Abstract
Background
Childhood obesity continues to be a substantial problem despite major public health efforts, and disproportionately impacts children from low-income families. Digital health tools and consumer technology offer promising opportunities for interventions, but few studies have evaluated how they might be incorporated into existing interventions or used to create new types of interventions. It remains unclear which approaches would be most beneficial for underserved pediatric populations.
Purpose
To describe the design and rationale of a single-center randomized, controlled trial evaluating the effects of personal activity tracker (PAT) use by parents on weight-status improvement in both parents and overweight children enrolled in BodyWorks (BW), a comprehensive behavioral family-lifestyle intervention program (CBFLI), in a primary-care clinic serving a predominantly low-income Latino population.
Methods
This study is being conducted in the AltaMed general pediatrics clinic at Children’s Hospital Los Angeles. Eligible participants are families (child and adult caregiver) in which the child is between 7–18 years of age, has a BMI ≥85th percentile for age and sex, and has been referred to BW by their AltaMed pediatrician. BW consists of one weekly, two-hour session for 7 weeks. In a given cycle, the program is offered on two separate nights: Monday (Spanish) and Wednesday (English). Families self sort into one of two groups based on language preference. To ensure balanced allocation of language preference groups and prevent in-group cross contamination, block randomization is used to assign whole groups to either the intervention or control arms of the study. The control arm consists of usual care, while the intervention arm adds assigning a Fitbit PAT to the parents and training them in its proper use. Study personnel are blinded to group assignment during the analysis phase. Study outcomes include attendance rate, program completion rate, and changes in weight-status improvement, defined as change in weight and BMI for adults and change in BMI z-score for children. We hypothesize that the intervention arm will have better weight-related outcomes than the control arm. Study completion is anticipated in 2017, after the enrollment of approximately 150 families.
Conclusions
The study aim is to evaluate the effects of PATs on weight-related outcomes in overweight children and parents participating in a CBFLI. The results will be important for determining whether wearable devices are an effective addition to weight loss interventions for overweight and obese children.
Keywords: Childhood obesity, Fitbit, physical activity, activity monitor, family-based intervention, wearable devices
Introduction
Childhood obesity continues to be a global public concern, with rates of childhood overweight and obesity rapidly rising, and in some cases tripling, in low-, middle-, and high-income countries [1–3]. New evidence suggests that this rise is plateauing in the US, but the problem is still substantial: 16.9% of children ages 2–19 are obese, and 31.8% are overweight or obese, despite major national public health efforts [4–10]. Overweight and obese children are at higher risk of remaining obese throughout their lives, have difficulty losing weight through traditional measures such as dietary change and physical activity, and have lower quality of life in terms of psychological and social health [11–13].
Certain groups of children are at particularly high risk of childhood obesity. Latino and Black children have a higher risk of obesity and related health problems [5,14]. Children from lower socioeconomic households have a 3.4–4.3 times higher rate of obesity than children from more affluent households [15]. In California, the income disparity in obesity prevalence is twofold in magnitude among children and adolescents, according to The National Health and Nutrition Examination Survey [16].
There is fair to good evidence that medium- to high-intensity comprehensive behavioral family-lifestyle intervention (CBFLI) programs result in the greatest, most sustained weight loss for children [17,18]. CBFLI involves the child and at least one caretaker, and addresses all three major areas of weight-loss interventions: dietary intake, physical activity (PA), and behavioral strategies [19]. Educating and engaging the caretaker enables changes in the home environment, which facilitates child weight-loss or stabilization. Due to their comprehensive nature, CBFLI programs can be expensive to run and difficult to implement in low-resource settings, putting them out of reach of low-income families.
A key component of pediatric weight loss programs in general, and CBFLIs in particular, is parent involvement. Parenting style and practices, family functioning, and family communication have all been linked to pediatric obesity [20–24]. Parents are crucial partners in the treatment of childhood obesity – they are role models, authority figures, providers, and they create the home environment that influences the energy balance and dietary composition of children [25,26]. Parenting interventions in early childhood can decrease the risk of obesity later in life [27–29]. And while parenting-only interventions have in general not been successful as treatment for childhood obesity [30,31], parent-targeted obesity interventions have delivered positive, long-lasting outcomes [25]. Taken together, the literature underscores the primacy of the parent in pediatric weight loss interventions.
Objectively measured physical activity (OMPA) methods, such as accelerometry and doubly labeled water, are more accurate and precise than subjective self--reports [32,33]. The use of accelerometers, or personal activity trackers (PAT), has been validated in adults and children as both feasible and effective in measuring PA [34]. Not enough is known, however, about whether PATs are effective in the management of childhood obesity [35]. In this study, we incorporate OMPA via PAT into BodyWorks (BW), a national CBFLI program at an urban federally qualified health center (FQHC). Uniquely, we provide the PAT intervention to the parents in the family, as well as the child.
This randomized, controlled trial is designed to 1) establish a baseline of enrollment, attendance, completion, and weight outcomes for BW, and 2) evaluate the effects of personal activity trackers on weight-related outcomes in overweight children and their parents. The primary study hypothesis is that children of families utilizing PAT will have improved weight-related outcomes, defined as either a stabilization or decrease of BMI z-scores, over the course of their participation in the BW program. Our secondary hypothesis is that participating parents who use PAT will have better weight-related outcomes at the end of the program. The trial will be open for 24 months. Herein we describe the design, rationale, and challenges related to this study.
Materials and Methods
This study was funded by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)-supported Academic Pediatric Association (APA) Research in Academic Pediatrics Initiative on Diversity (RAPID) program (NIH R25DK096944). BW is currently in its third year at our institution. We anticipate enrolling patients for 18–24 months (July 2015 – June 2017).
Conceptual framework
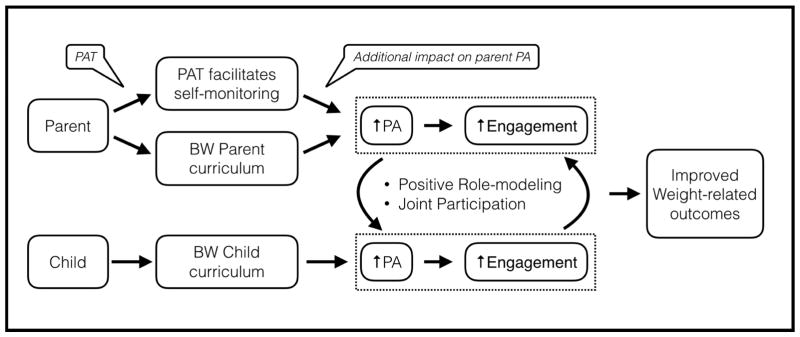
Physical Activity is an important part of both healthy lifestyle and weight loss programs [26]. Self-monitoring is an effective behavior change technique [36,37], and has been demonstrated to increase PA in adults [38,39]. Digital PATs enable self-monitoring of PA while eliminating the burden of manual data entry. Parents enrolled in BW can use PATs to self-monitor PA, which should lead to increases in PA. This increase in parent PA leads to better role modeling for their children and better engagement in the program. These should have an overall impact in the child’s PA and engagement. Joint participation and early successes will positively feedback to both parent and child, leading to overall improved weight-related outcomes for both members of the dyad (Figure 1).
Figure 1.
BW+PAT intervention conceptual framework. PAT= Physical activity monitor, BW= BodyWorks, PA= Physical Activity.
Human subjects
The Institutional Review Board of Children’s Hospital Los Angeles and AltaMed approved this study. Prospective participants were provided a thorough description of the protocol in either English or Spanish. Written consent and/or assent where appropriate was obtained from all participants.
Study setting, population, and enrollment
Setting
AltaMed at Children’s Hospital Los Angeles (AltaMed@CHLA) is an FQHC that serves as the medical home for a large, urban, medically underserved patient population. We care for approximately 21,000 children and provide upwards of 85,000 patient visits a year. Almost 90% of patients are insured by Medi-Cal, California’s Medicaid program.
The BodyWorks Program
Our clinic has three different weight-loss intervention programs predicated on the family’s readiness for change. BW is the only CBFLI program offered in the primary care setting for overweight and obese children and their families. Primary care providers refer patients and their families based on family needs and motivation level. BW is typically selected by those with an interest in group participation, ability to commit two hours per week for seven weeks, and have multiple overweight children in one family. Once patients are referred, they are contacted by the program coordinator, who gives the family an overview of the program, explains the time commitment, and verifies the family’s intent to participate. If the family is still interested, they are enrolled in the next available cycle of BW; the wait time is typically 2 months. The program is offered at no cost to the family.
Population of BodyWorks patients
BW is available to all overweight children seen in the AltaMed@CHLA primary care clinic. Overweight is defined as a BMI ≥85th percentile for age and gender. The adult enrollee may be a parent or other influential adult in the child’s life. There are no age, developmental or health restrictions for participating in BW, except to the extent that they affect the child’s ability to participate in the program.
In addition to the primary child and adult enrollees, additional enrollees are permitted and encouraged. All siblings, whether patients of the clinic or not, are eligible to participate in BW. Other adults connected to the primary enrollee may also participate, including relatives, family friends, or parental friends. No other children beyond the primary enrollee’s siblings, however, are permitted. Additional enrollees are generally 6 years or older to participate, engage, and tolerate the duration of the BW programs, but accommodations are made for families for whom child care is a barrier to participation.
Study Design, Randomization, Blinding, and Addressing Bias
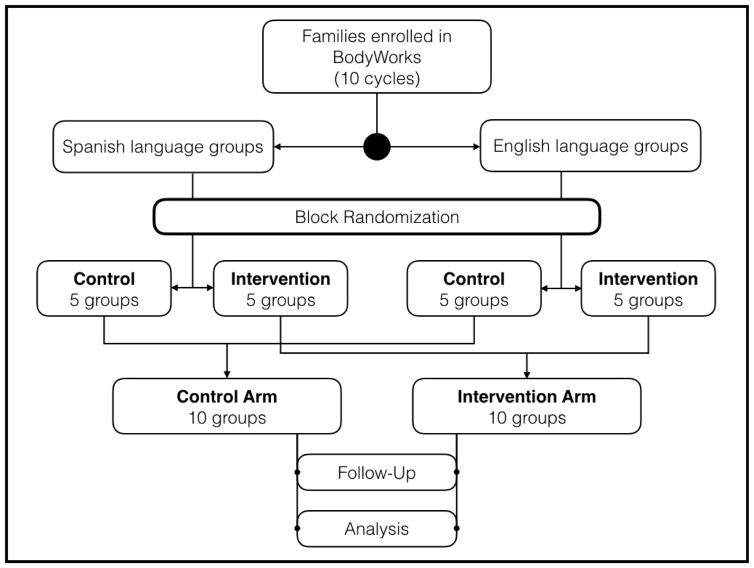
This is a prospective, open-label randomized controlled trial that follows the CONSORT reporting guidelines (Figure 2) [40].
Figure 2.
CONSORT flow diagram.
There are two groups within every BW cycle, conducted on two separate nights. The Monday group is taught in Spanish and the Wednesday group is taught in English. This is based on staff availability. Patients and families choose their BW session based on convenience and language preference. To ensure balanced allocation of language groups and prevent in-group informational and behavioral cross-contamination, block randomization was used to assign whole groups to either the intervention or control arms of the study. Hence in cycles 1, 3, 5, 7, and 9, the English-preference group receives the intervention, while in cycles 2, 4, 6, 8, and 10, the Spanish-preference group receives the intervention.
Once in a group, families are asked to attend all 7 sessions on the same night to reduce cross-contamination and maintain group cohesion. When a family has a scheduling conflict in a given week, however, they can attend the alternate night group. This is to ensure that they receive the full BW curriculum. Aside from the intervention (described below), the curriculum is identical for both groups.
Because the intervention involves wearing a device, there is no practical way to blind participants or providers. The data is blinded to the data analysis team via a randomly assigned 7 study identifier to minimize bias. Participants are instructed to limit potential co-interventions by refraining from herbal supplements or other “weight-loss” supplements/medications. They were also instructed to not purchase any other PAT during the 7-week period of the study.
Control Arm: BodyWorks with self-reported physical activity (SRPA)
Control participants in BW completed a paper SRPA journal each week. The physician provides personalized feedback about their physical activity to each family based on the contents of the SRPA journal.
Intervention Arm: BodyWorks with PAT + SRPA
Intervention participants are given a Fitbit Flex PAT in addition to a paper SRPA journal. During each week’s class, data are downloaded from PATs to a clinic computer. Charts showing the prior week’s daily steps and daily active minutes from the Fitbit PAT software are printed and given to participants with PATs. The physician provides personalized feedback on activity based on the combined information from the SRPA journal and PAT charts.
Study Recruitment, Enrollment and Eligibility
Participants consist of overweight children and their family members who are enrolled in the AltaMed@CHLA BW. Enrollment began in July 2015 and is projected to continue through June 2017. Families are approached to participate in the study during their first session of BW. Because new families can join the group up to and including session 3, we actively recruit during the first 3 sessions. Families that join after week 3 are not approached to participate. All parents are approached to enroll in the study, whereupon the study goal, design, procedure, and incentives are explained. Bilingual study assistants are available to answer any questions. Incentives are provided at completion of the program and return of the PAT. Program completion is defined as attendance to 4 of 7 weekly BW sessions, including the final session. This definition corresponds with the threshold of involvement at which point behavioral changes have been observed based on the experience and opinion of the BW team. Study participants are eligible for a $15 gift card upon program completion. Participants with PATs receiv a $50 gift card per family upon PAT return.
Inclusion criteria were kept broad and exclusion criteria were kept to a minimum so that our study would be reflective of the actual clinical program. We enroll families that meet the following eligibility criteria: the primary child is 7–18 years old, has a BMI ≥85% for age and gender, and has at least one parent or caretaker able to attend the sessions with the child. BW patients are excluded from the study if they are <7 years old, unable to wear a PAT as described in the study protocol, unwilling to wear a PAT, or unwilling to participate in the study. All adult participants in the intervention arm received a PAT. Children in the intervention arm had to be ≥13 years old in order to receive a Fitbit per federal regulations. If not, only the parent received a Fitbit.
Personal Activity Trackers (PATs)
Fitbit® (Fitbit, San Francisco, CA, USA) has been used in multiple studies of adults and has been shown to be reliable, economical, easy to use, and to provide substantial amounts of data [41–43]. In one study, Fitbits were shown to have mean absolute percent error values (computed as the average absolute value of the group-level errors) of 10%, compared with a research grade portable metabolic system [41]. In contrast, self-reported PA questionnaires have limited reliability and validity [44]. Although there is no clear consensus at this time, more recent literature suggests that wrist-worn PATs should be worn on the non-dominant hand for up to 24 hours a day [33].
Sixty Fitbit Flex PATs were purchased for the study and were loaned to the intervention-arm participants for the 7-week duration of each program cycle (MSRP $79 as of October 2016). PATs are assigned to intervention groups at the beginning of the program during the first lesson, whereupon participants were instructed on PAT use, including charging and usage practices. Specifically, participants were instructed to wear PAT devices on the wrist of the non-dominant arm for a minimum of 20 hours per day. Participants were informed that they should remove PATs for certain activities that may damage the device, such as showering and swimming.
Fitbit Flex PATs are charged using a device-specific USB charging cable, which was provided to intervention participants with a USB-to-outlet converter along with the PAT. The data from the PATs may be downloaded wirelessly using either a USB cable connected to a computer or via Bluetooth 4.0 to many iOS, Android, and Windows mobile devices using the Fitbit application [45]. As many BW participants do not own compatible mobile devices, PAT data was downloaded weekly during each BW session. Although participants were asked to not download the Fitbit mobile application, BW staff permitted and aided those with compatible mobile devices with installation and configuration of the Fitbit application. Participants were then asked to share data from their personal Fitbit account with the study team through the HexCare platform (HexCare, Los Angeles, CA, USA).
Data Collection
A BW nurse records anthropometric measurements onto paper forms at the beginning of every BW session and include weight, height, blood pressure, heart rate, and BMI. Weight is recorded using one of three clinic HealthOMeter Professional 2500 KL (500) scales (Pelstar, McCook, IL, USA) to the nearest 0.01 kg. Participants are weighed in light indoor clothing without shoes. These scales are calibrated weekly using the same technique. Height is measured without shoes to the nearest 0.1 cm using a wall-mounted Seca Height-Rite model 225 stadiometer (Seca, Chino, CA, USA). BMI is calculated for each session using the following formula: [patient weight (kg)/height2 (m2)]. Heart rate and blood pressure measurements are taken using a Welch Allyn Spot Vital Signs LXi device (Welch Allyn, Skaneateles Falls, NY, USA). Measurements are taken at rest while the patient is in a seated position with the back supported, feet flat on the floor, and arm at heart level.
After measurement of resting heart rate and blood pressure, participants are asked to perform a 3-minute step test during the first and last sessions. A BW staff member demonstrates the step cadence and times participants, who are asked to vigorously step on and off an aerobics block with alternating steps for three minutes. Within 15 seconds of completion, repeat heart rate and blood pressure measurements are taken.
Clinic staff enter data from the paper forms into the medical record. Study personnel enter data into an electronic database on a weekly basis. The presence of recorded anthropometric data serves as a record of attendance.
PA is assessed using a standardized SRPA form (Appendix 1) and, in the intervention group, with a PAT. The SRPA form is available in English and Spanish and prompts participants to log activity and duration each day of the week. Patients fill out the form at each session based on the previous week’s activity. For intervention participants, minutes of activity and number of steps were downloaded from each PAT and grouped into 24-hour blocks corresponding to each calendar day.
Parent and child attitudes, knowledge and perspective on diet, nutrition, and PA were measured using the BW standard entrance and exit surveys, administered at the first and last sessions, respectively. Child sleep was assessed using the Children’s Sleep Habits Questionnaire (abbreviated) (CSHQ-A) during the first and last sessions [46,47].
PAT satisfaction was measured using a 5-point Likert scale to assess 1) PAT comfort (“The Fitbit was comfortable to wear”), 2) ease of charging (“The Fitbit was easy to charge”), 3) enjoyment (“I enjoyed using the Fitbit”), 4) motivation to do more activity (“I was motivated to do more activity by wearing the Fitbit”), 5) utility of weekly reports and feedback (“The weekly Fitbit reports and feedback were useful for me”), and 6) consideration of PAT purchase (“I will consider getting my own Fitbit, or a similar device, in the future”).
Families are scheduled for monthly follow-up visits for 6 months post-program completion. BW staff calls families to remind them of upcoming appointments. Data for the 3- and 6-month follow up visit is collected via chart extraction using the electronic medical record. If a patient does not attend follow-up visits, the height, weight, and BMI recorded from any available encounter within one month of the follow-up interval will be used used. The complete data collection schedule is shown in Table 1.
Table 1.
Data collection schedule. Entries marked with an asterisk (*) indicate data is retrieved from medical records.
| Meeting | Anthropo metrics | 3-minute step test | Activity log | Entry survey and demographics | Sleep survey | Exit survey and PAT satisfaction |
|---|---|---|---|---|---|---|
| Orientation | X | X | ||||
| Week 1 | X | X | X | X | ||
| Week 2 | X | X | ||||
| Week 3 | X | X | ||||
| Week 4 | X | X | ||||
| Week 5 | X | X | ||||
| Week 6 | X | X | ||||
| Week 7 | X | X | X | X | X | |
| 3 month follow up | X* | |||||
| 6 month follow up | X* |
Description of BodyWorks
Curriculum
After several years of formative research, the Office of Women’s Health of the Department of Health and Human Services (DHHS) launched BW in 2006 as a response to the obesity epidemic in the US, and subsequently updated the content in 2010 and 2012, based on new research [48]. The evidence-based dietary and PA recommendations originate from the 2010 Dietary Guidelines for Americans and 2008 Physical Activity Guidelines for Americans, respectively [49,50]. The sessions were designed using components of several validated theories of learning and behavior change, including the Transtheoretical Model, Social Cognitive Theory, Motivational Interviewing, and Adult Learning Principles [51–53]. BW has undergone several single- and multi-site evaluations by the DHHS, typically with positive results [54]. Other institutions have also published their experience using the BW curriculum.
BW intervenes in three key areas: diet, exercise, and behavioral change. Most importantly, the program requires that a parent or other influential adult attend the program with the primary child, under the theory that adults must be willing and invested in enacting changes in the home for children to successfully lose weight. In response to participant feedback and our experience, AltaMed@CHLA runs a modified, 7-week protocol instead of the 8-week standard. Additionally, we have modified the curriculum to promote in vivo activities with trained session leaders. Notably, we have incorporated grocery store tours that include a produce exhibit where children select produce to taste test.
Class Schedule
The 8-week program consists of an orientation and seven classes. The orientation occurs the weekend prior to the first class and lasts approximately 3 hours. During orientation, participants are informed about the study, questions are answered, and consent obtained. Families complete the BW entrance survey, a demographics survey, and the initial CSHQ-A (Appendix 1). Baseline anthropometric data and 3-minute step test are recorded for all participants. Each class lasts approximately 2 hours, and meets once a week. A typical class schedule is described in Table 2.
Table 2.
Class schedule.
| Time | Activity |
|---|---|
| 4:30pm (30 min) | Clinic check-in, anthropometric data, and physician consult |
| 5:00pm (30 min) | Physical activity and exercise education |
| 5:30pm (30 min) | Snack and nutrition education |
| 6:00pm (60 min) |
|
Sessions largely adhere to the BW standard, but are condensed to accommodate the shorter program duration as well as new topics added at the discretion of the BW instructor. Topics covered include nutrition, PA, stress, sleep, body image, bullying, the role of the media, and how to continue lessons learned. An overview of the curriculum schedule with each week’s focus is listed in Table 3.
Table 3.
Weekly curriculum schedule.
| Week | Weekly curriculum focus | Details |
|---|---|---|
| 1 | Healthy Weight, Healthy Habits |
|
| 2 | Basics of Healthy Eating |
|
| 3 | Portions, Snacks, and Fast Foods |
|
| 4 | Meal Planning and Preparation |
|
| 5 | Physical Activity and Screen Time |
|
| 6 | Cooking, Shopping, and Eating Together |
|
| 7 | Review, Keeping It Up, Media Influences, and Graduation |
|
Bullying is a traumatic experience that many families discuss when body image is taught. If bullying arises prior to that session, program leaders follow up by phone with the family to provide additional support and linkage. When appropriate, these patients are referred to individual mental health services through AltaMed@CHLA.
Follow-up Visits
Once the program is completed, families continue follow up with their primary care provider. In addition, families are offered the opportunity to come back for monthly BW check-in visits. During these visits, families speak one-on-one with BW staff, and participate in an hour-long BW group session to discuss successes, struggles, and strategies to continue a healthy lifestyle. If families request additional support, BW staff connects them to other community programs, or participants may attend another cycle of BW. In the latter scenario, only the first cycle of participation is included in this study.
Outcomes and Analysis Plan
The primary hypothesis is that, compared with controls (BW alone), children in the intervention arm (BW+PAT) will experience better weight-related outcomes, defined as BMI z-score decrease or stabilization versus increase. As a secondary hypothesis, we expect parents in the intervention arm will also experience better weight-related outcomes compared to controls. The analysis will be based on intention-to-treat principles. The primary outcome will be assessed at program completion. A pediatric z-score calculator will be used to calculate z-scores for children 2–19 years of age [55]. The most commonly reported adult weight loss metrics for non-surgical interventions are absolute weight lost (ΔKg, calculated as initial weight in Kg – final weight in Kg), BMI units lost, (ΔBMI, calculated as initial BMI – final BMI), and percent weight loss (%WL, calculated as [initial weight – final weight]/initial weight x 100) [56–58].
A mixed ANOVA will be conducted for the primary analysis to examine the changes from the pretest to the posttest to three months follow-up and the differences between the intervention and control groups. Potential covariates may include age, gender, race/ethnicity, income per household, federal poverty level, and limited English proficiency
Intergroup differences will be assessed for baseline-to-follow-up changes in blood pressure, heart rate, and 3-minute step test post-recovery heart rate. SPSS will be used for all analyses.
Descriptive statistics will be used to report other study outcomes, including demographics, sleep behavior, attitudes and knowledge regarding nutrition and PA, overall satisfaction with PAT use, and associations between parent activity levels and child weight-status outcomes.
Power and Sample Size
To address the primary study outcome at baseline, intervention completion, and 3 months post-intervention, assuming a medium effect size of f=0.25, with an a priori alpha of 0.05, a power of 0.80, two groups, and three measurements, and using a mixed ANOVA, a sample size of at least 86 parent-child dyads is needed [59]. Accounting for up to 20% attrition, a minimum sample size of 104 parent-child dyads (or 52 per group) is needed. We plan to enroll a total of 150 parent-child dyads or about 15 per cycle, with a total of 10 cycles.
The effect size is estimated based on clinical significance. Healthy weight loss for adults is typically described as ~0.5–1 kg/week by the CDC and the DHHS [53,60,61]. Because child weight and growth velocity vary greatly by age, there are no standardized recommendations for weekly weight loss. Common practice targets no weight gain or, for older children, 1% of body weight/week or 0.5 kg/week, whichever is smaller.
Discussion
Adult studies have assessed the utility of Fitbit and other PATs in a variety of contexts, including weight loss, with mixed but promising results [38,62,63]. There are far fewer studies evaluating PATs in children. A 2016 systemic review by Ridgers et al [35] found just 5 studies that examined the feasibility and effectiveness of PATs as tools to increase PA in youth age 5–19 years. All 5 were relatively small (n=6 to 87) and none of these studies looked at weight-related outcomes. Our study has a series of unique features, including 1) the use of PATs within a large, rigorous pediatric CBFLI program, 2) the inclusion of parents as PAT users, and 3) the use of digital health interventions with low-income, minority populations.
Given the high prevalence of obesity and its disproportionate impact on underserved communities, there is an urgent need to implement and evaluate weight-management interventions for these high-risk populations. As financial, clinical, and temporal resources are limited, improving the effectiveness of existing weight loss interventions is valuable from a personal, professional, and societal perspective. As noted by the Children’s Health Fund, mobile health technologies enable new care models, and such disruptive innovations have the potential to reduce pediatric health disparities [64]. Technology as an adjunct to behavior change may improve weight loss, but the evidence base is still growing, and the underlying mechanisms are still being explored.
Challenges and solutions
The BW program and this study have evolved in response to participant needs, interests, and characteristics.
Challenges related to the BodyWorks program
As noted previously, the AltaMed@CHLA BW program was a shortened variation of the standard BW program. Due to significant participant dropout with an 8-week program length, BW was shortened to 7 weeks. In condensing the curriculum, however, addressing important topics was difficult, especially with the addition of new health topics. In practice, this manifested as difficulty staying within each class meeting’s time limits. The BW program adapted by focusing on operational improvements for more efficient sessions and training staff to ensure a positive participant experience. For example, nutrition and occupational therapy (OT) students were given explicit instructions for class session and role-playing activities days in advance. Before, students were given instructions the day of class sessions; the new structure resulted in greater student familiarity with class activities. Additionally, front desk staff and medical assistants were trained to emphasize good patient interaction and bedside manner. Obtaining consent was also shifted to the orientation session so that there was more time to complete the educational activities, as the process can be time-consuming.
By adjusting not only lesson content, but also how it was delivered, BW improved the overall curriculum to better engage participants. For example, participants showed little enthusiasm for the BW video, preferring in vivo activities during the sessions. In response, BW provided the video as a DVD in each participant’s program packet, and utilized the time instead for a grocery store tour. This tour was possible due not only to the reallocated time, but also due to the relationship developed between the BW team and grocery store management. The store was adjacent to CHLA, enabling the team to seamlessly incorporate the activity after didactic lessons. Additionally, management at the grocer accommodated activities that engage and excite the children, offering children opportunities to select produce to taste test in a produce exhibit. This encouraged participants to try new, healthy food options. BW also aims to enroll several families per group so as to promote group unity, cohesion, and accountability.
High coordinator turnover
The involvement of nutrition and OT student interns presented its own challenges in program continuity and consistency. OT student interns were actively involved in teaching BW during the school year, and could obtain class credit. During the summer, however, they conducted fieldwork, limiting their involvement in BW and restricting expertise in play-based learning. Additionally, nutrition interns were only involved with BW for several weeks. Each cohort of interns was trained for 2 hours prior to involvement, resulting in a relatively frequent, recurring time commitment from the BW lead.
Challenges related to serving an underserved population
BW participants came from predominantly low socioeconomic status families, which may have been a marker for decreased access to and familiarity with technology. Many of the participants did not own computers, nor had regular access to one, and some did not have smartphones. As a result, they were unable to use the Fitbit smartphone or desktop computer application to sync and review their activity data. For this reason, we synced Fitbits for all users once a week during class and provided them access to their data. This was a different user experience than intended by the manufacturer, and indeed from others who did have access to the necessary pairing technology. Over the course of the year, however, we noted that more and more families had smartphones, so future iterations of the intervention will incorporate allowing participants to sync Fitbits to their personal devices.
Although the BW program is a mature weight-management program that operates outside of the study, it continues to struggle with participation. It is estimated by the program lead that only 25% of contacted patients appear for the first class. Additionally, although classes are scheduled to occur outside of standard business hours, some parents had irregular work hours and were unable to attend the same weekday session from week to week. BW was first and foremost a community-based intervention meant to support these families, so every attempt was made to accommodate this variability in schedule even at the expense of clear segregation and blinding between the intervention and control groups.
Challenges related to PAT usage
Improper usage and loss of PATs is observed among some participants. Adult participants lose or damage PATs during work or everyday life. To date, our 60 devices have been loaned to participants 101 times; 1 has been lost; 3 have been damaged; and 14 are accounted for but have not been returned to program coordinators. Additionally, some participants have difficulty wearing PATs as instructed or keeping PATs charged. Gaps in PAT data typically indicated a lack of charging, and in such instances, the participant is typically unaware that the PAT was inactive. The frequency of such behavior is being recorded, and is an interesting area for future study to further understand the mechanism by which PATs interact with behavior. It is likely that if patients had access to their PAT data on mobile devices, they may have been more aware of whether the PAT was charged or not. Unfortunately, funding mobile devices for participants is beyond the scope of this study.
Legal restrictions on pediatric research
The active intervention was limited to children ≥13 years old due to factors outside of the control of the BW team. Federal law in the US prevents the creation of online accounts for children < 13 years old without parental permission [65]. Although the Children’s Online Privacy Protection Act (COPPA) has been amended since its original passage in 1998, it still requires parental permission for certain online registrations and activities, and Fitbit policies prohibit the creation of accounts for children < 13 years old [66,67]. This regulation creates some additional difficulties in studying any technology with an online component in a pediatric population. The interpretation and enforcement of the terms of use by manufacturers and research institutions, however, has been highly variable, with several published studies reporting the use of Fitbit and other devices in children < 13 years old [35,68,69]. Until there is further clarification of this law, researchers, IRBs, and manufacturers must continue to clarify roles and agreements on a study-by-study basis.
Our institution has decided that COPPA applies to this and other device studies, which required that our original study design (having all parents and all children, regardless of age, use PATs) be amended after obtaining funding and IRB approval to limit device use to children 13 years of age or older. While this creates some heterogeneity in the intervention group (some parent-child dyads have two devices users while other have one), because we believe the primary mechanism of action of PATs is through the parent, it is reasonable to analyze all intervention families as one group. Over time, a sufficiently large n may allow us to conduct sub-group analyses to evaluate the impact of one vs. two PAT users within the parent-child dyad.
Future directions
Our conceptual framework is informed by theory and anecdotal experience, but lacks empiric evidence. We intend to conduct additional qualitative research to better understand how PATs affect weight loss-related behavior change in this patient population. Our current study limits the participants’ ability to track their own data to just using the LED display on the device; future studies will give full, real-time data access to participants, thereby enabling comprehensive self-monitoring. Expanding to other sites and enrolling different populations would improve the generalizability of our intervention. As digital health interventions proliferate, several studies and clinical programs have started combining multiple modalities: wearable devices, text messaging, mobile applications, etc. We hope to study whether a more comprehensive “digital ecosystem” may have a larger effect than just a single device. Understanding if there is a “dose effect” with technology interventions will be valuable for intervention design and budget allocation, particularly in resource-limited settings. Finally, working closely with our institution, manufacturers, and legal counsel, we hope to arrive at a reasonable approach to studying digital health interventions in pediatric populations that is both in keeping with federal regulation and reflective of real world use.
Conclusions
Pediatric weight management is a complex and multidisciplinary endeavor, and there is an array of new, affordable technologies that may augment and improve existing clinical programs. Although research in the efficacy of these technologies is still early, it is important to ensure that researchers and clinicians consider how they may be evenly applied to all patients, including the underserved. Working with this population entails its own unique challenges regarding resource availability, technology familiarity, and language barriers. As the existing BW program demonstrates, however, proper preparation and staffing enable programs to operate effectively. The BW study has implications for the use of PATs in conjunction with weight-management programs as objective measures of activity as well as potential sources of motivation, which may influence program completion rates and weight-related outcomes. With our planned sample size, age range, and patient diversity, we are well positioned to gain valuable insights regarding the effects of PATs on weight outcomes for overweight children enrolled in a weight-management program at an FQHC.
Supplementary Material
Figure 1: Intervention conceptual framework
Figure 2: CONSORT flow diagram
Table 1: Data collection schedule
Table 2: Class schedule
Table 3: Weekly curriculum schedule
Appendix 1: Collected surveys
Acknowledgments
The authors would like to acknowledge Aric Ponce, Lisa Lopez, Audrey Lai, Sneha Annamaneni, and Monica Thukral for their participation in the BodyWorks program and data collection efforts. We would also like to acknowledge the Academic Pediatric Association for the creation and ongoing support of RAPID, without which this project would not have been possible.
Abbreviations
- BW
BodyWorks
- CBFLI
Comprehensive Behavioral Family Lifestyle Intervention
- PAT
Personal Activity Tracker
- PA
physical activity
- SRPA
Self Reported Physical Activity
- OMPA
Objectively Measured Physical Activity
- CSHQ-A
Children’s Sleep Habits Questionnaire (Abbreviated)
- RAPID
Research in Academic Pediatrics Initiative on Diversity
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- FQHC
Federally Qualified Health Center
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Juan Espinoza, Email: jespinoza@chla.usc.edu.
Alexander Chen, Email: chen190@usc.edu.
Jazminne Orozco, Email: jaorozco@chla.usc.edu.
Alexis Deavenport-Saman, Email: adeavenport@chla.usc.edu.
Larry Yin, Email: lyin@chla.usc.edu.
References
- 1.World Health Organization. Global status report on noncommunicable diseases 2010. Geneva: 2011. http://whqlibdoc.who.int/publications/2011/9789240686458{_}eng.pdf. [Google Scholar]
- 2.de Onis M, Blössner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92:1257–1264. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- 3.Finkelstein EA, Trogdon JG. Public health interventions for addressing childhood overweight: analysis of the business case. Am J Public Health. 2008;98:411–5. doi: 10.2105/AJPH.2007.114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dietz WH. Are we making progress in the prevention and control of childhood obesity? It all depends on how you look at it. Obesity. 2016;24:991–992. doi: 10.1002/oby.21518. [DOI] [PubMed] [Google Scholar]
- 5.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lytle LA. Dealing with the childhood obesity epidemic: a public health approach. Abdom Radiol. 2012;37:719–724. doi: 10.1007/s00261-012-9861-y. [DOI] [PubMed] [Google Scholar]
- 7.Let’s Move. [accessed November 30, 2016];2011 http://www.letsmove.gov/
- 8.National Collaborative on Childhood Obesity Research (NCCOR) [accessed November 30, 2016];2016 http://www.nccor.org/
- 9.Khan LK, Sobush K, Keener D, Goodman K, Lowry A, Kakietek J, Zaro S. Centers for Disease Control and Prevention, Recommended community strategies and measurements to prevent obesity in the United States. Morb Mortal Wkly Rep. 2009;58:1–26. http://www.ncbi.nlm.nih.gov/pubmed/19629029. [PubMed] [Google Scholar]
- 10.Committee on Accelerating Progress in Obesity Prevention, Food and Nutrition Board, Institute of Medicine. Accelerating Progress in Obesity Prevention: Solving the Weight of the Nation. National Academies Press; Washington, D.C: 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goisis A, Sacker A, Kelly Y. Why are poorer children at higher risk of obesity and overweight? A UK cohort study. Eur J Public Health. 2016;26:7–13. doi: 10.1093/eurpub/ckv219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buttitta M, Iliescu C, Rousseau A, Guerrien A. Quality of life in overweight and obese children and adolescents: a literature review. Qual Life Res. 2014;23:1117–39. doi: 10.1007/s11136-013-0568-5. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization. Population-based prevention strategies for childhood obesity. Rep. WHO Forum Tech. Meet; Geneva. 15–17 December 2009; Geneva: World Health Organization; 2010. p. 40. http://www.who.int/iris/handle/10665/44312. [Google Scholar]
- 14.Neckerman KM, Bader MDM, Richards CA, Purciel M, Quinn JW, Thomas JS, Warbelow C, Weiss CC, Lovasi GS, Rundle A. Disparities in the food environments of New York City public schools. Am J Prev Med. 2010;39:195–202. doi: 10.1016/j.amepre.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Singh GK, Siahpush M, Kogan MD. Rising Social Inequalities in US Childhood Obesity, 2003–2007. Ann Epidemiol. 2010;20:40–52. doi: 10.1016/j.annepidem.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Fradkin C, Wallander JL, Elliott MN, Tortolero S, Cuccaro P, Schuster MA. Associations between socioeconomic status and obesity in diverse, young adolescents: Variation across race/ethnicity and gender. Heal Psychol. 2015;34:1–9. doi: 10.1037/hea0000099. [DOI] [PubMed] [Google Scholar]
- 17.Oude Luttikhuis H, Baur L, Jansen H, Shrewsbury VA, O’Malley C, Stolk RP, Summerbell CD. Interventions for treating obesity in children. In: Oude Luttikhuis H, editor. Cochrane Database Syst Rev. John Wiley {&} Sons, Ltd; Chichester, UK: 2009. p. CD001872. [DOI] [PubMed] [Google Scholar]
- 18.Whitlock EP, O’Connor EA, Williams SB, Beil TL, Lutz KW. Effectiveness of Weight Management Interventions in Children: A Targeted Systematic Review for the USPSTF. Pediatrics. 2010;125:e396–e418. doi: 10.1542/peds.2009-1955. [DOI] [PubMed] [Google Scholar]
- 19.Janicke DM, Steele RG, Gayes La, Lim CS, Clifford LM, Schneider EM, Carmody JK, Westen S. Systematic Review and Meta-Analysis of Comprehensive Behavioral Family Lifestyle Interventions Addressing Pediatric Obesity. J Pediatr Psychol. 2014:1–17. doi: 10.1093/jpepsy/jsu023. [DOI] [PubMed] [Google Scholar]
- 20.Hennessy E, Hughes SO, Goldberg JP, Hyatt RR, Economos CD. Parent behavior and child weight status among a diverse group of underserved rural families. Appetite. 2010;54:369–377. doi: 10.1016/j.appet.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Darling N, Steinberg L. Parenting style as context: An integrative model. Psychol Bull. 1993;113:487. doi: 10.1037/0033-2909.113.3.487. [DOI] [Google Scholar]
- 22.Chen JL, Kennedy RNC. Family Functioning, Parenting Style, and Chinese Children’s Weight Status. JFN Weight Status J Fam Nurs. 2004;10:262–279. doi: 10.1177/1074840704264021. [DOI] [Google Scholar]
- 23.Cyril S, Halliday J, Green J, Renzaho aMN. Relationship between body mass index and family functioning, family communication, family type and parenting style among African migrant parents and children in Victoria, Australia : a parent-child dyad study. BMC Public Health. 2016:1–10. doi: 10.1186/s12889-016-3394-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Halliday A, Palma JA, Green CL, Renzaho J. The relationship between family functioning and child and adolescent overweight and obesity: a systematic review. Int J Obes. 2014;38:480–493. doi: 10.1038/ijo.2013.213. [DOI] [PubMed] [Google Scholar]
- 25.Golan M, Crow S. Targeting Parents Exclusively in the Treatment of Childhood Obesity: Long-Term Results. Obes Res. 2004;12:357–361. doi: 10.1038/oby.2004.45. [DOI] [PubMed] [Google Scholar]
- 26.Barlow SE. Expert Committee Recommendations Regarding the Prevention , Assessment , and Treatment of Child and Adolescent Overweight and Obesity: Summary Report. Pediatrics. 2007;120:S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 27.Brotman LM, Dawson-McClure S, Huang K-Y, Theise R, Kamboukos D, Wang J, Petkova E, Ogedegbe G. Early Childhood Family Intervention and Long-term Obesity Prevention Among High-risk Minority Youth. Pediatrics. 2012;129:e621–e628. doi: 10.1542/peds.2011-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller AL, Horodynski Ma, Herb HEB, Peterson KE, Contreras D, Kaciroti N, Staples-Watson J, Lumeng JC. Enhancing self-regulation as a strategy for obesity prevention in Head Start preschoolers: the growing healthy study. BMC Public Health. 2012;12:1040. doi: 10.1186/1471-2458-12-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waters E, de Silva-Sanigorski A, Hall BJ, Brown T, Campbell KJ, Gao Y, Armstrong R, Prosser L, Summerbell CD. Interventions for preventing obesity in children. Cochrane Database Syst Rev. 2011:CD001871. doi: 10.1002/14651858.CD001871.pub3. www.cochranelibrary.com. [DOI] [PubMed]
- 30.Gerards SMPL, Dagnelie PC, Gubbels JS, Van Buuren S, Hamers FJM, Jansen MWJ, Van Der Goot OHM, De Vries NK, Sanders MR, Kremers SPJ. The effectiveness of lifestyle triple P in the Netherlands: A randomized controlled trial. PLoS One. 2015;10:1–18. doi: 10.1371/journal.pone.0122240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robertson W, Fleming J, Kamal A, Hamborg T, Khan Ka, Griffiths F, Stewart-Brown S, Stallard N, Petrou S, Simkiss D, Harrison E, Kim SW, Thorogood M. Randomised controlled trial evaluating the effectiveness and cost-effectiveness of “Families for Health”, a family-based childhood obesity treatment intervention delivered in a community setting for ages 6 to 11 years. Health Technol Assess (Rockv) 2017;21:1–180. doi: 10.3310/hta21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilks DC, Besson H, Lindroos AK, Ekelund U. Objectively measured physical activity and obesity prevention in children, adolescents and adults: a systematic review of prospective studies. Obes Rev. 2011;12:e119–e129. doi: 10.1111/j.1467-789X.2010.00775.x. [DOI] [PubMed] [Google Scholar]
- 33.Troiano RP, McClain JJ, Brychta RJ, Chen KY. Evolution of accelerometer methods for physical activity research. Br J Sports Med. 2014;48:1019–1023. doi: 10.1136/bjsports-2014-093546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guinhouya BC, Samouda H, De Beaufort C. Level of physical activity among children and adolescents in Europe: a review of physical activity assessed objectively by accelerometry. Public Health. 2013;127:301–311. doi: 10.1016/j.puhe.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 35.Ridgers ND, Mcnarry MA, Mackintosh KA. Feasibility and Effectiveness of Using Wearable Activity Trackers in Youth: A Systematic Review. JMIR Mhealth Uhealth. 2016;4 doi: 10.2196/mhealth.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, Eccles MP, Cane J, Wood CE. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: Building an international consensus for the reporting of behavior change interventions. Ann Behav Med. 2013;46:81–95. doi: 10.1007/s12160-013-9486-6. [DOI] [PubMed] [Google Scholar]
- 37.Mercer K, Li M, Giangregorio L, Burns C, Grindrod K. Behavior Change Techniques Present in Wearable Activity Trackers: A Critical Analysis. JMIR mHealth uHealth. 2016;4:e40. doi: 10.2196/mhealth.4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evenson KR, Goto MM, Furberg RD. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int J Behav Nutr Phys Act. 2015;12:159. doi: 10.1186/s12966-015-0314-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lewis ZH, Lyons EJ, Jarvis JM, Baillargeon J. Using an electronic activity monitor system as an intervention modality: A systematic review. BMC Public Health. 2015;15:585. doi: 10.1186/s12889-015-1947-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schulz KF, Altman DG, Moher D. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JM, Kim Y, Welk GJ. Validity of Consumer-Based Physical Activity Monitors. Med Sci Sports Exerc. 2014:1840–1848. doi: 10.1249/MSS.0000000000000287. [DOI] [PubMed] [Google Scholar]
- 42.Adam Noah J, Spierer DK, Gu J, Bronner S. Comparison of steps and energy expenditure assessment in adults of Fitbit Tracker and Ultra to the Actical and indirect calorimetry. J Med Eng Technol. 2013;37:456–62. doi: 10.3109/03091902.2013.831135. [DOI] [PubMed] [Google Scholar]
- 43.Montgomery-Downs HE, Insana SP, Bond JA. Movement toward a novel activity monitoring device. Sleep Breath. 2012;16:913–917. doi: 10.1007/s11325-011-0585-y. [DOI] [PubMed] [Google Scholar]
- 44.Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med. 2003;37:197–206. doi: 10.1136/bjsm.37.3.197. discussion 206. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1724653&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fitbit Supported Devices. [accessed November 30, 2016];2016 https://www.fitbit.com/devices.
- 46.Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–1051. http://www.ncbi.nlm.nih.gov/pubmed/11145319. [PubMed] [Google Scholar]
- 47.National Institutes of Health, National Institute of Child Health and Human Development. Vandell DL, Pierce K. Children’s Sleep Habits Questionnaire (Abbreviated) documentation. 2000:1–2. http://www.education.uci.edu/childcare/pdf/instrumental{_}docs/Childrens.
- 48.Office on Women’s Health, BodyWorks. [accessed November 30, 2016];2014 http://womenshealth.gov/bodyworks/index.html.
- 49.U.S. Department of Agriculture, U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7. Washington, D.C: 2010. https://health.gov/dietaryguidelines/ [Google Scholar]
- 50.U.S. Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. Washington, D.C: 2008. http://www.health.gov/paguidelines. [Google Scholar]
- 51.National Institutes of Health, National Cancer Institute. Theory at a Glance: A Guide for Health Promotion Practice. Washington, D.C: 2005. [Google Scholar]
- 52.Prochaska JO, Velicier WF, Rossi JS, Goldstein MG, et al. Stages of change and decisional balance for 12 problem behaviors. Heal Psychol. 1994;13:39–46. doi: 10.1037//0278-6133.13.1.39. [DOI] [PubMed] [Google Scholar]
- 53.National Institutes of Health, National Heart Lung and Blood Institute. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. Bethesda: 1998. https://www.ncbi.nlm.nih.gov/books/NBK2003/pdf/Bookshelf{_}NBK2003.pdf. [Google Scholar]
- 54.Borden VM, Labiner-Wolfe J, Blake SM, Marr A, Rowe J, Wasserman J. BodyWorks: A Parent-Focused Program to Promote Healthful Eating and Physical Activity for Children and Adolescents. J Nutr Educ Behav. 2012;44:192–193. doi: 10.1016/j.jneb.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 55.The Children’s Hospital of Philadelphia Research Institute. [accessed November 30, 2016];Pediatric Z-Score Calculator. 2016 http://zscore.research.chop.edu/
- 56.Millstein Ra. Measuring outcomes in adult weight loss studies that include diet and physical activity: a systematic review. J Nutr Metab. 2014;2014:421413–421423. doi: 10.1155/2014/421423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hatoum IJ, Kaplan LM. Advantages of percent weight loss as a method of reporting weight loss after Roux-en-Y gastric bypass. Obesity. 2013;21:1519–1525. doi: 10.1002/oby.20186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van De Laar A. Bariatric outcomes longitudinal database (BOLD) suggests excess weight loss and excess BMI loss to be inappropriate outcome measures, demonstrating better alternatives. Obes Surg. 2012;22:1843–1847. doi: 10.1007/s11695-012-0736-7. [DOI] [PubMed] [Google Scholar]
- 59.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 60.National Institutes of Health, National Heart Lung and Blood Institute. Aim for a Healthy Weight. Bethesda: 2005. https://www.nhlbi.nih.gov/files/docs/public/heart/aim{_}hwt.pdf. [Google Scholar]
- 61.Blackburn G. Effect of Degree of Weight Loss on Health Benefits. Obes Res. 1995;3:211s–216s. doi: 10.1002/j.1550-8528.1995.tb00466.x. [DOI] [PubMed] [Google Scholar]
- 62.Wang JB, Cadmus-Bertram LA, Natarajan L, White MM, Madanat H, Nichols JF, Ayala GX, Pierce JP. Wearable Sensor/Device (Fitbit One) and SMS Text-Messaging Prompts to Increase Physical Activity in Overweight and Obese Adults: A Randomized Controlled Trial. Telemed J E-Health Assoc. 2015;21:782–792. doi: 10.1089/tmj.2014.0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jakicic JM, Davis KK, Rogers RJ, King WC, Marcus MD, Helsel D, Rickman AD, Wahed AS, Belle SH. Effect of Wearable Technology Combined With a Lifestyle Intervention on Long-term Weight Loss. Jama. 2016;316:1161. doi: 10.1001/jama.2016.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Redlener I, Grant R, Gracy D, Weisman J, Johnson D. The Persistent Health Care Access Gap for Children in Poverty: Will the Health Technology Revolution Level the Playing field? New York: 2015. https://ms01.childrenshealthfund.org/wp/wp-content/uploads/2015/05/WHITE-PAPER-4-22.pdf. [Google Scholar]
- 65.Murse T. Does Facebook Have Age Restrictions? Why You Can’t Sign Up For Facebook, GMail or Yahoo! Accounts if You're Too Young. [accessed November 30, 2016];About.com News. 2013 http://uspolitics.about.com/od/Technology/a/Facebook-Age-Requirement-Set-In-Federal-Law.htm.
- 66.Barr A, Winkler R. Google Is Planning to Offer Accounts to Kids Under 13. [accessed November 30, 2016];Wall Str J. 2014 http://blogs.wsj.com/digits/2014/08/18/google-moves-to-target-kids-under-13/
- 67.Fitbit Terms of Service. [accessed November 30, 2016];2015 https://www.fitbit.com/legal/terms-of-service.
- 68.Meltzer LJ, Hiruma LS, Avis K, Montgomery-Downs H, Valentin J. Comparison of a Commercial Accelerometer with Polysomnography and Actigraphy in Children and Adolescents. Sleep. 2015;38:1323–1330. doi: 10.5665/sleep.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hayes LB, Van Camp CM. Increasing physical activity of children during school recess. J Appl Behav Anal. 2015;48:690–695. doi: 10.1002/jaba.222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure 1: Intervention conceptual framework
Figure 2: CONSORT flow diagram
Table 1: Data collection schedule
Table 2: Class schedule
Table 3: Weekly curriculum schedule
Appendix 1: Collected surveys