Abstract
It is unclear whether African Americans with versus without chronic kidney disease (CKD) taking antihypertensive medication have an increased risk for apparent treatment resistant hypertension (aTRH). We analyzed 1,741 Jackson Heart Study participants without aTRH taking antihypertensive medication at baseline. aTRH was defined as uncontrolled blood pressure while taking 3 antihypertensive medication classes or taking ≥4 antihypertensive medication classes, regardless of blood pressure level. CKD was defined as albumin-to-creatinine ratio (ACR)≥30 mg/g or estimated glomerular filtration rate (eGFR)<60 ml/min/1.73m2. Over 8 years, 20.1% and 30.5% of participants without and with CKD, respectively, developed aTRH. The multivariable-adjusted hazard ratio (HR) for aTRH comparing participants with versus without CKD was 1.45 (95%CI: 1.12–1.86). Participants with ACR ≥30 versus <30 mg/g (HR 1.44; 95%CI 1.04–2.00) and eGFR of 45-59 and <45 versus ≥60 ml/min/1.73m2 (HR 1.60; 95%CI: 1.16–2.20 and 2.05; 95%CI: 1.28–3.26, respectively) were more likely to develop aTRH.
Keywords: chronic kidney disease, treatment-resistant hypertension, blood pressure
Introduction
Apparent treatment-resistant hypertension (aTRH) is defined as uncontrolled blood pressure (BP; systolic/diastolic BP ≥ 140/90 mmHg) with concurrent use of 3 or more classes of antihypertensive medication, or controlled BP (systolic/diastolic BP < 140/90 mmHg) with use of 4 or more classes of antihypertensive medication.1 Ideally, one of these antihypertensive medication classes should be a diuretic, and all of the drugs should be prescribed at optimal doses. Most adults with chronic kidney disease (CKD) have hypertension requiring treatment with multiple classes of antihypertensive medication.2 Also, the prevalence of aTRH is high among adults with CKD.2-6
CKD is associated with increased salt and water retention, excessive activation of the renin-angiotensin-aldosterone system, and over-activation of the sympathetic nervous system.5,7,8 Additionally, antihypertensive treatment has been associated with smaller reductions in systolic and diastolic BP among adults with lower eGFR and higher ACR, suggesting that more severe CKD may contribute to a diminished effectiveness of antihypertensive medication.5,9 Therefore, it is plausible that adults with CKD have an increased risk for developing aTRH. Determining the association between CKD and aTRH may inform treatment strategies aimed at improving BP control in patients with CKD. This may be particularly useful among African Americans, given their high prevalence of both CKD and aTRH and increased risk for cardiovascular outcomes associated with aTRH.6,10,11 The goal of the current analysis was to determine whether African-American adults with CKD have an increased risk for incident aTRH. Additionally, we determined risk factors for incident aTRH among participants with CKD. To address these aims, we conducted an analysis of adults participating in the Jackson Heart Study (JHS).
Methods
Study participants
The JHS is a community-based observational study of African-American adults recruited from urban and rural areas of three counties (Hinds, Madison, and Rankin) comprising the Jackson, Mississippi metropolitan area. Details of the study design and recruitment have been published previously.12-14 Participants were recruited from the Atherosclerosis Risk in Communities (ARIC) site in Jackson, Mississippi, and a representative sample of tri-county residents, study volunteers, randomly contacted individuals, and eligible family members of participants in any one of the ARIC, volunteer, or random samples. The final JHS cohort of 5,306 African-American adults 21 years and older was enrolled between 2000 and 2004. The study protocol was approved by the Institutional Review Boards governing research in human subjects at the participating centers and all participants provided written consent.
Participants with hypertension who were taking antihypertensive medication at baseline (n=2,462) formed the base population for the current analysis. We excluded 289 participants with prevalent aTRH or for whom aTRH status could not be determined at baseline, and 409 participants who did not attend any follow-up study visits. Additionally, participants who self-reported end-stage renal disease (ESRD) at baseline (n=23) were excluded from the analyses. After these exclusion criteria were applied, we included 1,741 JHS participants in all analyses.
Data collection
Of relevance to the current analysis, data were collected during a baseline study visit in 2000-2004 and follow-up visits in 2005-2008 (visit 2) and 2009-2012 (visit 3). Baseline data were collected during an in-home interview and a study examination conducted in the JHS clinic after an overnight fast. Information on age, sex, education, cigarette smoking, physical activity, history of myocardial infarction, and self-reported use of antihypertensive and antidiabetes medication was collected during the study interview. During the clinic visit, a standardized protocol was followed to measure BP, height, and weight, and collect blood and urine samples. Information was recorded by reviewing the pill bottles for all medications taken within the 2 weeks prior to the clinic visit. Height and weight were measured and used to calculate body mass index (BMI). Fasting serum glucose was measured using a glucose oxidase method on a Vitros 950 or 250 analyzer (Ortho-Clinical Diagnostics, Raritan, NJ). Hemoglobin A1C was measured using a TOSOH high performance liquid chromatography system. Diabetes was defined as hemoglobin A1C ≥ 6.5%, fasting plasma glucose ≥ 126 mg/dL, or use of antidiabetes medication.13
Using specimens collected during the baseline study visit, urinary albumin was measured with the Dade Behring BN II nephelometer (Newark, Delaware). Serum and urine creatinine were measured using a multi-point enzymatic spectrophotometric assay on a Vitros 950 Ortho-Clinical Diagnostics analyzer (Raritan, New Jersey). Creatinine values were biochemically calibrated to Cleveland Clinic-equivalent Minnesota Beckman CX3 assay for analysis purposes.15 We calculated the urinary albumin-to-creatinine ratio (ACR). Estimated glomerular filtration rate (eGFR) was calculated via the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation,16 and CKD was defined as an ACR ≥ 30 mg/g or an eGFR < 60 ml/min/1.73m2.
BP measurement and definition of aTRH
At each visit, BP was measured after a 5 minute rest on the participant's right arm using one of four cuff sizes selected following measurement of the arm circumference. The average of 2 measures taken 1 minute apart was used to define clinic BP. Quality control was assured by technician recertification, procedural checklists, and data review.13,17 BP was measured using a random-zero sphygmomanometer (Hawksley and Sons Limited, Lansing, UK) at visits 1 and 2 and a semi-automatic oscillometric device (Omron HEM-907XL, Omron Healthcare Inc., Lake Forest, IL) at visits 2 and 3. Among the 4,182 JHS participants who attended visit 2 and had their BP measured, 2,115 participants were included in a BP comparability sub-study, for which BP was assessed simultaneously by random zero sphygmomanometer and the Omron HEM-907XL device using a Y connector. As described elsewhere,18 the random zero BP measurements were calibrated to the semi-automated device using robust regression. When available, BP from the semi-automated device was used. In the current analysis, the calibrated BP measurements were used for 1,741 participants at visit 1 and 849 participants at visit 2 who did not have their BP measured using the semi-automatic oscillometric device.
Medication names recorded during the pill bottle review were coded into generic drug names and subsequently grouped into drug classes. One-pill combinations were classified into multiple medication classes. Antihypertensive medication classes were defined using the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7),19 with updates based on review of medications in each class by study investigators. Medication adherence was defined based on participant self-report of whether they took each of their prescribed antihypertensive medications in the prior 24 hours. Those who reported not taking one or more of their antihypertensive medications were classified as non-adherent, and those who reported taking all of their antihypertensive medications were classified as adherent. Hypertension was defined as systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg and/or self-reported use of antihypertensive medication. Uncontrolled BP was defined as systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg, and controlled BP was defined as systolic BP < 140 mmHg and diastolic BP < 90 mmHg. aTRH was defined as uncontrolled BP with concurrent use of ≥ 3 antihypertensive medication classes including a diuretic or use of ≥ 4 antihypertensive medication classes including a diuretic with controlled BP.1
Statistical analysis
We used multiple imputation (n=10 data sets) and chained equations to impute variables with missing data. The number and percentage of participants missing each variable are presented in Supplemental Table 1. Characteristics of JHS participants at baseline were calculated by CKD status. The statistical significance of differences across groups was calculated using t-tests and chi-square tests, as appropriate. We then calculated the cumulative percentage of participants with and without CKD who developed aTRH at visit 2 and 3. Using interval-censored Cox regression models, we calculated crude and multivariable adjusted hazard ratios for aTRH comparing participants with versus without CKD. For participants not developing aTRH, follow up ended at the last study visit they attended. Initial adjustment included age and sex. Full multivariable adjustment included age, sex, education, current smoking, physical activity, body mass index, diabetes status, and history of myocardial infarction. Next, we calculated the percentage of participants developing aTRH and crude and multivariable adjusted hazard ratios for incident aTRH associated with baseline level of ACR (< 10, 10-29, and ≥ 30 mg/g) and eGFR (≥ 60, 45-59, and < 45 ml/min/1.73m2), separately. In sensitivity analyses, we conducted the above analyses limited to individuals who were adherent to antihypertensive medication and also conducted a complete case (i.e., unimputed) analysis. We calculated multivariable adjusted hazard ratios for incident aTRH associated with CKD, eGFR, and ACR in subgroups defined by age (< 65 versus ≥ 65 years), sex, diabetes status, history of myocardial infarction, use of each antihypertensive medication class, and number of classes of antihypertensive medications being taken at baseline. Due to the limited number of participants developing aTRH in some sub-groups, we grouped ACR and eGFR into two categories (i.e., ACR < 10 versus ≥ 10 mg/g, consistent with the threshold for normal albuminuria from a spot urine according to the 2009 National Kidney Foundation and US Food and Drug Administration report on proteinuria as a surrogate outcome in CKD,20 and eGFR ≥ 60 versus < 60 ml/min/1.73m2) for these analyses. Finally, among participants with CKD, we calculated hazard ratios for incident aTRH associated with each study covariate included in the multivariable model described above. Analyses were conducted using SAS software Version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Study characteristics
Overall, 410 (23.6%) participants included in this analysis had CKD. Participants with versus without CKD were older and more likely to have less than a high school education, a body mass index ≥ 30 kg/m2, diabetes, a history of myocardial infarction, and reduced left ventricular ejection fraction (Table 1). Mean left ventricular mass and systolic BP were higher and mean physical activity score and diastolic BP were lower among participants with versus without CKD. A higher percentage of participants with versus without CKD were taking 3 classes of antihypertensive medication at baseline.
Table 1. Baseline characteristics of Jackson Heart Study participants with hypertension by chronic kidney disease status.
| Characteristic | No CKD (n=1,331) | CKD (n=410) | p-value |
|---|---|---|---|
|
| |||
| Age, years | 58.5 (10.1) | 63.0 (11.0) | <0.001 |
|
| |||
| Male | 29.0% | 29.4% | 0.661 |
|
| |||
| Less than high school education | 20.1% | 29.6% | <0.001 |
|
| |||
| Current smoking | 9.1% | 9.8% | 0.238 |
|
| |||
| Total physical activity score, exercise units† | 8.3 (2.5) | 7.4 (2.5) | <0.001 |
|
| |||
| Body mass index ≥ 30 kg/m2 | 59.5% | 62.3% | 0.001 |
|
| |||
| Diabetes | 26.0% | 43.5% | <0.001 |
|
| |||
| History of myocardial infarction | 4.7% | 10.9% | <0.001 |
|
| |||
| Left ventricular ejection fraction < 50% | 2.1% | 4.4% | <0.001 |
|
| |||
| Left ventricular mass, g | 150.3 (42.6) | 164.7 (98.2) | <0.001 |
|
| |||
| Systolic blood pressure, mmHg | 128.7 (14.6) | 132.8 (16.7) | <0.001 |
|
| |||
| Diastolic blood pressure, mmHg | 76.1 (8.3) | 74.8 (9.6) | <0.001 |
|
| |||
| Serum creatinine, mg/dL | 1.0 (0.2) | 1.2 (0.4) | <0.001 |
|
| |||
| Albumin-to-creatinine ratio, mg/g | 5.9 (4.2, 9.5) | 41.4 (11.8, 118.1) | <0.001 |
|
| |||
| eGFR, ml/min/1.73m2 | 84.0 (13.9) | 66.6 (21.6) | <0.001 |
|
| |||
| CKD stage‡ | <0.001 | ||
| 1 | 30.0% | 16.4% | |
| 2 | 70.0% | 33.1% | |
| 3a | 0% | 38.2% | |
| 3b | 0% | 10.1% | |
| 4 | 0% | 2.0% | |
| 5 | 0% | 0.2% | |
|
| |||
| Antihypertensive medication classes | |||
| ACE inhibitor | 34.8% | 38.9% | <0.001 |
| Aldosterone antagonist | 1.5% | 1.8% | 0.134 |
| Alpha blocker | 4.6% | 4.9% | 0.440 |
| Angiotensin II receptor blocker | 15.0% | 19.7% | <0.001 |
| Beta blocker: Cardioselective and nonselective | 18.1% | 20.4% | <0.001 |
| Beta blocker: Intrinsic sympathomimetic activity | 0.2% | 0% | 0.002 |
| Calcium channel blocker | 35.2% | 36.9% | 0.049 |
| Central acting agent | 3.2% | 4.9% | <0.001 |
| Combined alpha and beta blocker | 0.4% | 1.0% | <0.001 |
| Direct vasodilator | 0.3% | 0.5% | 0.074 |
| Diuretic: Loop | 6.3% | 10.4% | <0.001 |
| Diuretic: Potassium-sparing | 14.1% | 13.7% | 0.470 |
| Diuretic: Thiazide | 55.5% | 55.6% | 0.912 |
|
| |||
| Number of antihypertensive medication classes | <0.001 | ||
| 1 | 32.9% | 24.6% | |
| 2 | 47.7% | 46.7% | |
| 3 | 19.4% | 28.7% | |
Numbers in table are mean (standard deviation) or percent, except for albumin-to-creatinine ratio, which is presented as median (25th and 75th percentiles)
eGFR: estimated glomerular filtration rate; CKD: chronic kidney disease, ACE: angiotensin-converting enzyme
Physical activity score ranges from 1-20, with a higher score indicating higher physical activity.
CKD classified according to eGFR (ml/min/1.73m2) as follows-- stage 1: ≥ 90, stage 2: 60-89, stage 3a: 45-59, stage 3b: 30-44, stage 4: 15-29, stage 5: <15
Chronic kidney disease was defined as an eGFR < 60 ml/min/1.73 m2 or an albumin-to-creatinine ratio ≥ 30 mg/g
aTRH associated with CKD and levels of ACR and eGFR
Over a median follow-up of 8.0 years (maximum: 12.2 years), 392 participants developed aTRH. Among study participants with and without CKD, 22.8% and 12.3%, respectively, developed aTRH by visit 2 (median 4.6 years follow-up; Table 2). By visit 3, 30.5% with CKD and 20.1% without CKD developed aTRH. The crude hazard ratio for aTRH comparing those with and without CKD was 1.73 (95% confidence interval [CI]: 1.39 – 2.16). After full multivariable adjustment, the hazard ratio was 1.45 (95% CI: 1.12 – 1.86). Results were similar when restricted to participants who were adherent to their antihypertensive medications (n=1,309) and in the complete case analysis (data not shown).
Table 2. Percentage of participants developing apparent treatment-resistant hypertension and hazard ratios for incident apparent treatment-resistant hypertension associated with chronic kidney disease.
| No CKD | CKD | |
|---|---|---|
|
| ||
| N cases of incident aTRH / number attending study visit | ||
| Visit 2 | 151 / 1,229 | 86 / 377 |
| Visit 2 or Visit 3 | 267 / 1,331 | 125 / 410 |
|
| ||
| Percent developing aTRH | ||
| Visit 2 | 12.3% | 22.8% |
| Visit 2 or Visit 3 | 20.1% | 30.5% |
|
| ||
| Hazard ratio (95% CI) for aTRH | ||
| Crude | 1 (ref) | 1.73 (1.39 – 2.16) |
| Age and sex adjusted | 1 (ref) | 1.58 (1.23 – 2.03) |
| Multivariable adjusted† | 1 (ref) | 1.45 (1.12 – 1.86) |
CKD: chronic kidney disease; aTRH: apparent treatment-resistant hypertension; CI: confidence interval
Adjusted for age, sex, less than high school education, current smoking, physical activity, body mass index ≥30 kg/m2, diabetes, and history of myocardial infarction
Apparent treatment-resistant hypertension defined as uncontrolled BP with concurrent use of ≥ 3 antihypertensive medication classes including a diuretic or use of ≥ 4 antihypertensive medication classes including a diuretic with controlled BP.
Median time between visits-- visit 1 to visit 2: 4.6 years, visit 2 to visit 3: 3.1 years, visit 1 to visit 3: 8.0 years
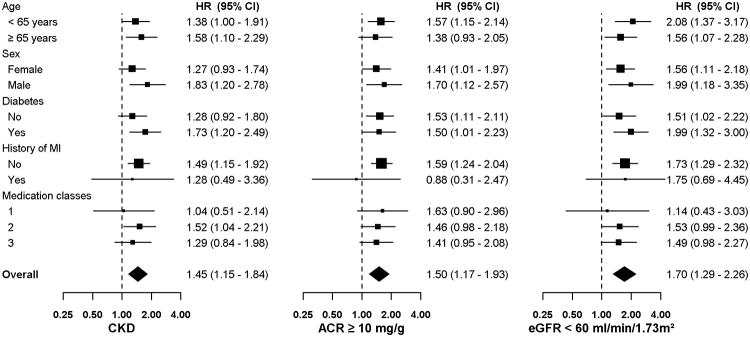
The percentage of participants developing aTRH increased with higher ACR and lower eGFR (Table 3). The multivariable adjusted hazard ratio for incident aTRH was 1.54 (95% CI: 1.15 – 2.07) and 1.44 (95% CI: 1.04 – 2.00) for participants with ACR 10-29 and ≥ 30 mg/g, respectively, each compared to those with ACR < 10 mg/g. The multivariable adjusted hazard ratio for incident aTRH was 1.60 (95% CI: 1.16 – 2.20) and 2.05 (95% CI: 1.28 – 3.26) for participants with eGFR 45-59 and < 45 ml/min/1.73m2, respectively, each compared to those with eGFR ≥ 60 ml/min/1.73m2. Results were similar when restricted to participants who were adherent to their antihypertensive medications and in a complete case analysis (data not shown). The association between CKD and, separately, ACR ≥ 10 mg/g and eGFR < 60 ml/min/1.73 m2 with incident aTRH was consistent across sub-groups (Figure 1 and Supplemental Figure 1).
Table 3. Percentage of participants developing apparent treatment-resistant hypertension and hazard ratios for incident apparent treatment-resistant hypertension associated with baseline albumin-to-creatinine ratio and estimated glomerular filtration rate.
| N cases of incident aTRH / N attending visit 2 or 3 | Percent developing aTRH at visit 2 or 3 | Age, sex adjusted hazard ratio (95% CI) | Multivariable adjusted† hazard ratio (95% CI) | |
|---|---|---|---|---|
|
| ||||
| ACR, mg/g | ||||
| < 10 | 209 / 1,112 | 18.8% | 1 (ref) | 1 (ref) |
| 10-29 | 105 / 361 | 29.2% | 1.61 (1.21 – 2.16) | 1.54 (1.15 – 2.07) |
| ≥ 30 | 78 / 268 | 29.1% | 1.66 (1.22 – 2.26) | 1.44 (1.04 – 2.00) |
|
| ||||
| eGFR, mL/min/1.73m2 | ||||
| ≥ 60 | 321 / 1,534 | 20.9% | 1 (ref) | 1 (ref) |
| 45-59 | 50 / 157 | 31.8% | 1.53 (1.12 – 2.09) | 1.60 (1.16 – 2.20) |
| < 45 | 21 / 50 | 42.2% | 2.45 (1.56 – 3.86) | 2.05 (1.28 – 3.26) |
aTRH: apparent treatment-resistant hypertension; CI: confidence interval; ACR: albumin-to-creatinine ratio; eGFR: estimated glomerular filtration rate
Adjusted for age, sex, less than high school education, current smoking, physical activity, body mass index ≥30 kg/m2, diabetes, and history of myocardial infarction
Figure 1.
Multivariable adjusted hazard ratios for incident apparent treatment-resistant hypertension associated with chronic kidney disease, albumin-to-creatinine ratio, and estimated glomerular filtration rate among participants, in subgroups
CKD: chronic kidney disease, defined as an ACR ≥ 30 mg/g or an eGFR < 60 ml/min/1.73m2; ACR: albumin-to-creatinine ratio; eGFR: estimated glomerular filtration rate.
aTRH associated with study covariates
Among participants with CKD, the multivariable adjusted hazard ratio for incident aTRH was 1.67 (95% CI: 1.10 – 2.52) for men versus women and 1.66 (95% CI: 1.12 – 2.48) for participants with versus without diabetes (Table 4). Additionally, the multivariable adjusted hazard ratio was 1.70 (95% CI: 1.04 – 2.79) for participants with versus without eGFR < 60 ml/min/1.73m2.
Table 4.
Hazard ratios for incident apparent treatment-resistant hypertension associated with study covariates among participants with chronic kidney disease (n=410).
| Age, sex adjusted hazard ratio (95% CI) | Multivariable adjusted† hazard ratio (95% CI) | |
|---|---|---|
|
| ||
| Age, per 10 years | 1.11 (0.93 – 1.33) | 1.08 (0.88 – 1.34) |
|
| ||
| Male sex | 1.55 (1.04 – 2.30) | 1.67 (1.10 – 2.52) |
|
| ||
| Less than high school education | 0.96 (0.62 – 1.49) | 0.90 (0.58 – 1.40) |
|
| ||
| Current smoking | 0.98 (0.46 – 2.08) | 1.08 (0.52 – 2.26) |
|
| ||
| Total physical activity, per exercise unit†† | 0.95 (0.87 – 1.04) | 0.98 (0.90 – 1.07) |
|
| ||
| Body mass index ≥ 30 kg/m2 | 1.33 (0.87 – 2.04) | 1.24 (0.81 – 1.91) |
|
| ||
| Diabetes | 1.74 (1.20 – 2.52) | 1.66 (1.12 – 2.48) |
|
| ||
| History of myocardial infarction | 0.82 (0.43 – 1.54) | 0.77 (0.40 – 1.46) |
|
| ||
| ACR, mg/g | ||
| < 10 | 1 (ref) | 1 (ref) |
| ≥ 10 | 1.06 (0.64 – 1.77) | 1.23 (0.68 – 2.22) |
|
| ||
| eGFR, mL/min/1.73m2 | ||
| ≥ 60 | 1 (ref) | 1 (ref) |
| < 60 | 1.44 (0.94 – 2.19) | 1.70 (1.04 – 2.79) |
CI: confidence interval; ACR: albumin-to-creatinine ratio; eGFR: estimated glomerular filtration rate
Adjusted for all variables in the left column
Physical activity score ranges from 1-20, with a higher score indicating higher physical activity.
Discussion
In the current study of a large, community-based study of African-American adults being treated for hypertension, CKD was associated with an increased risk for incident aTRH, which was present after multivariable adjustment. Over 30% of participants with CKD developed aTRH during follow-up compared with approximately 20% of those without CKD. Additionally, higher ACR and lower eGFR were each associated with an increased risk for aTRH. Among participants with CKD, male sex, diabetes, and reduced eGFR were associated with higher risk for aTRH.
Prior studies have reported a high prevalence of aTRH among adults with CKD. For example, 40% of participants with hypertension in the Chronic Renal Insufficiency Cohort (CRIC) Study had aTRH.5 In CRIC, the prevalence of aTRH was higher in participants with lower eGFR. The multivariable adjusted odds ratio for aTRH associated with each 5 ml/min/1.73m2 lower eGFR was 1.14 (95% CI: 1.10 – 1.17).5 Older age, male sex, black race, diabetes, and a higher body mass index were each associated with a higher odds of having aTRH.5 Also, in the REasons for Geographic And Racial Differences in Stroke (REGARDS) study, there was a strong, graded association between higher ACR and lower eGFR with a higher prevalence of aTRH.6 Male sex, black race, diabetes, larger waist circumference, history of myocardial infarction or stroke, and lower eGFR and higher ACR levels were each associated with aTRH.6
Laboratory studies demonstrate that chronic elevations in blood pressure promote damage to the renal vasculature, leading to intimal and medial thickening, renal ischemia, and glomerulosclerosis.21,22 Also, epidemiologic studies suggest that adults with aTRH have an increased risk for the development of incident CKD. In an analysis of claims data from two health plans within the Cardiovascular Research Network hypertension registry, Daugherty et al. reported that individuals with aTRH were more likely to develop CKD compared to those with non-resistant hypertension (14.5% versus 10.4%).23 After multivariable adjustment, aTRH was associated with a higher risk of a composite endpoint including CKD and cardiovascular events [hazard ratio (95% CI): 1.47 (1.33 – 1.62)].23 Additionally, among individuals with prevalent CKD, aTRH has been associated with CKD progression.5,24 In the aforementioned CRIC analysis, aTRH was associated with an increased risk for a composite outcome including 50% reduction in eGFR or ESRD [multivariable adjusted hazard ratio (95% CI): 1.28 (1.11 – 1.46)].5
Although there are few data on whether adults with CKD have an increased risk for incident aTRH, this association is biologically plausible. CKD is associated with excessive activation of the renin-angiotensin-aldosterone and sympathetic nervous systems, which promotes increased sodium retention and increased peripheral resistance.7 In an analysis of the Multi-Ethnic Study of Atherosclerosis, early kidney dysfunction (indicated by serum cystatin C levels) was associated with incident hypertension among participants without clinically apparent kidney or cardiovascular disease.22 Additionally, in a clinic-based study of adults with uncontrolled BP on antihypertensive medication at a baseline study visit, ACR of 30 to 300 mg/g and > 300 mg/g were associated with a 5.1 mmHg and 10.3 mmHg smaller systolic BP reduction, respectively, over a median of 5 years of follow-up when compared to patients with ACR < 30 mg/g.9 Similarly, compared to patients with an eGFR ≥ 60 ml/min/1.73 m2, those with reduced eGFR (< 60 ml/min/1.73 m2) experienced an 8.4 mmHg smaller reduction in systolic BP over follow-up.9 The presence of albuminuria and reduced eGFR also delayed the time to attainment of goal BP.9 These data suggest that individuals with CKD may have attenuated responses to antihypertensive pharmacotherapy, making them more susceptible to developing aTRH.
African Americans are less likely than whites to have impaired kidney function, but more likely to develop ESRD.25-27 For example, in the REGARDS study, the prevalence ratio for eGFR < 60 mL/min/1.73m2 comparing African Americans to whites was 0.51 (95% CI: 0.48 – 0.54).25 However, the United States Renal Data System recently reported that African Americans are three times more likely than whites to develop incident ESRD.27 This racial difference was also present in the REGARDS study.26 Data have shown that African Americans have a higher prevalence of hypertension compared to whites and are less likely to have their BP controlled to < 140/90 mm Hg.28 Also, African Americans with CKD are more likely than their white counterparts to have aTRH.6 Along with the findings of the current study, these data emphasize the importance of achieving BP control in African Americans with CKD.
Achieving BP control is a major challenge in the management of patients with CKD.29 However, prior studies suggest that, with appropriate interventions, BP goals can be achieved and maintained in populations where BP has historically been difficult to control.30-33 For example, in the African American Study of Kidney Disease and Hypertension (AASK), African Americans with hypertension and reduced eGFR were randomized to a goal mean arterial pressure (MAP) of either 102 – 107 mmHg (usual MAP goal) or < 92 mmHg (low MAP goal).30 In the low MAP goal group, the percentage of participants who achieved a BP < 140/90 mmHg increased from 20% at baseline to 79% by 14 months post-randomization, while these percentages increased from 22% to 42% in the usual MAP goal group.30 The findings of the current study emphasize the need for intensive clinical management strategies to lower BP early in the course of CKD in an effort to prevent aTRH. This is especially important given that aTRH is associated with increased risk for cardiovascular disease, ESRD, and all-cause mortality.23,24,34,35
In the current study, misclassification of aTRH was minimized by the use of standardized BP measurements and a pill bottle review to identify the number of antihypertensive medication classes being taken. Other strengths include the large community-based sample of African Americans and the availability of both albuminuria and eGFR measurements. However, the findings of the current study should be considered in the context of certain limitations. Albuminuria and eGFR were assessed at a single time point, making misclassification of CKD status possible. Additionally, although highly correlated, JHS used two different methods of measuring BP across study visits, making misclassification of aTRH status possible. We do not have medication dosing information or a validated measure of antihypertensive medication adherence. Some participants may have been non-adherent to therapy or on an inadequate treatment regimen and not truly treatment-resistant.
In conclusion, data from the current study suggest African-American adults with versus without CKD have an increased risk for developing aTRH. Furthermore, higher ACR and lower eGFR were each associated with an increased risk for aTRH. Intensive BP monitoring and early therapeutic interventions aimed at preventing the development of aTRH should be a high priority in African Americans with CKD.
Supplementary Material
Supplemental Figure 1. Multivariable adjusted hazard ratios for incident apparent treatment-resistant hypertension associated with chronic kidney disease, albumin-to-creatinine ratio, and estimated glomerular filtration rate among participants, in subgroups defined by antihypertensive medication class
Acknowledgments
The JHS is supported by contracts HHSN268201300046C, HHSN268201300047C, HHSN268201300048C, HHSN268201300049C, and HHSN268201300050C from the National Heart, Lung, and Blood Institute and the National Institute on Minority Health and Health Disparities. Additional support for the current analysis was provided by contract K24-HL125704 from the National Institutes of Health. The authors thank the participants and data collection staff of the JHS. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Footnotes
Disclosure Statement: This manuscript is being submitted as an original investigation. All authors have read the manuscript and approve its submission. The manuscript has not been previously published and is not being considered for publication in another journal. Additionally, we have no conflicts of interest to report.
References
- 1.Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403–1419. doi: 10.1161/HYPERTENSIONAHA.108.189141. [DOI] [PubMed] [Google Scholar]
- 2.Muntner P, Anderson A, Charleston J, et al. Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2010;55(3):441–451. doi: 10.1053/j.ajkd.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parikh NI, Hwang SJ, Larson MG, Meigs JB, Levy D, Fox CS. Cardiovascular disease risk factors in chronic kidney disease: overall burden and rates of treatment and control. Arch Intern Med. 2006;166(17):1884–1891. doi: 10.1001/archinte.166.17.1884. [DOI] [PubMed] [Google Scholar]
- 4.Coresh J, Wei GL, McQuillan G, et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988-1994) Arch Intern Med. 2001;161(9):1207–1216. doi: 10.1001/archinte.161.9.1207. [DOI] [PubMed] [Google Scholar]
- 5.Thomas G, Xie D, Chen HY, et al. Prevalence and prognostic significance of apparent treatment resistant hypertension in chronic kidney disease: report from the Chronic Renal Insufficiency Cohort Study. Hypertension. 2016;67(2):387–396. doi: 10.1161/HYPERTENSIONAHA.115.06487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanner RM, Calhoun DA, Bell EK, et al. Prevalence of apparent treatment-resistant hypertension among individuals with CKD. Clin J Am Soc Nephrol. 2013;8(9):1583–1590. doi: 10.2215/CJN.00550113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Julius S. The evidence for a pathophysiologic significance of the sympathetic overactivity in hypertension. Clin Exp Hypertens. 1996;18(3-4):305–321. doi: 10.3109/10641969609088965. [DOI] [PubMed] [Google Scholar]
- 8.Campese VM, Mitra N, Sandee D. Hypertension in renal parenchymal disease: why is it so resistant to treatment? Kidney Int. 2006;69(6):967–973. doi: 10.1038/sj.ki.5000177. [DOI] [PubMed] [Google Scholar]
- 9.Flack JM, Duncan K, Ohmit SE, et al. Influence of albuminuria and glomerular filtration rate on blood pressure response to antihypertensive drug therapy. Vasc Health Risk Manag. 2007;3(6):1029–1037. [PMC free article] [PubMed] [Google Scholar]
- 10.Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011;124(9):1046–1058. doi: 10.1161/CIRCULATIONAHA.111.030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muntner P, Davis BR, Cushman WC, et al. Treatment-resistant hypertension and the incidence of cardiovascular disease and end-stage renal disease: results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Hypertension. 2014;64(5):1012–1021. doi: 10.1161/HYPERTENSIONAHA.114.03850. [DOI] [PubMed] [Google Scholar]
- 12.Sempos CT, Bild DE, Manolio TA. Overview of the Jackson Heart Study: a study of cardiovascular diseases in African American men and women. The Am J Med Sci. 1999;317(3):142–146. doi: 10.1097/00000441-199903000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Taylor HA, Jr, Wilson JG, Jones DW, et al. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart Study. Ethn Dis. 2005;15(4 Suppl 6):S6-4–17. [PubMed] [Google Scholar]
- 14.Fuqua SR, Wyatt SB, Andrew ME, et al. Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis. 2005;15(4 Suppl 6):S6-18–29. [PubMed] [Google Scholar]
- 15.Wang W, Young BA, Fulop T, et al. Effects of serum creatinine calibration on estimated renal function in african americans: the Jackson Heart Study. Am J Med Sci. 2015;349(5):379–384. doi: 10.1097/MAJ.0000000000000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wyatt SB, Akylbekova EL, Wofford MR, et al. Prevalence, awareness, treatment, and control of hypertension in the Jackson Heart Study. Hypertension. 2008;51(3):650–656. doi: 10.1161/HYPERTENSIONAHA.107.100081. [DOI] [PubMed] [Google Scholar]
- 18.Abdalla M, Booth JN, III, Seals SR, et al. Masked hypertension and incident clinic hypertension among blacks in the Jackson Heart Study. Hypertension. 2016;68(1):220–226. doi: 10.1161/HYPERTENSIONAHA.115.06904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Cattran D, Friedman A, et al. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am J Kidney Dis. 2009;54(2):205–226. doi: 10.1053/j.ajkd.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 21.Harvey JM, Howie AJ, Lee SJ, et al. Renal biopsy findings in hypertensive patients with proteinuria. Lancet. 1992;340(8833):1435–1436. doi: 10.1016/0140-6736(92)92624-o. [DOI] [PubMed] [Google Scholar]
- 22.Kestenbaum B, Rudser KD, de Boer IH, et al. Differences in kidney function and incident hypertension: the Multi-Ethnic Study of Atherosclerosis. Ann Intern Med. 2008;148(7):501–508. doi: 10.7326/0003-4819-148-7-200804010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125(13):1635–1642. doi: 10.1161/CIRCULATIONAHA.111.068064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanner RM, Calhoun DA, Bell EK, et al. Incident ESRD and treatment-resistant hypertension: the reasons for geographic and racial differences in stroke (REGARDS) study. Am J Kidney Dis. 2014;63(5):781–788. doi: 10.1053/j.ajkd.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McClellan W, Warnock DG, McClure L, et al. Racial differences in the prevalence of chronic kidney disease among participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Cohort Study. J Am Soc Nephrol. 2006;17(6):1710–1715. doi: 10.1681/ASN.2005111200. [DOI] [PubMed] [Google Scholar]
- 26.McClellan WM, Warnock DG, Judd S, et al. Albuminuria and racial disparities in the risk for ESRD. J Am Soc Nephrol. 2011;22(9):1721–1728. doi: 10.1681/ASN.2010101085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.USRDS 2015 United States Renal Data System annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2015. [Google Scholar]
- 28.Lackland DT. Racial differences in hypertension: implications for high blood pressure management. Am J Med Sci. 2014;348(2):135–138. doi: 10.1097/MAJ.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khosla N, Kalaitzidis R, Bakris GL. The kidney, hypertension, and remaining challenges. Med Clin North Am. 2009;93(3):697–715. doi: 10.1016/j.mcna.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Wright JT, Jr, Agodoa L, Contreras G, et al. Successful blood pressure control in the African American Study of Kidney Disease and Hypertension. Arch Intern Med. 2002;162(14):1636–1643. doi: 10.1001/archinte.162.14.1636. [DOI] [PubMed] [Google Scholar]
- 31.Sarnak MJ, Greene T, Wang X, et al. The effect of a lower target blood pressure on the progression of kidney disease: long-term follow-up of the modification of diet in renal disease study. Ann Intern Med. 2005;142(5):342–351. doi: 10.7326/0003-4819-142-5-200503010-00009. [DOI] [PubMed] [Google Scholar]
- 32.Appel LJ, Wright JT, Jr, Greene T, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363(10):918–929. doi: 10.1056/NEJMoa0910975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ACCORD Study Group. Cushman WC, Evans GW, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Nicola L, Gabbai FB, Agarwal R, et al. Prevalence and prognostic role of resistant hypertension in chronic kidney disease patients. J Am Coll Cardiol. 2013;61(24):2461–2467. doi: 10.1016/j.jacc.2012.12.061. [DOI] [PubMed] [Google Scholar]
- 35.Kumbhani DJ, Steg PG, Cannon CP, et al. Resistant hypertension: a frequent and ominous finding among hypertensive patients with atherothrombosis. Eur Heart J. 2013;34(16):1204–1214. doi: 10.1093/eurheartj/ehs368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Multivariable adjusted hazard ratios for incident apparent treatment-resistant hypertension associated with chronic kidney disease, albumin-to-creatinine ratio, and estimated glomerular filtration rate among participants, in subgroups defined by antihypertensive medication class