Abstract
Introduction
We quantified clinical and imaging characteristics associated with childhood arteriopathy subtypes to facilitate their diagnosis and classification in research and clinical settings.
Methods
The “Vascular effects of Infection in Pediatric Stroke” (VIPS) study prospectively enrolled 355 children with arterial ischemic stroke (AIS) (2010–2014). A central team of experts reviewed all data to diagnose childhood arteriopathy and classify subtypes, including arterial dissection, focal cerebral arteriopathy-inflammatory type (FCA-i, which includes transient cerebral arteriopathy, TCA), moyamoya, and diffuse/multifocal vasculitis. Only children whose stroke etiology could be conclusively diagnosed were included in these analyses. We constructed logistic regression models to identify characteristics associated with each arteriopathy subtype.
Results
Among 127 children with definite arteriopathy, the arteriopathy subtype could not be classified in 18 (14%). Moyamoya (n=34) occurred mostly in children <8 years old, FCA-i (n=25) in 8–15 year olds, and dissection (n=26) at all ages. Vertigo at stroke presentation was common in dissection. Dissection affected cervical arteries, while moyamoya involved supraclinoid internal carotid arteries. A banded appearance of the M1 segment of the middle cerebral artery was pathognomonic of FCA-i, but present in <25% of FCA-i cases; a small lenticulostriate distribution infarct was a more common predictor of FCA-i, present in 76%. It remained difficult to distinguish FCA-i from intracranial dissection of the anterior circulation (FCA-d). We observed only secondary forms of diffuse/multifocal vasculitis, mostly due to meningitis.
Conclusions
Childhood arteriopathy subtypes have some typical features that aid diagnosis. Better imaging methods, including vessel wall imaging, are needed for improved classification of FCA.
Introduction
Approximately 2,500 U.S. children suffer an arterial ischemic stroke (AIS) each year.1 Childhood arteriopathies are the most common identifiable cause of AIS in a previously healthy child, present in up to 64%.2–6 They represent a strong predictor of recurrent stroke, with rates exceeding 30% within 12 months for some arteriopathy subtypes,2,7,8 and published guidelines for prevention of recurrence are specific to type (e.g., dissection, moyamoya, transient cerebral arteriopathy, etc.).9 Nonetheless, childhood arteriopathies remain difficult not only to diagnose but to classify; they are rare but heterogeneous, and MRA imaging, frequently substituted for conventional angiography, is technically limited. Publication of consensus-based definitions of childhood arteriopathy in 2004 (adapted for the VIPS study in 2009) and the development of the CASCADE system in 2012 (which provided a novel approach to classifying the “anatomic site of disease” in childhood AIS) have largely been addressed to pediatric stroke specialists. These tools are less useful for non-experts, however, who are frequently responsible for making timely decisions crucial to the prevention of stroke recurrence. It is this gap that, in large part, we seek to address. In the prospective, international, NIH-funded “Vascular effects of Infection in Pediatric Stroke” (VIPS) study, a four-person team of pediatric stroke experts classified the etiology of 355 cases of pediatric AIS based on rigorous central review of neuroimaging and clinical data. Using this “expert opinion” as the gold standard, the goal of the current analysis was to guide the classification of childhood arteriopathies by non-experts as well as experts by quantifying the prevalence and odds ratios for clinical and imaging biomarkers that were used in the expert review to (1) distinguish arteriopathy from cardioembolism, and (2) distinguish between the most common subtypes of childhood arteriopathy. In other words, we aimed to identify and quantify biomarkers that could allow trained neuroradiologists and neurologists who are non-experts in childhood arteriopathies to generate a reasonable differential diagnosis for a child with a stroke.
Material and Methods
Study Design
Ethics committee approvals were obtained at all sites. From 2009–2014, the VIPS study enrolled 355 children (age 29d–18y) with arterial ischemic stroke at 37 international sites, collected detailed clinical data (e.g., past medical history such as cardiac disease and sickle cell anemia; recent exposures such as infection and head trauma), and performed central review of brain and cerebrovascular imaging (by MW, HJF, GDV and AJB). Details of VIPS methods have been published.12 As a part of the VIPS study, an exhaustive and systematic centralized review of baseline and follow-up vascular imaging and clinical data was performed to first arrive at a diagnosis of arteriopathy, and then classify arteriopathy subtype.13 For this study, we included all children with abnormal vascular imaging that could be definitively classified as due to arteriopathy or cardioembolism.
Imaging Review
In our review of brain parenchymal imaging, we recorded infarct size (using ABC/2),14 laterality, location, acuity, and associated hemorrhage. Vascular imaging was first classified as normal or abnormal, and then completely described with respect to type of abnormality (e.g., hypoplasia, irregularity, banding, stenosis, occlusion, etc.), vascular territories and sides affected, number and type of arterial segments affected and degree of collateral flow. Details of the VIPS imaging review have been published.15
Childhood Arteriopathy Classification
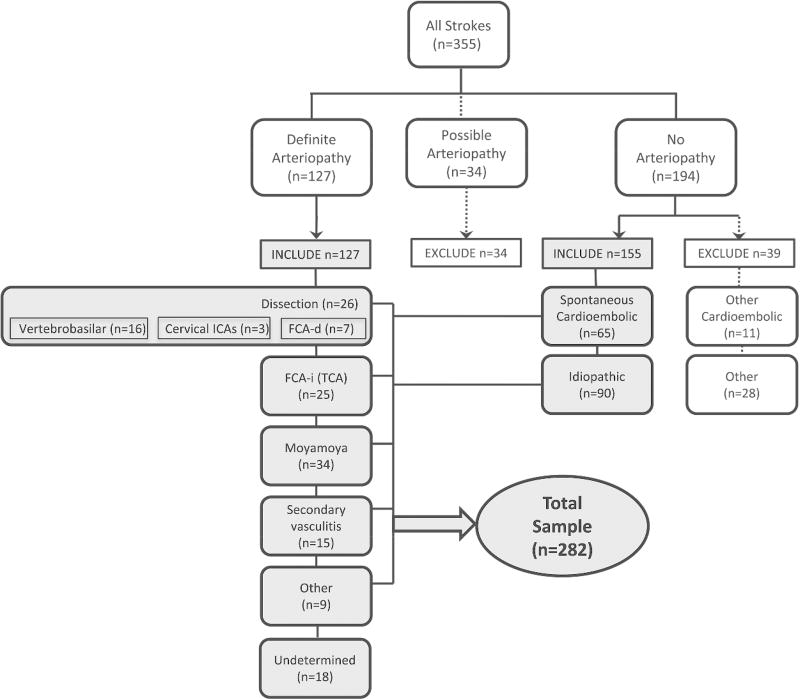
Two primary reviewers (MW, HJF) independently used clinical data and parenchymal and vascular imaging features to determine a diagnosis of either definite, possible, or no arteriopathy (“primary diagnosis”).13 Disagreements were resolved through consensus discussion by the full review team (MW, HJF, GDV and AJB). We defined arteriopathy as “the imaging appearance of an in situ arterial abnormality (stenosis, irregularity, occlusion, banding, pseudoaneurysm, dissection flap) not attributable to an exogenous thrombus (e.g., cardioembolism) and not considered a normal developmental variant.”13 The imaging finding of an isolated arterial occlusion could be classified as “no arteriopathy” (e.g., if the clinical history and/or the parenchymal imaging typified cardioembolism), “possible arteriopathy” (e.g., if the differential diagnosis included both cardioembolism and arterial dissection), or “definite arteriopathy” (e.g., if the imaging was definitive for moyamoya or dissection). The reviewers then classified the arteriopathies into subtypes (“secondary diagnosis”) using pre-established definitions for childhood arteriopathies10,11: arterial dissection, including unilateral focal cerebral arteriopathy-dissection type (FCA-d, further defined below); unilateral focal cerebral arteriopathy-inflammatory type (FCA-i), which includes transient cerebral arteriopathy (TCA); primary and secondary moyamoya (bilateral cerebral arteriopathy of childhood), genetic or syndromic arteriopathies such as PHACE syndrome,16,17 primary and secondary diffuse/multifocal vasculitis, fibromuscular dysplasia,18 iatrogenic, and others. The primary reviewers independently classified the secondary diagnosis; disagreements were resolved through consensus discussion by the full review team. The final conclusion (the “expert opinion” regarding that case’s stroke etiology) constituted the gold standard diagnosis. The diagnoses in the children included in this study are shown in Figure 1.
Figure 1.
Classification of stroke subtype among 355 children with arterial ischemic stroke enrolled in the VIPS study. The cases used for the current study are highlighted in grey.
The original definition of focal cerebral arteriopathy of childhood (FCA) consisted of “stenosis [of intracranial arteries] on vascular imaging not otherwise classified as dissection, moyamoya, sickle cell arteriopathy, post-varicella arteriopathy, vasculitis, or other specific diagnoses (such as post-irradiation arteriopathy)” and included “unifocal or multifocal, unilateral or bilateral lesions of the large and/or medium-sized vessels visualized on angiography.”7 Use of this term has evolved in the pediatric stroke literature, and in North American pediatric stroke centers is typically used to describe a specific angiographic appearance of unilateral stenosis and/or irregularity of the intracranial anterior circulation; it has a differential diagnosis including TCA, intracranial dissection, unilateral moyamoya, and the other diagnoses listed above.19 Hence, we implemented an updated definition of FCA: unifocal and unilateral stenosis/irregularity of the large intracranial arteries of the anterior circulation (distal internal carotid artery [ICA] and/or its proximal branches). FCA-dissection type (FCA-d) referred to intracranial arterial dissection of the anterior circulation, typically in the setting of trauma.20 FCA-inflammation type (FCA-i) referred to FCA that is presumed inflammatory, i.e. thought to represent a focal vasculitis. This could be diagnosed, for instance, because of marked enhancement of the abnormal arterial segment on vessel wall imaging (VWI)21 or preceding varicella zoster infection (if considered clinically relevant by the local pediatric stroke neurologist for a diagnosis of post-varicella arteriopathy).22,23 FCA-i was also diagnosed when the evolution of the arteriopathy was typical of TCA: a stereotyped, monophasic natural history characterized by frequent early progression (over days to weeks), plateau with non-progression by six months, and subsequent improvement in some with complete resolution in a minority.10,24 FCA that could not be further classified was considered “undetermined” (FCA-u) arteriopathy subtype (in which case the reviewers created a differential diagnosis).
Statistical Analysis
The outcome variables for our analyses were the stroke etiology (primary and secondary diagnoses) as classified by the VIPS team. In children with abnormal vascular imaging, arteriopathy must first be distinguished from cardioembolism (primary diagnosis); to this end, we first developed a predictive model for cardioembolic stroke. We then addressed our primary goal, modeling clinical and imaging biomarkers associated with the most commonly diagnosed arteriopathy subtypes (secondary diagnosis): dissection, FCA-i, moyamoya and secondary diffuse/multifocal vasculitis. By design, we evaluated as predictors biomarkers that were used by the reviewers in the classification process; although circular, this allowed the quantification of the prevalence of the biomarker, and the strength of its association with a specific subtype.
For our preliminary model (cardioembolic vs. arteriopathic stroke), we compared 65 children classified as having spontaneous cardioembolism (excluding strokes attributed to cardiac surgery) to 109 with definite arteriopathy (excluding those with “possible arteriopathy”, but including those whose definite arteriopathy could not be further classified) (Figure 1). We first utilized univariate logistic regression models to identify clinical and/or parenchymal and/or vascular imaging characteristics associated either positively or negatively with these two broad categories. We then constructed a multivariable model by entering all predictors significant at the 0.10 level in univariate analysis. Backward-selection logistic regression analysis was used to estimate adjusted odds ratios, with a significance level of 0.05 specified for removal of a variable from the model.
We followed a similar process to create models predictive of each individual arteriopathy subtype. Univariate logistic regression models were first utilized to identify characteristics associated with each subtype individually. For these models, we compared each subtype to the group of all other subtypes combined (excluding the 18 cases with definite arteriopathy that could not be further classified). In addition to calculating odds ratios and 95% confidence intervals for each potential predictor, we determined the frequency with which the predictor was observed within the subtype. We then constructed multivariable models for each subtype as described for the preliminary model, above. All models were assessed using post-estimation techniques, and c-statistics were compared between potential models. Adjustments were made where necessary to improve model fit before a final model was determined. All analyses were done using Stata v14 (Stata Corp., College Station, TX).
Results
All 355 VIPS patients had initial brain vascular imaging—MRA (91%), CTA (24%), and/or conventional angiography (14%); 53% had cervical vascular imaging and 3.9% had VWI. Overall, 41% had at least one follow-up brain vascular imaging study; the last follow-up was a median of 277 days (interquartile range 172, 408 days) post-stroke. Figure 1 demonstrates the results of the stroke subtype classification. Characteristics that distinguish cardioembolism from arteriopathic stroke (with a p-value <0.10 on univariate analysis) are shown in Table 1. Characteristics associated with arteriopathy subtype (with a p-value <0.10 on univariate analysis) are shown Tables 2–4. All the variables tested are shown in Online Tables 1–5. Independent predictors are summarized in Table 5, and shown in detail in Online Table 5.
Table 1.
Cardioembolism: clinical and imaging characteristics that distinguish cardioembolism (N=65) from definite arteriopathy (N=109).*
| Prevalence in Cardioembolic Cases (N=65) |
Prevalence in Arteriopathic Cases (N=109) |
Univariate Analyses | Multivariable Analysis‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| Characteristic | n | (%) | n | (%) | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Demographics | ||||||||||
| Age by category | ||||||||||
| 0–3 years | 29 | (44.6) | 30 | (27.5) | Reference | |||||
| 4–7 years | 8 | (12.3) | 37 | (33.9) | 0.22 | (0.09, 0.56) | 0.001 | |||
| 8–11 years | 7 | (10.8) | 17 | (15.6) | 0.43 | (0.15, 1.18) | 0.10 | |||
| 12–15 years | 11 | (16.9) | 14 | (12.8) | 0.81 | (0.32, 2.08) | 0.67 | |||
| 16–18 years | 10 | (15.4) | 11 | (10.1) | 0.94 | (0.35, 2.55) | 0.90 | |||
| Stroke presentation | ||||||||||
| Any focal signs (vs. non-focal only) | 54 | (83.1) | 101 | (92.7) | 2.67 | (0.96, 7.42) | 0.059 | |||
| Focal signs | ||||||||||
| Ataxia | 4 | (6.2) | 24 | (22.0) | 0.23 | (0.08, 0.74) | 0.01 | |||
| Hemiparesis | 52 | (80.0) | 91 | (83.5) | 0.91 | (0.39, 2.16) | 0.84 | |||
| Dysarthria | 14 | (21.5) | 33 | (30.3) | 0.75 | (0.35, 1.59) | 0.45 | |||
| Aphasia | 12 | (18.5) | 29 | (26.6) | 0.62 | (0.27, 1.45) | 0.27 | |||
| Visual field deficit | 10 | (15.4) | 11 | (10.1) | 1.68 | (0.66, 4.30) | 0.28 | |||
| Non-focal signs | ||||||||||
| Headache | 12 | (18.5) | 40 | (36.7) | 0.47 | (0.22, 1.03) | 0.06 | |||
| Nausea/vomiting | 5 | (7.7) | 28 | (25.7) | 0.26 | (0.09, 0.72) | 0.009 | |||
| Decreased level of consciousness | 20 | (30.8) | 30 | (27.5) | 1.37 | (0.68, 2.75) | 0.37 | |||
| Seizures at presentation | 15 | (23.1) | 27 | (24.8) | 0.97 | (0.47, 2.00) | 0.93 | |||
| Vertigo | 4 | (6.2) | 11 | (10.1) | 0.60 | (0.18, 2.01) | 0.41 | |||
| Diplopia | 1 | (1.5) | 1 | (0.9) | 1.87 | (0.11, 30.6) | 0.66 | |||
| Risk factors or co-morbidities (not mutually exclusive) | ||||||||||
| Cardiac disease | 65 | (100.0) | 6 | (5.5) | <0.0001† | |||||
| Congenital heart disease (CHD) only | 42 | (64.6) | 6 | (5.5) | 101.6 | (17.3, 596) | <0.0001 | |||
| Acquired heart disease (AHD) only | 19 | (29.2) | 0 | |||||||
| Both CHD and AHD | 4 | (6.2) | 0 | |||||||
| Other chronic disorders | ||||||||||
| Sickle cell anemia | 0 | 9 | (8.3) | - | 0.03† | |||||
| Indwelling catheter | 5 | (7.7) | 1 | (0.9) | 9.00 | (1.03, 78.8) | 0.05 | |||
| Acute systemic illness | ||||||||||
| Meningitis | 0 | 11 | (10.1) | - | 0.008† | |||||
| Vascular Imaging Findings | ||||||||||
| Pathologic finding | ||||||||||
| Occlusion | 31 | (47.7) | 67 | (61.5) | 0.57 | (0.31, 1.06) | 0.08 | |||
| Stenosis | 7 | (10.8) | 74 | (67.9) | 0.06 | (0.02, 0.14) | <0.0001‡ | 0.07 | (0.02, 0.28) | <0.0001 |
| Irregularity | 6 | (9.2) | 57 | (52.3) | 0.09 | (0.04, 0.23) | <0.0001‡ | 0.09 | (0.02, 0.34) | <0.0001 |
| Banding | 0 | 7 | (6.4) | - | 0.05† | |||||
| Pathologically affected vessels | ||||||||||
| Arterial segments affected | ||||||||||
| More than one | 24 | (36.9) | 94 | (86.2) | Reference | |||||
| One | 41 | (63.1) | 15 | (13.8) | 10.7 | (5.10, 22.5) | <0.0001 | |||
| Vascular territories affected | ||||||||||
| More than one | 7 | (10.8) | 49 | (45.0) | Reference | |||||
| One | 58 | (89.2) | 60 | (55.0) | 6.77 | (2.83, 16.2) | <0.0001‡ | 6.63 | (1.40, 31.3) | 0.02 |
| Sides affected | ||||||||||
| Bilateral | 6 | (9.2) | 49 | (45.0) | Reference | |||||
| Unilateral | 59 | (90.8) | 60 | (55.0) | 4.08 | (1.57, 10.6) | 0.004 | |||
| Segments affected | ||||||||||
| Supraclinoid ICA | 12 | (18.5) | 68 | (62.4) | 0.14 | (0.07, 0.29) | <0.0001 | |||
| Proximal MCA (M1) | 25 | (38.5) | 86 | (78.9) | 0.17 | (0.08, 0.33) | <0.0001 | |||
| Vertebrobasilar | 7 | (10.8) | 30 | (27.5) | 0.32 | (0.13, 0.77) | 0.01 | |||
| Cervical arteries | 9 | (13.8) | 27 | (24.8) | 0.49 | (0.21, 1.12) | 0.09 | |||
Full list of variables tested shown in Online Table 1.
p-value calculated using Fisher's exact test

Table 2.
Dissection: clinical and imaging characteristics that distinguish arterial dissection (N=26), compared to all other definite arteriopathy subtypes (N=83).*
| Prevalence (N=26) |
Univariate Analyses | Multivariable Analysis‡ | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Characteristic | n | (%) | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Stroke presentation | ||||||||
| Focal signs | ||||||||
| Hemiparesis | 17 | (65.4) | 0.23 | (0.08, 0.70) | 0.01 | |||
| Ataxia | 10 | (38.5) | 2.91 | (1.06, 8.02) | 0.04 | |||
| Non-focal signs | ||||||||
| Headache | 15 | (57.7) | 3.30 | (1.23, 8.87) | 0.02 | |||
| Nausea/vomiting | 13 | (50.0) | 4.96 | (1.86, 13.2) | 0.001 | |||
| Seizures at presentation | 3 | (11.5) | 0.31 | (0.08, 1.13) | 0.08 | |||
| Vertigo | 8 | (30.8) | 11.2 | (2.64, 47.9) | 0.001 | |||
| Risk factors or co-morbidities (not mutually exclusive) | ||||||||
| Acute systemic illness | ||||||||
| Meningitis | 0 | - | 0.06† | |||||
| Head trauma | 10 | (38.5) | 51.3 | (6.13, 428.8) | <0.0001‡ | 162 | (10.7, 3435) | <0.0001 |
| Infarct Characteristics at Baseline | ||||||||
| Location | ||||||||
| Vascular distribution of infarction | ||||||||
| Anterior circulation | 11 | (42.3) | 0.05 | (0.01, 0.15) | <0.0001 | |||
| ACA | 2 | (7.7) | 0.45 | (0.09, 2.13) | 0.31 | |||
| Lenticulostriate | 9 | (34.6) | 0.60 | (0.24, 1.49) | 0.27 | |||
| Superficial MCA | 10 | (38.5) | 0.20 | (0.08, 0.51) | 0.001 | |||
| Anterior choroidal | 1 | (3.8) | 0.62 | (0.07, 5.60) | 0.67 | |||
| Posterior circulation | 12 | (46.2) | 34.7 | (7, 172) | <0.0001 | |||
| Basilar | 3 | (11.5) | - | 0.01† | ||||
| SCA | 7 | (26.9) | 30.2 | (3.51, 260) | 0.002 | |||
| AICA | 1 | (3.8) | - | 0.24† | ||||
| PICA | 9 | (34.6) | 21.4 | (4.25, 108) | <0.0001 | |||
| PCA | 6 | (23.1) | 2.81 | (0.87, 9.04) | 0.08 | |||
| Other location | 0 | 0.34† | ||||||
| Vascular Imaging Findings | ||||||||
| Pathologic finding | ||||||||
| Occlusion | 21 | (80.8) | 3.38 | (1.16, 9.82) | 0.03 | |||
| Stenosis | 14 | (53.8) | 0.45 | (0.18, 1.11) | 0.08 | |||
| Irregularity | 12 | (46.2) | 0.72 | (0.30, 1.75) | 0.47 | |||
| Ectasia or fusiform (pseudo)aneurysm | 3 | (11.5) | 2.03 | (0.45, 9.17) | 0.36 | |||
| Intimal flap or mural hematoma | 3 | (11.5) | - | 0.01† | ||||
| Pathologically affected vessels | ||||||||
| Vascular territories affected | ||||||||
| More than one | 4 | (15.4) | Reference | |||||
| One | 22 | (84.6) | 6.51 | (2.06, 20.6) | 0.001 | |||
| Sides affected | ||||||||
| Bilateral | 4 | (15.4) | Reference | |||||
| Unilateral | 22 | (84.6) | 6.51 | (2.06, 20.6) | 0.001 | |||
| Segments affected | ||||||||
| Supraclinoid ICA | 9 | (34.6) | 0.22 | (0.08, 0.55) | 0.001 | |||
| Proximal MCA (M1) | 10 | (38.5) | 0.06 | (0.02, 0.17) | <0.0001 | |||
| Vertebrobasilar | 16 | (61.5) | 7.89 | (2.97, 20.9) | <0.0001 | |||
| Cervical arteries | 18 | (69.2) | 18.5 | (6.27, 54.6) | <0.000‡ | 29.3 | (5.43, 159) | <0.0001 |
Full list of variables tested shown in Online Table 1.
p-value calculated using Fisher's exact test

Table 4.
Moyamoya: clinical and imaging characteristics that distinguish moyamoya (N=34) compared to all other definite arteriopathy subgroups (N=75)*
| Prevalence (N=34) |
Univariate Analyses | Multivariable Analysis‡ | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Characteristic | n | (%) | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Demographics | ||||||||
| Race | ||||||||
| White | 16 | (47.1) | Reference | |||||
| Black | 10 | (29.4) | 7.65 | (2.11, 27.8) | 0.002‡ | 43.2 | (2.38, 781) | 0.01 |
| Indian/South Asian | 2 | (5.9) | 0.51 | (0.10, 2.53) | 0.41 | |||
| East Asian | 1 | (2.9) | 1.53 | (0.13, 18.0) | 0.74 | |||
| Middle Eastern | 0 | - | ||||||
| First Nations/Aboriginal | 0 | - | ||||||
| Mixed or other | 3 | (8.8) | 1.53 | (0.34, 6.84) | 0.58 | |||
| Unknown | 2 | (5.9) | 6.13 | (0.52, 72.1) | 0.15 | |||
| Stroke presentation | ||||||||
| Non-focal signs | ||||||||
| Headache | 3 | (8.8) | 0.08 | (0.02, 0.30) | <0.0001 | |||
| Decreased level of consciousness | 4 | (11.8) | 0.27 | (0.09, 0.87) | 0.03* | 0.01 | (0.0009, 0.19) | 0.002 |
| Vertigo | 0 | - | 0.01† | |||||
| Risk factors or co-morbidities (not mutually exclusive) | ||||||||
| Other chronic disorders | ||||||||
| Sickle cell anemia | 9 | (26.5) | - | <0.0001† | ||||
| Downs syndrome | 6 | (17.6) | 15.9 | (1.83, 138) | 0.01‡ | 2099 | (134, 328K) | 0.003 |
| Acute systemic illness | ||||||||
| Infection reported in prior 7 days | 8 | (23.5) | 3.54 | (1.12, 11.2) | 0.03‡ | 30.0 | (1.58, 569) | 0.02 |
| Meningitis | 0 | - | 0.02† | |||||
| Dehydration | 4 | (11.8) | 9.87 | (1.06, 91.9) | 0.04 | |||
| Infarct Characteristics at Baseline | ||||||||
| Location | ||||||||
| Vascular distribution of infarction | ||||||||
| Anterior circulation | 31 | (91.2) | 3.03 | (0.82, 11.1) | 0.10 | |||
| ACA | 5 | (14.7) | 1.12 | (0.35, 3.57) | 0.85 | |||
| Lenticulostriate | 8 | (23.5) | 0.27 | (0.11, 0.67) | 0.005 | |||
| Superficial MCA | 30 | (88.2) | 5.58 | (1.79, 17.4) | 0.003 | |||
| Anterior choroidal | 0 | - | 0.17† | |||||
| Posterior circulation | 0 | - | 0.005† | |||||
| Basilar | 0 | - | 0.55† | |||||
| SCA | 0 | - | 0.06† | |||||
| AICA | 0 | - | 1.00† | |||||
| PICA | 0 | - | 0.02† | |||||
| PCA | 0 | - | 0.005† | |||||
| Other location | 4 | (11.8) | 9.87 | (1.06, 91.9) | 0.04 | |||
| Vascular Imaging Findings | ||||||||
| Pathologic finding | ||||||||
| Occlusion | 25 | (73.5) | 2.18 | (0.90, 5.30) | 0.09 | |||
| Vascular territories affected | ||||||||
| More than one | 31 | (91.2) | 32.7 | (8.93, 120) | <0.0001‡ | 325.0 | (19.1, 5522) | <0.0001 |
| One | 3 | (8.8) | Reference | |||||
| Segments affected | ||||||||
| Supraclinoid ICA | 32 | (94.1) | 17.3 | (3.87, 77.6) | <0.0001‡ | 61.0 | (2.32, 1602) | 0.01 |
| Proximal MCA (M1) | 32 | (94.1) | 6.22 | (1.37, 28.3) | 0.02 | |||
| Vertebrobasilar | 7 | (20.6) | 0.59 | (0.22, 1.54) | 0.28 | |||
| Cervical arteries | 6 | (17.6) | 0.55 | (0.20, 1.52 | 0.25 | |||
Full list of variables tested shown in Online Table 1.
p-value calculated using Fisher's exact test

Table 5.
Summary results of independent predictors of arteriopathy subtypes
| Arteriopathy subtype | Cardioembolic | Dissection | FCA-i | Moyamoya | Secondary Vasculitis |
Other Definite |
|---|---|---|---|---|---|---|
| Demographic characteristics | ||||||
| Black race vs. other | +++ | |||||
| Clinical characteristics | ||||||
| Congenital or Acquired Heart Disease | +++ | |||||
| Downs syndrome | +++ | |||||
| Meningitis | +++ | |||||
| Sepsis/bacteremia | +++ | |||||
| Head trauma | +++ | |||||
| Presentation | ||||||
| Dysarthria | ++ | |||||
| Nausea/vomiting | + | |||||
| Vertigo | +++ | |||||
| Decreased level of consciousness | − | +++ | ||||
| Infarct Characteristics: Location | ||||||
| Lenticulostriate territory | +++ | |||||
| Infarct Characteristics: Volume | ||||||
| Infarct Volume (smaller) | ++ | |||||
| Vascular Imaging Abnormality | ||||||
| Occlusion | − | |||||
| Stenosis | − | |||||
| Irregularity | − | |||||
| Banding | +++ | |||||
| More than one 1 vascular territory | − | +++ | ||||
| More than one arterial segment | − | |||||
| Affected Artery | ||||||
| Proximal MCA (M1) | +++ | |||||
| Distal ICA (supraclinoid) | +++ | +++ | ||||
| Cervical artery | +++ | |||||
ACA=anterior cerebral artery; MCA=middle cerebral artery; PCA=posterior cerebral artery; ICA=internal carotid artery
Positive association: +++ indicates odds ratio (OR)>20, ++ indicates OR 5–15, + iindicates 1<OR <5; P<0.05 in all
Negative association: − indicates OR<1 and P<0.05
Cardioembolic versus Arteriopathic Stroke
In multivariable analysis, characteristics that were determined to best distinguish cardioembolic from arteriopathic stroke were the presence of congenital heart disease and involvement of multiple vascular territories (both positively associated with cardioembolic stroke); the presence of vascular stenosis or irregularity spoke against the possibility of cardioembolic stroke (Table 5 and Online Table 5). All cases of cardioembolic stroke had underlying congenital or acquired cardiac disease (Table 1 and Online Table 1). Having multiple or bilateral arterial segments affected unexpectedly decreased the odds of cardioembolism because these features were seen more frequently in arteriopathy. On vascular imaging, the most common abnormality in cardioembolic stroke was arterial occlusion, present in almost half; however, arterial occlusion was a nonspecific finding that was observed commonly in the arteriopathy group (61.5% of arteriopathy patients). Arterial irregularities and stenosis reduced the odds of cardioembolism, although each was observed in about 10% of cardioembolic strokes.
Characteristics of Childhood Arteriopathy Subtypes
Our final multivariable model to distinguish arterial dissection (intracranial, which includes FCA-dissection, or extracranial) from other arteriopathy subtypes included a history of head trauma and involvement of the cervical arteries (Table 5 and Online Table 5). Arterial dissection was associated with a history of head trauma in 39% of cases (Table 2 and Online Table 2). Dissections were equally distributed between the anterior and posterior circulation. Dissections tended to show as unilateral occlusions.
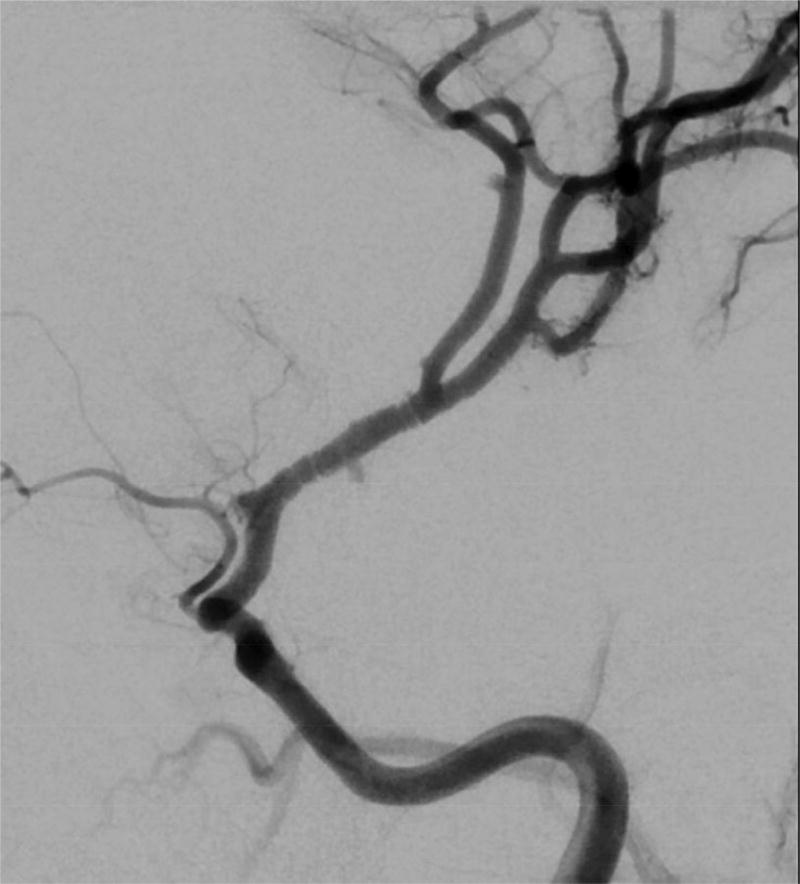
Characteristics which were associated with FCA-i in multivariable analysis included presentation with dysarthria, smaller infarcts in the lenticulostriate territory, infarct volume <25cm3, arterial banding, and isolated involvement of the M1 segment of the middle cerebral artery (MCA) (Table 5 and Online Table 5). FCA-i tended to occur more often in children between 8 and 15 years of age, (Table 3 and Online Table 3a). The banding pattern (Figure 2), while pathognomonic, was uncommon (24% of FCA-I patients).
Table 3.
FCA-i: clinical and imaging characteristics that distinguish FCA-i (N=25), compared to all other definite arteriopathy subgroups (N=84).*
| Prevalence (N=25) |
Univariate Analyses | Multivariable Analysis* | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Characteristic | n | (%) | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Demographics | ||||||||
| Age by category | ||||||||
| 0–3 years | 2 | (8.0) | Ref | |||||
| 4–7 years | 7 | (28.0) | 3.27 | (0.63, 17.1) | 0.16 | |||
| 8–11 years | 6 | (24.0) | 7.64 | (1.33, 43.8) | 0.02 | |||
| 12–15 years | 8 | (32.0) | 18.7 | (3.14, 111) | 0.001 | |||
| 16–18 years | 2 | (8.0) | 3.11 | (0.38, 25.4) | 0.29 | |||
| Stroke presentation | ||||||||
| Focal signs | ||||||||
| Dysarthria | 13 | (52.0) | 3.68 | (1.36, 9.96) | 0.01‡ | 8.34 | (1.50, 46.3) | 0.02 |
| Aphasia | 8 | (32.0) | 4.19 | (1.00, 17.6) | 0.05 | |||
| Non-focal signs | ||||||||
| Headache | 16 | (64.0) | 3.19 | (1.22, 8.30) | 0.02 | |||
| Risk Factors or co-morbidities (not mutually exclusive) | ||||||||
| Meningitis | 0 | - | 0.07† | |||||
| Head trauma | 0 | - | 0.07† | |||||
| Infarct Characteristics at Baseline | ||||||||
| Location | ||||||||
| Vascular distribution of infarction | ||||||||
| Anterior circulation | 25 | (100.0) | - | 0.006† | ||||
| ACA | 0 | - | 0.02† | |||||
| Lenticulostriate | 19 | (76.0) | 6.01 | (2.16, 16.7) | 0.001‡ | 45.6 | (4.81, 433) | 0.001 |
| Superficial MCA | 14 | (56.0) | 0.54 | (0.22, 1.35) | 0.19 | |||
| Anterior choroidal | 4 | (16.0) | 7.81 | (1.34, 45.6) | 0.02 | |||
| Posterior circulation | 0 | - | 0.04† | |||||
| Infarct side | 0.001† | |||||||
| Unilateral | 25 | (100.0) | ||||||
| Bilateral | 0 | |||||||
| Volume, largest infarct, cm3, median (IQR) | 13.8 | (3.3, 20.1) | ||||||
| Volume <25 cm3 | 20 | (80.0) | 6.84 | (2.33, 20.0) | <0.0001‡ | 5.48 | (1.31, 23.0) | 0.02 |
| Vascular Imaging Findings | ||||||||
| Pathologic finding | ||||||||
| Occlusion | 10 | (40.0) | 0.32 | (0.13, 0.79) | 0.01 | |||
| Stenosis | 15 | (60.0) | 0.64 | (0.25, 1.61) | 0.34 | |||
| Irregularity | 15 | (60.0) | 1.5 | (0.61, 3.72) | 0.38 | |||
| Banding | 6 | (24.0) | 26.2 | (2.98, 230) | 0.003‡ | 193 | (1.72, 22K) | 0.03 |
| Pathologically affected vessels | ||||||||
| Arterial segments affected | ||||||||
| More than one | 18 | (72.0) | Reference | |||||
| One | 7 | (28.0) | 3.69 | (1.19, 11.5) | 0.02‡ | 18.9 | (1.49, 240) | 0.02 |
| Vascular territories affected | <0.0001† | |||||||
| More than one | 0 | |||||||
| One | 25 | (100.0) | - | |||||
| Sides affected | <0.0001† | |||||||
| Bilateral | 0 | - | ||||||
| Unilateral | 25 | (100.0) | - | |||||
| Segments affected | ||||||||
| Supraclinoid ICA | 10 | (40.0) | 0.3 | (0.12, 0.75) | 0.01 | |||
| Proximal MCA (M1) | 24 | (96.0) | 8.52 | (1.09, 66.7) | 0.04* | 24.2 | (1.45, 405) | 0.03 |
| Vertebrobasilar | 0 | - | ||||||
| Cervical arteries | 1 | (4.0) | 0.09 | (0.01, 0.72) | 0.02 | |||
Full list of variables tested shown in Online Table 1.
p-value calculated using Fisher's exact test

Figure 2.
Banding pattern observed in 24% of patients with FCA-I.
A total of 41 children met criteria for FCA: seven FCA-d, 25 FCA-i, and nine that could not be further classified. Our analysis of characteristics that distinguish FCA-i from FCA-d Online Tables 3b and 3c) was limited by small sample sizes and availability of vessel wall imaging in only 8 of the 25 children with FCA-i. The expert review team used a history of head trauma to make a diagnosis of FCA-d; it was present in 5 of the 7 FCA-d patients (and in none of the FCA-i patients). Infarct volumes were larger for FCA-d (median 88 cm3; IQR 3.3, 20 cm3) than for FCA-i (median 14 cm3; IQR 8.6, 99 cm3; p=0.05). Arterial occlusion was seen in 86% of FCA-d versus 40% of FCA-i (p=0.06). Banding was seen in 24% of FCA-i but none of FCA-d (p=0.28). In FCA-i, 28% of patients had involvement of only one arterial segment (typically M1), while 72% had involvement of multiple arterial segments (supraclinoid ICA plus M1, or M1 plus M2). In contrast, all seven cases of FCA-d had involvement of both the supraclinoid ICA and M1. A coincident cervical artery abnormality was more suggestive of FCA-d (4/7 FCA-d versus 1/25 FCA-i, p=0.007).
Our multivariable model for a moyamoya diagnosis included both primary and secondary forms of moyamoya (Table 5 and Online Table 5). Of 34 children with moyamoya, 17 were diagnosed with primary (idiopathic) moyamoya disease and 17 with secondary moyamoya syndrome, most commonly caused by sickle cell anemia (N=9) or Down syndrome (n=6). An association between black race and moyamoya was almost entirely explained by sickle cell anemia; Asian race did not affect risk of moyamoya in our cohort (Table 4 and Online Table 4). Imaging characteristics included bilateral distal ICA occlusion or stenosis, and infarcts involving multiple vascular territories. Involvement of the posterior circulation was present in 21%. Although moyamoya is an intracranial arteriopathy, abnormalities of the cervical arteries were noted in six (18%), mostly representing the MRA finding of small cervical internal carotid arteries due to reduced intracranial flow. Patients with moyamoya typically did not present with decreased level of consciousness, differentiating them from children with secondary vasculitis, who had similar distal ICA involvement.
There were no cases of primary diffuse/multifocal vasculitis in VIPS, but 15 cases of secondary diffuse/multifocal vasculitis due to meningitis (n=11), other infection (cavernous sinus thrombophlebitis, n=1; mycotic aneurysm, n=1) or autoimmune disease (n=1). Hence, clinical characteristics associated with this diagnosis included meningitis and bacteremia/sepsis, and presentation with decreased level of consciousness (Table 5 and Online Table 5). Complete occlusion of the affected artery reduced the odds of diffuse/multifocal vasculitis. Diffuse/multifocal vasculitis was seen more frequently in Asian patients: there were 6 cases of stroke due to tubercular meningitis enrolled in the Philippines.
Among seven children with Down syndrome in our cohort, six had moyamoya, and one had a cardioembolic stroke.
Discussion
The diagnosis of childhood arteriopathy is complex, and pediatric stroke experts develop their diagnostic acumen over the years through the cumulative exposure to a large number of cases. However, because these diseases are rare, pediatric patients with childhood arteriopathy are often seen by healthcare professionals who have not developed this expertise. The VIPS study presented a unique opportunity to help non-experts not only in accurately diagnosing pediatric patients with childhood arteriopathy, but also in distinguishing among specific types, based on objectively defined clinical and imaging parameters. The VIPS study previously demonstrated that arteriopathies can be more accurately classified when clinical data are utilized than when imaging findings are used alone, and when follow-up vascular imaging is performed.15 The current analysis adds to our prior publication by defining the prevalence and predictive value of the individual characteristics that a pediatric stroke expert uses to diagnose a childhood arteriopathy. In addition, it allows identification of patterns, i.e. combinations of characteristics that distinguish certain arteriopathies, and also assessment of the relative importance of each of these characteristics. This analysis assumes the availability of complete and accurate clinical and imaging data at the time of the arteriopathy classification; in real clinical situations, arteriopathy classification should be revisited as new data become available over time.
As a first step in the approach to vascular imaging abnormalities in a child with AIS, radiologists and clinicians should consider whether the abnormality represents inherent arterial disease of the artery (i.e., arteriopathy) versus thrombus from a proximal source (cardioembolism, or artery-to-artery embolism). Underlying cardiac disease strongly favors cardioembolism; however, four children with congenital heart disease had an arteriopathic stroke (one a dissection, three moyamoya), indicating that arteriopathic stroke should be considered even in patients with cardiac disease.
Arterial stenosis or irregularity reduces the odds of cardioembolism, but can be seen with a recanalizing thrombus. Complete arterial occlusion appeared to reduce odds of cardioembolism in our model, but only because it is a common feature of arteriopathies like moyamoya and dissection; it was still the most common vascular imaging finding in cardioembolism. Distinguishing arteriopathy from thrombus remains challenging; our expert team could not make the distinction in 34 cases (designated “possible” arteriopathy; Figure 1), highlighting the need for an echocardiogram as part of the work-up of pediatric patients suspected of childhood arteriopathy.
The most common childhood arteriopathies in our cohort of children presenting with acute AIS were moyamoya, arterial dissection (intracranial and extracranial), and FCA-i. This distribution would likely be different in a cohort including all children with cerebral or cervical arteriopathy; primary small vessel CNS vasculitis, for example, typically presents with only with headache or cognitive decline, and no focal signs or symptoms. Although atherosclerosis may begin in childhood, it was not seen as a cause of stroke in VIPS, consistent with prior reports.25,26
Age was the one demographic characteristic that helped distinguish between arteriopathy subtypes. FCA-i tended to affect older school-aged children, while moyamoya affected younger children; dissection had no age predilection. Gender and race did not correlate with arteriopathy subtype (after accounting for sickle cell disease). Although primary moyamoya occurs more commonly in Korean and Japanese populations,27 we had no enrolling sites in those countries, and saw a broad distribution of ethnicities amongst our moyamoya cases.
A diagnosis of dissection is suggested by the involvement of cervical arteries. Based on current definitions, FCA-i does not include arteriopathies affecting the posterior circulation. Moyamoya predominantly affects the anterior circulation; posterior circulation involvement, when present, is rarely symptomatic. Hence, dissection is high on the differential in a previously healthy child presenting with a posterior circulation arterial ischemic stroke. In addition, FCA-i, moyamoya, and secondary vasculitis are intracranial arteriopathies; involvement of the cervical arteries is strongly suggestive of dissection.
Distinguishing the etiologies of FCA—focal stenosis or irregularity of the distal ICA or proximal MCA—remains a challenge even to pediatric stroke experts. The differential diagnosis includes FCA-i, FCA-d (intracranial dissection of the anterior circulation), and early, unilateral moyamoya disease. All typically present with hemiparesis, but headache at the stroke ictus is common in FCA-i and FCA-d, but not moyamoya. Banding was considered a pathognomonic feature for FCA-i, but was present in less than a quarter of cases (being more conspicuous on conventional angiograms compared to CTAs and MRAs); hence, it was useful when present, but not a sensitive feature of FCA-i. Infarct location in the lenticulostriate territory and smaller infarct size correlated with FCA-i, and were more prevalent biomarkers. However FCA-i and FCA-d are in general difficult to distinguish from each other as showed in post-mortem cases.28
An infarct in the superficial middle cerebral artery territories (i.e., cerebral convexities) was more suggestive of moyamoya. Chronic deep borderzone infarcts, also common in moyamoya, do not result in focal deficits; because this is a cohort of children with acute AIS, such infarcts were not included in this analysis. A history of head trauma and/or coincident cervical artery abnormalities suggests dissection. Improved neuroimaging techniques, including vessel wall imaging29, are needed to distinguish forms of FCA with greater certainty, although VWI may not be 100% specific and there may be some overlap with FCA-d showing minimal enhancement on VWI, and FCA-i typically presenting marked enhancement on VWI. This is particularly important because their management strategies differ. FCA-i and FCA-d are currently treated with anti-platelet therapy. In addition, lifelong restriction of activities (e.g., no contact sports) is often recommended after an arterial dissection30, and clinical trials of corticosteroids for the treatment of FCA-i are under development.
In this paper, the definition of FCA-i was restricted to focal disease of the distal ICA and its proximal branches, including but not limited to TCA.31,32 However, we anticipate that increased utilization of vessel wall imaging29, allowing the delineation of enhancing arterial segments, will necessitate a broader definition of FCA. For example, we observed cases of focal stenosis of the petrous carotid or posterior circulation arteries that we diagnosed as having a “definite arteriopathy” that could not be further classified. If such cases had vessel wall imaging demonstrating enhancement of the affected vessel, it may be reasonable to expand the definition of FCA-i to include these cases. In addition, we identified one case of FCA-i that demonstrated arteriopathy progression after six months, contrary to the traditional definition of TCA; this highlights the fact that, while FCA-i includes TCA, not all FCA-I are TCA.
Our study’s most significant limitation is that there is no true gold standard for the diagnosis of childhood arteriopathies. Our expert review team was uncertain about the classification in 52 cases: 34 with “possible” arteriopathy and 18 with a definite arteriopathy that could not be further classified (Figure 1). Even among the arteriopathies that the review team classified with high certainty, there was likely some misclassification that cannot be measured. Because all of the imaging was performed on a clinical basis, there was variability in both the type and timing of imaging performed. As noted in our prior study, follow-up vascular imaging was helpful for classification, yet was available in only a minority of patients.13 The circularity of some analyses—biomarkers used to classify a subtype, and then evaluated as predictors of that subtype—must be emphasized; head trauma, for example, was an anticipated predictor of arterial dissection because it was used in the classification process. In such cases, the value of the analysis is in the prevalence of the predictor, such as noting that a minority of dissection cases had trauma, so an absence of trauma does not rule out this diagnosis. Lastly, analyses of arteriopathy subtypes were underpowered (as reflected by large confidence intervals of coefficients in the multivariable models), so should be interpreted with caution. However, advantages of our study include a prospectively collected cohort, a large sample size relative to most pediatric stroke studies, and rigorous classification methods based on independent, central expert reviews and adjudication. It allows the quantification of the prevalence of the predictors, the strength of their correlations with specific diagnoses, and patterns of multiple predictors. These results should provide a guide for clinicians and neuroradiologists to generate a reasonable differential for an arteriopathic stroke in a child, and prioritize diagnoses on that list. The application of these findings will depend, however, on the accurate characterization of the imaging biomarkers by the interpreting neuroradiologist.
In conclusion, the different types of childhood arteriopathies are associated with typical clinical and parenchymal and vascular imaging features that can help narrow the differential diagnosis in pediatric stroke patients with vascular anomalies (Table 5).
Supplementary Material
Acknowledgments
The authors wish to acknowledge the important contributions of the research coordinators at VIPS sites, and of the patients and their families.
FUNDING: NIH R01 NS062820 (PIs Fullerton, DeVeber); Bellaflies Foundation and Marc and Lynne Benioff for statistical support.
APPENDIX
Co-authors within the VIPS Investigators group
Dowling, Michael M(University of Texas Southwestern Medical Center, Dallas), Benedict, Susan L (Primary Children's Medical Center, Salt Lake City), Bernard, Timothy J (Denver Children's Hospital), Fox, Christine K (UCSF), DeVeber, Gabrielle A (The Hospital for Sick Children, Toronto), Friedman, Neil R (Cleveland Clinic Children's Hospital), Lo, Warren D (The Ohio State University and Nationwide Children's Hospital, Columbus OH), Ichord, Rebecca N (Children's Hospital of Philadelphia), Tan, Marilyn A (University of the Philippines-Philippine General Hospital, Manila), Mackay, Mark T (Royal Children's Hospital Melbourne), Kirton, Adam (Alberta Children's Hospital), Hernandez-Chavez, Marta I (Pontificia Universidad Catolica de Chile), Humphreys, Peter (Children's Hospital of Eastern Ontario), Jordan, Lori C (Vanderbilt University Medical Center, Nashville), Sultan, Sally (Columbia University Medical Center, New York), Rivkin, Michael J (Boston Children's Hospital), Rafay, Mubeen F (Children's Hospital, Winnipeg, University of Manitoba), Titomanlio, Luigi (Hôpital Robert Debré-Paris), Kovacevic, Gordana S (Mother and Child Health Care Institute, Serbia), Yager, Jerome Y (Stollery Children's Hospital), Amlie-Lefond, Catherine (Seattle Children's Hospital), Dlamini, Nomazulu (Evelina London Children's Hospital), Condie, John (Phoenix Children's Hospital), Yeh, Ann (Women and Children's Hospital of Buffalo), Kneen, Rachel (Alder Hey Children's Hospital), Bjornson, Bruce (British Columbia Children's Hospital), Pergami, Paola (West Virginia University), Zou, Li Ping (Chinese PLA General Hospital, Beijing), Elbers, Jorina M (Stanford Children’s Health, Palo Alto), Abdalla, Abdalla (Akron Children's Hospital), Chan, Anthony K (McMaster University, Hamilton), Farooq, Osman (Women & Children's Hospital of Buffalo), Lim, Mingming J (Evelina London Children's Hospital), Carpenter, Jessica L(Children's National Medical Center, Washington, D.C.), Pavlakis, Steven (Maimonides Medical Center, Brooklyn), Wong, Virginia C (Queen Mary Hospital, Hong Kong), Forsyth, Robert (Institute of Neuroscience, Newcastle University, UK)
References
- 1.Agrawal N, Johnston SC, Wu YW, Sidney S, Fullerton HJ. Imaging data reveal a higher pediatric stroke incidence than prior US estimates. Stroke. 2009;40:3415–21. doi: 10.1161/STROKEAHA.109.564633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fullerton HJ, Wu YW, Sidney S, Johnston SC. Risk of recurrent childhood arterial ischemic stroke in a population-based cohort: the importance of cerebrovascular imaging. Pediatrics. 2007;119:495–501. doi: 10.1542/peds.2006-2791. [DOI] [PubMed] [Google Scholar]
- 3.Ganesan V, Prengler M, McShane MA, Wade AM, Kirkham FJ. Investigation of risk factors in children with arterial ischemic stroke. Ann Neurol. 2003;53:167–73. doi: 10.1002/ana.10423. [DOI] [PubMed] [Google Scholar]
- 4.Strater R, Becker S, von Eckardstein A, et al. Prospective assessment of risk factors for recurrent stroke during childhood--a 5-year follow-up study. Lancet. 2002;360:1540–5. doi: 10.1016/S0140-6736(02)11520-0. [DOI] [PubMed] [Google Scholar]
- 5.Chabrier S, Husson B, Lasjaunias P, Landrieu P, Tardieu M. Stroke in childhood: outcome and recurrence risk by mechanism in 59 patients. J Child Neurol. 2000;15:290–4. doi: 10.1177/088307380001500504. [DOI] [PubMed] [Google Scholar]
- 6.Zimmer JA, Garg BP, Williams LS, Golomb MR. Age-related variation in presenting signs of childhood arterial ischemic stroke. Pediatr Neurol. 2007;37:171–5. doi: 10.1016/j.pediatrneurol.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Amlie-Lefond C, Bernard TJ, Sébire G, et al. Predictors of Cerebral Arteriopathy in Children With Arterial Ischemic Stroke Results of the International Pediatric Stroke Study. Circulation. 2009;119:1417–23. doi: 10.1161/CIRCULATIONAHA.108.806307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danchaivijitr N, Cox TC, Saunders DE, Ganesan V. Evolution of cerebral arteriopathies in childhood arterial ischemic stroke. Annals of neurology. 2006;59:620–6. doi: 10.1002/ana.20800. [DOI] [PubMed] [Google Scholar]
- 9.Roach ES, Golomb MR, Adams R, et al. Management of stroke in infants and children: a scientific statement from a Special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke. 2008;39:2644–91. doi: 10.1161/STROKEAHA.108.189696. [DOI] [PubMed] [Google Scholar]
- 10.Sebire G, Fullerton H, Riou E, deVeber G. Toward the definition of cerebral arteriopathies of childhood. Curr Opin Pediatr. 2004;16:617–22. doi: 10.1097/01.mop.0000144441.29899.20. [DOI] [PubMed] [Google Scholar]
- 11.Bernard TJ, Manco-Johnson MJ, Lo W, et al. Towards a consensus-based classification of childhood arterial ischemic stroke. Stroke. 2012;43:371–7. doi: 10.1161/STROKEAHA.111.624585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fullerton HJ, Elkind MS, Barkovich AJ, et al. The vascular effects of infection in Pediatric Stroke (VIPS) Study. J Child Neurol. 2011;26:1101–10. doi: 10.1177/0883073811408089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wintermark M, Hills NK, Barkovich AJ, et al. Arteriopathy Diagnosis in Childhood Arterial Ischemic Stroke Results of the Vascular Effects of Infection in Pediatric Stroke Study. Stroke. 2014;45:3597–605. doi: 10.1161/STROKEAHA.114.007404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sims JR, Gharai LR, Schaefer PW, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72:2104–10. doi: 10.1212/WNL.0b013e3181aa5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wintermark M, Hills NK, deVeber GA, et al. Arteriopathy diagnosis in childhood arterial ischemic stroke: results of the vascular effects of infection in pediatric stroke study. Stroke; a journal of cerebral circulation. 2014;45:3597–605. doi: 10.1161/STROKEAHA.114.007404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hess CP, Fullerton HJ, Metry DW, et al. Cervical and intracranial arterial anomalies in 70 patients with PHACE syndrome. AJNR Am J Neuroradiol. 2010;31:1980–6. doi: 10.3174/ajnr.A2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frieden IJ, Reese V, Cohen D. PHACE syndrome. The association of posterior fossa brain malformations, hemangiomas, arterial anomalies, coarctation of the aorta and cardiac defects, and eye abnormalities. Arch Dermatol. 1996;132:307–11. doi: 10.1001/archderm.132.3.307. [DOI] [PubMed] [Google Scholar]
- 18.Kirton A, Crone M, Benseler S, et al. Fibromuscular dysplasia and childhood stroke. Brain. 2013;136:1846–56. doi: 10.1093/brain/awt111. [DOI] [PubMed] [Google Scholar]
- 19.Tolani AT, Yeom KW, Elbers J. Focal Cerebral Arteriopathy: The Face With Many Names. Pediatric Neurology. 2015;53:247–52. doi: 10.1016/j.pediatrneurol.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Fullerton HJ, Johnston SC, Smith WS. Arterial dissection and stroke in children. Neurology. 2001;57:1155–60. doi: 10.1212/wnl.57.7.1155. [DOI] [PubMed] [Google Scholar]
- 21.Swartz R, Bhuta S, Farb R, et al. Intracranial arterial wall imaging using high-resolution 3-tesla contrast-enhanced MRI. Neurology. 2009;72:627–34. doi: 10.1212/01.wnl.0000342470.69739.b3. [DOI] [PubMed] [Google Scholar]
- 22.Lanthier S, Armstrong D, Domi T. Post-varicella arteriopathy of childhood Natural history of vascular stenosis. Neurology. 2005;64:660–3. doi: 10.1212/01.WNL.0000151851.66154.27. [DOI] [PubMed] [Google Scholar]
- 23.Chabrier S, Sébire G, Fluss J. Transient Cerebral Arteriopathy, Postvaricella Arteriopathy, and Focal Cerebral Arteriopathy or the Unique Susceptibility of the M1 Segment in Children With Stroke. Am Heart Assoc. 2016 doi: 10.1161/STROKEAHA.116.014606. [DOI] [PubMed] [Google Scholar]
- 24.Chabrier S, Rodesch G, Lasjaunias P, Tardieu M, Landrieu P, Sebire G. Transient cerebral arteriopathy: a disorder recognized by serial angiograms in children with stroke. J Child Neurol. 1998;13:27–32. doi: 10.1177/088307389801300105. [DOI] [PubMed] [Google Scholar]
- 25.Ganesan V, Prengler M, McShane MA, Wade AM, Kirkham FJ. Investigation of risk factors in children with arterial ischemic stroke. Annals of neurology. 2003;53:167–73. doi: 10.1002/ana.10423. [DOI] [PubMed] [Google Scholar]
- 26.Sträter R, Becker S, von Eckardstein A, et al. Prospective assessment of risk factors for recurrent stroke during childhood-a 5-year follow-up study. The Lancet. 2002;360:1540–5. doi: 10.1016/S0140-6736(02)11520-0. [DOI] [PubMed] [Google Scholar]
- 27.Kim JS. Moyamoya Disease: Epidemiology, Clinical Features, and Diagnosis. Journal of stroke. 2016;18:2. doi: 10.5853/jos.2015.01627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dlamini N, Freeman JL, Mackay MT, et al. Intracranial dissection mimicking transient cerebral arteriopathy in childhood arterial ischemic stroke. Journal of child neurology. 2011;26:1203–6. doi: 10.1177/0883073811408904. [DOI] [PubMed] [Google Scholar]
- 29.Mandell D, Mossa-Basha M, Qiao Y, et al. Intracranial Vessel Wall MRI: Principles and Expert Consensus Recommendations of the American Society of Neuroradiology. American Journal of Neuroradiology. 2016 doi: 10.3174/ajnr.A4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernard TJ, deVeber GA, Benke TA. Athletic participation after acute ischemic childhood stroke: a survey of pediatric stroke experts. Journal of child neurology. 2007;22:1050–3. doi: 10.1177/0883073807306271. [DOI] [PubMed] [Google Scholar]
- 31.Sébire G, Fullerton H, Riou E. Toward the definition of cerebral arteriopathies of childhood. Current opinion in pediatrics. 2004;16:617–22. doi: 10.1097/01.mop.0000144441.29899.20. [DOI] [PubMed] [Google Scholar]
- 32.Chabrier S, Rodesch G, Lasjaunias P, Tardieu M, Landrieu P, Sébire G. Transient cerebral arteriopathy: a disorder recognized by serial angiograms in children with stroke. Journal of child neurology. 1998;13:27–32. doi: 10.1177/088307389801300105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.