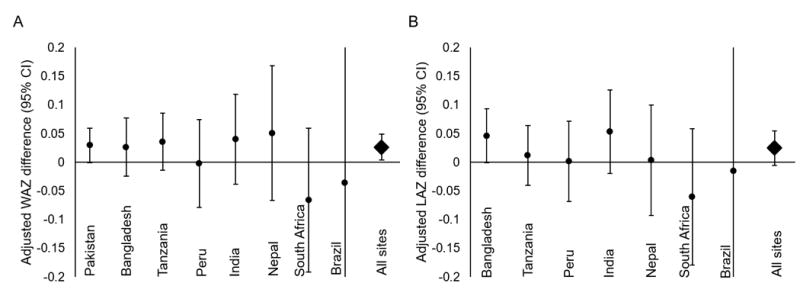
Adjusted weight-for-age (WAZ) and length-for-age (LAZ) z-score differences associated with a linear 7-day increase in duration of antibiotic exposure in the first 6 months of life among 1954 children followed in the MAL-ED birth cohort until at least 6 months of age with subsequent anthropometry. Sites ordered from greatest to least proportion exposed to antibiotics < 6 mo. (Pakistan: 261/265 (98.5%); Bangladesh: 236/240 (98.3%); Tanzania: 195/245 (79.6%); Peru: 211/269 (78.4%); India: 147/234 (62.8%); Nepal: 129/235 (54.9%); South Africa: 80/259 (13.3%); Brazil: 25/207 (12.1%); All sites: 1284/1954 (65.7%)).
The associations of duration of antibiotic exposure with LAZ were more varied across sites (Figure 1B). There was no difference in LAZ between children exposed to between 0 and 4 courses of antibiotics. Children exposed to 5 or more courses of antibiotics before 6 months had a slightly higher but non-significant increase in LAZ compared to unexposed children (Figure, Supplemental Digital Content 2B).
In the assessment of class-specific exposure, macrolide and metronidazole use on at least one day during the first 6 months were associated with an adjusted 0.14 (95% CI: 0.02, 0.25) and 0.17 (95% CI: 0.04, 0.31) increase in WAZ, respectively. Cephalosporins, fluoroquinolones, and penicillins were associated with smaller and non-significant increases in WAZ. Associations with LAZ were generally smaller and not statistically significant for any antibiotic class (Table 2). While the effects of macrolides and cephalosporins were close to null if only one course was received, two or more courses were associated with an adjusted increase of 0.23 (95% CI: 0.05, 0.42) and 0.19 (95% CI: 0.04, 0.35) in WAZ, respectively (Table 2). Metronidazole was associated with increases in WAZ both when children were exposed to only one course (WAZ difference: 0.14, 95% CI: -0.00, 0.29) and two or more courses (0.23, 95% CI: 0.05, 0.42). In contrast, a dose-response pattern was absent for the effects on LAZ, except for cephalosporins, in which two or more courses of cephalosporins were associated with a 0.19 (95% CI: 0.01, 0.37) increase in LAZ (Table 2). There was no statistical evidence for effect measure modification by malnutrition or pathogen burden in surveillance stools (not shown).
Table 2.
Adjusted weight-for-age and length-for-age z-score differences associated with class-specific antibiotic use in the first 6 months of life among 1954 children in the MAL-ED birth cohort.
| Antibiotic class exposure in first 6 months of life | Number exposed (%) (N=1954†) | Adjusted* WAZ difference (95% CI) | Number exposed (%) (N=1689†) | Adjusted* LAZ difference (95% CI) |
|---|---|---|---|---|
| Metronidazole | ||||
| 1 course | 170 (8.7) | 0.14 (-0.01, 0.29) | 109 (6.5) | 0.00 (-0.15, 0.16) |
| 2+ courses | 106 (5.4) | 0.24 (0.04, 0.43) | 11 (0.7) | 0.02 (-0.35, 0.40) |
| Macrolides | ||||
| 1 course | 258 (13.2) | 0.09 (-0.03, 0.21) | 206 (12.2) | 0.07 (-0.05, 0.18) |
| 2+ courses | 114 (5.8) | 0.23 (0.05, 0.42) | 87 (5.2) | -0.00 (-0.20, 0.19) |
| Cephalosporins | ||||
| 1 course | 244 (12.5) | 0.03 (-0.09, 0.16) | 182 (10.8) | 0.02 (-0.11, 0.14) |
| 2+ courses | 249 (12.7) | 0.19 (0.04, 0.35) | 106 (6.3) | 0.19 (0.01, 0.37) |
| Penicillins | ||||
| 1 course | 450 (23.0) | 0.09 (-0.02, 0.19) | 382 (22.6) | -0.04 (-0.13, 0.06) |
| 2+ courses | 457 (23.4) | 0.07 (-0.04, 0.18) | 373 (22.1) | -0.00 (-0.11, 0.10) |
| Fluoroquinolones (any) | 66 (3.4) | 0.08 (-0.14, 0.30) | 57 (3.4) | 0.05 (-0.16, 0.27) |
| Sulfonamides (any) | 228 (11.7) | 0.03 (-0.09, 0.15) | 155 (9.2) | -0.02 (-0.14, 0.11) |
Adjusted for other antibiotic classes included in the table, site, child sex, enrollment WAZ, WAMI score, crowding (people/room in household), maternal height, maternal education, and characteristics of the child’s first 6 months of life: percent days exclusively breastfed, number of diarrhea episodes, days with fever, vomiting, and respiratory illness, and presence of ALRI, bloody stools, and hospitalization. LAZ difference is also adjusted for enrollment LAZ
Children who were followed in the MAL-ED birth cohort until at least 6 months of age with subsequent anthropometry. LAZ difference estimates exclude Pakistan.
WAZ, weight-for-age z-score. LAZ, length-for-age z-score
The weight and length increases associated with antibiotic use before 6 months of age occurred during the exposure window and up to one year later at 18 months of age. By 6 months, children who were exposed to more than 3 courses of antibiotics had a 0.09 (95% CI: -0.04, 0.22) greater WAZ compared to children receiving 3 courses or less (Figure, Supplemental Digital Content 3A). These children had further gains of 0.08 (95% CI: -0.06, 0.06) and 0.04 (95% CI: -0.02, 0.05) in WAZ compared to children receiving 3 courses or less from 6-11 and 12-17 months, respectively. Adjusting for their WAZ at 18 months, there was no further difference in WAZ from 18-24 months associated with early antibiotic use (WAZ difference: 0.00, 95% CI: -0.04, 0.05). However, children who did not receive early antibiotics did not catch-up during this period such that the majority of the overall WAZ difference was maintained to 24 months. More than 3 courses of antibiotics was associated with a similar increase in LAZ by 6 months (0.08, 95% CI: -0.05, 0.21), but there were no associations in periods after 6 months (Figure, Supplemental Digital Content 3B) or overall as shown above.
In contrast to the antibiotic effects in early infancy, duration of antibiotic exposure after 6 months of age were not associated with cross-sectional WAZ or LAZ at two years (Table, Supplemental Digital Content 4), adjusting for exposure before 6 months and illnesses across the first two years of life. Among the antibiotic classes, only fluoroquinolones were associated with increases in size at two years of age. Two or more courses of fluoroquinolones after 6 months of age were associated with an adjusted increase of 0.21 (95% CI: 0.05, 0.37) in WAZ at two years (Table, Supplemental Digital Content 4). The associations with other antibiotic classes were near the null and/or did not show a dose-response trend.
Discussion
We demonstrate that the growth promoting phenomenon of early life antibiotic exposure among healthy children is not unique to high-resource settings and can also occur in populations with low average weights and lengths. Antibiotic exposure was associated with increases in weight in the MAL-ED cohort, and this effect was limited to early exposures in the first 6 months of life, which is consistent with previous studies (8,9,13,14). Associations of antibiotics with length were generally smaller and inconsistent across sites and drug classes. The greatest increases in WAZ were associated with more than 3 courses of exposure and when multiple treatment courses of macrolides, metronizadole, and cephalosporins were received.
A larger impact of macrolides compared to other antibiotics on BMI was similarly documented in a retrospective US study (12). Broad-spectrum antibiotics were associated with early childhood obesity in the US, while narrow-spectrum antibiotics (penicillins) were not.(11) Two previous trials of metronidazole treatment in the general pediatric population in Guatemala (1982) (29) and in malnourished children in Jamaica (1993) (30) found an association between metronidazole and improved growth. While metronidazole is considered narrow-spectrum, its anaerobic activity may be particularly destructive to the gut microbiota. Antibiotics with anaerobic activity have been associated with growth in a dose-dependent manner in the United Kingdom, while antibiotics without anaerobic activity were not (10).
Our results provide support for the hypothesized mechanism through the gut microbiota. The influence of antibiotics on the diversity and composition of the gut microbiota can persist long after treatment is completed (2,31), especially among infants (32,33). Altered microbiota can modify metabolism and intestinal inflammation and immunity resulting in increased energy harvest from the diet (4,6,34). Murine models suggest a causal association between the microbiota and obesity; when antibiotic-altered microbiota from overweight mice were transferred to germ-free mice, these mice experienced the same growth and immune response phenotypes as the donor mice (6).
However, because the effect of early life antibiotics did not affect growth rates after 18 months of age, the impact of an altered microbiota may be short lived. This evidence that the microbiota is not necessarily permanently “reprogrammed” to cause increased growth rates long after exposure is supported by a recent large study in the United States that found that early antibiotic use was not associated with higher rates of weight gain after the exposure period (15). Their results may be explained by their analytic exclusion of antibiotic-induced weight gain during the exposure period. Taken together, the evidence suggests that short-term boosts in growth due to antibiotics may be more relevant than long-term changes in growth rates.
Still, because the unexposed children in the MAL-ED cohort did not complete catch-up growth, such that the weight difference associated with antibiotic exposure was still present at 24 months, these exposures may have sustained impact on a child’s attained size. Short-term growth and attained size have been negatively associated with hospitalization and mortality (35), and positively with cognitive outcomes such as IQ, years of schooling, and income (36), respectively. The first two years of life are an especially critical period, (37) and even modest improvements in weight gain during this time may be beneficial. However, increased relative weight gain in the first two years of life, beyond what was expected from linear growth, was not associated with improvements in human capital in Brazil (36), which suggests linear growth may ultimately be more important for long-term cognitive outcomes.
The observed effects limited to exposure in the first 6 months suggest that antibiotic-related modifications of the microbiota, which are most detrimental early in microbiota development, may be more important than clearance of bacterial enteropathogens, which increase in prevalence across the first two years of life. If treatment of infections were the main mechanism, we would expect antibiotic exposure after 6 months of age to also have an impact. Further, we did not find that children with more bacterial pathogens before 6 months of age had larger improvements in WAZ associated with antibiotics than those without bacterial pathogens. However, pathogen prevalence in the MAL-ED study was high, especially for Campylobacter and EAEC in the first 6 months of life (38,39), and clearance of enteropathogens may also be contributing to improved growth in these children. Direct analysis of the microbiome in antibiotic exposed and unexposed children would clarify growth-promoting mechanisms.
This analysis improves on previous studies of the relationship between antibiotics and child growth in low-resource settings. The cross-sectional study conducted in 5 non-affluent countries (Nigeria, India, Indonesia, Thailand, and Syria) relied on caregiver recall of antibiotic exposures at a minimum of 4 years after exposure and caregiver report of anthropometry (21). A previous analysis of an observational birth cohort in India was unable to assess antibiotic class and had partially missing information on antibiotics for non-diarrheal illnesses (40). A systematic review of randomized controlled trials in low and middle-income countries only assessed antibiotic treatment for trial-specific indications and did not consider cumulative antibiotic exposure in early life (41). In contrast, MAL-ED included prospective follow-up for complete antibiotic information and monthly anthropometry measurement by trained fieldworkers.
This analysis was limited by the inability to assess long-term growth outcomes 5 to 10 years after exposure. This may explain why we did not see an effect of antibiotic exposure after 6 months of age, which may only become apparent later in childhood (9,12,14). This may also explain why the effects on length were smaller since length represents a longer-term growth process. In addition, the precision of estimates was highly dependent on frequency of use, which varied for different antibiotic classes across sites. Because MAL-ED was an observational study, we cannot eliminate the potential for unmeasured confounding, for example, by mode of delivery, which was not recorded. However, our analyses accounted for factors associated with antibiotic use previously identified in MAL-ED (22) as well as each child’s detailed illness history. Because we would expect children with more illnesses to be exposed to more antibiotics and have poorer growth, residual confounding by illnesses would likely bias our estimates towards the null, such that our estimates would be conservative.
An association between antibiotics and weight gain in high-income settings is often discussed as a negative side effect of treatment since obesity is a growing public health problem and particularly pernicious among children. However, the cost-benefit analysis may be different in low-resource settings where children may benefit from improvements in growth and are commonly infected with enteropathogens even in the absence of diarrhea (38). On the other hand, overuse of antibiotics is a major concern worldwide and can lead to adverse events, drug toxicity, and antimicrobial resistance. Antibiotic exposure can also cause antibiotic-associated diarrhea (42), alter intestinal immune function, increase intestinal permeability, and increase risk of systemic infections and subsequent diarrhea (32,43-46). Furthermore, increased weight gain may not be an unmitigated positive if antibiotic-induced changes to the microbiota lead to increased risk for obesity or metabolic syndrome later in life (6). It is unknown whether antibiotic-induced weight gain in these settings is equivalent in terms of developmental impact to similar gains achieved by appropriate nutrition and illness management. Therefore, the total impact of antibiotic exposure early in life among children in low-resource settings is unknown and may be mixed. Reduction of inappropriate antibiotic use must be a public health priority, though opportunities for rational and targeted antibiotic therapy may provide additional benefit by promoting weight gain in these children.
Supplementary Material
What is known
Antibiotics are growth-promoting in livestock, and early life exposure to antibiotics has been recently associated with obesity in several high-income countries.
The impact of antibiotic use may be different among children in highly contaminated environments in low-income settings where undernutrition is more common than obesity.
What is new
Antibiotic-associated growth promotion can also occur among children in low-resource settings who are often undernourished.
Antibiotic use in the first 6 months of life was associated with increased weight, especially among children with two or more exposures to macrolides, metronizadole, and cephalosporins.
Acknowledgments
The authors thank the staff and participants of the MAL-ED Network Project for their important contributions. The MAL-ED Network Investigators include representatives from the following organizations: A.B. PRISMA, Iquitos, Peru; Aga Khan University, Karachi, Pakistan; Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand; Christian Medical College, Vellore, India; Duke University, Durham, NC, USA; Fogarty International Center/National Institutes of Health, Bethesda, MD, USA; Foundation for the NIH, Bethesda, MD, USA; Haydom Lutheran Hospital, Haydom, Tanzania; icddr,b, Dhaka, Bangladesh; Institute of Medicine, Tribhuvan University, Kathmandu, Nepal; Johns Hopkins University, Baltimore, MD, USA; The Pennsylvania State University, University Park, PA, USA; Temple University, Philadelphia, PA, USA; Universidade Federal do Ceara, Fortaleza, Brazil; University of Bergen, Norway; University of Illinois at Chicago, IL, USA; University of Venda, Thohoyandou, South Africa; University of Virginia, Charlottesville, VA, USA; Walter Reed/AFRIMS Research Unit, Kathmandu, Nepal; Haukeland University Hospital, Bergen, Norway. A complete list of investigators is included in the online supplemental material.
Source of funding: The Etiology, Risk Factors and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development Project (MAL-ED) is carried out as a collaborative project supported by the Bill & Melinda Gates Foundation, the Foundation for the NIH and the National Institutes of Health/Fogarty International Center. This work was supported by the Fogarty International Center, National Institutes of Health (D43-TW009359 to ETR).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest relevant to this article to disclose.
Contributors’ Statement:
Dr Rogawski designed and carried out the analysis, drafted the initial manuscript, and approved the final manuscript as submitted.
Drs Platts-Mills and Seidman helped design the analysis, critically contributed to interpretation of the data, and approved the final manuscript as submitted.
Drs Houpt and Guerrant were lead investigators for the study, developed study protocols for the collection of data included in this analysis, reviewed and revised the manuscript, and approved the final manuscript as submitted.
Drs Kang and John (India), Shrestha (Nepal), Mduma and Svensen (Tanzania), Ahmed (Bangladesh), Lima (Brazil), Bhutta and Zaidi (Pakistan), Kosek (Peru), and Bessong (South Africa) were the lead investigators for each study site, critically reviewed the manuscript, and approved the final manuscript as submitted.
Drs Mahfuz, Ulak, Soofi, Yori coordinated and supervised data collection, critically reviewed the manuscript, and approved the final manuscript as submitted.
Drs Lang and Gottlieb centrally managed the study, critically reviewed the manuscript, and approved the final manuscript as submitted.
References
- 1.Lin J. Effect of Antibiotic Growth Promoters on Intestinal Microbiota in Food Animals: A Novel Model for Studying the Relationship between Gut Microbiota and Human Obesity? Front Microbiol. 2011;2:53. doi: 10.3389/fmicb.2011.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology (Reading, Engl) 2010 Nov;156(Pt 11):3216–23. doi: 10.1099/mic.0.040618-0. [DOI] [PubMed] [Google Scholar]
- 3.Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci USA. 2011 Mar 15;108(Suppl 1):4554–61. doi: 10.1073/pnas.1000087107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004 Nov 2;101(44):15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013 Sep 6;341(6150):1241214. doi: 10.1126/science.1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox LM, Yamanishi S, Sohn J, Alekseyenko AV, Leung JM, Cho I, et al. Altering the Intestinal Microbiota during a Critical Developmental Window Has Lasting Metabolic Consequences. Cell. 2014 Aug 14;158(4):705–21. doi: 10.1016/j.cell.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin R, Nauta AJ, Ben Amor K, Knippels LMJ, Knol J, Garssen J. Early life: gut microbiota and immune development in infancy. Benef Microbes. 2010 Nov;1(4):367–82. doi: 10.3920/BM2010.0027. [DOI] [PubMed] [Google Scholar]
- 8.Saari A, Virta LJ, Sankilampi U, Dunkel L, Saxen H. Antibiotic exposure in infancy and risk of being overweight in the first 24 months of life. Pediatrics. 2015 Apr;135(4):617–26. doi: 10.1542/peds.2014-3407. [DOI] [PubMed] [Google Scholar]
- 9.Trasande L, Blustein J, Liu M, Corwin E, Cox LM, Blaser MJ. Infant antibiotic exposures and early-life body mass. Int J Obes. 2013 Jan;37(1):16–23. doi: 10.1038/ijo.2012.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scott FI, Horton DB, Mamtani R, Haynes K, Goldberg DS, Lee DY, et al. Administration of Antibiotics to Children Before Age 2 Years Increases Risk for Childhood Obesity. Gastroenterology. 2016 Mar 18; doi: 10.1053/j.gastro.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey L, Forrest CB, Zhang P, Richards TM, Livshits A, DeRusso PA. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014 Sep 29;168(11):1063–9. doi: 10.1001/jamapediatrics.2014.1539. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz BS, Pollak J, Bailey-Davis L, Hirsch AG, Cosgrove SE, Nau C, et al. Antibiotic use and childhood body mass index trajectory. Int J Obes (Lond) 2016 Apr;40(4):615–21. doi: 10.1038/ijo.2015.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mbakwa CA, Scheres L, Penders J, Mommers M, Thijs C, Arts ICW. Early Life Antibiotic Exposure and Weight Development in Children. [2016 Jul 19];The Journal of Pediatrics [Internet] doi: 10.1016/j.jpeds.2016.06.015. Available from: http://www.sciencedirect.com/science/article/pii/S0022347616303754. [DOI] [PubMed]
- 14.Ajslev TA, Andersen CS, Gamborg M, Sørensen TIA, Jess T. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes (Lond) 2011 Apr;35(4):522–9. doi: 10.1038/ijo.2011.27. [DOI] [PubMed] [Google Scholar]
- 15.Gerber JS, Bryan M, Ross RK, Daymont C, Parks EP, Localio AR, et al. Antibiotic Exposure During the First 6 Months of Life and Weight Gain During Childhood. JAMA. 2016 Mar 22;315(12):1258–65. doi: 10.1001/jama.2016.2395. [DOI] [PubMed] [Google Scholar]
- 16.WHO. Guideline: Updates on the management of severe acute malnutrition in infants and children [Internet] Geneva: World Health Organization; 2013. Available from: http://www.who.int/nutrition/publications/guidelines/updates_management_SAM_infantandchildren/en/ [PubMed] [Google Scholar]
- 17.Trehan I, Goldbach HS, LaGrone LN, Meuli GJ, Wang RJ, Maleta KM, et al. Antibiotics as part of the management of severe acute malnutrition. N Engl J Med. 2013 Jan 31;368(5):425–35. doi: 10.1056/NEJMoa1202851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isanaka S, Langendorf C, Berthé F, Gnegne S, Li N, Ousmane N, et al. Routine Amoxicillin for Uncomplicated Severe Acute Malnutrition in Children. N Engl J Med. 2016 Feb 4;374(5):444–53. doi: 10.1056/NEJMoa1507024. [DOI] [PubMed] [Google Scholar]
- 19.Angelakis E, Merhej V, Raoult D. Related actions of probiotics and antibiotics on gut microbiota and weight modification. Lancet Infect Dis. 2013 Oct;13(10):889–99. doi: 10.1016/S1473-3099(13)70179-8. [DOI] [PubMed] [Google Scholar]
- 20.Million M, Lagier J-C, Yahav D, Paul M. Gut bacterial microbiota and obesity. Clinical Microbiology and Infection. 2013;19(4):305–13. doi: 10.1111/1469-0691.12172. [DOI] [PubMed] [Google Scholar]
- 21.Murphy R, Stewart AW, Braithwaite I, Beasley R, Hancox RJ, Mitchell EA. Antibiotic treatment during infancy and increased body mass index in boys: an international cross-sectional study. Int J Obes (Lond) 2013 Nov 21; doi: 10.1038/ijo.2013.218. [DOI] [PubMed] [Google Scholar]
- 22.Rogawski ET, Platts-Mills JA, Seidman JC, John S, Mahfuz M, Ulak M, et al. Use of antibiotics in children younger than two years in eight countries: a prospective cohort study. Bulletin of the World Health Organization. 2017;95:49–61. doi: 10.2471/BLT.16.176123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MAL-ED Network Investigators. The MAL-ED study: a multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource-poor environments. Clin Infect Dis. 2014 Nov 1;59(Suppl 4):S193–206. doi: 10.1093/cid/ciu653. [DOI] [PubMed] [Google Scholar]
- 24.Houpt E, Gratz J, Kosek M, Zaidi AKM, Qureshi S, Kang G, et al. Microbiologic Methods Utilized in the MAL-ED Cohort Study. Clin Infect Dis. 2014 Nov 1;59(suppl 4):S225–32. doi: 10.1093/cid/ciu413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health Organization. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age, Methods and development [Internet] [2016 May 9];2006 Available from: http://www.who.int/childgrowth/standards/Technical_report.pdf?ua=1.
- 26.Psaki SR, Seidman JC, Miller M, Gottlieb M, Bhutta ZA, Ahmed T, et al. Measuring socioeconomic status in multicountry studies: results from the eight-country MAL-ED study. Population Health Metrics. 2014 Mar 21;12(1):8. doi: 10.1186/1478-7954-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richard SA, Barrett LJ, Guerrant RL, Checkley W, Miller MA MAL-ED Network Investigators. Disease surveillance methods used in the 8-site MAL-ED cohort study. Clin Infect Dis. 2014 Nov 1;59(Suppl 4):S220–4. doi: 10.1093/cid/ciu435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999 Jan;10(1):37–48. [PubMed] [Google Scholar]
- 29.Gupta MC, Urrutia JJ. Effect of periodic antiascaris and antigiardia treatment on nutritional status of preschool children. Am J Clin Nutr. 1982 Jul;36(1):79–86. doi: 10.1093/ajcn/36.1.79. [DOI] [PubMed] [Google Scholar]
- 30.Heikens GT, Schofield WN, Dawson S. The Kingston Project. II. The effects of high energy supplement and metronidazole on malnourished children rehabilitated in the community: anthropometry. Eur J Clin Nutr. 1993 Mar;47(3):160–73. [PubMed] [Google Scholar]
- 31.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol. 2008 Nov 18;6(11):e280. doi: 10.1371/journal.pbio.0060280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faa G, Gerosa C, Fanni D, Nemolato S, van Eyken P, Fanos V. Factors influencing the development of a personal tailored microbiota in the neonate, with particular emphasis on antibiotic therapy. J Matern Fetal Neonatal Med. 2013 Oct;26(Suppl 2):35–43. doi: 10.3109/14767058.2013.829700. [DOI] [PubMed] [Google Scholar]
- 33.Saavedra JM, Dattilo AM. Early development of intestinal microbiota: implications for future health. Gastroenterol Clin North Am. 2012 Dec;41(4):717–31. doi: 10.1016/j.gtc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 34.Cox LM, Blaser MJ. Pathways in microbe-induced obesity. Cell Metab. 2013 Jun 4;17(6):883–94. doi: 10.1016/j.cmet.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Victora CG, Barros FC, Horta BL, Martorell R. Short-term benefits of catch-up growth for small-for-gestational-age infants. Int J Epidemiol. 2001 Dec 1;30(6):1325–30. doi: 10.1093/ije/30.6.1325. [DOI] [PubMed] [Google Scholar]
- 36.Horta BL, Victora CG, de Mola CL, Quevedo L, Pinheiro RT, Gigante DP, et al. Associations of Linear Growth and Relative Weight Gain in Early Life with Human Capital at 30 Years of Age. J Pediatr. 2017 Mar;182:85–91. e3. doi: 10.1016/j.jpeds.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, et al. Maternal and child undernutrition: consequences for adult health and human capital. The Lancet. Jan 26;371(9609):340–57. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Platts-Mills JA, Babji S, Bodhidatta L, Gratz J, Haque R, Havt A, et al. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED) Lancet Glob Health. 2015 Sep;3(9):e564–75. doi: 10.1016/S2214-109X(15)00151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amour C, Gratz J, Mduma E, Svensen E, Rogawski ET, McGrath M, et al. Epidemiology and impact of Campylobacter infection in children in eight low-resource settings: results from the MAL-ED study. Clin Infect Dis. 2016 Aug 7; doi: 10.1093/cid/ciw542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rogawski ET, Westreich DJ, Adair LS, Becker-Dreps S, Sandler RS, Sarkar R, et al. Early Life Antibiotic Exposure Is Not Associated with Growth in Young Children of Vellore, India. The Journal of Pediatrics. 2015 Nov;167(5):1096–102. e3. doi: 10.1016/j.jpeds.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gough EK, Moodie EEM, Prendergast AJ, Johnson SMA, Humphrey JH, Stoltzfus RJ, et al. The impact of antibiotics on growth in children in low and middle income countries: systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g2267. doi: 10.1136/bmj.g2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McFarland LV. Antibiotic-associated diarrhea: epidemiology, trends and treatment. Future Microbiol. 2008 Oct;3(5):563–78. doi: 10.2217/17460913.3.5.563. [DOI] [PubMed] [Google Scholar]
- 43.Wlodarska M, Finlay BB. Host immune response to antibiotic perturbation of the microbiota. Mucosal Immunol. 2010 Mar;3(2):100–3. doi: 10.1038/mi.2009.135. [DOI] [PubMed] [Google Scholar]
- 44.Ryan CA, Nickels MK, Hargrett-Bean NT, Potter ME, Endo T, Mayer L, et al. Massive outbreak of antimicrobial-resistant salmonellosis traced to pasteurized milk. JAMA. 1987 Dec 11;258(22):3269–74. [PubMed] [Google Scholar]
- 45.Rogawski ET, Westreich DJ, Becker-Dreps S, Adair LS, Sandler RS, Sarkar R, et al. Antibiotic treatment of diarrhoea is associated with decreased time to the next diarrhoea episode among young children in Vellore, India. Int J Epidemiol. 2015 Jun 1;44(3):978–87. doi: 10.1093/ije/dyv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogawski ET, Westreich D, Becker-Dreps S, Adair LS, Sandler RS, Sarkar R, et al. The Effect of Early Life Antibiotic Exposures on Diarrheal Rates Among Young Children in Vellore, India. Pediatr Infect Dis J. 2015 Mar 3;34:583–8. doi: 10.1097/INF.0000000000000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


