Abstract
Even when a diet has been successful, people often return to overeating when the diet ends. One potential reason is that behavioral inhibition learned while dieting might not transfer readily outside the context in which it is learned: Basic research indicates that after a behavior is inhibited, a return to the conditioning context or simple removal from the treatment context can cause it to relapse or return (“renewal”). Can hunger and satiety states play the role of context? In two experiments, rats learned a food-seeking response for sucrose or sweet-fatty food pellets while satiated. Responding was then inhibited (extinguished) while they were hungry. On return to the satiated state, food-seeking was renewed. Additional results suggest that associations with hunger/satiety stimuli were learned more readily than those with other potentially useful exteroceptive stimuli. The findings have implications for understanding the role of interoceptive contexts in controlling the inhibition of motivated behavior.
Keywords: Motivation, Relapse, Renewal, Context, Behavioral Inhibition
Obesity-related deaths and diseases have become a worldwide public health concern. The prevalence of obesity has doubled since 1980 and now accounts for more preventable deaths than malnutrition. Unfortunately, maintaining weight loss achieved through dieting is often difficult (Elfhag & Rössner, 2005). One possible contributor to the problem may be that behavioral inhibition learned while dieting may be expressed primarily in the context in which it is learned (e.g., Bouton, 2002, 2014; see below). Thus, an individual on a diet may learn to inhibit overeating in the context of hunger. If the inhibition learned were context-specific, then it would be lost to some extent if the individual encountered fullness cues. Interestingly, where overeating is prevalent, fullness cues themselves may also become a context for eating. Thus, dieting overeaters may paradoxically learn to eat when full and inhibit eating when hungry, leading to an unproductive cycle of inhibition and overeating.
Research on basic behavioral processes suggests that behavioral inhibition in the form of extinction is highly specific to the context in which it is learned (e.g., Bouton, 2002, 2004; Bouton & Todd, 2014). For example, renewal is a type of behavioral relapse that occurs when the context is changed after a behavior has been suppressed or inhibited (e.g., Bouton et al., 2011; Nakajima et al., 2000). In the instrumental-learning laboratory, renewal experiments often involve reinforcing an instrumental behavior in one context (Context A) and then extinguishing it in a second one (Context B). Interestingly, behavior that is inhibited through extinction typically returns to performance (i.e., renews) when the response is then tested back in the original context (ABA Renewal) or in a new context (ABC Renewal). Moreover, testing in a new context also promotes renewal when behavior was both acquired and extinguished in the same context (AAB Renewal). Overall, the findings suggest that behavioral inhibition is relatively specific to the context in which it is learned (Bouton & Todd, 2014).
Renewal experiments typically use contexts that differ in terms of exteroceptive stimuli (e.g., visual, tactile, and olfactory cues). However, many different types of stimuli may play the role of context, including but not limited to drug states, mood states, and time (e.g., Bouton, 2002, 2010). There is also evidence that interoceptive cues provided by hunger and satiety may play the role of context under some conditions (e.g., Davidson, 1993). In one example, Davidson (1987) found that rats could learn to use daily alternations in deprivation stimuli (i.e., 0 hr deprivation vs 24 hrs deprivation) to anticipate whether a footshock would occur at the end of an experimental session. More recently, research has found that animals can also learn to associate alternating deprivation conditions with the delivery of free food reinforcers (Sample et al., 2016). However, it is not yet clear that deprivation stimuli can play the role of context in renewal designs that involve relatively few shifts in the deprivation conditions and relatively extensive extinction treatments.
Our first experiment was therefore designed to determine whether interoceptive deprivation states can function as contexts in an ABA renewal design applied to instrumental food seeking. While satiated, rats learned to press a lever to obtain highly palatable sucrose or sweet-fatty pellets over a series of daily sessions. Then, after being made hungry by 23 hours of food deprivation on each of several days, the rats had the opportunity to learn that lever pressing no longer yielded food pellets. Lever pressing became suppressed during this extinction phase. In final tests, lever pressing was tested in the hungry and satiated states in a counterbalanced order. Our hypothesis was that responding when tested in the satiety state after extinction in the hungry state would renew. Although such a recovery of food-seeking while satiated (as opposed to hungry) would violate intuition as well as traditional ideas about how hunger motivates instrumental behavior (e.g., Hull, 1943), it would be consistent with the idea that the inhibition of food-seeking is specific to the deprivation context in which it is learned.
Method
Subjects
The subjects were 32 naïve female Wistar rats, purchased from Charles River Laboratories (St. Constance, Quebec), that were between 75 and 90 days old at the start of the experiment. The rats were individually housed in a room maintained on a 16:8-h light:dark cycle. The experiment was run each day during the light period of the cycle. A power analysis informed by data obtained from Bouton et al. (2011) determined that our sample size would be sufficient to provide .80 power to detect a small to medium-sized effect.
Apparatus
Two sets of four conditioning chambers housed in separate rooms of the laboratory were used. Each box was housed in its own sound attenuation chamber. All boxes were of the same design (Med Associates model ENV-008-VP, St. Albans, VT). The side walls and ceilings were made of clear acrylic plastic, while the front and rear walls were made of brushed aluminum. A recessed 5.1 cm × 5.1 cm food cup was centered in the front walls approximately 2.5 above the level of the floor. Retractable levers (Med Associates model ENV-112CM) were positioned to the left and right of the food cup. (The present experiments utilized the left lever.) The chambers were illuminated by one 7.5-W incandescent bulb mounted to the ceiling of the sound attenuation chamber, approximately 34.9 cm from the grid floor at the front wall of the chamber. Ventilation fans provided background noise of 65 dBA. Food rewards consisted of 45-mg sweet, high-fat pellets (38% kcal Omnitreat Tab 45 mg, TestDiet, Richmond, IN, USA) or 45-mg sucrose pellets (TestDiet, Richmond, IN, USA). The apparatus was controlled by computer equipment located in an adjacent room.
Procedure
Feeding schedules
Prior to each experimental session, the rats received one of two feeding schedules in the home cage. In the “deprived” condition, the rats had received 1 hr of access to chow that ended 23 hrs before the beginning of the session. In the “sated” condition, rats had received 23 hours of continuous ad lib access to chow before the session.
Magazine training
During each of the first 2 days, each rat received a 30-min session of magazine training, one in the sated condition and another in the deprived condition. The rats were placed in an operant chamber, and after a two-min delay, received free pellets on a random time 30-s (RT 30-s) schedule. This resulted in approximately 60 pellets being delivered. Half the rats received sucrose pellets, and the other half received sweet-fat pellets. The rats continued to receive the same pellet type throughout the experiment.
Acquisition
On each of the next 12 days, the rats then received 30-min sessions in which they were reinforced for lever pressing while satiated. Each session began when the lever was inserted into the chamber after a two-min delay. (The lever then remained in the chamber for the remainder of the session.) Reinforcers were available for lever presses on a variable interval (VI) schedule. On Days 1 and 2, a VI 10-s schedule was in place (pellets were delivered upon a response, on average every 10 s). On Days 3 and 4 the schedule was increased to VI 20-s. Finally, on Days 5 through 12 the schedule was VI 30-s. The rats learned to lever press without any further shaping. However, given the satiation conditions (i.e., the absence of hunger motivation during training or, indeed, very little experience with it), not all rats learned to lever press reliably. Ten were thus excluded following the 4th training session (3 in Group Sweet Fat and 7 in Group Sucrose) because they had failed to average a single response per minute (M = 0.22). The remaining 22 rats averaged 5.45 responses per minute in Session 4.
Extinction
Extinction then began when rats were now in the deprived condition. Extinction sessions were identical to acquisition sessions except that food pellets were no longer available for lever press responses (responses had no programmed consequences). There were 4 daily 30-min sessions of extinction.
Renewal Test
During the two days that followed the last session of extinction, each rat received one extinction test of lever pressing while sated and another while deprived (counterbalanced order). As usual, the lever was inserted after a 2-min delay; lever presses were then recorded for the next 10 min. Pellets were not delivered.
Results
The results are summarized in Figure 1. As suggested by the leftmost panel, rats learned to lever press at similar rates whether the reinforcer was sucrose or sweet-fat pellets. A Pellet Type × Session ANOVA found a main effect of session, F(11, 220) = 29.72, MSE = 3.37, p < .001, , but no group effect or group by session interaction, largest F = 1.09. The center panel shows that response rates also declined at similar rates for the two groups during extinction. A Pellet Type × Session ANOVA found a main effect of session, F(3, 60) = 73.13, MSE = .77, p < .001, , but no group effect or group by session interaction, Fs < 1.
Fig. 1.
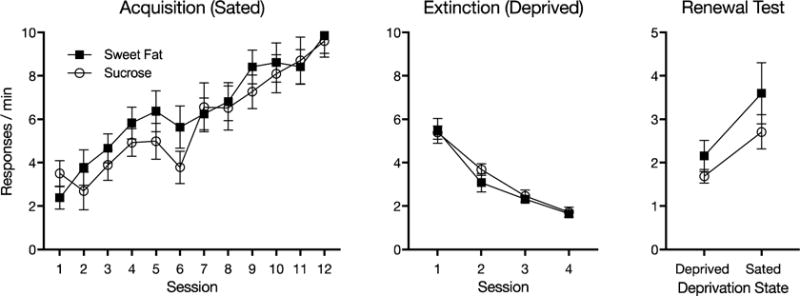
Results of Experiment 1. Mean lever responses per minute during each 30-min acquisition session when rats were sated (left) and extinction sessions when rats were food deprived (middle). The right panel shows the mean responses per minute during each 10-min test session when food deprived and when sated. Error bars represent ±1 SEM.
The right-most panel shows the focal results of the test sessions. The clear finding was that the rats made more lever presses when they were sated than when they were deprived. Further, the increase in responding in the sated session did not depend on the type of reinforcer that had been used during training. A Pellet Type × Session ANOVA found a main effect of session, F(1, 20) = 8.89, MSE = .26, p = .007, , confirming that rats made more responses in the sated session, Mdifference = 1.20, 95% CI [.36, 2.03]. However, the main effect of pellet type and a session by pellet type interaction did not approach significance, Fs < 1.
Discussion
Instrumental food-seeking that was acquired while the rats were sated (i.e., Context A) and then extinguished while the rats were deprived (i.e., Context B) renewed when the rats were sated again. This result suggests that satiety and hunger can play the role of contexts A and B in a simple ABA renewal design. Furthermore, the type of reinforcer had little effect on the rate of acquisition, extinction, or renewal of lever responding. For simplicity, the remaining experiments therefore used only sweet fat pellets.
Experiment 2
The purpose of Experiment 2 was to replicate the ABA renewal effect and to determine whether a simple shift in deprivation state after extinction could produce another form of renewal known with standard exteroceptive contexts, so-called AAB Renewal. As noted earlier, when behavior is learned and then extinguished in the same context (A), it can renew when the response is tested in a new context (B). Experiment 2 thus asked whether renewal also occurs when food-seeking is trained and extinguished in the context of deprivation (Context A) and then tested in the context of satiation (Context B). Such a counterintuitive increase in responding when shifted from hunger to satiation would be worth knowing about.
Subjects and Apparatus
The subjects were 32 naïve female Wistar rats of the same age and from the same stock as in Experiment 1. The apparatus was also the same.
Procedure
All rats received the sweet-fat pellets as reinforcers. In addition, all rats received only 1 hr of daily access to chow for the seven days before the beginning of the experiment. Magazine training then occurred in each deprivation state according to the procedure used in Experiment 1. The rats were then randomly assigned to one of two groups that differed based on their deprivation states during 12 daily lever-press acquisition and 4 extinction sessions. Like the groups in Experiment 1, rats in Group SDS were sated prior to acquisition and deprived prior to extinction sessions. In contrast, rats in Group DDS were deprived prior to each acquisition and extinction session. As in Experiment 1, all rats then received one session in which lever pressing was tested while sated and another while deprived (counterbalanced order). Acquisition, extinction, and testing sessions otherwise followed the procedures described in Experiment 1. Perhaps because they had received more extended experience with food restriction before the beginning of training, all rats learned to lever press while satiated in this experiment.
Results
The results are summarized in Figure 2. During acquisition (left panel), the deprived rats (Group DDS) made more responses than the sated rats (Group SDS). This was confirmed by a Group × Session ANOVA that found main effects of session, F(11, 330) = 33.46, MSE = 14.95, p < .001, , group, F(1, 30) = 14.06, MSE = 460.15, p = .001, , and a group by session interaction, F(11, 330) = 77.78, MSE = 14.95, p < .001, .
Fig. 2.
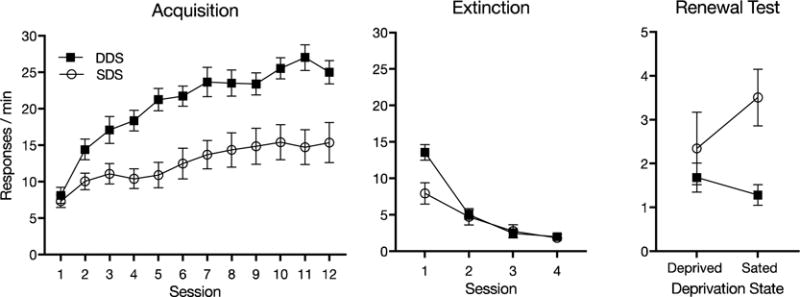
Results of Experiment 2. Mean lever responses per minute during each 30-min acquisition session when rats in Group SDS were sated and rats in Group DDS were deprived (left) and extinction sessions when both groups were food deprived (middle). The right panel shows the mean responses per minute during each 10-min test session when food deprived and when sated. Error bars represent ±1 SEM.
The center panel of the figure shows the four sessions of extinction. The group difference in response rate established during acquisition persisted, at least initially. A Group × Session ANOVA indicated an effect of session, F(3, 90) = 81.79, MSE = 6.30, p < .001, , as well as a group by session interaction, F(3, 90) = 62.64, MSE = 6.30, p < .001, . The main effect of group was not significant, F(1, 30) = 2.00, MSE = 33.09, p = .168. Follow-up comparisons that pursued the interaction indicated that Group DDS made more responses than Group SDS during the first extinction session (p < .05).
The right panel of the figure summarizes the results of the renewal test sessions. There was a clear renewal effect in Group SDS, replicating Experiment 1, but not in Group DDS. A Group × Session ANOVA found a significant group by session interaction, F(1, 30) = 7.4, MSE = 1.32, p = .011, . The main effect of group approached significance, F(1, 30) = 3.82, MSE = 8.74, p = .060, , whereas the effect of session did not, F(1, 30) = 1.76, MSE = 1.32, p = .194. Follow up comparisons confirmed that Group SDS responded more in the sated test than in the deprived test, p = .008, Mdifference = 1.16, 95% CI [.33, 1.99], η2=.22. There was no such effect in Group DDS (p = .333).
Discussion
Group SDS responded more in a test session in which they were sated than in an identical session in which they were deprived, successfully replicating the ABA renewal effect of Experiment 1. An AAB renewal effect was not observed in Group DDS. It is worth noting that although AAB renewal can be a robust effect, it is often smaller in magnitude and therefore more difficult to detect than ABA renewal when exteroceptive contexts are used (e.g., Bouton et al., 2011). Moreover, in the present design, where food deprivation played the role of Context A, deprivation demonstrably increased the amount of responding that occurred during extinction in the AAB (DDS) condition; an increased amount of responding during extinction may produce a more durable extinction effect (e.g., Rescorla, 1997; see also Bouton Trask, & Carranza-Jasso, 2016). We will discuss the lack of DDS renewal further in the General Discussion.
The ABA renewal effects in Experiments 1 and 2 provide strong evidence consistent with the idea that inhibitory extinction learning is especially dependent on the context in which it is learned. Moreover, the fact that satiation produced an increase rather than a decrease in food-seeking behavior continues to suggest that this is an especially interesting and robust effect.
Experiment 3
Experiment 3 was designed to ask whether the deprivation conditions that produced ABA renewal in the first two experiments worked as truly interoceptive contexts. The sated and deprived feeding schedules used in those experiments confounded the interoceptive states of satiety and hunger with whether food was present or absent in the home cage before the experimental session. That is, in the ABA (SDS) condition, chow was available in the homecage before sessions in which lever pressing was reinforced, but not when lever pressing was extinguished. This creates the possibility that the rats used the presence or absence of food cues in the homecage to predict whether lever pressing would be reinforced or not. Experiment 3 was therefore designed to separate contextual control of instrumental food-seeking by true interoceptive deprivation cues as opposed to exteroceptive food cues.
Method
Subjects and Apparatus
The subjects were 24 naïve female Wistar rats of the same age and from the same vendor as before. The apparatus was also the same. A power analysis using data from Experiments 1 and 2 indicated that this sample size would provide .8 power to observe a small to medium sized effect.
Procedure
As in Experiment 2, the rats received 1hr daily access to chow for the seven days prior to the beginning of the experiment. They were then randomly assigned to one of three groups (n = 8). The rats in each group received 40 daily sessions in the experimental chambers that alternated between sessions in which lever pressing was reinforced (“R+” sessions) and those when it was extinguished (“R−” Sessions). The groups received different feeding schedules that were designed to provide potential deprivation and/or homecage food cues that could signal the reinforcement conditions (Figure 3). Group Food Cue always received ad lib access to chow in the homecage except for the two hours prior to each R− session. In this way, the presence of chow immediately prior to a session signaled R+, and the absence of chow signaled R−. For Group Deprivation Cue, chow was removed from the homecage 23 hrs prior to each R− session and 2 hrs prior to the R+ sessions. Here, food was never present in the home cage immediately prior to R+ or R−, but differential interoceptive cues of satiety and hunger could still signal R+ and R−, respectively. Finally, Group Both Cues received a similar feeding schedule to Group Deprivation Cue except that their chow was not removed before R+ sessions. In this way, rats in Group Both cues could potentially use interoceptive deprivation cues and/or the presence/absence of food in the homecage to signal the upcoming R+ and R− condition.
Fig. 3.
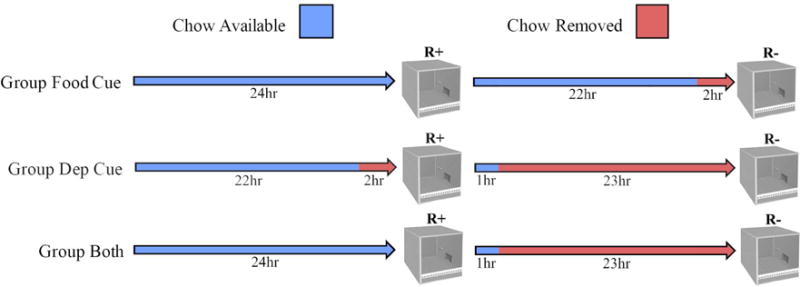
Design and illustration of food availability in the home cage before R+ (reinforced) and R− (nonreinforced) training sessions in Experiment 3.
As usual, on each of the first 2 days of training, the rats received 30-min sessions of magazine training. Here each group received one magazine training session following each of their previously-described feeding schedules. Over the next 40 days, there was one 30-min session in the operant chamber each day. As noted earlier, these alternated between R+ and R− sessions. During R+ sessions, lever pressing was reinforced following the procedure used in the previous experiments. During R− sessions, lever pressing was not reinforced. The rate of reinforcement gradually increased over the first several R+ sessions; on the first two, lever pressing was reinforced on a VI 10-s schedule, on the second two it was VI 20-s, and for the remainder of the experiment lever pressing was reinforced on VI 30-s. The reinforcer was the sweet-fatty pellet.
Results
Rats in Groups Deprivation Cue and Both Cues gradually learned to anticipate whether lever pressing would be reinforced or not. This was confirmed by analyzing the latency between insertion of the lever into the chamber and the first response on it in each session. Notice that the first response in each session occurred prior to the delivery (or nondelivery) of the reinforcer, and could thus differ only if the rat had learned to anticipate R+ and R−. (Analysis of response rates throughout the sessions would have been confounded by that factor.) Figure 4a summarizes the latencies collapsed over the final 24 sessions of training. The data suggest that Groups Deprivation Cue and Both Cues responded with a shorter latency on the R+ sessions than the R− sessions. A Group × Session Type ANOVA confirmed a main effect of session type, F(1, 21) = 8.73, MSE = .064, p = .008, , and a crucial group by session type interaction, F(2, 21) = 3.91, MSE = .064, p = .036, . Follow-up comparisons confirmed shorter log latencies in the R+ sessions in Group Deprivation Cue, p < .029, Mdifference = .30, 95% CI [.034, .559], η2=.31, and Group Both Cues, p < .004, Mdifference = .41, 95% CI [.152, .667], η2=.51. In contrast, rats in Group Food Cue responded with a similar latency in R+ and R− sessions, p =.61. Figure 4b provides a summary of how the differences in latencies to the first response on R+ and R− sessions actually developed over training.
Fig. 4.
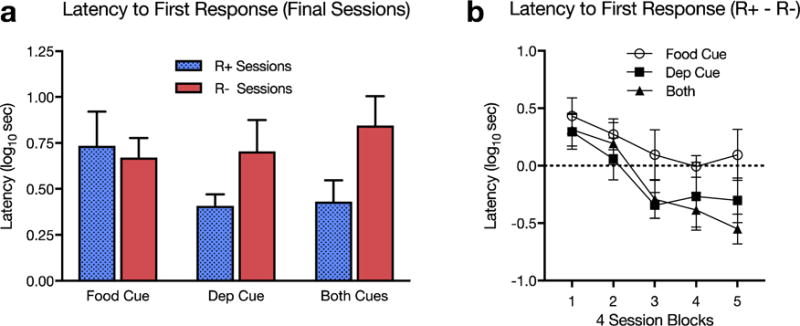
Experiment 3 (a) Mean latency (log10 sec) to the first response collapsed over the final 24 sessions of training for R+ (12 sessions) and R− (12 sessions). (b) The latency (log10 sec) to the first response in R+ minus R− sessions over the entire 40 sessions of the experiment. Each data point represents the average mean difference in latency (log10 sec) between each 4 R+ and 4 R− sessions. Error bars represent ±1 SEM.
Discussion
Rats that were given only the presence and absence of food in the homecage to signal reinforcement and extinction sessions (Group Food Cue) never learned to anticipate the reinforcement contingency. In contrast, rats that were sated before R+ and hungry before R− sessions did (Groups Deprivation Cues and Both Cues). Interestingly, the addition of homecage food cues to the deprivation cues did not make the discrimination more rapid in Group Both Cues. Apparently, the animals learned to discriminate satiation and deprivation with very little input from the availability of food in the homecage. The results strongly suggest that interoceptive states of hunger and satiety can control instrumental food seeking. Notice further that, as in Experiments 1 and 2, satiety once again served counterintuitively as a cue that increased the motivation to lever press for food.
General Discussion
The results suggest that interoceptive food deprivation stimuli can play the role of context in controlling food seeking. In Experiments 1 and 2, regardless of whether the reinforcer was sucrose or sweet-fatty pellets, food seeking that was learned while the animal was sated and then inhibited (extinguished) while hungry renewed when the rat was sated again. The results of Experiment 3 further confirmed that interoceptive cues connected with deprivation and satiation provide important discriminative cues. Interestingly, the presence/absence of food in the homecage was not effective at signaling the reinforcement contingency.
As previously mentioned, the lack of renewal in Experiment 2 for rats that had been deprived for acquisition and extinction training and sated for the first time at test could be attributed to the smaller magnitude of the AAB Renewal effect. It is also possible that the invigorated responding during extinction allowed the development of stronger response inhibition (Bouton et al., 2016; Rescorla, 1997). The failure to observe AAB renewal might also point to features of interoceptive satiety and deprivation cues that make them different from the exteroceptive contexts usually used in the renewal literature. The evidence that interoceptive hunger and thirst states can serve as discriminative cues was once controversial (e.g., Bolles, 1975); one complication among others might have been that the discriminative effects of deprivation and satiety states interact with their motivating effects (e.g., Capaldi et al., 1981). In our experiments, renewal always took the form of satiety increasing the level of food-motivated responding. In the rat’s prior experience, interoceptive satiety cues had presumably been associated with the cessation of feeding (e.g., Davidson, 1993), perhaps making renewal in their presence difficult without the more explicit discrimination between satiety and deprivation that is provided by the ABA procedure.
The results of Experiment 3 indicated that interoceptive deprivation cues rather than exteroceptive food cues were most likely responsible for the discriminations observed here. First, by exhibiting a shorter latency to the first response while sated (during R+ Sessions), the rats’ performance indicated that interoceptive satiety cues provided a stimulus signaling reinforcement. In contrast, the presence of homecage food alone did not enable similar anticipatory responding. And when both food cues and deprivation cues were available, the food cues provided little additional support in helping the rats learn the discrimination. It is worth noting that pharmacological ligands purported to induce satiety or hunger can also produce effects that generalize to satiety and deprivation states created through feeding manipulations. In such methods, after training with a hunger/satiety discrimination, animals receive tests under identical food deprivation conditions with and without the ligand. The fact that responding after ligand exposure generalizes to that shown during discrimination training suggests that generalization occurs along the dimension of interoceptive state (Kanoski et al., 2007; Davidson et al, 2005).
The present findings fit with a research literature pointing to the role of conditioning and learning processes in eating, appetite, and their disorders and treatment (e.g., Boutelle & Bouton, 2015; Bouton, 2011; Jansen, 2016). To our knowledge, however, the present results provide the first evidence that satiety can play the role of context and produce renewal in a typical ABA design that provides relatively few shifts between deprivation states. Some authors have proposed that food restriction itself may be a causal factor in the development of maladaptive overeating (e.g., Polivy & Herman, 1985; 2002). However, the present data and other recent analyses (Jansen, 2016) suggest that restrained or inhibited eating is not alone sufficient to cause excessive eating later: Rats that never learned to seek food while sated (Group DDS in Experiment 2) did not exhibit increased food seeking when they were switched from the extinction context of hunger to the context of satiety. Only behaviors that had been reinforced while satiated renewed when hunger was interrupted and satiety was resumed (Group SDS). Satiety and hunger states can come to control the excitation and inhibition of food-seeking in the ways that other kinds of “context” do (e.g., Bouton, 2010).
Acknowledgments
This research was supported by NIH Grant RO1 DA033123 to MEB.
Footnotes
Authors Contributions
S. T. Schepers and M.E. Bouton developed the experimental concept and designs. S.T. Schepers performed the experiments and collected and analyzed the data. Both authors drafted the manuscript, revised it, and approved the final version.
References
- Bolles RC. Theory of motivation. 2nd. New York: Harper & Row; 1975. [Google Scholar]
- Boutelle KN, Bouton ME. Implications of learning theory to develop programs to decrease overeating. Appetite. 2015;93:62–74. doi: 10.1016/j.appet.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: sources of relapse after behavioral extinction. Biological Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME. The multiple forms of “context” in associative learning theory. In: Mesquita B, Feldman Barrett L, Smith E, editors. The mind in context. New York: The Guilford Press; 2010. pp. 233–258. [Google Scholar]
- Bouton ME. Learning and the persistence of appetite: Extinction and the motivation to eat and overeat. Physiology & Behavior. 2011;103:51–58. doi: 10.1016/j.physbeh.2010.11.025. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Why behavior change is difficult to sustain. Preventive Medicine. 2014;68:29–36. doi: 10.1016/j.ypmed.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Todd TP. A fundamental role for context in instrumental learning and extinction. Behavioural Processes. 2014;104:13–19. doi: 10.1016/j.beproc.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Todd TP, Vurbic D, Winterbauer NE. Renewal after the extinction of free operant behavior. Learning & Behavior. 2011;39:57–67. doi: 10.3758/s13420-011-0018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Trask S, Carranza-Jasso R. Learning to inhibit the response during instrumental (operant) extinction. Journal of Experimental Psychology: Animal Learning and Cognition. 2016;42:246–258. doi: 10.1037/xan0000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi ED, Viveiros DM, Davidson TL. Deprivation stimulus intensity and incentive factors in the control of instrumental responding. Journal of Experimental Psychology: Animal Behavior Processes. 1981;7:140–149. [Google Scholar]
- Davidson TL. Learning about deprivation intensity stimuli. Behavioral Neuroscience. 1987;101:198–208. doi: 10.1037//0735-7044.101.2.198. [DOI] [PubMed] [Google Scholar]
- Davidson TL. The nature and function of interoceptive signals to feed: toward integration of physiological and learning perspectives. Psychological Review. 1993;100:640–657. doi: 10.1037/0033-295x.100.4.640. [DOI] [PubMed] [Google Scholar]
- Davidson TL, Kanoski SE, Tracy AL, Walls EK, Clegg D, Benoit SC. The interoceptive cue properties of ghrelin generalize to cues produced by food deprivation. Peptides. 2005;26:1602–1610. doi: 10.1016/j.peptides.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Elfhag K, Rössner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obesity reviews. 2005;6:67–85. doi: 10.1111/j.1467-789X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- Herman CP, Polivy J. Restrained eating. Obesity. 1980:208–225. [Google Scholar]
- Hull C. Principles of behavior. New York, NY: Appleton-Century-Crofts; 1943. [Google Scholar]
- Jansen A. Eating disorders need more experimental psychopathology. Behaviour Research and Therapy. 2016;86:2–10. doi: 10.1016/j.brat.2016.08.004. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Walls EK, Davidson TL. Interoceptive “satiety” signals produced by leptin and CCK. Peptides. 2007;28:988–1002. doi: 10.1016/j.peptides.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S, Tanaka S, Urushihara K, Imada H. Renewal of extinguished lever-press responses upon return to the training context. Learning and Motivation. 2000;31:416–431. [Google Scholar]
- Polivy J, Herman CP. Dieting and binging: A causal analysis. American Psychologist. 1985;40:193–201. doi: 10.1037//0003-066x.40.2.193. [DOI] [PubMed] [Google Scholar]
- Polivy J, Herman CP. Causes of eating disorders. Annual Review of Psychology. 2002;53:187–213. doi: 10.1146/annurev.psych.53.100901.135103. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Response inhibition in extinction. The Quarterly Journal of Experimental Psychology. 1997;50:238–252. [Google Scholar]
- Sample CH, Jones S, Hargrave SL, Jarrard LE, Davidson TL. Western diet and the weakening of the interoceptive stimulus control of appetitive behavior. Behavioural Brain Research. 2016;312:219–230. doi: 10.1016/j.bbr.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


