Over the past decade Intensive Care Medicine has led the way in identifying a group of critically ill patients who have both an underlying disease (linked to immune suppression), and an acute trigger (immune, malignancy, or infection related) that result in an increased TH1 response marked by cooperation between T cells and macrophages as well as the effectors of the interferon gamma response which activate the reticuloendothelial system.1,2 These patients have uncontrolled inflammation and high mortality that appears to be reduced by early treatment of ‘Hemophagocytic Lympho Histiocytosis’ within 24 hours of diagnosis (median 5 hours).1,2 These patients fulfill 5 of 8 criteria including 1) fever > 7 days, 2) splenomegaly, 3) bicytopenia (hemoglobin < 9 g/dL, platelet count < 100,000 mm3, neutrophil count < 1,100/mm3), 4) hypertriglyceridemia (> 3.0 mmol/L fasting value), or hyperferritinemia (> 500 ng/L), 6) low (< 10%) or absent NK cell activity, 6) hypofibrinemia (< 1.5 g/dL), 7) increased soluble CD25 levels (> 2400 IU/mL), and 8) histological hemophagocytosis in reticulo-endothelial system organs including spleen, liver, bone marrow, or lymph nodes. Authors have recently proposed adding aspartate aminotransferase and underlying immune suppression to the 8 criteria to craft an ‘H-score’ that facilitates early diagnostic certainty.3 These clinical criteria do not distinguish between patients with familial Hemophagocytic Lympho Histiocytosis (fHLH), reactive Hemophagocytic Lympho Histiocytosis (rHLH), Macrophage Activation Syndrome (MAS), or sepsis induced Multiple Organ Dysfunction Syndrome (MODS).4,5
Adults, and children over two years of age without a family history of a child dying of fever in the family, or consanguinity, or a primary central nervous system presentation, are less likely to have familial Hemophagocytic Lympho Histiocytosis.4 Etiologies for uncontrolled inflammation in adults and older children are often related to a) viral, intracellular, bacterial, fungal, and parasitic infections (sepsis induced MODS/reactive Hemophagocytic Lympho Histiocytosis) b) cancer or other immune deficiencies (reactive Hemophagocytic Lympho Histiocytosis), and c) systemic diseases including autoimmune disease and drug exposures (Macrophage Activation Syndrome).1,2,4,5 To help address diagnostic and therapeutic challenges in this regard, we illustrate five prototypical cases that provide a ‘consideration framework’ for potential clinical approaches (Figure 1), recognizing that the absence of any randomized trials is the basis of considerable controversy regarding treatment choices.
Figure 1.
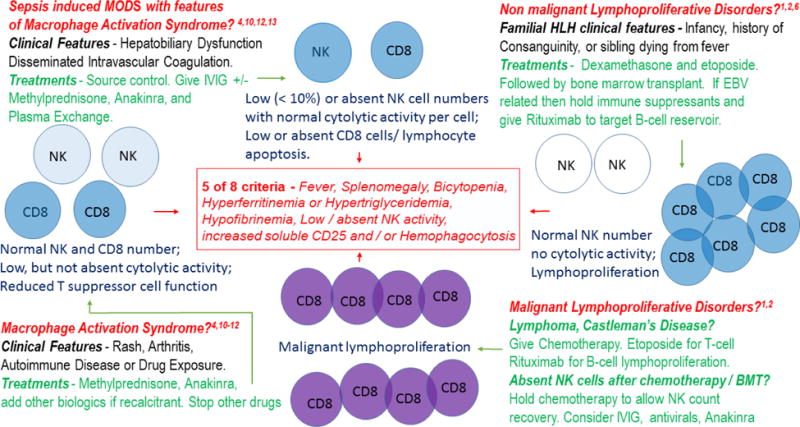
A hypothetical framework illustrating conditions seen in critically ill patients meeting 5 of 8 Hemophagocytic Lympho Histiocytosis criteria that require consideration for different consultative services and therapeutic approaches. Sepsis induced MODS has decreased NK cell numbers with normal cytolytic (
 ) function, decreased CD8 cell numbers, and activated macrophages. Non-malignant lymphoproliferative disorders have normal NK cell numbers without cytolytic activity (
) function, decreased CD8 cell numbers, and activated macrophages. Non-malignant lymphoproliferative disorders have normal NK cell numbers without cytolytic activity (
 ), with lymphoproliferation and macrophage activation. Malignant lymphoproliferative (
), with lymphoproliferation and macrophage activation. Malignant lymphoproliferative (
 ) disorders have failed cytolytic activity and macrophage activation. Macrophage Activation Syndrome disorders have normal NK cell numbers with reduced cytolytic activity (
) disorders have failed cytolytic activity and macrophage activation. Macrophage Activation Syndrome disorders have normal NK cell numbers with reduced cytolytic activity (
 ), decreased suppressor T cells, and activated macrophages.
), decreased suppressor T cells, and activated macrophages.
Abbreviations: MODS – Multiple Organ Dysfunction Syndrome; NK – natural killer; IVIG – intravenous immune globulin; AKI – acute kidney injury; HLH – Hemophagocytic Lymphohistiocytosis; EBV – Epstein Barr Virus; BMT – Bone Marrow Transplantation
A febrile infant with fHLH, a non-malignant lymphoproliferative disorder
The intensivist elicits a history of another child in the family dying from ‘fever’ and consanguinity in an infant who presents with central nervous system findings of seizures. A bone marrow aspirate is attained and the infant is started on dexamethasone, IVIG, and etoposide. The genetic analysis comes back 1 month later showing a homozygous UNC13D gene variant which leads to ineffective NK cell killing of virus infection and ineffective activated immune cell death. After recovery, the child is brought back for bone marrow transplantation. The etiology is commonly a viral infection in a host who has a genetically determined inability to induce granzyme - perforin mediated cytolytic killing. In these children, non-malignant lymphoproliferation leads to high levels of lymphocyte derived interferon γ that activates macrophages. Dexamethasone induces lymphocyte apoptosis and reduces macrophage activation, IVIG neutralizes the viral infection, and etoposide kills proliferating CD8 lymphocytes.6,7
A febrile immunocompromised patient with myelodyplasia or malignant lymphoproliferative disorder
The intensivist consults a hematologist who performs a bone marrow and finds hemophagocytosis. The hematologist tells the intensivist that she has seen this before in other lymphoproliferative disorders including Castleman’s Disease. The hematologist begins etoposide and a chemotherapeutic regimen to stop lymphoproliferation. Although lymphoproliferation is driven by malignant transformation in this patient, it still leads to interferon γ stimulated macrophage activation. Therapy is directed to stopping malignant transformation and killing cancer cells.1,2
A febrile immunocompromised patient with pancytopenia after chemotherapy and bone marrow transplant
A 28 year old patient with leukemia recalcitrant to rounds of chemotherapy has an absolute neutrophil count > 500/mm3; however, the NK cell count is zero from chemotherapy and failed bone marrow engraftment. The intensivist suspects florid DNA viremia, and obtains diagnostic tests for HSV, HHV6, HHV8, EBV, CMV, HIV, parvovirus, and BK virus to guide appropriate anti-viral therapy. Natural Killer cells recover from chemotherapy long after neutrophils.8,9 Macrophage activation is driven by CPG motifs of DNA viruses stimulating TLR9 mediated inflammasome activation that, in the absence of NK cells, is uncontrolled. When NK cells and blasts are absent, consideration can be given to holding chemotherapy until NK counts recover. The DNA viruses are treated with IVIG, and antivirals. Epstein Barr virus can be treated with anti CD20 monoclonal antibody (rituximab) to eradicate the B-cell reservoir. Anti-inflammatory biologics that decrease inflammation may also be considered while awaiting NK cell count recovery.
A febrile patient with rash, leukocytosis, arthritis, and Macrophage Activation Syndrome
The intensivist calls the rheumatologist who believes the presentation is consistent with Adult Onset Stills Disease (the adult form of systemic juvenile arthritis) related MAS. She starts the patient on corticosteroids and Anakinra.10,11,12 Other laboratory testing is sent to rule out Systemic Lupus Erythematosis, Sarcoidosis, Scleroderma, Sjogren’s, and Kawasaki’s disease.1,2 Patients with autoimmune rheumatologic disease have increased inflammasome activation and reduced NK activity without lymphoproliferation. Corticosteroids and Anakinra are indicated to control inflammasome activation. Other immune suppressants and chemotherapeutics such as cyclophosphamide, methotrexate, tocilizumab, or etoposide as well as plasma exchange are considered if the patient remains recalcitrant.10
A febrile patient with sepsis induced MODS and features of Macrophage Activation Syndrome
Antibiotics and source control are implemented. The intensivist treats shock, AKI, and ARDS in this patient who also has hepatobiliary dysfunction and disseminated intravascular coagulation. The patient is treated with plasma exchange (if AKI and thrombocytopenia are present), IVIG, and Anakinra.4,12,13 An exhaustive search ensues for bacteria including mycoplasma, rickettsia, legionella, chlamydia, brucella, and borrelia; fungi and parasites including histoplasmosis, babesia, leishmaniasis, pneumocystis, aspergillus, toxoplasmosis, cryptococcus, and candida; and viruses including EBV, CMV, HSV, HIV, HHV8, HHV6, parvovirus, adenovirus, and influenza so that appropriate anti-microbial therapy can be used.1,2 Corticosteroids are considered if the patient does not have a contra-indicated infection such as HSV. Septic patients have low NK and CD8 cell numbers. Improvement occurs when NK cell and CD8 lymphocyte counts recover.1,2, 4, 7, 12
Conclusion
Clinical history and presentation with new onset hyperferritinemia help the intensivist to recognize uncontrolled inflammation and need for subspecialty consultation. Determination of the best treatment approaches for these patients is in need of clinical trial evaluation.
References
- 1.Creput C, Galicier L, Buyse S, Azoulay E. Understanding organ dysfunction in hemophagocyticc lymphohistiocytosis. Intensive Care Med. 2088;34:1177–1187. doi: 10.1007/s00134-008-1111-y. [DOI] [PubMed] [Google Scholar]
- 2.Buyse S, Teixera L, Galicier L, Mariotte E, Lemiale V, Seguion A, Bertheau P, Canet E, de Labarthe A, Darmon M, Rybjoad M, Schlemmer B, Azoulay E. Critical care management of patients with hemophagocytic lymphohistiocytosis. Intensive Care Med. 2010;36:1695–1702. doi: 10.1007/s00134-010-1936-z. [DOI] [PubMed] [Google Scholar]
- 3.Debaugnies F, Mahadeb B, Ferster A, Meuleman N, Rozen L, Demudder A, Corazza F. Performance of the H-Score for diagnosis of hemophagocytic lymphohistiocytosis in adult and pediatric patients. Am J Clin Pathol. 2016;145:862–870. doi: 10.1093/ajcp/aqw076. [DOI] [PubMed] [Google Scholar]
- 4.Demirkol D, Yildizdas D, Bayrakci B, Karapinar B, Kendirli T, Koroglu TF, Dursun O, Erkek N, Gedik H, Citak A, Kesici S, Karabocuoglu M, Carcillo JA, Turkish Secondary HLH/MAS Critical Care Study Group Hyperferritinemia in the critically ill child with secondary hemophagocytic lymphohistiocytosis/sepsis/multiple organ dysfunction syndrome/macrophage activation syndrome: what is the treatment? Crit Care. 2012;16(2):R52. doi: 10.1186/cc11256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castillo L, Carcillo J. Secondary hemophagocytic lymphohistiocytosis and severe sepsis/systemic inflammatory response syndrome/multiorgan dysfunction syndrome/macrophage activation syndrome share common intermediate phenotypes on a spectrum of inflammation. (2009) Pediatr Crit Care Med. 2009;10(3):387–92. doi: 10.1097/PCC.0b013e3181a1ae08. [DOI] [PubMed] [Google Scholar]
- 6.Trottestam H, Horne A, Aricò M, Egeler RM, Filipovich AH, Gadner H, Imashuku S, Ladisch S, Webb D, Janka G, Henter JI, Histiocyte Society Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood. 2011;118(17):4577–84. doi: 10.1182/blood-2011-06-356261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson TS, Terrell CE, Millen SH, Katz JD, Hildeman DA, Jordan MB. Etoposide selectively ablates activated T cells to control the immunoregulatory disorder hemophagocytic lymphohistiocytosis. J Immunol. 2014;192(1):84–91. doi: 10.4049/jimmunol.1302282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pical-Izard C, Crocchiolo R, Granjeaud S, Kochbati E, Just-Landi S, Chabannon C, Frassati C, Picard C, Blaise D, Olive D, Fauriat C. Reconstitution of natural killer cells in HLA-matched HSCT after reduced-intensity conditioning: impact on clinical outcome. Biol Blood Marrow Transplant. 2015;21(3):429–39. doi: 10.1016/j.bbmt.2014.11.681. [DOI] [PubMed] [Google Scholar]
- 9.Rey J, Fauriat C, Kochbati E, Orlanducci F, Charbonnier A, D’Incan E, Andre P, Romagne F, Barbarat B, Vey N, Olive D. Kinetics of Cytotoxic Lymphocytes Reconstitution after Induction Chemotherapy in Elderly AML Patients Reveals Progressive Recovery of Normal Phenotypic and Functional Features in NK Cells. 2017;8:64. doi: 10.3389/fimmu.2017.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minoia F, Davì S, Horne A, Demirkaya E, Bovis F, Li C, Lehmberg K, Weitzman S, Insalaco A, Wouters C, Shenoi S, Espada G, Ozen S, Anton J, Khubchandani R, Russo R, Pal P, Kasapcopur O, Miettunen P, Maritsi D, Merino R, Shakoory B, Alessio M, Chasnyk V, Sanner H, Gao YJ, Huasong Z, Kitoh T, Avcin T, Fischbach M, Frosch M, Grom A, Huber A, Jelusic M, Sawhney S, Uziel Y, Ruperto N, Martini A, Cron RQ, Ravelli A, Pediatric Rheumatology International Trials Organization.; Childhood Arthritis and Rheumatology Research Alliance.; Pediatric Rheumatology Collaborative Study Group.; Histiocyte Society Clinical features, treatment, and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a multinational, multicenter study of 362 patients. Arthritis Rheumatol. 2014;66(11):3160–3169. doi: 10.1002/art.38802. [DOI] [PubMed] [Google Scholar]
- 11.Castañeda S, Blanco R, González-Gay MA. Adult-onset Still’s disease: Advances in the treatment. Best Pract Res Clin Rheumatol. 2016;30(2):222–238. doi: 10.1016/j.berh.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Haytoglu Z, Yazici N, Erbay A. Secondary Hemophagocytic Lymphohistiocytosis: Do We Really Need Chemotherapeutics for All Patients? J Pediatr Hematol Oncol. 2017;39(2):e106–e109. doi: 10.1097/MPH.0000000000000740. [DOI] [PubMed] [Google Scholar]
- 13.Shakoory B, Carcillo JA, Chatham WW, Amdur RL, Zhao H, Dinarello CA, Cron RQ, Opal SM. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit Care Med. 2016;44(2):275–81. doi: 10.1097/CCM.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]


