Summary
Objective
C57BL/6J mice exposed to eight flurothyl-induced generalized clonic seizures exhibit a change in seizure phenotype following a 28-day incubation period and subsequent flurothyl rechallenge. Mice now develop a complex seizure semiology originating in the forebrain and propagating into the brainstem seizure network (a forebrain→brainstem seizure). In contrast, this phenotype change does not occur in seizure-sensitive DBA/2J mice. The underlying mechanism(s) was the focus of these studies.
Methods
DBA2/J mice were exposed to eight flurothyl-induced seizures (1/day) followed by 24-hour video-electroencephalographic recordings for 28-days. Forebrain and brainstem seizure thresholds were determined in C57BL/6J and DBA/2J mice following one or eight flurothyl-induced seizures, or after eight flurothyl-induced seizures, a 28-day incubation period, and final flurothyl rechallenge.
Results
Similar to C57BL/6J mice, DBA2/J mice expressed spontaneous seizures. However, unlike C57BL/6J mice, DBA2/J mice continued to have spontaneous seizures without remission. Because DBA2/J mice do not express forebrain→brainstem seizures following flurothyl rechallenge after a 28-day incubation period, this indicated that spontaneous seizures were not sufficient for the evolution of forebrain→brainstem seizures. Therefore, we determined whether brainstem seizure thresholds were changing during this repeated-flurothyl model and whether this could account for the expression of forebrain→brainstem seizures. Brainstem seizure thresholds were not different between C57BL/6J and DBA/2J mice on day one or on the last induction seizure trial (day eight). However, brainstem seizure thresholds did differ significantly on flurothyl rechallenge (day 28) with DBA/2J mice showing no lowering of their brainstem seizure thresholds.
Significance
These results demonstrated that DBA/2J mice exposed to the repeated-flurothyl model develop spontaneous seizures without evidence of seizure remission and provide a new model of epileptogenesis. Moreover, these findings indicated that the transition of forebrain ictal discharge into the brainstem seizure network occurs due to changes in brainstem seizure thresholds that are independent of spontaneous seizure expression.
Keywords: epileptogenesis, forebrain seizure threshold, seizure semiology, brainstem seizures, brainstem seizure thresholds, DBA2/J and C57BL6/J mice
Introduction
Experimental evidence indicates that two largely independent seizure systems are responsible for the expression of generalized convulsive seizures.1–5 These two systems are known as the forebrain seizure network (responsible for the expression of clonic seizures) and the brainstem seizure network (responsible for the expression of brainstem (tonic) seizures).1–5 Clonic seizures are principally organized and modulated within forebrain circuitry. In contrast, neuronal networks within the brainstem are both necessary and sufficient for the expression of a constellation of tonic-brainstem seizure types. In seizure naïve rodents, these two seizure systems are largely independent and seizures that are elicited in one system do not readily propagate to the other.1–5 Interestingly, studies utilizing blood oxygen level–dependent functional magnetic resonance imaging (BOLD fMRI) and single-photon emission computed tomography (SPECT) imaging reveal the importance of brainstem structures in the expression of tonic seizures in humans and in experimental animal models.6–8 However, little is known regarding the reorganizations that occur in the brainstem seizure network, or at the intersection of the forebrain seizure network and brainstem seizure network, to give rise to brainstem (tonic) seizure expression.
The repeated-flurothyl model has been utilized to understand how seizures develop and become more complex over time, and to explore the mechanistic intersections of the forebrain seizure network and brainstem seizure network that may lead to more complex seizure types.9–14 In this model, C57BL/6J mice express clonic-forebrain seizures during eight flurothyl induction trials.13,15 Following a one month incubation period and a flurothyl rechallenge, C57BL/6J mice subsequently express a clonic-forebrain seizure that rapidly transitions uninterruptedly into a tonic-brainstem seizure.11,13 We refer to these seizures as forebrain→brainstem seizures denoting the ictal progression from the forebrain seizure network to the brainstem seizure network.15,16 In fact, there is a loss of ictal discharge in hippocampal electrodes when the behavioral seizure transitions from the clonic phase to the brainstem phase.17 Importantly, DBA2/J mice exposed to the same repeated-flurothyl model, though they are more seizure-sensitive (i.e., have low generalized seizure thresholds) never have forebrain→brainstem seizures upon flurothyl rechallenge.15 This indicates that DBA2/J mice have different mechanisms for mediating whether ictal discharge can propagate into the brainstem seizure network from those of C57BL6/J mice.
Interestingly, a similar behavioral seizure phenotype is found in developing rats administered various chemoconvulsants.18–22 These studies further support the hypothesis that the change in seizure phenotype to a forebrain→brainstem seizure is related to plasticity changes occurring in the brain of adult animals, but are also apparent in developing animals having substantial plasticity. Understanding the plasticity changes involved in this change of seizure semiology to forebrain→brainstem seizures will be critical for understanding how ictal discharge can propagate from the forebrain to the brainstem.
Previously, we have shown that the ventromedial nucleus of hypothalamus (VMH) displays increased bilateral neuronal activity, as determined by Fos immunolabeling, in C57BL/6J mice having forebrain→brainstem seizures.16,23 Functionally, bilateral ibotenic acid lesions of the VMH are able to block the expression of the brainstem seizure component of the forebrain→brainstem seizures.10 These results indicate that the VMH may be a critical node in the propagation of ictal discharge from the forebrain seizure network to the brainstem seizure network. Therefore, changes at the intersection of the forebrain seizure network and brainstem seizure network may be responsible for epileptogenesis and the refractoriness seen in epileptic individuals because this process may allow recruitment of new brain areas into the epileptic network causing more complex severe seizures.
C57BL/6J mice exposed to this repeated-flurothyl model rapidly develop spontaneous seizures that remit over a one month recording period.17 Whether these spontaneous seizures contribute to the occurrence of more complex seizures (forebrain→brainstem seizures) seen on flurothyl rechallenge is not known. However, valproate, but not phenytoin, blocks these spontaneous seizures in addition to blocking the development of forebrain→brainstem seizures upon flurothyl retest.17 Collectively, these data suggest that the spontaneous seizures observed during the one month recording period may contribute to the development of the more complex seizure presentation on flurothyl rechallenge.
Here, we show that DBA/2J mice given eight flurothyl seizure inductions develop spontaneous seizures. DBA/2J mice do not develop forebrain→brainstem seizures upon flurothyl induction, incubation, and flurothyl rechallenge. Therefore, these data indicate that spontaneous seizures are not directly a cause for the development of forebrain→brainstem seizures upon flurothyl retest in C57BL/6J mice. However, we provide evidence to suggest that the lack of change in seizure phenotype to a forebrain→brainstem seizure upon flurothyl rechallenge after a one month incubation/recording period in DBA2/J mice is a result of an inability of DBA2/J mice to decrease their brainstem seizure thresholds. This is in contrast to C57BL/6J mice that display both forebrain→brainstem seizures on rechallenge and decreases in brainstem seizure thresholds. Lastly, the presence of bilateral Fos expression in the VMH of either C57BL6/J or DBA2/J mice having brainstem seizures supports a role for the VMH in the expression of brainstem seizures.
Materials and methods
Animals
Male C57BL/6J and DBA/2J mice at six weeks of age were obtained from Jackson Laboratories and allowed to acclimate to the vivarium for up to 1 week. Fifteen DBA/2J mice were implanted with cortical and hippocampal electrodes to assess spontaneous seizure expression. Of these, eleven were exposed to the repeated-flurothyl model, while the remaining four were not exposed to flurothyl and served as controls. For determination of forebrain and brainstem seizure thresholds throughout the repeated-flurothyl paradigm, a second group of male mice (C57BL/6J (n=24) and DBA/2J (n=24)) were utilized and their generalized forebrain and brainstem seizure thresholds were determined at three time points in the repeated-flurothyl model: after 1 or 8 flurothyl induction trials, or following 8 flurothyl seizures, 28-day incubation, and final flurothyl rechallenge (n=8/time-point/strain). Mice were ~8 weeks old when seizure testing began, consistent with previous studies utilizing the repeated-flurothyl model.10–13,15,16,23–25
All mice were provided complete access to food and water on a standard 12 hour light/dark cycle with lights off at 7 PM. All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Albany Medical College and were in compliance with the National Institutes of Health’s Guide for the Use and Care of Laboratory Animals.
Electrode implantation for long-term video-electroencephalogram (EEG) monitoring
All surgical procedures were performed when mice were 6–7 weeks of age. Induction of anesthesia was attained with 3% isoflurane and was maintained with 1.5–2% isoflurane until completion of the surgical procedure. Electroencephalogram recordings were made in the hippocampus and cerebral cortex. Insulated twisted bipolar electrodes (Plastics One) with tips exposed were implanted into the hippocampus, targeting the hippocampal dentate gyrus (AP: 1.6; ML: 0.8; DV: 1.85),26 and cortical screws were placed contralaterally over the parietal, frontal, and occipital cortices. The screw in the occipital bone served as a common ground for both hippocampal and cortical electrodes (frontal and parietal screws serving as cortical electrodes). Post-surgical pain control was attained by subcutaneously administered buprenorphine (0.1mg/kg). Following surgery, mice were housed individually and allowed to recover for one week before seizure testing began.
Flurothyl seizure induction (repeated-flurothyl model)
At approximately 7–8 weeks of age, C57BL/6J and DBA/2J mice were exposed to the standardized repeated-flurothyl model.13,15 Mice were placed individually into a 1.5 liter Plexiglas chamber connected to a glass syringe loaded with 10% flurothyl (Sigma-Aldrich) made in 95% ethanol. Flurothyl was pumped at a rate of 100 µl per minute on to a fresh gauze pad suspended at the top of this closed Plexiglas chamber. Once mice lost postural control (start of the generalized seizure), the chamber was opened. The generalized seizure threshold (GST) was defined as the latency from the commencement of the infusion of flurothyl to the occurrence of loss of postural control. Seizures were induced once per day for a total of eight consecutive days (induction period). This was followed by a 28-day incubation period during which these mice were left undisturbed. A single flurothyl seizure was induced on day 28 (flurothyl rechallenge/retest) to determine the final seizure phenotype. Flurothyl-induced seizures were behaviorally graded on a scale of 1 to 7.13,25 Briefly, grades 1 and 2 were clonic-forebrain seizures, whereas grades 3 to 7 were tonic-brainstem seizure behaviors (Table 1).2–4
Table 1.
Seizure behavior classification grading scale.
| Seizure grade | Description of corresponding seizure behavior |
|---|---|
| Grade 1 | Loss of posture associated with facial clonus, including chewing, and clonus of forelimbs and/or hindlimbs |
| Grade 2 | Grade 1 seizure followed by recovery of the righting reflex and low intensity bouncing |
| Grade 3 | Grade 1 and/or 2 features with recovery of the righting reflex followed by wild running and popcorning |
| Grade 4 | Grade 3 followed by forelimb and/or hindlimb treading |
| Grade 5 | Grades 3 and/or 4 followed by bilateral tonic extension of the forelimbs |
| Grade 6 | Grade 5 followed by bilateral tonic extension of the hindlimbs |
| Grade 7 | Grade 6 followed by immediate death |
Long-term video-EEG monitoring
At the conclusion of the induction phase, long-term video EEG monitoring was performed for the duration of the incubation phase (28-days: 24 hours a day, 7 days a week) to ascertain whether spontaneous seizures were observed. Control mice, not exposed to flurothyl, were also monitored for spontaneous seizures. During long-term monitoring, mice were housed individually in custom made Plexiglas cylindrical cages, 25 cm high and 24 cm in diameter. The electrode terminals were connected to a headstage through a commutator (Plastics One) to permit unrestrained movement of the animal in the cage. The amplified signal then passed through an amplifier (A-M Systems), where it was further amplified, filtered (0.5Hz–1000Hz), and digitalized at a sampling rate of 2048 with a notch filter of 60Hz. All recordings of EEG activity were performed using data acquisition software (DataWave Technologies). At the end of the incubation period, all mice were rechallenged with flurothyl to determine if there was a change in seizure phenotype.
Brainstem seizure threshold determination
For determining brainstem seizure thresholds, separate groups of C57BL/6J and DBA/2J mice were utilized. At each time point, animals received a single continuous exposure to 10% flurothyl until experiencing brainstem seizures. The time from the infusion of flurothyl to the expression of a brainstem seizure was used to determine brainstem seizure thresholds. Once the animal had a brainstem seizure, the flurothyl pump was stopped and the chamber was opened to room air.
Fos Immunohistochemistry
Ninety minutes following the end of the last flurothyl-induced seizure, mice were deeply anesthetized with sodium pentobarbital and perfused transcardially with phosphate buffered saline (PBS) followed by 4% paraformaldehyde in PBS (4°C) and the brains extracted. To determine whether forced brainstem seizures resulted in VMH activation as detected by Fos immunolabeling, coronal sections of brain were cut at 40 µm thicknesses and processed for Fos immunohistochemistry. Free floating coronal sections were used for Fos immunolabeling using 3,3'-diaminobenzidine (DAB). Sections were incubated in 3% hydrogen peroxide for 30 minutes, followed by washes in PBS/Triton X-100 (0.04%; PBS-Tx). After blocking in 10% normal goat serum (Chemicon) in PBS-Tx for one hour, sections were incubated overnight with a rabbit polyclonal IgG antibody directed against Fos (1:1500; sc-52, Santa Cruz Biotechnology) at 4°C on a shaker. Following washes with PBS-Tx, sections were incubated with biotinylated goat anti-rabbit secondary antibodies (1:200) (Vector Laboratories) for one hour. Following subsequent washes in PBS-Tx, the tissue sections were incubated with an avidin-biotin-horseradish peroxidase solution (Vector Laboratories) for one hour. Following three washes in PBS, sections were then stained in 0.03% hydrogen peroxide and DAB with nickel intensification (Vector Laboratories). Tissue sections were then mounted onto slides and cover slipped with Cytoseal 60 (Richard-Allan Scientific). VMH sections were imaged using a Zeiss AxioImagerZ1 microscope (Carl Zeiss Microimaging) equipped with an AxioCam MRc camera (Carl Zeiss Microimaging) and processed with AxioVision Rel. 4.5 software (Carl Zeiss Microimaging).
Seizure detection and data analysis
EEG data files were manually analyzed for electrographic seizures. The electrographic seizures were verified by video and the behavioral seizure type determined. Some early spontaneous electrographic seizures observed had very subtle behavioral manifestations. Spontaneous seizures were defined electrographically as having both high frequency (>5 Hz) and high amplitude (> twice baseline) rhythmic epileptiform activity with a minimum duration of ten seconds. Collected data were further analyzed for seizure frequency, percent of mice expressing spontaneous seizures, duration of seizure episodes, and circadian pattern of seizure events (day versus night).
Statistical Analysis
Statistical analyses were performed with a minimum significance of p < 0.05. Repeated measures analysis of variance was used to analyze the effect of flurothyl between flurothyl-exposed and control groups across days and their interaction, and was used to analyze significance across the incubation/recording period. Comparisons of the percentages of animals expressing spontaneous seizures or when spontaneous seizures occurred during the light/dark cycle utilized Fisher Exact tests or Χ2 tests, respectively. Comparisons were made using t-tests to determine significance between flurothyl-exposed and control groups within weeks. One way analysis of variance (ANOVA) with Newman-Keuls post-hoc tests were done to determine significance between flurothyl-exposed and control groups between weeks. One way ANOVA with Tukey HSD tests were done to determine significance within flurothyl exposed groups between weeks. One-sample t-tests were also used to determine the significance between proportions of spontaneous seizures occurring during the day and night. One way ANOVA with Tukey HSD tests were performed to determine significance between forebrain and brainstem seizure thresholds across the repeated-flurothyl paradigm.
Results
DBA/2J mice had no changes in generalized seizure thresholds over 8 flurothyl-induced seizure trials
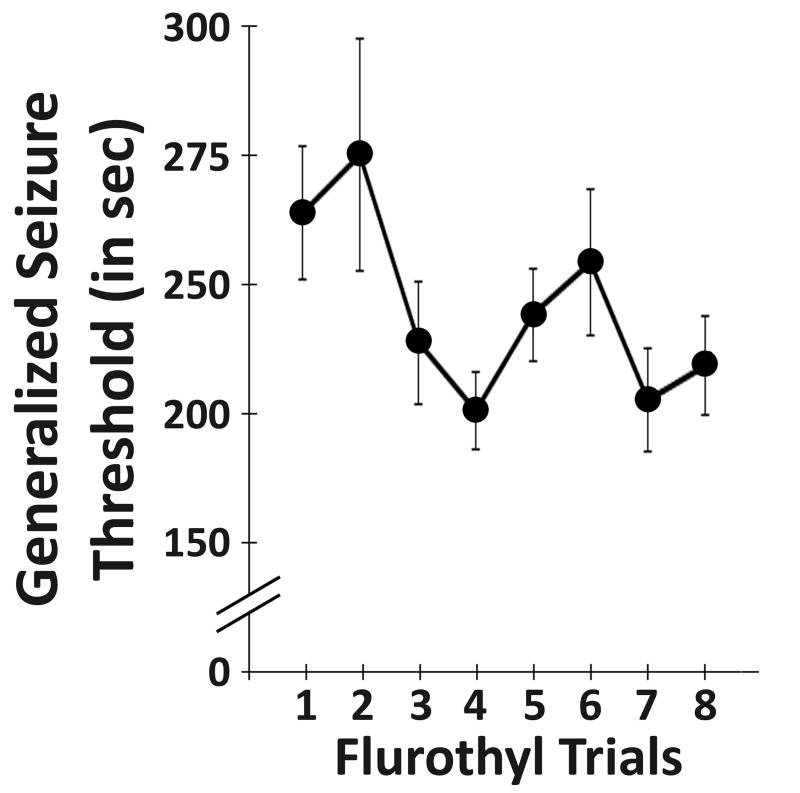
DBA/2J mice with cortical and hippocampal electrodes demonstrated no reductions in generalized seizure threshold (GST) with repeated flurothyl-induced seizures (F7,77=1.93, p > 0.05 by repeated measures ANOVA; Fig. 1), similar to previous findings in DBA2/J mice not having electrodes.15,16 Conversely, we previously reported that C57BL/6J mice showed decreases in their GST across eight flurothyl-induced seizure induction trials.15 All seizures observed during the flurothyl-induced seizure induction phase in DBA/2J (and C57BL/6J) mice were clonic-forebrain seizures.15
Figure 1.
DBA2/J mice showed no decrease in generalized clonic seizure thresholds with repeated flurothyl-induced seizures. The latency to a generalized clonic seizure (generalized seizure threshold) on each seizure induction trial was determined in DBA2/J mice by exposure to flurothyl across eight seizure trials (once/day). No significant decreases in generalized seizure thresholds were observed across the eight induction trials (p > 0.05). Note the expanded y-axis.
Eight flurothyl-induced seizures resulted in the appearance of spontaneous seizures in DBA/2J mice during the incubation recording period
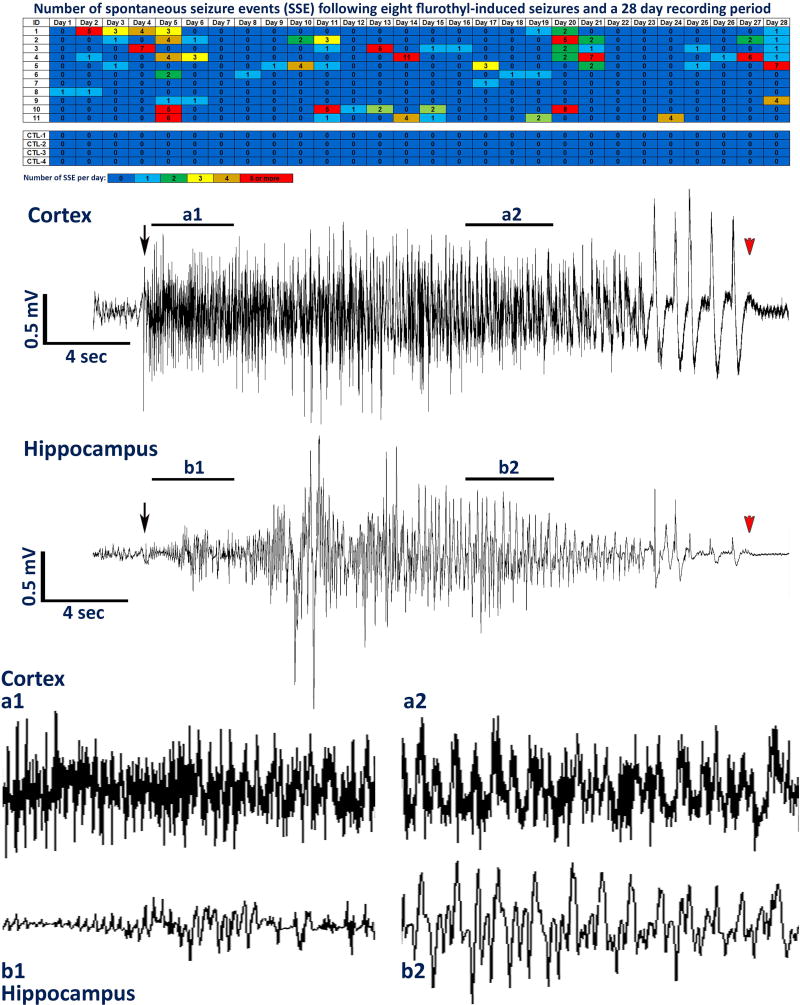
Exposure of DBA/2J mice to eight flurothyl-induced seizure induction trials resulted in the appearance of spontaneous seizures in all mice during the incubation/recording period (Fig. 2). In contrast, control DBA/2J mice expressed no spontaneous seizures indicating that electrode implantation alone had no effect on the expression of spontaneous seizures (Fig. 2). These results were significant in comparing spontaneous seizure occurrence in control vs. flurothyl-exposed DBA/2J mice (Fisher Exact test: p=0.0007). Significant differences in the average number of spontaneous seizure events during the incubation/recording period between flurothyl-exposed and control groups were found using repeated measures ANOVA (Fig. 2; F1,27 = 7.3, p < 0.018). Representative cortical and hippocampal EEGs of a spontaneous seizure from a DBA/2J mouse exposed previously to eight flurothyl-induced seizures are shown and illustrate typical synchronous ictal discharges seen during a clonic-forebrain seizure (Fig. 2). The majority of the flurothyl-exposed mice developed spontaneous seizures with little to no latent period (Fig. 2). In DBA/2J mice, significant differences between flurothyl-exposed and control groups within weeks of the incubation/recording period were evident (F1,52 = 16.8, p < 0.0002; week 1, t(13) = 2.2, p < 0.03; week 2, t(13) = 1.9, p < 0.04; week 3, t(13) = 2.3, p < 0.03; week 4, t(13) = 1.80, p = 0.05; Fig. 2 and 3). Over the course of the recording period, spontaneous seizures continued to occur without any significant decrease in the number of spontaneous seizures (Fig. 2 and 3), which is in contrast to our previous observations showing that C57BL/6J mice enter spontaneous seizure remission within one month.17
Figure 2.
Total number of spontaneous seizure events recorded from DBA/2J mice following 8 flurothyl-induced seizures and 28-days of 24-hour video-EEG monitoring. Spontaneous seizures were detected in DBA/2J mice implanted with cortical and hippocampal electrodes. Numbers in the first column indicate animal identifiers (controls (CTL) were simply implanted with cortical and hippocampal electrodes having no flurothyl-induced seizures). Subsequent columns indicate the day during the recording period following exposure to eight flurothyl-induced seizures. Each individual box is color-coded according to the legend. Each of the numbers in these boxes indicates the total number of seizures per day. DBA/2J mice not given flurothyl-induced seizures (CTL1-4) do not express spontaneous seizures during the recording period. Also presented are low and high resolution representative electroencephalograms (EEG, local field potentials) from the cerebral cortex and hippocampus recorded from the same DBA2/J mouse having a spontaneous generalized clonic seizure. Black arrows denote the beginning of the seizure and red arrowheads indicate the end of the seizure.
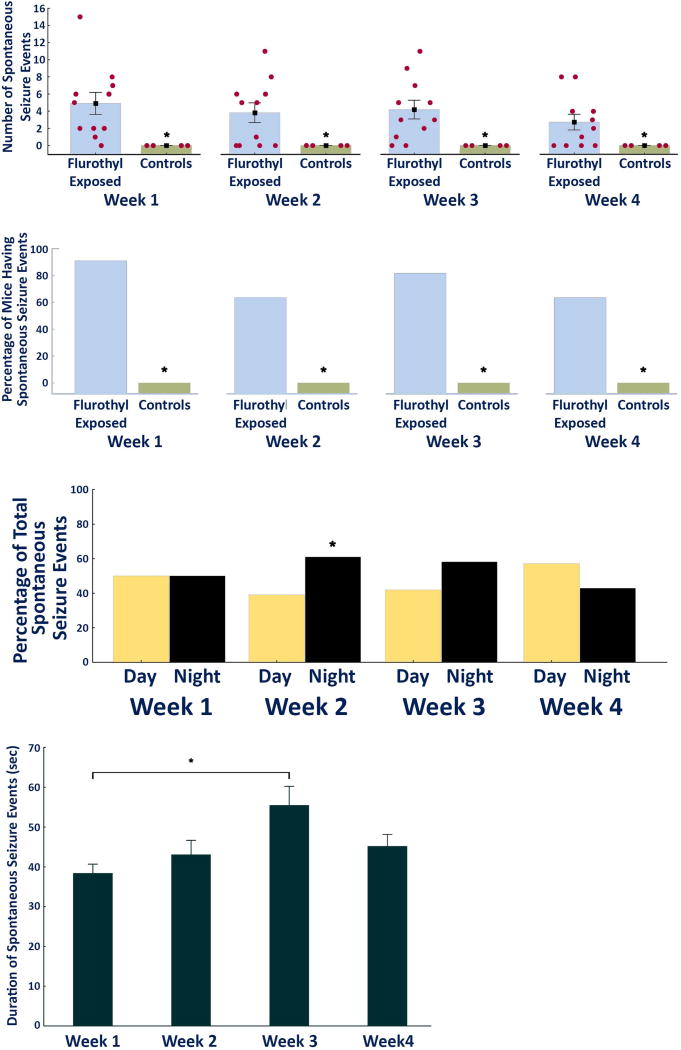
Figure 3.
Characteristics of the spontaneous seizures recorded during a four week long video-EEG recording period in DBA2/J mice following exposure to repeated flurothyl-induced seizures. (A) Average and total number of spontaneous seizures that occurred during the four week recording period in DBA/2J mice exposed to flurothyl as compared to controls grouped according to weeks. Significant differences exist between flurothyl exposed and control mice (week 1: p < 0.004; week 2: p < 0.009; week 3: p < 0.004; week 4: p < 0.02). The x-axis represents the duration of the recording period in weeks, whereas the y-axis represents both the average and SEM, and individual number of seizures expressed by the DBA2/J mice exposed to flurothyl (red circles). (B) Percentage of DBA/2J mice expressing spontaneous seizures during the four week recording period binned by weeks. Significant differences were observed between flurothyl exposed and control mice (Fisher Exact tests: week 1: p < 0.004; week 2: p=0.05; week 3: p < 0.02; week 4: p=0.05). (C) Circadian pattern of seizure distribution in DBA/2J mice during the four week recording period grouped by weeks. Significant differences exist between day and night only during week two of the recording period (p < 0.05). 7AM to 7PM represents day (the animals’ normal sleep cycle) during which the lights are turned on in the animal facility. (D) Average duration of spontaneous seizures in DBA/2J mice across the four week recording period. The duration of these seizure events were significantly different only between weeks one and three of the recording period (p < 0.002).
Approximately, 90% of DBA/2J mice expressed spontaneous seizures during the first week of the recording period. For weeks 2–4, 63%, 81%, and 63% of mice, respectively, expressed spontaneous seizures (Fig. 3). These percentage differences were significant compared to the control groups (Fisher Exact tests: week 1, p < 0.004; week 2, p=0.05, week 3, p < 0.02; week 4, p=0.05; Fig. 3). Taken together, at least 60% of DBA/2J mice continued to express spontaneous seizures by the end of the one month recording period.
To determine diurnal (circadian) effects on spontaneous seizures, comparisons were made using one-sample t-tests between the proportion of seizures occurring during the night versus the day. This analysis revealed significant differences only during week two of the incubation/recording period (t(41)=2.0, p < 0.05 or Χ2 =3.95, p < 0.05; Fig. 3) with significantly more seizures occurring during the dark cycle (the animals’ awake state). Analysis of the duration of spontaneous seizures across the recording period revealed that seizure durations were only significantly different between week one and week three (F3,162=4.6, p < 0.004; post-hoc comparisons using Tukey HSD, p < 0.0015; Fig. 3). Overall, these results indicated that although there was a slight tendency for the spontaneous seizures to occur during the night cycle, these differences were not demonstrable across four weeks of recording. Similarly, there was a trend toward prolongation of the spontaneous seizure duration across the recording period; however, the differences were only significant between weeks one and three.
These findings indicate that DBA2/J mice exposed to repeated flurothyl develop spontaneous seizures, similar to C57BL6/J mice.17 However, there is an inability for DBA2/J mice to change their seizure phenotype following repeated flurothyl exposure, a 28-day incubation period, and final flurothyl rechallenge, unlike C57BL6/J mice.17 That DBA2/J mice do not change their seizure phenotype despite the presence of spontaneous seizures suggests altered mechanisms for the expression of forebrain→brainstem seizures besides the presence of spontaneous seizures. We next investigated whether C57BL6/J and DBA2/J mice have differences in their forebrain and brainstem seizure thresholds that could account for the differences in the expression of forebrain→brainstem seizures.
Forebrain seizure thresholds in C57BL/6J and DBA/2J mice
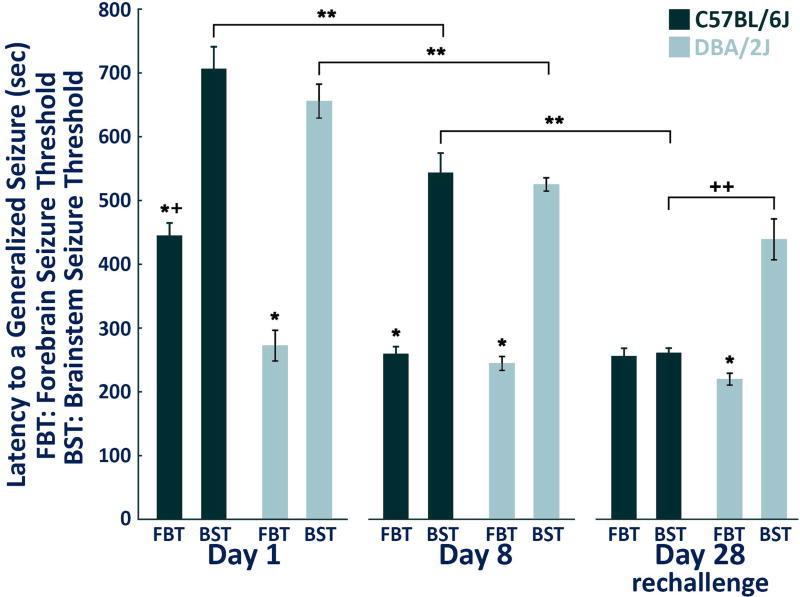
In agreement with our previous results,15 no significant differences in forebrain seizure thresholds existed within the DBA/2J strain with repeated flurothyl-induced seizures. In contrast, C57BL/6Jmice had significant differences in their forebrain seizure thresholds between day one and day eight of the induction phase (p < 0.0002; Fig. 4). One factor ANOVA revealed significant differences in the forebrain seizure thresholds between C57BL/6J and DBA/2J mice only on day one of the repeated-flurothyl model (p < 0.0002) with DBA/2J mice having significantly lowered forebrain seizure thresholds. No significant differences existed for forebrain seizure thresholds within and between strains (C57BL/6J & DBA/2J) either on day eight or on rechallenge (Fig. 4).
Figure 4.
Summary of forebrain and brainstem seizure thresholds in C57BL/6J and DBA/2J mice exposed to the repeated-flurothyl model. One-way analysis of variance (ANOVA) followed by Tukey HSD post-hoc comparisons were used to determine significance between day one, day eight and flurothyl rechallenge (eight flurothyl induction trials plus a 28-day incubation) within and between strains (C57BL/6J and DBA/2J mice). * Significantly different comparing forebrain seizure threshold (FBT) and brainstem seizure threshold (BST) within strain (p < 0.0002), ** significantly different comparing BST within strain (p < 0.004), + significantly different comparing FBT between strains (p < 0.0002), ++ significantly different comparing BST between strains (p < 0.0002).
Brainstem seizure thresholds in C57BL/6J and DBA/2J mice
In C57BL/6J mice, significant differences were found for brainstem seizure thresholds when comparing day one, day eight and rechallenge using one factor ANOVA (p < 0.0002; Fig. 4). In contrast, DBA/2J mice had significant differences only for brainstem seizure thresholds between day one and day eight (p < 0.004; Fig. 4). Importantly, for DBA/2J mice, brainstem seizure thresholds were not significantly different between day eight and the one month flurothyl rechallenge test (Fig. 4).
Comparison of forebrain and brainstem seizure thresholds in C57BL/6J and DBA/2J mice
In C57BL/6J mice, significant differences existed between forebrain and brainstem seizure thresholds on day one and day eight (p < 0.0002), but not on flurothyl retest (Fig. 4). Whereas in DBA/2J mice, significant differences between forebrain and brainstem seizure thresholds were found, not only on day one and day eight, but also at retest/rechallenge (p < 0.0002; Fig. 4). Comparison of the brainstem seizure thresholds between C57BL/6J and DBA/2J mice on day one and day eight found no significant differences. Importantly, similar comparisons of brainstem seizure thresholds between C57BL/6J and DBA/2J mice on rechallenge revealed significant differences (p < 0.0002; Fig. 4) with DBA2/J mice having higher rechallenge brainstem seizure thresholds.
Brainstem seizure expression and ventromedial nucleus of the hypothalamus (VMH) Fos immunolabeling
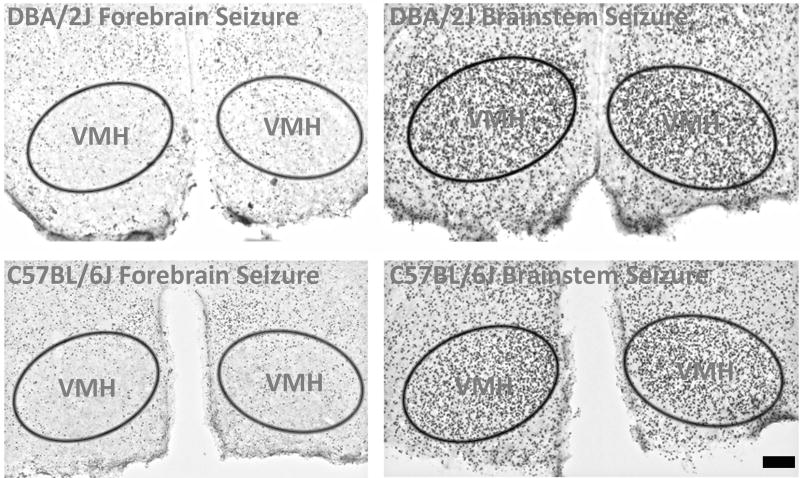
Fos immunolabeling was never bilaterally observed in the VMH of C57BL/6J or DBA/2J mice having only forebrain-clonic seizures (Fig. 5). Unlike C57BL/6J mice, DBA/2J mice never experienced tonic-brainstem seizures following eight flurothyl induction trials and a 28-day incubation period. However, all C57BL/6J and DBA/2J mice forced to experience forebrain→brainstem seizures showed significant bilateral Fos immunolabeling in the VMH compared to mice having only forebrain seizures (Fig. 5). These results indicated that, at least in the repeated-flurothyl model, VMH activation was critical for brainstem seizure expression.
Figure 5.
Induction of a flurothyl-induced brainstem seizure results in VMH activation. Immunohistochemical analysis examining Fos protein expression 90 minutes after the seizure ending time shows robust, bilateral Fos protein expression in the VMH in both C57BL/6J and DBA/2J mice only after brainstem seizure expression. Scale bar = 200 µm.
Discussion
Our current results confirmed that repeated exposure to flurothyl resulted in the appearance of spontaneous seizures during the subsequent one month recording period and demonstrated the generalizability of the repeated-flurothyl model to a second inbred strain of mice. DBA2/J mice began expressing spontaneous seizures following the last of eight flurothyl-induced seizures suggesting a brief latent period, similar to C57BL6/J mice17. There was a tendency for the spontaneous seizures to cluster in DBA2/J mice and these clusters spanned the recording period. Unlike C57BL/6J mice, where the spontaneous seizures remitted over time, DBA/2J mice have spontaneous seizure events throughout the one month recording period. However, DBA/2J mice did not have as many seizures over the one month time frame as C57BL/6J mice.17 These data suggested differences in the total number of spontaneous seizures and eventual seizure remission between these two strains. These observed differences between C57BL/6J and DBA/2J mice are likely under genetic control.
Under physiological conditions, there are two independent seizure systems in the brain, the forebrain and the brainstem seizure systems.2,3,27–29 Previous studies showed that bilateral lesions of the VMH resulted in blockade of forebrain→brainstem seizure expression in C57BL/6J mice.10 Therefore, the forebrain→brainstem seizures expressed in C57BL/6J mice upon flurothyl rechallenge likely result from a reorganizational process that occurs over the incubation period. Interestingly, in the repeated-flurothyl model, DBA/2J mice do not express forebrain→brainstem seizures on rechallenge. However, eliciting forebrain→brainstem seizures in both C57BL/6J and DBA/2J mice acutely on day one through continued exposure to flurothyl resulted in bilateral VMH neuronal activation in all animals tested, regardless of strain. Together, these results indicate that although DBA/2J mice express spontaneous seizures, these spontaneous seizures are likely not sufficient to drive the reorganization needed to transition to forebrain→brainstem seizures. Therefore, other factors conceivably contribute to the transition to the more complex forebrain→brainstem seizure phenotype. Such factors may be related to genetic, neurotrophic, or circuitry differences between mouse strains.16,30–34 Lastly, the number of spontaneous seizures and the times at which they occur could affect this reorganizational process, since C57BL6/J and DBA2/J mice differ in their spontaneous seizure characteristics.17 However, our current data indicate that the changes in seizure phenotype may be controlled by mechanisms related to changes in brainstem seizure thresholds over the incubation phase.
In C57BL/6J mice, significant differences were found for forebrain seizure thresholds across the induction phase, indicating that C57BL/6J mice were susceptible to flurothyl kindling. DBA/2J mice did not demonstrate this flurothyl kindling phenomenon. Although forebrain seizure thresholds were different between C57BL/6J and DBA/2J mice, brainstem seizure thresholds were not significantly different between C57BL/6J and DBA/2J mice at baseline or on the last flurothyl induction seizure (day eight). Typically, forebrain seizure thresholds are intrinsically lower than the inherently higher brainstem seizure thresholds. As such, this serves to separate the forebrain and brainstem seizure networks. In the event that the brainstem seizure threshold is lowered, moving closer to the forebrain seizure threshold, it becomes possible for the forebrain seizure network to interact with the brainstem seizure network in producing brainstem seizure expression. Forebrain→brainstem seizures may evolve due to mechanisms which lower brainstem seizure thresholds to forebrain seizure threshold levels. In this instance, when mice express forebrain seizures, they continue seizing and express brainstem seizures as long as the seizure discharge propagates to the brainstem seizure network.
Although there were decreases in brainstem seizure thresholds in C57BL/6J and DBA2/J mice during the induction trials, significant differences between the forebrain and brainstem seizures thresholds at the conclusion of the induction trials (higher brainstem seizure thresholds than forebrain seizure thresholds) indicated that flurothyl-induced seizures have minimal effect on brainstem seizure threshold reductions (to forebrain seizure threshold). However, upon a 28-day post-induction rechallenge with flurothyl, C57BL6/J mice have significantly reduced brainstem seizure thresholds that are now similar to their forebrain seizure thresholds thereby allowing for the expression of forebrain→brainstem seizures. Importantly, DBA2/J mice did not show a brainstem seizure threshold decrease to forebrain seizure threshold levels resulting in the inability to express forebrain→brainstem seizures upon flurothyl retest.
Although spontaneous seizures occur in DBA/2J mice repeatedly exposed to flurothyl, they do not express forebrain→brainstem seizures on retest. This suggests that spontaneous seizures are not sufficient for DBA/2J mice to express forebrain→brainstem seizures on retest. The failure of spontaneous seizures to lower brainstem seizure thresholds during the incubation period in these mice could be secondary to insufficient downstream signaling cascades. For example, we have recently shown that in DBA/2J mice, Fos (a neural activity dependent marker) expression was an unreliable marker of neuronal depolarization in the hippocampus following a single flurothyl seizure; this was not the case with C57BL/6J mice.16 We further determined that the low Fos levels in DBA/2J mice were due to low levels of phosphorylated extracellular signal-regulated kinases (p-ERK) upstream of Fos. This was overcome by repeated flurothyl-induced seizures, which resulted in Fos expression levels comparable to C57BL/6J mice. In summary, these findings support the idea that potential deficits in downstream signaling mechanisms may exist in DBA/2J mice. The fact that upon flurothyl rechallenge, brainstem seizure thresholds in DBA2/J mice are not significantly different from those on day 8 of induction, further supports our hypothesis. Studying the epileptogenic processes in these two inbred lines, each expressing different seizure characteristics with the repeated-flurothyl model, may provide valuable insights into mechanisms of epileptogenesis that are highly influenced by genetic background.
KEY POINTS.
Eight daily repeated flurothyl-induced seizures result in the appearance of recurrent, unprovoked seizures in DBA2/J mice
Decreased brainstem seizure thresholds in C57BL/6J mice may account for the change to a more complex seizure overtime, independent of spontaneous seizures
The differences in spontaneous seizure expression profiles between DBA2/J and C57BL/6J mice indicate dependence on genetic background
Acknowledgments
This work was supported by an NIH/NINDS R01NS064283 grant to RJF.
Footnotes
Disclosure of Conflicts of Interest: None of the authors has any conflict of interest to disclose.
Ethical Publication Statement: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Applegate CD, Samoriski GM, Burchfiel JL. Evidence for the interaction of brainstem systems mediating seizure expression in kindling and electroconvulsive shock seizure models. Epilepsy Res. 1991;10:142–147. doi: 10.1016/0920-1211(91)90006-2. [DOI] [PubMed] [Google Scholar]
- 2.Browning RA, Nelson DK. Modification of electroshock and pentylenetetrazol seizure patterns in rats after precollicular transections. Exp Neurol. 1986;93:546–556. doi: 10.1016/0014-4886(86)90174-3. [DOI] [PubMed] [Google Scholar]
- 3.Browning RA, Turner FJ, Simonton RL, et al. Effect of midbrain and pontine tegmental lesions on the maximal electroshock seizure pattern in rats. Epilepsia. 1981;22:583–594. doi: 10.1111/j.1528-1157.1981.tb04130.x. [DOI] [PubMed] [Google Scholar]
- 4.Kreindler A, Zuckermann E, Steriade M, et al. Electro-clinical features of convulsions induced by stimulation of brain stem. J Neurophysiol. 1958;21:430–436. doi: 10.1152/jn.1958.21.5.430. [DOI] [PubMed] [Google Scholar]
- 5.Magistris MR, Mouradian MS, Gloor P. Generalized convulsions induced by pentylenetetrazol in the cat: participation of forebrain, brainstem, and spinal cord. Epilepsia. 1988;29:379–388. doi: 10.1111/j.1528-1157.1988.tb03735.x. [DOI] [PubMed] [Google Scholar]
- 6.Blumenfeld H, Varghese GI, Purcaro MJ, et al. Cortical and subcortical networks in human secondarily generalized tonic-clonic seizures. Brain. 2009;132:999–1012. doi: 10.1093/brain/awp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeSalvo MN, Schridde U, Mishra AM, et al. Focal BOLD fMRI changes in bicuculline-induced tonic-clonic seizures in the rat. Neuroimage. 2010;50:902–909. doi: 10.1016/j.neuroimage.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varghese GI, Purcaro MJ, Motelow JE, et al. Clinical use of ictal SPECT in secondarily generalized tonic-clonic seizures. Brain. 2009;132:2102–2113. doi: 10.1093/brain/awp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Applegate CD, Samoriski GM, Ozduman K. Effects of valproate, phenytoin, and MK-801 in a novel model of epileptogenesis. Epilepsia. 1997;38:631–636. doi: 10.1111/j.1528-1157.1997.tb01231.x. [DOI] [PubMed] [Google Scholar]
- 10.Ferland RJ, Applegate CD. The role of the ventromedial nucleus of the hypothalamus in epileptogenesis. Neuroreport. 1998;9:3623–3629. doi: 10.1097/00001756-199811160-00013. [DOI] [PubMed] [Google Scholar]
- 11.Ferland RJ, Applegate CD. Decreased brainstem seizure thresholds and facilitated seizure propagation in mice exposed to repeated flurothyl-induced generalized forebrain seizures. Epilepsy Res. 1998;30:49–62. doi: 10.1016/s0920-1211(97)00093-4. [DOI] [PubMed] [Google Scholar]
- 12.Ferland RJ, Applegate CD. Bidirectional transfer between electrical and flurothyl kindling in mice: evidence for common processes in epileptogenesis. Epilepsia. 1999;40:144–152. doi: 10.1111/j.1528-1157.1999.tb02067.x. [DOI] [PubMed] [Google Scholar]
- 13.Samoriski GM, Applegate CD. Repeated generalized seizures induce time-dependent changes in the behavioral seizure response independent of continued seizure induction. J Neurosci. 1997;17:5581–5590. doi: 10.1523/JNEUROSCI.17-14-05581.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samoriski GM, Piekut DT, Applegate CD. Differential spatial patterns of Fos induction following generalized clonic and generalized tonic seizures. Exp Neurol. 1997;143:255–268. doi: 10.1006/exnr.1996.6368. [DOI] [PubMed] [Google Scholar]
- 15.Papandrea D, Anderson TM, Herron BJ, et al. Dissociation of seizure traits in inbred strains of mice using the flurothyl kindling model of epileptogenesis. Exp Neurol. 2009;215:60–68. doi: 10.1016/j.expneurol.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kadiyala SB, Papandrea D, Tuz K, et al. Spatiotemporal differences in the c-fos pathway between C57BL/6J and DBA/2J mice following flurothyl-induced seizures: A dissociation of hippocampal Fos from seizure activity. Epilepsy Res. 2015;109:183–196. doi: 10.1016/j.eplepsyres.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadiyala SB, Yannix JQ, Nalwalk JM, et al. Eight flurothyl-induced generalized seizures lead to the rapid evolution of spontaneous seizures in mice: a model of epileptogenesis with seizure remission. J Neurosci. 2016;36:7485–7496. doi: 10.1523/JNEUROSCI.3232-14.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klioueva IA, van Luijtelaar EL, Chepurnova NE, et al. PTZ-induced seizures in rats: effects of age and strain. Physiol Behav. 2001;72:421–426. doi: 10.1016/s0031-9384(00)00425-x. [DOI] [PubMed] [Google Scholar]
- 19.Velisek L, Veliskova J, Moshe SL. Developmental seizure models. Ital J Neurol Sci. 1995;16:127–133. doi: 10.1007/BF02229085. [DOI] [PubMed] [Google Scholar]
- 20.Velisek L, Veliskova J, Ptachewich Y, et al. Effects of MK-801 and phenytoin on flurothyl-induced seizures during development. Epilepsia. 1995;36:179–185. doi: 10.1111/j.1528-1157.1995.tb00978.x. [DOI] [PubMed] [Google Scholar]
- 21.Veliskova J, Velisek LS. Picrotoxin-induced tonic-clonic seizures and lethality are decreased by MK-801 in developing rats. Pharmacol Biochem Behav. 1992;43:291–295. doi: 10.1016/0091-3057(92)90670-b. [DOI] [PubMed] [Google Scholar]
- 22.Weller A, Mostofsky DI. Ontogenetic development and pentylenetetrazol seizure thresholds in rats. Physiol Behav. 1995;57:629–631. doi: 10.1016/0031-9384(94)00347-5. [DOI] [PubMed] [Google Scholar]
- 23.Samoriski GM, Piekut DT, Applegate CD. Regional analysis of the spatial patterns of Fos induction in brain following flurothyl kindling. Neuroscience. 1998;84:1209–1222. doi: 10.1016/s0306-4522(97)00571-x. [DOI] [PubMed] [Google Scholar]
- 24.Ferland RJ, Gross RA, Applegate CD. Increased mitotic activity in the dentate gyrus of the hippocampus of adult C57BL/6J mice exposed to the flurothyl kindling model of epileptogenesis. Neuroscience. 2002;115:669–683. doi: 10.1016/s0306-4522(02)00514-6. [DOI] [PubMed] [Google Scholar]
- 25.Kadiyala SB, Papandrea D, Herron BJ, et al. Segregation of seizure traits in C57 black mouse substrains using the repeated-flurothyl model. PLoS One. 2014;9:e90506. doi: 10.1371/journal.pone.0090506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Academic Press; San Diego, CA: 2001. [Google Scholar]
- 27.Browning RA. Role of the brain-stem reticular formation in tonic-clonic seizures: lesion and pharmacological studies. Fed Proc. 1985;44:2425–2431. [PubMed] [Google Scholar]
- 28.Browning RA, Nelson DK, Mogharreban N, et al. Effect of midbrain and pontine tegmental lesions on audiogenic seizures in genetically epilepsy-prone rats. Epilepsia. 1985;26:175–183. doi: 10.1111/j.1528-1157.1985.tb05402.x. [DOI] [PubMed] [Google Scholar]
- 29.Browning RA, Simonton RL, Turner FJ. Antagonism of experimentally induced tonic seizures following a lesion in the midbrain tegmentum. Epilepsia. 1981;22:595–601. doi: 10.1111/j.1528-1157.1981.tb04131.x. [DOI] [PubMed] [Google Scholar]
- 30.Applegate CD, Pretel S, Piekut DT. The substantia nigra pars reticulata, seizures and Fos expression. Epilepsy Res. 1995;20:31–39. doi: 10.1016/0920-1211(94)00064-4. [DOI] [PubMed] [Google Scholar]
- 31.Gaglani SM, Lu L, Williams RW, et al. The genetic control of neocortex volume and covariation with neocortical gene expression in mice. BMC Neurosci. 2009;10:44. doi: 10.1186/1471-2202-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mhyre TR, Applegate CD. Persistent regional increases in brain-derived neurotrophic factor in the flurothyl model of epileptogenesis are dependent upon the kindling status of the animal. Neuroscience. 2003;121:1031–1045. doi: 10.1016/s0306-4522(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 33.Rosen GD, Williams RW. Complex trait analysis of the mouse striatum: independent QTLs modulate volume and neuron number. BMC Neurosci. 2001;2:5. doi: 10.1186/1471-2202-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwab C, Bruckner G, Castellano C, et al. Different levels of acetylcholinesterase and choline acetyltransferase activities in C57Bl/6 and DBA/2 mice are not accompanied with different density of cortical acetylcholinesterase reactive fibers. Neurochem Res. 1990;15:1127–1133. doi: 10.1007/BF01101715. [DOI] [PubMed] [Google Scholar]