Abstract
Background
Live-attenuated influenza vaccines (LAIVs) are not licensed in children <2 years because of a wheezing safety signal that has not been fully elucidated. In 2000, the Kaiser Permanente Vaccine Study Center conducted a placebo controlled randomized clinical trial (RCT) of LAIV in children. As many of these children were still enrolled in Kaiser Permanente in 2014, we could assess the possible long-term association between LAIV and subsequent asthma diagnosis.
Methods
We identified all children who were originally enrolled into the LAIV RCT at < 3 years of age. We followed subjects until disenrollment from the health plan, a first diagnosis of asthma, or through the end of the study period in 2014. Asthma was defined by a first ICD-9-CM code (493.*) assigned at an outpatient or emergency department encounter. We performed a survival analysis of time to first asthma diagnosis among children receiving LAIV or placebo with a Cox proportional hazards model.
Results
We identified 1151 children in the original RCT who were aged 12 through 35 months at time of enrollment and who had received 2 doses of LAIV or placebo. A total of 767 (66.7%) RCT participants were still KPNC members in 2014. There was no evidence of differential drop-out by treatment group. The hazard ratio for new onset asthma for LAIV recipients compared to placebo was 1.1 (95% confidence interval 0.88–1.41, p=0.38).
Conclusions
We found no evidence of increased risk of subsequent asthma diagnosis among children < 3 years who received LAIV compared to placebo.
Keywords: Influenza Vaccines, Vaccine Safety, Live-attenuated Influenza vaccines, asthma
Introduction
Live-attenuated influenza vaccines (LAIVs) can be an important intervention to prevent severe influenza illness globally. LAIVs have several potential advantages over injectable influenza vaccines. Favorable production speed and yields, ease of administration, and demonstrated superiority to inactivated influenza vaccines in children in head-to-head randomized clinical trials (RCTs) indicate that LAIVs should be considered for use in routine national immunization programs.1,2 For these reasons, the World Health Organization (WHO) has supported transfer of LAIV manufacturing technology to increase developing country vaccine production capacity for use against seasonal and pandemic influenza.1
LAIVs are not currently licensed for use in children < 2 years,3 given concerns of a possible association between LAIV and wheezing in this age group.2,4,5 The lack of available LAIVs for children <2 years is a major limitation to disease prevention in low- and middle-income countries (LMICs), given that this age group has the greatest pediatric influenza disease burden and the strongest vaccine delivery infrastructure and systems.3
There are currently two different LAIV technologies in use: 1) Ann Arbor backbone-LAIV produced by AstraZeneca in the United Kingdom, licensed under the names FluMist® (United States / Canada) and Fluenz® (European Union / European Economic Area) which is licensed in North America and Europe respectively; and 2) Russian backbone-LAIV produced in Russia (Ultravac®) and India (Nasovac-S®) and used primarily in those two countries. The wheezing signal is specific to the Ann Arbor vaccines.
In 2014, WHO convened an expert consultation to assess LAIV’s potential to prevent pediatric influenza disease in LMICs.3 The consultation identified prevention of severe influenza illness in children <2 years as an unmet global health need. Participants noted that the mechanism for the safety signal was unknown and needed further elucidation, but could be related to reports of early life respiratory virus infection causing subsequent asthma illness in children.6 If the safety signal is real, the risk of LAIV receipt in <2 years may not outweigh the potential benefits. As the expert consultation recommended careful age de-escalation of LAIVs into <2 years age groups as a potential strategy to address the unmet global health need, more date are needed regarding the long term respiratory health of young children vaccinated with LAIVs. To inform decisions regarding LAIV age de-escalation trials, we conducted this study to assess whether early childhood LAIV vaccination was associated with long term asthma illness.
Materials and Methods
In 2000, the Kaiser Permanente Vaccine Study Center conducted a placebo controlled RCT of Ann Arbor LAIV in children.5 Children received two doses of study vaccine in a 2:1 ratio with placebo. A history of asthma was an exclusion criterion for this trial. Post hoc analyses showed elevated risk ratios in some comparisons of LAIV receipt and asthma, all in children 18 to 35 months of age. For our current analysis, we identified all children who were originally enrolled into the LAIV RCT at < 3 years of age. Three children were not included because they were “live-in” members of another Kaiser Region but living in Northern California at the time of the RCT. Tracking membership over time for those 3 children is problematic, since we may not have had a precise date if they returned to their “home region”. Our exposure of interest was receipt of LAIV or placebo. We followed subjects from enrollment through 2014 for asthma outcomes. We defined asthma by a first ICD-9-CM code (493.*) assigned at an outpatient or emergency department encounter in the electronic medical record. We performed a survival analysis of time to first asthma diagnosis with a Cox proportional hazards model. We estimated product-limit survival functions with corresponding 95% Hall-Wellner confidence bands. Analyses used SAS software version 9.2 (SAS Institute Inc., Cary, NC, USA). The study was reviewed and approved by the KPNC Institutional Review Board and the WHO Ethical Review Committee.
Results
We identified 1151 children in the original RCT who were aged 12 through 35 months at time of enrollment, who had received 2 doses of the same treatment (LAIV or placebo), and who were enrolled in KPNC at the time of the trial. These 1151 subjects were followed until they dropped from membership or until they received a first asthma diagnosis. Of included subjects, 503 (43.6%) were 12–23 months and 651 (56.4%) were 24–35 months old. A total of 762 (66.2%) subjects received LAIV, and 389 (33.8%) subjects received placebo. Subjects included 564 (51.0%) males and 587 (49.0%) females. Two-thirds (767/1151) of the study population were still KPNC members in 2014. There was no evidence of differential drop-out by treatment group. In any given year there was never more than 0.8% deviation from the 2:1 randomization ratio. Screening for a history of asthma in the original RCT was done by in-person parent interview. Despite this, some subjects had a history of an asthma diagnosis code identified after the trial. However, the proportion was equal between the LAIV and placebo groups, with 70 (9.2%) LAIV recipients and 35 (9.0%) placebo recipients having an asthma diagnosis at any time prior to study entry.
We graphed the total numbers of LAIV and placebo recipients who had a new (incident) asthma diagnosis by year, adjusting for the 2:1 enrollment ratio (Figure 1). There were similar asthma diagnosis rates between study groups. A log-rank homogeneity test did not find evidence for non-proportional hazards over time (p = 0.41). A proportional hazards model was fit, including only gender and treatment group as independent variables. The hazard ratio for new onset asthma for LAIV recipients compared to placebo recipients was 1.1 (95% confidence interval 0.88–1.41, p=0.38). Female sex was modestly protective, HR=0.8 (0.64, 0.99) p =0.044. Figure 2 shows an estimated survival curve with time to first diagnosis of asthma by study group. The 95% confidence intervals overlapped substantially. We found no evidence of increased risk of subsequent asthma diagnosis among children who received LAIV compared to placebo after 14 years of follow up.
Figure 1.
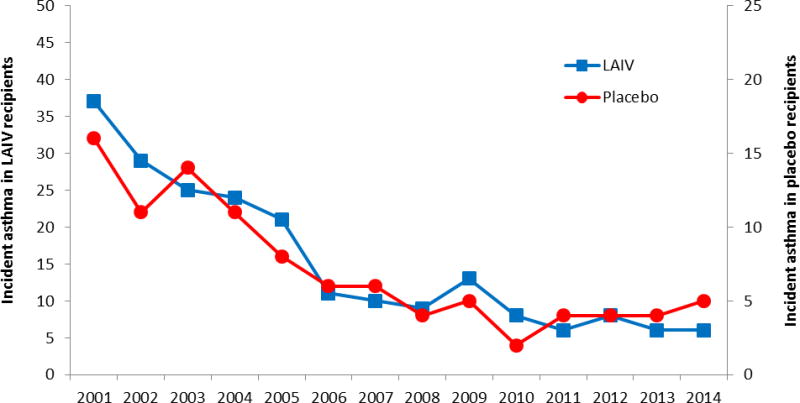
Incident Asthma cases in continuously enrolled subjects by LAIV and placebo group, Kaiser Permanente Northern California, 2001–2014
Note: Axes have been adjusted to reflect the 2:1 enrollment ratio.
Figure 2.
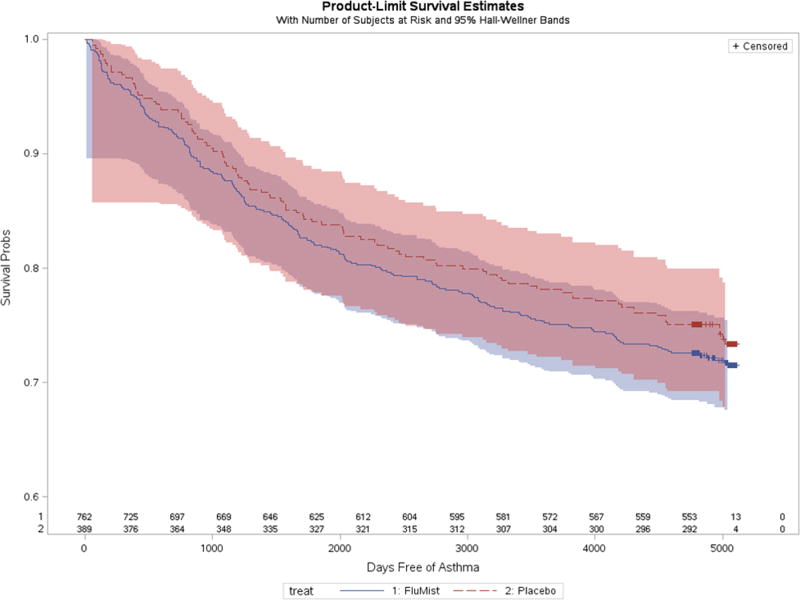
Estimated survival curves of time to first diagnosis of asthma by LAIV and placebo groups, Kaiser Permanente Northern California, 2001–2014
Note: Asthma-free survival among LAIV recipients is shown by the dark blue line, with surrounding 95% confidence interval in light blue. Asthma-free survival among placebo recipients is shown by the red line, with surrounding 95% confidence interval in light red. The substantial overlap of 95% confidence intervals is purple in color.
Discussion
Our study demonstrates that children receiving a dose of LAIV prior to the age of 3 years are no more likely to receive an asthma diagnosis in the subsequent 14 years than children receiving placebo. Previously, researchers have noted that acute wheezing during early childhood respiratory virus infections is a risk factor for subsequent asthma diagnoses.6 Despite the studies identifying a safety signal linking LAIV and acute wheezing among children receiving Ann Arbor LAIV,4,5,7 it is reassuring that we did not find evidence of association between LAIV exposure and subsequent asthma diagnoses.
The 2000 Kaiser Permanente Vaccine Study Center study was a randomized, double blind, placebo-controlled clinical trial of LAIV in healthy children 12 months to 17 years of age.5 A total of 9,689 children were enrolled in the study from October 2000 through December 2000. LAIV was found to be safe in all pre-specified safety analyses. Overall, asthma diagnoses in this trial were observed in 0.9% of LAIV recipients and 0.9% of controls. However, elevated risk ratios were observed in 4 of 31 separate post hoc comparisons for asthma among children aged 18 to 35 months of age. A subsequent open-label, nonrandomized trial of LAIV among healthy children 18 months to 18 years of age found a significantly increased risk of asthma (RR:2.85; 95% CI: 1.01–8.03) 15–42 days after LAIV among children aged 18 months to 4 years only in the first year of the study.7 However, a randomized-controlled trial comparing the safety of LAIV with inactivated influenza vaccine among children 6–59 months of age found that among previously unvaccinated children, wheezing within 42 days was more common following the first dose of LAIV than that of inactivated influenza vaccine,4 and this was most pronounced in children < 12 months of age. Wheezing illness following vaccine receipt was generally mild and self-limited in all of the above studies. Notably, the Russian LAIV has not been associated with an increase in wheezing illness. Early clinical studies in young children did not include wheezing as a solicited adverse event,8 but recent RTCs have not identified a similar signal.9–11
Our study provided the unique opportunity to follow children randomized to receive LAIV or placebo for 14 years in an electronic patient record for asthma diagnoses. The study is not without its limitations, however. Our analysis did not assess long term safety outcomes aside from asthma. We relied on ICD-9 diagnostic codes for asthma which have variable accuracy in children (sensitivity: 44%–92%; specificity: 80%–94%) depending on disease severity, remission and relapse, and the reference standard.12 However, incorrect asthma diagnosis would have resulted in non-differential misclassification, given the randomization in the original RCT. Our analyses did not account for subsequent LAIV or other influenza vaccine receipt. However, as LAIV was not licensed for use until three years after this study,13 none of the study participants in either study group had a second exposure to LAIV until age four at the earliest, an age thought to be outside the risk window for potential respiratory virus caused asthma illness.6 While we were unable to detect any association between LAIV use and asthma, we could not prove that no such association exists. It has been estimated that over 99% of global pediatric influenza deaths occur in LMICs,14 settings where influenza vaccines are not routinely used.15 Given this unmet public health need, WHO has developed Preferred Product Characteristics for Next-Generation Influenza Vaccines.16 LAIVs may have characteristics which would make them suitable for use in LMICs to prevent severe influenza disease, but more work needs to be done. Recent observational research from the United States indicate decreased relative effectiveness of Ann Arbor LAIV compared to injectable influenza vaccines in children.17 However, the United Kingdom and Finland report that Ann Arbor LAIV performance in the same years and age groups met program expectations.17 Similarly, Russian LAIV has also experienced mixed results, with clinical efficacy demonstrated in an RCT among children in Bangladesh but not in a similar study in Senegal.11 Better understanding about issues affecting LAIV performance is needed, and corrections, if necessary, must be made before advancing the product into LMICs. While wheezing signals have been found with Ann Arbor LAIV in the past, the benefit-risk calculation may be different with this vaccine in high disease burden settings where severe illness prevention could outweigh the risk of mild wheezing.16 This study supports the 2014 WHO consultation which recommended that careful age de-escalation studies of children <2 years to assess the benefit-risk of LAIVs in LMICs are unlikely to put the children at increased risk of chronic respiratory disease.3
Acknowledgments
The authors thank Niranjan Bhat, Mark Katz, and Margaret Rennels who provided peer-review of the study protocol for the WHO. The authors would like to acknowledge the contributions of the Centers for Disease Control and Prevention (CDC), which provides financial support to the World Health Organization Initiative for Vaccine Research (U50 CK000431).
Funding for this study: Funding for this study came from a technical service agreement from the World Health Organization Initiative for Vaccine Research.
Financial Disclosures: Roger Baxter and Nicola Klein report research grants for other studies from CDC, MedImmune, Sanofi Pasteur, GSK, Novartis (now GSK), Pfizer, Merck, and Protein Sciences.
Footnotes
Reprints are not available.
Ned Lewis, Bruce Fireman, John Hansen, and Justin R. Ortiz declare no conflicts.
Disclaimer
Justin R. Ortiz is an employee of the World Health Organization. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the decisions, policy, or views of the World Health Organization.
References
- 1.Friede M, Palkonyay L, Alfonso C, et al. WHO initiative to increase global and equitable access to influenza vaccine in the event of a pandemic: supporting developing country production capacity through technology transfer. Vaccine. 2011;29(Suppl 1):A2–7. doi: 10.1016/j.vaccine.2011.02.079. [DOI] [PubMed] [Google Scholar]
- 2.Grohskopf LA, Sokolow LZ, Broder KR, et al. Prevention and Control of Seasonal Influenza with Vaccines. MMWR Recomm Rep. 2016;65(5):1–54. doi: 10.15585/mmwr.rr6505a1. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Meeting to Assess Live-Attenuated Influenza Vaccines to Prevent Paediatric Influenza Disease in Low and Middle Income Countries: December 16–17, 2014: Executive Summary. 2014 http://www.who.int/influenza_vaccines_plan/Executive_Summary_LAIV_meeting_FINAL.pdf.
- 4.Belshe RB, Ambrose CS, Yi T. Safety and efficacy of live attenuated influenza vaccine in children 2–7 years of age. Vaccine. 2008;26(Suppl 4):D10–16. doi: 10.1016/j.vaccine.2008.06.083. [DOI] [PubMed] [Google Scholar]
- 5.Bergen R, Black S, Shinefield H, et al. Safety of cold-adapted live attenuated influenza vaccine in a large cohort of children and adolescents. The Pediatric infectious disease journal. 2004;23(2):138–144. doi: 10.1097/01.inf.0000109392.96411.4f. [DOI] [PubMed] [Google Scholar]
- 6.Busse WW, Lemanske RF, Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet (London, England) 2010;376(9743):826–834. doi: 10.1016/S0140-6736(10)61380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piedra PA, Gaglani MJ, Riggs M, et al. Live attenuated influenza vaccine, trivalent, is safe in healthy children 18 months to 4 years, 5 to 9 years, and 10 to 18 years of age in a community-based, nonrandomized, open-label trial. Pediatrics. 2005;116(3):e397–407. doi: 10.1542/peds.2004-2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rudenko LG, Slepushkin AN, Monto AS, et al. Efficacy of live attenuated and inactivated influenza vaccines in schoolchildren and their unvaccinated contacts in Novgorod, Russia. The Journal of infectious diseases. 1993;168(4):881–887. doi: 10.1093/infdis/168.4.881. [DOI] [PubMed] [Google Scholar]
- 9.Brooks WA, Zaman K, Lewis KD, et al. Efficacy of a Russian-backbone live attenuated influenza vaccine among young children in Bangladesh: a randomised, double-blind, placebo-controlled trial. The Lancet Global health. 2016;4(12):e946–e954. doi: 10.1016/S2214-109X(16)30200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ortiz JR, Goswami D, Lewis KD, et al. Safety of Russian-backbone seasonal trivalent, live-attenuated influenza vaccine in a phase II randomized placebo-controlled clinical trial among children in urban Bangladesh. Vaccine. 2015;33(29):3415–3421. doi: 10.1016/j.vaccine.2015.04.048. [DOI] [PubMed] [Google Scholar]
- 11.Victor JC, Lewis KD, Diallo A, et al. Efficacy of a Russian-backbone live attenuated influenza vaccine among children in Senegal: a randomised, double-blind, placebo-controlled trial. The Lancet Global health. 2016;4(12):e955–e965. doi: 10.1016/S2214-109X(16)30201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desai JR, Wu P, Nichols GA, Lieu TA, O’Connor PJ. Diabetes and Asthma Case Identification, Validation, and Representativeness When Using Electronic Health Data to Construct Registries for Comparative Effectiveness and Epidemiologic Research. Methods. 2012 doi: 10.1097/MLR.0b013e318259c011. Paper 1. http://repository.academyhealth.org/methods_resources/1. [DOI] [PMC free article] [PubMed]
- 13.FluMist [package insert] 2017 https://www.fda.gov/downloads/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm294307.pdf.
- 14.Nair H, Brooks WA, Katz M, et al. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet (London, England) 2011;378(9807):1917–1930. doi: 10.1016/S0140-6736(11)61051-9. [DOI] [PubMed] [Google Scholar]
- 15.Ortiz JR, Perut M, Dumolard L, et al. A global review of national influenza immunization policies: Analysis of the 2014 WHO/UNICEF Joint Reporting Form on immunization. Vaccine. 2016;34(45):5400–5405. doi: 10.1016/j.vaccine.2016.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Preferred Product Characteristics for Next-Generation Influenza Vaccines. 2017. [Google Scholar]
- 17.Penttinen PM, Friede MH. Decreased effectiveness of the influenza A(H1N1)pdm09 strain in live attenuated influenza vaccines: an observational bias or a technical challenge? Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2016;21(38) doi: 10.2807/1560-7917.ES.2016.21.38.30350. [DOI] [PMC free article] [PubMed] [Google Scholar]


