Abstract
Purpose
To noninvasively evaluate gliomas with magnetic resonance elastography (MRE) to characterize the relationship of tumor stiffness with tumor grade and mutations in the IDH1 gene.
Materials and Methods
With institutional review board approval and following written, informed consent, tumor stiffness properties were prospectively quantified in 18 patients (mean age 42, 6 female) with histologically proven gliomas using MRE from 2014–2016. Images were acquired on a 3T MR unit with a vibration frequency of 60 Hz. Tumor stiffness was compared with unaffected contralateral white matter, across tumor grade and by IDH1 mutation status. The performance of the use of tumor stiffness to predict tumor grade and IDH1 mutation was evaluated by using Wilcoxon rank sum, one-way ANOVA and Tukey-Kramer tests.
Results
Gliomas were softer than healthy brain parenchyma, 2.2 kPa compared to 3.3 kPa (p < .0001) with grade IV tumors softer than grade II. Tumors with an IDH1 mutation were significantly stiffer than those with wild-type IDH1, 2.5 kPa vs. 1.6 kPa respectively (p = .007).
Conclusions
MRE demonstrated that gliomas were not only softer than normal brain but the degree of softening was directly correlated with tumor grade and IDH1 mutation status. Noninvasive determination of tumor grade and IDH1 mutation may result in improved stratification of patients for different treatment options and the evaluation of novel therapeutics. This work reports on the emerging field of mechanogenomics – the identification of genetic features such as IDH1 mutation using intrinsic biomechanical information.
Introduction
While gliomas are rare when compared with other cancers, they have a high mortality rate. Despite improvements in five-year survival rates of many cancers, outcomes for brain tumors remain relatively unchanged over the last 30 years, improving less than 2%1. Median survival is 12–15 months for glioblastomas (GBMs) and 2–5 years for lower grade gliomas. As our understanding of cancer biology, genetics, and treatment resistance mechanisms improves, the ability to stratify patients early with predictive biomarkers will be critical in the development of new therapies and the evaluation of treatment response2. Gliomas are histopathologically typed and graded as outlined with the World Health Organization (WHO) criteria, which provide important prognostic information as well as potential guidance on the clinical treatment of the tumor3. The WHO classification was updated in 2016 to include molecular markers, which have significant implications for patient outcome and may be critical information in the selection of a treatment strategy. Recent efforts in the area of radiogenomics have explored the potential of utilizing MR imaging phenotypes to noninvasively determine tumor genotypes, including the detection of three common genomic alterations in glioma4. Mutations in the gene responsible for encoding a metabolic enzyme called isocitrate dehydrogenase 1 (IDH1) frequently occur in low grade gliomas, exhibiting different genetic and epigenetic etiology when compared to IDH1 wild-type gliomas and are considered a distinct disease entity with a poorer prognosis, independent of tumor grade5, 6. While only 6% of GBMs have mutations in IDH1, it is hypothesized that these tumors have evolved from lower grade gliomas, while low grade gliomas that lack a mutation in IDH1 could be considered “pre-glioblastomas”5, 7. IDH1 mutations may also be predictive of therapeutic outcome from specific treatments such as increased radiosensitivity in vitro and differentiating patients who benefited from alkylating-agent chemotherapy in combination with radiation therapy8, 9. Recent efforts have investigated noninvasive biomarkers to identify IDH1-mutant tumors in humans including magnetic resonance spectroscopy, utilizing the association between mutations in IDH1 and 2-hydroxygluterate in the tumor10. However, challenges related to long scan time, complex data processing and low spectral resolution have limited clinical applications11.
Tumors are characterized by altered tissue- and cellular-level mechanics, and the stiffness of the extracellular matrix (ECM) in gliomas may be associated with a mutation in IDH112, 13. A recent study by Miroshnikova et al. demonstrated an overall correlation between tumor grade and IDH1 mutational status with the ECM stiffness of human glioma brain biopsies. Utilizing stiffness measurements from an atomic force microscope, they demonstrated increased ECM stiffness with tumor grade, where the ECM from GBMs was stiffer than lower grade gliomas. Additionally, the ECM of gliomas with a mutation in IDH1 was softer than wild-type IDH1, regardless of histologic grade. These results demonstrate a microscopic mechanical correlation between ECM stiffness and tumor genotype. Additional work is needed to determine if this finding correlates to macroscopic mechanical properties of gliomas.
Magnetic resonance elastography (MRE) is a technique used to noninvasively quantify the mechanical properties of tissue14–16. Previous studies have demonstrated the feasibility of using MRE to evaluate the viscoelastic properties of brain tumors including gliomas, where brain tumors were mainly softer than normal brain and benign variants, however some tumors are stiffer than normal brain17 and GBMs were the softest brain tumors when compared to meningiomas, vestibular schwannomas, and metastases18, 19. Additional work demonstrated that viscoelastic properties of GBMs were dependent on composition (e.g., necrosis or cystic cavities) and that the mechanical properties were heterogeneous with both stiff and soft regions17, 20. Recent work investigated the stiffness of four common brain tumors and stated that MRE may reflect the collagenous content of tumors19.
The purpose of this study was to noninvasively evaluate gliomas with MRE to characterize the relationship of tumor stiffness with tumor grade and mutations in the IDH1 gene. We hypothesize that glioma stiffness will vary across tumor grade and gliomas with an IDH1 mutation will exhibit different mechanical properties than IDH1 wild-type gliomas.
Materials and methods
Patient Recruitment
This prospective study was approved by our Institutional Review Board, and informed written consent was obtained from each subject. Inclusion criteria for the study consisted of subjects over the age of 18 with biopsy confirmed glioma and a minimum tumor diameter of 2 cm. Subjects with contraindications to MRI (cardiac pacemaker, implanted metallic object, or claustrophobia) and lesions with extensive necrosis were excluded. Eighteen patients (mean age 44 years, range 25–68 for male (n = 12) and mean age 40 years, range 28–40 for female (n = 6)) with a presumed or previous needle biopsy diagnosed glioma scheduled for surgical resection were recruited for a MRE examination prior to tumor resection from April 2014 to December 2016. Diagnosis was confirmed following surgery by an experience pathologist as part of clinical standard of care, and included determination of tumor grade, histologic subtype, the presence of 1p/19q co-deletion, and IDH1-R132H mutations.
MR Image Acquisition
Preoperative imaging was performed with a 3T MR scanner (SIGNA Excite, GE Healthcare, Waukesha, WI). The MRI protocol for each subject included an anatomical T1-weighted inversion recovery spoiled gradient echo (IR-SPGR) acquisition with the following imaging parameters: repetition time/ echo time = 6.3/2.8 msec, inversion recovery time = 400 msec; flip angle = 11°; 256 × 256 acquisition matrix; field of view = 27 cm; slice thickness = 1.2 mm; 200 sagittal slices; bandwidth = 31.25 kHz; parallel imaging acceleration factor = 1.75. MRE imaging utilized a modified single-shot, flow-compensated, spin-echo, echo-planar imaging (SE-EPI) pulse sequence21, 22.
MR Elastography Image Acquisition
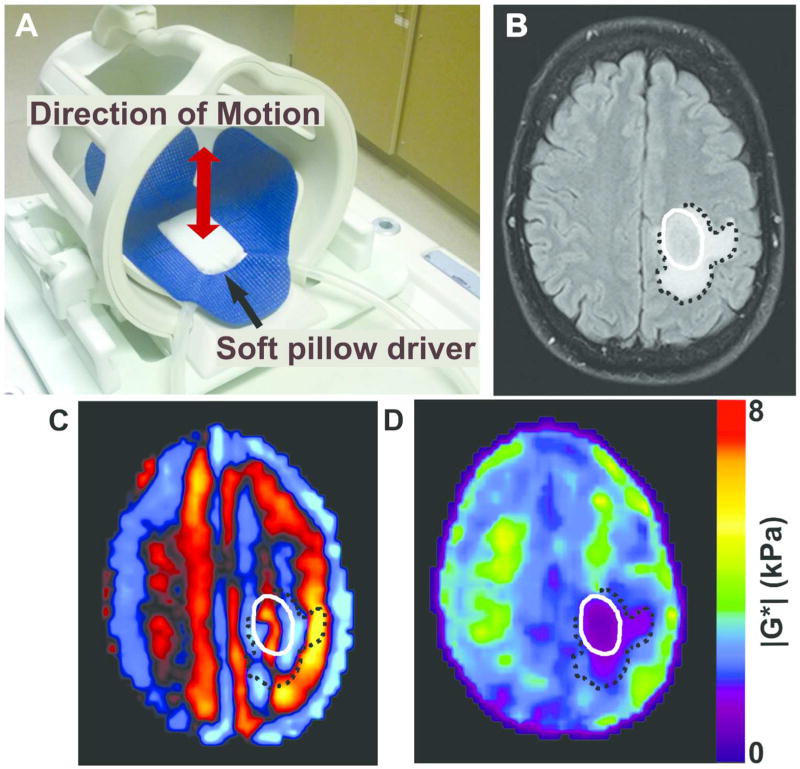
Low-amplitude mechanical vibrations in the form of shear waves were introduced into the brain at a frequency of 60 Hz as previously described23. A custom-built soft, pillow-like passive driver was positioned beneath the subject’s head in a standard eight-channel receive-only MR head coil (Figure 1). A long flexible tube connected the passive driver to the active component located outside the scan room which was comprised of a waveform generator, an amplifier, and an acoustic speaker. The resulting shear wave motion was imaged with the SE-EPI MRE pulse sequence by synchronizing motion-encoding gradients to the applied mechanical vibrations. The imaging parameters included: repetition time/echo time = 3600/62 ms; 72×72 acquisition matrix reconstructed to 80 × 80; field of view = 24 cm, slice thickness = 3 mm; 48 contiguous axial slices; bandwidth = 250 kHz; parallel imaging acceleration factor = 3; motion encoding in the positive and negative x, y, and z directions; and 8 phase offsets sampled over one period of motion at 60 Hz. The MRE acquisition time was less than 7 minutes.
Figure 1.
Brain MRE experimental setup and image procesing. (A) Brain MRE soft pillow driver placed within the 8-channel MRI head coil and positioned beneath the head to induce shear waves into the brain. (B) Axial T2 FLAIR image of a glioblastoma, IDH1 wild-type (male, age 51) with tumor denoted by solid white line and peritumoral edema by black dotted line. (C) MRE shear wave image and (D) elastogram or stiffness map displaying a soft tumor with a stiffness of 1.1kPa in the tumor compared to 3.5 kPa in a size-matched region of unaffected white matter on the contralateral hemisphere.
Image and Data Processing
Tissue viscoelastic shear properties were quantified from the measured displacement fields14, 24, 25. Assuming the tissue to be linear, isotropic, locally homogeneous, and viscoelastic, the complex shear modulus was quantified using previously described direct inversion (DI) methods (Figure 1)22, 26–28. Prior to DI, several post-processing steps were taken. First, the complex phase-difference images were calculated in the x, y, and z motion-encoding directions. Then, the curl of the input displacement field was calculated in order to reduce effects from the tissue boundaries and longitudinal wave propagation. A two-dimensional low pass filter was applied to reduce slice-to-slice phase discontinuities. A three-dimensional DI algorithm was used to calculate the complex shear modulus G*22. Shear stiffness was reported as the magnitude of the complex shear modulus (|G*|). A tumor region-of-interest (ROI) was manually drawn on each imaging slice from T1-maps registered to MRE space utilizing information from all available imaging sequences including T1-, T2-, diffusion-, and contrast-enhanced T1-weighted images as previously described (ME and NF, research trainees, 1 year experience under supervision of JH, 26 years’ experience)22. Tumor stiffness was calculated as the median |G*| of all voxels contained in the ROI volume and was compared to a size-matched ROI in the unaffected white matter on the contralateral hemisphere to serve as a control. Group results are reported as mean ± standard deviation (range).
Tumor volume was defined as the tumor ROI volume (cm3), calculated as the number of voxels contained in the ROI multiplied by the voxel volume. Contrast enhancement was assigned a label of non-enhancing (N), partially enhancing (P) or completely enhancing (C), determined from contrast-enhanced T1-weighted images performed during a standard diagnostic MRI (J.H., 26 years’ experience in neuroradiology).
Statistical Analysis
For each tumor ROI volume, the mean difference in tumor shear stiffness and unaffected contralateral normal white matter was analyzed using the Wilcoxon rank sum test. The mean differences in mean shear stiffness of IDH1+ and IDH1− tumors were analyzed with the Wilcoxon rank sum test. One-way ANOVA and Tukey-Kramer tests were used to compare the mean tumor shear stiffness between different tumor grades. A p-value of <0.05 was considered statistically significant. All calculations were performed using MATLAB (2016, MathWorks, Natick, Massachusetts, USA) and R Core Team (2015R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient Recruitment
MRE was performed on eighteen patients. Following surgery, tumor grade was determined by clinical pathology and included 5 grade II, 7 grade III, and 6 grade IV tumors (Table 1). Twelve patients had tumors with a mutation in IDH1-R132H; 5/5 grade II, 5/7 grade III, and 2/6 grade IV. Following the revision of the WHO classification of gliomas in 2016, the eighteen histopathology results were re-classified to reflect the new definitions.
Table 1.
Patient and tumor characteristics.
| # | Sex | Age yrs |
Tumor size cm3 |
Location | Contrast Enhancement |
IDH1 mutated? |
1p/19q co- deleted? |
Histologic and genetic classification | Grade |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 36 | 16.8 | Left parietal | None | Yes | Yes | Oligodendroglioma, IDH mutant and 1p19q codeleted | 2 |
| 2 | M | 39 | 60.6 | Right temporal | None | Yes | No | Diffuse astrocytoma, IDH mutant | 2 |
| 3 | M | 34 | 14.7 | Left frontal | Partial | Yes | No | Diffuse astrocytoma, IDH mutant | 2 |
| 4 | M | 31 | 54.7 | Right frontal | None | Yes | Yes | Oligodendroglioma, IDH mutant and 1p19q codeleted | 2 |
| 5 | M | 65 | 4.1 | Left frontal | None | Yes | Yes | Oligodendroglioma | 2 |
| 6 | M | 31 | 37.5 | Left frontal | None | Yes | Yes | Oligodendroglioma, IDH mutant and 1p19q codeleted | 3 |
| 7 | F | 35 | 66.9 | Right frontal | None | Yes | No | Diffuse astrocytoma, IDH mutant | 3 |
| 8 | F | 37 | 71.0 | Left temporal | Partial | Yes | No | Diffuse astrocytoma, IDH mutant | 3 |
| 9 | M | 51 | 59.9 | Left temporal | None | Yes | Yes | Oligodendroglioma, IDH mutant and 1p19q codeleted | 3 |
| 10 | F | 60 | 117.0 | Right frontal | Partial | Yes | No | Diffuse astrocytoma, IDH mutant | 3 |
| 11 | F | 44 | 75.6 | Right frontal | None | No | No | Diffuse astrocytoma, IDH wild-type | 3 |
| 12 | M | 33 | 38.9 | Left frontal | Partial | No | No | Diffuse astrocytoma, IDH wild-type | 3 |
| 13 | F | 28 | 5.5 | Left frontal | Partial | Yes | -- | Glioblastoma | 4 |
| 14 | M | 25 | 98.3 | Right frontal | Partial | Yes | -- | Glioblastoma | 4 |
| 15 | M | 51 | 27.6 | Left postcentral gyrus | Complete | No | -- | Glioblastoma | 4 |
| 16 | M | 46 | 9.5 | Left temporal | None | No | -- | Glioblastoma | 4 |
| 17 | M | 68 | 7.3 | Left frontal | Complete | No | -- | Glioblastoma | 4 |
| 18 | M | 55 | 37.1 | Right thalamus | Complete | No | -- | Glioblastoma | 4 |
Shear stiffness and tumor grade
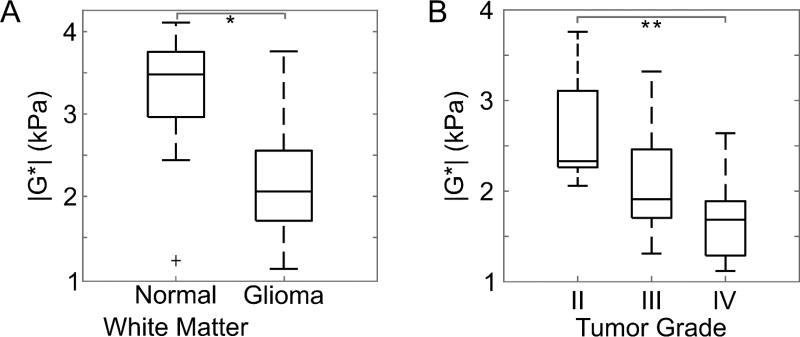
The mean shear stiffness of all gliomas was 2.2±0.7 kPa (1.1 – 3.8 kPa) compared to 3.3±0.7 kPa (1.2 – 4.1 kPa) in the contralateral unaffected white matter. In all but two cases, the tumor tissues were softer than normal brain tissue (p < .0001). Tumor stiffness displayed an inverse relationship with tumor grade, where high grade tumors were softer than lower grade tumors (Figure 2). For grades II, III, and IV, tumor stiffness was 2.7±0.7 kPa (2.1 – 3.8 kPa), 2.2±0.6 kPa (1.7 – 3.4 kPa), and 1.7±0.5 kPa (1.3 – 2.1 kPa), respectively. Grade IV GBMs were significantly softer than grade II gliomas (p = .03) but no statistically significant difference between grades II and III (p = 0.19) or between grades III and IV (p = 0.23) was observed. Additional correlations of tumor stiffness were investigated including anatomical location, patient age, and tumor volume, but no significant trends were observed.
Figure 2.
(A) Gliomas were softer than normal brain tissue, compared to size-matched regions of interest in the unaffected contralateral white matter (*p < .001). An outlier is indicated by a plus sign, and whiskers on the boxplot indicate the 25th and 75th percentiles. (B) Glioma stiffness decreased with increasing tumor grade (**p < .05).
Shear stiffness and IDH1 mutations
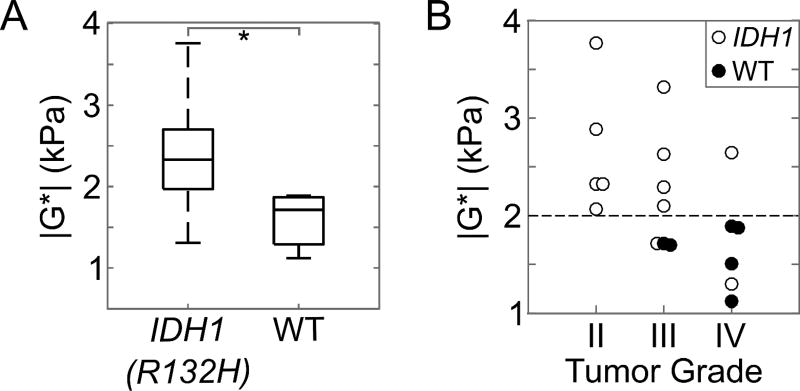
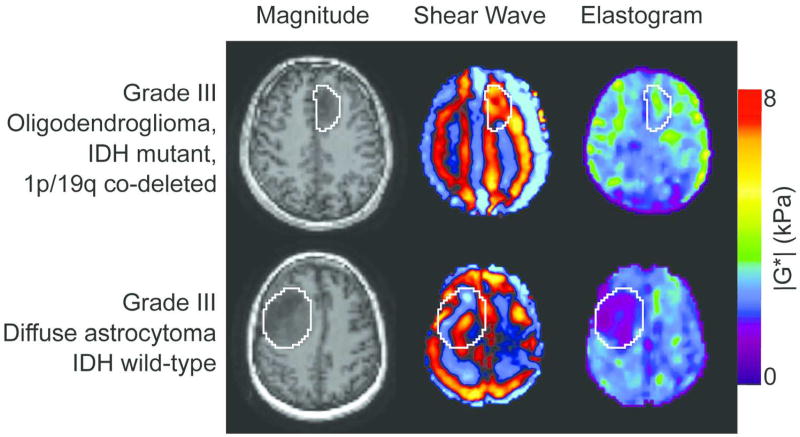
Tumors with a mutation in IDH1 (n = 12) were significantly stiffer than wild-type IDH1 (n = 6), with a shear stiffness of 2.5±0.6 kPa (1.5 – 3.8 kPa) and 1.6±0.3 kPa (1.1 – 1.9 kPa), respectively (p = .007; Figure 3). This observation was independent of tumor grade. There were two outliers including a secondary GBM with a positive IDH1 mutation and a shear stiffness of 1.5 kPa, and a grade III infiltrating anaplastic glioma with a positive IDH1 mutation and a shear modulus of 1.7 kPa. To demonstrate the large stiffness heterogeneity between IDH1 mutant and wild-type tumors, the MRE results from two grade III tumors are shown in Figure 4. While both grade III gliomas, the mechanical properties are drastically different between the two tumors, with tumor stiffness equal to 3.3 kPa for the IDH1 mutant tumors and 1.7 kPa for the wild-type tumor.
Figure 3.
(A) Comparison of the tumor stiffness (|G*|) between IDH1-R132H (n = 12) and wild-type (WT) gliomas (n = 6). Gliomas with wild-type IDH1 were significantly softer than gliomas with a mutation in IDH1-R132H (*p = 0.007). The whiskers on the boxplot indicate the 25th and 75th percentiles. (B) Tumor shear stiffness by tumor grade for all patients in this study including IDH1-R132H mutated tumors (white circles) and wild-type IDH1 (WT, black circles). The horizontal dotted line at 2.0 kPa separates the IDH1-mutated and wild-type gliomas with a sensitivity and specificity of 83% and 100%. There is one secondary IDH1-mutated GBM with a low |G*| = 1.5 kPa and may be unique due to the secondary disease subtype.
Figure 4.
Stiffness heterogeneity of gliomas. Non-contrast, axial MRE magnitude image (left column), shear wave images (middle column), and elastograms (right column) for two patients with grade III gliomas. Images in the top row are from an oligodendroglioma with an IDH1-R132H mutation with |G*| = 3.3 kPa (male, age 31) while the bottom row is from a diffuse astrocytoma with wild-type IDH1 where |G*| = 1.7 kPa (female, age 44).
Discussion
This study demonstrates that gliomas are softer than normal brain and that the stiffness of gliomas decreases with increasing tumor grade, consistent with previous MRE results of brain tumors. One study reported that primary brain tumors have a uniform loss of dissipative behavior and that the tumor mechanical properties are altered with increasing malignancy18. Similarly, another study investigated the mechanical properties of GBMs using MRE and found that the majority of GBMs were softer than normal brain20. In these studies, low grade gliomas were not included and no statistical analysis was reported for the relationship between tumor mechanical properties and tumor grade or IDH1 mutation status. A recent study demonstrated good correlation between glioma stiffness measurements and surgical assessment19. The results of this study are consistent with the quantitative stiffness values of gliomas reported in the literature.
The results presented in this work suggest that glioma stiffness may be a biomarker of IDH1 mutation status, with softer tumors being indicative of a wild-type IDH1 irrespective of tumor grade. IDH1 mutations in glioma are associated with improved outcome5. The stiffness of the grade III gliomas with wild-type IDH1 was more comparable to the stiffness of grade IV tumors than the IDH1-mutated grade III tumors. One outlier was an IDH1-mutated GBM with a relatively low stiffness (1.5 kPa compared to a mean of 2.5 kPa for IDH-mutated gliomas). This tumor was a secondary GBM, where 76% of secondary GBMs are IDH1-mutated compared to 6% of primary GBMs5, 6. The considerable softness of this GBM may be a result arising from previous radiation therapy. Further investigation is needed to understand the heterogeneity in glioma stiffness between primary and secondary malignancies for all tumor grades and different histological subtypes. These data provide the evidence to support the concept of mechanogenomics – the identification of genetic features such as IDH1 mutation using intrinsic biomechanical information and that MRE-derived shear stiffness can be used as a biomarker to both identify and spatially resolve genetically induced alterations of tissue biomechanical properties.
The results of this study found an inverse relationship between tumor stiffness, where GBMs were softer than lower grade tumors and gliomas with wild-type IDH1 were softer than mutated IDH1, regardless of tumor grade. This is the opposite relationship found in the recent study by Miroshnikova et al. of ECM stiffness in gliomas, where the ECM of gliomas with an IDH1 mutation were associated with a softer ECM, independent of histologic grade13. Macroscopic tumor stiffness is comprised of more than one constituent part, and in that study, ECM stiffness was not correlated with the levels or distribution of type I collagen, vasculature, or cellularity. Additional factors that may affect macroscopic tumor stiffness include cellularity, increased vessel density, and interstitial fluid pressure29, 30. Each of these factors may contribute to the overall tumor stiffness and potentially explain the opposite relationship of whole tumor stiffness with tumor grade and IDH1 mutation status observed in this study. While the opposite trends were observed in this study, the same correlations were found where stiffness was correlated with tumor grade and IDH1 mutations, irrespective of tumor grade. Further work is needed to understand the relationship between the microscopic ECM stiffness and the macroscopic whole tumor stiffness in gliomas.
The mechanisms behind these mechanogenomic differences are not well understood and therefore require further investigation to determine the diagnostic accuracy of this technique and to investigate the relationship between tumor mechanical properties and progression free survival and overall survival. Additionally, the role of other common somatic driver mutations, including the codeletion of the 1p and 19q chromosomal arms, methylguanine methyltransferase (MGMT) methylation status, and TERT promotor mutations need to be investigated. In the case of low grade gliomas, there is an important need for a noninvasive technique capable of detecting malignant transformation to a higher grade. The serial assessment of tumor mechanical properties using MRE may help identify these events prior to imaging changes on standard anatomical MRI. Previous results that suggest the completeness of non-enhancing tumor resection is an important prognostic factor in IDH1 mutant tumors and a priori knowledge of IDH1 status may help guide the extent of planned resection31. The potential of mechanogenomics with MRE to reliably and prospectively identify IDH1 mutation pre-operatively may have a large impact on surgical planning and postoperative patient management.
There are several limitations in this pilot study, including sample size and representation of lower-grade tumors. The inclusion criteria for this study required a minimum tumor diameter of 2 cm. Improvements in the MRE acquisition and data processing could allow for the quantification of mechanical properties in smaller tumors. Imaging plays an invaluable role in the treatment and monitoring of gliomas, but there still remains room for improvement. Common critiques of imaging techniques are low specificity and lack of histological correlation. For instance in the area of therapeutic response, the development of new targeted chemotherapy and radiation therapies may result in complicated imaging changes (either pseduoprogression or pseudoresponse) which are not adequately assessed with morphologic or anatomic imaging techniques. While our understanding of IDH1 mutations and glioma biology has increased dramatically over the last few years, the optimal strategies for therapeutic interventions remain unclear. The ability to noninvasively detect this mutation may have important implications for stratifying patients for treatment and the monitoring of response. Future work is needed to confirm these results and investigate additional tumoral genotypes with prognostic and therapeutic significance for gliomas including the 1p19q codeletion and MGMT methylation status, as well as stiffness differences with histopathological subtype.
Practical Applications
In conclusion, our study confirms that gliomas of all grades are softer than normal brain tissue and that tumor stiffness decreases with increasing tumor grade. In addition, gliomas with a mutation in IDH1 are stiffer than wild-type IDH1. The quantitative analysis of brain tumor mechanical properties may aid in the initial clinical assessment, surgical management and postoperative monitoring of gliomas.
Acknowledgments
The authors would like to thank NF and JK for their technical contributions. We would also like to thank SW and AM for their assistance in editing this manuscript.
This work was supported in part by grants from the National Institute of Health RO1 EB001981 and the Center for Individualizing Medicine, Imaging Biomarker Discovery Program, Mayo Clinic.
Abbreviations
- MRE
magnetic resonance elastography
- GBM
glioblastoma
- WHO
World Health Organization
- IDH1
isocitrate dehydrogenase 1
- ECM
extracellular matrix
- IR-SPGR
inversion recovery spoiled gradient echo
- SE-EPI
spin-echo, echo-planar imaging
- DI
direct inversion
- ROI
region of interest
References
- 1.AIHW. Cancer in Australia: an overview 2014. Cancer series. 2014;90 [Google Scholar]
- 2.Miles K. Can imaging help improve the survival of cancer patients? Cancer Imaging. 2011;11(Spec No A):S86–92. doi: 10.1102/1470-7330.2011.9022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 4.Smits M, van den Bent MJ. Imaging Correlates of Adult Glioma Genotypes. Radiology. 2017;284:316–331. doi: 10.1148/radiol.2017151930. [DOI] [PubMed] [Google Scholar]
- 5.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Labussiere M, Sanson M, Idbaih A, et al. IDH1 gene mutations: a new paradigm in glioma prognosis and therapy? Oncologist. 2010;15:196–199. doi: 10.1634/theoncologist.2009-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gorovets D, Kannan K, Shen R, et al. IDH mutation and neuroglial developmental features define clinically distinct subclasses of lower grade diffuse astrocytic glioma. Clin Cancer Res. 2012;18:2490–2501. doi: 10.1158/1078-0432.CCR-11-2977. [DOI] [PubMed] [Google Scholar]
- 8.Li S, Chou AP, Chen W, et al. Overexpression of isocitrate dehydrogenase mutant proteins renders glioma cells more sensitive to radiation. Neuro Oncol. 2013;15:57–68. doi: 10.1093/neuonc/nos261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cairncross JG, Wang M, Jenkins RB, et al. Benefit from procarbazine, lomustine, and vincristine in oligodendroglial tumors is associated with mutation of IDH. J Clin Oncol. 2014;32:783–790. doi: 10.1200/JCO.2013.49.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi C, Ganji SK, DeBerardinis RJ, et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat Med. 2012;18:624–629. doi: 10.1038/nm.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin G, Chung YL. Current opportunities and challenges of magnetic resonance spectroscopy, positron emission tomography, and mass spectrometry imaging for mapping cancer metabolism in vivo. Biomed Res Int. 2014;2014:625095. doi: 10.1155/2014/625095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar S, Weaver VM. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 2009;28:113–127. doi: 10.1007/s10555-008-9173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miroshnikova YA, Mouw JK, Barnes JM, et al. Tissue mechanics promote IDH1-dependent HIF1alpha-tenascin C feedback to regulate glioblastoma aggression. Nat Cell Biol. 2016;18:1336–1345. doi: 10.1038/ncb3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muthupillai R, Lomas DJ, Rossman PJ, et al. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science. 1995;269:1854–1857. doi: 10.1126/science.7569924. [DOI] [PubMed] [Google Scholar]
- 15.Muthupillai R, Lomas DJ, Rossman PJ, et al. Visualizing propagating transverse mechanical waves in tissue-like media using magnetic resonance imaging. Acoustical Imaging. 1996;22:279–283. [Google Scholar]
- 16.Pepin KM, Ehman RL, McGee KP. Magnetic resonance elastography (MRE) in cancer: Technique, analysis, and applications. Prog Nucl Magn Reson Spectrosc. 2015;90–91:32–48. doi: 10.1016/j.pnmrs.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon M, Guo J, Papazoglou S, et al. Non-invasive characterization of intracranial tumors by magnetic resonance elastography. New Journal of Physics. 2013;15:1–15. [Google Scholar]
- 18.Reiss-Zimmermann M, Streitberger KJ, Sack I, et al. High Resolution Imaging of Viscoelastic Properties of Intracranial Tumours by Multi-Frequency Magnetic Resonance Elastography. Clin Neuroradiol. 2014 doi: 10.1007/s00062-014-0311-9. [DOI] [PubMed] [Google Scholar]
- 19.Sakai N, Takehara Y, Yamashita S, et al. Shear Stiffness of 4 Common Intracranial Tumors Measured Using MR Elastography: Comparison with Intraoperative Consistency Grading. AJNR Am J Neuroradiol. 2016 doi: 10.3174/ajnr.A4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Streitberger KJ, Reiss-Zimmermann M, Freimann FB, et al. High-resolution mechanical imaging of glioblastoma by multifrequency magnetic resonance elastography. PLoS One. 2014;9:e110588. doi: 10.1371/journal.pone.0110588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy MC, Huston J, Glaser KJ, et al. Preoperative assessment of meningioma stiffness using magnetic resonance elastography. JOURNAL OF NEUROSURGERY. 2013;118:643–648. doi: 10.3171/2012.9.JNS12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murphy MC, Huston J, Jack CR, et al. Measuring the characteristic topography of brain stiffness with magnetic resonance elastography. PLoS One. 2013;8:e81668. doi: 10.1371/journal.pone.0081668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy MC, Huston J, Jack CR, et al. Decreased brain stiffness in Alzheimer's disease determined by magnetic resonance elastography. Journal of Magnetic Resonance Imaging. 2011;34:494–498. doi: 10.1002/jmri.22707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinkus R, Tanter M, Xydeas T, et al. Viscoelastic shear properties of in vivo breast lesions measured by MR elastography. Magnetic Resonance Imaging. 2005;23:159–165. doi: 10.1016/j.mri.2004.11.060. [DOI] [PubMed] [Google Scholar]
- 25.Papazoglou S, Hamhaber U, Braun J, et al. Algebraic Helmholtz inversion in planar magnetic resonance elastography. Physics in Medicine and Biology. 2008;53:3147–3158. doi: 10.1088/0031-9155/53/12/005. [DOI] [PubMed] [Google Scholar]
- 26.Manduca A, Oliphant TE, Dresner MA, et al. Magnetic resonance elastography: Non-invasive mapping of tissue elasticity. Medical Image Analysis. 2001;5:237–254. doi: 10.1016/s1361-8415(00)00039-6. [DOI] [PubMed] [Google Scholar]
- 27.Clayton EH, Genin GM, Bayly PV. Transmission, attenuation and reflection of shear waves in the human brain. Journal of the Royal Society. 2012;9:2899–2910. doi: 10.1098/rsif.2012.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sack I, Beierbach B, Hamhaber U, et al. Non-invasive measurement of brain viscoelasticity using magnetic resonance elastography. NMR in Biomedicine. 2008;21:265–271. doi: 10.1002/nbm.1189. [DOI] [PubMed] [Google Scholar]
- 29.Juge L, Doan BT, Seguin J, et al. Colon tumor growth and antivascular treatment in mice: complementary assessment with MR elastography and diffusion-weighted MR imaging. Radiology. 2012;264:436–444. doi: 10.1148/radiol.12111548. [DOI] [PubMed] [Google Scholar]
- 30.Heldin CH, Rubin K, Pietras K, et al. High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer. 2004;4:806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- 31.Beiko J, Suki D, Hess KR, et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol. 2014;16:81–91. doi: 10.1093/neuonc/not159. [DOI] [PMC free article] [PubMed] [Google Scholar]