Abstract
Luteolysis is characterized by angioregression, luteal cell apoptosis, and remodeling of the extracellular matrix characterized by deposition of collagen 1. Transforming growth factor beta 1 (TGFB1) is a potent mediator of wound healing and fibrotic processes through stimulation of the synthesis of extracellular matrix components. We hypothesized that TGFB1 stimulates profibrotic activities of luteal fibroblasts. We examined the actions of TGFB1 on luteal fibroblast proliferation, extracellular matrix production, floating gel contraction, and chemotaxis. Fibroblasts were isolated from the bovine corpus luteum. Western blot analysis showed that luteal fibroblasts expressed collagen 1 and prolyl 4-hydroxylase but did not express markers of endothelial or steroidogenic cells. Treatment of fibroblasts with TGFB1 stimulated the phosphorylation of SMAD2 and SMAD3. [3H]thymidine incorporation studies showed that TGFB1 caused concentration-dependent reductions in DNA synthesis in luteal fibroblasts and significantly (P < 0.05) reduced the proliferative effect of FGF2 and fetal calf serum. However, TGFB1 did not reduce the viability of luteal fibroblasts. Treatment of luteal fibroblasts with TGFB1 induced the expression of laminin, collagen 1, and matrix metalloproteinase 1 as determined by Western blot analysis and gelatin zymography of conditioned medium. TGFB1 increased the chemotaxis of luteal fibroblasts toward fibronectin in a transwell system. Furthermore, TGFB1 increased the fibroblast-mediated contraction of floating bovine collagen 1 gels. These results suggest that TGFB1 contributes to the structural regression of the corpus luteum by stimulating luteal fibroblasts to remodel and contract the extracellular matrix.
Keywords: corpus luteum, cytokines, extracellular matrix, fibroblast, luteolysis, TGFB1, transforming growth factor beta 1
INTRODUCTION
During each reproductive cycle, in the absence of fertilization, the corpus luteum loses functionality and undergoes extensive structural remodeling to become a small avascular fibrous tissue vestige, the corpus albicans [1]. Such structural remodeling involves an increase in extracellular matrix proteins and matrix metalloproteinases (MMPs) [2–4], cell death [5], vascular regression [6–8], and an influx of circulating immune cells into the regressing corpus luteum [9].
In luteolytic remodeling, the extracellular matrix is characterized by deposition of collagen 1 [8,10,11]. Alterations in the expression of the MMPs and tissue inhibitors of MMPs (TIMPs) have been hypothesized to have functional implications for the decrease in progesterone and structural remodeling in luteolysis. An increase in the expression (mRNA) and activity of several MMPs, including MMPs 1, 2, 3, 7, 9, and 14, was observed in sheep corpus luteum after in vivo treatment with a luteolytic dose of prostaglandin F2alpha (PGF2α) [12]. An increase in the MMP:TIMP ratio during PGF2α-induced luteolysis may be important for the structural involution of the corpus luteum. For instance, treatment resulted in a decline in TIMP1 mRNA and protein in ovine luteal tissue [13]. In the same study, TIMP2 and TIMP3 mRNA was not affected by PGF2α treatment [13]. Increased mRNA for MMP1, MMP2, MMP9, and MMP14 and decreased mRNA for TIMP2 were reported in bovine corpus luteum at different time points after PGF2α-induced luteolysis [14].
Transforming growth factor beta 1 (TGFB1) mRNA is induced in the bovine corpus luteum in vivo by a luteolytic dose of PGF2α [15–17]. In addition, treatment with PGF2α in vitro induced TGFB1 mRNA and protein secretion in primary cultures of bovine luteal cells [17] and reduced progesterone secretion, implicating TGFB1 in luteolysis [17]. Fibroblasts are responsible for the regulation of extracellular matrix production and turnover for the preservation of tissue architecture. Fibroblasts are the main effector cells in the process of fibrogenesis in multiple organ systems. TGFB1 induces fibroblast proliferation and transformation into myofibroblasts and stimulates the accumulation of matrix proteins, including laminin, collagens 1 and 3, as well as fibronectin [18–20]. Furthermore, it induces the expression of the MMPs, TIMPs, and plasmin activator inhibitor 1, thus fine-tuning the extent of matrix remodeling [21]. TGFB1 inhibits proliferation in most cells, including epithelial and endothelial cells [22]. Numerous articles describe promitogenic effects of TGFB1 on fibroblasts of different origin. TGFB1 promotes proliferation of renal fibroblasts [23], adult human skin fibroblasts [24, 25], dermal fibroblasts [26], and pulmonary fibroblasts [27]. However, TGFB1 reduces proliferation of serum-deprived murine cardiac fibroblasts [28], oral fibroblasts [26], and fetal human skin fibroblasts [24, 25]. During fibrogenesis, TGFB1 activates resident fibroblasts, which undergo functional and phenotypic changes, acquiring a myofibroblastic phenotype. Myofibroblasts in vitro express contractile proteins, including α-smooth muscle actin, vimentin, and desmin and are able to effectively contract collagen gels. In addition to participating in tissue fibrosis, myofibroblasts are associated with normal wound healing and are cleared through apoptosis in the progression of granulomatous tissue to a mature scar [29].
The involvement of TGFB1 in the regulation of tissue remodeling that occurs during luteolysis has not been examined in detail. The present study examined the effects of TGFB1 on the function of luteal fibroblasts. Our working hypothesis was that TGFB1 stimulates profibrotic activity during luteal regression. We found that treatment of luteal fibroblasts with TGFB1 results in increased matrix synthetic capability, migratory potential, and capacity to contract collagen matrices but reduced cell proliferation. Our findings suggest that luteal fibroblasts contribute to the accumulation and contraction of a collagen 1-rich extracellular matrix, which are mediated by increased expression of TGFB1 in the regressing corpus luteum.
MATERIALS AND METHODS
Materials
Dulbecco modified Eagle medium (DMEM) was obtained from Mediatech, and fetal calf serum (FCS) was from Cambrex. Type II collagenase was obtained from Atlantic Biologicals. Dynabeads M-450 Epoxy was purchased from Invitrogen. Griffonia (Bandeiraea) Simplicifolia Lectin 1 (BSL-I) and mounting medium VECTASHIELD were from Vector Laboratories. Recombinant human TGFB1, recombinant human Fas ligand (FASLG), recombinant bovine basic fibroblast growth factor (FGF2), and recombinant human tumor necrosis factor alpha (TNF) were from R&D Systems. Recombinant bovine interferon gamma (IFNG) was from Thermo Fisher Scientific. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), bovine fibronectin, bovine gelatin, bovine casein, rabbit antifibronectin, and monoclonal anti-β-actin antibody were from Sigma Chemical Co. Phospho-SMAD2 (Ser465/467), phospho-SMAD3 (Ser423/425), SMAD2, and SMAD3 antibodies were purchased from Cell Signaling Technology. Endothelial nitric oxide synthase (eNOS; also called NOS3) antibody was from BD Transduction Laboratories, and VE-cadherin (VE-cad; also called CDH5) antibody was obtained from Pierce. Laminin and collagen 1 antibodies were from Rockland. Monoclonal anti-prolyl-4-hydroxylase antibody was from Acris. MMP1, MMP2, MMP9, TIMP1, and TIMP2 antibodies were from R&D Systems. Alexa 488-conjugated immunoglobulin G was from Molecular Probes, Inc. [3H]thymidine was from MP Biomedicals. Bovine collagen 1 and cell culture inserts (transparent polyethylene terephthalate membrane; pore size, 8 μm) were purchased from BD Biosciences. The 3-beta-hydroxysteroid dehydrogenase (HSD3B) antibody was a gift from Ian Mason [30]. Western Lightning ECL was from PerkinElmer Life Sciences, Inc. Kodak x-ray film was purchased from Fisher Scientific, Inc.
Luteal Fibroblast Isolation and Culture
Ovaries were collected at a local slaughterhouse from first-trimester pregnant cows (fetal crown-rump length, <15 cm). Fibroblasts were isolated from the corpus luteum by repeated enzymatic digestion (twice for 40 min each time) with type II collagenase (~100 U/ml, 5 ml/g tissue) followed by affinity purification with magnetic beads. The magnetic beads (Dynabeads M-450 Epoxy) were coated with BSL-I following the manufacturer’s instructions. The beads (1 × 106 beads/ml) were incubated for 30 min with dispersed luteal cells. The bead-adherent cells were washed and cultured in growth medium (DMEM supplemented with 10% FCS and antibiotics [100 IU/ml of penicillin, 100 μg/ml of streptomycin, and 50 μg/ml of amphotericin]). We found that luteal fibroblasts and endothelial cells, but not steroidogenic cells, bind to BSL1-beads. Luteal endothelial cells required specific culture conditions and did not survive the long isolation process. Fibroblasts associated with the beads grew rapidly and took over the culture before colonies of endothelial cells were visible. Luteal fibroblasts were passed and expanded four times until no steroidogenic or endothelial cells were visible in the culture. At this passage, cells were stored and characterized. Luteal fibroblasts were used for all experiments between passages 5 and 10. The characteristics of passage 6 fibroblasts are shown in Figure 1.
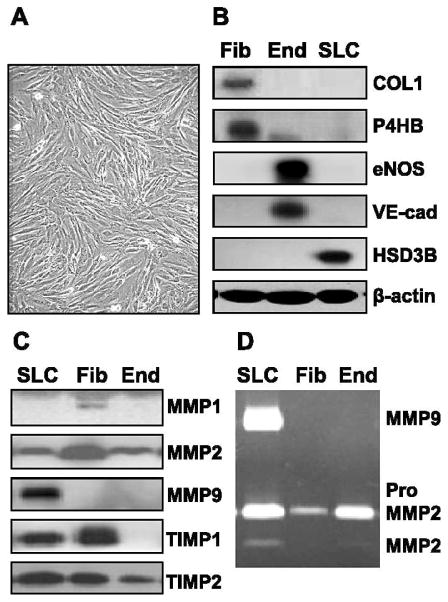
FIG. 1.
Characterization of fibroblasts isolated from the bovine corpus luteum. Luteal fibroblasts were isolated from bovine corpus luteum and characterized by their morphology and expression of cell markers. Fibroblasts were cultured in monolayer in growth medium (DMEM supplemented with 10% FCS). Supernatant medium was harvested, and the expression of matrix-remodeling proteins was examined by Western blot analysis and zymography. A) Luteal fibroblasts display typical fibroblast morphology; they are spindle-shaped, with numerous cytoplasmic processes forming a network of intercellular contacts. Original magnification ×100. B) Characterization of luteal fibroblasts by Western blot analysis of cell lysates (40 μg protein/lane). Luteal endothelial cell (End) and steroidogenic luteal cell (SLC) protein extracts were prepared as previously described [30, 37]. Luteal fibroblasts (Fib) expressed fibroblast cell markers collagen 1 (COL1) and prolyl 4-hydroxylase (P4HB) but did not express endothelial cell (End) markers VE-cad and eNOS. In addition, luteal fibroblasts did not express the SLC marker HSD3B. C) The expression of matrix-remodeling proteins by luteal fibroblasts was examined by Western blot analysis of conditioned medium. Luteal fibroblasts secreted MMP1, MMP2, TIMP1, and TIMP2 but did not express MMP9. D) Gelatin zymography of conditioned medium (30 μl/lane) shows the presence of MMP2 zymogen in conditioned medium from cultured luteal fibroblasts.
Western Blot Analysis
Cells were harvested from culture plates with ice-cold cell lysis buffer (20 mM Tris-HCl [pH 7], 150 mM NaCl, 1 mM disodium ethylenediaminetetra-acetic acid, 1 mM ethyleneglycoltetra-acetic acid, 1% Triton X-100, and protease and phosphatase inhibitor cocktails). Equal amounts of protein (30 or 40 μg) from cell lysates were subjected to electrophoresis under reducing conditions on 10% SDS-PAGE and electrotransferred to polyvinylidene fluoride membranes. The membranes were blocked for 1 h with TBS-T (Tris-buffered saline, 0.1% Tween 20) containing 5% nonfat dried milk at room temperature and incubated overnight at 4°C with a 1:1000 dilution of primary antibodies in TBS-T/5% nonfat dried milk. After washing in TBS-T, membranes were incubated for 1 h with horseradish peroxidase-coupled secondary antibody diluted 1:2500 in TBS-T/2% dried milk at room temperature. After an additional washing in TBS-T, the membranes were developed with Western Lightning ECL detection system. Detection was performed by exposure to blue light-sensitive autoradiography film.
[3H]Thymidine Incorporation
Luteal fibroblasts were seeded in 24-well plates at a density of 2 × 104 cells/well in growth medium (DMEM medium supplemented with 5% FCS) and cultured for 24 h. Cells were rinsed, serum-starved in DMEM for 2 h, and treated in serum-free DMEM with control or TGFB1 (0–10 ng/ml) for 24 h. [3H]thymidine (4 μCi/ml) was added 6 h before the end of the incubation period. To terminate the incubations, unincorporated radioactivity was removed by washing cells once with ice-cold PBS followed by the addition of 10% trichloroacetic acid for 30 min at 4°C. Next, wells were washed with ice-cold PBS and solubilized with 0.2 M NaOH at room temperature. The radioactivity was determined by liquid scintillation counting.
MTT Assay for Cell Viability
Luteal fibroblasts were seeded in growth medium (DMEM medium supplemented with 5% FCS) at low density (2 × 104 cells/well) in 24-well plates or at high density (3 × 104 cells/well) in 48-well plates. After 24 h of culture, cells were rinsed and serum-starved in DMEM for 2 h. Cells were treated in serum-free DMEM for 24 or 48 h as indicated in figure legends. MTT (0.5 mg/ml) was added to each well 4 h before the end of the incubation period. Next, the medium was removed, and dimethyl sulfoxide was added to each well. Optical density was read in a spectrophotometer (SPECTRAmax PLUS; Molecular Devices) using a wavelength of 570 nm.
Chemotaxis Assay
Chemotaxis assays were performed using transwell inserts (pore size, 8 μm), which were precoated with 100 μl of a solution of bovine gelatin (1 mg/ml in 0.1% acetic acid). Wells of a 24-well plate were coated with 100 μl of a 20 μg/ml solution of bovine fibronectin and dried at room temperature. Luteal fibroblasts were suspended at 4 × 105 cells/ml in DMEM and layered (150 μl) in the transwell insert. DMEM (0.5 ml) was added to the fibronectin-coated wells, and the inserts were placed in the wells. At this time, fibroblasts were treated with control, TGFB1 (1 ng/ml), or FGF2 (10 ng/ml) and incubated at 37°C. After 6 h, the cells on the top of the filter were removed by scraping. Migrated cells were fixed and stained with methanol/0.04% crystal violet for 30 min. Cells were photographed (100× magnification), and three pictures per condition tested were quantified. Quantification of cell numbers was done using MicroSuite FIVE software for imaging applications from Olympus Soft Imaging Solutions. The experiment was repeated in three separate occasions, and three inserts were used for each treatment.
Immunofluorescence
For the study of extracellular matrix synthesis, luteal fibroblasts were seeded on six-well plates containing glass coverslips at a density of 2.5 × 105 cells/well in growth medium (DMEM medium supplemented with 5% FCS) and cultured for 24 h. Cells were rinsed, serum-starved in DMEM for 2 h, and treated in serum-free DMEM with control or TGFB1 (0–10 ng/ml) for 24 h. After treatment, cells were washed with cold PBS and fixed with ice-cold 4% paraformaldehyde for 30 min. Cells were permeabilized in PBS/0.4% Triton X-100 for 10 min at room temperature and then blocked for 1 h in blocking buffer (PBS, 0.2% Triton X-100, and 10% FCS). Primary antibody was diluted 1:200 in blocking buffer and incubated overnight at 4°C. Cells were washed with PBS and incubated for 1 h at room temperature with appropriate secondary fluorophore-conjugated antibodies diluted 1:400 in blocking buffer. Cells were next washed three times with PBS and mounted with VECTASHIELD. Controls included cells probed with secondary antibody alone.
Gelatin and Casein Zymography
Fibroblasts were cultured in six-well plates and treated with TGFB1 (1 ng/ml) in serum-free DMEM and incubated for up to 48 h. Conditioned medium was collected for zymography. Equal volumes (30 μl) of conditioned medium samples were mixed with six-fold-concentrated sample loading buffer and subjected to electrophoresis on 10% SDS-PAGE containing 1% gelatin under nonreducing conditions. SDS was removed from the gel by washing twice for 30 min each time in washing buffer (2.5% Triton X-100 and 50 mM Tris-HCl [pH 7.5]). Enzymatic activity was initiated by incubation in digestion buffer (50 mM Tris-HCl [pH 7.6], 150 mM NaCl, and 5 mM CaCl2) for an optimized length of time at 37°C. Gels were developed by staining with Coomassie Brilliant Blue (0.1% Coomassie Brilliant Blue R-250 (Sigma Chemical Co.) in 50% methanol and 10% glacial acetic acid). Thereafter, gels were destained in 10% methanol and 5% glacial acetic acid. The gelatinolytic activities were detected as clear bands on a uniform blue background. For casein zymography, gelatin was replaced by casein (final concentration, 0.1%), and the gels were processed in the same manner.
Collagen Matrix Contraction Assay
Luteal fibroblasts were trypsinized, washed, resuspended in DMEM, and counted. Bovine collagen 1 (0.8 ml; protein concentration, 3 mg/ml) was prepared by neutralizing with 0.001 M NaOH (100 μl) and 10× DMEM (100 μl) following the manufacturer’s instructions. Fibroblasts (final concentration, 1 × 106 cells/ml) were mixed with bovine collagen 1 solution (final concentration, 1.5 mg/ml) in DMEM. Aliquots of this solution (200-μl aliquot containing 2 × 105 cells) were added into each well (containing a 12-mm circular inscription made with a pap pen) of a 24-well culture plate. The plate was incubated for 1 h at 37°C, during which time each aliquot would polymerize and form a disk. DMEM (0.5 ml) was added to each well after polymerization, and the fibroblast-populated matrices were released with the aid of a rounded metal spatula. Matrices (three per 35-mm dish) were floated in DMEM control medium or in medium treated with TGFB1 (1 ng/ml) and photographed daily during 3 days of incubation at 37°C. Microscopy of matrices was also performed daily. Quantification of matrix area was done using MicroSuite FIVE software for imaging applications from Olympus Soft Imaging Solutions. The experiment was repeated on three separate occasions, and three matrices were prepared for each condition tested.
Effect of Fibrillar Collagen 1 on Cell Viability
Bovine collagen 1 was diluted in DMEM and pipetted into 48-well plates (0.15 ml/well, ~2.4 mg/ml), polymerized for 1 h at 37°C, and dried overnight at room temperature. Luteal fibroblasts were plated (5 × 104 cells/well) in growth medium (DMEM medium supplemented with 5% FCS). Cells were treated as described in the figure legends. The effects on morphogenesis of cells were photographed after 24 h with an inverted microscope. One picture (40× magnification) was taken in the central area of each well. The experiment was repeated in three separate occasions, and three wells were used for each condition tested.
Apoptosis Assay
The effect of fibrillar bovine collagen 1 and TGFB1 on luteal fibroblasts was examined. Fibroblasts were plated on collagen 1 gels and treated with TGFB1 as described above. Caspase 3/7 activity was determined at 8 and 24 h of incubation using the Caspase-Glo 3/7 assay kit (catalog no. G8091; Promega) following the manufacturer’s instructions. Briefly, Caspase-Glo 3/7 reagent was added to each well in a 1:1 ratio and incubated with gentle shaking for 30 min at room temperature before measuring luminescence using a FLUOstar OPTIMA microplate reader (BMG Labtech, Inc.).
Statistical Analyses
All data are presented as the mean ± SEM. Three experiments were conducted on separate occasions (n = 3) unless otherwise noted, and each experiment was performed in triplicate for each condition tested. Comparisons for differences between groups were made by ANOVA with Tukey post-test. In all analyses, a value of P < 0.05 was considered to be significant. Statistical analysis of experimental data was performed using GraphPad Prism software (GraphPad Software, Inc.).
RESULTS
Characterization of Luteal Fibroblasts
Luteal fibroblasts were isolated using lectin-coated magnetic beads and expanded before they were used for experiments. During the initial passages, fibroblasts grew rapidly, whereas other cell types did not survive, resulting in a highly enriched culture. Luteal fibroblasts displayed typical fibroblast morphology, being spindle-shaped with numerous cytoplasmic processes forming a network of intercellular contacts (Fig. 1A). Characterization of luteal fibroblasts by Western blot analysis showed that these cells express the fibroblast cell markers collagen 1 and prolyl 4-hydroxylase. Luteal fibroblasts did not express the endothelial cell markers VE-cad and eNOS or the steroidogenic luteal cell marker HSD3B (Fig. 1B). We also examined the expression of MMPs and TIMPs in luteal fibroblasts by Western blot analysis and gelatin zymography of conditioned medium. Luteal fibroblasts secreted MMP1, MMP2, TIMP1, and TIMP2 but did not express MMP9 (Fig. 1C). Gelatin zymography showed the presence of the latent form of MMP2 in fibroblast-conditioned medium (Fig. 1D).
TGFB1 Induces Phosphorylation of SMAD2/3 in Luteal Fibroblasts
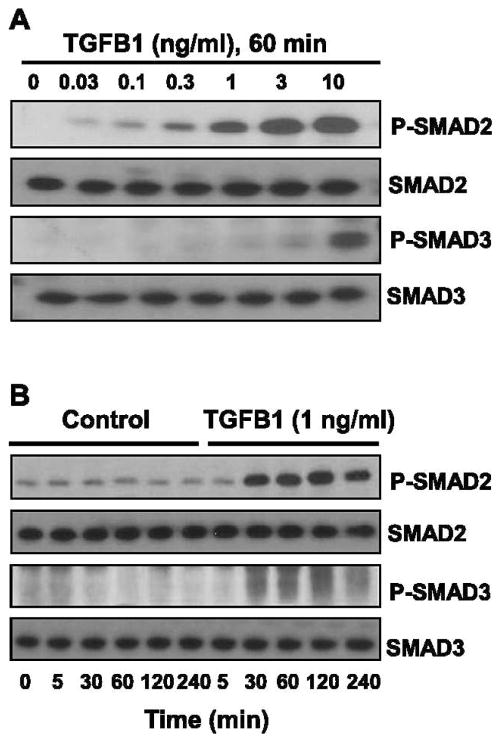
To examine whether TGFB1 signaling is active in luteal fibroblasts, we performed time-course and concentration-dependent response experiments. Treatment with TGFB1 (1 ng/ml) for 60 min produced a concentration-dependent increase in the phosphorylation of SMAD2 and SMAD3 (Fig. 2A). The physiologic concentration of 1 ng/ml of TGFB1 [31–34] appeared to be maximally effective for phosphorylation of SMAD2, whereas higher concentrations of TGFB1 (10 ng/ml) were required for phosphorylation of SMAD3. Time-course experiments revealed that SMAD2 was phosphorylated within 30 min following treatment with TGFB1 (1 ng/ml) and that phosphorylation was sustained after 4 h of treatment (Fig. 2B). Only slight increases in the phosphorylation of SMAD3 were observed following treatment with 1 ng/ml of TGFB1 over the 4-h time course (Fig. 2B).
FIG. 2.
TGFB1 induces phosphorylation of SMAD2 and SMAD3 in luteal fibroblasts. A) Concentration response to TGFB1. Cells were serum-starved and treated with TGFB1 (0–10 ng/ml) for 60 min under serum-free conditions. B) Time-course response to TGFB1. Cells were serum-starved and treated with TGFB1 (1 ng/ml) for up to 4 h under serum-free conditions. Levels of phosphorylated SMAD2 and SMAD3 were determined by Western blot analysis of cell lysates (30 μg protein/lane). Levels of total SMAD2 and SMAD3 proteins are also shown.
TGFB1 Reduces DNA Synthesis in Luteal Fibroblasts
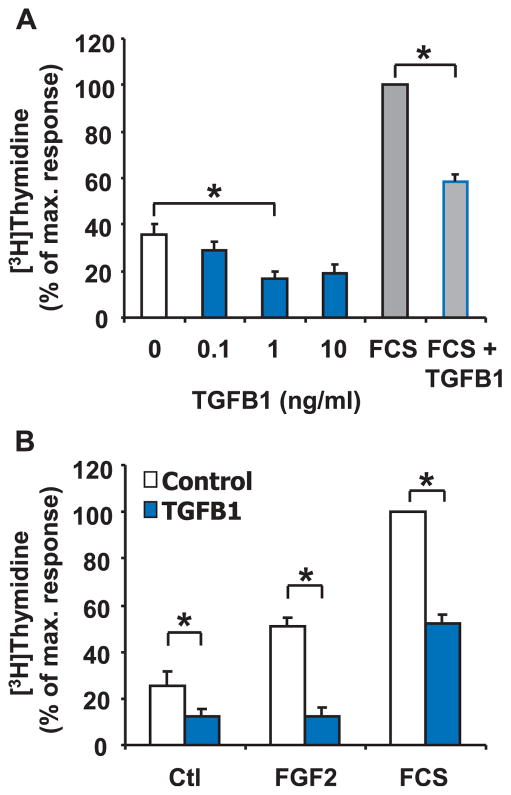
Because TGFB1 is a strong mitogen for renal fibroblasts, we speculated that TGFB1 would induce proliferation of luteal fibroblasts. To investigate whether TGFB1 affects luteal fibroblast proliferation, we monitored DNA synthesis by measuring [3H]thymidine incorporation in response to increasing concentrations of TGFB1 (0–10 ng/ml). Although all three concentrations tested reduced DNA synthesis in luteal fibroblasts, 1.0 ng/ml had the most robust effect and significantly (P < 0.05) reduced basal DNA synthesis (50% ± 3% inhibition, n = 6) (Fig. 3). Treatment of fibroblasts with 5% FCS routinely caused a 3-fold increase in [3H]thymidine incorporation (Fig. 3). FGF2 (10 ng/ml) also significantly (P < 0.05) increased basal DNA synthesis in fibroblasts (Fig. 3B). TGFB1 (1 ng/ml) significantly (P < 0.05) decreased the stimulatory effect of FGF2 (75% ± 5% inhibition, n = 3) and 5% FCS (55% ± 2% inhibition, n = 6) (Fig. 3B). An additional experiment examined whether the TGFB1-induced reduction in DNA synthesis was associated with a reduction in cell viability, as measured using the MTT assay. In a single experiment performed in triplicate under the same conditions used for analysis for [3H]thymidine incorporation, treatment of fibroblasts with TGFB1 (1 ng/ml) did not reduce cell viability in cells treated for 24 h with control medium, FGF2, or FCS (3% ± 2.9%, 1% ± 0.2%, 2 ± 0.2% inhibition, respectively). These results indicate that TGFB1 inhibits luteal fibroblast proliferation without reducing cell viability.
FIG. 3.
TGFB1 reduces DNA synthesis in luteal fibroblasts. A) [3H]thymidine incorporation assay of fibroblasts plated at low density and treated with or without TGFB1 (0–10 ng/ml) for 24 h. Some cells were cultured in the presence of 5% FCS alone or in combination with TGFB1 (1 ng/ml) for 24 h. Data are expressed as the percentage incorporation of [3H]thymidine compared to the maximal response group (5% FCS). Data represent three independent experiments, each performed in triplicate (mean ± SEM, n =3, *P < 0.05). B) [3H]thymidine incorporation assay of fibroblasts plated at low density and treated for 24 h with or without TGFB1 (1 ng/ml) in the absence (Ctl) or presence of FGF2 (10 ng/ml) and 5% FCS. Data are expressed as the percentage incorporation of [3H]thymidine compared to the maximal response group (5% FCS). Data represent three independent experiments, each performed in triplicate (mean ± SEM, n = 3, *P < 0.05).
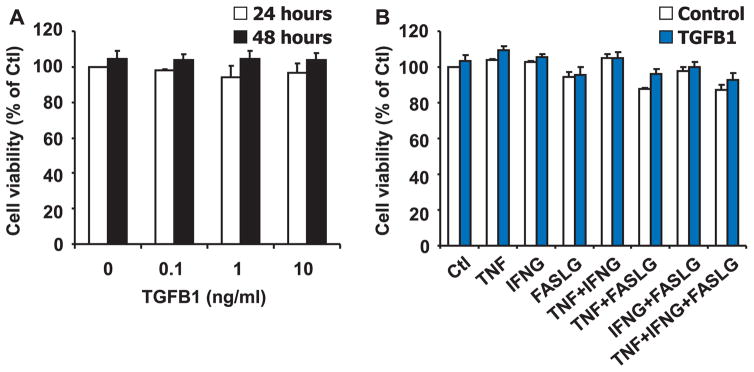
TGFB1 Does Not Affect Luteal Fibroblast Viability When Plated on Plastic
We examined if the TGFB1-induced reduction in DNA synthesis was associated with a reduction in cell viability, as measured using the MTT assay. To perform these experiments, fibroblasts were plated at higher density and treated when the cells were at approximately 80% density. Under these experimental conditions, treatment of luteal fibroblasts with TGFB1 (0–10 ng/ml) did not reduce cell viability after 24 or 48 h when plated on plastic (n = 3, P > 0.05) (Fig. 4A). We also examined the effect of cytokines known to induce cell death in luteal endothelial cells [35–37]. Treatment with TNF (50 ng/ml), IFNG (50 ng/ml), or their combinations for 24 or 48 h had no significant effect on cell viability (Fig. 4B). Treatment with FASLG (100 ng/ml) had a consistent but slight (~10%) inhibitory effect on fibroblast viability (Fig. 4B). In addition, under these culture conditions, treatment with TGFB1 (1 ng/ml) in combination with these cytokines did not alter cell viability (n = 3, P > 0.05). These results demonstrate that luteal fibroblasts are not a primary target for cytotoxic cytokines and that TGFB1 does not alter luteal fibroblast viability.
FIG. 4.
TGFB1 does not reduce viability of luteal fibroblasts. A) MTT assay of fibroblasts plated on plastic at high density and treated for 24 and 48 h without or with TGFB1 (0–10 ng/ml) in the absence of FCS. Data represent three independent experiments, each performed in triplicate (mean ± SEM, n = 3, P > 0.05 vs. 0 ng/ml of TGFB1). B) MTT assay of fibroblasts plated on plastic at high density and treated for 48 h with control medium (Control) or TGFB1 (1 ng/ml), alone or in combination with control medium (Ctl), TNF (50 ng/ml), IFNG (50 ng/ml), or FASLG (100 ng/ml) in the absence of FCS. Results are expressed as the percentage absorbance observed in the control group. Data shown represent three independent experiments, each performed in triplicate (mean ± SEM, n = 3, P > 0.05 vs. Ctl).
TGFB1 Increases Chemotaxis of Luteal Fibroblasts Toward Fibronectin
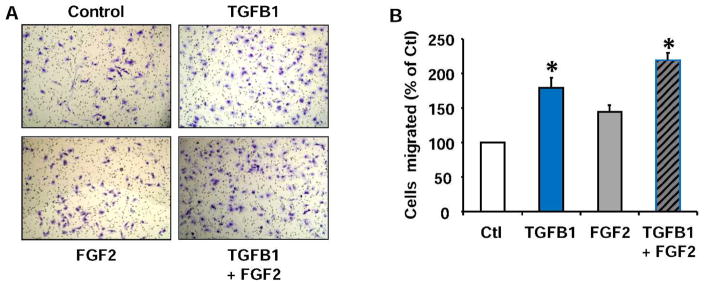
To determine whether TGFB1 affects luteal fibroblast migration, we used a transwell system with cell culture inserts (pore size, 8 μm). The membrane of the inserts was coated with a solution of bovine gelatin (100 μg/insert), and the wells of the culture plate were coated with human fibronectin (2 μg/well) to act as a chemoattractant. Fibroblasts were allowed to migrate over a 6-h time period. Using fibronectin as the chemoattractant [38], TGFB1 (1 ng/ml) significantly increased chemotaxis compared to control (80% ± 10% increase, n = 3, P < 0.05) (Fig. 5). FGF2 also increased luteal fibroblast chemotaxis, but to a lesser extent than TGFB1.
FIG. 5.
TGFB1 increases chemotaxis of luteal fibroblasts toward fibronectin. Fibroblasts were harvested with trypsin and used for chemotaxis in the blind-well Boyden chamber assay. Fibroblasts were treated with control medium (Ctl), TGFB1 (1 ng/ml), or FGF2 (10 ng/ml) alone or in combination in the upper well. Fibronectin (20 μg/ml) was used as the chemoattractant. Chemotaxis was measured as the number of migrated cells after a 6-h incubation period. A) Migrated fibroblasts found on the bottom of the transwell membrane were fixed, stained, and photographed. Original magnification ×100. B) Quantification of cell migration. Cell migration is represented as a percentage of the number of fibroblasts migrated in the control treatment group. Data shown represent three independent experiments, each performed in triplicate (mean ± SEM, n = 3, *P < 0.05 vs. Ctl).
TGFB1 Induces Extracellular Matrix Protein Synthesis
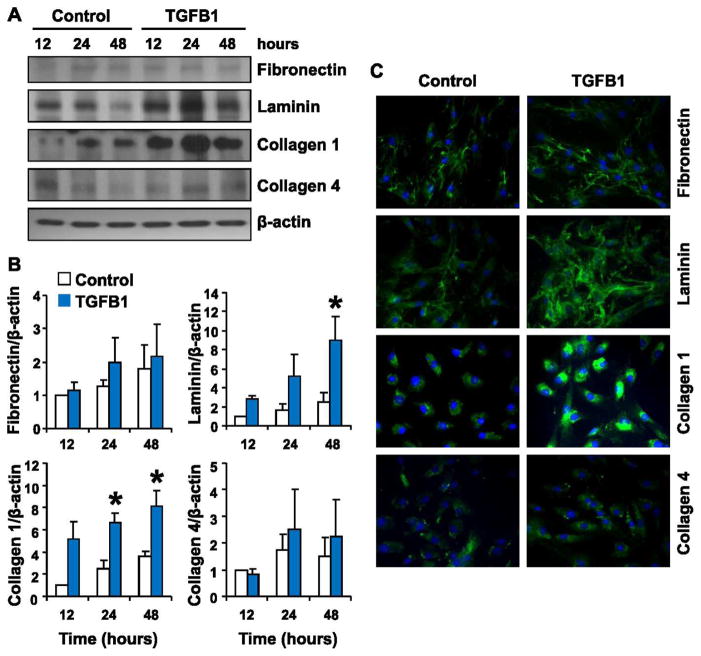
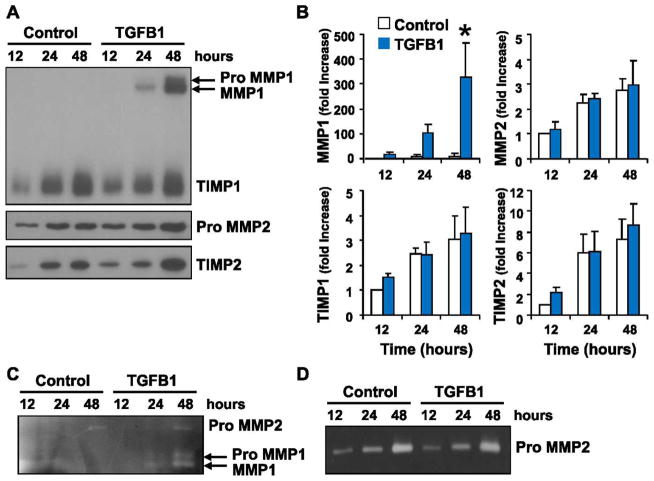
We examined the ability of luteal fibroblasts to produce extracellular matrix proteins. TGFB1 induced marked up-regulation of laminin and collagen 1 protein in luteal fibroblasts that peaked after 24 h of stimulation (Fig. 6). Luteal fibroblasts also produced fibronectin and collagen 4, but these proteins were not appreciably regulated by TGFB1 (Fig. 6). These results suggest that luteal-derived fibroblasts could directly contribute to the deposition of collagen 1 in the regressing corpus luteum. We also examined the ability of luteal fibroblasts to secrete MMPs by Western blot analysis (Fig. 7A) and zymography (Fig. 7, C and D) of conditioned medium. Western blot analysis showed no detectable level of MMP1 release under control conditions. Treatment with TGFB1 (1 ng/ml) significantly up-regulated the release of MMP1, which was detected in both the latent and active forms (Fig. 7, A and B). The presence and up-regulation of active MMP1 was confirmed by casein zymography (Fig. 7C). The release of TIMP1 was not appreciably regulated by TGFB1 (Fig. 7A). Western blot analysis showed that TGFB1 (1 ng/ml) slightly up-regulated the release of TIMP2 and latent MMP2 (Fig. 7A). Gelatin zymography of conditioned medium confirmed that MMP2 was present only in the latent form (Fig. 7D).
FIG. 6.
Effect of TGFB1 treatment on the expression of extracellular matrix proteins by luteal fibroblasts. Luteal fibroblasts were treated with control medium (Control) or TGFB1 (1 ng/ml) in serum-free medium for up to 48 h, and extracellular matrix protein expression was examined by Western blot analysis and immunofluorescence. A) Western blot analysis of cell lysates (40 μg protein/lane). A representative Western blot shows levels of fibronectin, laminin, collagen 1, and collagen 4, with β-actin as loading control. B) Data from densitometric analysis are shown as fold-increases over the untreated control samples after normalization to β-actin. Treatment with TGFB1 up-regulated the expression of laminin and collagen 1. Data represent three separate experiments (mean ± SEM, n = 3. *P < 0.05 vs. Control). C) Analysis of protein expression by immunofluorescence of luteal fibroblast treated with TGFB1 for 24 h. TGFB1 treatment increased the intensity of the staining for laminin and collagen 1. Original magnification ×200.
FIG. 7.
Effect of TGFB1 on MMP release by luteal fibroblasts. Fibroblasts were cultured in monolayer and treated with TGFB1 (1 ng/ml) in serum-free medium. Supernatant medium was harvested, and the expression of matrix-remodeling proteins was examined by Western blot analysis and zymography. A) Western blot analysis (30 μl medium/lane) for MMP1, TIMP1, MMP2, and TIMP2. B) Data from densitometric analysis shown as fold-increases over the untreated control samples (12 h). TGFB1 up-regulated the expression of latent and active MMP1. Data represent three separate experiments (mean ± SEM, n = 3, *P < 0.05 vs. Control). C) Casein zymography (30 μl medium/lane) for MMP1. The presence of the active form of MMP1 was detected in medium samples from TGFB1-treated cells only. D) Gelatin zymography (30 μl medium/lane) for MMP2. Luteal fibroblasts produced only inactive MMP2.
TGFB1-Mediated Contraction of Fibroblast-Populated Collagen Matrices
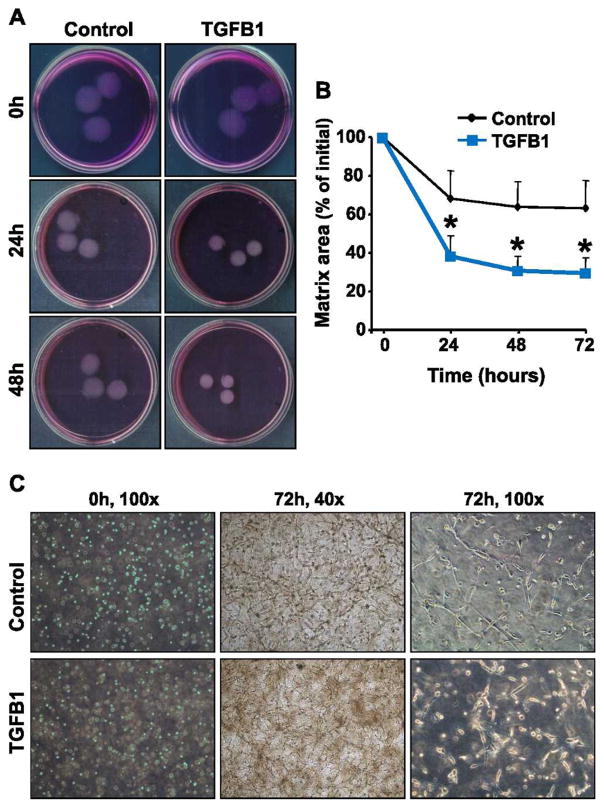
To examine the effects of TGFB1 on luteal fibroblast function, we assessed the ability of the cells to elicit extracellular matrix contraction using fibroblast-populated bovine collagen 1 matrices (Fig. 8A). Fibroblasts were harvested with trypsin and cast into collagen matrices, which floated in DMEM medium with or without TGFB1 (1 ng/ml). Fibroblasts embedded in a collagen matrix interact with collagen fibrils and produce cytoplasmic extensions to migrate through the matrix. This migratory activity of fibroblasts results in contraction of the matrix [39, 40]. In accordance, luteal fibroblasts within collagen matrices produced a network of cytoplasmic extensions (Fig. 8C). In the absence of serum, fibroblasts were able to contract the matrices (30% reduction of original size). Stimulation with TGFB1 (1 ng/ml) significantly (P < 0.05) increased matrix contraction compared to controls (63% reduction of original size) (Fig. 8, A and B).
FIG. 8.
TGFB1 enhanced contraction of fibroblast-populated collagen 1 matrices. The role of TGFB1 in luteal fibroblast contractile function was investigated using fibroblast-populated bovine collagen 1 matrices. Fibroblasts were harvested with trypsin and cast into collagen matrices, which were then floated in DMEM with or without TGFB1 (1 ng/ml). A) Representative experiment using control (top) and TGFB1 (1 ng/ml; bottom)-treated collagen matrices. B) Matrix size is expressed as the percentage of initial area. Quantitative analysis demonstrated that matrix area was significantly smaller in matrices treated with TGFB1. Data represent three independent experiments, each performed in triplicate (mean ± SEM, n =3, *P < 0.05 vs. Control). C) Morphology of fibroblasts cast into floating collagen matrices. Fibroblasts within collagen matrices produce a dendritic network of cytoplasmic extensions. Pictures were taken using a phase-contrast microscope at the indicated culture time points and magnification.
Effect of TGFB1 and Fibrillar Collagen 1 on Fibroblast Viability
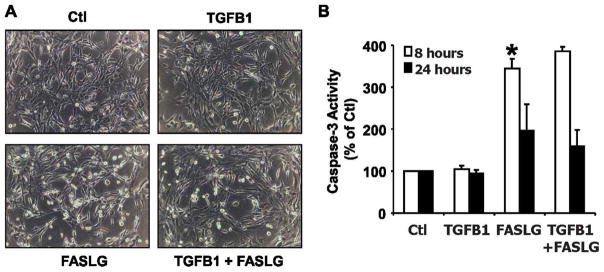
The behavior and function of cells is regulated by changes in the composition of the extracellular matrix [41]. During luteolysis, the expression of collagen 1 increases dramatically [8, 42, 43]. We demonstrated that TGFB1 induces luteal endothelial cell death when plated on collagen 1 gels, as shown by a significant increase in caspase 3 activity after treatment with TGFB1 for 8 and 24 h [37]. We wanted to determine if TGFB1 had the same effect on luteal fibroblasts. To answer this question, we plated luteal fibroblasts using the same experimental conditions as previously described for luteal endothelial cells [37]. TGFB1 had no effect on fibroblast morphology at 8 or 24 h of treatment (Fig. 9A). Treatment with FASLG (100 ng/ml) caused cell rounding and detachment that was evident at 24 h (Fig. 9A). Treatment of fibroblasts with FASLG (100 ng/ml) also caused a significant (P < 0.05) increase in caspase 3 activity at 8 and 24 h (Fig. 9B). In contrast to the proapoptotic effect of TGFB1 on bovine luteal endothelial cells [37], TGFB1 had no effect on the level of caspase activation of fibroblasts at 8 or 24 h of treatment in the absence or presence of FASLG (Fig. 9B).
FIG. 9.
Effect of TGFB1 on fibroblasts plated on collagen 1 gels. A) Morphology of fibroblasts plated (5 × 104 cells/well) in growth medium on 48-well plates coated with bovine collagen 1 gels (0.15 ml, ~2.4 mg/ml). Plated cells were treated with control medium (Ctl), TGFB1 (1 ng/ml), FASLG (100 ng/ml), or their combination. Pictures were taken under a phase-contrast microscope after 24 h of incubation at 37°C. Original magnification ×200. B) Caspase 3 activity was measured after 24 h of treatment using the Caspase-Glo 3/7 assay kit. Data are expressed as a percentage of the caspase activity observed in controls and represent three independent experiments, each performed in triplicate with similar results (mean ± SEM, n = 3, *P < 0.05).
DISCUSSION
The regulation of tissue remodeling that occurs during luteolysis is not completely understood. However, the process evidently involves effects on cell types in the corpus luteum that either express or do not express PGF2α receptors, like steroidogenic cells and fibroblasts, respectively, suggesting that steroidogenic cell products act in a paracrine fashion to regulate the function of other cell types. TGFB1 is produced by bovine luteal steroidogenic cells, and its expression is induced by PGF2α in vitro and in vivo during luteal regression [15–17]. In the present study, we show that TGFB1 regulates the function of luteal fibroblasts. The results suggest that TGFB1 may regulate the remodeling of extracellular matrix observed during luteolysis.
Fibroblasts are the principal cell type responsible for collagen synthesis and the production of connective tissue that occurs in normal tissue remodeling, such as wound healing in response to injury. Activation of fibroblasts, with their differentiation into myofibroblasts, is helpful during wound healing and tissue repair but turns harmful when the process becomes uncontrolled, as occurs in remodeling associated with fibrotic diseases [44]. The structural hallmarks of progressive fibrosis are enlarged extracellular matrix, parenchymal cell atrophy (i.e., epithelial tubular cells in renal fibrosis), and inflammatory response. The cells primarily involved in the pathological changes in fibrosis are the fibroblasts. In fact, the number of renal interstitial myofibroblasts increases dramatically during tubulointerstitial fibrosis, and their long-term activation results in massive extracellular matrix accumulation and the progression of renal failure [18, 45]. TGFB1 is the primary cytokine associated with disorders characterized by inflammation and fibrosis. The significant amount of TGFB1 normally produced under basal conditions by fibroblasts is augmented in fibrosis and correlates with clinical and histological markers of the pathology. In the present study, we demonstrate that TGFB1 stimulates luteal fibroblast chemotaxis, collagen contraction, and extracellular matrix production in vitro, suggesting that TGFB1 induces fibroblast-mediated profibrotic responses.
Although evidence suggests that TGFB1 exerts growth-inhibitory effects on an array of cell types, its role in fibroblast proliferation appears to depend on the distinctive properties of fibroblasts residing in different tissues. Accordingly, TGFB1 induced proliferation in dermal fibroblasts but attenuated the proliferative response in oral fibroblasts [26]. Our experiments demonstrated antiproliferative effects of TGFB1 on luteal fibroblasts in vitro. TGFB1 stimulation reduced proliferation of serum-deprived luteal fibroblasts in a concentration-dependent manner. FGF2, a potent mitogen for human renal fibroblasts [45], induced luteal fibroblast proliferation, which was blocked by TGFB1.
Despite decreasing the proliferative activity of luteal fibroblasts, TGFB1 induced fibroblast activation and increased floating matrix contraction and extracellular matrix production. Using a transwell migration assay, we found that stimulation with TGFB1 significantly increased migration in luteal fibroblasts. Interstitial fibroblasts of adult tissue are normally stationary despite the absence of spatial boundaries, such as basement membranes. Such motile restraint is lost during embryogenesis, wound healing, and pathologic processes like cancer metastasis and fibrosis [46]. TGFB1 signaling regulates fibroblast migration in normal and pathologic conditions [19, 38]. The effect of TGFB1 on the migratory response of luteal fibroblasts could have a physiological role in luteolysis by stimulating fibroblasts normally present in the wall of blood vessels to migrate into the parenchyma of the gland to deposit new extracellular matrix.
In normal tissues, resident fibroblasts are quiescent, producing limited amounts of extracellular matrix proteins [47]. Tissue injury induces cytokine and growth factor-mediated pathways that result in the activation of fibroblasts [29, 44]. Activated fibroblasts produce significant amount of matrix proteins, including fibronectin, which is thought to form a scaffold for the deposition of other proteins and function as a fibroblast chemoattractant to amplify the fibrotic process [48, 49]. Our in vitro studies demonstrated that TGFB1 markedly enhanced the matrix-synthetic ability of luteal fibroblasts. Laminin and collagen 1 synthesis were significantly up-regulated in luteal fibroblasts on stimulation with TGFB1. Furthermore, TGFB1 induced the expression of active MMP1 by luteal fibroblasts. These findings may explain the marked increase in collagen 1 mRNA expression [43] and deposition [8, 11] as well as the increase in MMP1 expression [12,14], observed in regressing corpus luteum. This allows fibroblasts to modulate both production and destruction of extracellular matrix during structural luteolysis as well as the response of other cells.
The floating matrix contraction assay was employed to study the process of matrix remodeling [50]. In the assay, the matrix is released after polymerization, at which point the embedded fibroblasts start to remodel the matrix. Fibroblasts within collagen matrices associate with collagen fibrils and produce a dendritic network of cytoplasmic extensions. Matrix remodeling occurs as a result of the migratory activity of cells, which are moving throughout the matrix to form connections with neighboring cells [39, 40]. As fibroblasts move, they exert mechanical forces on their surroundings that cause contraction of the matrix [39, 40]. TGFB1, along with platelet-derived growth factor and lysophosphatidic acid, stimulate contraction of floating collagen matrices by fibroblasts [51]. The present experiments utilized type I collagen gels populated with luteal fibroblasts to mimic the extracellular matrix present during luteolysis. Exposure to TGFB1 significantly increased gel contraction compared with that of gels floated in control medium. The ability of luteal fibroblasts to contract collagen matrices may be important to achieve efficient reduction in size of the corpus luteum as it is cleared from the ovary.
The present results indicate that luteal fibroblasts are relatively resistant to the cytotoxic effects of cytokines (TNF, FASLG, and IFNG) associated with induction of apoptosis of luteal steroidogenic cells [5, 6] and endothelial cells [35–37]. We observed that FASLG alone increased caspase 3/7 activity and slightly reduced fibroblast viability, a response that was not enhanced in the presence of TNF, IFNG, or TGFB1. The relative insensitivity to cytokines may provide a mechanism for survival of fibroblasts during the extensive matrix remodeling that occurs during luteal regression. Our laboratory reported that TGFB1 induced apoptosis of luteal endothelial cells when plated on fibrillar collagen 1 gels [37]. However, TGFB1 did not induce apoptosis of luteal fibroblasts when plated on fibrillar collagen 1 gels. These findings are consistent with the known cell- and context-specific actions of TGFB1. The observation that TGFB1 inhibits fibroblast proliferation but does not sensitize the fibroblast to apoptosis-inducing cytokines may provide a mechanism that prevents overt expansion of the fibrotic matrix to allow formation of a corpus albicans.
Our findings contribute new information essential for understanding the role of the TGFB1 pathway in matrix remodeling during luteal regression. Despite its antiproliferative effects, TGFB1 induced luteal fibroblast migration and collagen matrix contraction and promoted synthesis of extracellular matrix proteins. The antiproliferative effects of TGFB1 on resident fibroblasts may serve to attenuate adverse fibrotic remodeling of the regressing corpus luteum.
Footnotes
Supported by the VA Biomedical Laboratory R&D Merit Review Research Program, USDA NRI Competitive Grant 2011-67015-20076, the Olson Center for Women’s Health, University of Nebraska Medical Center (UNMC), and a UNMC Graduate Studies Fellowship (D.M.).
References
- 1.Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW. Mechanisms controlling the function and life span of the corpus luteum. Physiol Rev. 2000;80:1–29. doi: 10.1152/physrev.2000.80.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Duncan WC, McNeilly AS, Illingworth PJ. The effect of luteal “rescue” on the expression and localization of matrix metalloproteinases and their tissue inhibitors in the human corpus luteum. J Clin Endocrinol Metab. 1998;83:2470–2478. doi: 10.1210/jcem.83.7.4950. [DOI] [PubMed] [Google Scholar]
- 3.Smith MF, Ricke WA, Bakke LJ, Dowb MPD, Smith GW. Ovarian tissue remodeling: role of matrix metalloproteinases and their inhibitors. Mol Cell Endocrinol. 2002;191:45–56. doi: 10.1016/s0303-7207(02)00054-0. [DOI] [PubMed] [Google Scholar]
- 4.Ribeiro LA, Turba ME, Zannoni A, Bacci ML, Forni M. Gelatinases, endonuclease and vascular endothelial growth factor during development and regression of swine luteal tissue. BMC Dev Biol. 2006;6:58–66. doi: 10.1186/1471-213X-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007;28:117–149. doi: 10.1210/er.2006-0022. [DOI] [PubMed] [Google Scholar]
- 6.Davis JS, Rueda BR. The corpus luteum: an ovarian structure with maternal instincts and suicidal tendencies. Front Biosci. 2002;7:d1949–d1978. doi: 10.2741/davis1. [DOI] [PubMed] [Google Scholar]
- 7.Davis JS, Rueda BR, Spanel-Borowski K. Microvascular endothelial cells of the corpus luteum. Reprod Biol Endocrinol. 2003;1:89–103. doi: 10.1186/1477-7827-1-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vonnahme KA, Redmer DA, Borowczyk E, Bilski JJ, Luther JS. Vascular composition, apoptosis, and expression of angiogenic factors in the corpus luteum during prostaglandin F2alpha-induced regression in sheep. Reproduction. 2006;131:1115–1126. doi: 10.1530/rep.1.01062. [DOI] [PubMed] [Google Scholar]
- 9.Townson DH, Liptak AR. Chemokines in the corpus luteum: implications of leukocyte chemotaxis. Reprod Biol Endocrinol. 2003;1:94–100. doi: 10.1186/1477-7827-1-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duncan WC. The human corpus luteum: remodelling during luteolysis and maternal recognition of pregnancy. Rev Reprod. 2000;5:12–17. doi: 10.1530/ror.0.0050012. [DOI] [PubMed] [Google Scholar]
- 11.Irving-Rodgers HF, Roger J, Luck MR, Rodgers RJ. Extracellular matrix of the corpus luteum. Semin Reprod Med. 2006;24:242–250. doi: 10.1055/s-2006-948553. [DOI] [PubMed] [Google Scholar]
- 12.Ricke WA, Smith GW, Smith MF. Matrix metalloproteinase expression and activity following prostaglandin F(2α)-induced luteolysis. Biol Reprod. 2002;66:685–691. doi: 10.1095/biolreprod66.3.685. [DOI] [PubMed] [Google Scholar]
- 13.Ricke WA, Smith GW, McIntush EW, Smith MF. Analysis of luteal tissue inhibitor of metalloproteinase-1, -2, and -3 during prostaglandin F(2alpha)-induced luteolysis. Biol Reprod. 2002;66:1387–1394. doi: 10.1095/biolreprod66.5.1387. [DOI] [PubMed] [Google Scholar]
- 14.Kliem H, Welter H, Kraetzl WD, Steffl M, Meyer HHD, Schams D, Berisha B. Expression and localization of extracellular matrix degrading proteases and their inhibitors during the estrous cycle and after induced luteolysis in the bovine corpus luteum. Reproduction. 2007;134:535–547. doi: 10.1530/REP-06-0172. [DOI] [PubMed] [Google Scholar]
- 15.Stocco C, Callegari E, Gibori G. Opposite effect of prolactin and prostaglandin F(2α) on the expression of luteal genes as revealed by rat cDNA expression array. Endocrinology. 2001;142:4158–4161. doi: 10.1210/endo.142.9.8493. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Tamura K, Yoshie M, Tamura H, Imakawa K. Prostaglandin F2alpha-induced functional regression of the corpus luteum and apoptosis in rodents. J Pharmacol Sci. 2003;92:19–27. doi: 10.1254/jphs.92.19. [DOI] [PubMed] [Google Scholar]
- 17.Hou X, Arvisais EW, Jiang C, Chen DB, Roy SK, Pate JL, Hansen TR, Rueda BR, Davis JS. Prostaglandin F2alpha stimulates the expression and secretion of transforming growth factor B1 via induction of the early growth response 1 gene (EGR1) in the bovine corpus luteum. Mol Endocrinol. 2008;22:403–414. doi: 10.1210/me.2007-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi W, Chen X, Poronnik P, Pollock CA. The renal cortical fibroblast in renal tubulointerstitial fibrosis. Int J Biochem Cell Biol. 2006;38:1–5. doi: 10.1016/j.biocel.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007;132:1311–1321. doi: 10.1378/chest.06-2568. [DOI] [PubMed] [Google Scholar]
- 20.Coward WR, Saini G, Jenkins G. The pathogenesis of idiopathic pulmonary fibrosis. Ther Adv Respir Dis. 2010;4:367–388. doi: 10.1177/1753465810379801. [DOI] [PubMed] [Google Scholar]
- 21.Qi W, Twigg S, Chen X, Polhill TS, Poronnik P, Gilbert RE, Pollock CA. Integrated actions of transforming growth factor-beta1 and connective tissue growth factor in renal fibrosis. Am J Physiol Renal Physiol. 2005;288:F800–F809. doi: 10.1152/ajprenal.00179.2004. [DOI] [PubMed] [Google Scholar]
- 22.Massagué J. TGFβ in cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Strutz F, Zeisberg M, Renziehausen A, Raschke B, Becker V, van Kooten C, Müller G. TGF-beta 1 induces proliferation in human renal fibroblasts via induction of basic fibroblast growth factor (FGF-2) Kidney Int. 2001;59:579–592. doi: 10.1046/j.1523-1755.2001.059002579.x. [DOI] [PubMed] [Google Scholar]
- 24.Pratsinis H, Giannouli CC, Zervolea I, Psarras S, Stathakos D, Kletsas D. Differential proliferative response of fetal and adult human skin fibroblasts to transforming growth factor-beta. Wound Repair Regen. 2004;12:374–383. doi: 10.1111/j.1067-1927.2004.12305.x. [DOI] [PubMed] [Google Scholar]
- 25.Giannouli CC, Kletsas D. TGF-beta regulates differentially the proliferation of fetal and adult human skin fibroblasts via the activation of PKA and the autocrine action of FGF-2. Cell Signal. 2006;18:1417–1429. doi: 10.1016/j.cellsig.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Meran S, Thomas DW, Stephens P, Enoch S, Martin J, Steadman R, Phillips AO. Hyaluronan facilitates transforming growth factor-beta1-mediated fibroblast proliferation. J Biol Chem. 2008;283:6530–6545. doi: 10.1074/jbc.M704819200. [DOI] [PubMed] [Google Scholar]
- 27.Khalil N, Xu YD, O’Connor R, Duronio V. Proliferation of pulmonary interstitial fibroblasts is mediated by transforming growth factor-beta1-induced release of extracellular fibroblast growth factor-2 and phosphorylation of p38 MAPK and JNK. J Biol Chem. 2005;280:43000–43009. doi: 10.1074/jbc.M510441200. [DOI] [PubMed] [Google Scholar]
- 28.Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada G, Mendoza LH. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107:418–428. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 30.Hou X, Arvisais EW, Davis JS. Luteinizing hormone stimulates mammalian target of rapamycin signaling in bovine luteal cells via pathways independent of AKT and mitogen-activated protein kinase: modulation of glycogen synthase kinase 3 and AMP-activated protein kinase. Endocrinology. 2010;151:2846–2857. doi: 10.1210/en.2009-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukuchi M, Miyazaki T, Fukai Y, Nakajima M, Sohda M, Masuda N, Manda R, Tsukada K, Kato H, Kuwano H. Plasma level of transforming growth factor beta1 measured from the azygos vein predicts prognosis in patients with esophageal cancer. Clin Cancer Res. 2004;10:2738–2741. doi: 10.1158/1078-0432.ccr-1096-03. [DOI] [PubMed] [Google Scholar]
- 32.Kiliç ZM, Ayaz S, Ozin Y, Nadir I, Cakal B, Ulker A. Plasma transforming growth factor-beta1 level in inflammatory bowel disease. Turk J Gastroenterol. 2009;20:165–170. doi: 10.4318/tjg.2009.0002. [DOI] [PubMed] [Google Scholar]
- 33.Ouellette Y, Price CA, Carrière PD. Follicular fluid concentration of transforming growth factor-beta1 is negatively correlated with estradiol and follicle size at the early stage of development of the first-wave cohort of bovine ovarian follicles. Domest Anim Endocrinol. 2005;29:623–633. doi: 10.1016/j.domaniend.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Villar AV, Cobo M, Llano M, Montalvo C, González-Vílchez F. Plasma levels of transforming growth factor-b1 reflect left ventricular remodeling in aortic stenosis. PLoS ONE. 2009;4:e8476. doi: 10.1371/journal.pone.0008476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Friedman A, Weiss S, Levy N, Meidan R. Role of tumor necrosis factor alpha and its type I receptor in luteal regression: induction of programmed cell death in bovine corpus luteum-derived endothelial cells. Biol Reprod. 2000;63:1905–1912. doi: 10.1095/biolreprod63.6.1905. [DOI] [PubMed] [Google Scholar]
- 36.Pru JK, Lynch MP, Davis JS, Rueda BR. Signaling mechanisms in tumor necrosis factor alpha-induced death of microvascular endothelial cells of the corpus luteum. Reprod Biol Endocrinol. 2003;1:17–27. doi: 10.1186/1477-7827-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maroni D, Davis JS. TGFB1 disrupts the angiogenic potential of microvascular endothelial cells of the corpus luteum. J Cell Sci. 2011;124:2501–2510. doi: 10.1242/jcs.084558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li YJ, Wang XQ, Sato T, Kanaji N, Nakanishi M, Kim M, Michalski J, Nelson AJ, Sun JH, Farid M, Basma H, Patil A, et al. Prostaglandin E2 inhibits human lung fibroblast chemotaxis through disparate actions on different E-prostanoid receptors. Am J Respir Cell Mol Biol. 2011;44:99–107. doi: 10.1165/rcmb.2009-0163OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang H, Grinnell F. Cell-matrix entanglement and mechanical anchorage of fibroblasts in three-dimensional collagen matrices. Mol Biol Cell. 2005;16:5070–5076. doi: 10.1091/mbc.E05-01-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang H, Rhee S, Ho CH, Grinnell F. Distinguishing fibroblast promigratory and procontractile growth factor environments in 3-D collagen matrices. FASEB J. 2008;22:2151–2160. doi: 10.1096/fj.07-097014. [DOI] [PubMed] [Google Scholar]
- 41.Berrier AL, Yamada KM. Cell-matrix adhesion. J Cell Physiol. 2007;213:565–573. doi: 10.1002/jcp.21237. [DOI] [PubMed] [Google Scholar]
- 42.Zhao Y, Luck MR. Gene expression and protein distribution of collagen, fibronectin and laminin in bovine follicles and corpora lutea. J Reprod Fertil. 1995;104:115–123. doi: 10.1530/jrf.0.1040115. [DOI] [PubMed] [Google Scholar]
- 43.Casey OM, Morris DG, Powell R, Sreenan JM, Fitzpatrick R. Analysis of gene expression in nonregressed and regressed bovine corpus luteum tissue using a customized ovarian cDNA array. Theriogenology. 2005;64:1963–1976. doi: 10.1016/j.theriogenology.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 44.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 45.Strutz F, Zeisberg M, Hemmerlein B, Sattler B, Hummel K, Becker V, Müller GA. Basic fibroblast growth factor (FGF-2) expression is increased in human renal fibrogenesis and may mediate autocrine fibroblast proliferation. Kidney Int. 2000;57:1521–1538. doi: 10.1046/j.1523-1755.2000.00997.x. [DOI] [PubMed] [Google Scholar]
- 46.Shieh AC, Rozansky HA, Hinz B, Swartz MA. Tumor cell invasion is promoted by interstitial flow-induced matrix priming by stromal fibroblasts. Cancer Res. 2011;71:790–800. doi: 10.1158/0008-5472.CAN-10-1513. [DOI] [PubMed] [Google Scholar]
- 47.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechanoregulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 48.Yokoi H, Sugawara A, Mukoyama M, Mori K, Makino H, Suganami T, Nagae T, Yahata K, Fujinaga Y, Tanaka I, Nakao K. Role of connective tissue growth factor in profibrotic action of transforming growth factor-B: a potential target for preventing renal fibrosis. Am J Kidney Dis. 2001;38:S134–S138. doi: 10.1053/ajkd.2001.27422. [DOI] [PubMed] [Google Scholar]
- 49.Weston BS, Wahab NA, Mason RM. CTGF mediates TGF-β-induced fibronectin matrix deposition by up-regulating active α5β1 integrin in human mesangial cells. J Am Soc Nephrol. 2003;14:601–610. doi: 10.1097/01.asn.0000051600.53134.b9. [DOI] [PubMed] [Google Scholar]
- 50.Grinnell F. Fibroblast mechanics in three-dimensional collagen matrices. J Bodyw Mov Ther. 2008;12:191–194. doi: 10.1016/j.jbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rhee S, Grinnell F. P21-activated kinase 1: convergence point in PDGF-and LPA-stimulated collagen matrix contraction by human fibroblasts. J Cell Biol. 2006;172:423–432. doi: 10.1083/jcb.200505175. [DOI] [PMC free article] [PubMed] [Google Scholar]