Abstract
Background
Psychosis is among the most disabling complications of Parkinson’s disease (PD). The chronicity of PD psychosis remains understudied and the relative importance of dopaminergic therapy versus the disease process itself in engendering psychosis remains unclear.
Objectives
To examine pharmacologic and motoric correlates of PD psychosis onset and remission in a longitudinally monitored PD cohort.
Methods
We analyzed data from 165 participants enrolled in a longitudinal PD study through the Morris K. Udall Parkinson’s Disease Research Center of Excellence at Johns Hopkins University. Evaluations included formal psychiatric assessment and were conducted at two-year intervals. Regression with generalized estimated equations (GEE) was used to produce unadjusted and adjusted estimates for time-varying longitudinal associations between psychosis and putative risk factors.
Results
Sixty-two participants (37.6%) were diagnosed with psychosis during at least one evaluation. Of forty-nine participants with psychosis followed over multiple evaluations, 13 (26.5%) demonstrated remission despite significant Hoehn & Yahr stage increase (p=0.009); two of these cases later relapsed. Multivariable regression with GEE identified dementia diagnosis, akinesia-rigidity, anticholinergic usage, and levodopa-carbidopa dose to be significantly associated with psychosis, while disease duration was not. A sub-analysis of 30 incident psychosis cases suggested that dopamine agonist dose was lowered after psychosis onset with a compensatory increase in levodopa-carbidopa dosage.
Conclusions
Our findings suggest that in the context of standard therapy, PD-related psychotic disorder can remit at a frequency of approximately 27%. Additionally, akinetic-rigid motor impairment was more strongly associated with psychosis than disease duration, independent of cognitive impairment and medications.
Keywords: Parkinson’s disease, Psychosis, Motor subtypes, Remission, Hallucinations
Introduction
The point-prevalence of psychosis in patients with Parkinson’s disease (PD) is approximately 30%1 and it is the strongest predictor of nursing home placement in PD.2 The most common PD-related psychotic symptom is visual hallucinations, which 50% of patients experience at some point during the disease course.3 Even minor psychotic symptoms are strongly predictive of subsequent severe cognitive decline and behavioral disturbances.4 However, the typical course of psychotic phenomenology in PD has only been indirectly or partially addressed—for example, in prospective studies5,6 with few assessments and follow-up periods that do not capture the temporal dynamics of the disorder.
Historical accounts from the pre-levodopa era7 suggest that psychosis may be a “natural” component of PD, though it is typically thought to be largely related to dopaminergic medication—especially dopamine agonists.1,8,9 However, most cross-sectional studies have found no link between psychosis and levodopa dose, levodopa equivalent daily dose (LEDD), or a particular “treatment profile.”1,10 Changes in pharmacologic management prompted by the onset of psychotic symptoms have not been addressed in the context of longitudinal analyses; this information may help to understand how methodological differences in prior studies have produced conflicting findings on the relationship between psychosis and medication profile.
Identifying clinical correlates of psychosis that are more specific to the disease process itself may also help formulate more unifying prognostic indicators of vulnerability to psychosis in PD. For example, one study11 used cluster analysis to show that hallucinations—measured with a corresponding item in the Unified Parkinson’s Disease Rating Scale (UPDRS)—are more common among akinetic-rigid (AR) than tremor-dominant (TD) patients.12 However, the majority of patients convert to the AR subtype over time,13 making it unclear how psychotic phenomena relate to AR motor impairment longitudinally, as opposed to overall disease severity and duration. It may be more appropriate to treat AR and TD motor impairment as dimensional traits to differentially relate them to risk for undesirable outcomes such as psychosis.14
In the present report, we analyzed patterns in the onset and remission of PD psychosis in 165 patients who were longitudinally assessed approximately every two years. Psychiatric diagnoses were guided by the Diagnostic and Statistical Manual of Mental Disorders, version IV, (DSM-IV). Additionally, we incorporated a risk-factor analysis to identify clinical markers of PD psychosis and analyzed medication regimens by LEDD contribution. Regression analysis was conducted using GEE, which allows for correlation within subjects to be estimated; this feature facilitates analysis of longitudinal repeated-measures data with time-varying measures.15 We hypothesized that DSM-IV psychotic disorder—determined by psychiatrist interview to be directly due to PD—would be associated over time with increased akinesia-rigidity—measured according to convention16 using neurologist-rated UPDRS-III (motor examination) scores. Additionally, we performed secondary analyses to test the hypothesis that incident psychosis results in reductions of dopaminergic medications.
Methods
Participants and Study Design
The Morris K. Udall Parkinson’s Disease Research Center of Excellence, located at the Johns Hopkins School of Medicine, recruits individuals with PD, patients with related movement disorders, and healthy controls for a number of research studies. Participants included in this analysis were drawn from the Center’s Longitudinal Study, a Parkinson’s disease study initiated in 1998, which has enrolled 269 individuals since its inception. Of these 269 participants, 182 have a diagnosis of idiopathic PD by UK Brain Bank criteria,17 19 have related movement disorders, and 68 are controls. At the time of enrollment and every two years subsequently, subjects undergo a full psychiatric evaluation per DSM-IV criteria. These visits also included a neurologist-conducted UPDRS interview with Hoehn & Yahr stage18 determination and medication list updates.
We aimed to evaluate the longitudinal association between diagnosis of psychosis and medications or motor impairment phenotype. Inclusion criteria for this analysis were a clinical diagnosis of idiopathic PD and complete psychiatric assessment data, UPDRS-III data, medications, and demographic information. Of the 182 enrolled idiopathic PD participants, 15 were excluded for incomplete DSM-IV diagnosis data, one was excluded for incomplete Hoehn & Yahr stage and UPDRS-III data, and one was excluded for incomplete medication information. Therefore, 165 patients were included in the present analysis. Patient age was defined as age at enrollment and disease duration was defined as the time that elapsed between diagnosis date and that evaluation. The levodopa equivalent daily dose (LEDD) contributed by each medication was calculated for each subject based on their medication lists in accordance with convention.19 Where carbidopa/levodopa was prescribed to be used “as needed,” we estimated its LEDD contribution as the midpoint between the typical maximum (3 doses per day) and minimum (0 doses per day) daily values.
Measures
The dependent variable for the present analysis was diagnosis of psychotic disorder in accordance with DSM-IV criteria. These criteria require that (1) prominent hallucinations or delusions are present; (2) historical or examination-based indication that the disturbance is directly related to PD and not medications, alcohol, or substance abuse; (3) the disturbance is not better explained by another mental disorder (e.g., major depression with psychosis), (4) the disturbance does not exclusively occur during the course of delirium.20 The presence of insight is not a part of the diagnostic algorithm.
A total of 17 independent variables were entered into a saturated model for backward stepwise regression (described in Statistical Analysis), including two other DSM-IV diagnoses (dementia, major depression). Neurologist-rated Hoehn & Yahr stage18 and mean akinesia-rigidity and tremor motor impairment scores were included, the latter being calculated from UPDRS-III motor examination findings as previously described.16 Age, sex, years of adult education, MMSE score, and disease duration (defined as the time between PD diagnosis and enrollment in the study) were included. LEDD contributions from levodopa-carbidopa formulations (immediate-release separate from controlled-release), dopamine agonists, monoamine-oxidase inhibitors (MAOIs), amantadine, and entacapone were included separately for analyses as continuous variables. Each was divided by 100 for regression to facilitate interpretation of odds ratios.
Statistical Analysis
Logistic regression with GEE was used to model the longitudinal association between a binary outcome (psychosis diagnosis) and independent variables of interest.15 GEE allows for correlation within subjects to be estimated, facilitating analysis of repeated-measures longitudinal data.15 Additionally, time-varying changes in the measures of interest over time are captured by GEE. An exchangeable correlation structure was employed in all GEE analyses and robust parameter estimates are reported to ensure against biased or unstable estimates. The scale parameter was fixed at the default value of 1. The saturated model (described in Measures) was subjected to backward stepwise variable removal guided by a variant of Akaike’s Information Criterion (AIC) that is compatible with quasi-likelihood estimation.21 We report odds ratios (ORs) with corresponding 95% confidence intervals and p-values for each GEE model. ORs were calculated as the exponentiated regression coefficients and confidence interval endpoints.
In our secondary analysis, significance estimates were produced using tests appropriate for paired data: the Wilcoxon signed rank test for continuous and ordinal variables and McNemar’s test for count variables. For all statistical tests reported in this manuscript, statistical significance was accepted at p < 0.05.
Standard Protocol Approvals, Registrations, and Patient Consents
The Johns Hopkins Institutional Review Board approved the study; participants—and when available, informants—provided written informed consent.
Results
Baseline characteristics for the sample are presented in Table 1. The majority of participants were male (61.8%) and the average age at enrollment (± standard deviation) was 67.9 ± 10.1 years. Mean disease duration was 7.6 ± 5.5 years, ranging from 0.7 to 31.2 years. The duration and frequency of follow-up psychiatric and neurologic evaluations are presented in Table 2. A total of 111/165 participants were evaluated longitudinally (i.e., at least once after their baseline assessment) for up to 15 years. The median total follow-up time for participants who were evaluated longitudinally was 6.1 years (interquartile range = 2.3–8.1), and the number of serial evaluations per participant ranged from 2 to 8.
Table 1.
Baseline clinical and demographic measures of participants (n=165)
| Characteristic | Mean or n (%) | Standard deviation | Range |
|---|---|---|---|
| Age at enrollment, years | 67.9 | 10.1 | 42.1–90.6 |
| Age at PD diagnosis, years | 60.3 | 10.3 | 38–86 |
| Disease duration, years | 7.6 | 5.5 | 0.7–31.2 |
| MMSE | 26.9 | 3.8 | 0–30 |
| Adult education, years | 10.2 | 4.5 | 2–27 |
| Male sex | 102 (61.8%) | — | |
| Hoehn & Yahr (%) 1/1.5/2/2.5/3/4/5 | 7 / 4 / 36 / 24 / 18 / 8 /4 | ||
Table 2.
Overview of patient participation and psychosis, depression, and dementia diagnoses throughout longitudinal follow-up evaluations (n=165)
| Variable | Statistic |
|---|---|
|
| |
| Length of follow-up, years (IQR) (range) | 6.1 (2.3–8.1) (1.4–14.8) |
|
| |
| Time between serial evaluations, years (IQR) (range) | 2.1 (1.9–2.5) (0.9–6.5) |
|
| |
| No. serial evaluations (1/2/3/4/5/6/7/8) | 54/36/23/26/11/6/7/2 |
|
| |
| Total evaluations included in analysis | 455 |
|
| |
| Psychosis | |
| - Baseline prevalence | 32 (19.4%) |
| - Incidence during follow-up | 30 |
| - Unique cases followed over multiple evaluations | 49 |
| ○ Remission without detected relapse | 11 |
| ○ Remission with later detected relapse | 2 |
| - Persistent cases | 24 |
| ○ Persistence length, years | 2.1 (1.9–4.1) (1.2–10.4) |
| - Total evaluations with psychosis present | 101 (22.2%) |
IQR = interquartile range. Length of follow-up and average time between evaluations only calculated for patients with at least two serial observations (n=111).
Baseline prevalence is the number of unique participants diagnosed with the corresponding psychiatric diagnosis at baseline; incidence is the number of unique participants who were diagnosed for the first time at a subsequent visit; remission indicates cases where a diagnosis was not made for a participant who was diagnosed at a prior evaluation; for which the number of relapsing cases is also provided. Percentage is not provided for incidence due to subject attrition (changing sample size).
At baseline, the prevalence of psychosis was 19.4% (32 participants). During longitudinal follow-up, an additional 30 participants were diagnosed with psychosis. Of these 62 unique cases, 24 were persistent (i.e., present at multiple consecutive evaluations). This persistence ranged from 1.2 to 10.4 years. We possessed data for multiple evaluations for 49 of these 62 participants; among these 49 cases, there were 13 instances of remission, suggesting an estimated remission frequency of 26.5% in our cohort. For 9 of the 13 remissions, psychosis had only been diagnosed once at the evaluation immediately prior. For the remaining 4 remission cases, psychosis had been long-standing across multiple prior evaluations spanning 1.8–6.7 years. Of the 13 remission cases, 4 were demented and 4 were using antipsychotic medications both before and after remission. Hoehn & Yahr stage significantly progressed despite remission (p=0.009) from 2.2 ± 0.3 to 3.0 ± 1.1. Notably, two of the participants in remission exhibited relapse of psychosis at a subsequent evaluation. However, for 5 of the 13 remission cases, we did not have data for an evaluation after remission due to participant attrition.
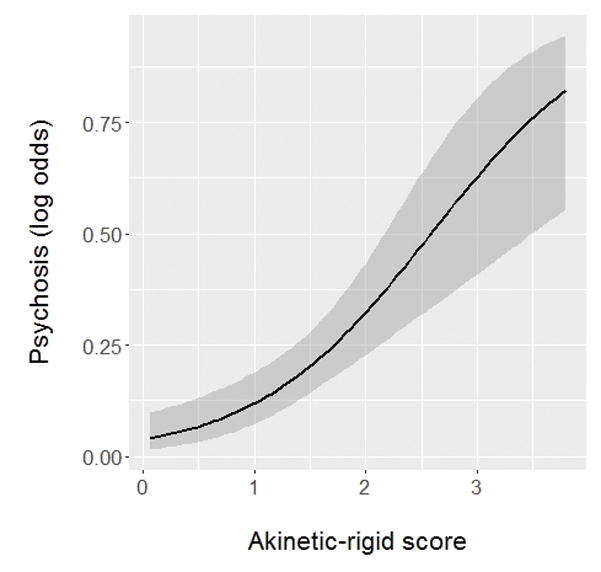
A psychosis diagnosis was made at 101 of the 455 total evaluations included in our analysis. Backward stepwise regression produced a final model that included the variables listed in Table 3. Unadjusted and adjusted OR, 95% confidence intervals, and significance measures are provided for each of these variables. Results from the multivariable model demonstrate that the presence of psychosis was associated with dementia, higher akinesia-rigidity score, higher levodopa-carbidopa dose (immediate- and controlled-release separately), and anticholinergic medication usage for overactive bladder. Notably, disease duration and depression were only significantly associated with psychosis in univariable analysis. A higher DA agonist LEDD was significantly associated with a lower likelihood of psychosis in univariable, but not multivariable regression. To visually represent the strength of the relationship between psychosis and akinesia-rigidity score, we graphed a binomial logistic regression model (Figure 1). We included only baseline evaluations (n=165) for this model, which was not produced using GEE.
Table 3.
Logistic regression with GEE for stepwise-selected variables associated with psychosis
| Independent Variable | Univariable | Multivariable | ||
|---|---|---|---|---|
| OR (95% CI), unadjusted | p | OR (95% CI), adjusted | p | |
| Dementia | 6.84 (3.86–12.11) | <0.001*** | 3.13 (1.71–5.70) | <0.001*** |
| Mean akinetic-rigid score | 3.15 (2.14–4.64) | <0.001*** | 2.38 (1.48–3.83) | <0.001*** |
| Immediate-release Levodopa-Carbidopa LEDD (mg/day) | 1.17 (1.10–1.23) | <0.001*** | 1.16 (1.08–1.26) | <0.001*** |
| Anticholinergic medication usage | 2.25 (1.20–4.24) | 0.012* | 2.83 (1.43–5.59) | 0.003** |
| Controlled-release Levodopa-Carbidopa LEDD (mg/day) | 1.02 (0.95–1.10) | 0.530 | 1.09 (1.00–1.19) | 0.040* |
| Disease duration (years) | 1.13 (1.07–1.18) | <0.001*** | 1.04 (0.99–1.10) | 0.140 |
| Dopamine agonist LEDD (mg/day) | 0.73 (0.60–0.89) | 0.002** | 0.86 (0.71–1.04) | 0.110 |
| Mean tremor score | 1.30 (0.86–1.95) | 0.210 | 0.67 (0.40–1.11) | 0.121 |
| Depression | 1.96 (1.18–3.26) | 0.009** | 1.50 (0.81–2.78) | 0.198 |
LEDD: Levodopa Equivalent Daily Dosage; MMSE: Mini-Mental State Exam. All LEDD variables were divided by 100 to facilitate OR interpretation.
p<0.05,
p<0.01,
p<0.001
Figure 1.
To further characterize correlates of psychosis onset, we conducted a secondary analysis of 30 participants with incident psychosis during follow-up (Table 4). Our criteria for inclusion in this analysis were two consecutive evaluations where a psychosis diagnosis was absent in the first but present in the second. We compared the characteristics of this sample before and after psychosis diagnosis. This comparison demonstrated an increased arithmetic difference between carbidopa/levodopa immediate-release LEDD and DA agonist LEDD (p=0.044), but a comparable arithmetic sum of these LEDD values across the consecutive evaluations, suggesting that an increase in levodopa dose compensated for the decrease in DA agonist dose. The mean AR score was significantly higher after psychosis diagnosis (p=0.007). We also tested this using the conventional motor subtype approach, which showed that a significantly higher number of participants were classified as akinetic-rigid after psychosis diagnosis (p<0.001). Additionally, MMSE score decreased (p=0.002) and a significant proportion of participants converted to dementia (p<0.001). Finally, both anticholinergic medication (p<0.001) and antipsychotic medication use (p=0.025) were increased at psychosis diagnosis. Quetiapine (9 participants), clozapine (2 participants), and olanzapine (1 participant) were the antipsychotic medications used by these participants, with daily dosage ranges of 25–200 mg, 25–37.5 mg, and 10 mg, respectively. No study participants were found to be using a typical antipsychotic.
Table 4.
Correlates of psychosis onset (n=30)
| Variable | Pre-psychosis | Diagnosis of psychosis and later | p |
|---|---|---|---|
| LEDD: (Levodopa IR) − (DA Agonist) | 299 (115–483) | 513 (372–653) | 0.044* |
| LEDD: (Levodopa IR) + (DA Agonist) | 738 (600–876) | 752 (579–926) | 0.994 |
| MMSE score | 26.9 (25.7–28.1) | 24.4 (22.2–26.6) | 0.002** |
| Mean akinetic-rigid motor score | 1.2 (0.9–1.4) | 1.6 (1.2–1.9) | 0.007** |
| Hoehn & Yahr (0/1/1.5/2/2.5/3/4/5) | 0/0/18/5/4/2/1 | 0/0/2/5/2/8/5 | <0.001*** |
| Dementia | 2 (6.7%) | +16 −0 |
<0.001*** |
| Akinetic-rigid motor subtype | 23 (76.7%) | +7 −1 |
<0.001*** |
| Depression | 14 (46.7%) | +5 −2 |
1.000 |
| Anticholinergic medication | 2 (6.7%) | +4 −1 |
<0.001*** |
| Antipsychotic medication | 3 (10%) | +11 −2 |
0.025* |
IR = Immediate Release; LEDD = Levodopa equivalent daily dose. Mean group values are provided for continuous variables with 95% confidence intervals in parentheses. “Pre-psychosis” refers to the evaluation where a diagnosis of psychotic disorder was not made, whereas “Post-psychosis” refers to the subsequent evaluation where psychosis was present. Median length of the intervals between these evaluations was 2.2 years with an interquartile range of (2.0–2.6 years). The full range was (0.9–6.5 years). For count variables at diagnosis, the number of new positive cases/medication users (+) is compared to the number of patients who discontinued the corresponding medication or who carried the corresponding diagnosis at the pre-psychosis evaluation but not at the time of psychosis (−). Significance estimates were produced using tests appropriate for paired data: the Wilcoxon signed rank test (continuous and ordinal variables) and McNemar’s test (count variables).
Discussion
In this report, we demonstrate that PD psychosis can have an unstable course characterized by remission and relapse. To our knowledge, these temporal dynamics have not been previously characterized. While a 4-year prospective study5 showed that remission occurred in 14% of patients with hallucinations at baseline, our analysis allows for a more temporally encompassing description. Additionally, the aforementioned study5 utilized a non-standard definition of psychosis based on a custom scale and did not describe the use of antipsychotic medications. In our study, 26.5% of the longitudinally monitored participants with DSM-IV-indicated psychosis exhibited remission; only two of these relapsed to psychosis again at a later visit. The true frequencies of remission and relapse could potentially be somewhat higher because our study only evaluated participants at two-year intervals, which may have missed relapsing and remitting psychotic states between visits. Importantly, our analysis is the first to utilize a diagnosis of psychotic disorder per DSM-IV criteria to examine the chronicity of PD psychosis. This remains the gold standard of psychiatric diagnostics and emphasizes the attribution of prominent hallucinations and/or delusions to PD itself as opposed to a transient or apparent medication-induced phenomenon.
It is noteworthy that the frequency of antipsychotic medication use at the time of psychosis onset was approximately 33%, which might be seen as low, although there are two important explanations. First, antipsychotic medications are relatively contraindicated in the presence of dementia, which is highly comorbid with psychosis in PD—over half of our cases with a new-onset psychosis diagnosis also carried a DSM-IV dementia diagnosis. Secondly, given the variable efficacy profile and adverse drug reactions (e.g., sedation, motor worsening, granulocytosis, and sialorrhea) carried by even newer atypical antipsychotics,22 both clinicians and patients are often hesitant to initiate antipsychotic treatment until symptoms become psychologically distressing or impair functioning. Even in the absence of comorbid dementia, antipsychotic medications may engender increased mortality risk in PD.23 The potential benefits of starting antipsychotic medication early in PD psychosis remains an area for investigation.
Psychosis is also marked by remission and relapse in other neurodegenerative disorders, such as Alzheimer’s disease (AD). Psychosis is reported to remit frequently in advanced AD, but impaired verbal communication and the consequential inability to relate psychotic phenomena complicate the assessment of psychosis in these patients.24,25 Alternatively, a certain degree of neuronal integrity may be required for psychosis in AD. A recent study of patients with Dementia with Lewy bodies (DLB) found the severity of hallucinations to be positively correlated with neuronal density in the stratum griseum superficiale of the superior colliculus.26 This finding lends credulity to the notion that structure-specific neurodegeneration can effect cessation of psychotic phenomena.27 Notably, among our participants with psychosis remission, there was still significant Hoehn & Yahr progression observed; however, potential pathologic correlates of remission remain in PD.
Levodopa-carbidopa dosage was significantly higher among participants with psychosis in our study after adjusting for other factors that might lead to increased medication dosage. Despite the common clinical intuition that high LEDD can induce psychosis, studies examining this relationship have not consistently found evidence for this hypothesis.1 Our secondary analyses among incident psychosis cases suggests that DA agonists were decreased or removed and replaced with carbidopa-levodopa, which is believed to be less hallucinogenic. However, we also noted a significant association between anticholinergic medications and psychosis in both our multivariable and secondary psychosis onset analyses. Cholinergic deficits in PD are thought to increase the likelihood of psychosis, which often dictates a change in the use of anticholinergics.8,33 The participants in our study with new-onset psychosis were taking oxybutynin (4 participants) or tolterodine (1 participant). Among all participants with psychosis, these two medications were the most common anticholinergics used. It is unclear why their use was continued in the presence of psychotic symptoms, as medication regimens were not under the control of study investigators.
Limitations of our study design include variability in the consistency of participant follow-up and attrition. Variability in the time separating serial evaluations was not dramatic, with a median of 2.1 (IQR = 1.9–2.5) years. GEE models are considered flexible enough to remain robust even when measures are not equally spaced in time.15 Additionally, GEE permits for changing outcome values to be representatively related to changes in independent variables, suggesting that the remission and relapse recorded in our study are not statistically concerning.15 Our analysis used a binary outcome to represent the presence or absence of psychosis, which may be objectionable because psychotic symptoms exist on a clinical continuum of severity in PD. The DSM-IV criteria for psychotic disorder require prominent hallucinations or delusions, which makes our findings less generalizable to patients with minor illusions/hallucinations. Importantly, these criteria do exclude cases of apparently medication-induced symptoms, such as hallucinosis that began soon after initiating a DA agonist, which may partly explain the negative association noted between DA agonist use and psychosis. This exclusion may reduce the remission rate observed, as drug-induced hallucinosis would be expected to remit after adjusting the therapeutic regimen, which was not under the control of the study investigators, but rather participants’ individual clinicians. The psychiatric diagnoses themselves were made based on the clinical discretion and expertise of psychiatrists participating in the research effort, but we lack detailed data for the basis on which those decisions were made (e.g., types of hallucinations or delusions). In other words, 27% of patients with PD psychosis not attributable to hallucinogenic medication exposure would be expected to remit, acknowledging the difficulty in determining the medication relatedness of psychosis. Another limitation of our approach is that we did not have data on the duration of dopamine replacement therapy in our patients, which could be important given the putative role of medications in precipitating psychosis. However, it seems reasonable to assume that most individuals with PD begin treatment soon after diagnosis, which we used as the starting time-point for disease duration. Finally, because psychosis is associated with advanced disease and dementia, high attrition among these cases in a longitudinal study is expected. This is demonstrated by our lack of follow-up data for several participants who exhibited remission.
Conclusion
In this report, we identified psychotic remission and relapse as important possibilities in the typical course of PD. This may have importance for our understanding of disease progression and underlying pathology. We also provide novel evidence that the inconsistently noted associations between LEDD and psychosis in PD may be due to changes in specific treatment strategy rather than changes in total DA equivalents. Finally, we show that akinesia-rigidity is a stronger independent marker of psychosis likelihood than disease duration in multivariable regression. Assuming a fundamentally dopaminergic etiology of PD psychosis, this finding is consistent with the generally accepted notion that AR patients exhibit more severe dopaminergic dysfunction and disease severity.34 Given the incredibly heterogeneous nature of PD,12 utilizing akinesia-rigidity and tremor as dimensional traits may facilitate refinements in our understanding of how they relate to associated non-motor comorbidities.
Acknowledgments
Funding sources for study: NIH/NINDS P50 NS38377 (T.M.D.); NIH/NIA K23 AG044441 (G.P.); NIH/NIGMS 5 T32 GM007309 (J.H. & D.S.); NIH/NEI P30 EY01765 (J.W.); NIH 1U54RR023561-01A1 (J.W.)
Author Roles
Research Project: A. Conception, B. Organization, C. Execution
Statistical analysis: A. Design, B. Execution, C. Review and Critique
Manuscript: A. Writing of the first draft, B. Review and Critique
J.H.: 2A, B; 3A
K.P.: 2C; 3B
C.B.: 1B, C; 3B
M.P.G.B.: 3B
K.C.: 3B
T.M.D.: 1A, B; 3B
V.J.: 1C; 3B
Z.M.: 3B
K.M.: 2C; 3B
C.M.: 3B
A.P.: 1C; 3B
O.P.: 1C
L.R.: 1A, B, C; 3B
M.S.: 3B
D.S.: 3B
J.T.:1C
J.W.: 2A, C; 3B
G.P.: 1A, B; 2C; 3B
Compliance with Journal Ethical Publication Guidelines Statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. All co-authors have been substantially involved in the study and/or the preparation of the manuscript. No undisclosed groups or persons have had a primary role in the study and/or in manuscript preparation. All co-authors have seen and approved the submitted version of the paper, accept responsibility for its content, and agree to the order of author names.
Financial Disclosures (past 12 months)
J.T.H.: Supported by the Medical Scientist Training Program at the Johns Hopkins School of Medicine, NIH/NIGMS 5 T32 GM007309
K.P. reports no disclosures.
C.B.: Supported by P50 NS38377.
M.P.G.B.: reports no disclosures.
K.C.: reports no disclosures.
T.M.D.: Dr. Dawson acknowledges the Adrienne Helis Malvin and Diana Henry Helis Medical Research Foundations and their research partnership with The Johns Hopkins Hospital, The Johns Hopkins University School of Medicine, and the Foundation’s Parkinson’s Disease Programs. He is supported NIH/NINDS P50NS038377, NIH/NINDS U01NS082133, NIH/NINDS R37NS067525, NIH/NIDA P50 DA00266, the JPB Foundation. Dr. Dawson is the Leonard and Madlyn Abramson Professor in Neurodegenerative Diseases, chair of the Dystonia Prize committee of the Bachmann Strauss Dystonia and Parkinson’s Disease Foundation and the Michael J. Fox Foundation. He is on the Board of Directors of the Bachmann Strauss Dystonia and Parkinson’s Disease Foundation and on the Scientific Advisory Board of CurePSP and a member of American Gene Technologies International Inc. advisory board. The terms of this arrangement are being managed by The Johns Hopkins University in accordance with its conflict of interest policies. Dr. Dawson is a founder of Valted, LLC and holds an ownership equity interest in the company. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies.
V.J.: Supported by P50 NS38377.
Z.M.: Dr. Mari is supported by the National Parkinson’s foundation with a Center of Excellence Grant and is supported by NIH/NINDS U01 NS082133. Dr. Mari is a founder of Neuraly, Inc. and holds ownership equity interest in the company. This arrangement has been reviewed and approved by Johns Hopkins University to be in accordance with its conflict of interest policies.
C.M.: Dr. Marvel is supported by NIH/NIDA R01 DA041264, The Margaret Q. Landenberger Research Foundation, and the Marilyn and Edward Macklin Foundation.
K.M.: Dr. Mills receives salary support through the NIH NCATS (KL2TR001077, PI Daniel Ford). He has received funding from Northwestern University.
AP: Dr. Pantelyat is supported for this project by NIH/NINDS U01 NS082133 and P50 NS38377.
O.P.: Dr. Pletnikova receives funding from NIH through NIA (U19AG033655, P50AG005146), through NINDS (P50NS38377), and from NIH (R01MH096636, R01NS086074). She receives support from a NIH sub-contract with Emory University (U01AG046161). She also receives funding through the BrightFocus Foundation.
L.R.: Dr. Rosenthal has received support from NIH/NINDS P50NS038377, Marilyn and Edward Macklin Foundation, and the Michael J. Fox Foundation. She also received an honorarium from the Edmond J. Safra Foundation and Functional Neuromodulation.
M.S.: Nothing to disclose.
D.S.: Supported by the Medical Scientist Training Program at the Johns Hopkins School of Medicine, NIH/NIGMS 5 T32 GM007309
J.T.: Dr. Troncoso receives funding from NIH through NIA (U19AG033655, P50AG005146), through NINDS (P50NS38377, R25NS079185), and from NIH (R01MH096636, R01NS086074). He receives support from a NIH sub-contract with Emory University (U01AG046161). He also receives funding through the BrightFocus Foundation. He also has NIH funding through collaborations (RO1NS086888, R01HL091541).
J.W.: Receives salary support through the Wilmer Ophthalmological Institute Core Grant (NIH/NEI P30 EY01765) and the Institute for Clinical and Translational Science Award (NIH 1U54RR023561-01A1)
GP: Dr. Pontone receives funding through the NIH/NIA as part of a K23 award (AG044441-01A1).
References
- 1.Fénelon G, Alves G. Epidemiology of psychosis in Parkinson’s disease. J Neurol Sci. 2009;289(1–2):12–7. doi: 10.1016/j.jns.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 2.Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson’s disease: a population-based, prospective study. J Am Geriatr Soc. 2000;48(8):938–42. doi: 10.1111/j.1532-5415.2000.tb06891.x. [DOI] [PubMed] [Google Scholar]
- 3.Williams DR, Lees AJ. Visual hallucinations in the diagnosis of idiopathic Parkinson’s disease: A retrospective autopsy study. Lancet Neurol. 2005;4(10):605–10. doi: 10.1016/S1474-4422(05)70146-0. [DOI] [PubMed] [Google Scholar]
- 4.Goetz CG, Fan W, Leurgans S, Bernard B, Stebbins GT. The malignant course of “benign hallucinations” in Parkinson disease. Arch Neurol. 2006;63(5):713–6. doi: 10.1001/archneur.63.5.713. [DOI] [PubMed] [Google Scholar]
- 5.Goetz CG, Leurgans S, Pappert EJ, Raman R, Stemer AB. Prospective longitudinal assessment of hallucinations in Parkinson’s disease. Neurology. 2001;57:2078–82. doi: 10.1212/wnl.57.11.2078. [DOI] [PubMed] [Google Scholar]
- 6.Holroyd S, Currie L, Wooten GF. Prospective study of hallucinations and delusions in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2001;70(6):734–8. doi: 10.1136/jnnp.70.6.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fénelon G, Goetz CG, Karenberg A. Hallucinations in Parkinson disease in the prelevodopa era. Neurology. 2006;66(1):93–8. doi: 10.1212/01.wnl.0000191325.31068.c4. [DOI] [PubMed] [Google Scholar]
- 8.Poewe W. When a Parkinson’s disease patient starts to hallucinate. Pr Neurol. 2008;8(4):238–41. doi: 10.1136/jnnp.2008.152579. [DOI] [PubMed] [Google Scholar]
- 9.Stowe R, Ives N, Ce C, et al. Dopamine agonist therapy in early Parkinson’s disease. Cochrane Database Syst Rev. 2008;(2):1–3. doi: 10.1002/14651858.CD006564.pub2. [DOI] [PubMed] [Google Scholar]
- 10.De Deurwaerdère P, Di Giovanni G, Millan MJ. Expanding the repertoire of L-DOPA’s actions: A comprehensive review of its functional neurochemistry. Prog Neurobiol. 2017;151:57–100. doi: 10.1016/j.pneurobio.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Reijnders JSAM, Ehrt U, Lousberg R, Aarsland D, Leentjens AFG. The association between motor subtypes and psychopathology in Parkinson’s disease. Park Relat Disord. 2009;15(5):379–82. doi: 10.1016/j.parkreldis.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Von Coelln R, Shulman LM. Clinical subtypes and genetic heterogeneity: of lumping and splitting in Parkinson disease. Curr Opin Neurol. 2016;29(6):727–34. doi: 10.1097/WCO.0000000000000384. [DOI] [PubMed] [Google Scholar]
- 13.Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson’s disease. Mov Disord. 2006;21(8):1123–30. doi: 10.1002/mds.20897. [DOI] [PubMed] [Google Scholar]
- 14.Kotagal V. Is PIGD a legitimate motor subtype in Parkinson disease? Ann Clin Transl Neurol. 2016;3(6):473–7. doi: 10.1002/acn3.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Locascio JJ, Atri A. An overview of longitudinal data analysis methods for neurological research. Dement Geriatr Cogn Dis Extra. 2011;1(1):330–57. doi: 10.1159/000330228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang G, Bronstein JM, Masterman DL, Redelings M, Crum J, Ritz B. Clinical characteristics in early Parkinson’s disease in a central California population-based study. Mov Disord. 2005;20(9):1133–42. doi: 10.1002/mds.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology. 1967;17:427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 19.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord. 2010;25:2649–53. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. DSM-IV. 2000.
- 21.Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57(1):120–5. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 22.Yuan M, Sperry L, Alexandra NM, et al. Atypical antipsychotic therapy in Parkinson’s disease psychosis: A retrospective study. Brain Behav. 2017;7(December 2016):e00639. doi: 10.1002/brb3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weintraub D, Chiang C, Kim HM, et al. Association of Antipsychotic Use With Mortality Risk in Patients With Parkinson Disease. JAMA Neurol. 2016;73(5):535. doi: 10.1001/jamaneurol.2016.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeste, Dilip V, Finkel SI. Psychosis of Alzheimer’s disease and related dementias. Am J Geriatr Psychiatry. 2000;8:29–34. doi: 10.1097/00019442-200002000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Jeste DV, Wragg RE, Salmon DP, Harris MJ, Thal LJ. Cognitive deficits of patients with Alzheimer’s disease with and without delusions. American J Geriatr Psychiatry. 1992;149:184–9. doi: 10.1176/ajp.149.2.184. [DOI] [PubMed] [Google Scholar]
- 26.Erskine D, Thomas AJ, Taylor J-P, et al. Neuronal Loss and A-Synuclein Pathology in the Superior Colliculus and Its Relationship to Visual Hallucinations in Dementia with Lewy Bodies. Am J Geriatr Psychiatry. 2017:1–10. doi: 10.1016/j.jagp.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Hinkle J, Pontone GM. Visual Hallucinations in Neurodegenerative Diseases: Focus on Selective Tissue Vulnerability. Am J Geriatr Psychiatry. 2017:2–6. doi: 10.1016/j.jagp.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Isaias IU, Marzegan A, Pezzoli G, et al. A role for locus coeruleus in Parkinson tremor. Front Hum Neurosci. 2011;5(January):179. doi: 10.3389/fnhum.2011.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doder M, Rabiner EA, Turjanski N, Lees AJ, Brooks DJ. Tremor in Parkinson’s disease and serotonergic dysfunction. Neurology. 2003;60(4):601–5. doi: 10.1212/01.wnl.0000031424.51127.2b. [DOI] [PubMed] [Google Scholar]
- 30.Asenbaum S, Pirker W, Angelberger P, Bencsits G, Pruckmayer M, Brücke T. [123I]β-CIT and SPECT in essential tremor and Parkinson’s disease. J Neural Transm. 1998;105(10–12):1213–28. doi: 10.1007/s007020050124. [DOI] [PubMed] [Google Scholar]
- 31.Benamer HTS, Patterson J, Wyper DJ, Hadley DM, Macphee GJA, Grosset DG. Correlation of Parkinson’s disease severity and duration with 123I-FP-CIT SPECT striatal uptake. Mov Disord. 2000;15(4):692–8. doi: 10.1002/1531-8257(200007)15:4<692::aid-mds1014>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 32.Koller W, Hubble J. Levodopa therapy in Parkinson’s disease. Neurology. 1990;40(10 Suppl 3):suppl 40–7. discussion 47–9. [PubMed] [Google Scholar]
- 33.Hoeh N, Gyulai L, Weintraub D, Streim J. Pharmacologic management of psychosis in the elderly: a critical review. J Geriatr Psychiatry Neurol. 2003;16(4):213–8. doi: 10.1177/0891988703258663. [DOI] [PubMed] [Google Scholar]
- 34.Rajput A, Voll A, Rajput M, Robinson C, Rajput A. Course in Parkinson disease subtypes: A 39-year clinicopathologic study. Neurology. 2009;73(3):206–12. doi: 10.1212/WNL.0b013e3181ae7af1. [DOI] [PubMed] [Google Scholar]


