Abstract
The clinical successes of immune checkpoint therapies for cancer make it important to identify mechanisms of resistance to anti-tumor immune responses. Numerous resistance mechanisms have been identified employing studies of single genes or pathways, thereby parsing the tumor microenvironment complexity into tractable pieces. However, this limits the potential for novel gene discovery to in vivo immune attack. To address this challenge, we developed an unbiased in vivo genome-wide RNAi screening platform that leverages host immune selection in strains of immunocompetent and immunodeficient mice to select for tumor cell-based genes that regulate in vivo sensitivity to immune attack. Utilizing this approach in a syngeneic Triple-Negative Breast Cancer (TNBC) model, we identified 709 genes that selectively regulated adaptive anti-tumor immunity, and focused on five genes (CD47, TGFβ1, Sgpl1, Tex9 and Pex14) with the greatest impact. We validated the mechanisms that underlie the immune-related effects of expression of these genes in different TNBC lines, as well as tandem synergistic interactions. Furthermore, we demonstrate the impact of different genes with previously unknown immune functions (Tex9 and Pex14) on anti-tumor immunity. Thus, this innovative approach has utility in identifying unknown tumor-specific regulators of immune recognition in multiple settings to reveal novel targets for future immunotherapies.
Keywords: In Vivo Genome-wide RNAi Screen, Triple-Negative Breast Cancer, Cancer-Derived Resistance to Immunotherapy, Functional Genomics
Introduction
The recent success of PD-1/PD-L1 immune checkpoint inhibition, along with the trial results using chimeric antigen receptor (CAR) T cell therapies has prompted a renewed interest in the use of immunotherapy in cancer [1–5]. For example, the impact of checkpoint inhibition has proven successful in melanoma and lung cancer and is in Phase III trials for many malignancies, such as Triple-Negative Breast Cancer (TNBC) [6]. The use of immunotherapy strategies has been limited, since there is accumulating evidence that multiple mechanisms of immune-resistance may limit the efficacy of immunotherapy in different tumor microenvironments (TMEs), resulting in only a significant minority of patients with benefits [7]. While some of these resistance mechanisms are known, it is likely that many others contribute to immunosuppression in the TME [8–10]. Therefore, it is essential to identify the molecular mechanisms within the tumor microenvironment to overcome resistance to immunotherapy.
The tumor microenvironment is composed of a heterogeneous population of cell types, whose cross-talk defines the mechanisms of pro- and anti-tumor immunity. This heterogeneous microenvironment makes it challenging to unravel these pathways using traditional models, especially with in vitro models that incompletely replicate tumor complexity [11]. As such, studies of tumor immunosuppression have focused on in vivo models, which have revealed that tumors can directly modulate immune activation and recognition through a variety of mechanisms. Some of these include: modulation of cell surface receptors such as MHC class I, PD-L1, B7 family members, CD40, ICOSL, and OX40L, as well as the expression of immune-related soluble factors such as IL-4, indoleamine-pyrrole 2,3 dioxygenase (IDO) and TGF-β [11–13]. The expression of these genes in the local TME can disrupt the recruitment, activation, and survival of cytotoxic immune cells, thereby limiting the magnitude and effectiveness of the anti-tumor immune response [14, 15]. As such, while focused studies of single genes in tumor cells using in vivo models have determined and validated specific immunosuppressive mechanisms, it is unclear how many mechanisms exist or which ones are dominant in specific cancers.
The recent widespread use of pooled shRNA- and CRISPR-based gene targeting libraries has enabled the rapid screening of genes to identify those involved in different cellular processes, including: proliferation, attachment, metastasis [16–23]. To date, no studies have explored if this approach could interrogate the response of malignant cells to immune selection in an in vivo setting. Such an approach should allow for higher-order dissection of dominant pathways, enabling prioritization of critical nodes that can be therapeutically targeted. These screens have largely been performed on infected cells in vitro, although they have expanded to studies using immunodeficient mice in vivo [1, 17–23]. In these in vivo studies, several groups have discovered determinants of resistance to small molecules [2, 24–26], while others have identified genes important in T cell infiltration [3, 16]. Most recently, Zhou et al infused genome-wide library-infected T cells into tumor bearing mice to identify T cell genes that are relevant to the in vivo anti-cancer immune response [4, 5, 16]. While these approaches demonstrate the utility of in vivo screening, no studies to date have utilized the approach we describe here to interrogate the impact of tumor-based genes in the context of host immunity.
We hypothesized that an in vivo screen utilizing an shRNA-transduced syngeneic TNBC line would facilitate the discovery of genes that impact anti-tumor immunity through screen-based comparisons of tumors in different immune competent mouse models. As such, we performed the first in vivo genetic screen to identify tumor-gene mediators of the anti-tumor adaptive immune response. Screening studies were performed using EO771 cells, a murine TNBC cell line that arose in a C57Bl/6 background. Using a whole genome shRNA library, we first generated pooled knockdown (KD) EO771 cell populations that were pre-screened in vitro to eliminate genes that intrinsically regulate cell proliferation and survival. These EO771-library populations were then engrafted in syngeneic immunocompetent or immunocompromised C57Bl/6 mice. Tumor growth in these mice reflected the differences in host immunity, suggesting immune selection of tumor cell populations. Differential analyses of the high throughput screening data revealed that the silencing of 709 individual genes significantly impacted anti-tumor immunity. Through a combination of bioinformatics analysis and literature review, we selected five candidates for individual validation. Through shRNA mediated gene knockdown, we validated the importance of three previously identified immune regulatory pathway genes (TGFβ1, CD47 and Sgpl1), as well as two novel genes (Pex14 and Tex9) that had no prior known immune functions. This general approach thus identifies tumor cell-based molecules that regulate adaptive anti-tumor immunity to TNBC, some of which can be potentially targeted in conjunction with existing and evolving immunotherapies.
Methods
Cell Lines
EO771 (gift from Dr. Peter Goedegeburre, Washington University in St. Louis, MO), HEK293T (Georgetown Tissue Culture Shared Resource (TCSR)), 4T1 (Duke University), and JC (Duke University) cells were cultured at 37°C with 5% CO2 in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum, 1x L-glutamine, and 100 units/ml of penicillin and streptomycin. All cell lines were periodically tested and determined to be free of Mycoplasma and other rodent pathogens.
Library Transduction
EO771 cells were transduced with lentivirus containing the pre-packaged shRNA library and Transdux (SBI) in 5 separate T-75 flasks. Infection was performed at an MOI of 3.75:1 in order to integrate shRNAs into 54.5% of cells (according to a positive-control empty vector infection assay), reducing the probability of multiple shRNAs per cell. After 72 hrs, the cells were selected using 5 μg/ml of puromycin for 10 days, whereupon they were cultured without puromycin for 11 more days. Library cells were cultured in vitro for 21 days, to ensure loss of cells containing shRNAs that inhibited in vitro proliferation.
Animal xenograft studies
2 × 106 EO771 cells were engrafted in the right flank of female C57Bl/6 (Jackson), C57Bl/6-SCID (Jackson), and NSG (Jackson) mice all aged 6–8 weeks. 5 × 10 5 JC cells and 104 4T1 cells were engrafted in the right flank of female Balb/c mice, all aged 6–12 weeks. Tumors were measured twice-weekly using calipers. Volume was calculated as length × width2/2. Once tumors reached 1 cm3, the mouse was euthanized using CO2 inhalation, and the tumors were excised under sterile conditions. For culturing, 3.75 mg (75μl at 50 μg/μl) gentamycin was added. After one or two passages cells were cryopreserved and RNA or supernatant was isolated. For immune infiltrate analysis, a section of the tumor was minced and digested in 1% Collagenase D (Sigma) at 37°C for 30 minutes while shaking vigorously. All studies with animals were approved by the Georgetown University Institutional Animal Care and Use Committee or the Duke University Institutional Animal Care and Use Committee.
Library shRNA amplification and hybridization
Tumor shRNAs were amplified and biotinylated according manufacturer’s instructions (SBI). Briefly, mRNA was reverse transcribed into cDNA, shRNAs were amplified and biotinylated using nested primers (SI691B-1; SIB), and non-shRNA DNA was degraded using a lambda exonuclease (NEB M0262L). For the first two in vivo screens, two tumor samples were combined (4.5 μg of each) and hybridized to one Affymetrix GeneChip Mouse Genome 430 2.0 array at the Genomics Core at George Washington University Medical Center. In the first screen, 4 arrays (corresponding to 8 tumors) for tumors grown in C57Bl/6 mice, 5 arrays (corresponding to 10 tumors) for tumors in SCID mice, and 5 arrays (corresponding to 10 tumors) for tumors in NSG mice. In the second screen, 4 arrays (corresponding to 8 tumors) for tumors grown in C57Bl/6 mice, 5 arrays (corresponding to 10 tumors) for tumors in SCID mice, and 5 arrays (corresponding to 5 biological replicates) for cultured EO771-library cells. For the initial library representation, 9 ug of shRNA DNA from each EO771-library freezedown was utilized and hybridized to one Affymetrix array. Each array was analyzed using R version 2.15.2.
Differential shRNA Analysis
Expression values for each shRNA were obtained using proprietary software from System Biosciences. Log transformed expression values were compared between immune-competent and immune-deficient samples using LIMMA version 3.14.4 [27]. shRNAs with an absolute Log2 fold change greater than 1 and FDR values for empirically Bayes moderated t-statistics below 0.05 were considered to have statistically significant differential expression. An shRNA was defined as existing in a population if it had a 2-fold higher expression than the background.
Real-time PCR
RNA was isolated using an RNA isolation kit (Ambion; Catalog 12183018A). RNA concentration was identified spectrophotometrically using the NanoDrop 1000 (Thermo Scientific, Waltham, MA). RNA was reverse-transcribed in 25 μl using Omniscript RT (Qiagen). Quantitative PCR was performed using QuantiTect SYBR Green with predesigned Quantitech Primer Assays for CD47, Pex14, TGFβ1, Tex9, Sgpl1 and GAPDH (Qiagen) according to the manufacturer’s instructions (Qiagen). The reactions were performed using the MastercyclerRealPlex2 Real-Time PCR system (Eppendorf). Analysis was performed by using the ΔΔCT method, with GAPDH as the endogenous loading control.
Western Blotting & Flow Cytometry
Protein expression was performed by immunoblotting or flow cytometry. Protein was isolated using a modified boiling technique and protein concentration was determined by the BCA assay (Bio-Rad). Cell pellets were lysed by heating to 100°C for 10 minutes in 100 μl of Boiling Buffer (1% SDS, 10 mM Tris, 1 mM Na3NO4, ddH20, and 1 protease inhibitor tablet (Sigma: S8820)). Approximately 30 μg of protein was run on Tris-glycine gels under reducing conditions. After transfer to a nitrocellulose membrane, the membrane was blocked with 5% milk for 1 hour (Bio-Rad). Primary blotting for proteins was performed overnight at 4°C in PBS-T with 5% milk using the following antibodies: Tex9 (Sigma; SAB2103771; 1:750), Sgpl1 (Abcam; ab56183), Pex14 (Sigma; SAB4502176; 1:500), TGFβ1 (Abcam; ab66043) and beta-actin (Sigma; clone AC-74, 1:2500). Membranes were exposed to a secondary antibody in PBS-T for 1 hour at room temperature (horseradish peroxidase (HRP)-labeled anti-rabbit or anti-mouse IgG; 1:5000; GE Healthcare). Supersignal West Femto high-sensitivity substrate and Supersignal West Pico (Thermo Scientific) were utilized for visualization of the western blots. The phycoerythrin (PE)-conjugated antibody was used for flow cytometry analysis of CD47 (Biolegend; clone Miap301).
ELISAs
Cell supernatants were obtained by passaging 105 cells in 2 ml of media in 6 well plates for 48 hrs or 72 hrs. TGFβ1 protein concentration was determined using the TGFβ1 quantikine ELISA Kit (R&D Systems Catalog: DB100B). Following the manufacturer’s recommendations, latent TGFβ1 was activated using HCl and NaCl and TGFβ1 existing in base-media was subtracted from the analysis.
shRNA Knockdown
shRNA plasmids, validated through the RNAi consortium (TRC), were purchased from Sigma or Open Biosystems. Lentivirus was created by transfecting HEK293T cells at 60% confluence in 10 cm plates with 6 μg shRNA DNA, 3.0 μg psPAX2 (Addgene plasmid 12260), 0.3 μg pCMV-VSV-G (gift from Dr. Todd Waldman, Georgetown University Medical Center), and 18 μl of Fugene 6 (Promega; Madison, WI). 24 hours after transfection, media was replaced with normal growth medium. 48 hours after transfection, virus-containing media was isolated, centrifuged at 250 μg, and filtered using a 0.45-μm syringe filter. Virus media was frozen at −80°C or exposed to target cells immediately. EO771 cells were transduced at 50% confluency in a T-25 flask with 3.5 ml virus media, 0.5 ml fresh media, and 3.2 μg polybrene (Sigma; St. Louis, MO). Cells were selected after 48 hours with 7.5 μg/ml of Puromycin (Sigma). After 10 days of selection, cells were assessed for protein knockdown.
Overexpression
For CD47 overexpression, EO771 cells were transfected at 50% confluency in a 12 well dish with 1μg cDNA plasmids (Origene) and Fugene 6 (Promega; E2691) to induce expression of the desired transcript. After 48 hrs of exposure, EO771 cells were selected using 0.5 μg/ml neomycin (G418) for 10 days. Transfected cells were maintained in culture with 0.1 μg/ml of G418. For TGFβ1 overexpression, murine TGFβ1 was cloned into the CDH vector (no selection marker) and lentivirus was created using the method described above. Cells were infected with TGFβ1 virus using the same conditions used for shRNA introduction.
Anti-CD47 Antibody In Vivo Study
The antagonistic anti-murine CD47 antibody, VX-1000R, was provided by Tioma Therapeutics, Inc. (St. Louis, MO) for preclinical testing in our in vivo model system. 10 EO771 engrafted C57Bl/6 mice (106 cells in the right flank) were intraperitoneally injected with 133 μg of VX-1000R three times a week. Tumors were measured twice weekly and sacrificed once tumors reached 1000 mm3.
Illumina Array Analysis
20 μl at 45 ng/μl for each sample was hybridized to Illumina MouseWG-6 v2.0 Expression BeadChip. The Genomics and Epigenomics Shared Resource at Georgetown University performed the hybridization and obtained expression values. Three biological replicates of EO771-shScramble, -shSgpl1, -shPex14, -shCD47, and –shTex9 were all analyzed. Illumina data was preprocessed using the R package lumi version 2.16.0 using log2-variance stabilization and quantile normalization [28, 29]. Gene annotations were found using the R package LumiMouseIDMapping version 1.10.0 [30]. SVA analysis was performed to eliminate batch effects [31]. Log transformed expression values were compared between cell types using LIMMA version 3.14.4 [27]
Survival Analysis
Survival analysis for all mouse xenograft studies was performed using Prism 5 (GraphPad). Survival plots were considered significantly different if the log-rank (Mantel-Cox) test resulted in a p ≦ 0.05.
Pathway Analysis
Pathway analysis of significant genes targeted by shRNAs was performed using Pathway Studio [32]. Briefly, all statistically significant shRNAs, targeting the annotated genes (157 in total), and their aliases, were uploaded to Pathway Studio for pathway creation. Selecting for direct connections between gene candidates identified enriched pathways. Statistically significant pathways were identified if the Jaccard index was ≦ 0.05 or the p ≦ 0.05.
Statistical Analysis
All statistical analysis, unless otherwise noted, was performed using GraphPad Prism version 5 (GraphPad Software; La Jolla, CA). Marks for significance include: *, for p-value < 0.05; **, for p-value < 0.01; and ***, for p-value < 0.001.
Results
Creation of the TNBC shRNA Library Model System
As TNBC has shown a high degree of immune infiltration and some responsiveness to checkpoint antibody inhibition, we chose to conduct our proof-of-principle study using the murine TNBC cell line, EO771. EO771 is a long-used syngeneic breast adenocarcinoma cell line that spontaneously arose in a C57Bl/6 mouse in 1939 [7, 33–35]. The C57Bl/6 EO771 cell line grows aggressively, subcutaneously and in the mammary fat pad of immune-tolerant syngeneic C57Bl/6 mice, mimicking analogous human tumor:host immune interactions. Additionally, EO771 cells resemble human triple-negative/basal breast cancer, as they are estrogen-insensitive, HER2-negative, and contain a mutated p53 [8–10, 36]. After preparation of an shRNA library profiling 150,000 unique shRNA constructs (targeting 39,000 mRNA transcripts), we infected EO771 cells using a multiplicity of infection (MOI) of 3.75, which resulted in a successful copy number integration of 0.545, thereby limiting the possibility of multiple shRNA integrations per cell. At this MOI/integration efficiency, the relative expression of each shRNA across two representative libraries was well preserved (Fig. 1b). Transduced cells were cultured in vitro for 21 days, thereby creating an initial library containing shRNAs with limited effect on in vitro cell fitness. Therefore, we expected a significant loss in shRNA representation compared to the pooled shRNA library input. This necessarily reduced the sensitivity of the screen, as the likelihood of having multiple independent shRNAs targeting a single gene was diminished. Specifically, the pooled library contained 150,000 shRNAs, while our infected and cultured EO771-library cells have only about 20,000 integrated shRNAs. However, this strategy limited the impact of genes that principally mediated cell growth. Additionally, having a smaller number of unique shRNA transcripts in the pooled EO771-library cells enhanced target representation in the in vivo EO771-library samples. This approach allowed us to focus on immunity-relevant genes and to essentially increase the representation of non-synthetically lethal genes 7.5-fold in the subsequent in vivo screens. We considered the possibility that a given shRNA-mediated knockdown might enhance the proliferation of its targeted EO771 cell, leading to that cell’s overrepresentation in the library. Accordingly, we analyzed the shRNA expression of four randomly chosen semi-independent freeze-downs of the same initial EO771-library cells 21 days post-transduction and did not find an overrepresentation of a few shRNAs (Fig. 1c). Occurring across all four replicates, 20,408 shRNAs targeted 14,679 unique genes. An shRNA was defined as existing in the cell population if it had a relative expression value two-fold above the background, in order to ensure that shRNA was truly there. It is possible some shRNAs were lost while using this cutoff threshold. While this approach eliminated many shRNAs due to their impacts on tumor cell proliferation, plating efficiency or poor transduction efficiency, the resulting ‘pre-screened’ population allowed for a focused examination of immune-relevant genes in the subsequent screening experiments described below.
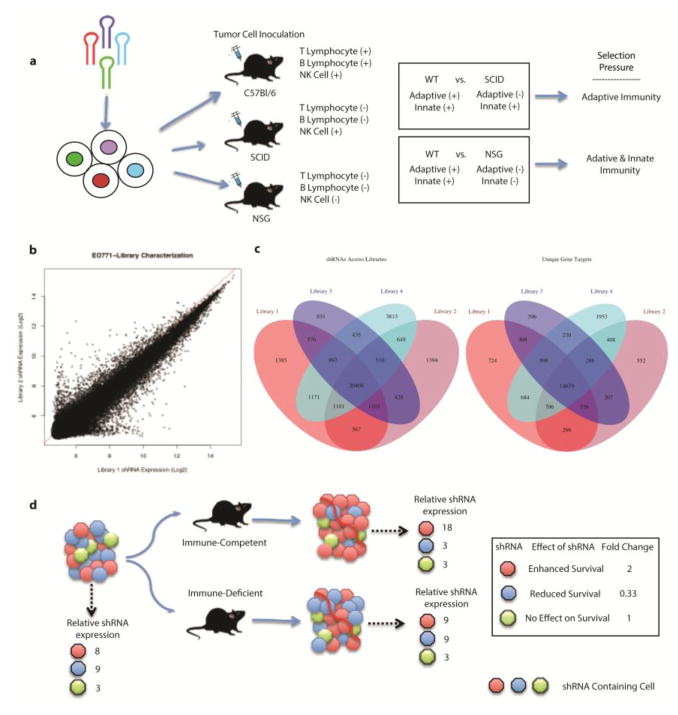
Figure 1.
Schematic of in vivo RNAi screening approach to identify tumor-based modulators of immune selection. a) EO771 cells were infected with a lentivirus delivered genome-wide shRNA library in order to have only 1 shRNA integration per cell. EO771-library cells were engrafted in mice with varying levels of immunity; C57Bl/6, C57Bl/6-SCID, and NSG mice. Differential analysis of barcoded was used to identify functional mediators of the anti-tumor immune response. b) Raw shRNA expression values from two separate replicates of EO771-library cells. The similarity of relative expression values for each shRNA is represented by the identity line y=x, represented in red. c) Integration of shRNA and gene targets from four separate replicates of EO771-library cells describes the overlap of gene targets found in each. d) EO771-library cells that contain an shRNA that enhances survival in WT but not in immune-deficient mice are represented as red containing cells. Blue shRNA containing cells are preferentially eliminated in immune-competent mice. shRNAs with no effect on survival from the immune system will expand at similar rates in both mice (green).
In vivo shRNA pooled screen to identify tumor-based immune modulators
Having produced an shRNA library for immune-directed approaches, primary and secondary in vivo screens were performed. Three separate strains of mice were utilized: wild-type (WT) C57Bl/6, which possess intact adaptive and innate immune systems, C57Bl/6-SCID (PrkdcSCID), which lack mature T and B lymphocytes, and NSG (NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ) which lack functional T and B lymphocytes, natural killer cells, complement and defective macrophages and dendritic cells. Comparisons of the representation of cells with specific gene knockdowns amongst these different mouse strains allowed us to determine genes whose KD regulated cell survival in the face of adaptive immunity (WT vs. SCID or vs. NSG), as well as those that played a role in innate immunity (SCID vs. NSG). Figure 1d provides a schematic of the in vivo screen design to demonstrate how we prioritized candidate shRNAs. To ensure the validity of identified candidates, a repeat in vivo screen was performed using only the WT and SCID mice, as well as 5 biological replicates of the initial EO771-library.
When the library cells were engrafted in each strain of mice (n=10 per strain), tumor growth corresponded inversely to intensity of immune selection pressure, with median survival of 17 days in NSG mice, 21 days in SCID mice and 31 days in wild-type mice (Fig. 2a). Wild-type, empty vector (EV), and library cells grew at equivalent rates in C57Bl/6 mice, suggesting that lentiviral transduction had little effect on the induction of an immune response (Supp. Fig. 1). The repeat screen was performed using C57Bl/6-SCID and C57Bl/6 mice (n=10 per strain), with median survivals of 20 and 36 days, respectively. For both screens, as expected, the combined shRNA repertoire derived after in vivo growth was significantly reduced compared to the size of the repertoire in the initial EO771-library inoculum, suggesting the presence of in vivo selection pressure (Fig. 2b).
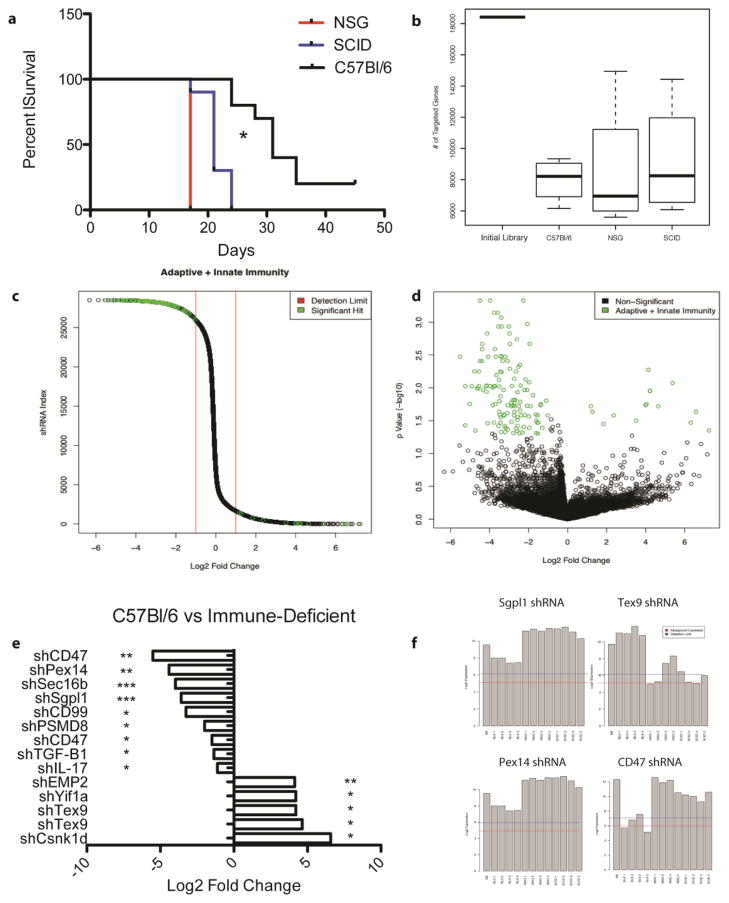
Figure 2.
In vivo RNAi screen identifies shRNAs gained or lost when EO771-library tumor cells are grown in an immune competent host. All results shown are from the initial in vivo screen and include analysis of all arrays run for each in vivo condition (4 arrays for C57BL/6, 5 for SCID, 5 for NSG). a) Survival of mice engrafted with EO771-library cells. Both BL6 vs. SCID and BL6 vs. NSG were statistically significant. * = Mantel-Cox test with p <0.05. b) Total number of genes targeted by shRNAs across each tumor strain. c) Waterfall plot of relative fold change of each shRNA in the C57Bl/6 vs. NSG analysis. Green circles represent those shRNAs with an adjusted p value (q value) < 0.05. d) Volcano plot of each shRNA by log2 fold change and q value. Green circles represent those hits with a absolute log2 fold change > 1 and q < 0.05. e) Combined results of significant shRNAs from the two independent RNAi screens. f) Relative levels of shRNAs from the first screen in each individual tumor found through the Affymetrix arrays. B8 initial represents the starting shRNA repertoire. The red line represents background array expression. The blue line is considered to be the detection limit for an shRNA in the tumor. Each screen was performed with 10 mice per group and two tumors (equal amounts) were combined for each array. WT= wild-type mouse, SCID = severe combined immunodeficient mouse, NSG = NOD SCID Gamma mouse. * indicates a q < 0.05; ** indicates a q < 0.01; *** indicates a q < 0.001.
We next performed differential expression analysis to identify significantly over- or under-represented shRNAs between the C57Bl/6 WT versus SCID and between the C57Bl/6 WT versus NSG strains (Fig. 2c, Fig. 2d). To do this, we combined tumor samples from two mice onto a single array, in an effort to identify those shRNAs with the most consistent changes. As the most well described differences between C57Bl/6 WT mice and the immune deficient strains are the constitution of the immune system, we considered these hits to be potential tumor cell-based regulators of response to immune attack. The significant gene candidate results of both screens and both comparisons were combined to facilitate analysis and significant hits are presented in Table 1. A full listing of the pairwise comparison significant hits is included in Supplemental Tables 1 and 2.
Table 1.
Combined results of significant shRNAs from two independent in vivo screens
| Average # in C57B1/6 tumors | Average # in SCID tumors | Average # in NSG tumors | Gained in C57B1/6 vs. SCID + NSG | Lost in C57B1/6 vs. SCID + NSG | |
|---|---|---|---|---|---|
| Screen 1 | |||||
| Genes targeted | 7978 | 9253 | 8609 | 14 | 196 |
| Unique shRNAs | 9828 | 11457 | 10834 | 13 | 184 |
| Screen 2 | |||||
| Genes targeted | 15186 | 11264 | N/A | 3 | 565 |
| Unique shRNAs | 23813 | 14416 | N/A | 3 | 541 |
| Enriched pathways | Significant genes in indicated pathway | ||||
| TGF-βR signaling | Tgfb1, Elk1, Rps6ka5, Smad3, Smad5, Mef2d | ||||
| Immune regulation | Cd47 (2), Iglc2 (2)*, Il17, Ifnb1, H2-Q5, H2-Q6 | ||||
| Sphingolipid metabolism | Sgpl1*, Smpd2, Glt8d2, B4galnt2 | ||||
| Leukocyte adhesion to endothelial cells | Cd99, Vcam1, Cxcl11, Sele | ||||
| MHC class I antigen presentation | Psmd8 (2)*, Uba52, Ubc, Rps27a | ||||
Genes highlighted by a * were significant in both screens. A (2) following the gene name indicates two unique shRNAs were identified as significant
Target Selection and Validation
To further select candidates for validation, we performed a pathway analysis of significant hits from the two screens. Utilizing Pathway Studios, we created a compartmentalized map of known connections between each of the significant targets. This identified the selective under-representation of shRNAs targeting the TGFβ receptor, sphingosine metabolism, and immune regulation (Table 1). Of the over-represented shRNAs, testis expressed gene 9 (Tex9), was of particular interest, as it was targeted by multiple significant shRNAs. More than 700 significantly contracted shRNAs were identified across both screens but sphingosine phosphate lyase 1 (Sgpl1), peroxisomal biogenesis factor 14 (Pex14), TGFβ1, and CD47 were identified in both screens or because they were targeted by multiple unique shRNAs (Fig. 2e). Figure 2f describes the relative expression of four selected shRNAs across the different strains of mice. Interestingly, the peroxisome has been implicated as having a role in the biology of triple-negative breast cancer, strengthening the likelihood that a peroxisome-related hit is a true gene candidate [11, 37]. CD47, also known as integrin-associated protein, is an important receptor involved in numerous physiological processes, most importantly as an inhibitor of phagocytosis [11–14, 38, 39]. Recently, CD47 has been identified as a promising immune checkpoint, whose inhibition can lead to anti-tumor immune responses [15, 39]. To further select gene candidates, those with no known human homolog (eg., VR13) or were uncharacterized genes (eg. RIKEN or ENSEMBLE genes) were not selected for functional validation. Therefore, based on multiple significant shRNAs targeting the same genes (CD47 and Tex9), significant shRNAs occurring in multiple screens (Sgpl1 and Pex14), and a pathway analysis (TGFB1), we chose CD47, TGFβ1, Pex14, Tex9, and Sgpl1 for functional validation studies.
To validate the findings from our in vivo screens, EO771 cells were transduced with lentivirus encoding a unique shRNA for each target gene. It should be noted that the shRNAs utilized were chosen to differ from those used in the genome-wide library. After protein knockdown was validated (Supp. Fig. 2a–i, Supp. Fig. 3a–f), EO771 shRNA cells were engrafted in C57Bl/6 or C57Bl/6-SCID mice. Tumor growth rates and surviving cell populations were analyzed for each shRNA KD-validated cell line. Each individual validation was performed at least 3 times with equivalent results for each. The following figures are representative of one of the in vivo validations. Of the gene candidates, Tex9 KD cells exhibited growth enhancement and Pex14, Sgpl1, CD47, and TGFβ1 KD cells exhibited growth reduction when exposed to an intact adaptive immune system, as compared with a control cell line (WT or scramble shRNA) (Fig. 3, Fig. 4, Fig. 5). It should also be noted that two other significant shRNAs targeting Yif1a and EMP2 were selected for functional validation but their in vivo responses did not validate our screening results, therefore they were excluded from further analysis.
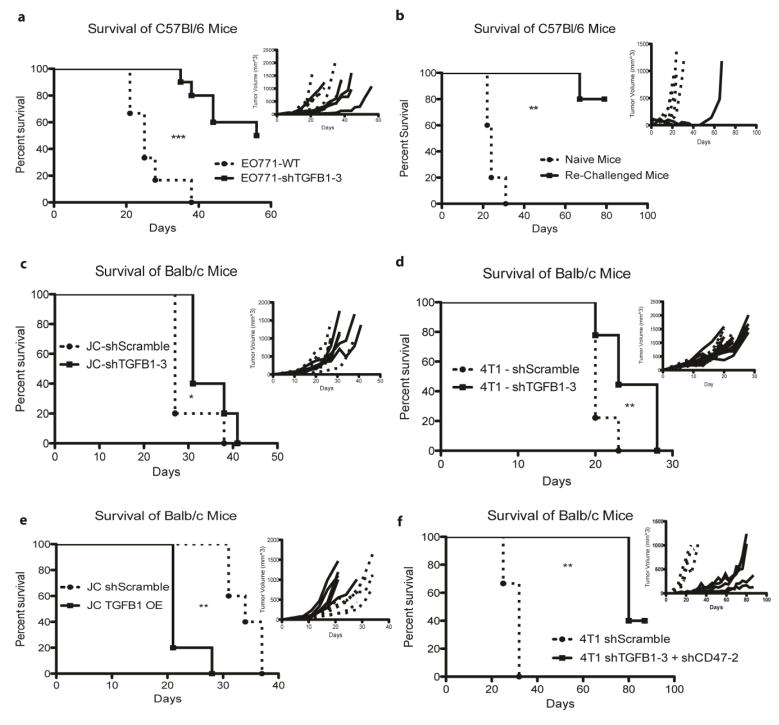
Figure 3.
Loss of TGFB1 enhances in vivo adaptive immune response to Triple Negative Breast Cancer cell lines in multiple mouse strains. a) In vivo analysis of C57Bl/6 mice engrafted with EO771 (2*106) cells with KD of TGFB1. b) Surviving mice from a) and naïve C57Bl/6 mice were challenged at day 90 post initial tumor engraftment with 2.5 times the initial inoculum of EO771-WT cells (5*106 cells) to assess for an anti-tumor adaptive memory response. Representative in vivo analysis of Balb/c mice engrafted with 5*105 JC TGFβ1 KD cells (c) and 104 4T1 TGFβ1 KD cells (d). e) Overexpression of TGFβ1 in JC cells results in rapid growth of tumors in immune competent Balb/c mice. f) Engraftment of 4T1 cells with double KD of TGFβ1 and CD47 in WT Balb/c mice results in the retardation of tumor growth. N = 5 mice per group. Each panel is a representative survival and growth kinetics analysis (panel insert) from one animal study and was repeated two times with equivalent results. The dotted lines and circle markers indicate cells expressing the shScramble, while solid lines and square markers represent the cells with the indicated shRNA knockdown or protein overexpression.
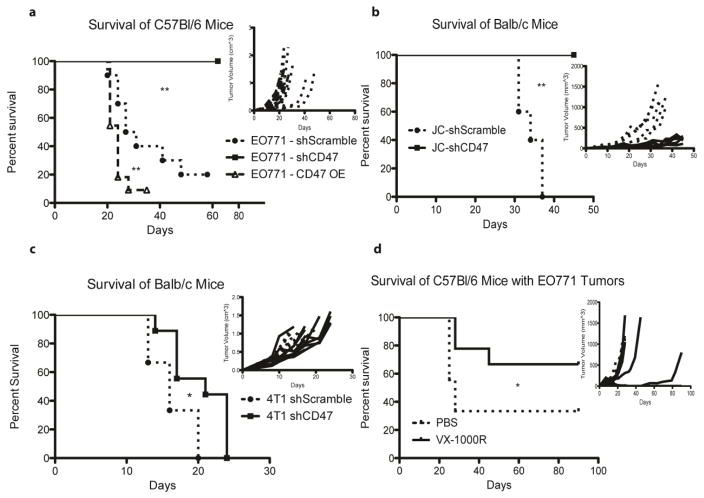
Figure 4.
Abrogation of CD47 signaling enhances in vivo adaptive immune response to Triple Negative Breast Cancer cell lines in multiple mouse strains. a) In vivo analysis of WT C57Bl/6 mice engrafted with 2*106 EO771 cells with CD47 shRNA KD, a scrambled shRNA control, or CD47 overexpression (OE). b) and c) In vivo analysis of WT Balb/c mice engrafted with 5*105 JC cells with CD47 KD and 104 4T1 cells with CD47 KD, respectively. d) Representative in vivo analysis of C57Bl/6 mice engrafted with 106 EO771-WT cells treated with PBS or the anti-CD47 antibody VX-1000R. N = 10 mice per group for a) and d). N = 5 mice per group for b) and c). Each panel is a representative survival and growth kinetics analysis (panel insert) from one animal study and was repeated at least once with equivalent results. The dotted lines and circle markers indicate mice engrafted with cells expressing the shScramble, solid lines and square markers represent those mice engrafted with cells with CD47 shRNA knockdown, dashed lines and triangle markers indicate mice engrafted with cells with CD47 overexpression.
Figure 5.
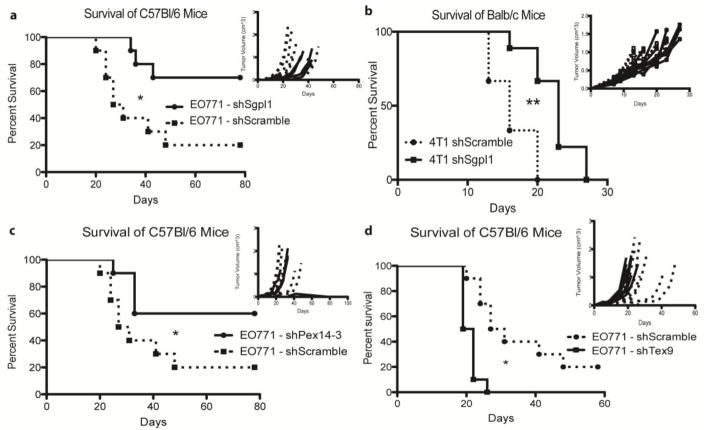
Knockdown of three novel genes (Sgpl1, Pex14, and Tex9) augment the antitumor immune response in multiple models of TNBC in an immune competent setting. a) and b) In vivo analysis of C57Bl/6 and Balb/c mice, respectively, engrafted with 2*106 EO771 or 104 4T1 cells, with scrambled shRNA or Sgpl1 shRNA integration. N = 10 mice per group for a) and b). c) and d) In vivo analysis of C57Bl/6 mice, engrafted with 2*106 EO771 cells, with scrambled shRNA, Pex14 shRNA (c), or Tex9 shRNA integration (d). N = 10 mice per group. Each panel is a representative survival and growth kinetics analysis (panel insert) from one animal study and was repeated at least once with equivalent results (3 times for EO771 C57Bl/6 studies, 2 times for 4T1 Balb/c studies). The dotted lines and circle markers indicate mice engrafted with cells expressing the shScramble, solid lines and square markers represent those mice engrafted with cells with the targeted gene shRNA knockdown.
Previously Validated Immune Regulator: TGFβ1
Transforming growth factor β1, or TGFβ1, has been observed to have paradoxical roles in tumor development and progression of a variety of cancers [16–23, 40]. In the EO771 cell line, knockdown of TGFβ1 resulted in a significant increase in the survival of only engrafted immune-competent mice, with 50% of the mice rejecting the tumor (Fig. 3a, Supp. Fig. 4). To determine if the inhibition of TGFβ1 signaling in the surviving mice was sufficient to induce an adaptive memory response to EO771 cells, mice were re-challenged with 5*106 WT EO771 cells (2.5 times the initial inoculum) at day 90-post initial engraftment. As shown in Figure 3b, naïve C57Bl/6 mice succumbed due to this tumor challenge rapidly, while 4 of the 5 re-challenged mice remained tumor free. The fifth mouse developed a quickly growing tumor around day 50 post re-challenge, suggesting the selective survival of an immune-shielded variant.
To assess the importance of TGFβ1 on the adaptive immune response of alternative TNBC model systems, TGFβ1 shRNA was transduced in 4T1 and JC TNBC cells syngeneic to Balb/c mice. Engraftment of KD cell lines resulted in the modest reduction in survival of immune competent mice (Supp. Fig. 3 c & e, Fig. 3c–d). The decrease in growth suggests that while tumor cell-based TGFβ1 might have some effect on adaptive immune responses, its knockdown alone is not capable of inducing a potent anti-cancer response. To determine if TGFβ1 overexpression (OE) resulted in tumor growth in these models, JC-TGFβ1 OE and 4T1-TGFβ1 OE cells were engrafted in immune competent mice (Supp. Fig. 3 b & d). While overexpression of TGFβ1 did not significantly influence the growth or survival of 4T1 engrafted mice, JC-TGFβ1 OE engrafted mice exhibited significantly reduced survival (Fig. 3e).
Previously Validated Immune Checkpoint: CD47
To further clarify the role of CD47 in our model system, we over-expressed CD47 on the surface of EO771 cells and found an accelerated growth of engrafted tumors in C57Bl/6 mice (Fig. 4a). The differential effects of CD47 on survival were only evident when CD47 OE or KD cells were grown in immune-competent mice, suggesting that CD47 manipulation affects more than the shielding of target cells from macrophage phagocytosis, since SCID mice have functional macrophages (Supp. Fig. 5). Global RNA analysis, utilizing an Illumina expression array, identified a subset of genes differentially regulated by the knockdown of CD47 expression in EO771 cells (Supp. Fig. 6). Of interest is the upregulation of genes previously found to be associated with the chemotaxis and migration of immune cells; namely Cathepsin H, CCL4, ENPP2, and SEMA7a. Therefore, it is likely that abrogation of CD47 signaling in tumor cells not only enhances macrophage-mediated phagocytosis but also the enhanced infiltration of immune cells into the tumor microenvironment.
We then attempted to validate the significance of CD47 signaling in the syngeneic TNBC cell lines 4T1 and JC by reducing CD47 expression through shRNA introduction followed by inoculating these cells in syngeneic immune competent mice. Interestingly, in the JC cell line, knockdown of CD47 resulted in a significant increase in the survival of Balb/c mice, further highlighting the importance of CD47 signaling in an immune competent setting (Fig. 4b). Reduced expression of CD47 in the 4T1-Balb/c model system resulted in a modest but significant increase in the survival of immune-competent mice (Fig. 4c). In an effort to validate the translational significance of our findings, we performed an in vivo CD47 antibody study with immune-competent C57Bl/6 mice. Beginning on the day of tumor engraftment, mice were treated thrice weekly with a murine antibody (VX-1000R) that binds to murine CD47. In the EO771 model system, VX-1000R treated mice were able to completely reject tumor growth in 60% of mice, with reduced growth of the remaining tumors (Fig. 4d). Each experiment was repeated at least once (5 mice per group), with equivalent results.
With TGFβ1 and CD47 validated as critical to the anti-tumor immune response in the EO771-C57Bl/6 and JC-Balb/c triple negative model systems, we further interrogated the effect of combined knockdown in the highly aggressive line 4T1. When both TGFβ1 and CD47 were knocked down in 4T1 cells, an apparent enhanced anti-tumor effect was noticed, as the double-KD tumors grew more slowly than either individual KD cell line (n=5, repeated once) (Fig. 3f). The apparent benefits of simultaneously targeting these two systems highlight the potential of the screening method described here to identify therapeutically targetable resistance mechanisms to adaptive immune selection.
Novel Immune Regulators: Sgpl1, Pex14, and Tex9
As TGFβ1 and CD47 have previously been shown to influence the adaptive immune response against tumors, we sought to validate identified genes with little or no prior known immune regulatory function. Sgpl1, or sphingosine-1 phosphate lyase, is a member of the sphingosine metabolism pathway that directly and irreversibly degrades sphingosine-1 phosphate (S1P) [41]. shRNA-transduced Sgpl1 knockdown in EO771 cells led to a significant reduction in survival in both wild-type and SCID mice, with 70% of mice surviving tumor challenge at day 80 post tumor engraftment in immune competent mice (Fig. 5a, Supp. Fig. 7a). Interestingly, EO771-shSgpl1 cells grew slower in C57Bl/6-SCID mice, suggesting that a component of the innate immune system is affected by lower Sgpl1 levels (and higher S1P levels) in the tumor microenvironment (Supp. Fig. 7a). Similar to the other validated targets, knockdown of Sgpl1 in the 4T1-Balb/c model system resulted in a modest but significant enhancement in survival of engrafted mice (Figure 5b). Interestingly, knockdown of Sgpl1 resulted in the overexpression of immune related genes; including Enpp2, Cck, and CeaCam2 (Supp. Fig. 8).
Pex14 and Tex9, both of which have no previously validated function in tumor-based adaptive immunity, also were identified in these screens. Knockdown of Pex14 in EO771 cells resulted in a significant increase in survival of WT C57Bl/6 mice challenged with these tumor cells (Fig. 5c). In contrast, knockdown of the previously understudied gene, Tex9, in EO771 cells resulted in a significant decrease in the survival of immune competent C57Bl/6 mice (Fig. 5d). In both of these cell lines, the effect was only seen in immune competent mice, with little to no effect in SCID mice (Supp. Fig 7b–c). Global RNA analysis, utilizing an Illumina expression array, identified a subset of genes differentially regulated by the knockdown of Pex14 and Tex9 expression in EO771 cells (Supp. Fig. 9a–b). Of interest is the upregulation of genes previously found to be associated with the immune response; namely Tiam1, Enpp2, and IL13ra1. Therefore, this screening method can identify tumor-based genes that enhance or reduce the adaptive immune response against TNBC. Each experiment was performed three times with equivalent results using 10 mice per immune competent group and 5 per immune deficient group.
Discussion
This is the first description of a high-throughput in vivo screening approach to identify tumor genes that regulate the anti-tumor immune responses. In our study, we utilized a pre-screening strategy to first eliminate shRNAs targeting genes essential for cell growth, and then subjected this population to in vivo immune selection pressures through their implantation into different immune-competent mouse models. Differential analysis revealed 709 genes that were potentially relevant to the anti-tumor adaptive immune response in TNBC. Pathway analysis of these genes revealed the over-representation of multiple interconnected processes/pathways (TGF-β signaling, MHC Class I antigen presentation, immune regulation, sphingolipid metabolism, and leukocyte adhesion) that are essential to tumor immune modulation. To confirm that these targets were important for immune function in TNBC, we validated selected hits using shRNAs in multiple mouse TNBC lines. These studies validated the importance of these pathways in suppressing anti-tumor immunity across different lines and mouse strains.
TGFβ1 signaling was validated as a critical regulator of anti-tumor immunity in these studies, and its reduction induced immune memory, as cured mice were able to resist tumor-rechallenge (Fig. 3b). Attempts to pharmacologically inhibit TGFβ1 have been attempted clinically, but have not yet elicited striking anti-tumor immunity on a consistent basis [42]. This could be due to a variety of factors that include the stage of tumor being treated (our studies employed tumor cells with suppressed TGFβ1 expression at the time of implantation), as well as heterogeneity within tumors. To this point, our studies also revealed a less pronounced anti-tumor phenotype after TGFβ1 suppression in certain TNBC lines, such as the highly aggressive 4T1 line. In 4T1 cells, TGFβ1 inhibition is not sufficient to elicit anti-tumor immunity but is highly effective when combined with CD47 inhibition. As these pathways have been linked in different settings, it is likely that compensatory signaling could offset the anti-tumor effect of single pathway suppression in certain contexts [43, 44]. This suggests that combinatorial approaches may be needed for effective TGF-β targeted immunotherapy. Thus, this work suggests that TGFβ1 may be an excellent target for combinatorial approaches to elicit anti-tumor adaptive immunity. The dual KD of CD47 and TGFB1 likely works through the perturbation of the interaction of both proteins with the secreted protein, thrombospondin 1 (TSP1). TSP1 is a direct binding partner of CD47 and has been shown to inhibit angiogenesis, modulate extracellular matrix formation and cell migration, suppress to tumor, and most importantly, activate latent TGFB1 [45, 46]. Thus, a reduction in CD47 on the cell surface of cells allows for higher micro environmental levels of TSP1, potentially leading to the activation of more TGFB1, which has been directly shown in murine osteoblasts [44]. Therefore, in our model system, co-inhibition of both TGFB1 after CD47 inhibition, likely inhibits the potential for compensatory TGFB1 mechanisms from occurring.
This work also revealed that a cluster of genes with known immune regulatory function could have a profound effect on anti-tumor adaptive immunity. CD47 is one of these genes and has previously been shown to inhibit macrophage-mediated lysis through binding to SIRPα on macrophages. In previous studies, antagonism of CD47 led to enhancement in tumor cell lysis by macrophages as well as an increase in CD8+ T cells in a syngeneic murine model [47]. However, the impact of CD47 blockade on immunity in our studies was largely T-cell mediated, as knockdown or forced overexpression of CD47 did not alter the growth of EO771 tumors in C57Bl/6-SCID mice, which contain macrophages but lack T-cells. As CD47 is a highly expressed cell surface molecule on many tumors [38], we also tested if antibody-mediated blockade could elicit an anti-tumor phenotype. We found that while one CD47-directed antibody had an anti-tumor effect, it was less effective compared to knockdown of CD47, suggesting functions for CD47 that cannot be blocked by antibodies, or a need for more intense antibody dosing to overcome the large in vivo CD47 sink in mice.
Another critical pathway revealed in this work is that of sphingolipid metabolism. While studies have demonstrated that this metabolic process impacts immunity, its relation to the anti-tumor immune response is less clear. Our studies focused on sphingosine-1-phosphate lyase 1 (Sgpl1), which has been shown to cleave phosphorylated sphingoid bases, mainly S1P, which influence T-cell migration [48, 49]. We found that suppression of Sgpl1 had a significant, selective effect on resistance to anti-tumor immune attack in immune competent mice and that this effect is present in multiple TNBC lines and mouse strains. A recent report by van der Weyden et al identified a number of genes important for the metastasis of melanoma and TNBC cells in an immune competent setting [50]. They employed an in vivo RNA-seq metastasis screen in immune competent mice across a variety of cell lines and mouse strains, and identified the importance of sphingosine-1-phosphate (S1P) in local immune cell trafficking. Blocking the release of S1P through the genetic deletion of its extracellular transporter, S1P transporter spinster homologue 2 (Spns2) led to an increased infiltration of effector T cells and natural killer (NK) cells into the metastatic tumor site, lowering the metastatic burden. Since the target Sgpl1, identified and validated in our in vivo screens, directly and irreversibly degrades S1P and results in a similar phenotype, our findings are consistent with an impact of Sgpl1 knockdown on its immune stimulated phenotype [50]. Therefore, we hypothesize that knockdown of Sgpl1 works to enhance immune cell tumor destruction by increasing the microenvironment levels of S1P, leading to an increase in NK cell and CD8 T cell trafficking into the tumor. It should also be noted that EO771-shSgpl1 cells grown in SCID mice have a significant delay in growth as compared with NSG mice, suggesting that NK cells are capable of slowing the growth of these tumors alone (Supp. Fig. 7a). While this pathway is known to have inflammatory and immune involvement, it has not yet been targeted in tumor immunotherapeutic approaches. As such, these findings suggest that pharmacologic inhibition of this metabolic pathway could enhance anti-tumor immunity. In line with the previous effect of CD47 and TGFB1 co-KD, it is likely that combining TGFB1 KD with Sgpl1 knockdown will also have a dramatic effect on tumor growth, since similar compensatory mechanisms exist between S1P and TGFB signaling. Both TGFB1 and Sgpl1’s binding target S1P, activate the phosphorylation of SMAD3 in cultured cells [51–53]. On top of this TGFB1 has been shown to increase S1P mRNA expression and S1P can transactivate the TGFBR pathway [54], suggesting a level of crosstalk between the two pathways. In our model system, KD of Sgpl1 enhances S1P expression and can potentially lead to an increase in TGFBR signaling. Therefore, activation of the TGFB1 pathway might be a compensatory mechanism in TNBC cancer to Sgpl1 elimination, suggesting the combination as potentially synergistic.
Finally, this screening approach also identified a plethora of genes now implicated in immunity-related control of tumor growth that have not yet been linked with any specific immune related functions. We explored this class of genes by focusing on the impact of two genes, Tex9 and Pex14, which were shown to play contrasting roles in tumor immunosuppression. Tex9 has no known function, only being first identified in the Mammalian Gene Collection [55]. We found that knockdown of Tex9 enhanced growth in immunocompetent but not immunocompromised mice. This pattern suggests that Tex9 could function as a tumor antigen in EO771 tumors, but this possibility has not been formally explored. In contrast, Pex14 knockdown reduces the representation of targeted cells in immunocompetent mice, and thus may play a role in tumor immunosuppression. Pex14 is a peroxisomal membrane protein that plays a role in peroxisomal import machinery and can also act as a transcriptional co-repressor [56, 57]. While Pex14 has been implicated in TNBC [37], it has not been associated with any role in tumor immunosuppression. Our results suggest that it may be important for tumor immunosuppression in TNBC. These validation studies demonstrate the ability of this screening approach to identify novel regulators of tumor immunity, thereby providing a path to the identification of new targets for immunotherapy.
By utilizing an RNAi screening approach in an in vivo setting, we have identified multiple conserved pathways that underlie tumor-based immune evasion, without introducing bias towards an individual cell type or interaction, and have identified genes whose knockdown promotes tumor rejection in immunocompetent, but not immunodeficient syngeneic mice. The potential of this functional screening approach is underscored by the identification of several major pathways and novel molecules that regulate the response to anti-tumor immune attack. This screening approach can be used with more powerful immune selection imposed by immune checkpoint inhibition, with the potential to identify novel resistance mechanisms in vivo; such studies are ongoing in our laboratory. Alternatively, some genes that regulate anti-tumor immune responses in these and related screens might serve as prognostic or predictive biomarkers when tested in the human setting. Therefore, the preclinical and clinical utility of this screening method could have important implications for the discovery and development of novel immune therapeutic strategies.
Supplementary Material
Acknowledgments
This research was supported by the following Shared Resources at Lombardi Comprehensive Cancer Center: The Genomics & Epigenomics Shared Resource, the Flow Cytometry & Cell Sorting Shared Resource, and the Tissue Culture Shared Resource. All Lombardi Comprehensive Cancer Center Shared Resources are partially supported by National Institutes of Health (NIH)/National Cancer Institute (NCI) grant P30-CA051008. An antibody to CD47 was kindly provided by Dr. Robert Karr, Tioma Therapeutics, Inc (St. Louis, MO).
Funding Sources:
This manuscript was supported by NIH Grants CA50633 (Louis M Weiner) and CA51880 (Louis M Weiner), and Susan G. Komen Career Catalyst Research Grant CCR14299200 (Zachary C Hartman)
Abbreviations
- BCA
Bicinchoninic acid assay
- CaeCam2
Carcinoembryonic Antigen related cell adhesion molecule 2
- CCK
Cholecystokinin
- CCL4
C-C motif chemokine ligand 4
- CO2
Carbon Dioxide
- CRISPR
Clustered regularly interspaced short palindromic repeats
- ENPP2
Ectonucleotide Pyrophosphatase/phosphodiesterase 2
- FDR
False Discovery Rate
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
- HCL
Hydrogen Chloride
- IDO
Indoleamine-pyrrole 2,3 dioxygenase
- IgG
Immunoglobulin G
- IL-4
Interleukin 4
- IL13Ra1
interleukin 13 receptor subunit alpha 1
- KD
Knocked Down
- LIMMA
Linear Models for Microarray Data
- MOI
Multiplicity of Infection
- NSG
NOD-SCID-IL2 Gamma Chain knock-out
- NaCl
Sodium Chloride
- OE
Overexpression
- OX40L
Tumor Necrosis Factor (ligand) superfamily, member 4
- P53
Tumor Protein P53
- Pex14
Peroxisomal Membrane Protein 14
- RNAi
RNA interference
- S1P
Sphingosine-1-phosphate
- SBI
System Biosciences
- SEMA7a
Semaphorin 7a
- Sgpl1
Sphingosine-1-phosphate ligase 1
- shRNA
Short Harpin RNA
- SMAD3
SMAD Family member 3
- Spns2
S1P transporter spinster homologue 2
- Tex9
Testis Expressed Gene 9
- Tiam1
T-Cell Lymphoma invasion and metastasis 1
- TME
Tumor Microenvironment
- TNBC
Triple-Negative Breast Cancer
- TSP1
Thrombospondin 1
- WT
Wild-Type
Footnotes
Conflict of Interest:
The authors have no conflicts of interest to report or disclose.
References
- 1.Wolchok JD, Kluger H, Callahan MK, et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N Engl J Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodi FS, O’Day SJ, McDermott DF, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol. 2012;30:2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 4.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porter DL, Levine BL, Kalos M, et al. Chimeric Antigen Receptor–Modified T Cells in Chronic Lymphoid Leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liedtke C, Mazouni C, Hess KR, et al. Response to Neoadjuvant Therapy and Long-Term Survival in Patients With Triple-Negative Breast Cancer. J Clin Oncol. 2008;26:1275–1281. doi: 10.1200/JCO.2007.14.4147. [DOI] [PubMed] [Google Scholar]
- 7.Creelan BC. Update on immune checkpoint inhibitors in lung cancer. Cancer Control. 2014;21:80–9. doi: 10.4161/21624011.2014.967147. [DOI] [PubMed] [Google Scholar]
- 8.Leto SM, Trusolino L. Primary and acquired resistance to EGFR-targeted therapies in colorectal cancer: impact on future treatment strategies. J Mol Med. 2014;92:709–722. doi: 10.1007/s00109-014-1161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Napolitano S, Martini G, Rinaldi B, et al. Primary and Acquired Resistance of Colorectal Cancer to Anti-EGFR Monoclonal Antibody Can Be Overcome by Combined Treatment of Regorafenib with Cetuximab. Clin Cancer Res. 2015;21(13):2975–83. doi: 10.1158/1078-0432.CCR-15-0020. [DOI] [PubMed] [Google Scholar]
- 10.Hiba Zahreddine KLBB. Mechanisms and insights into drug resistance in cancer. Front Pharmacol. 2013;4(28):1–8. doi: 10.3389/fphar.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunn GP, Bruce AT, Ikeda H, et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. doi: 10.1038/ni1102-991. [DOI] [PubMed] [Google Scholar]
- 12.Driessens G, Kline J, Gajewski TF. Costimulatory and coinhibitory receptors in anti-tumor immunity. Immunol Rev. 2009;229:126–144. doi: 10.1111/j.1600-065X.2009.00771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shuptrine CW, Surana R, Weiner LM. Monoclonal antibodies for the treatment of cancer. Sem Cancer Biol. 2012;22:3–13. doi: 10.1016/j.semcancer.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Surana R, Wang S, Xu W, et al. IL4 Limits the Efficacy of Tumor-Targeted Antibody Therapy in a Murine Model. Cancer Immunol Res. 2014;2:1103–1112. doi: 10.1158/2326-6066.CIR-14-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou P, Shaffer DR, Alvarez Arias DA, et al. In vivo discovery of immunotherapy targets in the tumour microenvironment. Nature. 2014;506:52–57. doi: 10.1038/nature12988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zender L, Xue W, Zuber J, et al. An Oncogenomics-Based In Vivo RNAi Screen Identifies Tumor Suppressors in Liver Cancer. Cell. 2008;135:852–864. doi: 10.1016/j.cell.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen S, Sanjana NE, Zheng K, et al. Genome-wide CRISPR Screen in a Mouse Model of Tumor Growth and Metastasis. Cell. 2015;160:1246–1260. doi: 10.1016/j.cell.2015.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolf J, Müller-Decker K, Flechtenmacher C, et al. An in vivo RNAi screen identifies SALL1 as a tumor suppressor in human breast cancer with a role in CDH1 regulation. Oncogene. 2013;33:4273–4278. doi: 10.1038/onc.2013.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrysik Z, Kim J, Tan AC, Espinosa JM. A Genetic Screen Identifies TCF3/E2A and TRIAP1 as Pathway-Specific Regulators of the Cellular Response to p53 Activation. Cell Rep. 2013;3:1346–1354. doi: 10.1016/j.celrep.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeung ML, Houzet L, Yedavalli VSRK, Jeang KT. A Genome-wide Short Hairpin RNA Screening of Jurkat T-cells for Human Proteins Contributing to Productive HIV-1 Replication. J Biol Chem. 2009;284:19463–19473. doi: 10.1074/jbc.M109.010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hattori H, Zhang X, Jia Y, et al. RNAi screen identifies UBE2D3 as a mediator of all-trans retinoic acid-induced cell growth arrest in human acute promyelocytic NB4 cells. Blood. 2007;110:640–650. doi: 10.1182/blood-2006-11-059048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berens EB, Sharif GM, Schmidt MO, et al. Keratin-associated protein 5-5 controls cytoskeletal function and cancer cell vascular invasion. Oncogene. 2016;36:593–605. doi: 10.1038/onc.2016.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seyhan AA, Varadarajan U, Choe S, et al. A genome-wide RNAi screen identifies novel targets of neratinib resistance leading to identification of potential drug resistant genetic markers. Mol BioSyst. 2012;8:1553. doi: 10.1039/c2mb05512k. [DOI] [PubMed] [Google Scholar]
- 25.Berns K, Horlings HM, Hennessy BT, et al. A Functional Genetic Approach Identifies the PI3K Pathway as a Major Determinant of Trastuzumab Resistance in Breast Cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 26.Brummelkamp TR, Fabius AWM, Mullenders J, et al. An shRNA barcode screen provides insight into cancer cell vulnerability to MDM2 inhibitors. Nat Chem Biol. 2006;2:202–206. doi: 10.1038/nchembio774. [DOI] [PubMed] [Google Scholar]
- 27.Smyth GK, Michaud J, Scott HS. Use of within-array replicate spots for assessing differential expression in microarray experiments. Bioinformatics. 2005;21:2067–2075. doi: 10.1093/bioinformatics/bti270. [DOI] [PubMed] [Google Scholar]
- 28.Du P, Zhang X, Huang C-C, et al. Comparison of Beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinformatics. 2010;11:587. doi: 10.1186/1471-2105-11-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin SM, Du P, Huber W, Kibbe WA. Model-based variance-stabilizing transformation for Illumina microarray data. Nucleic Acids Res. 2007;36:e11–e11. doi: 10.1093/nar/gkm1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan Du WAKSML. nuID: a universal naming scheme of oligonucleotides for Illumina, Affymetrix, and other microarrays. Biol Direct. 2007;2:16. doi: 10.1186/1745-6150-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leek JT, Johnson WE, Parker HS, et al. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28:882–883. doi: 10.1093/bioinformatics/bts034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nikitin A, Egorov S, Daraselia N, Mazo I. Pathway studio--the analysis and navigation of molecular networks. Bioinformatics. 2003;19:2155–2157. doi: 10.1093/bioinformatics/btg290. [DOI] [PubMed] [Google Scholar]
- 33.Sugiura K, Stock CC. Studies in a tumor spectrum.I. Comparison of the action of methylbis(2-chloroethyl)amine and 3-bis(2-chloroethyl)aminomethyl-4-methoxymethyl-5-hydroxy-6-methylpyridine on the growth of a variety of mouse and rat tumors. Cancer. 1952;5:382–402. doi: 10.1002/1097-0142(195203)5:2<382::AID-CNCR2820050229>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 34.Sugiura K, Hitchings GH, Cavalieri LF, Stock CC. The Effect of 8-Azaguanine on the Growth of Carcinoma, Sarcoma, Osteogenic Sarcoma, Lymphosarcoma and Melanoma in Animals. Cancer Res. 1950;10:178–185. [PubMed] [Google Scholar]
- 35.Snell GD, Cloudman AM. The Effect of Rate of Freezing on the Survival of Fourteen Transplantable Tumors of Mice. [Accessed 22 Jul 2016];Cancer Res. http://cancerres.aacrjournals.org/content/canres/3/6/396.full.pdf.
- 36.Johnstone CN, Smith YE, Cao Y, et al. Functional and molecular characterisation of EO771.LMB tumours, a new C57BL/6-mouse-derived model of spontaneously metastatic mammary cancer. Dis Model Mech. 2015;8:237–251. doi: 10.1242/dmm.017830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purrington KS, Slager S, Eccles D, et al. Genome-wide association study identifies 25 known breast cancer susceptibility loci as risk factors for triple-negative breast cancer. Carcinogenesis. 2014;35:1012–1019. doi: 10.1093/carcin/bgt404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Willingham SB, Volkmer J-P, Gentles AJ, et al. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc Natl Acad Sci USA. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X, Pu Y, Cron K, et al. CD47 blockade triggers T cell–mediated destruction of immunogenic tumors. Nat Med. 2015;21:1209–1215. doi: 10.1038/nm.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Massagué J. TGFβ in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spiegel S, Milstien S. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol. 2011;11:403–415. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neuzillet C, Tijeras-Raballand A, Cohen R, et al. Targeting the TGFβ pathway for cancer therapy. Pharmacol Ther. 2015;147:22–31. doi: 10.1016/j.pharmthera.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-β by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev. 2000;11:59–69. doi: 10.1016/S1359-6101(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 44.Shimada K, Nakajima A, Ikeda K, et al. CD47 regulates the TGF-β signaling pathway in osteoblasts and is distributed in Meckel’s cartilage. J Oral Sci. 2011;53:169–175. doi: 10.2334/josnusd.53.169. [DOI] [PubMed] [Google Scholar]
- 45.Rogers NM, Yao M, Novelli EM, et al. Activated CD47 regulates multiple vascular and stress responses: implications for acute kidney injury and its management. AJP: Renal Physiology. 2012;303:F1117–F1125. doi: 10.1152/ajprenal.00359.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daniel C, Wiede J, Krutzsch HC, et al. Thrombospondin-1 is a major activator of TGF-β in fibrotic renal disease in the rat in vivo. Kidney Int. 2004;65:459–468. doi: 10.1111/j.1523-1755.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 47.Tseng D, Volkmer J-P, Willingham SB, et al. Anti-CD47 antibody–mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci USA. 2013;110(27):11103–11108. doi: 10.1073/pnas.1305569110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar A, Saba JD. Lyase to live by: Sphingosine phosphate lyase as a therapeutic target. Expert Opin Ther Targets. 2009;13:1013–1025. doi: 10.1517/14728220903039722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alvarez SE, Milstien S, Spiegel S. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metab. 2007;18:300–307. doi: 10.1016/j.tem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 50.Weyden LVD, Arends MJ, Campbell AD, et al. Genome-wide in vivo screen identifies novel host regulators of metastatic colonization. Nature. 2017;541:233–236. doi: 10.1038/nature20792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao J, Liu J, Lee J-F, et al. TGF-β/SMAD3 Pathway Stimulates Sphingosine-1 Phosphate Receptor 3 Expression. J Biol Chem. 2016;291:27343–27353. doi: 10.1074/jbc.M116.740084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Radeke HH, Wenckstern von H, Stoidtner K, et al. Overlapping Signaling Pathways of Sphingosine 1-Phosphate and TGF- in the Murine Langerhans Cell Line XS52. J Immunol. 2005;174:2778–2786. doi: 10.4049/jimmunol.174.5.2778. [DOI] [PubMed] [Google Scholar]
- 53.Xin C, Ren S, Kleuser B, et al. Sphingosine 1-Phosphate Cross-activates the Smad Signaling Cascade and Mimics Transforming Growth Factor- -induced Cell Responses. J Biol Chem. 2004;279:35255–35262. doi: 10.1074/jbc.M312091200. [DOI] [PubMed] [Google Scholar]
- 54.Miller AV, Alvarez SE, Spiegel S, Lebman DA. Sphingosine Kinases and Sphingosine-1-Phosphate Are Critical for Transforming Growth Factor -Induced Extracellular Signal-Regulated Kinase 1 and 2 Activation and Promotion of Migration and Invasion of Esophageal Cancer Cells. Mol Cell Biol. 2008;28:4142–4151. doi: 10.1128/MCB.01465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.The MGC Project Team. The Status, Quality, and Expansion of the NIH Full-Length cDNA Project: The Mammalian Gene Collection (MGC) Genome Res. 2004;14:2121–2127. doi: 10.1101/gr.2596504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dammai V, Subramani S. The Human Peroxisomal Targeting Signal Receptor, Pex5p, Is Translocated into the Peroxisomal Matrix and Recycled to the Cytosol. Cell. 2001;105:187–196. doi: 10.1016/S0092-8674(01)00310-5. [DOI] [PubMed] [Google Scholar]
- 57.Albertini M, Rehling P, Erdmann R, et al. Pex14p, a Peroxisomal Membrane Protein Binding Both Receptors of the Two PTS-Dependent Import Pathways. Cell. 1997;89:83–92. doi: 10.1016/S0092-8674(00)80185-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.