Abstract
Purpose
To quantify the burden and complexity associated with treatment of Medicare beneficiaries with stage I non–small-cell lung cancer (NSCLC).
Methods
Using the SEER-Medicare database, we conducted a retrospective cohort study of Medicare beneficiaries who were diagnosed with stage I NSCLC from 2007 to 2011 and who were treated with surgery, stereotactic body radiation therapy, or external beam radiation therapy. Main outcome measures were the number of days a patient was in contact with the health care system (encounter days), the number of physicians involved in a patient's care, and the number of medications prescribed. Logistic regression modeled the association between patient characteristics, treatment type, and high treatment burden (defined as ≥ 66 encounter days).
Results
On average, 7,955 patients spent 1 in 3 days interacting with the health care system during the initial 60 days of treatment. Patients experienced a median of 44 encounter days with high variability (interquartile range [IQR], 29 to 66) in the 12 months after treatment initiation. The median number of physicians involved was 20 (IQR, 14 to 28), and the median number of medications prescribed was 12 (IQR, 8 to 17). Patients who were treated with surgery had high treatment burden (predicted probability, 21.6%; 95% CI, 20.2 to 23.1) compared with patients who were treated with stereotactic body radiation therapy (predicted probability, 16.1%; 95% CI, 12.9 to 19.3), whereas patients who were treated with external beam radiation therapy had the highest burden (predicted probability, 46.8%; 95% CI, 43.3 to 50.2).
Conclusion
The treatment burden imposed on patients with early-stage NSCLC was substantial in terms of the number of encounters, physicians involved, and medications prescribed. Because treatment burden varied markedly across patients and treatment types, future work should identify opportunities to understand and ameliorate this burden.
Introduction
Treatment burden is an important patient-centered quality measure with increasing importance for a rapidly growing geriatric oncology population in the era of value-based cancer care. The definition of value in health care equals the health outcome per cost.1 In cancer care, the desired outcome is a cure of cancer in exchange for the cost of undergoing treatment. Treatment burden is an emerging concept that is distinct from disease burden or symptom burden that describes the work of being a patient and the impact this workload has on a patient's functioning and well-being.2,3 Although treatment burden is an important concept for all aspects of medical care, it is particularly important for cancer, which is associated with increasing age, intensive treatment regimens, and a variable prognosis. Prior efforts to understand treatment burden among patients with cancer have focused on treatment-related adverse effects and the time patients spend undergoing treatment.4 Yet time is only one consideration for older adults with a new cancer diagnosis.
Treatment burden matters to patients and affects compliance and satisfaction with care.5,6 Operationalizing treatment burden within large claims databases is a necessary first step in its development as a cancer-specific quality measure. Furthermore, understanding treatment burden will identify strategies for decreasing the work of being a patient and minimizing health care disruptions, a concept known as minimally disruptive medicine.5
The median age at diagnosis for lung cancer is age 70 years,7 and 66% of all Medicare patients have multiple chronic conditions—multimorbidity. Patients with significant multimorbidity often describe caring for their health as full-time work when receiving care from a poorly coordinated health care system. Treatment burden that is attributable to early-stage lung cancer care is important to define and understand among older patients who often have competing health conditions and functional limitations. The magnitude and variability of treatment burden associated with lung cancer care of Medicare beneficiaries with early-stage disease has yet to be described in a rigorous quantitative manner.
Care of older patients with early-stage non-small-cell lung cancer (NSCLC) is especially relevant as the incidence of the disease is predicted to increase as a consequence of new screening guidelines. Although the number of curative treatment strategies has expanded in recent years, a clear best strategy for each patient is not always certain. Conflicting evidence regarding the mortality difference between surgery and radiation creates uncertainty in treatment decision-making, particularly among adults who may not tolerate surgery.9-13 Informing patients about the treatment burden of different treatment options is actionable information that clinicians can incorporate into shared decision-making.
To address these knowledge gaps, we evaluated treatment burden in a sample of Medicare beneficiaries who were diagnosed with early-stage NSCLC. We used Medicare claims to operationalize the quantity and variability of treatment burden, which we defined as total encounter days, physicians seen, and medications prescribed. We also determined the probability of experiencing high treatment burden on the basis of treatment type and comorbidity.
Methods
Study Design and Data Source
Using the SEER-Medicare linked database, we performed a retrospective cohort study to determine cancer-related treatment burden among patients who received definitive treatment of early-stage NSCLC from 2007 to 2011. We incorporated a matched, non-cancer comparison group to estimate the magnitude of treatment burden that was attributable to lung cancer treatment. We used Medicare data to operationalize treatment burden as encounter days, physicians seen, and medications prescribed, by using the following fee-for-service claims files: MedPAR (inpatient hospitalizations, including skilled nursing facilities), Outpatient, Carrier (physician and supplier services), Home Health Agency, and Part D Drug Events.14
Study Sample
We identified patients age 67 to 94 years who were diagnosed with stage I NSCLC from 2007 to 2011 and who received surgery—lobectomy, wedge resection, or sublobar resection—or radiation—stereotactic body radiation therapy (SBRT) or external beam radiation therapy (EBRT), including intensity-modulated radiation therapy. Patients must have initiated treatment within 6 months of diagnosis, and the date of treatment initiation must have been on or before December 31, 2011, to allow for a full year of follow-up claims. Patients had to be enrolled in Medicare fee-for-service Parts A and B for 24 months before diagnosis through 12 months after treatment initiation or death, whichever occurred first. Patients with an unknown month of diagnosis or with a diagnosis from autopsy or death certificate were excluded. Patients were excluded if they received both surgery and radiation or multiple types of radiation to describe the treatment burden associated with each curative treatment modality. We excluded patients who did not receive treatment or who had treatment claims with a diagnosis code for metastasis as this study focused on curative treatment. SBRT and surgery were presumed for curative intent as this cohort was restricted to stage I NSCLC.
We selected a matched, non-cancer comparison group from the 5% random sample of Medicare beneficiaries to determine the treatment burden associated with the usual (non-cancer) care of Medicare beneficiaries. Each member of the comparison group was randomly assigned to an index date during each year (2007 to 2011) during which they were alive and fulfilled the same enrollment criteria as did the patients with cancer. To assess treatment burden among controls who were similar to the matched patients with cancer on the basis of several matching covariates, we assigned a pseudo treatment date of 50 days after their index date, because the median length of time between diagnosis and cancer treatment initiation was 50 days. Each patient with cancer was matched with two patients without cancer without replacement. Each patient without cancer only appeared in the final data set once, using the following factors: age, sex, year, number of comorbid conditions, SEER region, and number of doctor visits in the year before the index date.
Outcome Variables
Treatment burden was defined as the cumulative number of days that adults were in contact with the health care system (encounter days), number of physicians seen, and the number of medications prescribed. We categorized encounter days into two categories: acute care (emergency department visits and short-stay hospitalizations) and outpatient care (all outpatient visits, including diagnostic testing and radiation therapy visits). We aggregated the total number of encounter days for each patient and divided the number of encounter days across patients into quartiles. We defined a priori high treatment burden as having total encounter days in the top quartile, which corresponded to ≥ 66 encounter days. We created a dichotomous outcome that represented whether a patient was in the highest quartile of overall encounter days. We tallied the number of physicians who were involved in each patient's care on the basis of the National Provider Identifier. We excluded providers who would not interact directly with the patient, such as pathologists. We determined the number of unique prescription medications prescribed by using the generic drug name in Medicare Part D data. Only patients with Part D coverage were included in the prescription analysis—approximately one half of the sample.
We measured encounter days during two time periods, each lasting 365 days: pretreatment (18 through 6 months before treatment initiation) and post-treatment (the year after treatment initiation). Treatment initiation was defined as the date of surgery or first radiation treatment. We did not assess encounter days in the 6 months before treatment initiation, as we did not want to capture the NSCLC diagnostic period. To calculate cancer-related encounter days we subtracted the pretreatment encounter days from the post-treatment encounter days. We calculated 30-day and 90-day mortality starting from the last day of the treatment burden assessment period, and we calculated 1-year mortality from the date of diagnosis.
Covariates
Comorbid conditions were assessed by using Medicare claims in the 24 through 3 months before diagnosis. We searched for International Classification of Diseases, 9th Revision, diagnosis codes for the conditions recommended by Elixhauser et al15 that were previously found to be significantly associated with survival in a sample of patients without cancer. We selected International Classification of Diseases, 9th Revision, codes that appeared on one or more inpatient claim or on two or more outpatient and/or physician claims billed ≥ 30 days apart. To estimate life expectancy, we used a sample of patients without cancer from the Medicare 5% random sample and constructed age- and comorbidity-specific life tables using the annual mortality rates.16
Statistical Analysis
To quantify cancer-related treatment burden, we calculated the difference in total, acute, and outpatient encounter days, physicians, and medications for each patient between the two time periods. For patients with and without cancer, separately, we calculated the median difference of encounter days and 95% CI of this difference to determine whether the difference was significantly different from zero.
We calculated the median and interquartile range (IQR) for each outcome variable during each time period for patients with and without cancer. For the number of encounter days, physicians seen, acute care days, and medications, we also calculated the median in the post-treatment period across strata of age, sex, comorbidity, life expectancy, and treatment type. We used the Kruskal-Wallis test for nonparametric data to test for differences in each outcome variable across the strata of covariates.
By using logistic regression, we determined the association between patient characteristics, treatment type, and high treatment burden—defined as the highest quartile of encounter days, ≥ 66 encounter days. For the final adjusted model, we only retained covariates that were significantly associated with high treatment burden (P < .05). We also included an interaction term for comorbidity group and treatment type. Logistic regression models accounted for clustering at the level of hospital referral region by using the SAS GLIMMIX procedure with hospital referral region as a random effect. We used the –margins– command in STATA (STATA, College Station, TX; Computing Resource Center, Santa Monica, CA) to calculate the predicted probability of high treatment burden on the basis of treatment type and number of comorbidities. All analyses were conducted using SAS (SAS/STAT User's Guide, Version 9.4; SAS Institute, Cary, NC) and STATA version 14. The Yale Human Investigations Committee determined that this study was not human participants research.
Results
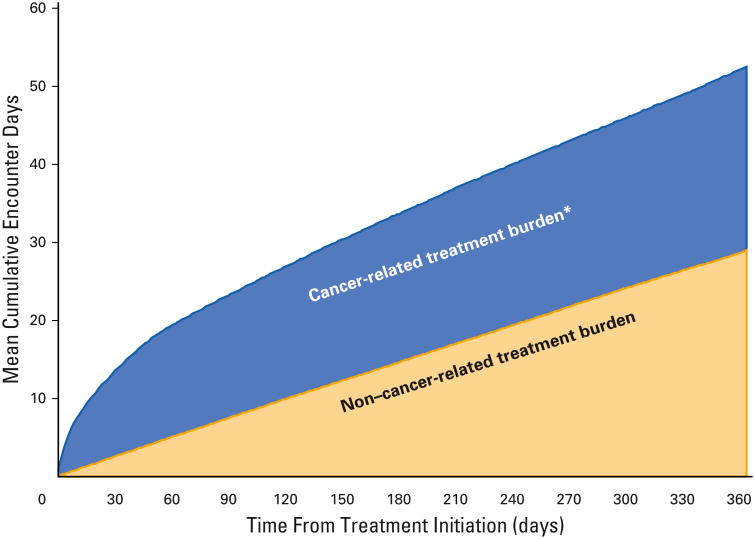
Wec%), SBRT (10.1%), or EBRT (13.5%). Overall, 30.4% of patients had three or more comorbidities and > 80% had a life expectancy of ≥ 5 years. During the first 60 days of treatment, an average of 1 in 3 days was spent interacting with the health care system. Cumulative encounter days for adults without cancer increased at a steady rate, spending 1 in 12 days interacting with the health care system in the same 60-day time period (Fig 1). During the 12 months post-treatment, the NSCLC group spent 44 encounter days (IQR, 29 to 66) interacting with the health care system compared with 19 encounter days (IQR, 10 to 34) in the pretreatment period (Table 2). Patients spent a median of 9 days (IQR, 5 to 20) in the hospital. Adults with NSCLC had a median number of 20 physicians (IQR, 14 to 28) who were involved in their care (Table 2). In the 12 months post-treatment, patients received care from a median of three general practitioners (IQR, 1 to 5), one cancer treatment specialist (IQR, 1 to 2), three non-cancer specialists (IQR, 1 to 5), and seven radiologists (IQR, 4 to 9). The median number of medications prescribed was 12 (IQR, 8 to 17), of which three (IQR, 3 to 3) were during the 12 months post-treatment.
Fig 1.
Medicare encounter days 1 year after curative treatment of stage I non–small cell lung cancer. *Cancer-related treatment burden was determined by subtracting the non–cancer-related encounter days in the year before treatment initiation from the total encounter days in the year after treatment initiation.
Table 2. Median Encounter Days After Diagnosis Stratified by Treatment Type and Care Category.
| Variable | Full Cancer Sample | Surgery | SBRT | EBRT | ||||
|---|---|---|---|---|---|---|---|---|
| Total (IQR) | Cancer Related (IQR)* | Total (IQR) | Cancer Related (IQR)* | Total (IQR) | Cancer Related (IQR)* | Total (IQR) | Cancer Related (IQR)* | |
| Total days | 44 (29-66) | 22 (21-22) | 41 (27-62) | 20 (8-38) | 39 (26-59) | 13 (2-30) | 64 (50-87) | 42 (24-61) |
| Acute days | 9 (5-16) | 7 (7-8) | 9 (6-16) | 8 (5-15) | 3 (0-9) | 1 (0-7) | 5 (0-13) | 2 (0-10) |
| Outpatient days | 30 (17-46) | 10 (9-10) | 26 (16-40) | 8 (-1-19) | 30 (20-44) | 9 (0-19) | 53 (41-67) | 35 (18-48) |
| Physicians | 20 (14-28) | 14 (13-14) | 22 (16-29) | 15 (9-23) | 16 (11-24) | 7 (2-14) | 16 (11-24) | 9 (4-17) |
| Medications | 12 (8-17) | 3 (3-3) | 12 (8-16) | 3 (0-7) | 14 (9-18) | 2 (22 to 5) | 13 (9-19) | 3 (21 to 7) |
Abbreviations: EBRT, external beam radiation therapy; IQR, interquartile range; SBRT, stereotactic body radiation therapy.
Calculated by subtracting pretreatment health care utilization from post-treatment health care utilization.
Treatment burden varied by treatment type. Patients who received surgery had a median of 20 (IQR, 8 to 38) cancer-related encounter days compared with 13 (IQR, 2 to 30) and 42 (IQR, 24 to 61) for SBRT and EBRT, respectively (P < .001; Table 2). The number of physicians seen was 15 (IQR, 9 to 23) for surgery and was lower for each type of radiation (P < .001). The number of medications was similar across treatment types. In the adjusted multivariable model, patients who were treated with surgery had a 21.6% (95% CI, 20.2 to 23.1) predicted probability of experiencing high treatment burden compared with 16.1% (95% CI, 12.9 to 19.3) and 46.8% (95% CI, 43.3 to 50.2) with SBRT and EBRT, respectively. High treatment burden and treatment type varied by the number of comorbidities (interaction P < .001). Patients with three or more comorbidities who were treated with surgery had a 35.8% predicted probability of experiencing high treatment burden compared with 29.0% for adults who were treated with SBRT (Appendix Fig A1, online only). There was no significant difference in treatment burden between surgery and SBRT among adults with fewer than three comorbidities (Fig A1). Patients who were treated with EBRT had the highest probability of high treatment burden as a result of the increased number of required treatment days.
In the multivariable model, a greater number of comorbid conditions was associated with higher treatment burden. The predicted probability of experiencing high treatment burden for adults with three or more comorbidities was 37.3% (95% CI, 34.8% to 39.7%) compared with 22.0% (95% CI, 20.3 to 23.7) with one to two comorbidities and 14.5% (95% CI, 12.7 to 16.4) with no comorbidities.
Observed 1-year mortality was 13.4% for all patients who were treated for early-stage NSCLC and 19.4% for those with multimorbidity. Seventeen percent of patients had a life expectancy of < 5 years. After the treatment burden assessment period, patients' 30-day and 90-day mortality was 16.6% and 18.5%, respectively. For patients who died and survived the 12-month post-treatment period, the IQR of encounter days was wide. Patients with NSCLC who survived experienced a median of 41 encounter days (IQR, 28 to 60) compared with 55 encounter days (IQR, 34 to 83) for patients who died.
Discussion
Adults who were treated for early-stage NSCLC experienced substantial treatment burden related to cancer care. Medicare beneficiaries spent an average of 1 in 3 days during the first 60 days of treatment interacting with the health care system. There was significant variation in treatment burden among patients who survived the full year and among those who died within 12 months post–treatment initiation. Among adults with multimorbidity, SBRT had significantly lower treatment burden compared with surgery in terms of total encounter days, hospital days, and physicians involved in care. Patients with multimorbidity have competing risks, which will result in higher treatment burden if a more invasive treatment option, such as surgery, is chosen overaless invasive treatment option, such as SBRT. EBRT was associated with the highest treatment burden because of the increased number of outpatient treatments required. EBRT requires several more treatment days compared with SBRT or surgery, which explains the higher number of encounter days. The probability of experiencing high treatment burden was greatest among patients with three or more comorbidities and among patients who were treated with EBRT. This is the first study, to our knowledge, to characterize treatment burden in terms of touches with the health care system among patients who were treated for early-stage NSCLC. With an aging, worldwide, cancer patient population, understanding the work of being a patient will become increasingly important.
Treatment burden is an important patient-centered quality measure, as it has the potential to guide clinician and patient treatment decision-making.9–11 Even with the best, newest, and most expensive cancer treatments, clinicians often cannot substantially improve mortality, but minimizing treatment burden is actionable and can have a dramatic impact on a patient's quality of life. Risks and costs of therapeutic choices have traditionally been part of discussions with patients; we believe that treatment burden should also be discussed, as it allows for tailoring the treatment delivery plan to aligned with the patient's priorities and functional capacity. Each additional complexity, whether it is an extra medication or additional outpatient visit, increases the risk of potential errors and adverse events. Future studies should prospectively validate treatment burden measures among patients with early-stage and advanced lung cancer, particularly among older patients with multimorbidity and functional limitations. Treatment burden should also be correlated with health outcomes, such as medication adverse events and quality of life.
The goal of minimally disruptive medicine is to alleviate treatment burden on patients and their caregivers by tailoring treatment regimens to fit the realities of people's daily lives and at the same time preserve their health goals.5 Even though this study was restricted to patients with early-stage disease, 17% of patients had a life expectancy of, 5 years and 19.4% of those with multimorbidity were deceased at 1 year. Many patients with multimorbidity will have a limited amount of lifetime post-treatment, which underscores the need for actionable strategies to minimize treatment burden. Patients should spend less time interacting with the health care system and more time on higher priority experiences. Expecting patients to spend 1 in 3 days interacting with the health care system may not be reasonable, particularly older patients with functional limitations. This study demonstrates a more complete understanding of treatment burden by using administrative claims, challenging physicians and health care systems to improve coordinated health care delivery and patient-centered care.
Minimizing treatment burden will improve value-based cancer care. The encounter days, physician visits, and medications currently required for cancer care are not only burdensome but can affect adherence to the treatment program and/or the ability of patients to face life demands that are unrelated to health care. When clinicians better understand the treatment burden that is placed on the patient and the limitations in addressing treatment demands, the health care community can identify how best to streamline care and to practice minimally disruptive medicine.5,17,18
Similar to chronic illness and a decreased life expectancy, the presence and complexity of treatment burden has important implications for screening decisions in the aging population.19 New screening guidelines will likely result in increased detection and subsequent treatment of early-stage lung cancers among older adults.20-22 In the National Lung Screening Trial, 50% of cancers diagnosed with computed tomography scan were early stage.23 Older adults need to make decisions about screening with a clear understanding of the overall burden that is associated not only with screening and diagnosis, but the consequences of treatment21,23
There are several limitations to this study. Medicare claims data do not contain covariates that impact encounter days (cognition and severity of competing comorbidities), nor does the data differentiate care delivered by multidisciplinary teams. Further more, our results may not be generalizable to patients with health insurance other than fee-for-service Medicare. Our data source does not include qualitative measures of treatment burden. Only recently have tools that are based on the burden of treatment theory become available to directly measure qualitative treatment burden.3 Not all encounter days are equivalent; for example, an EBRT visit is relatively short compared with an emergency department or hospitalization day. Treatment burden is a multidimensional framework that includes additional domains, such as financial and psycho-social demands, that were not included in this study.24
In conclusion, we demonstrated a substantial treatment burden exists among older patients with stage INSCLC, which varies on the basis of treatment type and comorbidity. Interventions to minimize treatment burden are needed to improve the value of early-stage NSCLC treatment. Future work should focus on shared decision-making interventions for treatment selection, comparison of different treatment modalities and their impact on patient-reported treatment burden in early-stage NSCLC, and identifying interventions that will streamline care delivery. As our health care system advances toward value-based and patient-centered care, treatment burden will be an important quality measure in addition to traditional clinical outcomes.
Supplementary Material
Fig A1. Predicted probability of high treatment burden by treatment type and comorbidity. High treatment burden was defined as the highest quartile of encounter days (≥ 66 encounter days). EBRT, external beam radiation therapy; SBRT, stereotactic body radiation therapy.
Table 1. Sample Characteristics: Cancer Cohort.
| Characteristic | No. (%) |
|---|---|
| Total | 7,955 |
|
| |
| Age, years | |
| 67-69 | 1,335 (16.8) |
| 70-74 | 2,327 (29.3) |
| 75-79 | 2,199 (27.6) |
| 80-84 | 1,448 (18.2) |
| 85-94 | 646 (8.1) |
|
| |
| Sex | |
| Male | 3,592 (45.2) |
| Female | 4,363 (54.9) |
|
| |
| Race | |
| White | 7,166 (90.1) |
| Black | 447 (5.6) |
| Other | 342 (4.3) |
|
| |
| Marital status | |
| Married | 4,270 (53.7) |
| Unmarried | 3,419 (43.0) |
| Unknown | 266 (3.3) |
|
| |
| Median household income, USD | |
| < 33,000 | 1,746 (22.0) |
| 33,000 to < 40,000 | 1,182 (14.9) |
| 40,000 to < 50,000 | 1,646 (20.7) |
| 50,000 to < 63,000 | 1,563 (19.7) |
| ≥ 63,000 | 1,818 (22.9) |
| Unknown | 0 (0.0) |
|
| |
| Comorbidity | |
| 0 | 2,013 (25.3) |
| 1-2 | 3,528 (44.4) |
| ≥ 3 | 2,414 (30.4) |
|
| |
| Life expectancy, years | |
| < 5 | 1,390 (17.5) |
| ≥ 5 | 6,565 (82.5) |
|
| |
| Treatment | |
| Surgery | 6,081 (76.4) |
| SBRT | 803 (10.1) |
| EBRT | 1,071 (13.5) |
Abbreviations: EBRT, external beam radiation therapy; SBRT, stereotactic body radiation therapy.
Acknowledgments
Supported by the Robert Wood Johnson Foundation Veterans Affairs Affiliation and the John A. Hartford Foundation. C.J.P. and C.P.G. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute's Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, Grant No. N01-PC-35139 awarded to the University of Southern California, and Grant No. N02-PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention's National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The authors of this report are responsible for its content. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. The authors acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare & Medicaid Servies; Information Management Services; and the SEER Program tumor registries in the creation of the SEER-Medicare database. The interpretation and reporting of the SEER-Medicare data are the sole responsibility of the authors.
Footnotes
Author Contributions: Conception and design: Carolyn J. Presley, Mary Tinetti, Victor M. Montori, James B. Yu, Cary P. Gross
Financial support: Carolyn J. Presley
Collection and assembly of data: Carolyn J. Presley, Pamela R. Soulos
Data analysis and interpretation: Carolyn J. Presley, Pamela R. Soulos, James B. Yu
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
Authors' Disclosures of Potential Conflicts of Interest: Treatment Burden of Medicare Beneficiaries With Stage I Non–Small-Cell Lung Cancer: The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jop/site/misc/ifc.xhtml.
Carolyn J. Presley, Research Funding: Celgene (Inst)
Pamela R. Soulos, Research Funding: 21st Century Oncology (Inst)
Mary Tinetti, No relationship to disclose
Victor M. Montori No relationship to disclose
James B. Yu, Research Funding: 21st Century Oncology (Inst)
Cary P. Gross, Research Funding: 21st Century Oncology (Inst), Johnson (Inst), Pfizer (Inst)
References
- 1.Tinetti ME, Naik AD, Dodson JA. Moving from disease-centered to patient goals–directed care for patients with multiple chronic conditions: Patient value-based care. JAMA Cardiol. 2016;1:9–10. doi: 10.1001/jamacardio.2015.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eton DT, Ramalho de Oliveira D, Egginton JS, et al. Building a measurement framework of burden of treatment in complex patients with chronic conditions: A qualitative study. Patient Relat Outcome Meas. 2012;3:39–49. doi: 10.2147/PROM.S34681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.May CR, Eton DT, Boehmer K, et al. Rethinking the patient: Using burden of treatment theory to understand the changing dynamics of illness. BMC Health Serv Res. 2014;14:281. doi: 10.1186/1472-6963-14-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henry DH, Viswanathan HN, Elkin EP, et al. Symptoms and treatment burden associated with cancer treatment: Results from a cross-sectional national survey in the U.S. Support Care. Cancer. 2008;16:791–801. doi: 10.1007/s00520-007-0380-2. [DOI] [PubMed] [Google Scholar]
- 5.May C, Montori VM, Mair FS. We need minimally disruptive medicine. BMJ. 2009;339:b2803. doi: 10.1136/bmj.b2803. [DOI] [PubMed] [Google Scholar]
- 6.Mair FS, May CR. Thinking about the burden of treatment. BMJ. 2014;349:g6680. doi: 10.1136/bmj.g6680. [DOI] [PubMed] [Google Scholar]
- 7.Presley C, Lilenbaum R. The treatment of advanced lung cancer in the elderly: The role of a comprehensive geriatric assessment and doublet chemotherapy. Cancer J. 2015;21:392–397. doi: 10.1097/PPO.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 8.Lochner KA, Goodman RA, Posner S, et al. Multiple chronic conditions among Medicare beneficiaries: State-level variations in prevalence, utilization, and cost, 2011. Medicare Medicaid Res Rev. 2013;3:3E1–E18. doi: 10.5600/mmrr.003.03.b02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Oncol. 2015;16:630–637. doi: 10.1016/S1470-2045(15)70168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shirvani SM, Jiang J, Chang JY, et al. Comparative effectiveness of 5 treatment strategies for early-stage non-small cell lung cancer in the elderly. Int J Radiat Oncol Biol Phys. 2012;84:1060–1070. doi: 10.1016/j.ijrobp.2012.07.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu JB, Soulos PR, Cramer LD, et al. Comparative effectiveness of surgery and radiosurgery for stage I non-small cell lung cancer. Cancer. 2015;121:2341–2349. doi: 10.1002/cncr.29359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palma D, Visser O, Lagerwaard FJ, et al. Treatment of stage I NSCLC in elderly patients: A population-based matched-pair comparison of stereotactic radiotherapy versus surgery. Radiother Oncol. 2011;101:240–244. doi: 10.1016/j.radonc.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 13.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. doi: 10.1001/jama.2010.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Cancer Institute. SEER cancer statistics review, 1975-2012. http://seer.cancer.gov/archive/csr/1975_2012/
- 15.Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Gross CP, Soulos PR, Ross JS, et al. Assessing the impact of screening colonoscopy on mortality in the medicare population. J Gen Intern Med. 2011;26:1441–1449. doi: 10.1007/s11606-011-1816-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shippee ND, Shah ND, May CR, et al. Cumulative complexity: A functional, patient-centered model of patient complexity can improve research and practice. J Clin Epidemiol. 2012;65:1041–1051. doi: 10.1016/j.jclinepi.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Leppin AL, Montori VM, Gionfriddo MR. Minimally disruptive medicine: A pragmatically comprehensive model for delivering care to patients with multiple chronic conditions. Healthcare (Basel) 2015;3:50–63. doi: 10.3390/healthcare3010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gross CP, McAvay GJ, Krumholz HM, et al. The effect of age and chronic illness on life expectancy after a diagnosis of colorectal cancer: Implications for screening. Ann Intern Med. 2006;145:646–653. doi: 10.7326/0003-4819-145-9-200611070-00006. [DOI] [PubMed] [Google Scholar]
- 20.US Department of Health and Human Services. Profile of older Americans: 2013–Administration on Aging. http://www.aoa.acl.gov/Aging_Statistics/Profile/2013/index.aspx.
- 21.Moyer VA. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160:330–338. doi: 10.7326/M13-2771. [DOI] [PubMed] [Google Scholar]
- 22.American Cancer Society. Cancer facts & figures 2016. http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/
- 23.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sav A, King MA, Whitty JA, et al. Burden of treatment for chronic illness: A concept analysis and review of the literature. Health Expect. 2013;18:312–324. doi: 10.1111/hex.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig A1. Predicted probability of high treatment burden by treatment type and comorbidity. High treatment burden was defined as the highest quartile of encounter days (≥ 66 encounter days). EBRT, external beam radiation therapy; SBRT, stereotactic body radiation therapy.