Summary
Background
One previous pilot study suggested the association of low plasma glucosylceramide (GlcCer) levels with venous thrombosis (VTE) risk.
Objective
We aimed to confirm and evaluate the association of low plasma GlcCer levels with VTE and myocardial infarction (MI) occurrence, respectively.
Patients and Methods
We evaluated the association of GlcCer in two independent case-control studies of Caucasian VTE populations (N = 210 and 636) and one case-control study of Caucasian MI patients (N = 345).
Result
Plasma GlcCer levels in VTE patients were lower compared to controls in two independent VTE populations (5.0 vs 5.8 μg/mL, p = 0.003 for the Scripps registry, and 5.6 vs 6.0 μg/mL, p = 0.001 for the Valencia registry, respectively). A low plasma GlcCer level (below 10th percentile of controls) was associated with increased VTE occurrence [odds ratio (OR) = 3.7 (95%CI, 1.8–7.9) for Scripps registry and OR = 2.1 (95%CI, 1.3–3.3) for Valencia registry, respectively). For the MI study, the median GlcCer plasma level was lower in MI patients than in controls (4.3 vs 5.6 μg/mL, p<0.001), and a low level of GlcCer (below 10th percentile of control) was associated with higher MI occurrence [OR = 7.7, (95%CI, 4.3–13.8)].
Conclusion
Lower concentration of GlcCer was associated with VTE occurrence in two independent studies and also with MI occurrence in one study.
Keywords: venous thrombosis, myocardial infarction, glucosylceramide, risk factor, epidemiology
Introduction
Plasma lipids and lipoproteins are associated with the development of atherosclerosis and arterial and venous thrombotic disease [1, 2]. Although the imbalance of certain sphingolipids is suggested to be associated with thrombotic diseases [3, 4], there are only a few clinical studies on the association of sphingolipid imbalance with thrombosis. Glucosylceramide (GlcCer) is a glycosphingolipid possessing a variety of biological activities, including an anticoagulant cofactor activity for activated protein C which can downregulate thrombin generation [5, 6] and an immune regulatory activity [7–9]. In a pilot study, low plasma GlcCer levels were associated with the risk of venous thromboembolism (VTE) [5]; to date, this finding has not been replicated. Three genetic variants in the ATPase, class IV, type 10D gene (ATP10D) were associated with higher GlcCer level and may be protective against myocardial infarction (MI) [4]. However, there are no studies on association of plasma GlcCer levels with MI. Here we have measured plasma levels of GlcCer in subjects from VTE and MI populations in order to replicate our pilot study showing the association of low GlcCer with VTE [5] and to investigate the association of plasma GlcCer levels with the risk of MI. Phosphatidylethanolamine (PtdEtn), which, like GlcCer [5, 6], is known as an APC anticoagulant cofactor [10], was also measured on the same chromatograms used for GlcCer determination to evaluate its association with VTE and MI.
Material and Methods
Patient and control groups
Plasma samples from the Scripps VTE registry (105 Caucasian VTE patients and 105 age matched controls) [11, 12], the Valencia VTE registry (320 VTE Caucasian cases and 316 Caucasian controls) [14], and one MI cohort (209 MI patients from Genetic and Viral Attributes of Myocardial Infarction study and 197 healthy controls from Genetic Attributes of Thrombosis Epidemiology study) [15, 16] were studied. The subjects taking lipid lowering medications including statins were excluded to avoid any interaction of dynamic lipid metabolism including glycolipids [11, 13] for VTE studies. Further, to address the GlcCer independent association with VTE for known risk factors of VTE, subjects with known thrombophilic defects, including factor V Leiden and prothrombin G20210A mutations and anyone currently taking oral anticoagulants or oral contraceptives were excluded in the Valencia VTE study. To minimize the age effect on the association analysis, older VTE patients (> 55 years old) were excluded from both VTE registries. To eliminate the warfarin influence on the association of GlcCer levels with Scripps VTE or MI occurrences, the association of GlcCer levels were also analyzed for cases vs. controls after excluding warfarin users. The detailed information for the patient and control groups are presented in the supporting information.
Plasma GlcCer analysis
Plasma GlcCer and PtdEtn levels were measured with minor modification as described [5, 17]. Briefly, plasma lipids extracted from citrated plasma with chloroform/methanol (2:1, v/v) were analyzed using a high performance liquid chromatography (HPLC) system (Waters Corp., Milford, MA) coupled to a Sedex-55 evaporative light scattering detector system (SEDERE, Alfortville Cedex, France). A μPorosil column (300 mm × 3.9 mm) was used with isocratic chloroform/methanol/water (88:11:1). Mean intra-assay coefficients of variation from 8 independent determinations of GlcCer and PtdEtn (N = 8 pooled plasma aliquots) (George King) were 8.7% and 7.0%, respectively, and GlcCer was stable at room temperature in whole blood, EDTA-plasma and citrated plasma for up to 10 hours after blood draw.
Other lipid and lipoprotein analysis
Serum lipid profile data were obtained from the routine clinical laboratory with the use of standard techniques. Lipoprotein particle concentrations of 10 lipoprotein subclasses in EDTA plasma were determined by proton NMR spectroscopy LipoScience as described [11].
DNA Analyses
Genomic DNA was extracted from EDTA-blood with the use of Puregene DNA Purification Kits (Gentra Systems). Factor V Leiden and prothrombin G20210A mutations were assayed as described [18].
Statistical Analysis
Mann Whitney test was used to compare the median values (e.g., GlcCer and PtdEtn) between case and control groups using Prism™ 4.03 software (Graph Pad Software Inc., San Diego, CA). The correlation of GlcCer or PtdEtn with lipid/lipoproteins was determined by two-tailed Spearman with 95% confidence interval using Prism™ 4.03 software. Logistic regression was used to evaluate the association between GlcCer level and VTE or MI occurrence using STATA (StataCorp LLC, College Station, TX) and SAS version 9.3 (SAS Institute Inc, Cary, NC). Plasma GlcCer levels were evaluated for association with VTE and MI by calculating the odds ratio (OR) as a measure of relative risk comparing participants above and below the 10th percentile and by tertiles. Adjusted models produced for VTE included risk factors for VTE, e.g., obesity (BMI > 30), factor V Leiden, prothrombin G20210A mutation, hormone use, and HDL and LDL levels. Adjusted models included risk factors for MI and factors that were significantly associated with GlcCer levels among controls, including obesity (BMI > 30), hypertension, current smoking, family history of MI, alcohol consumption (never drinking, drinking less than once per week, 1–7 times per week, or more than 8 times per week). All percentiles were defined based on values for controls. A p value <0.05 was considered statistically significant.
Results
Previously reported plasma GlcCer and PtdEtn levels in VTE pilot study [5]
Plasma levels of GlcCer and PtdEtn were previously reported for 70 Caucasian VTE patients referred for evaluation and 70 Caucasian healthy blood donors (50 of each from the University of Vienna, Austria, and 20 of each from the Mayo Clinic, Rochester, MN) [5]. Low plasma GlcCer levels were associated with the occurrence of VTE. The mean GlcCer level was lower for VTE patients versus controls [4.9 vs 6.5 μg/mL (p = 0.0007)] (Table S1). As a measure of relative risk, the odds ratio (OR) for VTE in subjects with GlcCer levels below the 10th percentile of controls was 5.7 (95% CI, 2.3–14).
The association of established VTE risk factors or lipid/lipoproteins with VTE in the Scripps registry
The association of known VTE risk factors with VTE occurrence in the Scripps registry and with plasma GlcCer levels was evaluated. VTE was associated with established risk factors for VTE, factor V Leiden mutation, prothrombin G20210A mutation and BMI (Table S1). None of these risk factors was significantly associated with GlcCer levels among controls (Table 1). Only hormone therapy at the time of blood draw was associated with PtdEtn levels among controls (Table 1), and the plasma levels of GlcCer were lower in the group of hormone users.
Table 1.
Association between covariates and GlcCer and PtdEtn plasma levels in controls of the Scripps VTE Registry.
| Covariate | GlcCer median (IQR), μg/mL |
PtdEtn median (IQR), μg/mL |
|---|---|---|
| Sex | ||
| female | 5.6 (4.9–6.6) | 51.3 (38.2–64.6) |
| male | 6.0 (5.3–7.1) | 57.2 (43–75.1) |
| p value | 0.06 | 0.24 |
| Age | ||
| < 35 | 5.5 (5.1–6.4) | 45.7 (35.5–59.1) |
| 35–45 | 5.8 (4.8–6.6) | 53.6 (38.7–70.5) |
| 45–55 | 5.9 (5.0–7.3) | 60.6 (44.6–70.1) |
| ≥ 55 | 5.6 (5.1–6.7) | 56.7 (44.3–66.4) |
| p value | 0.39 | 0.13 |
| Obesity | ||
| Not obese | 5.9 (4.9–6.9) | 53.6 (44.2–65.0) |
| obese | 5.6 (4.4–6.6) | 56.2 (46.2–67.5) |
| p value | 0.12 | 0.47 |
| Factor V Leiden | ||
| Yes | 5.1 (4.8–6.6) | 42.0 (35.5–91.5) |
| No | 5.8 (5.0–6.7) | 56.2 (42.0–66.9) |
| p value | 0.21 | 0.59 |
| Prothrombin G20210A | ||
| Yes | 5.5 (N = 3) | 42.0 (N = 3) |
| No | 5.8 (5.0–6.8) | 56.3 (42.0–67.5) |
| p value | 0.41 | 0.15 |
| Hormone use | ||
| Yes | 5.5 (5.0–6.4) | 46.2 (37.0–59.1) |
| No | 5.7 (4.7–6.7) | 59.0 (47.2–67.3) |
| p value | 0.62 | 0.02 |
HDL particles levels determined by NMR were lower in VTE cases than controls. In contrast, levels of LDL particles and serum LDL-C levels were higher in VTE cases (Table S1).
The correlation of plasma levels of GlcCer and PtdEtn with plasma levels of other lipids and lipoproteins in control subjects in the Scripps Registry (Tables S2 and S3) showed that serum HDL-C and plasma HDL particles levels positively correlated with plasma GlcCer levels (r = 0.21, p = 0.03 and r= 0.30, p = 0.002, respectively). Plasma GlcCer levels positively correlated with LDL-C levels (r = 0.31, p = 0.001). Plasma GlcCer levels correlated weakly with plasma PtdEtn levels (r = 0.28, p = 0.005), and this correlation was also observed in the Valencia control group (r = 0.17, p = 0.007) and in the MI control group (r = 0.28, p = 0.0007) group.
Plasma GlcCer and PtdEtn levels in the Scripps VTE Registry
The median plasma GlcCer level was lower in VTE cases than in controls (Table S1, Figure 1A). Low plasma GlcCer levels (below the 10th percentile) were associated with VTE [OR = 3.7 (95% CI, 1.8–7.9)] (Figure 1A). When VTE and control subjects were categorized by tertile of GlcCer levels, the OR for the lowest vs. highest tertile was increased [OR = 3.9 (95% CI,1.9–7.8)] (Table 2).
Figure 1. Plasma GlcCer and PtdEtn levels in VTE and MI patients and controls.
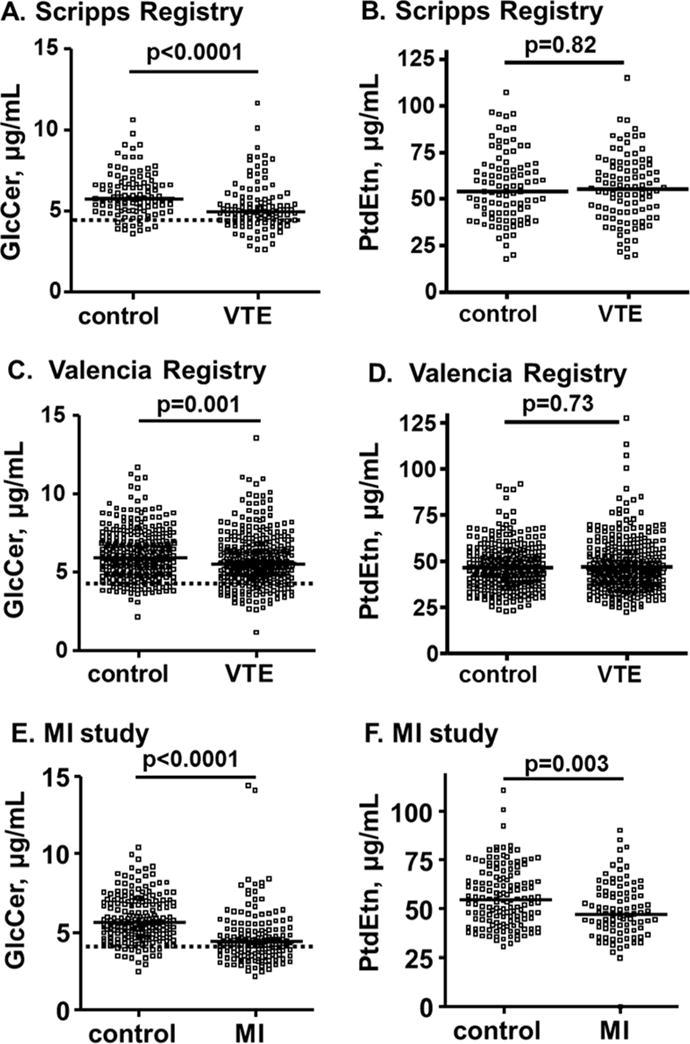
Plasma GlcCer and PtdEtn levels were determined for the Scripps VTE registry (A, B), the Valencia VTE registry (C, D), and the MI population (E, F). The solid thick lines indicate median values and the dotted lines indicate the values for the 10th percentile of the controls. The p-values shown are for the difference of median values between VTE patients and controls which was analyzed by Mann-Whitney test using Prism™ 4.03 software (Graph Pad Software Inc., San Diego, CA).
Table 2.
Odds ratios (OR) (95% CI) for VTE according to tertiles of plasma GlcCer levels.
| GlcCer tertiles | |||
|---|---|---|---|
| Scripps VTE registry | lowest | middle | highest |
| GlcCer levels (μg/mL) | < 5.3 | ≥ 5.3 and < 6.5 | ≥ 6.5 |
| Adjustment | |||
| I. none | 3.9 (1.9–7.8) | 1.1 (0.50–2.5) | 1 |
| II. FV Leiden, prothrombin G20210A, BMI, Age, Sex | 3.2 (1.5–6.7) | 1.1 (0.45–2.6) | 1 |
| III. model II plus HDL-C, LDL-C | 4.6 (2.0–11) | 1.4 (0.52–3.8) | 1 |
| IV. model II plus HDL particles, LDL particles | 2.8 (1.3–6.2) | 0.90 (0.34–2.4) | 1 |
| Valencia VTE Registry* | lowest | middle | highest |
| GlcCer levels (μg/mL) | < 5.4 | ≥ 5.4 and < 6.6 | ≥ 6.6 |
| Adjustment | |||
| I. none | 1.8 (1.2–2.6) | 1.3 (0.86–2.0) | 1 |
| II. BMI, Age, Sex | 1.8 (1.2–2.7) | 1.4 (0.93–2.1) | 1 |
The tertile-based odds ratios (OR) (95% CI) for VTE based on the plasma GlcCer levels in controls are shown. Tertile cut points were defined in controls. The subjects with the highest tertile of GlcCer served as reference. Models II to IV were adjusted by variables indicated in the Table. BMI, HDL-C, HDL particles, LDL-C and LDL particles were used as continuous variables.
The carriers of FV Leiden and/or prothrombin G20210A and the hormone and/or anticoagulant users were excluded from the subjects available for GlcCer analysis for the Valencia VTE Registry study.
The association of low plasma GlcCer levels (below the 10th percentile) and of the lowest vs. highest tertile with VTE occurrence remained significant after the adjustment for known risk factors (i.e., factor V Leiden, prothrombin G20210A mutation, and BMI) [OR = 3.5 (95%CI, 1.6–7.7) and OR = 3.2 (95%CI, 1.5–6.7) (Table S4 and Table 2), respectively], including additional adjustment for lipid/lipoprotein parameters (Table S4 and Table 2, models III to V).
The median plasma PtdEtn level among VTE cases was not significantly different than controls [54.7 μg/mL (IQR, 41.2–67.5 μg/mL) vs. 53.6 μg/mL (IQR, 42.0‒67.3 μg/mL), p = 0.82] (Figure 1B) as previously observed [5]. There was no association for low plasma PtdEtn or for the lowest vs. the highest tertile of PtdEtn with VTE occurrence (Table S5).
Plasma GlcCer and PtdEtn levels in the Valencia Registry
The median plasma GlcCer level was lower in Valencia VTE cases than controls (Table S1, Figure 1C). Low plasma GlcCer levels (below the 10th percentile) were associated with higher risk of VTE [OR = 2.1 (95%CI, 1.3–3.3)] (Figure 1C). The OR for the lowest vs highest GlcCer tertile was 3.9 (95%CI, 1.9–7.8). These ORs remained elevated after additional adjustment for lipid/lipoprotein parameters.
There was no difference in the median PtdEtn level between VTE and controls in the Valencia registry [44.9 μg/mL (IQR, 36.9–54.7μg/mL) vs. 44.6 μg/mL (IQR, 37.3–53.7 μg/mL)] (Table S1, Figure 1D), and no increased OR of VTE by PtdEtn level (Table S5).
The association of MI with established MI risk factors in the MI registry
MI was associated with established risk factors for MI: hypertension, diabetes, current smoking, obesity, alcohol consumption (assessed by comparing distribution of those never drinking, drinking less than once per week, 1–7 times per week, and more than 8 times per week among cases and controls) and family history in our study group (Table S6). None of the MI-risk-modifying medications (e.g., statin use and aspirin use) being used at the time of blood draw were significantly associated with GlcCer levels among controls (Table 4). Only hormone therapy at the time of blood draw was significantly associated with PtdEtn levels among controls, and the plasma level of GlcCer was lower in the group of hormone users.
Table 4.
Association between medication use and GlcCer and PtdEtn plasma levels.
| GlcCer | PtdEtn | |||
|---|---|---|---|---|
| Covariate | Median (IQR), μg/mL | p | Median (IQR), μg/mL | p |
| Hormone Use | ||||
| Yes | 5.3 (4.8–7.1) | 0.73 | 68.2 (59.5–75.0) | <0.0001 |
| No | 5.7 (4.8–6.8) | 51.3 (41.7–63.0) | ||
| Warfarin use | ||||
| Yes | 5.1 (n = 1) | 0.56 | 60.9 (n = 1) | 0.63 |
| No | 5.6 (4.8–6.9) | 54.1 (44.2–66.2) | ||
| Statin Use | ||||
| Yes | 5.1 (4.7–6.3) | 0.15 | 50.8 (44.2–67.0) | 0.70 |
| No | 5.7 (4.8–6.9) | 54.9 (44.4–65.7) | ||
| Aspirin Use | ||||
| Yes | 5.6 (4.7–6.5) | 0.62 | 49.3 (43.8–69.3) | 0.23 |
| No | 5.6 (4.8–6.9) | 55.7 (44.9–65.7) | ||
Plasma GlcCer and PtdEtn levels in the MI registry
The median GlcCer plasma level was lower in MI cases than in controls (Table S6, Figure 1E). Low plasma GlcCer levels (below 10th percentile) was associated with MI [OR = 6.9 (95%CI, 3.9–12.2)] (Table 5). The OR for the lowest vs highest tertile was also increased [OR = 6.4 (95%CI, 3.5–11.9)] The relationship between low plasma GlcCer and MI remained significant even after adjustments for age and gender plus MI risk factors (Table 5).
Table 5.
Odds ratios (OR) (95% CI) for MI according to tertiles of GlcCer and PtdEtn.
| MI study | tertiles | |||
|---|---|---|---|---|
| lowest | middle | highest | ||
| GlcCer | GlcCer levels (μg/mL) | < 5.1 | ≥ 5.1 and < 6.4 | ≥ 6.4 |
| Model I | 6.4 (3.5–11.9) | 1.5 (0.7–3.0) | 1 | |
| Model II | 5.3 (1.9–14.7) | 2.0 (0.7–5.9) | 1 | |
| PtdEtn | PtdEtn levels (μg/mL) | < 47.6 | ≥ 47.6 and < 61.6 | ≥ 61.6 |
| Model I | 2.7 (1.4–5.3) | 1.6 (0.8–3.3) | 1 | |
| Model II | 1.6 (0.4–6.1) | 2.9 (0.7–12.3) | 1 | |
The tertile-based odds ratios (OR) (95% CI) for MI based on the plasma GlcCer are shown. Tertile cut points were defined in controls. The control subjects with the highest tertile of GlcCer served as the reference group.
Model I: unadjusted OR
Model II GlcCer: ORs were adjusted by age, gender, diabetes, obesity, hypertension, current smoking, family history for MI, alcohol consumption.
Model II PtdEtn: ORs were adjusted by age, gender, diabetes, obesity, hypertension, current smoking, family history for MI, alcohol consumption, hormone therapy use.
The median values of plasma PtdEtn were also lower in the MI patients than in controls (Table S6, Figure 1F). Low plasma levels of PtdEtn (below 10th percentile) were significantly linked with MI [OR = 2.4 (95%CI, 1.1–5.1), p = 0.02]. The OR for the lowest vs. highest tertile of plasma level of PtdEtn was also increased [OR = 2.7 (95%CI, 1.4–5.3)] (Table 5). However, after adjustment for hormone use, the association was attenuated [OR = 1.6 (95%CI, 0.4–6.1)] (Table5, model II).
Plasma GlcCer levels in the Scripps VTE and MI registries without warfarin user
To test the warfarin influence on the association of GlcCer levels with Scripps VTE or MI occurrences, we made an analysis excluding warfarin users and analyzed the association of GlcCer levels with cases vs. controls. When the warfarin users are eliminated from Scripps VTE registry cases, the median GlcCer plasma level remained significantly lower in VTE cases than in controls [4.6 μg/mL (IQR, 4.0–5.5 μg/mL) vs 5.8 μg/mL (IQR, 5.0–6.7 μg/mL), p = 0.0001, (data not shown)]. Similarly, when the warfarin users were eliminated for the MI study, the median GlcCer plasma level remained significantly lower for MI cases vs. controls [4.4 μg/mL (IQR, 3.5–5.5 μg/mL) vs 5.6 μg/mL (IQR, 4.8–6.9 μg/mL), p < 0.0001 (data not shown)].
Discussion
Low plasma GlcCer levels were associated with the occurrence of VTE in a previous pilot study [5]. Major limitations of that pilot study were that a younger control population was compared with cases and that there was a lack of analyses using other known VTE risk factors, which may affect the association analysis. We confirmed this previous result using plasma from VTE subjects and age and sex matched controls from two independent VTE registries. Using these two VTE populations, we replicated that plasma GlcCer deficiency is associated with VTE occurrence. Deficiency of plasma PtdEtn, known as another APC anticoagulant cofactor [10], was not associated with VTE as reported in the pilot study [5].
Based on the analysis of the association of VTE with known risk factors in our study groups, the factor V Leiden and prothrombin G20210A mutations were included as covariates in the analysis. Since none of the VTE-risk-modifying medications being used at the time of blood draw were significantly associated with GlcCer levels among the Scripps registry controls, these factors were not included in the adjusted models for analysis of GlcCer association with VTE. The association of low plasma GlcCer with VTE (below the 10th percentile) and the tertile-based OR for VTE remained significant after adjustments (i.e., factor V Leiden, prothrombin G20210A mutation, BMI, age and Sex).
Further, VTE is suggested to be associated with dyslipidemia and dyslipoproteinnemia (e.g., lower HDL particles in VTE and higher LDL particles levels, especially in male populations) [11, 19]. The observed correlations between plasma GlcCer levels and lipids and lipoproteins suggest that low GlcCer’s association with VTE merely reflects the association of low HDL particle levels with VTE risk [5, 11]. However, the correlation between HDL particles levels and GlcCer was mainly derived from the correlations of small HDL particle levels with plasma GlcCer levels (r = 0.23, p = 0.02) and medium HDL particles levels for plasma PtdEtn levels (r = 0.27, p = 0.009), whereas GlcCer and PtdEtn levels were independent of large HDL particles levels whose deficiency is associated with the risk of VTE [11]. This implies that association of lower plasma GlcCer levels with VTE risk is not simply reflective of low HDL levels. Importantly, association of lower plasma GlcCer with VTE (below the 10th percentile) remained significant after further adjustments for lipid/lipoprotein parameters (e.g., HDL particles, and LDL particles), implying low plasma GlcCer associated to VTE does not depend on lipoprotein associations. These findings suggest that low plasma GlcCer is associated with increased occurrence of VTE independent of previously known risk factors.
To confirm the association of lower GlcCer levels with VTE, we also measured plasma GlcCer levels using the Valencia Registry with the exclusion of two major genetic VTE risk factors, factor V Leiden and prothrombin G20210A mutation. Those using other major medications seen in Scripps registry (i.e., oral contraceptives and warfarin), which may have an influence on lipoprotein and glycosphingolipid metabolism [17, 20], were also excluded. In this Valencia registry, we observed a similar association of low GlcCer levels with VTE as in the pilot study [5] and in the Scripps registry shown in this article. In addition, this confirmed that the association of low GlcCer levels with VTE occurrence is not caused by the factor V Leiden or prothrombin 20210A mutations. Thus, here we confirmed our previous finding of the association of low levels of plasma GlcCer with increased occurrence of VTE in two independent Caucasian populations, and this association was independent of factor V Leiden, prothrombin G20210A mutations, anticoagulant use or oral contraceptive use, HDL, LDL or BMI levels.
Epidemiological studies suggest some overlap of risk factors for venous and arterial thrombosis [19, 21–23]. For instance, atherosclerosis and venous thrombosis share several common risk factors, such as age, obesity, cigarette smoking, and metabolic syndrome [19, 21, 22]. Consistent with this, we observed that low plasma GlcCer levels (below 10th percentile) was associated with MI. The relationship between low plasma levels of GlcCer and MI remained significant even after adjustments for these known MI risk factors. Further, we demonstrated that low plasma levels of GlcCer were independently associated with MI. Further, none of the MI-risk-modifying medications being used at the time of blood draw were significantly associated with GlcCer levels among controls. So these medications do not explain this association.
The median values of plasma PtdEtn were also lower in the MI patients than in controls and low plasma levels of PtdEtn (below 10th percentile) were significantly associated with MI [OR = 2.4 (95%CI = 1.1–5.1)]. However, when the OR for the lowest vs. highest tertile of plasma PtdEtn level was adjusted for hormone use, it appeared that the association of low PtdEtn levels with MI occurrence was caused by the unequal number of hormone users between MI and control groups.
Since plasma GlcCer levels positively correlated with HDL levels and since low HDL level is a well known risk factor for coronary artery disease [1], one might think that low plasma GlcCer level association with MI could merely reflect the level of HDL-C as discussed above for VTE. In coronary artery disease, low plasma levels of large HDL particles are associated with increased MI risk among HDL subfactions [1]. As noted above, large HDL particles levels did not correlate with GlcCer levels in the Scripps VTE registry controls. This suggests that the association of low plasma GlcCer levels with the increased risk of MI is not likely to reflect the association of low HDL with MI risk.
This study has multiple limitations. The timing of a blood drawn in relation to a clinical event can raise an issue of validity of conclusions. Some plasma was drawn > 5 years after the event in our registries. When the Scripps VTE group was sub grouped by the time since VTE (< 1 year, 1 to 3 years, ≥ 3 years), the median GlcCer levels were not different among sub groups (Figure S1). This observation does support the validity of the findings for GlcCer, but it may remain an issue to consider. The use of warfarin at the time of blood collection is a potential issue, as the Scripps VTE and MI registries included some warfarin users among cases. However, the results were robust among those not on warfarin in both Scripps VTE and MI registries. These findings thus appear to confirm that warfarin use does not affect the association of low GlcCer with VTE and MI occurrence. The retrospective studies of GlcCer plasma levels from these case-control studies cannot distinguish whether the observed differences are causal, consequential, or incidental although the anticoagulant cofactor activity of GlcCer for APC [5, 6] provides a notable degree of biologic plausibility for potential antithrombotic actions of GlcCer. For the MI cohort, the lack of the information of other lipid/lipoprotein parameters is a limitation. Finally, these studies included only Caucasians; thus, it should note that the results may not be valid for other racial groups.
How might low plasma GlcCer levels be associated with the pathology of VTE and MI? GlcCer is a minor plasma lipid, but it possesses various biological activities involved in the coagulation [5, 6] and immune systems [7–9]. In the coagulation system, GlcCer acts as an anticoagulant lipid cofactor by enhancing APC anticoagulant activity to reduce thrombin generation [5, 6]. Excess amounts of thrombin generation, namely hypercoagulability, are associated with VTE and atherosclerosis [23–28]. Low plasma APC levels are an independent risk factor of both venous [29] and arterial thrombosis [30]. Therefore, lower APC anticoagulant cofactor activity due to low plasma GlcCer level might contribute to hypercoagulability and increase risk for thrombotic diseases. Decreased immuno-modulatory and anti-inflammatory functions of GlcCer [7, 8] might also cause a link to increased thrombosis risk. Additionally or alternatively, the association of low plasma GlcCer level with VTE and MI could be a secondary effect. It might reflect the level of other sphingolipids which contribute to thrombosis risk. For instance, ceramide, which is converted into GlcCer by GlcCer synthase or produced from GlcCer by β-glucosidase, is considered to be an intracellular apoptosis inducer causing endothelial cell damage and the development of thrombosis [31, 32]. Deficiency of GlcCer synthase, which decreases GlcCer but increases ceramide levels, is associated with ceramide-dependent cell apoptosis [33]. Further, an increase of ceramide levels in LDL particles induce LDL aggregation on the cell wall [32]. Thus, increased production of ceramide is likely to be atherogenic and the ceramide levels may be reflected by the plasma levels of GlcCer.
In summary, a previous finding of an association of plasma GlcCer deficiency with VTE [5] was replicated in two independent Caucasian populations, and furthermore, we found that lower plasma GlcCer level is associated with MI in one Caucasian population.
Supplementary Material
Table 3.
Association between covariates and GlcCer and PtdEtn levels in controls of the MI study.
| Covariate | GlcCer median (IQR), μg/mL | PtdEtn median (IQR), μg/mL |
|---|---|---|
| Sex | ||
| female | 5.4 (4.8–7.0) | 61.7 (52.4–72.4) |
| male | 5.7 (4.7–6.8) | 49.5 (41.3–60.3) |
| p value | 0.65 | 0.0005 |
| Age, years old | ||
| < 40 | 5.3 (4.5–5.9) | 52.3 (40.5–61.6) |
| 40–50 | 5.5 (4.8–6.8) | 53.0 (41.3–71.7) |
| 50–60 | 5.7 (5.1–6.7) | 57.5 (44.2–67.5) |
| ≥ 60 | 5.8 (4.9–7.4) | 54.1 (44.9–66.2) |
| p value | 0.12 | 0.70 |
| Obesity | ||
| Not Obese | 5.6 (4.9–6.9) | 53.2 (44.2–65.0) |
| Obese | 5.1 (4.4–6.6) | 62.4 (46.2–67.5) |
| p value | 0.09 | 0.32 |
| Family History for MI | ||
| No Family History | 5.6 (4.8–6.9) | 53.2 (43.7–64.8) |
| Family History | 5.4 (4.9–6.5) | 77.2 (44.2–81.5) |
| p value | 0.80 | 0.04 |
| Alcohol Consumption | ||
| 0 | 5.7 (4.3–6.9) | 47.6 (39.5–56.5) |
| < 1 / week | 5.3 (4.5–6.0) | 60.9 (47.8–68.0) |
| 1–7 / week | 5.6 (4.8–7.0) | 52.3 (43.2–66.6) |
| ≥ 7 / week | 6.1 (5.2–6.9) | 57.5 (47.8–73.2) |
| p value | 0.32 | 0.15 |
| Smoking Status | ||
| No Smoking | 5.5 (4.6–6.8) | 48.3 (39.4–69.1) |
| Smoking | 5.9 (4.8–6.8) | 60.9 (45.9–74.1) |
| p value | 0.44 | 0.19 |
| Hypertension | ||
| No Hypertension | 5.6 (4.8–6.9) | 55.8 (45.1–69.8) |
| Hypertension | 5.3 (4.7–6.6) | 50.3 (43.7–61.8) |
| p value | 0.46 | 0.17 |
| Diabetes | ||
| No Diabetes | 5.6 (4.8–6.9) | 54.5 (44.0–67.1) |
| Diabetes | 4.9 (3.8–5.8) | 57.3 (44.8–64.1) |
| p value | 0.08 | 0.99 |
Brief summary.
Lower levels of the plasma lipid, glucosylceramide, are associated with venous thrombosis and myocardial infarction.
Essentials.
Minor abundance plasma lipids, e.g., glucosylceramide, can modulate blood coagulation reactions
This lipid was measured in plasmas of 1 myocardial infarction and 2 venous thrombosis populations
Low plasma glucosylceramide levels were found in each population compared to matched controls
Low plasma glucosylceramide levels are associated with venous and arterial thrombosis
Acknowledgments
Support was provided, in part, by grants HL021544, HL133728 and HL052246 from the National Institutes of Health and by Spanish grants PI12/00027, PI14/00079 and PI14/00512 from ISCIII and FEDER (Una manera de hacer Europa). We are grateful to Ms. Young Mee Lee for skillful technical assistance and to the RPTH reviewers for very helpful suggestions. P. Medina is a Miguel Servet researcher (FIS-CPII15/00002). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Authorship
J.H. Griffin and H. Deguchi participated in the conception of the study. S. Navarro and H. Deguchi were responsible for GlcCer and PtdEtn measurements. The statistical analysis was performed by H. Deguchi, A.B. Payne, H.D. Austin and S. Navarro. D.J. Elias was responsible for organizing the Scripps VTE Registry, consenting the patients, and obtaining blood specimens. S. Navarro, P. Medina and F. España were responsible for organizing the Valencia VTE Registry, consenting the patients and obtaining blood specimens. H.D. Austin, N.F. Dowling and W.C. Hooper were responsible for organizing the MI population, consenting the patients, and obtaining blood specimens. J.H. Griffin and H. Deguchi were responsible for writing the manuscript. All authors were involved in the interpretation of data and gave final approval.
References
- 1.Carmena R, Duriez P, Fruchart JC. Atherogenic lipoprotein particles in atherosclerosis. Circulation. 2004;109(23 Suppl 1):III2–7. doi: 10.1161/01.CIR.0000131511.50734.44. [DOI] [PubMed] [Google Scholar]
- 2.Griffin JH, Fernandez JA, Deguchi H. Plasma lipoproteins, hemostasis and thrombosis. Thromb Hemost. 2001;86:386–394. [PubMed] [Google Scholar]
- 3.Levade T, Auge N, Veldman RJ, Cuvillier O, Negre-Salvayre A, Salvayre R. Sphingolipid mediators in cardiovascular cell biology and pathology. Circ Res. 2001;89:957–968. doi: 10.1161/hh2301.100350. [DOI] [PubMed] [Google Scholar]
- 4.Richards JB, Waterworth D, O’Rahilly S, Hivert MF, Loos RJ, Perry JR, Tanaka T, Timpson NJ, Semple RK, Soranzo N, Song K, Rocha N, Grundberg E, Dupuis J, Florez JC, Langenberg C, Prokopenko I, Saxena R, Sladek R, Aulchenko Y, et al. GIANT Consortium A genome-wide association study reveals variants in ARL15 that influence adiponectin levels. PLoS Genet. 2009;5:e1000768. doi: 10.1371/journal.pgen.1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deguchi H, Fernandez JA, Pabinger I, Heit JA, Griffin JH. Plasma glucosylceramide deficiency as potential risk factor for venous thrombosis and modulator of anticoagulant protein C pathway. Blood. 2001;97:1907–1914. doi: 10.1182/blood.v97.7.1907. [DOI] [PubMed] [Google Scholar]
- 6.Yegnesswaran S, Deguchi H, Griffin JH. Glucosylceramide, a neutral glycosphingolipid anticoagulant cofactor, enhances the interaction of human and bovine activated protein C with negatively charged phospholipid vesicles. J Biol Chem. 2003;278:14614–14621. doi: 10.1074/jbc.M206746200. [DOI] [PubMed] [Google Scholar]
- 7.Margalit M, Shalev Z, Pappo O, Sklair-Levy M, Alper R, Gomori M, Engelhardt D, Rabbani E, Ilan Y. Glucocerebroside ameliorates the metabolic syndrome in OB/OB mice. J Pharmacol Exp Ther. 2006;319:105–110. doi: 10.1124/jpet.106.104950. [DOI] [PubMed] [Google Scholar]
- 8.Yeom M, Park J, Lim C, Sur B, Lee B, Han JJ, Choi HD, Lee H, Hahm DH. Glucosylceramide attenuates the inflammatory mediator expression in lipopolysaccharide-stimulated RAW264.7 cells. Nutr Res. 2015;35:241–250. doi: 10.1016/j.nutres.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Edsfeldt A, Dunér P, Ståhlman M, Mollet IG, Asciutto G, Grufman H, Nitulescu M, Persson AF, Fisher RM, Melander O, Orho-Melander M, Borén J, Nilsson J, Gonçalves I. Sphingolipids Contribute to Human Atherosclerotic Plaque Inflammation. Arterioscler Thromb Vasc Biol. 2016;36:1132–1140. doi: 10.1161/ATVBAHA.116.305675. [DOI] [PubMed] [Google Scholar]
- 10.Smirnov MD, Esmon CT. PE incorporation into vesicles selectively enhances factor Va inactivation by activated protein C. J Biol Chem. 1994;269:816–819. [PubMed] [Google Scholar]
- 11.Deguchi H, Pecheniuk NM, Elias DJ, Averell PM, Griffin JH. High-density lipoprotein deficiency and dyslipoproteinemia associated with venous thrombosis in men. Circulation. 2005;112:893–899. doi: 10.1161/CIRCULATIONAHA.104.521344. [DOI] [PubMed] [Google Scholar]
- 12.Deguchi H, Elias DJ, Navarro S, España F, Griffin JH. Elevated serum amyloid A is associated with venous thromboembolism. Thromb Haemost. 2013;109:358–359. doi: 10.1160/TH12-10-0722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Binnington B, Nguyen L, Kamani M, Hossain D, Marks DL, Budani M, Lingwood CA. Inhibition of Rab prenylation by statins induces cellular glycosphingolipid remodeling. Glycobiology. 2016;26:166–180. doi: 10.1093/glycob/cwv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navarro S, Medina P, Bonet E, Corral J, Martínez-Sales V, Martos L, Rivera M, Roselló-Lletí E, Alberca I, Roldán V, Mira Y, Ferrando F, Estellés A, Vicente V, Bertina RM, España F. Association of the thrombomodulin gene c.1418C>T polymorphism with thrombomodulin levels and with venous thrombosis risk. Arterioscler Thromb Vasc Biol. 2013;33:1435–1440. doi: 10.1161/ATVBAHA.113.301360. [DOI] [PubMed] [Google Scholar]
- 15.De Staerck C, Lally C, Austin H, Winston C, Dowling N, Williams B, Hooper WC. The lack of association between four point mutations in the promoter region of the toll-like 4 receptor gene and myocardial infarction. Thromb Res. 2007;119:105–110. doi: 10.1016/j.thromres.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Dowling NF, Austin H, Dilley A, Whitsett C, Evatt BL, Hooper WC. The epidemiology of venous thromboembolism in Caucasians and African-Americans: the GATE study. J Thromb Haemost. 2003;1:80–87. doi: 10.1046/j.1538-7836.2003.00031.x. [DOI] [PubMed] [Google Scholar]
- 17.Deguchi H, Bouma BN, Middeldorp S, Lee YM, Griffin JH. Decreased plasma sensitivity to activated protein C by oral contraceptives is associated with decreases in plasma glucosylceramide. J Thromb Haemost. 2005;3:935–938. doi: 10.1111/j.1538-7836.2005.01335.x. [DOI] [PubMed] [Google Scholar]
- 18.Xu X, Bauer KA, Griffin JH. Two multiplex PCR-based DNA assays for the thrombosis risk factors prothrombin G20210A and coagulation factor V G1691A polymorphisms. Thromb Res. 1999;93:265–269. doi: 10.1016/s0049-3848(98)00178-9. [DOI] [PubMed] [Google Scholar]
- 19.Franchini M, Mannucci PM. Association between venous and arterial thrombosis: clinical implications. Eur J Intern Med. 2012;23:333–337. doi: 10.1016/j.ejim.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Sundaram KS, Lev M. Warfarin administration reduces synthesis of sulfatides and other sphingolipids in mouse brain. J Lipid Res. 1988;29:1475–1479. [PubMed] [Google Scholar]
- 21.Riva N, Donadini MP, Ageno W. Epidemiology and pathophysiology of venous thromboembolism: similarities with atherothrombosis and the role of inflammation. Thromb Haemost. 2015;113:1176–1183. doi: 10.1160/TH14-06-0563. [DOI] [PubMed] [Google Scholar]
- 22.Mi Y, Yan S, Lu Y, Liang Y, Li C. Venous thromboembolism has the same risk factors as atherosclerosis: A PRISMA-compliant systemic review and meta-analysis. Medicine (Baltimore) 2016;95:e4495. doi: 10.1097/MD.0000000000004495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalz J, ten Cate H, Spronk HM. Thrombin generation and atherosclerosis. J Thromb Thrombolysis. 2014;37:45–55. doi: 10.1007/s11239-013-1026-5. [DOI] [PubMed] [Google Scholar]
- 24.Hron G, Kollars M, Binder BR, Eichinger S, Kyrle PA. Identification of patients at low risk for recurrent venous thromboembolism by measuring thrombin generation. JAMA. 2006;296:397–402. doi: 10.1001/jama.296.4.397. [DOI] [PubMed] [Google Scholar]
- 25.Lutsey PL, Folsom AR, Heckbert SR, Cushman M. Peak thrombin generation and subsequent venous thromboembolism: the Longitudinal Investigation of Thromboembolism Etiology (LITE) study. J Thromb Haemost. 2009;7:1639–1648. doi: 10.1111/j.1538-7836.2009.03561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ten Cate-Hoek AJ, Dielis AW, Spronk HM, van OR, Hamulyak K, Prins MH, ten CH. Thrombin generation in patients after acute deep-vein thrombosis. Thromb Haemost. 2008;100:240–245. [PubMed] [Google Scholar]
- 27.Tripodi A, Martinelli I, Chantarangkul V, Battaglioli T, Clerici M, Mannucci PM. The endogenous thrombin potential and the risk of venous thromboembolism. Thromb Res. 2007;121:353–359. doi: 10.1016/j.thromres.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Wallentin L. Prevention of cardiovascular events after acute coronary syndrome. Semin Vasc Med. 2005;5:293–300. doi: 10.1055/s-2005-916169. [DOI] [PubMed] [Google Scholar]
- 29.España F, Vayá A, Mira Y, Medina P, Estellés A, Villa P, Falcó C, Aznar J. Low level of circulating activated protein C is a risk factor for venous thromboembolism. Thromb Haemost. 2001;86:1368–1373. [PubMed] [Google Scholar]
- 30.Zorio E, Navarro S, Medina P, Estellés A, Osa A, Rueda J, Cubillo P, Aznar J, España F. Circulating activated protein C is reduced in young survivors of myocardial infarction and inversely correlates with the severity of coronary lesions. J Thromb Haemost. 2006;4:1530–1536. doi: 10.1111/j.1538-7836.2006.01996.x. [DOI] [PubMed] [Google Scholar]
- 31.Dimmeler S, Haendeler J, Zeiher AM. Regulation of endothelial cell apoptosis in atherothrombosis. Curr Opin Lipidol. 2002;13:531–536. doi: 10.1097/00041433-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Kinnunen PK, Holopainen JM. Sphingomyelinase activity of LDL: a link between atherosclerosis, ceramide, and apoptosis? Trends Cardiovasc Med. 2002;12:37–42. doi: 10.1016/s1050-1738(01)00143-8. [DOI] [PubMed] [Google Scholar]
- 33.Bleicher RJ, Cabot MC. Glucosylceramide synthase and apoptosis. Biochim Biophys Acta. 2002;1585:172–178. doi: 10.1016/s1388-1981(02)00338-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


