Abstract
Background
Diseases associated with coal mine dust continue to affect coal miners. Elucidation of initial pathological changes as a precursor of coal dust-related diffuse fibrosis and emphysema, may have a role in treatment and prevention.
Objective
To identify the precursor of dust-related diffuse fibrosis and emphysema.
Methods
Birefringent silica/silicate particles were counted by standard microscope under polarized light in the alveolar macrophages and fibrous tissue in 25 consecutive autopsy cases of complicated coal worker’s pneumoconiosis and in 21 patients with tobacco-related respiratory bronchiolitis.
Results
Coal miners had 331 birefringent particles/high power field while smokers had 4 (p<0.001). Every coal miner had intra-alveolar macrophages with silica/silicate particles and interstitial fibrosis ranging from minimal to extreme. All coal miners, including those who never smoked, had emphysema. Fibrotic septa of centrilobular emphysema contained numerous silica/silicate particles while only a few were present in adjacent normal lung tissue. In coal miners who smoked, tobacco-associated interstitial fibrosis was replaced by fibrosis caused by silica/silicate particles.
Conclusion
The presence of silica/silicate particles and anthracotic pigment-laden macrophages inside the alveoli with various degrees of interstitial fibrosis indicated a new disease: coal mine dust desquamative chronic interstitial pneumonia, a precursor of both dust-related diffuse fibrosis and emphysema. In studied coal miners, fibrosis caused by smoking is insignificant in comparison with fibrosis caused by silica/silicate particles. Counting birefringent particles in the macrophages from bronchioalveolar lavage may help detect coal mine dust desquamative chronic interstitial pneumonia, and may initiate early therapy and preventive measures.
Keywords: Pneumoconiosis, Pulmonary emphysema, Silicosis, Anthracosis, Anthracosilicosis, Tobacco, Lung diseases, Coal mining, Pulmonary fibrosis
Introduction
Recent advances in the understanding of multiple and complex pathophysiological effects of coal mine dust on lung parenchyma have introduced a new, broad generic term, “coal mine dust lung disease.”1 It encompasses interstitial lung diseases (coal worker’s pneumoconiosis, silicosis, and mixed dust pneumoconiosis), coal mine dust-related diffuse fibrosis, and chronic airway diseases including emphysema and chronic bronchitis.1 “Coal mine dust-related diffuse fibrosis” is also a rather new term coined four years ago,1 although first described 20 years ago.2 It denotes bridging fibrosis connecting the macular, nodular, or progressive massive fibrosis lesions of coal worker’s pneumoconiosis or silicosis.1,3 There are no data in the medical literature about the precursor of the coal mine dust-related diffuse fibrosis. The objective of this study was to find that precursor and to elucidate the sequence of events leading from coal dust exposure up to the end-stage coal mine dust-related diffuse fibrosis.
Materials and Methods
Hematoxylin and eosin-stained glass slides from 25 autopsies of patients with complicated coal worker’s pneumoconiosis and from 21 patients with tobacco-related respiratory bronchiolitis (wedge biopsies or lobectomy specimens) were examined. These autopsies were performed from 2001 to 2016, and lung wedge biopsies were obtained and for patients with tobacco-associated respiratory bronchiolitis from 2008 to 2016. Institutional Review Board approval for the autopsies and surgical pathology materials was obtained.
All the autopsies were performed with the standard protocol. Whole lungs were examined and representative sections from all lung lobes were submitted for histological examination. The emphysema was graded by comparison with pictures presented by A. Nagai’s, et al,4 in a way that their grades ‘1,’ ‘2,’ ‘3,’ ‘4,’ and ‘5′ were regarded as “mild emphysema,” grades ‘6,’ ‘7,’ and ‘8′ “moderate emphysema,” and grades ‘9′ and ‘10′ as “marked emphysema.” Diagnosis of chronic bronchitis was established by standard histological criteria.5 Patients’ electronic medical data were evaluated. The criterion used for diagnosis of complicated coal worker’s pneumoconiosis/progressive massive pulmonary fibrosis was as stated in the Federal Coal Mine Health and Safety Act of 1969 Public Law: coal dust-pigmented irregular or whorled deposition of collagen fibers measuring 1 cm or more in diameter (Federal Safety Act 1969).6
Silica, very small, varying in size from <1–2 μm faintly bright, dull birefringent particles, and larger elongated or platy very bright birefringent silicate particles were counted in a dark room at 400× magnification with Carl Zeiss microscope manufactured in West Germany. From each autopsy and from each patient with tobacco-associated respiratory bronchiolitis each glass slide was carefully examined and the most appropriate one high power field was chosen for counting silica/silicate particles. The pathologists were not blinded to diagnoses. We strived to select an area in which birefringent particles seem to be the most frequent and in which alveolar macrophages occupied essentially an entire high power field not disturbed by fibrous bands to be able to accurately express number of birefringent particles per high power field. The number of silica/silicate particles inside alveoli and in the areas of coal mine dust-related diffuse fibrosis was expressed as number (sum) of bright small birefringent dots (particles) and less bright dots (1–3 μm) per one high power field (400× magnification). The numbers of silica/silicate particles in the alveolar septa were counts of birefringent particles. Alveolar septa vary in thickness and occupy different percentages of high power field that are impossible to accurately calculate. Therefore, the number of birefringent particles in the alveolar septa per high power field can also not be accurately calculated. For this reason, the counted numbers of birefringent particles in the alveolar septa could not be usable for statistical comparison.
Although it was suggested by Mc Donald and Roggly7 that there is no correlation between the intensity of the light source used for polarized light and the differential ease of visualizing silica vs silicates, we were able to see and count silica and silicate particles more easily with stronger light than with less strong light. We used strong constant bright light produced by a bulb light source of 25 watts at 15 volts. Photographs were taken with the Cell Sense camera and software system attached to Olympus BX50 microscope. Student’s t for independent samples was used for statistical analysis.
Panels in Figures 1–3 were taken from only one patient to emphasize sequential continuity of the pathologic process in the lung from its beginning to its end in coal mine dust-related diffuse fibrosis, as well as the simultaneous presence of all these pathological changes. Panels in Figure 4 were taken from a non-smoker coal miner with marked emphysema caused by coal dust. In this Figure, an area of mild centrilobular emphysema was selected, because at the low magnification necessary to photograph marked emphysema, the birefringent particles were not visible. Panels in Figure 5 were taken from a patient with tobacco-related respiratory bronchiolitis.
Figure 1.
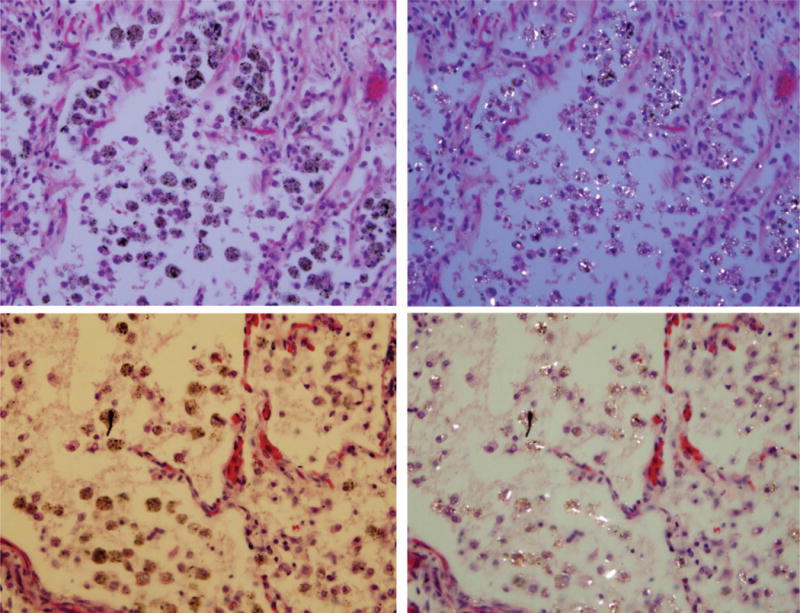
Coal mine dust desquamative chronic interstitial pneumonia. Alveoli are filled by macrophages laden with anthracotic pigment and birefringent silica/silicate particles. There is no fibrosis in the upper panels. Mild fibrosis of the alveolar septum presents in the left lower corner of photomicrographs in the lower panel. On the left side are photomicrographs of H&E-stained tissue (original magnification 400×); on the right side are same frames under polarized light.
Figure 3.
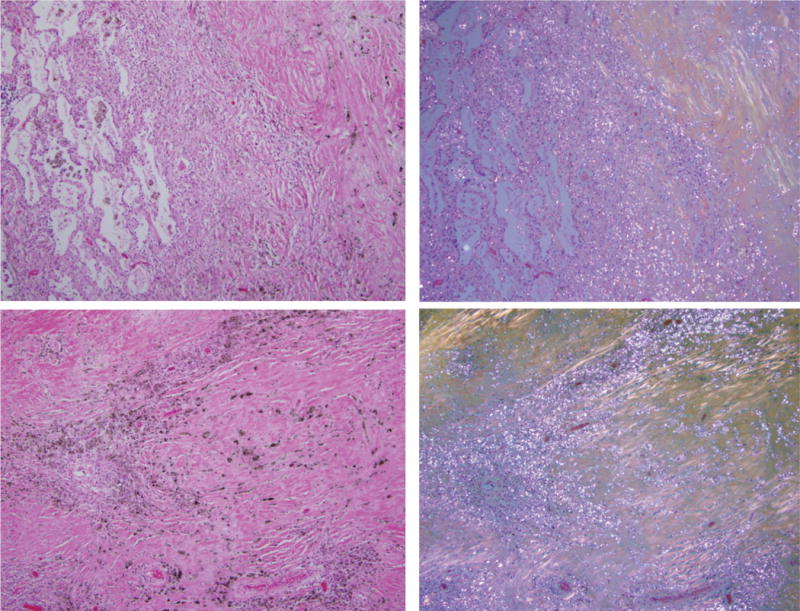
Coal mine dust desquamative chronic interstitial pneumonia (upper panel, left side of the photomicrograph) in a continuous gradual progression to coal mine dust-related diffuse fibrosis (upper panel, right side of the photomicrograph). Lower panels show coal mine dust-related diffuse fibrosis. Collagen fibers are more or less parallel to each other. On the left side are photomicrographs of H&E-stained tissue (original magnification 100×); on the right side are the same frames under polarized light.
Figure 4.
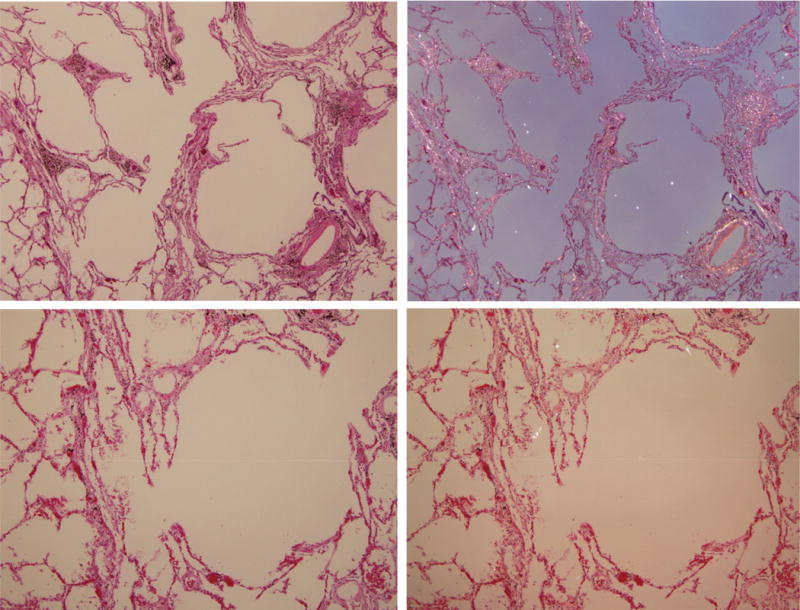
Centriacinar emphysema caused by coal mine dust. Numerous (in the upper panels) and frequent (in the lower panels) birefringent silica/silicate particles with anthracotic pigment are present in the fibrotic thickened alveolar septa. There are only few birefringent particles in the rather normal lung tissue in the left lower corner of the upper panels and in the left part of the photomicrograph in the lower panels. On the left side are photomicrographs of H&E-stained tissue (original magnification 40× for the upper panels and 100× for the lower panels); on the right side are the same frames under polarized light.
Figure 5.
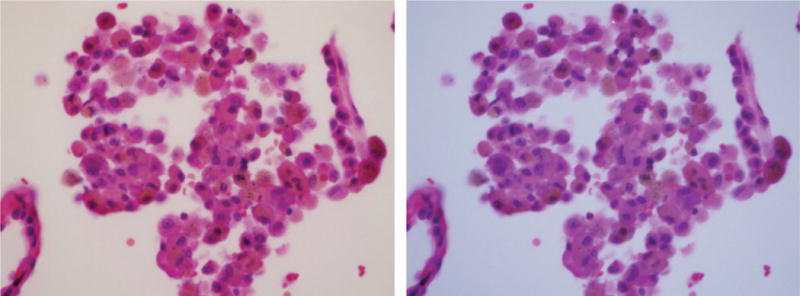
Tobacco-related respiratory bronchiolitis. Macrophages inside the alveolus contain brown smoker granules and black soot pigment. Image under polarized light demonstrated one bright birefringent silicate particle (at 12 o’clock position) and a faintly birefringent silica particle at 9 o’clock. On the left side is photomicrograph of H&E-stained tissue (original magnification 400×); on the right side is the same frame under polarized light.
Results
Figure 1 presents alveolar macrophages laden with birefringent silica/silicate particles and anthracotic pigment. There is no evidence of fibrosis in the upper panels. One of the alveolar septa in the lower panels exhibits mild fibrous thickening. We called these findings “coal mine dust desquamative chronic interstitial pneumonia.”
Figure 2 demonstrates again the presence of birefringent silica/silicate particles in the macrophages inside the alveoli as well as progressive thickening of the interalveolar septa (interstitial fibrosis). Significant numbers of silica/silicate particles are present in the interalveolar septa.
Figure 2.
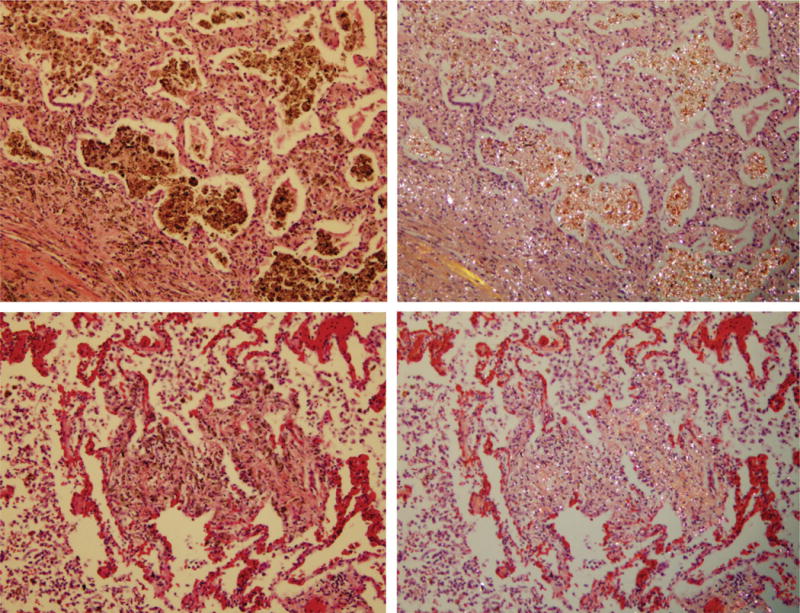
Coal mine dust chronic desquamative interstitial pneumonia with conspicuously thickened alveolar septa. In the lower panel, septa contain more collagen fibers. On the left side are photomicrographs of H&E-stained tissue (original magnification 200×); on the right side are the same frames under polarized light.
Figure 3 demonstrates simultaneous presence of coal mine dust desquamative chronic interstitial pneumonia and coal mine dust-related diffuse fibrosis, as well as continuous progression of interstitial fibrosis from former to the latter. Numerous birefringent silica/silicate particles are present in the alveolar macrophages and in the areas of dust-related diffuse fibrosis. Collagen fibers in the area of dust-related diffuse fibrosis are more or less parallel one to another and not whorled or irregular.
In brief, the above images illustrate the set of findings we describe as coal mine dust desquamative chronic interstitial pneumonia, and the subsequent progressive increase of interstitial fibrosis from no fibrosis and mild to marked in dust-related diffuse fibrosis, culminating in progressive massive pulmonary fibrosis/complicated coal worker’s pneumoconiosis. All these pathological changes were simultaneously present in each of the autopsied patients.
Figure 4 demonstrates interstitial fibrosis and emphysema in a non-smoking coal miner caused by coal mine dust, primarily silica/silicate particles. There are numerous (upper panels) and frequent (lower panels) birefringent silica/silicate particles as well as anthracotic pigment in the interalveolar septa. In the normal lung tissue in the left lower corner in the upper panel and in the left side of the photomicrograph in the lower panel, only a few silica/silicate particles are noted.
Figure 5 demonstrates a negligible number of birefringent silica/silicate particles in the macrophages inside the alveoli of patients with tobacco-related respiratory bronchiolitis.
The mean age of the coal miners was 73.5 (SD 9.2) years and of the patients with tobacco associated respiratory bronchiolitis was 53.9 (SD 13.9) years. The difference was statistically significant (p<0.001). Data in the medical records regarding number of cigarettes smoked per day for individual smokers varied among physicians taking patient’s history, but each of the smokers smoked at least one pack of 20 cigarettes per day. Data about number of cigarettes for patients with tobacco associated respiratory bronchiolitis were not available.
The degree of emphysema was moderate in 10 coal miners (40%), marked in 8 (32%), bullous in 5 (20%), and mild in 2 (8%) patients. All our patients with bullous emphysema also had marked emphysema, and thus, the percentage of patients with marked emphysema was 52%. Seventeen (68%) coal miners were smokers, 5 (20%) were not, and there were no data available for 3 (12%) coal miners. Four out of five (70%) coal miners with bullous emphysema were smokers and one (20%, 1/5) coal miner with bullous emphysema was a non-smoker. Thirty-three percent of our patients with marked emphysema were non-smokers (4 out of 12 patients; for one patient with marked emphysema information about smoking was not available). Only two out of 25 coal miners (8%) did not have chronic bronchitis. All nonsmoking coal miners had chronic bronchitis. Acute bronchopneumonia was present in 16 (64%) coal miners at their demise. All coal workers with acute bronchopneumonia had acute exacerbation of chronic bronchitis. Coal miners without acute exacerbation of chronic bronchitis did not have acute bronchopneumonia at the time of death. Twenty-four percent (6 out of 25) of coal miners with progressive massive pulmonary fibrosis had lung carcinoma.
The median number of birefringent particles in macrophages inside alveoli of coal mine workers was 301 (IQR 173 to 431) in comparison to 3 (IQR 1.5 to 6.5) in patients with tobacco-related respiratory bronchiolitis. The difference was statistically significant (p<0.001). The median number of birefringent particles in coal miners was 226 (IQR 195 to 412) per high power field in areas of dust related diffuse fibrosis, and the average number was 250 (IQR 166 to 389) in emphysema-associated septal fibrosis. In non-emphysematous areas, the number of birefringent silica/silicate particles was zero or very low, but since the number cannot be accurately quantified per high power field, it could not be used for statistical analysis. However, it is obvious that in areas of emphysema, thickened septa contained numerous or frequent birefringent silica/silicate particles and anthracotic pigment, whereas they were not present or only a few in number in alveolar septa of non-emphysematous areas of the same lung (Fig 4).
Discussion
Since electric plants fueled by coal are still the most important source of electricity worldwide, coal miners will continue to suffer and some will die from coal mine dust lung diseases. After passage of the Federal Coal Mine Health and Safety Act in 1969 (Federal Safety Act 1969),6 measures were adopted in coal mines to limit exposure to coal dust leading to a steady decline in the prevalence of coal worker’s pneumoconiosis in the USA from 1970 to about 2000.8 However, since then, the proportion of miners with pneumoconiosis has increased significantly, and severe forms including progressive massive fibrosis, silicosis, and rapidly progressive pneumoconiosis are increasing in their incidence.8 Modernization of the mining technology enabled pulverization of thousands of tons of coal per shift, which generates clouds of respirable dust particles containing toxic silica (quartz) and silicate particles and less toxic carbon (coal) particles. The vast majority of our patients were working in West Virginia coal mines where coal dust is more dangerous than in other states, since coal is present in thin seams, sandwiched by rock composed of silica and silicates.9 Silica and silicates are similar but chemically slightly different. The Earth’s crust is 90% made up of silicates. The basic chemical unit of silicates is the silicium (silicon) tetroxide (SiO4) in the form of a tetrahedron-shaped anion. Silicium tetrahedron anion can bind different cations and very importantly can bind to other tetrahedrons forming complicated structures (chains, rings, sheets, frameworks). Both bindings produce numerous types of silicates/minerals (cyclosilicates, phyllosilicates, tectosilicates, etc). Quartz (silica particle) consists of silicium dioxide that forms framework with an oxygen to silicium ratio of 2:1.
Inhaled coal mining dust particles travel by air-flow to respiratory bronchioles and alveoli where they are engulfed by alveolar macrophages and neutrophils. Macrophages and neutrophils activated by coal-dust, primarily by silica and silicate particles,8 release proteolytic enzymes that destroy collagen and elastic alveolar tissue, causing connective tissue breakdown and destruction (emphysema).10 This process is augmented by dust-catalyzed formation of oxidants (for example, quartz-generated hydrogen peroxide) that inactivate α1-antitrypsin (inactivator of proteolytic enzymes) by oxidizing the mercapto group (−SH) of methionine to methionine sulfoxide,10 in the same way that oxidants in the cigarette smoke act. Coal mining dust particles at the same time also stimulate macrophages to produce transforming growth factor-β (TGF-β), platelet-derived growth factor, tumor necrosis factor-α (TNF-α), which all stimulate fibroblast growth and collagen production, thus causing interstitial fibrosis.11 We have noticed in the lungs of Appalachian coal miners (mainly from West Virginia) with complicated coal worker’s pneumonoconiosis a characteristic sign—macrophages laden with silica/silicate particles and anthracotic pigment inside alveoli with associated fibrosis. The degree of alveolar septal (interstitial) fibrosis was variable from no fibrosis and minimal fibrosis to significant alveolar wall thickening, varying in grade from ‘0′ to ‘3′ (in a scale of 0–3) according to McConnochie, et al,3 (1998) ending in coal dust-related diffuse fibrosis that surrounds or is adjacent to the area of coal mine dust desquamative chronic interstitial pneumonia (Figs 1–3). As visible in Figure 3, all these changes are present simultaneously, indicating an ongoing process continuously progressing even for years after exposure to coal dust had stopped. One of the coal miners stopped mining 10 years before his death. All pathological changes from accumulation of silica/silicate particles and anthracotic pigment inside the alveolar macrophages to end-stage fibrosis were present in all our coal miners with complicated coal worker’s pneumoconiosis as pictured in Figures 1–3.
This recurring set of findings that included the presence of anthracotic pigment and silica/silicate-laden macrophages inside alveoli, interstitial fibrosis, and the chronic nature of the process, prompted us to coin the term “coal mine dust desquamative chronic interstitial pneumonia.” The terminal outcome of the process is dust-related diffuse fibrosis. The term “desquamative” is not quite accurate because alveolar macrophages are not cells desquamated from the alveolar walls but egressed from circulation. However, this term is generally accepted and used in the well-known diagnosis of “desquamative interstitial pneumonia” associated with smoking or inorganic particulates12 or aluminum particles13. Therefore, in order to emphasize pathologic similarities between coal mine dust desquamative chronic interstitial pneumonia caused by silica/silicate particles and desquamative interstitial pneumonia caused by smoking or other inorganic particles, we prefer this “desquamative” well-established term. When dust-related diffuse fibrosis is ≥1 cm in greatest dimension, it fulfills the criterion for progressive massive pulmonary fibrosis/complicated coal worker’s pneumoconiosis (Federal Safety Act 1969).6 Although in dust-related diffuse fibrosis, collagen fibers (Fig 3) are parallel, not whorled and irregular, such type of dust-related diffuse fibrosis measuring 1 cm on glass slide will produce on x-ray shadow measuring 1 cm or more (due to dispersion of x-rays) and thus fulfills radiographic criterion for complicated coal worker’s pneumoconiosis.6
We have noted the coal mine dust desquamative chronic interstitial pneumonia in 100% of our autopsy cases with progressive massive pulmonary fibrosis. According to the literature, we could expect that on imaging studies, coal mine dust desquamative chronic interstitial pneumonia will present with irregular opacities14 and a network of septal lines surrounding pulmonary lobules due to diffuse interlobular septal thickening15. We have also confirmed the finding that coal mine dust causes centriacinar emphysema.10,16,17 All our coal miners had centriacinar emphysema and in 20% (5 out of 25) it progressed to bullous emphysema. Fifty-two percent of our patients with complicated coal worker’s pneumoconiosis had marked emphysema. Thirty-three percent of our patients with marked emphysema were non-smokers, confirming that coal dust can cause emphysema independent of smoking.16 In fact one coal miner with bullous emphysema was a non-smoker, while others were cigarette smokers. Our findings are in accordance with the previously established observation that smoking and coal dust simultaneously may cause pulmonary fibrosis and emphysema by similar pathological mechanisms (destruction of lung tissue and healing by fibrosis), so that in some patients emphysema is the dominant feature, in others interstitial fibrosis, and in some others a combined pulmonary fibrosis and emphysema syndrome may occur.18 We conclude according to our findings of emphysema in all non-smoking coal miners (Fig 4), the presence of numerous to frequent silica/silicate particles in thickened septa in emphysematous alveoli, and by deduction from literature10,16–18 that the first step in the genesis of emphysema is accumulation of macrophages laden with silica/silicate particles and anthracotic pigment inside the alveoli, ie, coal mine dust desquamative chronic interstitial pneumonia.
Macrophages in the alveoli are characteristically present in tobacco-related respiratory bronchiolitis and desquamative interstitial pneumonia. We present a basis for distinguishing coal mine dust desquamative chronic interstitial pneumonia from tobacco-related respiratory bronchiolitis and desquamative interstitial pneumonia. In the former, there are many birefringent silica/silicate particles in the macrophages inside the alveoli (about 331/high power field). In the latter, the number of silica/silicate particles is low, about four birefringent particles per high power field, in the range from 0 to 16. We have not studied patients with desquamative interstitial pneumonia, but since it is caused by smoking we can expect that the number of birefringent particles in the macrophages inside alveoli may be similar to that in tobacco-related respiratory bronchiolitis. In cigarette smokers macrophages contain fine brown dust-like smoker granules, which do not polarize light. Alveolar macrophages in smokers may also contain black pigment (soot from cigarette combustion) in the form of very small black, opaque rounded dots. Anthracotic pigment of coal-dust displays characteristic irregular angular contours. In our coal miners smokers, characteristic features of tobacco-caused respiratory bronchiolitis-associated interstitial lung disease with fibrosis19 were not present. It may suggest that in coal miners the contribution of smoking to interstitial fibrosis is insignificant in comparison to the contribution of silica/silicate particles from coal mine dust. In distinction to smokers, the thickened interalveolar septa in coal workers contain birefringent silica/silicate particles and anthracotic pigment. Twenty-four percent (6 out of 25) of our coal miners with progressive massive pulmonary fibrosis had lung carcinoma. Since all our patients with lung cancer were smokers, eventual contribution of coal mine dust to cancer development would be difficult to appraise.
The histological findings we described herein may be of considerable use clinically. We suggest based on our findings and in accordance with the literature that counting of silica/silicate particles under polarized light in the macrophages from the bronchoalveolar lavage specimens may detect coal mine dust desquamative chronic interstitial pneumonia, a precursor of dust-related diffuse fibrosis and of emphysema. Bronchoalveolar lavage has established its role in the pathogenesis, diagnosis, and management of interstitial lung diseases20 including occupational lung diseases21. In bronchoalveolar lavage birefringent silica and silicate particles can be quantified (semi-quantified) with polarized light microscopy by enumerating the percentage of macrophages containing birefringent particles.22 Polarized light microscopy was comparable in sensitivity, specificity, and overall accuracy to electron microscopy, while chemical analysis was less accurate than either microscopic technique.23
Finding of birefringent silica/silicate particles in the bronchoalveolar lavage by polarized light might be important in certain worker’s compensation cases. These cases involve uncertain or contested diagnoses obtained from radiographic imaging and functional respiratory testing studies. It is obvious from our findings that patients with coal mine dust diseases will have a significant number of birefringent silica/silicate particles in alveolar lavage specimens, since alveoli contain these particles in macrophages from the beginning of the disease to end-stage disease in the form of coal mine dust-related diffuse fibrosis. Since patients with tobacco-associated respiratory bronchiolitis with interstitial fibrosis do not have or have very small number of silica/silicate particles in their alveoli, examination of the bronchoalveolar lavage by polarized light might easily resolve whether interstitial lung fibrosis visible on imaging study is due to coal mine dust or cigarette smoking. This new disease—coal mine dust desquamative chronic interstitial pneumonia—contributes to the understanding of the genesis of coal dust-related diffuse fibrosis and provides histological evidence of simultaneous presence of coal dust-related fibrosis and birefringent silica/silicate particles in the macrophages inside alveoli. Our findings also provide morphologic differences between interstitial fibrosis caused by coal mine dust and that caused by cigarette smoking. Our finding that interstitial lung fibrosis caused by smoking is negligible in magnitude compared with that caused by silica/silicate particles, and our corroboration of previously known fact that emphysema is also caused by silica/silicate particles might be useful in worker’s compensation cases in disputes regarding the degree of contribution of coal mine dust particles vs smoking to interstitial lung fibrosis and emphysema.
Furthermore, while counting intracellular birefringent particles is time-consuming and inconvenient, given the degree of specificity conferred by identifying coal mine dust particles, it may be sufficient to create diagnostic criteria of either (1) yes—silica/silicate particles are present in significant numbers or (2) no—silica/silicate particles are few, or not present (Fig 5).
Detection of early pathological changes caused by coal mine dust may have a beneficial effect on coal miners regarding an early individual therapeutic intervention, for example, in the form of whole lung lavage24 and safety-precaution interventions in the working environment. Whole lung lavage can reduce the dust burden in the patients with pneumoconiosis,24,25 resulting in immediate symptomatic improvement,24 and thus be effective therapy for silicosis, acute silicoproteinosis26 and especially for early silicosis and accelerated silicosis27. Repeating whole lung lavage is reasonable and safe treatment with positive long-term effects in silicosis.28
Additionally, because the main toxic agents in coal dust (silica and silicate particles)8 are also present in the working environment of stone workers,29 denim sandblasters,30 and hydraulic fracturing workers,31 eventually the quantification of silica/silicate particles in the macrophages of bronchioloalveolar lavage specimens might have role in early therapy, occupational safety, and prevention in this population of workers, and in public health policy.
In conclusion, our study demonstrated the utility of quantification of silica/silicate particles in intra-alveolar macrophages to distinguish coal mine dust-related from cigarette smoking-related lung disease. Histopathological findings constituting the presence of anthracotic pigment and silica/silicate-laden macrophages inside alveoli, with chronic progressive interstitial fibrosis, occurring even decades after coal mine dust exposure, prompted us to coin the term coal mine dust desquamative chronic interstitial pneumonia as a novel disease entity and precursor of coal mine dust-related diffuse fibrosis and of emphysema. We conclude, based on a review of autopsy lungs from patients with complicated coal worker’s pneumoconiosis, that the role of smoking in causing fibrosis is insignificant in comparison to that of silica/silicate particles from coal mine dust.
We propose significant therapeutic and public health benefits may be provided by counting of silica/silicate particles in the macrophages of bronchioalveolar lavage specimens to allow early detection of coal mine dust desquamative chronic interstitial pneumonia, a precursor of dust-related diffuse fibrosis and of emphysema.
TAKE-HOME MESSAGE.
The first sign of exposure to coal mine dust is accumulation of silica/silicate particles and anthracotic pigment inside alveolar macrophages and associated interstitial fibrosis. These findings are the characteristic hallmark of a new disease—coal mine dust desquamative chronic interstitial pneumonia.
Coal mine dust desquamative chronic interstitial pneumonia is a precursor of coal dust-related diffuse fibrosis and of emphysema.
In coal miners with complicated coal worker’s pneumoconiosis, the role of smoking in causing fibrosis is insignificant in comparison with that of silica/silicate particles from coal mine dust.
Acknowledgments
Financial Support: None.
Footnotes
Conflicts of Interest: None declared.
References
- 1.Petsonk EL, Rose C, Cohen R. Coal mine dust lung disease. New lessons from old exposure. Am J Respir Crit Care Med. 2013;187:1178–85. doi: 10.1164/rccm.201301-0042CI. [DOI] [PubMed] [Google Scholar]
- 2.Brichet A, Wallaert B, Gosselin B, et al. “Primary” diffuse interstitial fibrosis in coal miners: a new entity? Study Group on Interstitial Pathology of the Society of Thoracic Pathology of the North. Rev Mal Respir. 1997;14:277–85. [PubMed] [Google Scholar]
- 3.McConnochie K, Green FHY, Vallyathan V, et al. Interstitial fibrosis in coal workers: experience in Wales and West Virginia. Annals of Occup Hygiene. 1988;32:553–60. [Google Scholar]
- 4.Nagai A, Yamawaki I, Thurlbeck WM, Takizawa T. Assessment of lung parenchymal destruction by using routine histologic tissue sections. Am Rev Respir Dis. 1989;139:313–19. doi: 10.1164/ajrccm/139.2.313. [DOI] [PubMed] [Google Scholar]
- 5.Cagle PhT. Color Atlas and Text of Pulmonary Pathology. Philadelhia Baltimore New York London Buenos Aires Hong Kong Sydney Tokyo: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2008. p. 299. [Google Scholar]
- 6.Federal Coal Mine Health and Safety Act of 1969 Public Law 91-173, 30 U.S.C. ch. 22, 801 et seq. 1969.
- 7.McDonald JW, Roggli VL. Detection of silica particles in lung tissue by polarizing light microscopy. Arch Pathol Lab Med. 1995;119:242–6. [PubMed] [Google Scholar]
- 8.Cohen RA, Petsonk EL, Rose C, et al. Lung Pathology in U.S. Coal Workers with Rapidly Progressive Pneumoconiosis Implicates Silica and Silicates. Am J Resp Crit Care Med. 2016;193:673–80. doi: 10.1164/rccm.201505-1014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollock DE, Potts JD, Joy GJ. Investigation into dust exposures and mining practices in mines in the southern Appalachian region. Min Engl. 2010;62:44. [Google Scholar]
- 10.Churg A, Zay K, Li K. Mechanisms of mineral dust-induced emphysema. Environ Health Perspect. 1997;105:1215–18. doi: 10.1289/ehp.97105s51215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schns RP, Borm PJ. Mechanisms and mediators in coal dust induced toxicity: a review. Ann Occup Hyg. 1999;43:7–33. doi: 10.1016/s0003-4878(98)00069-6. [DOI] [PubMed] [Google Scholar]
- 12.Abraham JL, Hertzberg MA. Inorganic particulates associated with desquamative interstitial pneumonia. Chest. 1981;80:67–70. doi: 10.1378/chest.80.1_supplement.67s. [DOI] [PubMed] [Google Scholar]
- 13.Herbert A, Sterling G, Abraham J, Corrin B. Desquamative interstitial pneumonia in an aluminum welder. Hum Pathol. 1982;13:694–9. doi: 10.1016/s0046-8177(82)80291-8. [DOI] [PubMed] [Google Scholar]
- 14.Young RC, Rachal RE, Carr PG, Press HC. Patterns of coal workers’ pneumoconiosis in Appalachian former coal miners. J Natl Med Assoc. 1992;84:41–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Thrumurthy SG, Kearney S, Sissons M, Haider Y. Diffuse interlobular septal thickening in a coal miner. Thorax. 2010;65:82–4. doi: 10.1136/thx.2009.121418. [DOI] [PubMed] [Google Scholar]
- 16.Santo T. Emphysema and chronic obstructive pulmonary disease in coal miners. Curr Opin Pulm Med. 2011;17:123–5. doi: 10.1097/MCP.0b013e3283431674. [DOI] [PubMed] [Google Scholar]
- 17.Cohen RA, Patel A, Green FH. Lung disease caused by exposure to coal mine and silica dust. Semin Respir Crit Care Med. 2008;29:651–61. doi: 10.1055/s-0028-1101275. [DOI] [PubMed] [Google Scholar]
- 18.Jankowich MD, Rounds SI. Combined pulmonary fibrosis and emphysema syndrome: a review. Chest. 2012;141:222–31. doi: 10.1378/chest.11-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yousem SA. Respiratory bronchiolitis-associated interstitial lung disease with fibrosis is a lesion distinct from fibrotic nonspecific interstitial pneumonia: a proposal. Mod Pathol. 2006;11:1474–9. doi: 10.1038/modpathol.3800671. [DOI] [PubMed] [Google Scholar]
- 20.Daniele RP, Elias JA, Epstein PE, Rossman MD. Bronchoalveolar lavage: role in the pathogenesis, diagnosis, and management of interstitial lung disease. Ann Intern Med. 1985;102:93–108. doi: 10.7326/0003-4819-102-1-93. [DOI] [PubMed] [Google Scholar]
- 21.Cordeiro CR, Jones JC, Alfaro T, Ferreira AJ. Bronchoalveolar lavage in occupational lung diseases. Semin Respir Crit Care Med. 2007;28:504–13. doi: 10.1055/s-2007-991523. [DOI] [PubMed] [Google Scholar]
- 22.Moreira VB, Ferreira AS, Soares PJ, et al. The role of bronchoalveolar lavage in quantifying inhaled particles in silicosis. Rev Port Pneumol. 2005;11:457–75. doi: 10.1016/s0873-2159(15)30522-5. [DOI] [PubMed] [Google Scholar]
- 23.Christman JW, Emerson RJ, Hemenway DR, et al. Effects of work exposure, retirement, and smoking on bronchoalveolar lavage measurements of lung dust in Vermont granite workers. Am Rev Respir Dis. 1991;144:1307–13. doi: 10.1164/ajrccm/144.6.1307. [DOI] [PubMed] [Google Scholar]
- 24.Mason GR, Abraham JL, Hoffman L, et al. Treatment of mixed-dust pneumoconiosis with whole lung lavage. Am Rev Resp Dis. 1982;126:1102–07. doi: 10.1164/arrd.1982.126.6.1102. [DOI] [PubMed] [Google Scholar]
- 25.Wilt JL, Banks DE, Weissman DN, et al. Reducion of lung dust burden in pneumoconiosis by whole-lung lavage. J Occup Environ Med. 1996;38:619–24. doi: 10.1097/00043764-199606000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Stafford M, Cappa A, Weyant M, et al. Treatment of acute silicoproteinosis by whole-lung lavage. Semin Cardiothorac Vasc Anesth. 2013;17:152–9. doi: 10.1177/1089253213486524. [DOI] [PubMed] [Google Scholar]
- 27.Zhang YM, Zhang HT, Wang Cy, et al. Long-term therapeutic effects of whole lung lavage in the management of silicosis. Zhonghua Lao Dong Eei Sheng Zhi Ye Bing Za Zhi. 2012;30:690–3. [in Chinese] [PubMed] [Google Scholar]
- 28.Zhang YM, Wang W, Wang CY, et al. The long – term therapeutic effects of silicosis by repeat the whole lung lavage. Zhonghua Lao Dong Eei Sheng Zhi Ye Bing Za Zhi. 2013;31:681–4. [in Chinese] [PubMed] [Google Scholar]
- 29.Kramer MR, Blanc PD, Fireman E, et al. Artificial stone silicosis: disease resurgence among artificial stone workers. Chest. 2012;142:419–24. doi: 10.1378/chest.11-1321. [DOI] [PubMed] [Google Scholar]
- 30.Bakan ND, Özkan G, Çamsari G, et al. Silicosis in denim sandblasters. Chest. 2011;140:1300–4. doi: 10.1378/chest.10-1856. [DOI] [PubMed] [Google Scholar]
- 31.Esswein EJ, Breitenstein M, Snawder J, et al. Occupational exposures to respirable crystalline silica during hydraulic fracturing. J Occup Environ Hyg. 2013;10:347–56. doi: 10.1080/15459624.2013.788352. [DOI] [PubMed] [Google Scholar]


