Abstract
The cytokines of the IL-17 family play a central role in the control of infections, especially extracellular fungi. Conversely, if unrestrained, these inflammatory cytokines contribute to the pathology in numerous autoimmune and chronic inflammatory conditions. Recent advances have led to the approval of IL-17A-blocking biologics for the treatment of moderate to severe plaque psoriasis, but much remains to be understood about the biological functions, regulation and signaling pathways downstream of these factors. In this review, we outline the current knowledge on signal transduction and known physiological activities of IL-17 family cytokines. We will highlight in particular the current understanding of these cytokines in the context of skin manifestations of disease.
1. INTRODUCTION TO IL-17 LIGANDS AND RECEPTORS
IL-17A, the founding and most studied member of the IL-17 family, was cloned in 1993 and initially named CTLA-8. Its sequence and predicted structure were markedly different from other known cytokines, but interestingly was homologous to an open reading frame (ORF) in the T cell tropic Herpesvirus saimiri virus [1]. A decade later IL-17A took central stage with the discovery of Th17 cells as a T helper (Th) subset distinct from Th1 and Th2 cells [2, 3]. Five additional family members have been described, designated IL-17B, C, D, E, and F. Of these, IL-17F shares the greatest degree of conservation to IL-17A (55%) and is commonly produced by the same cell types. IL-17F was the first member of this family for which a crystallographic structure was elucidated. Interestingly, structural analysis revealed the formation of a cysteine-knot fold, similar to that adopted by neurotrophins such as NGF [4]. IL-17E, also known as IL-25, displays the lowest degree of sequence conservation (16%) [5]. In turn, other family members derive from different cellular sources and are associated with varying functions. IL-17A, IL-17F, IL-17C and IL-17E function in host defense against pathogens and play various but not fully understood roles in mediating inflammation in autoimmune, allergic and chronic inflammatory conditions. Given the central role of IL-17A in autoimmunity, much effort has focused on the development of neutralizing antibodies for therapeutic use. Indeed, IL-17A-blocking antibodies secukinumab and ixekizumab recently received FDA-approval for the treatment of psoriasis, ankylosing sponylitis (AS) and psoriatic arthritis (PsA) [6, 7]. Nonetheless, many aspects of IL-17A function, and especially of other cytokines in this family, remain poorly defined.
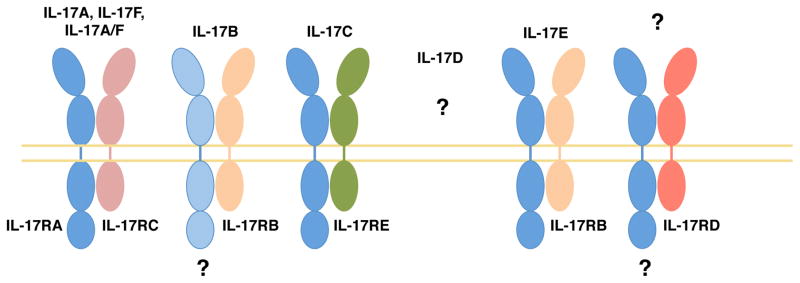
All known IL-17 family cytokines signal via a receptor family that is distinct from other known cytokine receptors [8]. The IL-17R family contains five members, IL-17RA-E, all of which are single-pass transmembrane receptors with conserved structural features [9]. Specifically, all family members encode two extracellular fibronectin II-like domains and an intracellular “SEFIR” domain; the name alludes to the presence of this domain in SEF/IL-17RD and other IL-17 Receptor proteins. The SEFIR is structurally related to the TIR domain found in the TLR/IL-1R family and is crucial for triggering downstream signaling events (see also section 3, “IL-17 CYTOKINE SIGNALING AND REGULATION”) [10]. The prevailing paradigm for most IL-17 cytokines is that signaling occurs through heterodimeric receptors composed of a common IL-17RA chain and a second chain that determines ligand or signaling specificity. The second receptor chains are as follows: IL-17RC for IL-17A and IL-17F [11], IL-17RB for IL-17E [12], and IL-17RE for IL-17C [13] (Figure 1). IL-17B is also reported to bind IL-17RB, albeit less strongly than IL-17E [14]. In addition, the requirement for IL-17RA in IL-17B signaling is still under debate, and the receptor for IL-17D remains undefined. Here, we review the current understanding of cellular sources of the IL-17 family of cytokines, signal transduction mechanisms that govern their function, and the cutaneous biological processes in which these cytokines participate.
Figure 1. IL-17 family cytokines and their receptors.
Most IL-17 family cytokines signal via a heterodimeric receptor composed of IL-17RA and a second chain that varies depending on ligand, as indicated. Despite advances in the characterization of receptor-ligand interactions, several questions remain. Namely, a role for IL-17RA in IL-17B signaling has not been fully demonstrated. In addition, the receptor for IL-17D, as well as the ligand for IL-17RD remain unknown.
2. CELLULAR SOURCES OF IL-17 FAMILY CYTOKINES
IL-17A and IL-17F
More than 30 years ago, the paradigm of T helper (Th) differentiation postulated that two discrete Th populations, Th1 and Th2 cells, acquired the ability to produce canonical thereby “tuned” to contain biologically dissimilar pathogens [15]. Although a useful model, there were numerous discrepancies that called this view into question [16]. Indeed, in 2005 a third Th cell subset was described that producing IL-17A, IL-17F, as well as IL-21, IL-22 and GM-CSF [3, 17–19], and hence came to be known as ‘Th17’. Like other Th subsets, naïve CD4+ T cells become committed to the Th17 lineage via cytokine cues received during antigen presentation in secondary lymphoid organs. For Th17 cells, this is a combination of IL-1b, IL-6, TGF-b and IL-21 for initial commitment [20–23], and IL-23 for full acquisition of their pathogenic capacity [24–26]. Like Th1 and Th2 cells, Th17 cells express a lineage-determining ‘master’ transcription factor, Rorgt, which directs the production of their hallmark cytokines [27].
More recently, it has become clear that additional populations of cells are also important sources of IL-17A and IL-17F. . These include CD8+ Tc cells [28, 29] and innate tissue-resident cells that are rapidly activated upon injury or pathogenic insult. Among these subsets are gd T cells (including Vg4+ and Vg6+ cells [30]) innate lymphoid cells (ILCs, specifically the ILC3 subset) [31], “natural” Th17 cells [32] and NKT cells [33]. These cells share a common dependence on IL-23 and on the transcription factor Rorgt for IL-17 production, and express the chemokine receptor CCR6 [30]. In addition, given their positioning at barrier sites and their fast responsiveness, these innate-like cells constitute important early sources of IL-17 during infection and tissue damage. Recent reports have also proposed the expression of IL-17A by myeloid cells, including macrophages, neutrophils and mast cells [30, 34, 35]. However, these findings remain controversial, especially given the low levels of IL-17 detected in these cells and their propensity for phagocytosis. Indeed, a recent article demonstrated that mast cells can take up IL-17A from the extracellular environment via receptor-mediated endocytosis and subsequently release it to promote inflammation [36]. Similarly, neutrophils and mast cells have been shown to release IL-17 via extracellular traps [37].
IL-17E (IL-25)
IL-17E, also known as IL-25, was discovered through a bioinformatics search for proteins homologous to IL-17A [38]. At the protein level, IL-17E bears 16–20% sequence similarity to IL-17A, B and C. IL-17E derives from both hematopoietic and non-hematopoietic cells [38]. In mice, IL-17E is expressed by innate immune cells such as mast cells and alveolar macrophages in response to allergic stimuli [39]. This also seems to be true in humans, as blood eosinophils and basophils from normal and allergic subjects expressed IL-17E mRNA, which was further boosted upon IL-5 treatment [40]. In addition, tissue stromal cells can express IL-17E. Human lung epithelial cells and murine primary type II alveolar epithelial cells express IL-17E following challenge with Aspergillus oryzae, ragweed allergens, and allergen proteases [41, 42]. Concordantly, IL-17E was detected at higher levels via immunohistochemistry (IHC) in the bronchial mucosa of asthmatics [43]. The triggers for IL-17E production in many of these cells remains an active area of investigation.
IL-17E is a pleiotropic cytokine, acting on stromal, innate immune and adaptive immune cells to orchestrate Th2-type inflammation. Consistent with the association of dysregulated Th2 responses with the development of allergy, IL-17E production is linked to the severity of chronic allergic conditions [44]. Thus, IL-17E-induced inflammation can be distinguished from IL-17A- and IL-17F-induced inflammation through the nature of the immune infiltrate, which mostly consists of eosinophils for the former and neutrophils for the latter [39]. However, IL-17E expression can be advantageous in some situations, as IL-17E can inhibit Th17 development through the induction of IL-13 by DCs and by inhibiting macrophage-derived IL-23 production [45]. In addition, IL-17E delivery ameliorates autoimmune diabetes [46, 47]. IL-17E therefore seems to be an atypical IL-17 family member, both in terms of low sequence homology and different biological actions.
IL-17C
IL-17C was also identified during the search for IL-17A-related cytokines [48]. IL-17C is mainly expressed by epithelial cells following stimulation with TLR2 and TLR5 ligands or with the proinflammatory cytokines IL-1β and TNF-a [13]. Its expression has also been reported to be induced in CD4+ T cells, dendritic cells and macrophages in inflamed tissues [48, 49]. IL-17C has been suggested to act via a heterodimeric receptor composed of IL-17RA and IL-17RE, mediating a seemingly overlapping function to that of IL-17A and IL-17F [13]. Indeed, intranasal delivery of IL-17C-expressing adenovirus triggers neutrophilia and drives the expression of set of proinflammatory molecules that overlaps considerably with IL-17A-dependent target genes [50]. Its role in mediating inflammation in several inflammatory and infection settings is just beginning to be unraveled.
IL-17B and IL-17D
IL-17B and D were also found through a search for IL-17A homologs [48, 51]. IL-17B is expressed at the transcriptional level in many cell types, including chondrocytes, neurons, intestinal epithelial cells and breast cancer cells. Like IL-17E, IL-17B can bind to IL-17RB, albeit with a lower affinity [52]. However, its function in the context of these cells is still enigmatic. IL-17D mRNA is detected in various tissues, including brain, heart, lung, pancreas, skeletal muscle and adipose tissue [51]. In the immune system, expression seems to be restricted to naïve CD4+ T cells and B cells. IL-17D most closely resembles IL-17B, with which it shares 27% homology. Its C-terminal motif is absent in other IL-17 family members [51]. To date, its receptor remains unknown.
3. IL-17 CYTOKINE SIGNALING AND REGULATION
Most IL-17 family members characterized to date mediate signaling through heterodimeric receptors composed of IL-17RA and a subunit that confers ligand or signaling specificity. IL-17RA is widely expressed among cells of both hematopoietic and non-hematopoietic compartments [8, 53]. Other IL-17R family receptors generally exhibit expression more restricted to specific cell types, which helps explain the target cell specificity of different ligands. This situation is analogous to signaling by IL-6 or βc family cytokines, which use the common gp130 subunit or the common β subunit for signaling [54, 55]. The existence of conserved mechanisms of receptor binding in the IL-17 family is reinforced by crystallographic analyses of IL-17RA in complex with IL-17A and IL-17F. These analyses revealed the acquisition of a similar conformation by the receptor upon cytokine binding, and the requirement for the same amino acid residues for receptor-ligand interactions [56]. Stoichiometry of the receptor complex seems to be dimeric. The lack of further receptor chains may be explained by the induction of conformational changes in the receptors upon cytokine binding, which disfavor binding to a second homotypic receptor chain [56].
Signaling pathways downstream of IL-17 cytokine family members are beginning to be unraveled, with IL-17A-targeted signaling mechanisms having been most thoroughly studied. In this section, we will focus on current knowledge regarding the molecular actions downstream of IL-17A, and point out commonalities, divergences and gaps in our understanding of IL-17 family cytokines.
IL-17A, IL-17F and IL-17A/F
IL-17A and IL-17F signal through the IL-17RA/RC heterodimer, evidenced by a complete loss of responsiveness in Il17ra−/− and Il17rc−/− mice or cell lines derived from them [57]. Importantly, this receptor can bind to three different covalent cytokine dimers: IL-17A homodimers, IL-17F homodimers or IL-17A/F heterodimers, albeit with varying affinities [58]. IL-17RA has a 100-fold weaker affinity for IL-17F and an intermediate affinity for the IL-17A/F heterodimer and bears weaker affinity for IL-17B, C, D and E. Conversely, IL-17RC has a higher affinity for IL-17F than for IL-17A [59]. Overall, IL-17A signaling induces stronger responses than IL-17F (10–30 times more potent, as assessed by downstream gene induction), which may explain its dominant role in driving autoimmunity [60]. Receptor expression patterns also differ between the two chains, with IL-17RA being expressed more highly in the immune compartment, and IL-17RC expression being largely restricted to non-immune cells [53, 59]. Whether varying expression patterns, coupled with the different affinity of each receptor chain for IL-17A or IL-17F underlies their diverging biological functions remains an open question.
Detailed sequence analysis of IL-17R family members revealed the presence of a conserved intracellular subdomain with homology to Toll-IL-1R (TIR) domains, which are essential for signaling downstream of the IL-1 receptor and TLRs. These motifs share sequence homology with boxes 1 and 2 of the TIR domain, but lack box 3. Interestingly, this motif was discovered in SEF proteins (an IL-17RD orthologue called “similar expression to fibroblast growth factor”) from zebrafish and chicken and hence became known as the SEF/IL-17R domain (SEFIR) [10]. Upon cytokine ligation, the IL-17 receptor complex is thought to undergo a conformational change enabling the establishment of homotypic interactions between the SEFIR domains of the receptor and the signaling adaptor Act1 [61]. Act1, also known as CIKS (connection to IkB kinase and stress-activated protein kinases), is an adapter required for all known downstream IL-17A signaling pathways. The canonical pathway relies on the E3 ligase activity of Act1, which mediates Lys63-linked ubiquitylation of TRAF6 [62]. This event leads to activation of the canonical NF-kB and MAPK pathways, which include ERK, p38 and JNK, as well as the CCAAT-enhancer-binding proteins (C/EBP) pathway [8, 63]. Together, these transcription factors drive transcriptional activation of IL-17A target genes, which play key roles in inflammation.
In contrast, a second, non-canonical pathway is elicited by IL-17A, which leads to the stabilization of mRNA transcripts, particularly those encoding for intrinsically unstable targets such as cytokines and chemokines. This mRNA stabilization pathway is dependent on IkB kinase (IKKi) and TBK1-mediated phosphorylation of Act1 at residue 311 [64, 65]. TRAF2 and TRAF5 are thereby recruited to the receptor complex, which results in the recruitment of molecules that control mRNA turnover [66]. In particular, TRAF2 and TRAF5 can sequester the RNA destabilizing factor ASF/SF2 and recruit the mRNA stabilizing factor HuR, thereby enhancing the half-life of various mRNAs [66, 67]. In addition, Act1 is reported to interact with Hsp90 to activate IL-17 activity [68]. A psoriasis-associated genetic variant in Act1 carrying the D10N mutation abrogates this interaction [69–71]. Together, IL-17-mediated events at both the transcriptional and post-transcriptional levels enhance production of genes that underlie its functions, including cytokines and chemokines, antimicrobial peptides (AMPs), acute phase proteins and other inflammatory effectors [72]. IL-17RA/RC signaling is summarized in Figure 2.
Figure 2. IL-17RA/RC signaling pathways.
IL-17A/IL-17F/IL-17A/F binding to the receptor complex enables homotypic interactions between the SEFIR domains in the receptor and in the adapter Act1/CIKS. The canonical IL-17 signaling pathway initiates signaling through Act1- induced K63-linked ubiquitylation of TRAF6, thereby activating the MAPK, C/EBPβ and NF-κB pathways. This triggers transcriptional activation of downstream target genes, including pro-inflammatory cytokines, chemokines and antimicrobial peptides. In turn, non-canonical signaling relies on Act1 phosphorylation at amino acid 311. This recruits TRAF2 and TRAF5, which sequesters the mRNA destabilizing factor ASF/SF2 and recruits the mRNA stabilizing factor HuR. Together, these two pathways mediate the pro-inflammatory functions of IL-17A, IL-17F and IL-17A/F.
IL-17E (IL-25)
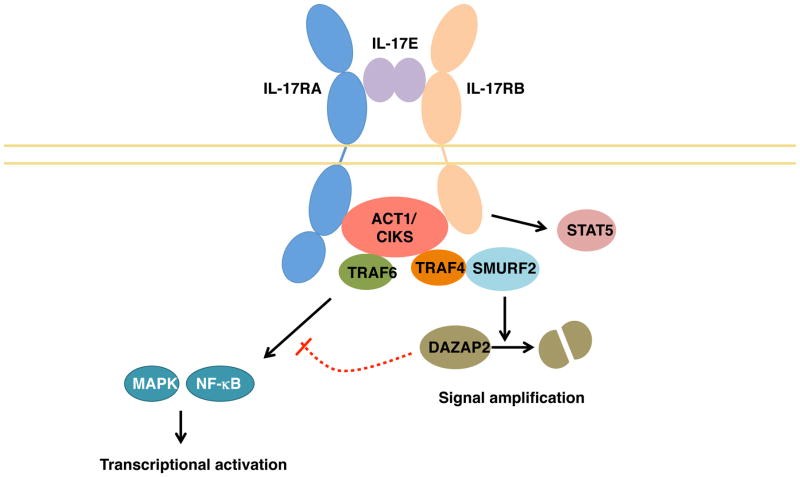
IL-17E signals through a heterodimer of IL-17RA and IL-17RB [12], as summarized in Figure 3. Unlike the relatively stromal-restricted activity of IL-17A and IL-17F, IL-17E acts mainly on immune cells, including Th2, Th9 and NKT cells. IL-17E induces the production of classical Type-2 cytokines, such as IL-4, IL-5, IL-9 and IL-13, in a GATA-3, c-MAF, and JunB-dependent fashion [40]. IL-17RB is also expressed on monocytes, certain populations of type 2 innate lymphocytes such as nuocytes, non-T/non-B cells, multipotent progenitor type 2 cells and innate type 2 helper cells [73–76]. In addition, stromal cells such as intestinal and pulmonary epithelial cells also respond to IL-17E. Similar to IL-17A/F signaling, IL-17RB interacts with Act1 via homotypic SEFIR interactions [77, 78]. Act1 recruits TRAF6, enabling NF-kB activation [79]. However, the pathways diverge, in that IL-17RB can recruit TRAF4 via Act1, leading to the further recruitment of the E3 ligase SMURF2 [80]. This leads to the ubiquitylation and subsequent degradation of the IL-17RB inhibitor DAZAP2, consequently reinforcing IL-17E-mediated signaling [80]. Further, IL-17E is reported to activate STAT5 in an Act1-independent manner, which further potentiates a Th2 response [81]. The precise stoichiometry of the receptor required for signaling via IL-17E is currently unclear, as there are reports of IL-17E being unable to bind IL-17RA in vitro [4]. Whether the nature of the receptor varies depending on the cell type is another area of inquiry.
Figure 3. IL-17RA/RB signaling.
Upon IL-17E binding to its receptor, homotypic interactions between the SEFIR domains in the receptor and in the adapter Act1/CIKS are established. This leads to the recruitment of TRAF6, activating the MAPK and NF-κB signaling pathways. In turn, Act1 can recruit TRAF4, which activates the E3 ligase SMURF2. This leads to the ubiquitylation and subsequent degradation of the inhibitor DAZAP2, amplifying IL-17E-mediated signaling. In addition, IL-17RB can elicit STAT5 activation in an Act1-independent manner.
IL-17B, C and D
Our current understanding of signaling downstream of IL-17 family members other than IL-17A, F and E remains very limited. IL-17B has been shown to induce proinflammatory cytokine secretion by the THP-1 acute monocytic leukemic cell line, and to enhance inflammation, survival and metastasis in breast and pancreatic cancer [82, 83]. IL-17RB engagement in these cells recruited the Act1-TRAF6-TAK1 complex to the receptor [83]. Interestingly, IL-17B and IL-17E seem to present antagonic activities, despite reportedly binding to the same receptor [84]. IL-17C has been reported to signal via a heterodimeric IL-17RA/RE complex [13]. Expression of IL-17RE is restricted to epithelial cells, specialized epithelial cells like keratinocytes and Th17 cells (reviewed in [85]). In line with other proinflammatory cytokines in the family, IL-17C signaling activates the NF-kB and MAPK pathways [86]. Similar to other cytokines in the family, IL-17C signaling also seems to be dependent on Act1, and Song et al have recently reported their unpublished findings that IL-17RE associates with Act1 [85]. As noted above, the action of IL-17D, its receptor and the signaling mechanisms it elicits, remain entirely obscure.
Regulation of IL-17 family cytokines
Given its central role in inflammation, numerous mechanisms have evolved to restrict the IL-17A signaling pathway, presumably to curtail bystander inflammation. For example, TRAF3 and TRAF4 interfere with early events in IL-17A signaling by competing with Act1 or TRAF6 for IL-17RA binding [87, 88]. The deubiquitinase A20 is induced downstream of IL-17A and dampens the activation of NF-κB and MAPK pathways by removal of K63-linked ubiquitin chains on TRAF6 [89]. Thus, A20 serves as a feedback regulator of the IL-17 pathway, analogous to its effect for TNFα and IL-1 signaling as well [90–92]. Similarly, the deubiquitinase USP25 acts on TRAF5 and TRAF6, suppressing IL-17A signaling [93]. GSK-3β-mediated phosphorylation of the transcription factor C/EBPβ inhibits IL-17 target gene expression [94]. GWAS analysis of psoriasis has revealed genetic associations with known regulators of immune signaling, including TNFAIP3 (A20), TNIP1 (ABIN-1, NAF1) and NFKBIA (IκBα) [95]. Importantly, ABIN-1 was recently shown to regulate IL-17A signaling in keratinocytes. Correspondingly, Tnip1-deficient mice develop cutaneous inflammation with psoriasiform characteristics, linking findings in this mouse model to the enhanced susceptibility to psoriasis of individuals with TNIP1 SNPs [96]. Finally, the endoribonuclease MCPIP1 (also known as Regnase-1, encoded by ZC3H12A) limits IL-17 signaling through the degradation of IL-17-driven genes, including Il6, Nfkbiz and Il17ra and Il17rc [97–99]. To date, 11 non-synonymous SNPs have been described for the ZC3H12A gene, but so far none are associated with human disease [100].
With an emerging role in inflammatory diseases [93], IL-17C may similarly be subject to robust control mechanisms, although little is currently known about this issue. We recently demonstrated that the action of MCPIP1 can also curb IL-17C-mediated inflammation in murine keratinocytes both in vitro and in vivo, thereby limiting skin inflammation in the imiquimod-driven psoriasis model [101]. Given the high degree of conservation across signaling pathways in the IL-17 family of cytokines, it is tempting to speculate that other members of the IL-17 will share common regulatory mechanisms.
4. IL-17 family cytokines in host protection and inflammation
IL-17A, F and A/F in infection
IL-17A and IL-17F evolved to protect from infection, and it is now clear that they orchestrate protective responses against infections at mucosal and epithelial surfaces, demonstrated in the intestine, skin, lung, and oral cavity. Their central role in mediating protective immunity relies on the induction of molecules that stimulate epithelial barrier function. Signaling downstream of IL-17RA/RC elicits the expression of antimicrobial peptides (AMPs), including b-defensins, S100 proteins and Lipocalin-2 (Lcn2, also known as NGAL) [102]. Lcn2 competes with bacterial siderophores for acquisition of free iron and thus limits bacterial growth [103]. In addition, IL-17A and IL-17F induce a proinflammatory milieu with enhanced cytokine and chemokine, and matrix metalloproteinase (MMP) production. These factors mediate the activation and recruitment of immune cells to the site of infection, promoting a potent immune response to the invading pathogen. One of the hallmarks of IL-17A-driven inflammation is neutrophil accumulation. Indeed, induction of G-CSF production regulates neutrophil production, while chemokines such as CXCL1, CXCL5 and CCL2 stimulate neutrophil chemotaxis [104]. In addition, IL-17 induces CCL20, which recruits CCR6-expressing cells such as Th17 and ILC3s [105]. In this manner, IL-17A and to a lesser extent IL-17F regulate the coordinated action of stromal, innate and adaptive immune cells.
The central role of IL-17A and IL-17F in protective immunity against infections is highlighted by the increased susceptibility of IL-17A or IL-17F-deficient mice to pathogens. For example, IL-17A−/− mice are unable to control lung infection with Klebsiella pneumoniae [106]. In addition, IL-17A stimulates macrophage-derived IL-12, which is required to promote protective Th1 responses against pulmonary infection with Francisella tularensis live vaccine strain [107]. Furthermore, IL-17 levels are elevated during acute lung infection with Pseudomonas aeruginosa, which contributes to neutrophil recruitment and bacterial containment [108, 109]. Similarly, a deficiency in IL-17 or the IL-17-promoting cytokine IL-23 renders mice more susceptible to Citrobacter rodentium intestinal infection [23], as well as to a number of other bacterial pathogens (reviewed in [110, 111]).
Candida albicans is a commensal fungal organism in about 70% of healthy individuals, residing in the skin, mouth, gastrointestinal tract and vagina without causing disease. However, following loss of immune control mechanisms, C. albicans can become an opportunistic pathogen. Chronic mucocutaneous candidiasis (CMC) can ensue in individuals with primary and acquired immunodeficiencies, leading to oropharyngeal candidiasis (OPC or thrush), or to cutaneous lesions. Importantly, defects in the IL-17/IL-23 axis render the host exquisitely susceptible to CMC, highlighting the importance of this pathway in controlling C. albicans infections. Genetic variants in IL-12Rb1 and STAT3, which compromise IL-23 signaling, have also been associated with diminished Th17 responses in humans and accordingly to CMC [112].
IL-17A is dominant in the requirement for control of C. albicans OPC infection in mice, although IL-17F and IL-17AF may also contribute to protection [113]. Notably, a mutation in the IL17F gene was recently reported in a family with CMC [114]. This point mutation at position 65 in the polypeptide chain leads to the production of a dominant negative variant, which abrogates IL-17F homodimer and IL-17A/IL-17F heterodimer signaling. Likewise, mutations in IL-17RA, IL-17RC and Act1 lead to CMC in humans. Mutations in the AIRE gene (autoimmune regulator) lead to the development of the multiorgan autoimmune disease APECED (autoimmune polyendocrinopathy candidiasis ectodermal dystrophy). One of the main manifestations of APECED is an enhanced susceptibility to CMC [112]. Interestingly, compromised negative selection in the thymus due to AIRE deficiency leads to the development of neutralizing autoantibodies against IL-17A, IL-17F and IL-22 [115]. Importantly, the dependence of the host on IL-17 for containment of Candida infections is dependent on colonization route and tissue. For instance, vulvovaginal candidiasis is associated with alterations in other host factors, such as pH and microbial flora composition. In turn, control of systemic candidiasis seems to be more reliant on Th1 and NK cell responses [116].
Staphylococcus aureus dermatitis has been reported in patients with ACT1 or IL17RA null variants [114, 117]. In line with a role for IL-17 in S. aureus control, Il17ra-deficient mice exhibit an increased susceptibility to cutaneous S. aureus infection [118, 119]. Given the emergence in recent years of methicillin-resistant S. aureus (MRSA) strains, harnessing the IL-17 axis in vaccination strategies may be of prophylactic promise.
IL-17A, F and A/F in chronic skin inflammatory and autoimmune diseases
Upregulation of inflammatory and tissue-remodeling molecules can lead to tissue damage if IL-17 activity is left uncontrolled. Indeed, IL-17A and related cytokines are upregulated in numerous autoimmune conditions, including psoriasis, rheumatoid arthritis, multiple sclerosis, scleroderma and lupus, among others. Similarly, GWAS studies have associated SNPs in genes of the IL-17 pathway with autoimmunity. A number of reviews have recently addressed the role of IL-17 in other autoimmune conditions [85, 120]. We will focus here on the role of IL-17A and IL-17F in driving cutaneous inflammation.
Psoriasis is a chronic inflammatory skin condition characterized by epidermal hyperplasia, affecting 2–3% of the world’s population. One of the hallmarks of disease is neutrophilic infiltration and formation of neutrophil microabscesses [121]. Elevated IL-17A and Th17-related cytokines such as IL-22 and IL-23 are found in human psoriasis skin lesions [93, 122, 123]. In addition, IL-17A can directly act on human keratinocytes stimulated to upregulate AMPs and neutrophil-attracting chemokines [19, 124]. Consistently, GWAS studies have identified psoriasis-associated variants in genes participating in Th17 differentiation and IL-17A signaling, such as IL23R and TRAF3IP2 (encoding Act1) [70, 71, 125–127]. Mouse preclinical models of psoriasis have confirmed a role for IL-17 family cytokines in mediating disease. In the imiquimod-driven dermatitis model (driven by a TLR7 agonist), IL-17RA-deficient mice show dramatically diminished skin involvement [128]. Interestingly, IL-17 signaling plays a dual role during imiquimod-driven psoriasis depending on cell type. Mice deficient in IL-17 signaling in keratinocytes present dampened keratinocyte proliferation and neutrophilic microabscess formation. In turn, Act1 deficiency in skin fibroblasts limits the recruitment of IL-17-producing cells, thereby controlling the amplification of skin inflammation [129]. Interestingly, intradermal injection of IL-22 or IL-23 into mouse ear also elicits the development of psoriasis-like disease, indicating that other cytokines in the IL-23/IL-17 axis can initiate disease [85]. The importance of IL-17A-mediated inflammation in psoriasis has been more recently highlighted by the clinical success of biologic drugs, including IL-17A-blocking antibodies secukinumab and ixekizumab and the IL-17RA-targeting antibody brodalumab [7, 130–133].
Atopic dermatitis affects 10–20% of children and 1–3% of adults in the Western world [134], and is characterized by chronic skin inflammation due to exacerbated responses to environmental antigens. The IL-17 axis has been reported to participate in allergic skin reactions, including atopic dermatitis and contact dermatitis. Serum levels of IL-17A and F are increased in children with atopic dermatitis and positively correlated with disease severity [135]. Expression of IL-17A at the mRNA level is increased in the skin of patients with nickel allergy [136]. In addition, increased Th17 cell infiltration was detected in a mouse model of contact dermatitis, and IL-17A-deficient mice displayed reduced pathology [137]. Whether the enhancement of IL-17 responses is a driver of pathology or reflects the immune efforts to limit colonization of skin lesions by bacteria remains an open question.
Strikingly, IL-17 signaling has been associated with the promotion of skin cancer development during chemical carcinogenesis in mouse models. Indeed, IL-17 or IL-17RA-deficient mice show considerably diminished incidence of DMBA/TPA-induced skin tumors [138, 139]. This pro-tumorigenic effect of IL-17 is thought to occur via the promotion of epithelial proliferation and the anti-apoptotic effect of STAT-3, which may be downstream of IL-17-indcued genes such as IL-6. Importantly, IL-17RA blocking in mice with established tumors blocked further tumor progression [139]. Thus, IL-17A blockade may be useful for controlling at least some cancers.
IL-17B
IL-17B is expressed by neutrophils in the synovial tissue of RA patients [140]. Treatment of human fibroblasts with IL-17B synergized with TNF-a to induce G-CSF and IL-6 [140]. Intriguingly, IL-17B is expressed in limb buds during mouse embryonic development, suggesting a role in chondrogenesis and osteogenesis that may be dysregulated in autoimmune processes affecting the joints [141]. IL-17B, like IL-17E (IL-25), has been shown to bind to IL-17RB. IL-17B can oppose IL-25-driven inflammation, and has been shown to play an antagonistic role. In a DSS-driven colitis mouse model, IL-25 administration exacerbated colonic damage [142]. In contrast, in a second report, IL-25-deficient mice exhibited reduced weight loss, inflammation and tissue damage [84], which may result from discrepancies in microbiome composition or animal housing between the two studies. Interestingly, IL-17B-deficient mice developed increased susceptibility to DSS colitis, with enhanced weight loss, proinflammatory cytokine production and colonic tissue destruction [84]. Similarly, IL-17B and IL-25 play opposing roles in the context of Citrobacter rodentium infection and OVA-induced lung inflammation [84]. In vitro, co-treatment of primary colonic epithelial cells with IL-17B diminished IL-25-, but not IL-17A-driven IL-6 production. IL-17B remains the most obscure of all IL-17 family members, with a role in skin immunity and pathology yet to be ascribed. The described antagonism to IL-25 function is interesting in this context, particularly given the association between IL-25 expression and skin atopy. The potential role of IL-17B in skin immunity and pathology should therefore be explored.
IL-17C
As noted above, IL-17C induces a similar pattern of gene expression to IL-17A, which poses the question of functional redundancy. However, despite the overlap in target gene induction, IL-17C-deficient mice do not exhibit a compromised ability to control oral, dermal or disseminated candidiasis, in contrast to IL-17RA deficient mice [143]. In addition, the IL-17RA-dependent gene signature associated with immunity against C. albicans was unchanged in IL-17C-deficient mice. Concordantly, IL-17RE deficiency did not lead to enhanced susceptibility to candidiasis. Interestingly, IL-17C can be induced in keratinocytes infected with S. aureus via a NOD2-dependent mechanism. Using this in vitro system, suppression of IL-17C expression rendered keratinocytes slightly more permissive to S. aureus survival [144]. Thus, IL-17C may contribute to the control of infections, potentially through the activation of common mechanisms with IL-17A and F.
A common feature for IL-17 family cytokines, or indeed, all inflammatory stimuli, is their propensity to promote protective immunity while simultaneously exacerbating tissue damage. IL-17C is the most highly expressed IL-17 family member in psoriatic lesions [93, 122, 123], and drives the expression of AMPs, proinflammatory cytokines and neutrophil-attracting chemokines in keratinocytes [19, 124]. Consistent with a role in mediating cutaneous pathology, intradermal delivery of recombinant IL-17C into mouse ears led to epidermal thickening and neutrophil recruitment, whereas IL-17C-deficient mice developed less skin inflammation upon imiquimod treatment [13]. In line with these findings, keratinocyte-specific IL-17C transgenic mice develop spontaneous psoriasiform dermatitis with epidermal hyperplasia, increased leukocytosis and overexpression of proinflammatory cytokines [93]. In concordance with manifestations in human psoriasis, these mice display an enhanced proclivity toward thrombotic arterial occlusion, indicating the potential systemic effects of a skin inflammatory process [145]. Therefore, IL-17C is clearly a driver of psoriasis that could be a safer target for blockade since its role in immunity to infection, at least in mice, appears to be less central [143].
IL-17D
As mentioned, the orphan cytokine IL-17D is poorly understood, with recent reports showing that this cytokine can induce IL-6, IL-8 and GM-CSF expression in endothelial cells [51] and IL-6 and IL-8 in chicken fibroblasts [146]. Interestingly, the stress-sensing protein NRF2 induces IL-17D expression in cancer cells. IL-17D-deficient mice displayed increased tumor growth when compared to wild type mice [147]. Recent studies have also linked IL-17D to the recruitment of NK cells into the tumor microenvironment and subsequent activation [148, 149]. Indeed, IL-17D plays a dual role in promoting human NK cell cytotoxicity and inducing NK-recruiting MCP-1 by tumor endothelial cells, thus placing this cytokine in a central role in tumor surveillance [149]. The potential involvement of IL-17D in cutaneous surveillance mechanisms remains an open question.
IL-17E
IL-17E is an interesting example of an IL-17-family cytokine that possesses a divergent function to its founding member IL-17A. Like IL-17A, IL-17E can activate NF-kB and induce the production of IL-8. In addition, transgenic mice overexpressing IL-17E develop common features of IL-17A-driven inflammation, including neutrophilia and elevated circulating G-CSF [150]. However, IL-17E functions mainly to stimulate Th2 responses, promoting Th2 cytokine secretion, class switch recombination to IgE, IgG1 and IgA, and the recruitment and activation of eosinophils, in both mice and humans. Concordantly, IL-17E transgenic mice present with eosinophilia, increased IgE and IgG1 and elevated serum IL-5 and IL-13 [150]. Given its role in promoting Th2-mediated immunity, IL-17E plays a central role in protection against helminth infection [151, 152]. In turn, IL17E mRNA expression is enhanced in the lungs of asthmatic patients [40] and IL-17E delivery promotes Th2 cytokine and IgE production, as well as eosinophil infiltration in a mouse model of asthma [153].
IL-17E expression has been reported in patients with several skin conditions. In particular, a SNP in the IL17E gene is positively correlated with severe forms of psoriasis in a Spanish cohort of patients [154]. However, the effect of this polymorphism on IL-17E expression and/or function remains unknown. Atopic dermatitis often presents in association with mutations in the gene encoding filaggrin [155–157]. IL-25 was overexpressed in the epidermis of atopic dermatitis patients and in corresponding mouse models [158, 159]. In cultured keratinocytes, IL-25 treatment inhibited the expression of filaggrin, which may account for the loss of skin barrier function associated with atopic dermatitis [158]. In addition, IL-25 can mediate the recruitment of “type 2” cytokine-producing ILC2 in atopic dermatitis [160]. Therefore, IL-25 plays a dual role in promoting atopic dermatitis via stimulation of type 2 responses and through its direct action on keratinocytes.
Concluding remarks
The IL-17 family of cytokines plays a central part in the induction of inflammation to limit numerous pathogenic insults. Here, we have reviewed the prominent role of IL-17A in orchestrating protective responses against cutaneous bacterial and fungal infections, and the emerging roles of other IL-17 family members in boosting immunity. Given the recent development of novel therapies to block IL-17A and IL-17RA signals in chronic inflammatory diseases, the potential long-term consequences of such treatments vis-à-vis the exacerbation of fungal and extracellular bacterial infections should be examined. In that light, dissecting the commonalities and divergences in signaling pathways that drive protective versus tissue disruptive functions could provide alternative therapeutic strategies for at-risk populations. Given the pleiotropic roles of IL-17 family members, an in-depth analysis of individual cytokines’ roles during infection and inflammation could provide insight into the advantages of the therapeutic alternatives that are currently under study, including IL-17A versus IL-17RA-blocking strategies.
Table 1.
Cytokines and receptors driving cutaneous inflammation
| Cytokine | Receptor | Infection | Skin inflammatory phenotype(s) |
|---|---|---|---|
| IL-17A and IL-17F | IL-17RA/RC | C. albicans, S. aureus | Psoriasis, atopic dermatitis, skin cancer |
| IL-17B | IL-17RB/? | Undefined | Undefined |
| IL-17C | IL-17RA/RE | S. aureus | Psoriasis |
| IL-17D | Undefined | Undefined | Undefined |
| IL-17E (IL-25) | IL-17RA/RB | Undefined | Psoriasis, atopic dermatitis |
Acknowledgments
SLG was supported by the NIH (AI107825, AR062546, DE022550).
Footnotes
Conflicts of Interest
SLG received research grants from Janssen and Novartis, and serves on the Scientific Advisory Board of Lycera Corporation. There are no other conflicts of interest.
References
- 1.Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150:5445–5456. [PubMed] [Google Scholar]
- 2.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hymowitz SG, Filvaroff EH, Yin JP, Lee J, Cai L, Risser P, et al. IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. The EMBO journal. 2001;20:5332–5341. doi: 10.1093/emboj/20.19.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang XD, Zhang H, He MX. Comparative and Evolutionary Analysis of the Interleukin 17 Gene Family in Invertebrates. PLoS One. 2015;10:e0132802. doi: 10.1371/journal.pone.0132802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gordon KB, Blauvelt A, Papp KA, Langley RG, Luger T, Ohtsuki M, et al. Phase 3 Trials of Ixekizumab in Moderate-to-Severe Plaque Psoriasis. N Engl J Med. 2016;375:345–356. doi: 10.1056/NEJMoa1512711. [DOI] [PubMed] [Google Scholar]
- 7.Langley RG, Elewski BE, Lebwohl M, Reich K, Griffiths CE, Papp K, et al. Secukinumab in plaque psoriasis--results of two phase 3 trials. N Engl J Med. 2014;371:326–338. doi: 10.1056/NEJMoa1314258. [DOI] [PubMed] [Google Scholar]
- 8.Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 9.Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. 2002;71:1–8. [PubMed] [Google Scholar]
- 10.Novatchkova M, Leibbrandt A, Werzowa J, Neubuser A, Eisenhaber F. The STIR-domain superfamily in signal transduction, development and immunity. Trends in biochemical sciences. 2003;28:226–229. doi: 10.1016/S0968-0004(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 11.Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, et al. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177:36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 12.Rickel EA, Siegel LA, Yoon BR, Rottman JB, Kugler DG, Swart DA, et al. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J Immunol. 2008;181:4299–4310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
- 13.Ramirez-Carrozzi V, Sambandam A, Luis E, Lin Z, Jeet S, Lesch J, et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nature immunology. 2011;12:1159–1166. doi: 10.1038/ni.2156. [DOI] [PubMed] [Google Scholar]
- 14.Shi Y, Ullrich SJ, Zhang J, Connolly K, Grzegorzewski KJ, Barber MC, et al. A novel cytokine receptor-ligand pair. Identification, molecular characterization, and in vivo immunomodulatory activity. J Biol Chem. 2000;275:19167–19176. doi: 10.1074/jbc.M910228199. [DOI] [PubMed] [Google Scholar]
- 15.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 16.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 17.Korn T, Oukka M, Kuchroo V, Bettelli E. Th17 cells: effector T cells with inflammatory properties. Seminars in immunology. 2007;19:362–371. doi: 10.1016/j.smim.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 19.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 22.Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 23.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 24.Awasthi A, Riol-Blanco L, Jager A, Korn T, Pot C, Galileos G, et al. Cutting edge: IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J Immunol. 2009;182:5904–5908. doi: 10.4049/jimmunol.0900732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 26.McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 28.Huber M, Heink S, Pagenstecher A, Reinhard K, Ritter J, Visekruna A, et al. IL-17A secretion by CD8+ T cells supports Th17-mediated autoimmune encephalomyelitis. J Clin Invest. 2013;123:247–260. doi: 10.1172/JCI63681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He D, Wu L, Kim HK, Li H, Elmets CA, Xu H. CD8+ IL-17-producing T cells are important in effector functions for the elicitation of contact hypersensitivity responses. J Immunol. 2006;177:6852–6858. doi: 10.4049/jimmunol.177.10.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 31.Villanova F, Flutter B, Tosi I, Grys K, Sreeneebus H, Perera GK, et al. Characterization of innate lymphoid cells in human skin and blood demonstrates increase of NKp44+ ILC3 in psoriasis. The Journal of investigative dermatology. 2014;134:984–991. doi: 10.1038/jid.2013.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marks BR, Nowyhed HN, Choi JY, Poholek AC, Odegard JM, Flavell RA, et al. Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nat Immunol. 2009;10:1125–1132. doi: 10.1038/ni.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- 34.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, et al. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120:331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoshino A, Nagao T, Nagi-Miura N, Ohno N, Yasuhara M, Yamamoto K, et al. MPO-ANCA induces IL-17 production by activated neutrophils in vitro via classical complement pathway-dependent manner. Journal of autoimmunity. 2008;31:79–89. doi: 10.1016/j.jaut.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 36.Noordenbos T, Blijdorp I, Chen S, Stap J, Mul E, Canete JD, et al. Human mast cells capture, store, and release bioactive, exogenous IL-17A. J Leukoc Biol. 2016;100:453–462. doi: 10.1189/jlb.3HI1215-542R. [DOI] [PubMed] [Google Scholar]
- 37.Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol. 2011;187:490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J, Ho WH, Maruoka M, Corpuz RT, Baldwin DT, Foster JS, et al. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J Biol Chem. 2001;276:1660–1664. doi: 10.1074/jbc.M008289200. [DOI] [PubMed] [Google Scholar]
- 39.Morita H, Arae K, Unno H, Toyama S, Motomura K, Matsuda A, et al. IL-25 and IL-33 Contribute to Development of Eosinophilic Airway Inflammation in Epicutaneously Antigen-Sensitized Mice. PLoS One. 2015;10:e0134226. doi: 10.1371/journal.pone.0134226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang YH, Angkasekwinai P, Lu N, Voo KS, Arima K, Hanabuchi S, et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J Exp Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, et al. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kouzaki H, Tojima I, Kita H, Shimizu T. Transcription of interleukin-25 and extracellular release of the protein is regulated by allergen proteases in airway epithelial cells. Am J Respir Cell Mol Biol. 2013;49:741–750. doi: 10.1165/rcmb.2012-0304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corrigan CJ, Wang W, Meng Q, Fang C, Eid G, Caballero MR, et al. Allergen-induced expression of IL-25 and IL-25 receptor in atopic asthmatic airways and late-phase cutaneous responses. J Allergy Clin Immunol. 2011;128:116–124. doi: 10.1016/j.jaci.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 44.Cheng D, Xue Z, Yi L, Shi H, Zhang K, Huo X, et al. Epithelial interleukin-25 is a key mediator in Th2-high, corticosteroid-responsive asthma. Am J Respir Crit Care Med. 2014;190:639–648. doi: 10.1164/rccm.201403-0505OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kleinschek MA, Owyang AM, Joyce-Shaikh B, Langrish CL, Chen Y, Gorman DM, et al. IL-25 regulates Th17 function in autoimmune inflammation. J Exp Med. 2007;204:161–170. doi: 10.1084/jem.20061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Emamaullee JA, Davis J, Merani S, Toso C, Elliott JF, Thiesen A, et al. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes. 2009;58:1302–1311. doi: 10.2337/db08-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saadoun D, Terrier B, Cacoub P. Interleukin-25: key regulator of inflammatory and autoimmune diseases. Current pharmaceutical design. 2011;17:3781–3785. doi: 10.2174/138161211798357872. [DOI] [PubMed] [Google Scholar]
- 48.Li H, Chen J, Huang A, Stinson J, Heldens S, Foster J, et al. Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proc Natl Acad Sci U S A. 2000;97:773–778. doi: 10.1073/pnas.97.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hwang SY, Kim HY. Expression of IL-17 homologs and their receptors in the synovial cells of rheumatoid arthritis patients. Molecules and cells. 2005;19:180–184. [PubMed] [Google Scholar]
- 50.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169:443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 51.Starnes T, Broxmeyer HE, Robertson MJ, Hromas R. Cutting edge: IL-17D, a novel member of the IL-17 family, stimulates cytokine production and inhibits hemopoiesis. J Immunol. 2002;169:642–646. doi: 10.4049/jimmunol.169.2.642. [DOI] [PubMed] [Google Scholar]
- 52.Chang SH, Dong C. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell Signal. 2011;23:1069–1075. doi: 10.1016/j.cellsig.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 54.Hercus TR, Dhagat U, Kan WL, Broughton SE, Nero TL, Perugini M, et al. Signalling by the betac family of cytokines. Cytokine & growth factor reviews. 2013;24:189–201. doi: 10.1016/j.cytogfr.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 55.Ozaki K, Leonard WJ. Cytokine and cytokine receptor pleiotropy and redundancy. J Biol Chem. 2002;277:29355–29358. doi: 10.1074/jbc.R200003200. [DOI] [PubMed] [Google Scholar]
- 56.Liu S, Song X, Chrunyk BA, Shanker S, Hoth LR, Marr ES, et al. Crystal structures of interleukin 17A and its complex with IL-17 receptor A. Nat Commun. 2013;4:1888. doi: 10.1038/ncomms2880. [DOI] [PubMed] [Google Scholar]
- 57.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wright JF, Guo Y, Quazi A, Luxenberg DP, Bennett F, Ross JF, et al. Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J Biol Chem. 2007;282:13447–13455. doi: 10.1074/jbc.M700499200. [DOI] [PubMed] [Google Scholar]
- 59.Kuestner RE, Taft DW, Haran A, Brandt CS, Brender T, Lum K, et al. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol. 2007;179:5462–5473. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zrioual S, Ecochard R, Tournadre A, Lenief V, Cazalis MA, Miossec P. Genome-wide comparison between IL-17A- and IL-17F-induced effects in human rheumatoid arthritis synoviocytes. J Immunol. 2009;182:3112–3120. doi: 10.4049/jimmunol.0801967. [DOI] [PubMed] [Google Scholar]
- 61.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8:247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 62.Schwandner R, Yamaguchi K, Cao Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J Exp Med. 2000;191:1233–1240. doi: 10.1084/jem.191.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, et al. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279:2559–2567. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- 64.Bulek K, Liu C, Swaidani S, Wang L, Page RC, Gulen MF, et al. The inducible kinase IKKi is required for IL-17-dependent signaling associated with neutrophilia and pulmonary inflammation. Nat Immunol. 2011;12:844–852. doi: 10.1038/ni.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qu F, Gao H, Zhu S, Shi P, Zhang Y, Liu Y, et al. TRAF6-dependent Act1 phosphorylation by the IkappaB kinase-related kinases suppresses interleukin-17-induced NF-kappaB activation. Molecular and cellular biology. 2012;32:3925–3937. doi: 10.1128/MCB.00268-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun D, Novotny M, Bulek K, Liu C, Li X, Hamilton T. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF) Nat Immunol. 2011;12:853–860. doi: 10.1038/ni.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herjan T, Yao P, Qian W, Li X, Liu C, Bulek K, et al. HuR is required for IL-17-induced Act1-mediated CXCL1 and CXCL5 mRNA stabilization. J Immunol. 2013;191:640–649. doi: 10.4049/jimmunol.1203315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang C, Wu L, Bulek K, Martin BN, Zepp JA, Kang Z, et al. The psoriasis-associated D10N variant of the adaptor Act1 with impaired regulation by the molecular chaperone hsp90. Nat Immunol. 2013;14:72–81. doi: 10.1038/ni.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Genetic Analysis of Psoriasis C, the Wellcome Trust Case Control C. Strange A, Capon F, Spencer CC, Knight J, et al. A genome-wide association study identifies new psoriasis susceptibility loci and an interaction between HLA-C and ERAP1. Nature genetics. 2010;42:985–990. doi: 10.1038/ng.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ellinghaus E, Ellinghaus D, Stuart PE, Nair RP, Debrus S, Raelson JV, et al. Genome-wide association study identifies a psoriasis susceptibility locus at TRAF3IP2. Nature genetics. 2010;42:991–995. doi: 10.1038/ng.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huffmeier U, Uebe S, Ekici AB, Bowes J, Giardina E, Korendowych E, et al. Common variants at TRAF3IP2 are associated with susceptibility to psoriatic arthritis and psoriasis. Nature genetics. 2010;42:996–999. doi: 10.1038/ng.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Onishi RM, Gaffen SL. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology. 2010;129:311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, et al. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- 74.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, Erle DJ, et al. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dolgachev V, Petersen BC, Budelsky AL, Berlin AA, Lukacs NW. Pulmonary IL-17E (IL-25) production and IL-17RB+ myeloid cell-derived Th2 cytokine production are dependent upon stem cell factor-induced responses during chronic allergic pulmonary disease. J Immunol. 2009;183:5705–5715. doi: 10.4049/jimmunol.0901666. [DOI] [PubMed] [Google Scholar]
- 77.Claudio E, Sonder SU, Saret S, Carvalho G, Ramalingam TR, Wynn TA, et al. The adaptor protein CIKS/Act1 is essential for IL-25-mediated allergic airway inflammation. J Immunol. 2009;182:1617–1630. doi: 10.4049/jimmunol.182.3.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Swaidani S, Bulek K, Kang Z, Liu C, Lu Y, Yin W, et al. The critical role of epithelial-derived Act1 in IL-17- and IL-25-mediated pulmonary inflammation. J Immunol. 2009;182:1631–1640. doi: 10.4049/jimmunol.182.3.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maezawa Y, Nakajima H, Suzuki K, Tamachi T, Ikeda K, Inoue J, et al. Involvement of TNF receptor-associated factor 6 in IL-25 receptor signaling. J Immunol. 2006;176:1013–1018. doi: 10.4049/jimmunol.176.2.1013. [DOI] [PubMed] [Google Scholar]
- 80.Zepp JA, Wu L, Qian W, Ouyang W, Aronica M, Erzurum S, et al. TRAF4-SMURF2-mediated DAZAP2 degradation is critical for IL-25 signaling and allergic airway inflammation. J Immunol. 2015;194:2826–2837. doi: 10.4049/jimmunol.1402647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu L, Zepp JA, Qian W, Martin BN, Ouyang W, Yin W, et al. A novel IL-25 signaling pathway through STAT5. J Immunol. 2015;194:4528–4534. doi: 10.4049/jimmunol.1402760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Huang CK, Yang CY, Jeng YM, Chen CL, Wu HH, Chang YC, et al. Autocrine/paracrine mechanism of interleukin-17B receptor promotes breast tumorigenesis through NF-kappaB-mediated antiapoptotic pathway. Oncogene. 2014;33:2968–2977. doi: 10.1038/onc.2013.268. [DOI] [PubMed] [Google Scholar]
- 83.Wu HH, Hwang-Verslues WW, Lee WH, Huang CK, Wei PC, Chen CL, et al. Targeting IL-17B-IL-17RB signaling with an anti-IL-17RB antibody blocks pancreatic cancer metastasis by silencing multiple chemokines. J Exp Med. 2015;212:333–349. doi: 10.1084/jem.20141702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Reynolds JM, Lee YH, Shi Y, Wang X, Angkasekwinai P, Nallaparaju KC, et al. Interleukin-17B Antagonizes Interleukin-25-Mediated Mucosal Inflammation. Immunity. 2015;42:692–703. doi: 10.1016/j.immuni.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Song X, He X, Li X, Qian Y. The roles and functional mechanisms of interleukin-17 family cytokines in mucosal immunity. Cellular & molecular immunology. 2016;13:418–431. doi: 10.1038/cmi.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Song X, Zhu S, Shi P, Liu Y, Shi Y, Levin SD, et al. IL-17RE is the functional receptor for IL-17C and mediates mucosal immunity to infection with intestinal pathogens. Nat Immunol. 2011;12:1151–1158. doi: 10.1038/ni.2155. [DOI] [PubMed] [Google Scholar]
- 87.Zhu S, Pan W, Shi P, Gao H, Zhao F, Song X, et al. Modulation of experimental autoimmune encephalomyelitis through TRAF3-mediated suppression of interleukin 17 receptor signaling. J Exp Med. 2010;207:2647–2662. doi: 10.1084/jem.20100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zepp JA, Liu C, Qian W, Wu L, Gulen MF, Kang Z, et al. Cutting edge: TNF receptor-associated factor 4 restricts IL-17-mediated pathology and signaling processes. J Immunol. 2012;189:33–37. doi: 10.4049/jimmunol.1200470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garg AV, Ahmed M, Vallejo AN, Ma A, Gaffen SL. The deubiquitinase A20 mediates feedback inhibition of interleukin-17 receptor signaling. Science signaling. 2013;6:ra44. doi: 10.1126/scisignal.2003699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Duong BH, Onizawa M, Oses-Prieto JA, Advincula R, Burlingame A, Malynn BA, et al. A20 restricts ubiquitination of pro-interleukin-1beta protein complexes and suppresses NLRP3 inflammasome activity. Immunity. 2015;42:55–67. doi: 10.1016/j.immuni.2014.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Luo H, Liu Y, Li Q, Liao L, Sun R, Liu X, et al. A20 regulates IL-1-induced tolerant production of CXC chemokines in human mesangial cells via inhibition of MAPK signaling. Scientific reports. 2015;5:18007. doi: 10.1038/srep18007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.He KL, Ting AT. A20 inhibits tumor necrosis factor (TNF) alpha-induced apoptosis by disrupting recruitment of TRADD and RIP to the TNF receptor 1 complex in Jurkat T cells. Molecular and cellular biology. 2002;22:6034–6045. doi: 10.1128/MCB.22.17.6034-6045.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johnston A, Fritz Y, Dawes SM, Diaconu D, Al-Attar PM, Guzman AM, et al. Keratinocyte overexpression of IL-17C promotes psoriasiform skin inflammation. J Immunol. 2013;190:2252–2262. doi: 10.4049/jimmunol.1201505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shen F, Li N, Gade P, Kalvakolanu DV, Weibley T, Doble B, et al. IL-17 receptor signaling inhibits C/EBPbeta by sequential phosphorylation of the regulatory 2 domain. Science signaling. 2009;2:ra8. doi: 10.1126/scisignal.2000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harden JL, Krueger JG, Bowcock AM. The immunogenetics of Psoriasis: A comprehensive review. Journal of autoimmunity. 2015;64:66–73. doi: 10.1016/j.jaut.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ippagunta SK, Gangwar R, Finkelstein D, Vogel P, Pelletier S, Gingras S, et al. Keratinocytes contribute intrinsically to psoriasis upon loss of Tnip1 function. Proc Natl Acad Sci U S A. 2016;113:E6162–E6171. doi: 10.1073/pnas.1606996113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sonder SU, Saret S, Tang W, Sturdevant DE, Porcella SF, Siebenlist U. IL-17-induced NF-kappaB activation via CIKS/Act1: physiologic significance and signaling mechanisms. J Biol Chem. 2011;286:12881–12890. doi: 10.1074/jbc.M110.199547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Garg AV, Amatya N, Chen K, Cruz JA, Grover P, Whibley N, et al. MCPIP1 Endoribonuclease Activity Negatively Regulates Interleukin-17-Mediated Signaling and Inflammation. Immunity. 2015;43:475–487. doi: 10.1016/j.immuni.2015.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Somma D, Mastrovito P, Grieco M, Lavorgna A, Pignalosa A, Formisano L, et al. CIKS/DDX3X Interaction Controls the Stability of the Zc3h12a mRNA Induced by IL-17. J Immunol. 2015;194:3286–3294. doi: 10.4049/jimmunol.1401589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cifuentes RA, Cruz-Tapias P, Rojas-Villarraga A, Anaya JM. ZC3H12A (MCPIP1): molecular characteristics and clinical implications. Clinica chimica acta; international journal of clinical chemistry. 2010;411:1862–1868. doi: 10.1016/j.cca.2010.08.033. [DOI] [PubMed] [Google Scholar]
- 101.Monin L, Gudjonsson JE, Childs EE, Amatya N, Xing X, Verma AH, et al. MCPIP1/Regnase-1 Restricts IL-17A- and IL-17C-Dependent Skin Inflammation. J Immunol. 2016 doi: 10.4049/jimmunol.1601551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 103.Yang J, Goetz D, Li JY, Wang W, Mori K, Setlik D, et al. An iron delivery pathway mediated by a lipocalin. Molecular cell. 2002;10:1045–1056. doi: 10.1016/s1097-2765(02)00710-4. [DOI] [PubMed] [Google Scholar]
- 104.Shen F, Ruddy MJ, Plamondon P, Gaffen SL. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-alpha-induced genes in bone cells. J Leukoc Biol. 2005;77:388–399. doi: 10.1189/jlb.0904490. [DOI] [PubMed] [Google Scholar]
- 105.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 106.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, et al. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am J Respir Cell Mol Biol. 2001;25:335–340. doi: 10.1165/ajrcmb.25.3.4424. [DOI] [PubMed] [Google Scholar]
- 107.Lin Y, Ritchea S, Logar A, Slight S, Messmer M, Rangel-Moreno J, et al. Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity. 2009;31:799–810. doi: 10.1016/j.immuni.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dubin PJ, Martz A, Eisenstatt JR, Fox MD, Logar A, Kolls JK. Interleukin-23-mediated inflammation in Pseudomonas aeruginosa pulmonary infection. Infect Immun. 2012;80:398–409. doi: 10.1128/IAI.05821-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu J, Feng Y, Yang K, Li Q, Ye L, Han L, et al. Early production of IL-17 protects against acute pulmonary Pseudomonas aeruginosa infection in mice. FEMS immunology and medical microbiology. 2011;61:179–188. doi: 10.1111/j.1574-695X.2010.00764.x. [DOI] [PubMed] [Google Scholar]
- 110.Curtis MM, Way SS. Interleukin-17 in host defence against bacterial, mycobacterial and fungal pathogens. Immunology. 2009;126:177–185. doi: 10.1111/j.1365-2567.2008.03017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Manni ML, Robinson KM, Alcorn JF. A tale of two cytokines: IL-17 and IL-22 in asthma and infection. Expert review of respiratory medicine. 2014;8:25–42. doi: 10.1586/17476348.2014.854167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Milner JD, Holland SM. The cup runneth over: lessons from the ever-expanding pool of primary immunodeficiency diseases. Nat Rev Immunol. 2013;13:635–648. doi: 10.1038/nri3493. [DOI] [PubMed] [Google Scholar]
- 113.Whibley N, Tritto E, Traggiai E, Kolbinger F, Moulin P, Brees D, et al. Antibody blockade of IL-17 family cytokines in immunity to acute murine oral mucosal candidiasis. J Leukoc Biol. 2016;99:1153–1164. doi: 10.1189/jlb.4A0915-428R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. 2011;332:65–68. doi: 10.1126/science.1200439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Conti HR, Gaffen SL. IL-17-Mediated Immunity to the Opportunistic Fungal Pathogen Candida albicans. J Immunol. 2015;195:780–788. doi: 10.4049/jimmunol.1500909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Boisson B, Wang C, Pedergnana V, Wu L, Cypowyj S, Rybojad M, et al. An ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity. 2013;39:676–686. doi: 10.1016/j.immuni.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. 2010;120:1762–1773. doi: 10.1172/JCI40891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chan LC, Chaili S, Filler SG, Barr K, Wang H, Kupferwasser D, et al. Nonredundant Roles of Interleukin-17A (IL-17A) and IL-22 in Murine Host Defense against Cutaneous and Hematogenous Infection Due to Methicillin-Resistant Staphylococcus aureus. Infect Immun. 2015;83:4427–4437. doi: 10.1128/IAI.01061-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 121.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 122.Johansen C, Usher PA, Kjellerup RB, Lundsgaard D, Iversen L, Kragballe K. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br J Dermatol. 2009;160:319–324. doi: 10.1111/j.1365-2133.2008.08902.x. [DOI] [PubMed] [Google Scholar]
- 123.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- 124.Nograles KE, Zaba LC, Guttman-Yassky E, Fuentes-Duculan J, Suarez-Farinas M, Cardinale I, et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. The British journal of dermatology. 2008;159:1092–1102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cargill M, Schrodi SJ, Chang M, Garcia VE, Brandon R, Callis KP, et al. A large-scale genetic association study confirms IL12B and leads to the identification of IL23R as psoriasis-risk genes. Am J Hum Genet. 2007;80:273–290. doi: 10.1086/511051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tsoi LC, Spain SL, Knight J, Ellinghaus E, Stuart PE, Capon F, et al. Identification of 15 new psoriasis susceptibility loci highlights the role of innate immunity. Nature genetics. 2012;44:1341–1348. doi: 10.1038/ng.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sonder SU, Paun A, Ha HL, Johnson PF, Siebenlist U. CIKS/Act1-mediated signaling by IL-17 cytokines in context: implications for how a CIKS gene variant may predispose to psoriasis. J Immunol. 2012;188:5906–5914. doi: 10.4049/jimmunol.1103233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 129.Ha HL, Wang H, Pisitkun P, Kim JC, Tassi I, Tang W, et al. IL-17 drives psoriatic inflammation via distinct, target cell-specific mechanisms. Proc Natl Acad Sci U S A. 2014;111:E3422–3431. doi: 10.1073/pnas.1400513111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Durham LE, Kirkham BW, Taams LS. Contribution of the IL-17 Pathway to Psoriasis and Psoriatic Arthritis. Current rheumatology reports. 2015;17:55. doi: 10.1007/s11926-015-0529-9. [DOI] [PubMed] [Google Scholar]
- 131.Sanford M, McKeage K. Secukinumab: first global approval. Drugs. 2015;75:329–338. doi: 10.1007/s40265-015-0359-0. [DOI] [PubMed] [Google Scholar]
- 132.Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190–1199. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- 133.Baeten D, Sieper J, Braun J, Baraliakos X, Dougados M, Emery P, et al. Secukinumab, an Interleukin-17A Inhibitor, in Ankylosing Spondylitis. N Engl J Med. 2015;373:2534–2548. doi: 10.1056/NEJMoa1505066. [DOI] [PubMed] [Google Scholar]
- 134.Schultz Larsen F. Atopic dermatitis: an increasing problem. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology. 1996;7:51–53. doi: 10.1111/j.1399-3038.1996.tb00395.x. [DOI] [PubMed] [Google Scholar]
- 135.Leonardi S, Cuppari C, Manti S, Filippelli M, Parisi GF, Borgia F, et al. Serum interleukin 17, interleukin 23, and interleukin 10 values in children with atopic eczema/dermatitis syndrome (AEDS): association with clinical severity and phenotype. Allergy and asthma proceedings. 2015;36:74–81. doi: 10.2500/aap.2015.36.3808. [DOI] [PubMed] [Google Scholar]
- 136.Albanesi C, Cavani A, Girolomoni G. IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: synergistic or antagonist effects with IFN-gamma and TNF-alpha. J Immunol. 1999;162:494–502. [PubMed] [Google Scholar]
- 137.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 138.Wang L, Yi T, Zhang W, Pardoll DM, Yu H. IL-17 enhances tumor development in carcinogen-induced skin cancer. Cancer research. 2010;70:10112–10120. doi: 10.1158/0008-5472.CAN-10-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.He D, Li H, Yusuf N, Elmets CA, Athar M, Katiyar SK, et al. IL-17 mediated inflammation promotes tumor growth and progression in the skin. PLoS One. 2012;7:e32126. doi: 10.1371/journal.pone.0032126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kouri VP, Olkkonen J, Ainola M, Li TF, Bjorkman L, Konttinen YT, et al. Neutrophils produce interleukin-17B in rheumatoid synovial tissue. Rheumatology. 2014;53:39–47. doi: 10.1093/rheumatology/ket309. [DOI] [PubMed] [Google Scholar]
- 141.You Z, DuRaine G, Tien JY, Lee C, Moseley TA, Reddi AH. Expression of interleukin-17B in mouse embryonic limb buds and regulation by BMP-7 and bFGF. Biochemical and biophysical research communications. 2005;326:624–631. doi: 10.1016/j.bbrc.2004.11.087. [DOI] [PubMed] [Google Scholar]
- 142.McHenga SS, Wang D, Li C, Shan F, Lu C. Inhibitory effect of recombinant IL-25 on the development of dextran sulfate sodium-induced experimental colitis in mice. Cellular & molecular immunology. 2008;5:425–431. doi: 10.1038/cmi.2008.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Conti HR, Whibley N, Coleman BM, Garg AV, Jaycox JR, Gaffen SL. Signaling through IL-17C/IL-17RE is dispensable for immunity to systemic, oral and cutaneous candidiasis. PLoS One. 2015;10:e0122807. doi: 10.1371/journal.pone.0122807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Roth SA, Simanski M, Rademacher F, Schroder L, Harder J. The pattern recognition receptor NOD2 mediates Staphylococcus aureus-induced IL-17C expression in keratinocytes. The Journal of investigative dermatology. 2014;134:374–380. doi: 10.1038/jid.2013.313. [DOI] [PubMed] [Google Scholar]
- 145.Golden JB, Wang Y, Fritz Y, Diaconu D, Zhang X, Debanne SM, et al. Chronic, not acute, skin-specific inflammation promotes thrombosis in psoriasis murine models. Journal of translational medicine. 2015;13:382. doi: 10.1186/s12967-015-0738-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hong YH, Lillehoj HS, Park DW, Lee SH, Han JY, Shin JH, et al. Cloning and functional characterization of chicken interleukin-17D. Veterinary immunology and immunopathology. 2008;126:1–8. doi: 10.1016/j.vetimm.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 147.Saddawi-Konefka R, Seelige R, Gross ET, Levy E, Searles SC, Washington A, Jr, et al. Nrf2 Induces IL-17D to Mediate Tumor and Virus Surveillance. Cell reports. 2016;16:2348–2358. doi: 10.1016/j.celrep.2016.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Saddawi-Konefka R, O'Sullivan T, Gross ET, Washington A, Jr, Bui JD. Tumor-expressed IL-17D recruits NK cells to reject tumors. Oncoimmunology. 2014;3:e954853. doi: 10.4161/21624011.2014.954853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.O'Sullivan T, Saddawi-Konefka R, Gross E, Tran M, Mayfield SP, Ikeda H, et al. Interleukin-17D mediates tumor rejection through recruitment of natural killer cells. Cell reports. 2014;7:989–998. doi: 10.1016/j.celrep.2014.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pan G, French D, Mao W, Maruoka M, Risser P, Lee J, et al. Forced expression of murine IL-17E induces growth retardation, jaundice, a Th2-biased response, and multiorgan inflammation in mice. J Immunol. 2001;167:6559–6567. doi: 10.4049/jimmunol.167.11.6559. [DOI] [PubMed] [Google Scholar]
- 151.Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, Hewett DR, et al. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, et al. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med. 2006;203:843–849. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/s1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 154.Batalla A, Coto E, Gonzalez-Lara L, Gonzalez-Fernandez D, Gomez J, Aranguren TF, et al. Association between single nucleotide polymorphisms IL17RA rs4819554 and IL17E rs79877597 and Psoriasis in a Spanish cohort. Journal of dermatological science. 2015;80:111–115. doi: 10.1016/j.jdermsci.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 155.Barker JN, Palmer CN, Zhao Y, Liao H, Hull PR, Lee SP, et al. Null mutations in the filaggrin gene (FLG) determine major susceptibility to early-onset atopic dermatitis that persists into adulthood. The Journal of investigative dermatology. 2007;127:564–567. doi: 10.1038/sj.jid.5700587. [DOI] [PubMed] [Google Scholar]
- 156.Weidinger S, Illig T, Baurecht H, Irvine AD, Rodriguez E, Diaz-Lacava A, et al. Loss-of-function variations within the filaggrin gene predispose for atopic dermatitis with allergic sensitizations. J Allergy Clin Immunol. 2006;118:214–219. doi: 10.1016/j.jaci.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 157.Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nature genetics. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 158.Hvid M, Vestergaard C, Kemp K, Christensen GB, Deleuran B, Deleuran M. IL-25 in atopic dermatitis: a possible link between inflammation and skin barrier dysfunction? The Journal of investigative dermatology. 2011;131:150–157. doi: 10.1038/jid.2010.277. [DOI] [PubMed] [Google Scholar]
- 159.Aktar MK, Kido-Nakahara M, Furue M, Nakahara T. Mutual upregulation of endothelin-1 and IL-25 in atopic dermatitis. Allergy. 2015;70:846–854. doi: 10.1111/all.12633. [DOI] [PubMed] [Google Scholar]
- 160.Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939–2950. doi: 10.1084/jem.20130351. [DOI] [PMC free article] [PubMed] [Google Scholar]