Abstract
Histone deacetylases (HDACs) are a family of enzymes that influence expression of genes implicated in tumor initiation, progression and anti-tumor responses. In addition to their canonical role in deacetylation of histones, HDACs regulate many non-canonical targets, such as Signal Transducer and Activator of Transcription 3 (STAT3). We hypothesize that tumors use epigenetic mechanisms to dysregulate CD1d-mediated antigen presentation, thereby impairing the ability of natural killer T (NKT) cells to recognize and destroy malignant cells. In this study, we pre-treated CD1d-expressing tumor cells with HDAC inhibitors (HDACi) and assessed CD1D-dependent NKT cell responses to mantle cell lymphoma (MCL). Pre-treatment with Trichostatin A, a pan-HDACi, rapidly enhanced both CD1d- and MHC class II-mediated antigen presentation. Similarly, treatment of MCL cells with other HDACi resulted in enhanced CD1d-dependent NKT cell responses. The observed changes are due, at least in part, to an increase in both CD1D mRNA and CD1D cell surface expression. Mechanistically, we found that HDAC2 binds to the CD1D promoter. Knockdown of HDAC2 in tumor cells resulted in a significant increase in CD1D-mediated antigen presentation. In addition, treatment with HDACi inhibited STAT3 and STAT3-regulated inflammatory cytokine secretion by MCL cells. We demonstrated that MCL-secreted IL-10 inhibits CD1d-mediated antigen presentation and pretreatment with TSA abrogates secretion of IL-10 by MCL. Taken together, our studies demonstrate the efficacy of HDACi in restoring anti-tumor responses to MCL through both cell-intrinsic and extrinsic mechanisms and strongly implicate a role for HDACi in enhancing immune responses to cancer.
Keywords: immunotherapy, cancer, epigenetics, HDAC inhibitors, NKT cells, CD1d
Introduction
A hallmark of cancer cell survival is their ability to evade immune destruction [1, 2]. This is essential for the tumor because the host immune system possesses the potential to eliminate malignancies through a multi-layered process that includes early recognition of transformation events by mediators of innate immunity, followed by the development of a strong and highly specific adaptive immune response [3]. Natural killer T (NKT) cells are a unique subset of T cells that recognize glycolipid antigens within the context of CD1d, a non-classical MHC class I-like molecule [4-6]. NKT cells have the capacity to mount strong anti-tumor responses and have thus become a major focus in the development of effective cancer immunotherapy [7-9]. In this respect, NKT cells have been shown to augment anti-tumor responses due, in part, to their capacity to rapidly produce large amounts of IFN-γ, which acts on NK cells to target MHC-negative tumors, and also, to promote CD8 cytotoxic T cells mediated killing of MHC-positive tumors [7, 8].
Given the importance of NKT cells in anti-tumor immunity, studies elucidating the mechanisms of tumor escape from NKT cell-mediated anti-tumor immunity are crucial. NKT cells have a well-established role in mediating anti-tumor responses [5, 10]. However, studies from our lab and others have shown that NKT cells are physically and functionally reduced in cancer patients [11-13], which suggests that tumors may utilize various mechanisms to evade NKT cell-mediated immune surveillance. Specifically, we posit that lymphomas utilize epigenetic mechanisms to rapidly undergo immunologic sculpting, allowing poorly immunogenic clones to survive. In light of data demonstrating that tumors dysregulate antigen presentation to escape anti-tumor immune responses [14-17], the focus of our studies is on epigenetic modulation of CD1d-mediated antigen presentation and mechanisms of tumor escape from NKT cell-mediated immune surveillance.
MCL presents a unique target for immunotherapy [18-20]. Although previously thought to be a homogeneous disease, the heterogeneity of MCL is now better understood, with many pathways discovered to be of prognostic value [19]. However, the mechanisms by which these pathways contribute to MCL pathogenesis are not well understood. For example, Signal Transducer and Activator of Transcription 3 (STAT3) is constitutively activated in MCL [21, 22]. Importantly, although STAT3 has been shown to be upregulated in MCL, as well as many other cancers [23], and to contribute to tumor progression and tumor immune evasion [24], the exact mechanisms are not well understood [25].
HDACs are a family of enzymes that regulate diverse cellular events such as gene expression, cell proliferation, and immune pathways through deacetylation of their protein targets [26, 27]. Moreover, HDAC activities are frequently dysregulated in cancer and have been implicated not only in tumor onset, but also tumor progression [26, 27]. Recent evidence demonstrates that HDACs facilitate tumor evasion from immune surveillance and HDAC inhibitors (HDACi) have thus garnered attention as a potential therapy in a variety of cancers [26, 28, 29]. HDACs possess many non-canonical targets, such as the mediator of inflammation STAT3 [30, 31]. Given the well-established role of inflammation in tumor progression [32], we investigated the cell intrinsic and extrinsic mechanisms by which treatment with HDACi regulate CD1d-mediated antigen presentation to NKT cells.
Materials and Methods
Cell Lines
The NKT cell hybridoma cell lines DN32.D3, N38-3C3, and N37-1A12 have been previously described [8, 33, 34]. The cells were cultured in IMDM medium supplemented with 5% FBS, 2 mM L-glutamine and Penicillin/Streptomycin. LCD1dwt cells are CD1d1-transfected mouse fibroblast cells, which were kindly provided by Dr. Randy Brutkiewicz (Indiana University School of Medicine, Indianapolis, IN, USA). The LCD1dwt cells were cultured in DMEM supplemented with 10% FBS, 2 mM L-glutamine, 500 μg/mL G418 as a selection agent and Penicillin/Streptomycin. DR4-restricted 17.9 CD4+ T cell hybridomas were generously provided by Dr. Janice Blum (Indiana University School of Medicine, Indianapolis, IN, USA) and cultured in RPMI supplemented with 10% FBS, 50 μM 2-mercaptoethanol and 2 mM L-glutamine. Mantle cell lymphoma lines, JeKo-1 and SP53, were graciously provided by Dr. Raymond Lai (University of Alberta, Edmonton, AB, Canada). The cell lines were authenticated by assessing expression of cell surface markers, such as CD19, CD20, kappa, and lambda, as described [35]. JeKo-1 and SP53 were cultured in RPMI 1640 medium supplemented with non-essential amino acids (Sigma-Aldrich, St. Louis, MO, USA), sodium pyruvate (Gibco, Carlsbad, CA, USA), 2-mercaptoethanol (Gibco), vitamin solution (Gibco), 10% fetal bovine serum (Gibco), and Penicillin/Streptomycin (Gibco).
Peripheral Blood Mononuclear Cells (PBMCs) and Primary NKT cells
Buffy coats were purchased from the New York Blood Bank (New York City, NY, USA) and PBMCs were isolated via centrifugation in SepMate tubes (Stem Cell Technologies, Vancouver, BC, Canada) containing Ficoll-Paque (GE Healthcare, Uppsala, Sweden). NKT were isolated and expanded as previously described [36]. Primary NKT cells were cultured and tested for functionality as previously described [36].
NKT Cell Assays
To measure NKT cell responses to lymphoma cells, MCL cells were treated with the indicated amounts of drugs for 4 h, pulsed with α-GalCer (100 ng/mL), washed extensively, and cocultured (1 × 105 cells/well) with primary human NKT cells (2 × 104 cells/well) in triplicate in 96-well microtiter plates. In assays using NKT cell hybridomas, MCL cells were pre-treated with drugs for 4 h, pulsed with α-GalCer (100 ng/mL), fixed in 0.05% paraformaldehyde for 20 minutes, washed extensively, and cocultured (5 × 105 cells/well) with the NKT cell hybridomas (5 × 104 cells/well) in triplicate in 96-well microtiter plates. In human NKT cell assays, after a 42- to 72 h coculture, supernatants were harvested, and IFN-γ was measured by ELISA kit purchased from BioLegend (San Diego, California, USA). In NKT cell hybridoma assays, after 16- to 24 h coculture, supernatants were harvested and IL-2 was measured by standard ELISA kit (BD Biosciences, San Jose, CA, USA).
RT-PCR
JeKo-1 and SP53 were treated with 1 μM TSA for 0-4 hours and mRNA was isolated from the cells using standard TRIzol reagent. cDNA was made using Bio-Rad iScript kit (Hercules, CA, USA). PCR was performed using CD1D primers designed with the help of Primer-BLAST website (NIH). 18S primers were used as a loading/positive control. Following amplification, the products were resolved on 1.5% agarose gels and images were captured using Syngene G:Box imaging module and GeneSnap software. Densitometry analyses were made using ImageJ software.
Western Blotting
Cells were lysed using radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich), supplemented with phenylmethylsulfonyl fluoride (PMSF) (Cell Signaling Technology, Beverly, MA, USA). Proteins were resolved by electrophoresis on a 4-12% gradient polyacrylamide gel and transferred to a polyvinylidene fluoride (PVDF) membrane using the Bolt Mini Blot Module (Life Technologies, Carlsbad, CA, USA). All polyacrylamide gels, gel boxes, Bolt Mini transfer modules, buffers and other materials were purchased from Life Technologies and were used according to the manufacturer's instructions. Membranes were probed with antibodies to HDACs 1-3, clones 10E2, 3F3, and 7G6C5, respectively, purchased from Cell Signaling Technology. GAPDH levels were assessed on the same blot as the test protein using an antibody (clone 14C10) from Cell Signaling Technology. STAT3 (clone 79D7) and phospho-STAT3 (Y705, clone D3A7) antibodies were purchased from Cell Signaling Technology. DyLight800-conjugated anti-rabbit secondary antibody was purchased from Thermo Scientific (Waltham, MA, USA) and DyLight800-conjugated anti-mouse antibody was purchased from Cell Signaling Technology. Membranes were scanned using the Odyssey Imaging System from Li-COR Biosciences (Lincoln, NE, USA).
Immunoprecipitation
STAT3 was immunoprecipitated from JeKo-1 lysates using Protein G PLUS-Agarose beads (Santa Cruz Biotechnology, Santa Cruz, CA, USA), following the manufacturer's instructions. Immunoprecipitation was performed with three STAT3 antibodies: D3Z2G (Cell Signaling Technology), 79D7 (Cell Signaling Technology), and C-20 (Santa Cruz). Immunoprecipitates were resolved by SDS-PAGE and HDAC2 detection was performed as described above.
Chromatin Immunoprecipitation
Chromatin immunoprecipation (ChIP) was performed by Active Motif (Carlsbad, CA, USA), with a validated and tailored protocol. Promoter probes were designed by Active Motif to span the known sequence of the CD1D promoter [37] and included appropriate positive controls: beta-actin (ACTB) and interferon response factor 1 (IRF1). Untr12 is the negative control, comprising a sequence from a gene desert on chromosome 12, to which no transcription factors are expected to bind. Positive signal was defined as at least 2-fold enrichment over Untr12.
Knockdown of HDAC2
HDAC2 knockdown was achieved using lentiviral particles purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), at multiplicity of infection (MOI) of 2. Polybrene based transduction was carried out according to the manufacturer's instructions. Stable transductants were cultured in medium containing the selection agent puromycin dihydrocholoride at a final concentration of 2.5 μg/mL (ready-made solution was purchased from Sigma-Aldrich, St. Louis, MO, USA). HDAC2 knockdown was confirmed by Western blotting.
Antigens and Inhibitors
α-Galactosylceramide, was purchased from Enzo Life Sciences (New York City, NY, USA) and used at a final concentration of 100 ng/mL. Human Serum Albumin was purchased from Sigma-Aldrich and used at 10μM final concentration. Trichostatin A was purchased from Cell Signaling Technology and reconstituted in ethanol. MC1568 (Sigma-Aldrich) was reconstituted in DMSO. Panobinostat/LBH589 (Biovision (Milpitas, CA, USA)) was reconstituted in DMSO.
Flow Cytometry
Cells were stained in PBS containing 0.5% bovine serum albumin and 2 mM EDTA for 30 min at 4°C with a PE-conjugated antibody to human CD1D (clone 51.1) and APC-conjugated antibody to human DR4 (clone L243) from BioLegend. Detection of mouse CD1d was accomplished using PE-conjugated antibody to murine CD1d (clone 1B1) from eBioscience. Intracellular cytokine staining was accomplished using PE-conjugated antibodies for STAT3 (clone M59-50) and phospho-STAT3 (pY703, clone 4/P-STAT3) from BD Biosciences, following the standard protocol by BD Biosciences. Multiplex assay for inflammatory cytokines was performed using LEGENDplex Human Inflammation Panel from BioLegend, following kit instructions. Data were collected on an LSR II from BD Biosciences and analyzed using FCS Express Version 5 from De Novo Software (Los Angeles, CA, USA). Geometric means of histograms were reported according to the calculations performed by the FCS Expression Version 5 software.
Statistical Analyses
All experiments were performed at least three times. Student's T tests were performed to test for significant differences. A p value of <0.05 was considered significant. All analyses were performed using GraphPad Prism software (La Jolla, CA, USA). * p<0.05, **<0.005, and *** p<0.0001.
Results
HDACi treatment enhances CD1d-mediated antigen presentation
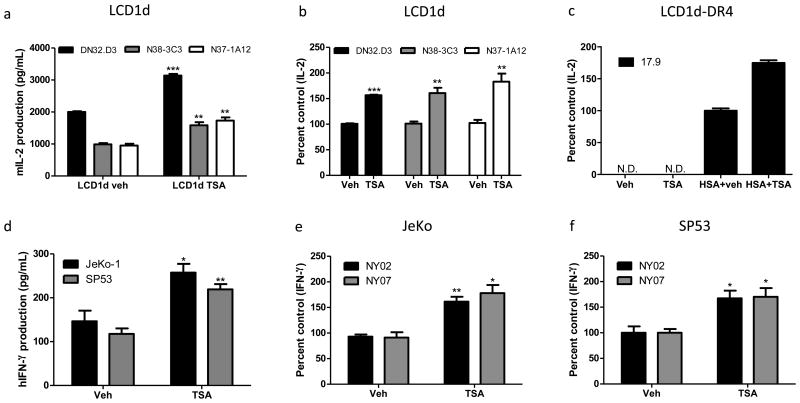
To examine the effects of HDACi on CD1d-mediated antigen presentation, LCD1d cells were pre-treated with Trichostatin-A (TSA) for 4 hours, washed extensively, and cocultured with NKT cell hybridomas, DN32.D3, N38-3C3, and N37-1A12. CD1d-mediated antigen presentation was enhanced (Fig. 1a, b). Similarly, we assessed MHC class II-mediated antigen presentation by co-culturing vehicle- and TSA-treated LCD1d cells expressing DR4 with DR4-specifc T cell line, 17.9. MHC class II-mediated antigen presentation was enhanced (Fig. 1c). Next, we sought to determine whether TSA treatment enhances CD1D-mediated antigen presentation by MCL cells. Thus, we pre-treated two MCL cell lines, JeKo-1 and SP53, with TSA for 4 hours and co-cultured the cells with DN32.D3 and primary human NKT cells expanded from healthy donor blood (Fig. 1d-f). We found that TSA treatment enhances CD1D-mediated antigen presentation by MCL cells. The table presented in Supp. Fig 1 c summarizes the effects of the panel of HDACi tested. We tested HDACi that inhibit both class I and II HDACs (TSA and LBH589) and only class II HDACs (MC1568) (Supp. Fig 1). TSA is the prototypical HDACi and it induced both CD1d and MHC class II mediated-antigen presentation; therefore, we used TSA to ascertain the mechanisms by which HDACi enhance antigen presentation.
Figure 1. HDAC inhibitor treatment enhances CD1d- and MHC class II-mediated antigen presentation.
a LCD1d cells were pretreated with 1 μM TSA for 4 hours, washed extensively and subsequently co-cultured with NKT cells, DN32.D3, N38-3C3, and N37-1A12 and IL-2 levels were measured by standard ELISA b Graphical representation of percent control of vehicle-treated cells. c LCD1d-DR4 cells were pretreated with TSA and co-cultured with DR4-specific T cells, 17.9 and IL-2 production was measured by ELISA d JeKo-1 and SP53 were treated with TSA, pulsed with α-GalCer (100 ng/mL) and co-cultured with primary human NKT cells derived from healthy donor blood. e and f Similar experiments were performed with primary human NKT cells; percent control IFN-γ production are shown for a representative experiment. * p<0.05, ** p<0.005, and *** p<.0001.
HDACi treatment induced CD1d mRNA and cell surface protein levels
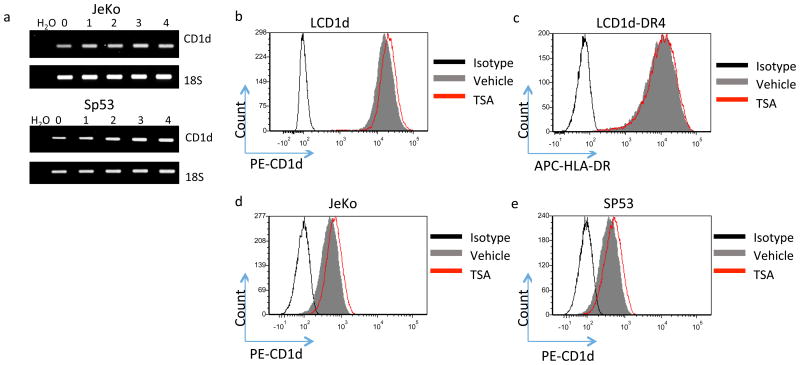
We sought to determine whether the increase in CD1D-mediated NKT cell activation following treatment with TSA was due to an increase in CD1D expression. In good agreement with Yang et al. [38], who demonstrated that TSA treatment results in an increase in CD1D mRNA levels, we found that CD1D mRNA is induced rapidly following TSA treatment (Fig. 2a). Interestingly, the rapid increase in CD1D mRNA followed by a slight decline in the mRNA is consistent with epigenetic changes, which are rapid and reversible. In addition, TSA treatment induced an increase in cell surface CD1d expression, as assessed by flow cytometry (Fig. 2b, d and e). However, TSA treatment did not alter MHC class II cell surface expression, as assessed by flow cytometry, suggesting that TSA may differentially regulate CD1d- and MHC class II-mediated antigen processing and presentation through recruitment of different HDACs to the CD1D and MHC class II promoters, or through regulation of antigen processing pathways. Moreover, different HDACs may be involved in regulating CD1d- and MHC class II-mediated antigen presentation.
Figure 2. HDAC inhibitor treatment rapidly induces CD1d mRNA and protein.
a JeKo-1 cells were treated with 1 μM TSA for 0, 1, 2, 3, and 4 hours and CD1d mRNA levels were assessed by RT-PCR. 18S serves as the loading control. Fold change in CD1D mRNA is calculated as CD1D/18S. The lower panel shows a graphical representation of CD1D/18S fold change vs time. The data are representative of three independent experiments. b LCD1d were treated with 1 μM TSA for 4 hours and cell surface expression of CD1d was assessed by flow cytometry. Vehicle-treated cells are represented by the shaded grey histogram. TSA-treated cells are represented by the red histogram. c LCD1d-DR4 were treated with 1 μM TSA for 4 hours and cell surface expression of MHC class II was determined by flow cytometry. d and e CD1D cell surface levels of vehicle and TSA-treated JeKo-1 (d) and SP53 (e) were assessed by flow cytometry. Geometric means (GM) were calculated and are representative of the changes seen in three independent experiments.
HDAC2 binds the CD1D promoter and is the main HDAC regulating CD1D transcription in MCL
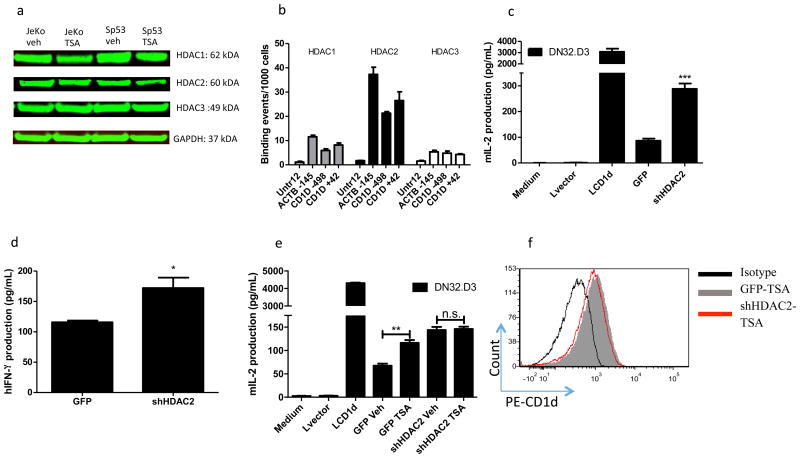
To identify which specific HDAC(s) are involved in CD1D-mediated antigen presentation, we examined HDAC expression in vehicle and TSA-treated JeKo-1 and SP53 cells by Western blotting. We found that TSA-treatment inhibited HDACs 1 and 2, without significantly affecting HDAC3 levels (Fig. 3a). Chromatin immunoprecipitation (ChIP) identified HDAC2 as binding at the CD1D promoter, at both proximal and distal regions, whereas HDACs 1 and 3 did not bind (Fig. 3b). Beta-actin (ACTB) served as a positive control for HDAC binding. The primers spanned the known proximal (+42) and distal (-498) regions of the CD1D promoter. Thus, we knocked down HDAC2 in JeKo-1 and assessed antigen presentation. HDAC2 knockdown (Supplementary Fig. 1a) enhanced antigen presentation to both NKT hybridomas and primary human NKT cells (Fig. 3c, d). Importantly, treatment of shHDAC2 JeKo-1 cells with TSA did not further induce CD1D-mediated antigen presentation (Fig. 3e). These data demonstrate that inhibition of HDAC2 is the main mechanism by which TSA treatment enhances CD1D-mediated antigen presentation by MCL. Notably, CD1D levels were similar in TSA-treated JeKo-1 control and shHDAC2 cells (Fig. 3f). These data suggest that HDAC2 is the main HDAC regulating CD1D-mediated antigen presentation.
Figure 3. HDAC2 binds the CD1d promoter and HDAC2 is the main regulator of CD1d-medianted antigen presentation.
a HDAC1-3 levels in TSA-treated JeKo-1 and SP53 cells were assessed by Western blot. GAPDH serves as a loading control. HDAC1-3 levels were normalized to GAPDH and then fold change was calculated relative to vehicle-treated cells. Bottom panel shows fold change in HDAC1-3 levels in TSA-treated JeKo-1 and SP53 cells, relative to vehicle-treated cells. The values were normalized to GAPDH. b ChIP was performed in JeKo-1, using primers for proximal and distal CD1d promoter, with beta-actin (ACTB) gene serving as a positive control. c Antigen presentation by JeKo-1 GFP control and shHDAC2 cells was assessed in a co-culture with NKT cell hybridomas. LCD1d is a positive control. IL-2 production is shown. d In a similar experiment, the GFP control and HDAC2 knockdown JeKo-1 cells were co-cultured with primary human NKT cells. e Vehicle and TSA-treated GFP control and shHDAC2 JeKo-1 cells were co-cultured with NKT cell hybridomas. * p<0.05, ** p<0.005, and *** p<.0001. f GFP control and shHDAC2 JeKo-1 cells treated with TSA and CD1d levels were assessed by flow cytometry. GFP control TSA-treated cells are represented by the shaded grey histogram. shHDAC2 TSA-treated cells are represented by the red histogram. GM values are shown for vehicle- and TSA-treated cells.
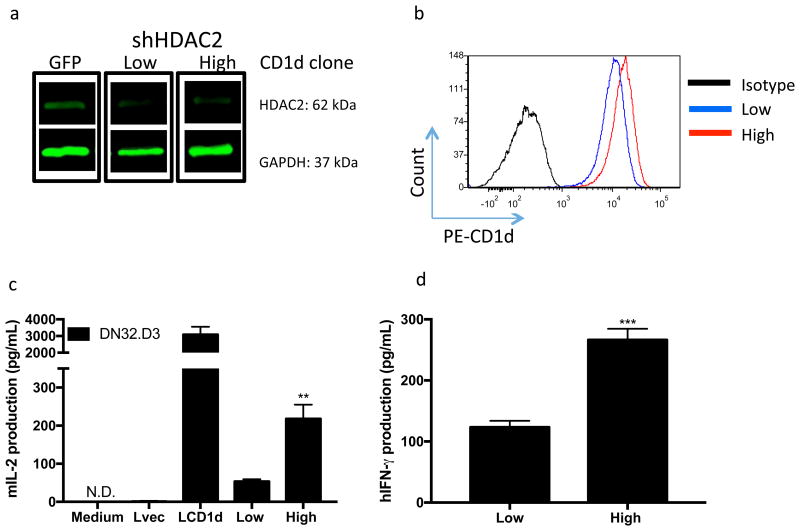
To confirm a role for HDAC2 in regulating CD1D cell surface expression and to demonstrate that minute changes in CD1D expression can have a profound effect on NKT cell responses, we selected shHDAC2 clones expressing relatively high and low levels of CD1D (termed Low and High clones). We confirmed HDAC2 knockdown in the clones and assessed CD1D surface expression by flow cytometry (Fig. 4a,b). Next, we assessed their antigen presentation capabilities by co-culturing the clones with NKT cell hybridomas (Fig. 4c) and primary human NKT cells (Fig. 4d). Importantly, we noted a dose-dependent relationship in CD1D levels and the ability of the JeKo-1 shHDAC2 clones to activate NKT cells. Specifically, the clone expressing higher CD1D levels displayed enhanced antigen presentation capabilities, compared to the clone expressing lower levels of CD1D. Thus, downregulation of CD1D levels via HDAC upregulation may serve as a mechanism by which tumors evade recognition by NKT cells. Notably, we showed that NKT cells are sensitive to minute changes in CD1d levels.
Figure 4. Dose dependence of CD1d levels and NKT cell activation.
a HDAC2 knockdown was confirmed in JeKo-1 displaying low and high CD1D levels. Bottom panel shows fold change in HDAC2 levels in shHDAC2 knockdown-containing JeKo-1 cells relative to GFP control JeKo-1 cells. Values were normalized to GAPDH b shHDAC2 clones displaying low (blue histogram) and high (red histogram) levels of cell surface CD1D.The GM values are shown for low and high CD1D clones. c Antigen presentation capabilities of low and high CD1D level expressing shHDAC2 clones were assessed in a coculture with NKT cell hybridomas. d Similar experiment was performed with primary human NKT cells and IFN-γ production was assessed by ELISA. * p<0.05, ** p<0.005, and *** p<0.0001.
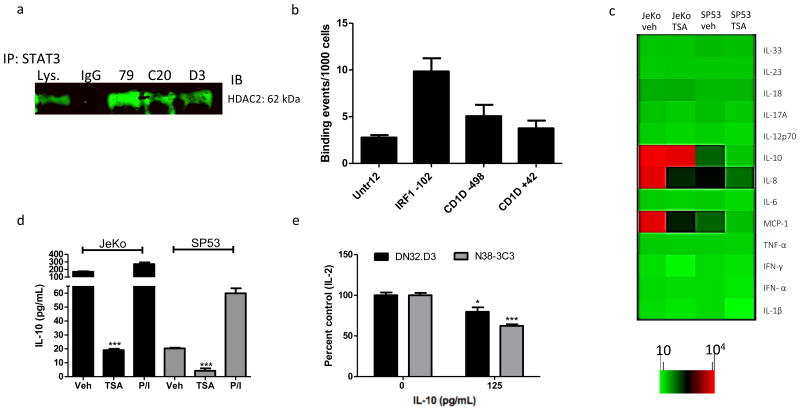
HDAC2 regulates STAT3 and thus alters STAT3-indicuble cytokine secretion
Given that TSA treatment induced CD1D transcription, we sought to identify transcription factors regulated by HDACs and immunoprecipitated STAT3, which we found to be in a complex with HDAC2 (Fig. 5a). However, we found that STAT3 does not bind to the CD1D promoter at the distal (-498) and proximal (+42) regions, as assessed by ChIP, with interferon response factor 1 (IRF1) serving as a positive control (Fig. 5b). Given that STAT3 levels can be modulated by HDACi treatment [22, 39], we hypothesized that TSA inhibits STAT3 in MCL and examined the effects of TSA on STAT3 expression. Following TSA treatment, both phosphorylated and total STAT3 were inhibited (Supplementary Fig. 2b). Thus, we sought to determine whether HDACi treatment inhibits secretion of STAT3-regulates inflammatory cytokines. We utilized a multiplex flow cytometric bead-based array to assess the levels of various inflammatory cytokines in the supernatants of vehicle- and TSA-treated JeKo-1 and SP53 cells. We found that TSA treatment inhibited secretion of MCP-1, IL-8 and IL-10 (Fig. 5c). We confirmed TSA-mediated inhibition of IL-10 by ELISA (Fig. 5d), with phorbol 12-myristate 13-acetate and ionomycin (P/I) serving as a positive control. We found that both JeKo-1 and SP53 secrete IL-10, even in absence of stimulation, and that TSA treatment inhibits IL-10 secretion (Fig. 5d). Given the multitude of cytokines secreted by MCL cells, we chose to focus on a cytokine—IL-10—that has been previously shown to negatively regulate antigen presentation [40]. Furthermore, we set up an LCD1d-based in vitro system using purified cytokines in order to eliminate confounding variables, such as the effects of other cytokines and inhibitory lipids that are produced by MCL cells. To elucidate the effects of IL-10 on antigen presentation, LCD1d cells were treated with purified recombinant IL-10 and cultured with NKT cell hybridomas. IL-10 pre-treatment suppressed CD1d-mediated antigen presentation to NKT cell hybridomas, DN32.D3 and N38-3C3. We confirmed that IL-10 activates STAT3 by performing a Western blot for STAT3 and phospho-STAT3 (Supplementary Fig. 2c). Overall, these studies demonstrate that HDAC2 regulates STAT3, which in turn modulates inflammatory cytokine secretion, which can suppress CD1d-mediated antigen presentation. Moreover, we demonstrated that treatment with HDACi resulted in a concomitant increase in CD1d cell surface expression and decrease in IL-10 production, thus increasing tumor cell immunogenicity (Fig. 6).
Figure 5. HDACs alter CD1d-mediated antigen presentation through cell-extrinsic mechanisms.
a STAT3 was immunoprecipitated from JeKo-1 cell lysates using three different STAT3 monoclonal antibodies (mAbs) and the immunoprecipitates were evaluated for presence of HDAC2. b ChIP was performed to determine whether STAT3 binds the CD1d promoter at proximal and distal sites, with interferon response factor 1 (IRF1) serving as a positive control. c JeKo-1 and Sp53 were treated with 1 μM TSA for 24 hours and the cytokine concentrations in supernatants were assessed by LEGENDPlex Human Inflammation Panel assay. d JeKo-1 and Sp53 cells were treated with 1 μM TSA for 24 hours and IL-10 levels in supernatants were confirmed by standard ELISA. Positive control is P/I. e LCD1d were treated with IL-10 to induce STAT3 and co-cultured with DN32.D3 and N38-3C3. T-tests were performed to demonstrate statistical significance: * p < 0.05 and ***p<0.0001.
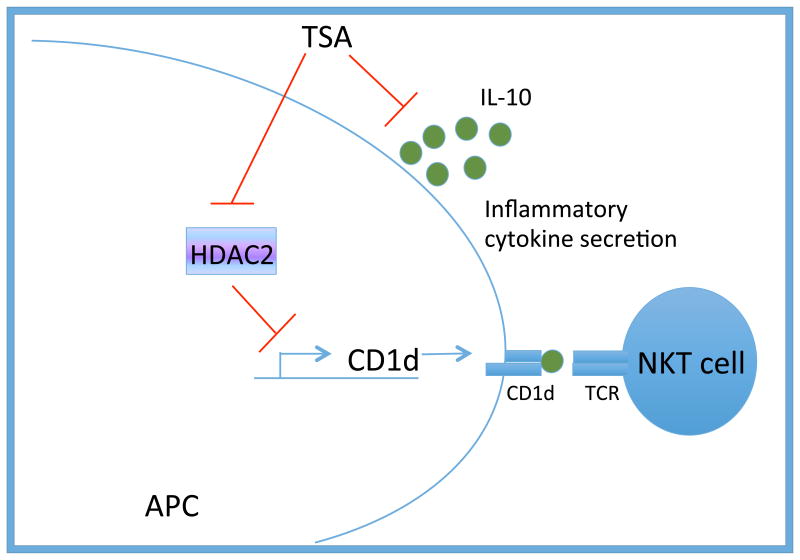
Figure 6. Proposed model by which TSA treatment enhances CD1d-mediated antigen presentation to NKT cells.
CD1d expression is enhanced following HDACi treatment, due, at least in part, to a dose-dependent increase in CD1d-cell surface expression. Treatment with HDACi results in a decrease in STAT3 as well as STAT3-inducible cytokine secretion by MCL cells. STAT3-inducible cytokines, such as IL-10, inhibit CD1d-mediated antigen presentation. Thus, HDACi treatment restores NKT cell responses through cell intrinsic and cell extrinsic mechanisms.
Discussion
HDACs exert their effects via modulation of numerous processes within the mammalian cell: they regulate cell processes of both healthy and cancerous cells, such as cell cycle progression and proliferation. Furthermore, they have important immunomodulatory roles, achieved through the alteration of cytokine profiles of immune cells [41] and differential gene expression.
While other groups have ascribed a role for HDACs in regulating CD1d transcription [38, 42], our study is the first to demonstrate that HDACi have a functional effect and enhance CD1d-mediated antigen presentation. In our studies, we tested the hypothesis that tumors use epigenetic mechanisms to dysregulate CD1d-mediated antigen presentation. We examined CD1d-mediated antigen presentation to NKT cells following treatment with HDACi. Consistent with previous studies [43-45], we found that treatment with TSA, a pan-HDACi, enhanced both CD1d and MHC class II-mediated antigen presentation. Furthermore, we assessed CD1d and MHC class II levels following TSA treatment and found that CD1d levels were induced, but MHC class II levels were unaffected, suggesting that different mechanisms are responsible for the functional effects.
While our studies suggest that HDACi positively impact antigen presentation, HDACi have been shown to inhibit antigen presentation by professional antigen presenting cells. HDACi can suppress the expression of costimulatory molecules on dendritic cells (DCs), change their metabolism as well as their cytokine profiles, and can impair their antigen uptake capabilities [46]. Specifically, valproic acid and LBH589 have been shown to reduce the expression of costimulatory molecules CD80, CD40, and CD83 and alter expression of adhesion molecules [46, 47]. As a result of these changes, both MHC class I and I-mediated antigen-specific immune responses relayed by DCs were impaired, limiting their ability to activate T cells and NKT cells [48]. The discrepancies in the effects of HDACi may be attributed to the fact that HDACs have normal functions, but due to their overexpression and aberrant recruitment in tumors, HDACi treatment of tumors restores the “normal” epigenomic program by fine-tuning HDAC activities. Thus, future studies are needed to determine cell-specific effects of HDACi on antigen presentation.
We found that HDACi enhance antigen presentation due, at least in part, to a dose-dependent rapid increase in CD1D mRNA and, subsequently, CD1D cell surface expression. Finally, treatment with HDACi resulted in a decrease in STAT3 as well as STAT3-inducible cytokine secretion by MCL. We found that HDAC2 binds to the CD1D promoter. However, we found that STAT3 did not bind to the CD1D promoter. There are several explanations for this finding. First, it is possible that STAT3 regulates CD1D gene expression through binding at distant enhancer regions. Future studies will determine whether STAT3 regulates CD1D gene expression through binding at distal enhancers or whether it binds at the promoter of a gene whose products regulates CD1D gene expression. Moreover, it is possible that STAT3 binds the CD1D promoter indirectly. For example, one study determined that, in a panel of lymphoma cell lines, STAT3 binds the GADD45G promoter indirectly, through association with NF-κB and that panobinostat inhibits the binding of the NF-κB-STAT3 complex [49].
It is possible that STAT3 plays a positive role in CD1d-mediated antigen presentation. Iyer et al. demonstrated that STAT3 inhibition reduced endogenous lipid antigen presentation to NKT cells [50]. Knockdown of STAT3 led to decreased levels of uridine diphosphate (UDP) glucose ceramide glucosyltransferase, an enzyme involved in glycosphingolipid biosynthesis [50]. Moreover, CD1D levels of HEK-293 cells containing STAT3 knockdown were not deemed significantly different [50]. However, it must be noted these studies used CD1d transfectants; thus, CD1d was not regulated by its natural, endogenous promoter. In contrast, our studies focused on CD1d regulation in lymphoma cells. The same signaling pathway can have different outcomes in fibroblasts, compared to cancer cells, due to cross-talk with other pathways that are uniquely upregulated in cancer. Thus, although STAT3 has been shown to enhance endogenous lipid antigen presentation through modulation of glycosphingolipid biosynthesis pathways, future studies are needed to delineate the cell-specific effects of STAT3 signaling. Moreover, future studies are needed to determine whether STAT3 is aberrantly recruited to the CD1D promoter in tumors, which would provide a rational explanation for discrepant findings in different systems.
Here, we present data that provides evidence that HDACi may be combined with NKT cell-based immunotherapy. Importantly, we demonstrate that treatment with HDACi not only induces CD1D-mediated antigen presentation, but also inhibits inflammatory cytokine secretion, which may contribute to suppression of anti-tumor NKT cell responses. Our studies demonstrate the efficacy of HDACi in restoring anti-tumor responses to MCL through both cell-intrinsic and extrinsic mechanisms. Collectively, these results suggest that HDACi may work to enhance the immune response by increasing antigen processing and presentation, while inhibiting inflammatory cytokine secretion. While various combinations of HDACi with other chemotherapeutic agents have been widely studied, the combination of HDACi with immunotherapy is a novel avenue, with mechanistic studies helping to build the foundation for the application of this combination in the clinic [51].
Supplementary Material
Acknowledgments
This work was supported by NIH/National Cancer Institute (NCI) 1R21CA162277, R21CA184469, and R21CA199544 grants to Tonya Webb and the American Association of Immunologists (AAI) Careers in Immunology Fellowship to Irina Tiper.
Abbreviations
- ACTB
beta-actin
- ChIP
chromatin immunoprecipitation
- GM
geometric mean
- HDAC
histone deacetylases
- HDACi
histone deacetylase inhibitor(s)
- IRF1
interferon response factor 1
- MCL
mantle cell lymphoma
- NKT
natural killer T
- P/I
phorbol 12-myristate 13-acetate and ionomycin
- TSA
trichostatin-A
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest.
- Immunology 2016 Annual Meeting, May 13-17, 2015, Seattle, WA, USA. Poster/Abstract.
- Sixth Annual Cancer Biology Research Retreat, May 18, 2015, UM BSMC Campus Center, University of Maryland, Baltimore, MD, USA. Poster/Abstract.
- Immunology 2015 Annual Meeting, May 8-12, 2015, New Orleans, LA, USA. Poster/Abstract
- 29th Annual Scientific Meeting of the Society for Immunotherapy of Cancer (SITC), November 6-9, 2014, Washington, DC, USA. Abstract.
Published abstracts discussing the findings in this study: Tiper I, Webb TJ. Epigenetic regulation of CD1d-mediated antigen presentation in B cell lymphoma. Journal for Immunotherapy of Cancer 2014, 2(Suppl 3):P177
Tiper I, Webb TJ. Epigenetic regulation of CD1d-mediated antigen presentation by B cell lymphomas. Journal of Immunology 2015, 194(Suppl 1): P211
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66:5527–36. doi: 10.1158/0008-5472.can-05-4128. [DOI] [PubMed] [Google Scholar]
- 3.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terabe M, Berzofsky JA. The immunoregulatory role of type I and type II NKT cells in cancer and other diseases. Cancer Immunol Immunother. 2014;63:199–213. doi: 10.1007/s00262-013-1509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Sun W, Subrahmanyam PB, Page C, Younger KM, Tiper IV, Frieman M, Kimball AS, Webb TJ. NKT Cell Responses to B Cell Lymphoma. Med Sci (Basel) 2014;2:82–97. doi: 10.3390/medsci2020082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brossay L, Kronenberg M. Highly conserved antigen-presenting function of CD1d molecules. Immunogenetics. 1999;50:146–51. doi: 10.1007/s002510050590. [DOI] [PubMed] [Google Scholar]
- 7.Terabe M, Berzofsky JA. The immunoregulatory role of type I and type II NKT cells in cancer and other diseases. Cancer Immunol Immunother. 2014;63(3):199–213. doi: 10.1007/s00262-013-1509-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robertson FC, Berzofsky JA, Terabe M. NKT cell networks in the regulation of tumor immunity. Front Immunol. 2014;5:543. doi: 10.3389/fimmu.2014.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12:239–52. doi: 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun W, Subrahmanyam PB, East JE, Webb TJ. Connecting the dots: artificial antigen presenting cell-mediated modulation of natural killer T cells. J Interferon Cytokine Res. 2012;32:505–16. doi: 10.1089/jir.2012.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tahir SM, Cheng O, Shaulov A, Koezuka Y, Bubley GJ, Wilson SB, Balk SP, Exley MA. Loss of IFN-gamma production by invariant NK T cells in advanced cancer. J Immunol. 2001;167:4046–50. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 12.Molling JW, Kolgen W, van der Vliet HJ, et al. Peripheral blood IFN-gamma-secreting Valpha24+Vbeta11+ NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int J Cancer. 2005;116:87–93. doi: 10.1002/ijc.20998. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu K, Hidaka M, Kadowaki N, et al. Evaluation of the function of human invariant NKT cells from cancer patients using alpha-galactosylceramide-loaded murine dendritic cells. J Immunol. 2006;177:3484–92. doi: 10.4049/jimmunol.177.5.3484. [DOI] [PubMed] [Google Scholar]
- 14.Igney FH, Krammer PH. Immune escape of tumors: apoptosis resistance and tumor counterattack. J Leukoc Biol. 2002;71:907–20. [PubMed] [Google Scholar]
- 15.Romero JM, Jimenez P, Cabrera T, Cozar JM, Pedrinaci S, Tallada M, Garrido F, Ruiz-Cabello F. Coordinated downregulation of the antigen presentation machinery and HLA class I/beta2-microglobulin complex is responsible for HLA-ABC loss in bladder cancer. Int J Cancer. 2005;113:605–10. doi: 10.1002/ijc.20499. [DOI] [PubMed] [Google Scholar]
- 16.Bubenik J. MHC class I down-regulation: tumour escape from immune surveillance? (review) Int J Oncol. 2004;25:487–91. [PubMed] [Google Scholar]
- 17.Khanna R. Tumour surveillance: missing peptides and MHC molecules. Immunol Cell Biol. 1998;76:20–6. doi: 10.1046/j.1440-1711.1998.00717.x. [DOI] [PubMed] [Google Scholar]
- 18.Gunnellini M, Falchi L. Therapeutic Activity of Lenalidomide in Mantle Cell Lymphoma and Indolent Non-Hodgkin's Lymphomas. Adv Hematol. 2012;2012:523842. doi: 10.1155/2012/523842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dreyling M, Ferrero S, Hermine O. How to manage mantle cell lymphoma. Leukemia. 2014;28:2117–30. doi: 10.1038/leu.2014.171. [DOI] [PubMed] [Google Scholar]
- 20.Pérez-Galán P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117:26–38. doi: 10.1182/blood-2010-04-189977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng F, Wang H, Horna P, et al. Stat3 inhibition augments the immunogenicity of B-cell lymphoma cells, leading to effective antitumor immunity. Cancer Res. 2012;72:4440–8. doi: 10.1158/0008-5472.can-11-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu K, Chen N, Zhou XX, Ge XL, Feng LL, Li PP, Li XY, Geng LY, Wang X. The STAT3 inhibitor WP1066 synergizes with vorinostat to induce apoptosis of mantle cell lymphoma cells. Biochem Biophys Res Commun. 2015;464:292–8. doi: 10.1016/j.bbrc.2015.06.145. [DOI] [PubMed] [Google Scholar]
- 23.Aggarwal BB, Kunnumakkara AB, Harikumar KB, Gupta SR, Tharakan ST, Koca C, Dey S, Sung B. Signal transducer and activator of transcription-3, inflammation, and cancer: how intimate is the relationship? Ann N Y Acad Sci. 2009;1171:59–76. doi: 10.1111/j.1749-6632.2009.04911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang Q, Bournazou E, Sansone P, et al. The IL-6/JAK/Stat3 Feed-Forward Loop Drives Tumorigenesis and Metastasis. Neoplasia (New York, NY) 2013;15:848–62. doi: 10.1593/neo.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carpenter RL, Lo HW. STAT3 Target Genes Relevant to Human Cancers. Cancers (Basel) 2014;6:897–925. doi: 10.3390/cancers6020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest. 2014;124:30–9. doi: 10.1172/jci69738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marks PA. Histone deacetylase inhibitors: a chemical genetics approach to understanding cellular functions. Biochim Biophys Acta. 2010;1799:717–25. doi: 10.1016/j.bbagrm.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woan KV, Sahakian E, Sotomayor EM, Seto E, Villagra A. Modulation of antigen-presenting cells by HDAC inhibitors: implications in autoimmunity and cancer. Immunol Cell Biol. 2012;90:55–65. doi: 10.1038/icb.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prince HM, Bishton MJ, Harrison SJ. Clinical studies of histone deacetylase inhibitors. Clin Cancer Res. 2009;15:3958–69. doi: 10.1158/1078-0432.ccr-08-2785. [DOI] [PubMed] [Google Scholar]
- 30.Ray S, Lee C, Hou T, Boldogh I, Brasier AR. Requirement of histone deacetylase1 (HDAC1) in signal transducer and activator of transcription 3 (STAT3) nucleocytoplasmic distribution. Nucleic Acids Res. 2008;36:4510–20. doi: 10.1093/nar/gkn419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Current Opinion in Genetics & Development. 2008;18:11–8. doi: 10.1016/j.gde.2007.12.007. http://dx.doi.org/10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brutkiewicz RR, Bennink JR, Yewdell JW, Bendelac A. TAP-independent, beta 2-microglobulin-dependent surface expression of functional mouse CD1.1. J Exp Med. 1995;182:1913–9. doi: 10.1084/jem.182.6.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts TJ, Sriram V, Spence PM, Gui M, Hayakawa K, Bacik I, Bennink JR, Yewdell JW, Brutkiewicz RR. Recycling CD1d1 molecules present endogenous antigens processed in an endocytic compartment to NKT cells. J Immunol. 2002;168:5409–14. doi: 10.4049/jimmunol.168.11.5409. [DOI] [PubMed] [Google Scholar]
- 35.Amin HM, McDonnell TJ, Medeiros LJ, Rassidakis GZ, Leventaki V, O'Connor SL, Keating MJ, Lai R. Characterization of 4 mantle cell lymphoma cell lines. Arch Pathol Lab Med. 2003;127:424–31. doi: 10.1043/0003-9985(2003)127<0424:comclc>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 36.East JE, Sun W, Webb TJ. Artificial antigen presenting cell (aAPC) mediated activation and expansion of natural killer T cells. J Vis Exp. 2012:70. doi: 10.3791/4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen QY, Jackson N. Human CD1D gene has TATA boxless dual promoters: an SP1-binding element determines the function of the proximal promoter. J Immunol. 2004;172:5512–21. doi: 10.4049/jimmunol.172.9.5512. [DOI] [PubMed] [Google Scholar]
- 38.Yang PM, Lin PJ, Chen CC. CD1d induction in solid tumor cells by histone deacetylase inhibitors through inhibition of HDAC1/2 and activation of Sp1. Epigenetics. 2012;7:390–9. doi: 10.4161/epi.19373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Batalo M, Bose P, Holkova B, Grant S. Targeting Mantle Cell Lymphoma with a Strategy of Combined Proteasome and Histone Deacetylase Inhibition. In: Dou PQ, editor. Resistance to Proteasome Inhibitors in Cancer: Molecular Mechanisms and Strategies to Overcome Resistance. Springer International Publishing; Cham: 2014. pp. 149–79. [Google Scholar]
- 40.Mittal SK, Roche PA. Suppression of antigen presentation by IL-10. Curr Opin Immunol. 2015;34:22–7. doi: 10.1016/j.coi.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alvarez-Breckenridge CA, Yu J, Price R, et al. The histone deacetylase inhibitor valproic acid lessens NK cell action against oncolytic virus-infected glioblastoma cells by inhibition of STAT5/T-BET signaling and generation of gamma interferon. J Virol. 2012;86:4566–77. doi: 10.1128/jvi.05545-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen QY, Zhang T, Pincus SH, Wu S, Ricks D, Liu D, Sun Z, Maclaren N, Lan MS. Human CD1D gene expression is regulated by LEF-1 through distal promoter regulatory elements. J Immunol. 2010;184:5047–54. doi: 10.4049/jimmunol.0901912. [DOI] [PubMed] [Google Scholar]
- 43.Magner WJ, Kazim AL, Stewart C, Romano MA, Catalano G, Grande C, Keiser N, Santaniello F, Tomasi TB. Activation of MHC class I, II, and CD40 gene expression by histone deacetylase inhibitors. J Immunol. 2000;165:7017–24. doi: 10.4049/jimmunol.165.12.7017. [DOI] [PubMed] [Google Scholar]
- 44.Khan AN, Gregorie CJ, Tomasi TB. Histone deacetylase inhibitors induce TAP, LMP, Tapasin genes and MHC class I antigen presentation by melanoma cells. Cancer Immunol Immunother. 2008;57:647–54. doi: 10.1007/s00262-007-0402-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manning J, Indrova M, Lubyova B, et al. Induction of MHC class I molecule cell surface expression and epigenetic activation of antigen-processing machinery components in a murine model for human papilloma virus 16-associated tumours. Immunology. 2008;123:218–27. doi: 10.1111/j.1365-2567.2007.02689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song W, Tai YT, Tian Z, Hideshima T, Chauhan D, Nanjappa P, Exley MA, Anderson KC, Munshi NC. HDAC inhibition by LBH589 affects the phenotype and function of human myeloid dendritic cells. Leukemia. 2011;25:161–8. doi: 10.1038/leu.2010.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frikeche J, Simon T, Brissot E, Gregoire M, Gaugler B, Mohty M. Impact of valproic acid on dendritic cells function. Immunobiology. 2012;217:704–10. doi: 10.1016/j.imbio.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 48.Frikeche J, Peric Z, Brissot E, Gregoire M, Gaugler B, Mohty M. Impact of HDAC inhibitors on dendritic cell functions. Exp Hematol. 2012;40:783–91. doi: 10.1016/j.exphem.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Scuto A, Kirschbaum M, Buettner R, Kujawski M, Cermak JM, Atadja P, Jove R. SIRT1 activation enhances HDAC inhibition-mediated upregulation of GADD45G by repressing the binding of NF-kappaB/STAT3 complex to its promoter in malignant lymphoid cells. Cell Death Dis. 2013;4:e635. doi: 10.1038/cddis.2013.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iyer AK, Liu J, Gallo RM, Kaplan MH, Brutkiewicz RR. STAT3 promotes CD1d-mediated lipid antigen presentation by regulating a critical gene in glycosphingolipid biosynthesis. Immunology. 2015;146:444–55. doi: 10.1111/imm.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ribas A, Wolchok JD. Combining cancer immunotherapy and targeted therapy. Curr Opin Immunol. 2013;25:291–6. doi: 10.1016/j.coi.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.