Abstract
IMPORTANCE
Prescription opioid misuse is a national crisis. Few interventions have improved adherence to opioid-prescribing guidelines.
OBJECTIVE
To determine whether a multicomponent intervention, Transforming Opioid Prescribing in Primary Care (TOPCARE; http://mytopcare.org/), improves guideline adherence while decreasing opioid misuse risk.
DESIGN, SETTING, AND PARTICIPANTS
Cluster-randomized clinical trial among 53 primary care clinicians (PCCs) and their 985 patients receiving long-term opioid therapy for pain. The study was conducted from January 2014 to March 2016 in 4 safety-net primary care practices.
INTERVENTIONS
Intervention PCCs received nurse care management, an electronic registry, 1-on-1 academic detailing, and electronic decision tools for safe opioid prescribing. Control PCCs received electronic decision tools only.
MAIN OUTCOMES AND MEASURES
Primary outcomes included documentation of guideline-concordant care (both a patient-PCC agreement in the electronic health record and at least 1 urine drug test [UDT]) over 12 months and 2 or more early opioid refills. Secondary outcomes included opioid dose reduction (ie, 10% decrease in morphine-equivalent daily dose [MEDD] at trial end) and opioid treatment discontinuation. Adjusted outcomes controlled for differing baseline patient characteristics: substance use diagnosis, mental health diagnoses, and language.
RESULTS
Of the 985 participating patients, 519 were men, and 466 were women (mean [SD] patient age, 54.7 [11.5] years). Patients received a mean (SD) MEDD of 57.8 (78.5) mg. At 1 year, intervention patients were more likely than controls to receive guideline-concordant care (65.9% vs 37.8%; P < .001; adjusted odds ratio [AOR], 6.0; 95% CI, 3.6–10.2), to have a patient-PCC agreement (of the 376 without an agreement at baseline, 53.8% vs 6.0%; P < .001; AOR, 11.9; 95% CI, 4.4–32.2), and to undergo at least 1 UDT (74.6% vs 57.9%; P < .001; AOR, 3.0; 95% CI, 1.8–5.0). There was no difference in odds of early refill receipt between groups (20.7% vs 20.1%; AOR, 1.1; 95% CI, 0.7–1.8). Intervention patients were more likely than controls to have either a 10% dose reduction or opioid treatment discontinuation (AOR, 1.6; 95% CI, 1.3–2.1; P < .001). In adjusted analyses, intervention patients had a mean (SE) MEDD 6.8 (1.6) mg lower than controls (P < .001).
CONCLUSIONS AND RELEVANCE
A multicomponent intervention improved guideline-concordant care but did not decrease early opioid refills.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT01909076
The United States is facing an opioid morbidity and mortality crisis.1 Legitimately prescribed opioid analgesics contribute to the availability of opioids, and they are then used for nonmedical purposes.2 To improve opioid prescribing, professional medical societies and the Centers for Disease Control and Prevention have released clinical guidelines for long-term opioid therapy.3–6 These guidelines call for use of patient-clinician agreements (agreements), urine drug testing (UDT), prescription drug monitoring programs (PDMPs), and assessment tools to mitigate risks of long-term opioid therapy.3 In addition, the guidelines recommend against prescribing high-dose opioids (eg, ≥100 mg morphine-equivalent daily dose [MEDD]).4 National approaches to improve opioid prescribing include voluntary continuing medical education for prescribers,5,6 mandatory online education courses,7 state regulatory interventions8 (eg, mandatory use of PDMPs),9–12 and limitations on insurance coverage for opioid analgesic prescriptions based on duration or dose.13–15
Despite national guidelines, educational programs, and regulatory requirements, most clinicians do not follow best practices for opioid prescribing.16–19 These strategies focus on changing individual prescriber behavior. Yet observational studies suggest that a systems-based approach may be more effective.20,21
In an effort to improve adherence to opioid-prescribing guidelines in primary care, we conducted a randomized clinical trial (RCT) to test a multicomponent intervention—TOPCARE (Transforming Opioid Prescribing in Primary Care)—combining individual components found to be potentially effective in observational studies: nurse care management, an electronic registry, academic detailing incorporating individual performance reports (eg, audit and feedback), and electronic decision tools.22 We hypothesized that the intervention would increase use of guideline-concordant strategies.
Methods
Study Design
We conducted a cluster RCT randomly assigning primary care clinicians (PCCs) in 4 safety-net primary care practices to receive either the TOPCARE intervention (nurse care management, electronic registry, academic detailing, and electronic decision tools) or electronic decision tools alone for long-term opioid therapy prescribing for a 1-year period. We randomized at the PCC level instead of at the patient level to mitigate potential contamination. Primary outcomes were patient receipt of guideline-concordant care (UDT and agreement) and reduction of early refills. We have described the study design in detail elsewhere.22 The Boston University Medical Center institutional review board and Boston HealthNet research committee approved the study procedures; written informed consent was obtained from all participating PCCs and waived for all patient participants.
Study Setting and Participants
This study took place from January 2014 through March 2016 at 4 urban primary care practices in Boston, Massachusetts. One site was a large primary care internal medicine practice affiliated with an academic safety-net hospital, and the other 3 sites were the internal medicine and family medicine practices at federally qualified community health centers. One health center focused on homeless populations; 1 served a primarily white working-class population; and 1 served a mix of Latino and Vietnamese populations. Eligible PCCs were attending physicians or nurse practitioners (NPs) who had at least 4 patients aged 18 years or older being treated with long-term opioid therapy (3 opioid prescriptions at least 21 days apart in a 6-month period) as documented in the electronic health record (EHR).17,23,24 We excluded prescriptions for opioid-containing cough medicine. We included patients of enrolled PCCs who received long-term opioid therapy with an active opioid prescription in the 60 days prior to the start of the intervention.
Clinical Champion
At each site, at least 1 PCC served as clinical champion. The clinical champions were not study participants; they pilot tested the intervention to determine feasibility at each practice. Clinical champions also served as liaisons to practice administration, facilitated study team contact with PCCs, and coauthored study articles.
Recruitment
To identify eligible PCCs, we extracted data for all sites from the EHR data repositories. We presented the study details at staff meetings at all sites, and in some cases met individually with eligible PCCs. Of the initial 72 PCCs considered for participation, 15 were ineligible owing to insufficient number of patients receiving long-term opioid therapy; 3 declined participation; and 1 did not respond to the invitation to participate. The Figure provides the CONSORT diagram for recruitment.22
Figure. Consolidated Standards of Reporting Trials Study Flow Diagram.
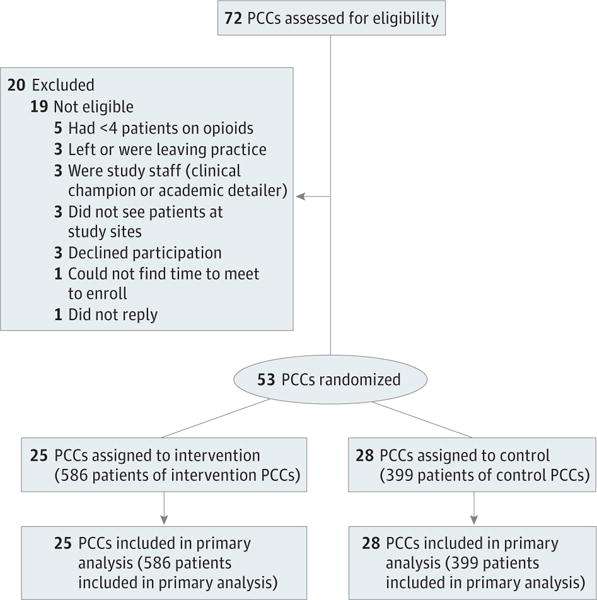
PCC, primary care clinician (ie, a physician or nurse practitioner).
Allocation Procedure
We stratified PCCs by site, training (physician vs NP), and whether they were waivered to prescribe buprenorphine for opioid-use disorders. We randomized individuals using random-number generators in SAS software, version 9.3, with allocation concealment to research assistants until after obtaining informed consent. PCCs were aware of assigned study arms but not study hypotheses or outcomes. Patients of intervention PCCs were aware that a new nurse (the study nurse care manager) was now working with their PCC on opioid management, and, in some sites, that the workflow for obtaining opioid refills had changed. Patients in the control group did not experience changes in their care team or workflow.
Intervention and Control Conditions
The development and rationale of the intervention is detailed elsewhere,22 and the trial protocol is provided in Supplement 1. Here, we summarize the 4 intervention components.
The first component of intervention is the nurse care manager, a registered or licensed practical nurse who performs initial and ongoing patient assessments for pain, addiction, and opioid misuse risk; prepares prescriptions for the PCC to sign or reminds the PCC to print prescriptions; collects UDTs; conducts pill counts (ie, counts pills between refills to monitor medication use); checks PDMPs on behalf of PCCs; and assesses for concerning patient issues (eg, unexpected UDT results) and collaborates with the PCC to develop appropriate clinical responses to these issues.
The second intervention component is a web-based electronic registry to facilitate population management by importing data from the EHR (eg, refill dates, UDT results) and producing reports used to direct work flow (eg, lists of patients with opioid prescriptions due on a certain day or week) and to support academic detailing (eg, provide feedback on PCC panel characteristics such as percentage of patients with an agreement).
The third intervention component is a single 1-on-1 academic detailing session25 between an opioid-prescribing expert (J.M.L., M.L. or D.P.A.) and the PCC. The participants in these sessions discuss principles of safe opioid prescribing and monitoring, registry reports, details on patient monitoring and risk level, and challenging patient cases.
Finally, all participating PCCs, both intervention and control, undergo orientation about and are given access to electronic decision tools through an online platform (http://mytopcare.org/) that includes evidence-based tools for assessment of patient opioid misuse risk (eg, the Opioid Risk Tool26) and interactive tools to assist with UDT ordering and interpretation. Control PCCs receive only this fourth component (ie, orientation and access to the electronic decision tools). See eAppendix B in Supplement 2 for more details.
Outcomes and Data Collection
Patient-Level Outcomes
Primary outcomes were observed over a 12-month period and included (1) PCC adherence to opioid therapy monitoring strategies and (2) early refills. Primary care clinician adherence to guidelines was defined as presence of a patient-clinician agreement and at least 1 completed UDT for controlled and illicit substances. Early refills were defined as 2 or more early refills, consistent with prior studies.27,28 An early refill was considered to be a similar opioid prescription (ie, same dose and directions) given more than 3 days prior to the next expected refill date.22 eAppendix A in Supplement 2 provides details on early refill calculation.
Secondary outcomes included the proportion of patients for whom treatment with opioids was discontinued and the proportion of patients who had a 10% reduction in opioid dose as measured in MEDD among nondiscontinued patients. eAppendix A in Supplement 2 provides details on discontinuation and MEDD calculation.
Covariates
Patient risk factors for opioid misuse included age younger than 45 years, substance use diagnosis, alcohol use diagnosis, current tobacco use, and mental health diagnosis, identified through billing and diagnosis codes in the EHR.29,30
Data Source
An EHR data repository provided deidentified data. The data reflected opioids prescribed, not dispensed.
Sample Size Calculation
Using an alpha of .05, a sample size of 50 PCCs with a mean of 24 patients undergoing long-term opioid therapy would achieve 80% power to detect a 15–percentage point difference in the proportions of patients having an agreement. Details of sample size calculation have been published elsewhere.22
Statistical Analysis
All outcomes were analyzed according to the intent-to-treat principle.31 We verified that demographic characteristics of the intervention and control patients were similar at baseline (Table 1). We compared the baseline and 12-month follow-up measures for each of the primary outcomes of the intervention vs control patients, stratified by intervention status, using 2-sided statistical significance at the P = .05 level. To analyze odds of receipt of agreement, we analyzed data only for patients without existing agreements because the outcome was measured as agreement ever in the EHR, and those with prior agreements would not be eligible for a new one. To control for potential confounders identified in bivariate analyses, we conducted a regression analysis of the 12-month follow-up outcomes, adjusting for baseline measures that differed between groups. We used a regression model with a logit link function for binary outcomes, and an identity link function for continuous outcomes. We used robust standard error estimates (generalized estimating equations method), adjusting for clustering of patients among PCCs. We used quasi-likelihood under the independence model criteria for generalized estimating equations model fit criteria. We reported adjusted odds ratios (AORs) with 95% confidence intervals for binary outcomes, and beta coefficients for continuous outcomes. In addition, we used Cox regression to evaluate the time to discontinuation of opioid treatment among patients, reporting hazard ratios of the relative risk of treatment discontinuation by intervention status. We evaluated proportional hazard assumption of the Cox model. No covariates in adjusted models were time-dependent variables.
Table 1.
Patient Participant Demographic and Clinical Characteristics by Intervention Statusa
| Characteristic | Overall (n = 985) |
Intervention (n = 586) |
Control (n = 399) |
P Value |
|---|---|---|---|---|
| Age, mean (SD), y | 54.7 (11.5) | 54.4 (11.51) | 55.25 (11.47) | .25 |
| Female | 466 (47.3) | 287 (49.0) | 179 (44.9) | .20 |
| Race or ethnic group | .78 | |||
| Non-Hispanic white | 512 (52.0) | 305 (52.1) | 207 (51.9) | |
| Non-Hispanic black | 361 (36.7) | 219 (37.4) | 142 (35.6) | |
| Hispanic | 91 (9.2) | 51 (8.7) | 40 (10.0) | |
| Other | 21 (2.1) | 11 (1.9) | 10 (2.5) | |
| Risk factorsb | ||||
| Age <45 years | 175 (17.8) | 107 (18.3) | 68 (17.0) | .62 |
| Alcohol use diagnosis | 120 (12.2) | 76 (13.0) | 44 (11.0) | .36 |
| Drug use diagnosis | 157 (15.9) | 82 (14.0) | 76 (18.8) | .04 |
| Mental health diagnosis | 611 (62.0) | 346 (59.0) | 265 (66.4) | .02 |
| Tobacco use | 415 (42.1) | 237 (40.4) | 178 (44.6) | .19 |
| English speaking | 914 (92.8) | 552 (94.2) | 362 (90.7) | .04 |
| Primary insurancec | ||||
| Medicaid | 442 (44.9) | 255 (43.5) | 187 (46.9) | .54 |
| Medicare | 296 (30.1) | 181 (31.4) | 112 (28.1) | |
| Private | 146 (14.8) | 90 (15.4) | 56 (14.0) | |
| Other | 101 (10.3) | 57 (9.7) | 44 (11.0) | |
| MEDD, mgd | .27 | |||
| 0 | 21 (2.1) | 16(2.7) | 5(1.3) | |
| >0 to <50 | 649 (65.9) | 392 (66.9) | 257 (64.4) | |
| 50–100 | 167 (17.0) | 93 (15.9) | 74 (18.6) | |
| >100 | 148 (15.0) | 85 (14.5) | 63 (15.8) |
Abbreviations: MEDD, morphine equivalent daily dose.
Unless otherwise noted, data are reported as number (percentage) of patient participants.
Opioid misuse risk factors identified through billing codes in the electronic health record.
Other types of insurance include Massachusetts insurance program for the uninsured, uninsured, and missing.
Mean MEDD within 30 days prior to start of the intervention.
Results
A total of 53 PCCs were enrolled in the study; 28 were randomized to the control group and 25 to the intervention condition. Twenty-eight percent of PCCs were aged 25 to 35 years (n = 16); 30% were aged 36 to 45 years (n = 16); 12% aged 46 to 50 years (n = 6); and 34% 51 years or older (n = 15). Two-thirds were female (n = 35), 66% white (n = 35), 9% African American/black (n = 5), 19% Asian (n = 10), and 6% other (n = 3). Ninety-one percent were physicians (n = 48), and 30% were certified to prescribe buprenorphine (n = 16). Intervention PCCs cared for a mean of 23 patients (median, 13; range, 1–92) with long-term opioid therapy, while control PCCs cared for a mean of 15 patients (median, 11; range 0–70) (P = .09). Regarding ranges below the prespecified minimum of 4 or more patients for enrollment, some of the originally eligible PCCs dropped below the threshold between the time we calculated eligibility via EHR algorithm and the time we enrolled them into the study (generally 4- to 6-week difference). We did not exclude anyone from the study on this basis because we conducted intent-to-treat analyses. There were no differences between the groups with respect to demographic or practice characteristics.22 At baseline, clinicians displayed no difference in their patient proportions of signed agreements, early refills, UDTs, or mean MEDD (Table 2).
Table 2.
Patient-Level Primary Outcomes at 12 Months by Intervention Statusa
| Variable | Baseline | Follow-up | ||||||
|---|---|---|---|---|---|---|---|---|
| Intervention (n = 586) | Control (n = 399) | P Value | Intervention (n = 586) | Control (n = 399) | P Valueb | OR (95% CI) | AOR (95% CI) | |
| Guideline-concordant care (agreement plus UDT) | 241 (41.1) | 168 (42.1) | .76 | 386 (65.9) | 151 (37.8) | <.001 | 3.3 (1.9–5.6) | 6.0 (3.6–10.2) |
| Signed agreement (ever) | 376 (64.2) | 233 (58.4) | .07 | 489 (83.5) | 243 (60.9) | <.001 | 2.5 (1.4–4.5) | Not converge |
| No baseline agreement | 210 (100) | 166 (100) | – | 133 (53.8) | 10 (6.0) | <.001 | 11.2 (4.1–30.7)c | 11.9 (4.4–32.2) |
| UDT (once in past 12 mo) | 348 (59.4) | 259 (64.9) | <.08 | 437 (74.6) | 231 (57.9) | <.001 | 2.4 (1.3–4.4) | 3.0 (1.8–5.0) |
| ≥2 early refillsc | 145 (24.7) | 94 (23.6) | .67 | 121 (20.7) | 80 (20.1) | .82 | 1.1 (0.6–1.9) | 1.1 (0.7–1.8) |
Abbreviations: agreement, patient-clinician agreement; AOR, adjusted odds ratio; OR, unadjusted odds ratio; SE, standard error; UDT, urine drug testing.
Unless otherwise noted, data are reported as number (percentage) of patient participants. Analyses included patients on active opioid prescriptions within 60 days prior to the start date of the intervention. Analyses adjusted for drug use diagnoses, mental health diagnoses, English-speaking patient, and baseline levels of outcome measures (UDT, agreement, early refills).
P values for the test of difference of the differences between groups were identical.
Early refill is defined as a prescription of the same opioid dose and directions given more than 3 days prior to the next expected fill date. This measure excludes potential prescription reprints (multiple prescriptions printed within 7 days).
Patient characteristics are summarized in Table 1. The 985 patients (586 intervention, 399 control) had a mean (SD) age of 54.7 (11.5) years, and 47.3% were female (n = 466). Fifty-two percent of patients were non-Hispanic white (n = 512); 36.7% were non-Hispanic black (n = 361); and 9.2% Hispanic (n = 91). More than three-quarters had Medicaid and/or Medicare insurance (n = 538). Relative to intervention patients, control patients were more likely to have a history of a substance use diagnosis (18.8% vs 14.0%; P = .04) and/or a mental health diagnosis (66.4% vs 59%; P = .02). Intervention patients were more likely to list English as their primary language (94.2% vs 90.7%; P = .04) (Table 1).
At 12-month follow-up, the TOPCARE intervention resulted in significant differences in all outcomes except early refills, favoring the intervention group (Table 2). In analyses controlling for substance use diagnosis, mental health diagnoses, and patient language, intervention patients were more likely that control patients to have guideline-concordant care (65.9% vs 37.8%; P < .001; AOR, 6.0; 95% CI, 3.6–10.2), to have an agreement (of the 376 without an agreement at baseline, 53.8% vs 6.0%; P < .001; AOR, 11.9; 95% CI, 4.4–32.2), and to undergo at least 1 UDT (74.6% vs 57.9%; P < .001; AOR, 3.0; 95% CI, 1.8–5.0). There was no difference in odds of early refill receipt between groups (20.7% vs 20.1%; AOR, 1.1; 95% CI, 0.7–1.8).
A greater proportion of intervention than control PCCs discontinued opioid treatment (21.3% vs 16.8%; P = .04, AOR, 1.4; 95% CI, 1.02–2.1) (Table 3). The mean (SD) time to discontinuation of opioids was shorter for intervention patients (127.1 [89.8] days; median, 136 days; n = 125) than for control patients (142.8 [91.1] days; median, 171.3 days; n = 67). Cox regression analysis of time to discontinuation showed a 40% greater likelihood of opioid discontinuation (adjusted hazard ratio, 1.40; P = .03) for the intervention patients vs the control group, with support of proportional hazard assumption (P = .32 for the group status by time interaction). Among patients still taking opioids in the last 60 days of the intervention period, a greater proportion in the intervention group had a 10% reduction in MEDD from baseline level compared with controls (32.8% vs 22.9%; P = .01; AOR, 1.6; 95% CI, 1.1–2.4). Intervention patients were more likely than controls to have either a 10% dose reduction or opioid discontinuation (47.1% vs 35.8%; P<.001; AOR, 1.6; 95% CI, 1.3–2.1). Of note, 60.4% of patients (116 of 192) had subsequent primary care visits after opioid treatment discontinuation. In adjusted analyses, during the last 30 days of the intervention period, intervention patients had a mean (SE) 6.8 (1.6)-mg lower mean MEDD than controls (P < .001) (Table 3).
Table 3.
Patient-Level Secondary Outcomes at 12 Months by Intervention Statusa
| Variable | Baseline | Follow-up | |||||
|---|---|---|---|---|---|---|---|
| Intervention (n = 586) | Control (n = 399) | P Value | Intervention (n = 586) | Control (n = 399) | P Value | AOR (95% CI) | |
| Discontinuation of opioid prescriptionb | NA | NA | NA | 125 (21.3) | 67 (16.8) | .04 | 1.5 (1.0–2.1) |
| Opioid dose reductionc,d | NA | NA | NA | 151 (32.8) | 76 (22.9) | .002 | 1.6 (1.1–2.4)e |
| Opioid dose reductionc,d or discontinuationb | NA | NA | NA | 276 (47.1) | 143 (35.8) | <.001 | 1.6 (1.3–2.1)e |
| MEDD, mean (SD), mgd,e | 61.1 (84.9) | 62.3 (75.6) | .84 | 60.8 (93.7) | 67.3 (80.4) | .31 | −6.8 (1.6)f,g |
Abbreviations: AOR, adjusted odds ratio; MEDD, morphine equivalent daily dose; NA, not applicable; SE, standard error.
Unless otherwise noted, data are reported as number (percentage) of patient participants. Analyses included patients with complete information on active opioid prescriptions within 60 days prior to the start date of the intervention. Analyses adjusted for drug use diagnosis, mental health problems, English-speaking, and baseline levels of outcome measures.
Definition of discontinuation: if the last day of the prescription (accounting for the days of supply) falls within 300th day after the start of the intervention, the prescription has been discontinued.
10% reduction in MEDD; this compares MEDD in the 30 days prior to the start of the intervention with the last 30 days of the 12-month follow-up of patients receiving opioids in those time periods.
This measure excludes patients who discontinued opioid treatment (n = 192): TOPCARE (n = 461), e-tools only (n = 332).
This measure compares the mean MEDD 30 days prior to the start of the intervention to the last 30 days of the 12-month follow-up of patients receiving opioids in those time periods.
P ≤ .01.
Beta coefficient (standard error).
Although not an a priori outcome, after the study was completed at the clinical sites, efforts to maintain and expand the TOPCARE intervention occurred. Two sites hired study nurses to continue the intervention and to expand services to all PCCs. The other 2 sites sought resources to sustain and expand the intervention to all PCCs.
Discussion
The multicomponent TOPCARE intervention tripled guideline-concordant opioid monitoring with patient-clinician agreements and UDT compared with electronic decision tools alone in 4 urban safety-net primary care practices. The intervention did not reduce the likelihood of obtaining early refills. Although not a primary study outcome, opioid dose reduction and opioid treatment discontinuation were both increased in the intervention group compared with the control group.
Although numerous efforts have targeted opioid-prescribing practices, TOPCARE is the only effort of which we are aware to be studied in a randomized clinical trial. Other health system innovations have shown improved guideline adherence when researchers have analyzed observational data using a pre-post design or comparison of different settings.21,32 Von Korff et al33 compared the outcomes of their group practice physicians who received a system innovation with the outcomes of community-based clinicians in the context of stricter state policies for opioid prescribing. The study intervention standardized care for patients treated with long-term opioid therapy through changes in the EHR, enhanced clinician education, and monetary incentives for adherence. When compared with clinicians exposed to state policies alone, the intervention clinicians reduced the number of patients who were prescribed high-dose opioids and received early refills, although both groups improved significantly during the study period.
Also using a multicomponent intervention, Westanmo et al21 decreased the number of patients taking high-dose opioids.
By conducting a cluster RCT, the present TOPCARE study accounted for local, state, and national pressures to improve the safety of opioid prescribing. The present study focused on improving guideline-concordant monitoring by implementing strategies such as UDT and patient-clinician agreements. The evidence base for these strategies is limited, without direct proof that these strategies result in decreased harms without worsening chronic pain.
The TOPCARE intervention aligns with the current movement toward patient-centered medical homes,34–36 using team-based care (nurse care managers), population management (electronic registry), care management and support (nurse care managers), self-care support (nurse interactions with patients and website), and performance measurement and quality (audit and feedback as a key element of academic detailing).34–36 Furthermore, the TOPCARE intervention shares characteristics of opioid-prescribing practices among 30 primary care clinics noted nationally for practice innovations37: leadership support through clinical champions, revision of prescribing workflows, population management through a registry, planned patient-centered visits (with the nurse care manager), and assessment of progress via data. Nurse care managers play key roles in coordinating the intervention, such as ensuring that monitoring (ie, UDT, pill counts) occurred, interfacing with patients and PCCs to resolve concerning behaviors or pain-relatedneeds, managing the registry (inputting data, printing reports for academic detailing) and directing patients and PCCs to the TOPCARE website (http://mytopcare.org/).
We posit that nurse care management is a critical component of the TOPCARE model, and it has been successfully applied to improve opioid prescribing and pain management. The nurse care manager model in office-based buprenorphine treatment for opioid use disorders38,39 shares characteristics of risk management and monitoring with opioid prescribing. Bair and colleagues40 demonstrated that nurse care managers using a stepped-care approach with medications and cognitive behavioral therapy improve pain-related disability in veterans with chronic pain. Chronic pain and substance use disorders have behavioral components and demand high levels of trust between patient and PCC for successful treatment. Opioid medications pose risks to the patient and society at large. Thus, nurse care manager–PCC partnership builds additional supports for patients and can ensure that PCCs meet the increasing regulatory demands related to opioid prescribing.4,8,41 The benefits of employing nurses to deliver pain-related and opioid-related care may relate to fundamental nursing functions, such as comprehensive assessments, patient education, and patient self-management, which contrast with PCCs’ focus on diagnosis and treatment.
We were surprised by the lack of difference in early refills between the 2 groups because of the close attention paid by the nurse care manager to patients requesting early refills. Our reliance strictly on EHR data limited our ability to measure early refills as a marker of opioid misuse because we lacked data on whether opioid prescriptions were filled. We were unable to use the state PDMP to verify refills owing to restrictions on its use for research. Furthermore, early preparation of prescriptions may result from patient vacation preparations or intensified monitoring (eg, 14-day refills), making it difficult to interpret this outcome. We chose early refills as a proxy for potential opioid misuse; however, opioid misuse determination requires patient-level assessments. Future iterations of the intervention should incorporate data generated by the state PDMP to report early refills, discontinuations, and dose reductions as part of the clinical dashboard for individual clinicians and nurse care managers.
In observational studies, opioid dose is correlated with risk of overdose.42,43 At the time of study initiation, no controlled trials tested whether lowering the dose improves overdose risk, so dose reduction and discontinuation were included as secondary study aims, consistent with national guideline recommendations to use lower doses and discontinue opioids when possible.4 We posit that closer scrutiny of patient function and risks may have contributed to these findings.
Limitations
Using the EHR as a sole source of patient data is a limitation. For example, the EHR did not capture the patient experience of the intervention, including its potential impact on pain control, function, and disability. Furthermore, EHR data do not provide accurate substance use and mental health diagnoses.44–46 We did not have prescription or visit data from outside health systems. Other limitations include inability to measure unintended consequences. It is unclear whether opioid dose reduction or discontinuation was due to more judicious or more fearful opioid prescribing. Fearful prescribing may deprive patients of indicated pain medication, concerns reflected in the medical and lay press describing patients’ barriers to obtaining pain medications with increased focus on opioid safety.43,44 In addition, opioid reduction and discontinuation may produce a rupture in the patient-PCC relationship and not necessarily a decrease in risk. Finally, the study’s generalizability to non–safety-net settings is unknown.
Conclusions
TOPCARE, a multicomponent primary care–based intervention, was successful in increasing PCC adherence to guidelines for monitoring patients treated with long-term opioid therapy for chronic pain but not at decreasing early opioid refills by these patients.
Supplementary Material
Key Points.
Question
Does a multicomponent intervention with a nurse care manager, electronic registry, data-driven academic detailing, and electronic decision tools improve adherence to opioid-prescribing guidelines and decrease early refills of opioids in patients with chronic pain compared with electronic decision tools alone?
Findings
The multicomponent intervention improved adherence to guideline-recommended monitoring but did not decrease early opioid refills.
Meaning
While the multicomponent intervention improved adherence to guideline-recommended monitoring of opioids in patients with chronic pain, further research is needed to determine whether guideline adherence reduces opioid-related risks.
Acknowledgments
Funding/Support: This study was funded in part by the National Institute on Drug Abuse, grant R01DA034252-01 (Drs Liebschutz and Lasser).
Role of the Funder/Sponsor: the funders had no role in the design and conduct of the study; collection, management, analysis or interpretation of data; preparation, review or approval of manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Liebschutz had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Liebschutz, Xuan, Shanahan, Alford, Parker, Samet, Lasser.
Acquisition, analysis, or interpretation of data: Liebschutz, Xuan, Shanahan, LaRochelle, Keosaian, Beers, Guara, O’Connor, Weiss, Crosson, Cushman.
Drafting of the manuscript: Liebschutz, Xuan, Shanahan, Keosaian.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Liebschutz, Xuan, Shanahan, LaRochelle.
Obtained funding: Liebschutz, Lasser.
Administrative, technical, or material support: Liebschutz, Shanahan, LaRochelle, Keosaian, Beers, Guara, O’Connor.
Supervision: Liebschutz, Shanahan, Keosaian.
Additional Contributions: The authors wish to acknowledge Linda Rosen, MSEE, for aiding database extraction, Vinay Hooloomann, BE/BS, for development of the registry, Jessie Gaeta, MD, and Mohamed Azzam Mehssen, MD, for help as clinical champions, and the entire clinical staff at all sites for support in this project. Contributors Rosen and Hooloomann were compensated in the normal course of their employment for their contributions; Drs Gaeta and Mehssen each received a stipend for serving as clinical champions.
Conflict of Interest Disclosures: Dr Weiss consulted to GW Pharmaceuticals, Alkermes, and Indivior. No other disclosures are reported.
Contributor Information
Jane M. Liebschutz, Section of General Internal Medicine, Boston Medical Center, Boston, MassachusettsBoston University School of Medicine, Boston, Massachusetts.
Ziming Xuan, Boston University School of Public Health, Boston, Massachusetts.
Christopher W. Shanahan, Section of General Internal Medicine, Boston Medical Center, Boston, MassachusettsBoston University School of Medicine, Boston, Massachusetts.
Marc LaRochelle, Section of General Internal Medicine, Boston Medical Center, Boston, MassachusettsBoston University School of Medicine, Boston, Massachusetts.
Julia Keosaian, Boston University School of Public Health, Boston, Massachusetts.
Donna Beers, Section of General Internal Medicine, Boston Medical Center, Boston, Massachusetts.
George Guara, Section of General Internal Medicine, Boston Medical Center, Boston, Massachusetts.
Kristen O’Connor, Section of General Internal Medicine, Boston Medical Center, Boston, Massachusetts.
Daniel P. Alford, Section of General Internal Medicine, Boston Medical Center, Boston, MassachusettsBoston University School of Medicine, Boston, Massachusetts.
Victoria Parker, Boston University School of Public Health, Boston, Massachusetts.
Roger D. Weiss, McLean Hospital, Belmont, MassachusettsHarvard Medical School, Boston, Massachusetts.
Jeffrey H. Samet, Section of General Internal Medicine, Boston Medical Center, Boston, MassachusettsBoston University School of Medicine, Boston, MassachusettsBoston University School of Public Health, Boston, Massachusetts.
Julie Crosson, Dorchester House Community Health Center, Boston, MassachusettsSection of General Internal Medicine, Boston Medical Center, Boston, Massachusetts.
Phoebe A. Cushman, Section of General Internal Medicine, Boston Medical Center, Boston, MassachusettsBoston University School of Medicine, Boston, Massachusetts.
Karen E. Lasser, Section of General Internal Medicine, Boston Medical Center, Boston, MassachusettsBoston University School of Medicine, Boston, MassachusettsBoston University School of Public Health, Boston, Massachusetts.
References
- 1.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64(50–51):1378–1382. doi: 10.15585/mmwr.mm6450a3. [DOI] [PubMed] [Google Scholar]
- 2.Volkow ND, McLellan AT. Opioid abuse in chronic pain: misconceptions and mitigation strategies. N Engl J Med. 2016;374(13):1253–1263. doi: 10.1056/NEJMra1507771. [DOI] [PubMed] [Google Scholar]
- 3.Nuckols TK, Anderson L, Popescu I, et al. Opioid prescribing: a systematic review and critical appraisal of guidelines for chronic pain. Ann Intern Med. 2014;160(1):38–47. doi: 10.7326/0003-4819-160-1-201401070-00732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm Rep. 2016;65(1):1–49. doi: 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 5.Alford DP, Zisblatt L, Ng P, et al. SCOPE of pain: an evaluation of an opioid risk evaluation and mitigation strategy continuing education program. Pain Med. 2016;17(1):52–63. doi: 10.1111/pme.12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Califf RM, Woodcock J, Ostroff S. A proactive response to prescription opioid abuse. N Engl J Med. 2016;374(15):1480–1485. doi: 10.1056/NEJMsr1601307. [DOI] [PubMed] [Google Scholar]
- 7.Saunders K, Shortreed S, Thielke S, et al. Evaluation of health plan interventions to influence chronic opioid therapy prescribing. Clin J Pain. 2015;31(9):820–829. doi: 10.1097/AJP.0000000000000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haegerich TM, Paulozzi LJ, Manns BJ, Jones CM. What we know, and don’t know, about the impact of state policy and systems-level interventions on prescription drug overdose. Drug Alcohol Depend. 2014;145:34–47. doi: 10.1016/j.drugalcdep.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paulozzi LJ, Kilbourne EM, Desai HA. Prescription drug monitoring programs and death rates from drug overdose. Pain Med. 2011;12(5):747–754. doi: 10.1111/j.1526-4637.2011.01062.x. [DOI] [PubMed] [Google Scholar]
- 10.Brady JE, Wunsch H, DiMaggio C, Lang BH, Giglio J, Li G. Prescription drug monitoring and dispensing of prescription opioids. Public Health Rep. 2014;129(2):139–147. doi: 10.1177/003335491412900207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li G, Brady JE, Lang BH, Giglio J, Wunsch H, DiMaggio C. Prescription drug monitoring and drug overdose mortality. Inj Epidemiol. 2014;1(1):9. doi: 10.1186/2197-1714-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baehren DF, Marco CA, Droz DE, Sinha S, Callan EM, Akpunonu P. A statewide prescription monitoring program affects emergency department prescribing behaviors. Ann Emerg Med. 2010;56(1):19–23.e1-3. doi: 10.1016/j.annemergmed.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Sullivan MD, Bauer AM, Fulton-Kehoe D, et al. Trends in opioid dosing among Washington state Medicaid patients before and after opioid dosing guideline implementation. J Pain. 2016;17(5):561–568. doi: 10.1016/j.jpain.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 14.Wachino V. Best practices for addressing prescription opioid overdoses, misuse and addiction. Centers for Medicare and Medicaid Services; 2016. https://www.medicaid.gov/federal-policy-guidance/downloads/cib-02-02-16.pdf. Accessed May 8, 2017. [Google Scholar]
- 15.Blue Cross Blue Shield of Massachusetts. New quality and safety measures in opioid management, effective. 2012 Jul 1; http://www.bluecrossma.com/bluelinks-for-employers/whats-new/special-announcements/opioid-management.html. Accessed May 8, 2017.
- 16.Morasco BJ, Duckart JP, Dobscha SK. Adherence to clinical guidelines for opioid therapy for chronic pain in patients with substance use disorder. J Gen Intern Med. 2011;26(9):965–971. doi: 10.1007/s11606-011-1734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Starrels JL, Becker WC, Weiner MG, Li X, Heo M, Turner BJ. Low use of opioid risk reduction strategies in primary care even for high risk patients with chronic pain. J Gen Intern Med. 2011;26(9):958–964. doi: 10.1007/s11606-011-1648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tournebize J, Gibaja V, Muszczak A, Kahn J-P. Are physicians safely prescribing opioids for chronic noncancer pain? a systematic review of current evidence. Pain Pract. 2016;16(3):370–383. doi: 10.1111/papr.12289. [DOI] [PubMed] [Google Scholar]
- 19.Sekhon R, Aminjavahery N, Davis CN, Jr, Roswarski MJ, Robinette C. Compliance with opioid treatment guidelines for chronic non-cancer pain (CNCP) in primary care at a Veterans Affairs Medical Center (VAMC) Pain Med. 2013;14(10):1548–1556. doi: 10.1111/pme.12164. [DOI] [PubMed] [Google Scholar]
- 20.Wiedemer NL, Harden PS, Arndt IO, Gallagher RM. The opioid renewal clinic: a primary care, managed approach to opioid therapy in chronic pain patients at risk for substance abuse. Pain Med. 2007;8(7):573–584. doi: 10.1111/j.1526-4637.2006.00254.x. [DOI] [PubMed] [Google Scholar]
- 21.Westanmo A, Marshall P, Jones E, Burns K, Krebs EE. Opioid dose reduction in a VA health care system: implementation of a primary care population-level initiative. Pain Med. 2015;16(5):1019–1026. doi: 10.1111/pme.12699. [DOI] [PubMed] [Google Scholar]
- 22.Lasser KE, Shanahan C, Parker V, et al. A multicomponent intervention to improve primary care provider adherence to chronic opioid therapy guidelines and reduce opioid misuse: a cluster randomized controlled trial protocol. J Subst Abuse Treat. 2016;60:101–109. doi: 10.1016/j.jsat.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larochelle MR, Liebschutz JM, Zhang F, Ross-Degnan D, Wharam JF. Opioid prescribing after nonfatal overdose and association with repeated overdose: a cohort study. Ann Intern Med. 2016;164(1):1–9. doi: 10.7326/M15-0038. [DOI] [PubMed] [Google Scholar]
- 24.Becker WC, Starrels JL, Heo M, Li X, Weiner MG, Turner BJ. Racial differences in primary care opioid risk reduction strategies. Ann Fam Med. 2011;9(3):219–225. doi: 10.1370/afm.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Avorn J. Academic detailing: “marketing” the best evidence to clinicians. JAMA. 2017;317(4):361–362. doi: 10.1001/jama.2016.16036. [DOI] [PubMed] [Google Scholar]
- 26.Zgierska A, Miller M, Rabago D. Patient satisfaction, prescription drug abuse, and potential unintended consequences. JAMA. 2012;307(13):1377–1378. doi: 10.1001/jama.2012.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reid MC, Engles-Horton LL, Weber MB, Kerns RD, Rogers EL, O’Connor PG. Use of opioid medications for chronic noncancer pain syndromes in primary care. J Gen Intern Med. 2002;17(3):173–179. doi: 10.1046/j.1525-1497.2002.10435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleming MF, Balousek SL, Klessig CL, Mundt MP, Brown DD. Substance use disorders in a primary care sample receiving daily opioid therapy. J Pain. 2007;8(7):573–582. doi: 10.1016/j.jpain.2007.02.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khalid L, Liebschutz JM, Xuan Z, et al. Adherence to prescription opioid monitoring guidelines among residents and attending physicians in the primary care setting. Pain Med. 2015;16(3):480–487. doi: 10.1111/pme.12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liebschutz JM, Saitz R, Weiss RD, et al. Clinical factors associated with prescription drug use disorder in urban primary care patients with chronic pain. J Pain. 2010;11(11):1047–1055. doi: 10.1016/j.jpain.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta SK. Intention-to-treat concept: a review. Perspect Clin Res. 2011;2(3):109–112. doi: 10.4103/2229-3485.83221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson D, Zlateva I, Khatri K, Ciaburri N. Using health information technology to improve adherence to opioid prescribing guidelines in primary care. Clin J Pain. 2015;31(6):573–579. doi: 10.1097/AJP.0000000000000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Von Korff M, Dublin S, Walker RL, et al. The impact of opioid risk reduction initiatives on high-dose opioid prescribing for patients on chronic opioid therapy. J Pain. 2016;17(1):101–110. doi: 10.1016/j.jpain.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith CS, Gerrish WG, Weppner WG. Interprofessional Education in Patient-Centered Medical Homes. Cham, Switzerland: Springer International Publishing; 2015. The argument for the patient-centered medical home: replicating good primary care; pp. 11–25. [Google Scholar]
- 35.Hoff T, Weller W, DePuccio M. The patient-centered medical home: a review of recent research. Med Care Res Rev. 2012;69(6):619–644. doi: 10.1177/1077558712447688. [DOI] [PubMed] [Google Scholar]
- 36.Nielsen M, Buelt L, Patel K, Nichols LM. The patient-centered medical home’s impact on cost and quality: annual review of evidence 2014–2015. Patient-Centered Primary Care Collaborative. 2016 https://www.pcpcc.org/resource/patient-centered-medical-homes-impact-cost-and-quality-2014-2015. Accessed July 21, 2016.
- 37.Parchman ML, Von Korff M, Baldwin L-M, et al. Primary care clinic re-design for prescription opioid management. J Am Board Fam Med. 2017;30(1):44–51. doi: 10.3122/jabfm.2017.01.160183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LaBelle CT, Han SC, Bergeron A, Samet JH. Office-based opioid treatment with buprenorphine (OBOT-B): statewide implementation of the Massachusetts Collaborative Care model in community health centers. J Subst Abuse Treat. 2016;60:6–13. doi: 10.1016/j.jsat.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alford DP, LaBelle CT, Kretsch N, et al. Collaborative care of opioid-addicted patients in primary care using buprenorphine: five-year experience. Arch Intern Med. 2011;171(5):425–431. doi: 10.1001/archinternmed.2010.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bair MJ, Ang D, Wu J, et al. Evaluation of stepped care for chronic pain (ESCAPE) in veterans of the Iraq and Afghanistan conflicts: a randomized clinical trial. JAMA Intern Med. 2015;175(5):682–689. doi: 10.1001/jamainternmed.2015.97. [DOI] [PubMed] [Google Scholar]
- 41.Massachusetts Medical Society. Fact sheet: an act relative to substance use treatment, education and prevention. http://www.massmed.org/Advocacy/Key-Issues/Opioid-Abuse/Fact-Sheet–An-Act-Relative-to-Substance-Use-Treatment,-Education-and-Prevention/#.WRD359y1sY0. Accessed May 8, 2017.
- 42.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010;152(2):85–92. doi: 10.7326/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bohnert ASB, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 44.Tournier M, Molimard M, Titier K, et al. Accuracy of information on substance use recorded in medical charts of patients with intentional drug overdose. Psychiatry Res. 2007;152(1):73–79. doi: 10.1016/j.psychres.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Walley AY, Farrar D, Cheng DM, Alford DP, Samet JH. Are opioid dependence and methadone maintenance treatment (MMT) documented in the medical record? a patient safety issue. J Gen Intern Med. 2009;24(9):1007–1011. doi: 10.1007/s11606-009-1043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joling KJ, van Marwijk HWJ, Piek E, et al. Do GPs’ medical records demonstrate a good recognition of depression? a new perspective on case extraction. J Affect Disord. 2011;133(3):522–527. doi: 10.1016/j.jad.2011.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


