Summary
Background
The inflammatory biomarker galectin-3 contributes to pathologic conditions such as heart failure and stimulates murine thrombogenesis. Its association with venous thromboembolism (VTE) has been sparsely studied.
Objectives
To assess the prospective association of plasma galectin-3 and the LGALS3 rs4644 SNP with VTE incidence.
Methods
We measured plasma galectin-3 in 9,916 participants in the Atherosclerosis Risk in Communities (ARIC) study cohort in 1996 – 1998 and identified VTEs through 2013. Using Cox regression, we estimated the hazard ratio associating galectin-3 with incident VTE over a median of 13.9 years. Replication was sought in the Cardiovascular Health Study (CHS).
Results
ARIC included 21.8% blacks and 56.2% females with mean baseline age of 62.7 years. The incidence rate of VTE (n=389 events) increased across quintiles of galectin-3, with hazard ratios (95% CI) of 1 (reference), 1.13 (0.80 – 1.61), 1.00 (0.70 – 1.43), 1.36 (0.96 – 1.91), and 1.55 (1.09 – 2.19) (p-trend = 0.005), adjusted for age, sex, race, body mass index, diabetes status, and renal function. Results did not replicate in the CHS (124 VTE), but meta-analysis of both studies yielded a pooled hazard ratio (95% CI) for 1 SD increment in log galectin-3 of 1.10 (1.00 – 1.22). In ARIC, the C allele of rs4644 in the LGALS3 gene was associated with higher galectin-3 level, and in whites, with an increased rate of VTE.
Conclusion
Galectin-3 levels were associated positively with VTE incidence.
Keywords: Galectin-3, Gene, Prospective, Thrombosis, Venous thromboembolism
INTRODUCTION
Venous thromboembolism (VTE), presenting as deep venous thrombosis (DVT) and/or pulmonary embolism (PE), is a major public health problem [1–4] with a multifactorial etiology [1, 2]. Risk of VTE is increased in patients with inflammatory diseases or with elevated levels of inflammation markers, such as C-reactive protein and interleukin-6 [5–8]. Among inflammatory pathways, galectin-3–binding protein (gal3bp) and its receptor/ligand, galectin-3, are secreted proteins that can interact with each other to promote cell-to-cell adhesion and initiate pathologic, proinflammatory signaling cascades [9]. Gal3bp and galectin-3 play important roles in a number of pathological conditions, such as cancer, diabetes, atherosclerosis, and heart failure, and some evidence suggests a role in VTE [9–15]. Specifically, a recent study showed that galectin-3 and gal3bp are involved in murine thrombogenesis, that galectin-3 and gal3bp interact at the thrombus–vein wall interface, and that galectin-3 may contribute to thrombosis through proinflammatory, interleukin-6–dependent mechanisms [9].Gal3bp is up-regulated in microparticles from DVT patients compared to control patients [10, 16]. Yet, to our knowledge, there has been no prospective epidemiological study of the association between galectin-3 and VTE. We, therefore, hypothesized that plasma galectin-3 concentration would be positively associated with incident VTE in a biracial cohort.
A genome wide association study identified a non-synonymous single-nucleotide polymorphism (SNP) (rs4644) in the galectin-3 encoding gene, LGALS3, to be strongly associated with plasma galectin-3 concentrations [17]. We, therefore, also explored whether the rs4644 in the LGALS3 gene is associated with incident VTE.
METHODS
Study Population
The Atherosclerosis Risk in Communities (ARIC) study is a prospective epidemiological study aimed at identifying the risk factors for atherosclerosis and cardiovascular diseases. Participants were recruited between 1987 and 1989 from four communities in the United States (Washington County, MD; the north-west suburbs of Minneapolis, MN; Jackson, MS; and Forsyth County, NC) [18]. The ARIC cohort comprised 15,792 men and women initially aged 45 to 64 years who were mostly white or black. Participants were examined at baseline and at 5 additional times: visit 2 (1990 – 1992), visit 3 (1993 – 1995), visit 4 (1996 – 1998), visit 5 (2011 – 2013) and visit 6 (2016 – 2017). Participants are also being followed by annual or semi-annual telephone interviews and surveillance of ARIC community hospitals. For replication we used the Cardiovascular Health Study (CHS). Briefly, CHS is prospective study of 5,888 participants aged 65 years and older enrolled from four US communities (Forsyth County, NC; Sacramento County, CA; Washington County, MD; and Pittsburgh, PA) from 1989 – 1990 and 1992 – 1993 (African Americans only). CHS contacted participants every six months for follow-up, alternating between a telephone interview and clinic visit for the first 10 years and by telephone interview only after that [19]. Both studies were approved by the institutional review board of each participating center, and all participants provided written informed consent.
In ARIC, galectin-3 was measured at visit 4, which serves as the baseline for our analysis. Among 11,656 visit 4 participants, we excluded those with a history of VTE or using anticoagulants (n = 523), those without galectin-3 measurements (n = 899), those who were not white or black (n = 30), those with no further follow-up (n = 2), and those with missing covariates (n = 286). This left a total of 9,916 ARIC participants for our analysis. Similar exclusions were used for the replication analysis using the baseline CHS study visit (1989 – 1990 and 1992 – 1993) as our study baseline. We excluded 419 CHS participants with a history of VTE or who were taking anticoagulants at baseline, 1,692 with missing galectin-3 measurements, 29 who self-identified as neither white nor black, and 39 with missing covariates. This left a total of 3,709 CHS participants at risk of VTE.
VTE Ascertainment
We ascertained incident hospitalized DVT and PE occurring from visit 4 (1996 – 1998) through December 31, 2013. Hospitalizations were identified by participant report and surveillance of local hospitals [20]. Medical records with possible International Classification of Diseases (ICD) discharge codes for VTE were abstracted. Two physicians reviewed the records using a standardized protocol and identified incident VTEs. Cases were confirmed in the presence of a positive imaging test [20, 21]. CHS ascertained VTE events from baseline CHS visit (1989 – 1993) through December 31, 2001 using similar methods [20].
Galectin-3 and Risk Factor Ascertainment
Plasma samples collected from ARIC participants at visit 4 (1996 – 98) were stored at −70°C. Galectin-3 was measured using a chemiluminescent microparticle immunoassay on an Architect i 2000sr instrument (Abbott, Abbott Park, IL). The measurements were performed July 2015 – February 2016. The Architect galectin-3 assay has a limit of detection of 1.1 ng/mL and a limit of quantitation of 4.0 ng/mL. Interassay coefficients of variation were 5.2%, 3.3%, and 2.3% at mean galectin-3 levels of 8.8 ng/mL, 19.2 ng/mL and 72.0 ng/ml, respectively. At the time of blood processing, 402 participants’ plasma specimens were split, masked, and sent to the laboratory to assess galectin-3 laboratory reliability. The reliability coefficient was r=0.92 and coefficient of variation (CV), using the split specimens, was 7.5%. After removing 7 potentially mislabeled “outlier” samples these respective values were r=0.95 and CV=5.7%. CHS measured galectin-3 at baseline from stored serum frozen at −70°C using an enzyme-linked immunosorbent assay (BG Medicine, Waltham, MA) [22].
In both studies, participants reported their age, sex, and race. Diabetes mellitus was defined as fasting glucose ≥126 mg/dL (7.0 mmol/L), nonfasting glucose ≥200 mg/dL (11.1 mmol/L), treatment for diabetes mellitus, or self-reported physician diagnosis of diabetes mellitus. Body mass index (BMI) was calculated as weight (kg) divided by height (m2). Estimated glomerular filtration rate (eGFR), C-reactive protein (CRP), troponin T (TnT), and N-terminal pro–B-type natriuretic peptide (NT-proBNP) were measured from baseline blood samples as described previously in ARIC [5, 23–26] and CHS [27–30]. The rs4644 SNP genotying was performed using the HumanExome BeadChip Array in ARIC participants only [31].
Statistical Analysis
Statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC) and STATA, version 12 (Stata, College Station, TX). Baseline characteristics of participants were described within galectin-3 quintiles. We summarized normally distributed variables by means and standard deviations, and skewed variables by medians with 25th to 75th percentiles. Categorical variables were presented as percentages. Multivariable Cox proportional hazards models were used to estimate the hazard ratios for the association between incident VTE and galectin-3. Galectin-3 was analyzed both as quintiles and as a natural log transformed continuous variable due to non-normality of galectin-3. We verified that the proportional hazards assumption was not violated by testing the unadjusted interactions of follow-up time with galectin-3 quintiles. In the proportional hazards model, we also tested for interactions of age, sex, and race with galectin-3 on VTE risk. Model 1 was unadjusted, while model 2 was minimally adjusted for participant age (years), sex (female, male), race (white, black) and in CHS, field center. Model 3 was additionally adjusted for several VTE risk factors identified in the ARIC cohort – diabetes status (yes, no), BMI (kg/m2), and eGFR (ml/min per 1.73 m2) [32]. Model 4 further adjusted for several plasma inflammatory and cardiac biomarkers previously associated with VTE – log CRP (log mg/L), log troponin T (log ng/L), and log NT-proBNP (log pg/mL) – which might be mediators of the galectin-3 association with VTE. Using the Cox regression, the test for linear trend of VTE risk across galectin-3 categories modeled the median value of each category of galectin-3 as a continuous variable.
In ARIC, restricted cubic splines adjusted for age, sex, and race with 7 knots at the 5th, 25th, 50th, 75th, 90th, 95th, and 99th percentiles, were used to further characterize the association between galectin-3 and incident VTE. We also repeated the main analysis excluding those with baseline cancer and cancer related VTE. In another sensitivity analysis, we split the follow-up time into three categories (0 – 6 years, >6 – 10.5 years, and >10.5 years). We examined the race-specific associations of the LGALS3 rs4644 genotype (AA, AC, and CC) with VTE using Cox models. Of the 9,916 included ARIC participants, we had genetic data on 9,509 participants. For each participant, we coded the SNP as having 0 (AA), 1 (AC), or 2 (CC) alleles and used an additive genetic model. For the analysis in blacks, we also adjusted for 10 principal components of ancestry.
RESULTS
Baseline characteristics
In ARIC, the study sample (n= 9,916) was 21.8% black and 56.2% were females; the mean (SD) age was 62.7 (5.7) years. Plasma galectin-3 concentration had a median (25th – 75th percentile) of 14.2 (12.0 – 16.8) ng/mL. As shown in Table 1, the proportion of females, blacks, and diabetics increased across galectin-3 quintiles (p-trend <0.001). BMI, CRP, TnT, and NT-proBNP were all positively associated with galectin-3 concentration, while eGFR showed a negative association.
Table 1.
Baseline characteristics of participants stratified by plasma galectin-3 quintiles, ARIC, 1996 – 1998
| Characteristics* | Galectin-3 Quintiles (ng/mL)
|
p-trend | ||||
|---|---|---|---|---|---|---|
| Q1 (4.4 – 11.4) |
Q2 (11.5 – 13.3) |
Q3 (13.4 – 15.1) |
Q4 (15.2 – 17.5) |
Q5 (17.6 – 114.0) |
||
| N | 1973 | 2052 | 1951 | 1954 | 1986 | – |
| Age (years) | 61.4 (5.4) | 62.1 (5.6) | 62.6 (5.6) | 63.1 (5.6) | 64.4 (5.7) | <0.0001 |
| Sex (% female) | 38.7 | 49.0 | 56.5 | 64.5 | 72.7 | <0.0001 |
| Race (% black) | 16.3 | 19.2 | 21.2 | 24.4 | 28.2 | <0.0001 |
| Diabetes | 13.9 | 13.7 | 15.7 | 17.0 | 21.8 | <0.0001 |
| BMI (kg/m2) | 27.9 (4.6) | 28.1 (5.1) | 28.5 (5.3) | 29.1 (5.7) | 29.9 (6.4) | <0.0001 |
| eGFR (ml/min per 1.73 m2) | 91 (13) | 89 (14) | 88 (14) | 86 (15) | 79 (21) | <0.0001 |
| CRP (mg/L)† | 1.6 (0.8 – 3.5) |
1.9 (0.9 – 4.5) |
2.3 (1.1 – 5.1) |
3.0 (1.3 – 6.1) |
4.0 (1.6 – 8.0) |
<0.0001 |
| Troponin T (ng/L)† | 5.0 (3.0 – 7.0) |
5.0 (3.0 – 8.0) |
5.0 (3.0 – 8.0) |
4.0 (3.0 – 8.0) |
6.0 (3.0 – 10.0) |
<0.0001 |
| NT-proBNP (pg/mL)† | 53 (26 – 105) |
62 (30 – 120) |
65 (33 – 121) |
69 (36 – 134) |
95 (47 – 179) |
0.0005 |
BMI, body mass index; eGFR, estimated glomerular filtration rate; CRP, C-reactive protein; NT-proBNP, N-terminal pro–B-type natriuretic peptide.
Values are mean (standard deviation) for continuous variables and percentages for categorical variables unless otherwise specified.
Data are expressed as median (25th – 75th percentile).
In CHS (n = 3,709), 17.2% were black and 58.5% were female. The mean (SD) age was 72.8 (5.6) years and the median (25th – 75th percentile) galectin-3 concentration was 15.6 (12.7 – 19.1) ng/mL. Except for race and diabetic status, baseline characteristics showed trends with galectin-3 similar to ARIC (supplemental table 1).
Association of Galectin-3 with VTE incidence
We identified 389 incident cases of VTE over a median of 13.9 years in ARIC (1996 – 2013), and 124 incident VTEs over a median of 11.5 years of follow-up in CHS (1989 – 2001).
In ARIC, the incidence rates of VTE per 1000 person-years increased across galectin-3 quintiles (2.2, 2.6, 2.5, 3.6, and 4.6 respectively), and this positive association between galectin-3 quintiles and VTE incidence did not differ by age, sex, or race. Figure 1 depicts the positive association of galectin-3 with VTE, adjusted for these three demographic variables. In the unadjusted model of our Cox regression (Model 1), the hazard ratios (95% CI) for VTE across the quintiles were 1 (reference), 1.19 (0.84 – 1.69), 1.11 (0.78 – 1.58), 1.62 (1.17 – 2.26), and 2.11 (1.54 – 2.91) (p-trend < 0.001). After adjusting for demographic variables, BMI, diabetes status, and renal function (Model 3), the hazard ratios (95% CI) for VTE across the quintiles were 1 (reference), 1.13 (0.80 – 1.61), 1.00 (0.70 – 1.43), 1.36 (0.96 – 1.91), and 1.55 (1.09 – 2.19) (p-trend = 0.005). The hazard ratio for VTE for the highest quintile compared to the lowest quintile was partly attenuated after further adjustment for log CRP, log NT-proBNP, and log TnT in model 4 [HR (95% CI) = 1.37 (0.96 – 1.94)], but the trend remained significant (p-trend = 0.04). The hazard ratio for VTE for 1-SD increment in log galectin-3 was 1.31 (p <0.001) in model 1 and 1.17 (p = 0.007) in model 3 (Table 2).
Fig 1.
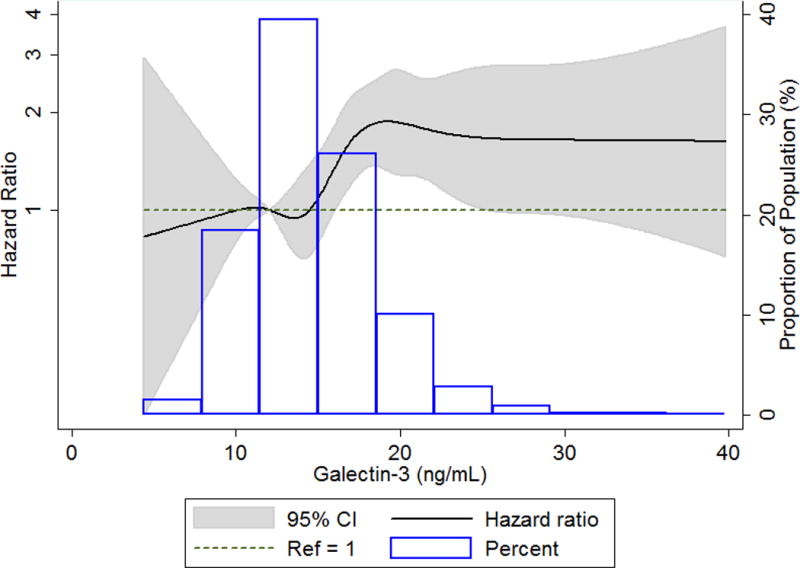
Cubic spline showing association of galectin-3 with venous thromboembolism incidence in ARIC, 1996 – 2013. High extreme values of galectin-3 (> 40 ng/ml) were excluded (n=26) from the spline analysis. The 25th percentile (12.2ng/mL) was used as reference in a Cox proportional hazards model adjusted for age, sex, and race. The knots were placed at the 5th, 25th, 50th, 75th, 90th, 95th, and 99th percentiles.
Table 2.
Incidence rates (95% CI) and hazard ratios (95% CI) of venous thromboembolism in relation to plasma galectin-3 concentration, ARIC, 1996 – 2013
| Galectin-3 Quintiles (ng/mL)
|
p-trend | Per 1-SD increment in log galectin-3 | |||||
|---|---|---|---|---|---|---|---|
| Q1 (4.4 – 11.4) |
Q2 (11.5 – 13.3) |
Q3 (13.4 – 15.1) |
Q4 (15.2 – 17.5) |
Q5 (17.6 – 114.0) |
|||
|
|
|||||||
| VTE, n | 58 | 71 | 63 | 89 | 108 | – | – |
| Person – years | 26075 | 26803 | 25600 | 24928 | 23676 | – | – |
| Incidence rate* | 2.2 (1.7 – 2.9) |
2.6 (2.1 – 3.3) |
2.5 (1.9 – 3.2) |
3.6 (2.9 – 4.4) |
4.6 (3.8 – 5.5) |
– | – |
| Model 1 | 1 (Referent) |
1.19 (0.84 – 1.69) |
1.11 (0.78 – 1.58) |
1.62 (1.17 – 2.26) |
2.11 (1.54 – 2.91) |
<0.0001 | 1.31 (1.19 – 1.44) |
| Model 2 | 1 (Referent) |
1.15 (0.81 – 1.63) |
1.04 (0.72 – 1.49) |
1.48 (1.06 – 2.08) |
1.81 (1.30 – 2.54) |
<0.0001 | 1.24 (1.12 – 1.38) |
| Model 3 | 1 (Referent) |
1.13 (0.80 – 1.61) |
1.00 (0.70 – 1.43) |
1.36 (0.96 – 1.91) |
1.55 (1.09 – 2.19) |
0.005 | 1.17 (1.04 – 1.31) |
| Model 4 | 1 (Referent) |
1.09 (0.77 – 1.55) |
0.95 (0.66 – 1.37) |
1.27 (0.90 – 1.79) |
1.37 (0.96 – 1.94) |
0.04 | 1.09 (0.98 – 1.22) |
SD, standard deviation.
SD log galectin-3 = 0.27 log ng/mL
Incidence rate is unadjusted and per 1000 person-years.
Model 1: crude model.
Model 2: adjusted for age (continuous), sex (female, male), and race (white, black).
Model 3: Model 2 plus diabetes (yes, no), BMI (continuous), and eGFR (continuous).
Model 4: Model 3 plus log CRP (continuous), log Troponin T (continuous), and log NT-proBNP (continuous).
As shown in Table 3, the unadjusted hazard ratios for VTE across galectin-3 quintiles in CHS were 1 (reference), 1.49 (0.86 – 2.57), 1.13 (0.64 – 2.02), 1.02 (0.56 – 1.86), and 1.39 (0.78 – 2.48) (p-trend = 0.60). There was no obvious association between galectin-3 and VTE in CHS. When the Model 3 results from the two studies were meta-analyzed (fixed effects model), the pooled hazard ratio (95% CI) of VTE for 1 SD increment in log galectin-3 was 1.10 (1.00 – 1.22). The test for between study heterogeneity was borderline significant (p = 0.05, I-squared = 75.1%).
Table 3.
Incidence rates (95% CI) and hazard ratios (95% CI) of venous thromboembolism in relation to plasma galectin-3 concentration, CHS, 1989 – 2001
| Galectin-3 Quintiles (ng/mL)
|
p-trend | Per 1-SD increment in log galectin-3 | |||||
|---|---|---|---|---|---|---|---|
| Q1 (3.1 – 12.1) |
Q2 (12.1 – 14.5) |
Q3 (14.5 – 16.8) |
Q4 (16.8 – 20.2) |
Q5 (20.2 – 94.8) |
|||
| VTE, n | 22 | 31 | 25 | 21 | 25 | – | – |
| Person – years | 7403 | 7074 | 7188 | 7009 | 6230 | – | – |
| Incidence rate* | 3.0 (2.0 – 4.5) |
4.4 (3.1 – 6.2) |
3.5 (2.4 – 5.1) |
3.0 (2.0 – 4.6) |
4.0 (2.7 – 5.9) |
– | – |
| Model 1 | 1 (Referent) |
1.49 (0.86 – 2.57) |
1.13 (0.64 – 2.02) |
1.02 (0.56 – 1.86) |
1.39 (0.78 – 2.48) |
0.60 | 1.03 (0.85 – 1.24) |
| Model 2 | 1 (Referent) |
1.53 (0.88 – 2.65) |
1.16 (0.65 – 2.08) |
1.05 (0.57 – 1.95) |
1.35 (0.74 – 2.47) |
0.69 | 1.01 (0.84 – 1.22) |
| Model 3 | 1 (Referent) |
1.46 (0.84 – 2.56) |
1.07 (0.60 – 1.93) |
0.92 (0.49 – 1.72) |
1.05 (0.55 – 2.01) |
0.63 | 0.92 (0.75 – 1.13) |
| Model 4 | 1 (Referent) |
1.47 (0.84 – 2.57) |
1.07 (0.59 – 1.94) |
0.93 (0.50 – 1.75) |
1.04 (0.53 – 2.02) |
0.61 | 0.91 (0.74 – 1.13) |
SD, standard deviation.
SD log galectin-3 = 0.34 log ng/mL
Incidence rate is unadjusted and per 1000 person-years.
Model 1: crude model.
Model 2: adjusted for age (continuous), sex (female, male), race (white, black), and center.
Model 3: Model 2 plus diabetes (yes, no), BMI (continuous), and eGFR (continuous).
Model 4: Model 3 plus log CRP (continuous), log Troponin T (continuous), and log NT-proBNP (continuous).
After excluding participants with baseline cancer or cancer related VTE (n = 1147), 8,769 ARIC study participants remained, and 252 of these had incident VTE over follow-up. The mean (SD) galectin-3 concentration of participants in this group was 14.8 (4.9) ng/mL, which was significantly lower than the galectin-3 concentration in participants with baseline cancer or cancer related VTE [mean (SD) = 15.1 (4.1) ng/mL, p = 0.01]. Regression results were qualitatively similar to the overall ARIC results. In model 3, the hazard ratios for VTE not due to cancer across quintiles were 1 (referent), 1.00 (0.65 – 1.55), 0.96 (0.62 – 1.49), 1.16 (0.76 – 1.77), and 1.50 (0.98 – 2.28) (p-trend = 0.02), and per 1-SD increment in log galectin-3 was 1.16 (1.004 – 1.33). These associations were no longer statistically significant after additional adjustment for covariates in model 4 (supplemental table 2).
In the ARIC cohort, when the follow-up period was split into three intervals, the association of galectin-3 with VTE remained positive throughout with no significant interaction of galectin-3 by time, though the association was slightly stronger for > 6 – 10.5 years of follow-up. The unadjusted hazard ratios (95% CI) per 1-SD increment in log galectin-3 were 1.32 (1.13 – 1.55), 1.40 (1.20 – 1.65), and 1.19 (0.99 – 1.42) for follow-up of 0 – 6 years, > 6 – 10.5 years, and > 10.5 years, respectively.
Association of LGALS3 rs4644 with VTE incidence
As shown in Table 4, the frequencies of having 0, 1, or 2 copies of the C allele were 17.0%, 48.4%, and 34.6% in whites and 7.7%, 39.8%, and 52.6% in blacks. The mean galectin-3 concentrations increased across those with 0, 1, or 2 copies: 11.2 ng/mL, 14.0 ng/mL, and 16.8 ng/mL (p-trend <0.0001) for whites and 12.2 ng/mL, 14.4 ng/mL, and 17.6 ng/mL (p-trend <0.0001) for blacks. The risk for VTE in whites was 34% (−9% – 98%) higher for the AC genotype and 52% (2% – 126%) higher for the CC genotype compared to the AA genotype. This association was not statistically significant in blacks (Table 4).
Table 4.
Race-specific incidence rates (95% CI) and hazard ratios (95% CI) of venous thromboembolism in relation to LGALS3 rs4644 genotype, ARIC, 1996 – 2013
| Variable | rs4644 genotype
|
P-trend | ||
|---|---|---|---|---|
| AA | AC | CC | ||
| Whites | ||||
| N (%) | 1273 (17.0) | 3624 (48.4) | 2590 (34.6) | – |
| Mean (SD) Galectin-3 (ng/mL) | 11.2 (2.7) | 14.0 (3.3) | 16.8 (4.3) | <0.0001 |
| VTEs, n | 32 | 122 | 98 | – |
| Incidence rate* (95% CI) | 1.9 (1.4 – 2.7) | 2.6 (2.2 – 3.1) | 3.0 (2.4 – 3.6) | – |
| Hazard ratios | 1 (Referent) | 1.34 (0.91 – 1.98) | 1.52 (1.02 – 2.26) | 0.04 |
| Blacks | ||||
| N (%) | 155 (7.7) | 804 (39.8) | 1063 (52.6) | – |
| Mean (SD) Galectin-3 (ng/mL) | 12.2 (4.4) | 14.4 (4.1) | 17.6 (8.2) | <0.0001 |
| VTEs, n | 8 | 46 | 63 | – |
| Incidence rate* (95% CI) | 4.0 (2.0 – 8.0) | 4.6 (3.4 – 6.1) | 4.7 (3.6 – 6.0) | – |
| Hazard ratios† | 1 (Referent) | 1.19 (0.56 – 2.52) | 1.20 (0.57 – 2.51) | 0.72 |
Unadjusted incidence rates and per 1,000 person-years.
Adjusted for 10 principal components of ancestry.
DISCUSSION
In the large population-based ARIC study, concentrations of plasma galectin-3 in ARIC were associated positively with the incidence of VTE after adjusting for several risk factors for VTE. There was no interaction by race, suggesting this association did not differ between whites and blacks. We were unable to replicate this association between galectin-3 and VTE in CHS, possibly due to the small number of VTE events in CHS (n = 124), or due to a competing risk bias whereby elderly participants were likely to suffer from severe morbid conditions or die from other causes, or because risk factors measured many years prior are not as indicative of future events in the elderly when compared to younger individuals. Nonetheless, a meta-analysis of ARIC and CHS results still showed a positive association between plasma galectin-3 and VTE.
In mouse models, elevated galectin-3 is correlated with thrombus size, and in human patients it correlated with acute VT [9]. The partial attenuation of the association between galectin-3 and VTE by other inflammatory and cardiac biomarkers suggests that galectin-3 level may also reflect the role of inflammation in VTE pathogenesis [6, 8]. This attenuation may be the result of the other biomarkers being mediators of the galectin-3 association with VTE or reflect similar pathways between these biomarkers in VTE pathogenesis. Galectin-3 has been reported to interact with gal3bp at the thrombus–vein wall interface, and may contribute to thrombosis through proinflammatory, interleukin-6–dependent mechanisms [9].
The LGALS3 rs4644 C allele was associated with higher mean galectin-3 levels in both whites and blacks. The rs4644 C allele was also associated with greater risk of incident VTE in whites (but not blacks). However, this association was likely due to chance, as the association of rs4644 with VTE was not detected (p>0.20) in the GWAS study of VTE in the INVENT consortium (7,507 VTE cases and 52,632 controls) [33]. On the other hand, the association between LGALS3 rs4644 and galectin-3 levels have been previously documented, and evidence suggests that this association may be partly artifact, as this SNP has been shown to lie within or near the epitopes of the binding region for the antibody used for the ARIC galectin-3 assay [17]. This might affect the affinity of the antibody and account for racial differences seen in plasma galectin-3 levels.
A limitation of our study is that we had a single measure of galectin-3 in both ARIC and CHS, and galectin-3 measurements were made on samples stored for years. Although the ARIC lab quality control and blinded replicate data indicated excellent assay performance, we cannot rule out that sample degradation may have affected galectin-3 measurements. However, if sample integrity was a factor this should lead to a significant reduction in the power of our analyses, and our findings would underestimate the true association. Also, galectin-3 concentrations have been suggested to remain stable after storage for at least 6 months [34]. In CHS, stability has been demonstrated for at least 2 years when samples were stored at −7°C [22]. Galectin-3 levels may have changed prior to the VTE occurrence, which we could not document with a single measure. Yet, our analysis by duration of follow-up in ARIC did not provide evidence that the association waned greatly over time.
We could not determine whether galectin-3 was associated with VTE in ARIC independently from other important hemostatic factors, such as D-dimer, since hemostatic factors were measured at a different study visit from galectin-3. VTE events were identified only through hospitalizations and we may have missed participants who did not present to the hospital. Despite this, pilot data suggests the vast majority of patients with first VTE in ARIC and CHS were hospitalized.
In conclusion, plasma galectin-3 concentration is associated positively with incidence of VTE.
Supplementary Material
Brief summary.
Galectin-3, an inflammatory biomarker, is involved in murine thrombogenesis.
Galectin-3–binding protein is up-regulated in microparticles from deep venous thrombosis patients compared to control patients.
In this prospective epidemiological study, participants with plasma galectin-3 concentrations in the highest quintile had a greater risk of incident venous thromboembolism than did those in the lowest quintile.
The association of galectin-3 and VTE was not replicated in a second prospective study, but the meta-analyzed hazard ratio still indicated a positive association.
Galectin-3 is associated positively with risk of venous thromboembolism.
Acknowledgments
The authors thank the staff and participants of the ARIC Study and CHS for their important contributions. We also thank the investigators of the INVENT consortium for analysis to potentially replicate our genetic finding.
Funding Sources: The National Heart, Lung, and Blood Institute (NHLBI) supported LITE via R01HL0597367, ARIC via contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C, and ARIC lab work via R01HL134320-01. NHLBI supported CHS via contracts HHSN268201200036C, HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and grant U01HL080295 and U01HL130114, with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS) and R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org.
Footnotes
AUTHOR CONTRIBUTIONS
All authors contributed to the writing of this manuscript and have approved the final version. O. Fashanu analyzed ARIC data and drafted the manuscript. S. Heckbert contributed to the conception and design of study, review and interpretation of results. D. Aguilar contributed to the interpretation of the genetic analysis and review of the manuscript. P. Jenson drafted the replication study in CHS and is responsible for its data analysis. C. Ballantyne contributed to the interpretation of the genetic analysis, the interpretation of the data and review of the manuscript. S. Basu contributed to the review of the data analysis methods, interpretation of the data and review of the manuscript. R. Hoogeveen contributed the galectin-3 measurement in ARIC, the interpretation of the data and review of the manuscript. C. deFilippi contributed to the galectin-3 measurment in CHS, interpretation of the data and review of the manuscript. M. Cushman contributed VTE validation, to the interpretation of the data and review of the manuscript. A. Folsom contributed to the conception and design of study, VTE validation, interpretation of results, and supervision of the study.
References
- 1.Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. J Thromb Thrombolysis. 2016;41:3–14. doi: 10.1007/s11239-015-1311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolberg AS, Rosendaal FR, Weitz JI, Jaffer IH, Agnelli G, Baglin T, Mackman N. Venous thrombosis. Nat Rev Dis Primers. 2015;1:15006. doi: 10.1038/nrdp.2015.6. [DOI] [PubMed] [Google Scholar]
- 3.Raskob GE, Angchaisuksiri P, Blanco AN, Büller H, Gallus A, Hunt BJ, Hylek EM, Konstantinides SV, McCumber M, Ozaki Y, Wendelboe A. Thrombosis: A major contributor to the global disease burden. J Thromb Haemost. 2014;12:1580–90. doi: 10.1111/jth.12698. [DOI] [PubMed] [Google Scholar]
- 4.Office of the Surgeon General. The Surgeon General’s call to action to prevent deep vein thrombosis and pulmonary embolism. Rockville, MD: Office of the Surgeon General; 2008. Available at: http://www.ncbi.nlm.nih.gov/books/NBK44178/ [PubMed] [Google Scholar]
- 5.Folsom AR, Lutsey PL, Astor BC, Cushman M. C-reactive protein and venous thromboembolism. A prospective investigation in the ARIC cohort. Thromb Haemost. 2009;102:615–9. doi: 10.1160/TH09-04-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saghazadeh A, Rezaei N. Inflammation as a cause of venous thromboembolism. Crit Rev Oncol Hematol. 2016;99:272–85. doi: 10.1016/j.critrevonc.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Wojcik BM, Wrobleski SK, Hawley AE, Wakefield TW, Myers DD, Jr, Diaz JA. Interleukin-6: a potential target for post-thrombotic syndrome. Ann Vasc Surg. 2011;25:229–39. doi: 10.1016/j.avsg.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Diaz JA. Inflammation and acute venous thrombosis. US Oncol Hematol. 2011;7:68–71. [Google Scholar]
- 9.DeRoo EP, Wrobleski SK, Shea EM, Al-Khalil RK, Hawley AE, Henke PK, Myers DD, Wakefield TW, Diaz JA. The role of galectin-3 and galectin-3–binding protein in venous thrombosis. Blood. 2015;125:1813–21. doi: 10.1182/blood-2014-04-569939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diaz JA, Ramacciotti E, Wakefield TW. Do galectins play a role in venous thrombosis? a review. Thromb Res. 2010;125:373–6. doi: 10.1016/j.thromres.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Velde AR, Meijers WC, Ho JE, Brouwers FP, Rienstra M, Bakker SJ, Muller Kobold AC, van Veldhuisen DJ, van Gilst WH, van der Harst P, de Boer RA. Serial galectin-3 and future cardiovascular disease in the general population. Heart. 2016;102:1134–41. doi: 10.1136/heartjnl-2015-308975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newlaczyl AU, Yu L-G. Galectin-3–a jack-of-all-trades in cancer. Cancer Lett. 2011;313:123–8. doi: 10.1016/j.canlet.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, Larson MG, Levy D. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60:1249–56. doi: 10.1016/j.jacc.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darrow AL, Shohet RV, Maresh JG. Transcriptional analysis of the endothelial response to diabetes reveals a role for galectin-3. Physiol Genomics. 2011;43:1144–52. doi: 10.1152/physiolgenomics.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papaspyridonos M, McNeill E, de Bono JP, Smith A, Burnand KG, Channon KM, Greaves DR. Galectin-3 is an amplifier of inflammation in atherosclerotic plaque progression through macrophage activation and monocyte chemoattraction. Arterioscler Thromb Vasc Biol. 2008;28:433–40. doi: 10.1161/ATVBAHA.107.159160. [DOI] [PubMed] [Google Scholar]
- 16.Ramacciotti E, Hawley AE, Wrobleski SK, Myers DD, Jr, Strahler JR, Andrews PC, Guire KE, Henke PK, Wakefield TW. Proteomics of microparticles after deep venous thrombosis. Thromb Res. 2010;125:e269–74. doi: 10.1016/j.thromres.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Boer RA, Verweij N, van Veldhuisen DJ, Westra H-J, Bakker SJL, Gansevoort RT, Muller Kobold AC, van Gilst WH, Franke L, Leach IM, van der Harst P. A genome-wide association study of circulating galectin-3. PLoS One. 2012;7:e47385. doi: 10.1371/journal.pone.0047385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 19.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, O’Leary DH. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 20.Cushman M, Tsai AW, White RH, Heckbert SR, Rosamond WD, Enright P, Folsom AR. Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology. Am J Med. 2004;117:19–25. doi: 10.1016/j.amjmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Bell EJ, Lutsey PL, Basu S, Cushman M, Heckbert SR, Lloyd-Jones DM, Folsom AR. Lifetime risk of venous thromboembolism in two cohort studies. Am J Med. 2016;129:339.e19–26. doi: 10.1016/j.amjmed.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bansal N, Katz R, Seliger S, DeFilippi C, Sarnak MJ, Delaney JA, Christenson R, de Boer IH, Kestenbaum B, Robinson-Cohen C, Ix JH, Shlipak MG. Galectin-3 and soluble ST2 and kidney function decline in older adults: the Cardiovascular Health Study (CHS) Am J Kidney Dis. 2016;67:994–6. doi: 10.1053/j.ajkd.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whelton SP, Roy P, Astor BC, Zhang L, Hoogeveen RC, Ballantyne CM, Coresh J. Elevated high-sensitivity C-reactive protein as a risk marker of the attenuated relationship between serum cholesterol and cardiovascular events at older age: The ARIC Study. Am J Epidemiol. 2013;178:1076–84. doi: 10.1093/aje/kwt086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Folsom AR, Lutsey PL, Astor BC, Wattanakit K, Heckbert SR, Cushman M, for the Atherosclerosis Risk in Communities S Chronic kidney disease and venous thromboembolism: a prospective study. Nephrol Dial Transplant. 2010;25:3296–301. doi: 10.1093/ndt/gfq179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Folsom AR, Nambi V, Bell EJ, Oluleye OW, Gottesman RF, Lutsey PL, Huxley RR, Ballantyne CM. Troponin T, N-terminal pro-B-type natriuretic peptide, and incidence of stroke: the atherosclerosis risk in communities study. Stroke. 2013;44:961–7. doi: 10.1161/STROKEAHA.111.000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro AF, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.deFilippi CR, Christenson RH, Gottdiener JS, Kop WJ, Seliger SL. Dynamic cardiovascular risk assessment in elderly people. The role of repeated N-terminal pro-B-type natriuretic peptide testing. J Am Coll Cardiol. 2010;55:441–50. doi: 10.1016/j.jacc.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tracy RP, Lemaitre RN, Psaty BM, Ives DG, Evans RW, Cushman M, Meilahn EN, Kuller LH. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly. Results from the Cardiovascular Health Study and the Rural Health Promotion Project. Arterioscler Thromb Vasc Biol. 1997;17:1121–7. doi: 10.1161/01.atv.17.6.1121. [DOI] [PubMed] [Google Scholar]
- 30.Shastri S, Katz R, Rifkin DE, Fried LF, Odden MC, Peralta CA, Chonchol M, Siscovick D, Shlipak MG, Newman AB, Sarnak MJ. Kidney function and mortality in octogenarians: Cardiovascular Health Study All Stars. J Am Geriatr Soc. 2012;60:1201–7. doi: 10.1111/j.1532-5415.2012.04046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grove ML, Yu B, Cochran BJ, Haritunians T, Bis JC, Taylor KD, Hansen M, Borecki IB, Cupples LA, Fornage M, Gudnason V, Harris TB, Kathiresan S, Kraaij R, Launer LJ, Levy D, Liu Y, Mosley T, Peloso GM, Psaty BM, et al. Best practices and joint calling of the HumanExome BeadChip: the CHARGE Consortium. PLoS One. 2013;8:e68095. doi: 10.1371/journal.pone.0068095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsai AW, Cushman M, Rosamond WD, Heckbert SR, Polak JF, Folsom AR. Cardiovascular risk factors and venous thromboembolism incidence: the longitudinal investigation of thromboembolism etiology. Arch Intern Med. 2002;162:1182–9. doi: 10.1001/archinte.162.10.1182. [DOI] [PubMed] [Google Scholar]
- 33.Germain M, Chasman DI, De Haan H, Tang W, Lindström S, Weng LC, De Andrade M, De Visser MC, Wiggins KL, Suchon P, Saut N. Meta-analysis of 65,734 individuals identifies TSPAN15 and SLC44A2 as two susceptibility loci for venous thromboembolism. Am J Hum Genet. 2015;96:532–42. doi: 10.1016/j.ajhg.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christenson RH, Duh S-H, Wu AHB, Smith A, Abel G, deFilippi CR, Wang S, Adourian A, Adiletto C, Gardiner P. Multi-center determination of galectin-3 assay performance characteristics: Anatomy of a novel assay for use in heart failure. Clin Biochem. 2010;43:683–90. doi: 10.1016/j.clinbiochem.2010.02.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


