Abstract
“Let’s Move!” is a comprehensive initiative, launched by the First Lady, Michelle Obama, dedicates to solving problems of obesity, which is growing in child. The life behaviors do affect obesity; however, the mechanistic insight in molecular level is still not clear. In this study, by continually monitoring mouse body weight under chow and high fat western diets as well as metabolic, physical activity and food intake behaviors assessed in a CLAMS Comprehensive Lab Animal Monitoring System, we demonstrated that the platelet-activating factor receptor (PTAFR) contributes to modification of life behaviors. PTAFR does not affect metabolism of ingested dietary fat and carbohydrate in young animals; however, Ptafr ablation dramatically increased weight gain without affecting adipose tissue accumulation. Ptafr−/− mice possess new habits that increased food intake and decreased movement. Our studies suggest that regulation of PTAFR activity may be a novel strategy to control obesity in children or young adults.
Keywords: Behavior, Food intake, Obesity, Physical activity, Platelet-activating factor
Introduction
Obesity is a growing worldwide health problem that is associated with an increased risk of cardiovascular diseases, type II diabetes as well as cancer, and is positively correlated with the presence of inflammatory factors.1 This population is becoming young and children obesity has been a serious public health concern to be solved. Platelet-activating factor (1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine, PAF) is a potent autocoid—locally signaling hormone—that initiates and localizes acute inflammation.2 PAF initiates signaling after engagement of a G-protein coupled receptor, the PAF receptor (PTAFR), with exceedingly high affinity.3–5 PTAFR is expressed by most cells of the innate immune and vascular systems, but is absent on hepatocytes.6 PTAFR is unique in recognizing PAF to the exclusion of other physiologic phospholipids, although a range of phospholipids generated by unregulated oxidative truncation of polyunsaturated choline phospholipids are PTAFR agonists and are biologically active.7
Despite the widespread roles of PAF and PTAFR in cell activation ex vivo, genetic ablation of Ptafr does not interfere with birth or viability, nor even induce marked changes to the inflammatory system in the absence of overt stimulation.3,4 We employed Ptafr−/− mice to assess circulating PAF and PAF-like oxidized phospholipids in blood of older animals,8 and noted that most of these aged mice were significantly obese when compared to age-matched wild type C57BL6 mice (WT) or Apoe null mice (data not shown). This phenotype was found in both genders. Our findings are identical to the recent studies which reported that loss of PTAFR increases adiposity over time, although the mechanism for this weight gain is not fully elucidated. 9,10 In this study, we provide additional evidence and mechanistic insights regarding the role of PTAFR in obesity. We found young Ptafr null mice were “couch potatoes” that ingested significantly more food and moved significantly less frequently. We conclude PTAFR signaling contributes to behavior control in early life through the dual actions of enhanced movement and feeding suppression separate from a metabolic role, implicating PTAFR signaling in behavior modification.
Material and methods
All procedures and manipulations of mice have been approved by the Institutional Animal Care and Use Committee (IACUC) of The Cleveland Clinic in accordance with the United States Public Health Service Policy on the Humane Care and Use of Animals, and the NIH Guide for the Care and Use of Laboratory Animals. WT mice were purchased from Jackson Laboratories. Ptafr−/− mice, which have been back-crossed to the C57/BL6 mouse more than 10 times, were from Jeffery Travers (University of Indiana) with permission of the strain’s originator Takao Shimizu (University of Tokyo). Animals were identified by ear tags and fed ad libitum with regular chow (CD, 8604 Teklad Rodent Diet) with the calorie contributions from protein, fat, and carbohydrate being 32%, 14% and 54%, respectively. Harlan TD.88137 “Western diets” (WD) was used to accelerate gain of body weight and the calorie contributions from protein, fat, and carbohydrate were 15.2%, 42% and 42.7%. TD.88137 also includes 0.2% cholesterol by weight. Mice were weighed weekly.
Behavior and metabolism assessment
Mouse metabolism was assessed using an Oxymax Lab Animal Monitoring System: CLAMS Comprehensive Lab Animal Monitoring System (Columbus Instruments, Columbus, OH). This system can continuously monitor food intake as well as movement detection by interruption of triple axis infrared beams. Interruption of a beam in any dimension accrues as a single “count”. This system also continuously monitors the volume of oxygen consumed and carbon dioxide exhaled, which can be used to calculate the energy content of the foodstuff utilized by the mouse. Mice were placed in individual isolator cages and allowed to acclimate to the environmental chamber for two days prior to data collection. The data were collected over a two-day period after acclimation.
Body composition
At the end of experiments, the mice were weighted and anesthetized with Ketamine/Xylazine (100/10 mg/kg, IP injection). The abdominal cavity was opened via middle incision and then abdominopelvic fat was isolated from gonadal organs, and its wet weight measured. Liver was isolated from surrounding tissue, all visible ligaments and vessels were removed, and then the wet weight was immediately measured. Data are presented as ratio of the weight of abdominopelvic fat or liver to the body weight.
Statistical analysis
Statistical analyses were performed using Prisim4 (Graph-Pad) software. One-way ANOVA (Bonferroni/Dunn) was used to determine the differences among groups. Unpaired t test was used to determine differences between groups. Data are presented as the mean ± SEM, with p < 0.05 considered significant.
Results and discussion
Ptafr−/− mice excessively gain weight irrespective the type of diet
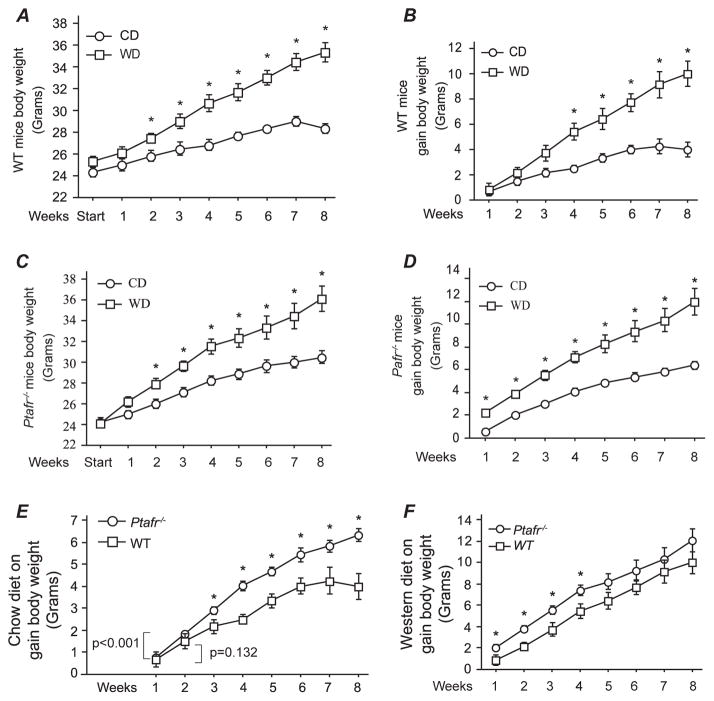
PAF is a potent inflammatory mediator,5 but PTAFR also participates in non-inflammatory events. PAF modulates complex physiologic events including blood pressure regulation, 11 matrix production by renal tubulointerstitial epithelial cells,12 vascular permeability in kidney,13 and reproduction.4 A western diet (WD) induces chronic and low level of inflammation,14,15 so to determine if potential dietmediated inflammation contributes to Ptafr-null-mediated gain of body weight, we fed the WT and Pafr−/− mice with control diet (CD) or WD and body weight were monitored continually up to 8 weeks. The study was initiated in 8-week-old mice as at this moment there is no difference in body weight was found between the two strains. The body weight of the 8-week-old WT mice ingesting CD increased linearly and reached a final weight of approximately 27 g after 8 weeks feeding period (Fig. 1A), and gain about 4 g body weight (Fig. 1B). As predicted, when WT mice were fed WD, they gained weight at a greater rate than that of CD-fed mice (Fig. 1A), and the difference became significant from week two of the trial, and increased over time without achieving a constant value. Ultimately, these WD-fed WT mice gained almost 10 g of weight over the eight weeks feeding period (Fig. 1B). Ptafr−/− mice ingesting CD also significantly gained weight starting from the second week onwards, which, in contrast to WT mice, continued over the full 8 weeks feeding trial (Fig. 1C). As anticipated, the weight gain by Ptafr−/− mice ingesting a WD was also robustly increased. The rates of weight change continued throughout the trial in the Ptafr−/− mice fed either a CD or WD, although the rate of change was slower for the CD-fed mice (Fig. 1D).
Fig. 1.
Ptafr deletion causes excessive weight gain. A & B. Body weight of 8-week old WT mice fed a chow diet (CD) or western style high fat diet (TD.88137, WD) over the subsequent 8 weeks was monitored. A is showing the total body weight and B is the differential gain in body weight over times. C & D. Body weight of 8-week-old Ptafr−/− mice fed a CD or WD over the subsequent 8 weeks was measured. C is the total body weight and D is the differential gain in body weight. E & F. The differential gain in body weight of WT and Ptafr−/− mice in the 8-week feeding trial ingesting a CD (E) or WD (F) was compared. *p < 0.05, between groups at each time point, n = 8 in each group.
Comparison of weight gain over time between WT and Ptafr−/− animals revealed that Ptafr−/− animals ingesting either a CD (Fig. 1E) or WD (Fig. 1F) gained weight at a significantly increased rate over WT animals. The largest divergence, however, occurred in the CD feeding arm of the experiment (Fig. 1E). WD consumption has been demonstrated inducing hypothalamic inflammation which contributes to obesity pathogenesis through the development of central leptin and insulin resistance.14,15 There was no difference in gain of weight in the late phase of WD fed WT and Ptafr−/− mice, suggesting that PTAFR does not contribute to WD-induced inflammation, but rather PTAFR plays a physiological role in controlling body weight.
Loss of PTAFR signaling did not promote aberrant fat accumulation
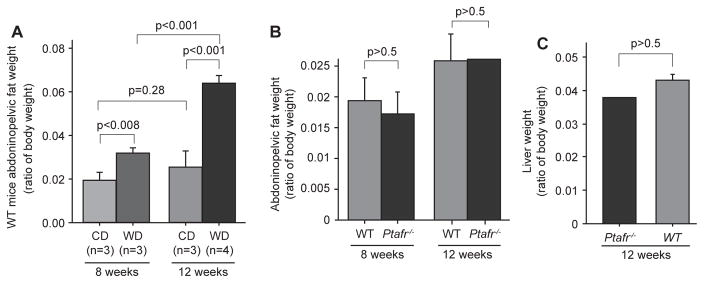
To understand how PTAFR affects body weight, we determined whether the enhanced weight gain in Ptafr−/− mice is due to accumulation of adipose tissue. As anticipated, in comparison to CD-fed animals, total abdominopelvic fat became significantly more abundant when fed a WD in WT mice (Fig. 2A) at both 8 and 12 weeks, although the abdominopelvic fat did not significantly increase in mice fed the CD over this time (p = 0.28). However, the ratio of abdominopelvic fat mass to total body weight was nearly equivalent between Ptafr−/− and WT mice fed a CD for both 8 and 12 weeks (Fig. 2B). The total liver weight also was comparable between the Ptafr−/− and WT mice feeding a CD for 12 weeks (Fig. 2C). This outcome implies that adiposity alone does not account for the increased body weight of Ptafr−/− mice and that this tissue grows in proportion with body size in animals lacking the PTAFR. This phenotype differs from weight gain through adiposity reported before.9
Fig. 2.
WT and Ptafr−/− mice have similar accumulation of adipose tissue under dietary trail. A. Eight-week old WT mice were fed with a CD or WD for 8 or 12 weeks and then abdominopelvic fat was isolated and wet weight was measured. Data were presented as ratio of fat mass to body weight. B & C. Eight-week old WT and Ptafr−/− mice were fed with a CD for 8 and 12 weeks, and abdominopelvic fat mass (B) and liver weight (C) were measured. n = 3 in the WT groups, and n = 3 at 8 weeks, n = 2 at 12 weeks in the Ptafr−/− groups.
Ptafr deletion altered behavior, not energy metabolism
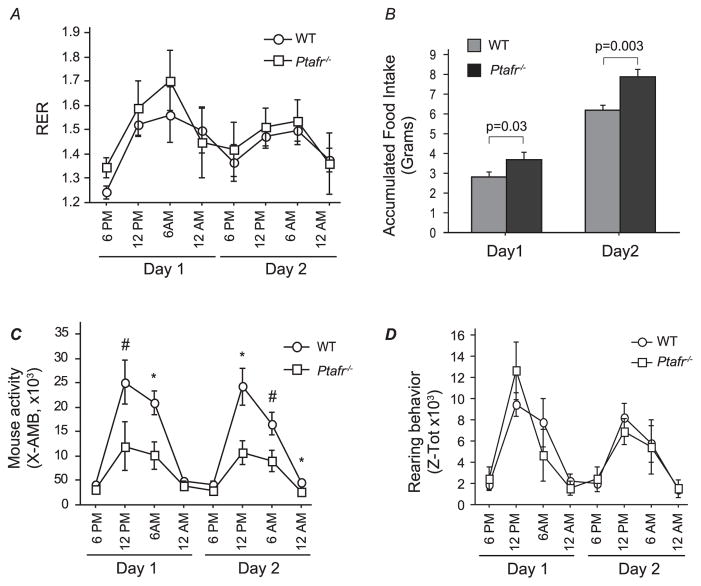
To determine if PAF and its receptor play a role in energy metabolism and thus control body weight, we used a CLAMS animal monitoring system to quantify O2 consumption and CO2 production as a measure of metabolism and energy production from the ingested foodstuff. We examined mice ingesting the CD since this diet generated the largest difference in body weight between the strains. We found a diurnal variation in the respiratory quotient that was maximal during the dark period in both WT and Ptafr−/− mice (Fig. 3A). The patterns of the respiratory exchange ratios overlaid one another, showing that there was no significant difference in energy expenditure between the two mouse strains. These data suggest that loss of Ptafr did not affect dietary fat or carbohydrate metabolism.
Fig. 3.
Ptafr deletion alters feeding and movement behaviors, but not energy metabolism monitored in a Comprehensive Lab Animal Monitoring System (CLAMS). Eight-week old WT and Ptafr−/− mice with same body weight ingesting a CD were monitored for 2 days after a 2 day acclimation period and the following data were harvested. A. The respiratory exchange rate (RER). B. Accumulated food consumption over 24 h by individual WT and Ptafr−/− animals. C. Ambulatory movement detected by interruption of an infrared beam in three dimensions over the stated interval. n = 3, *p < 0.05; #p < 0.01 Ptafr−/− vs. WT mice. D. Rearing behavior monitored simultaneously as mentioned in panel C.
The food weight was also monitored over the trail. The CLAMS system tracks both food removed from the sensor as well as food and the debris not ingested by the mice. Data would have been less accurate when food intake was measured weekly without specifically accounting for food waste. We found that Ptafr−/− mice ingested approximately 25% more diet than the WT mice over the two days of metabolic analysis (Fig. 3B). This difference in accumulated food intake was highly significant (p = 0.003), suggesting that PTAFR modifies food intake behavior.
Interestingly, when animal activities were monitored, we found a pattern of lateral diurnal movement of WT and Ptafr−/− mice that was maximal during the dark cycle from 6 pm to 6 am (Fig. 3C). Notably, however, the activity of the Ptafr−/− mice was significantly less during the dark cycle of each night than that of WT mice, with just as half as many lateral beam disruptions by the Ptafr−/− mice did. In contrast, the number of rearing motions did not differ between the two strains (Fig. 3D). Rearing behavior is one measure of interest in the novelty of the environment and vigilance, suggesting that these two strains have similar level of anxiety. In addition, these metabolic data were harvested from 8-week-old mice and at this moment the weight was not different between strains, indicating that less movement of the Ptafr−/− mice was not related to its weight. We thus conclude that Ptafr−/− mice were less active, but also prone to ingest larger amounts of food.
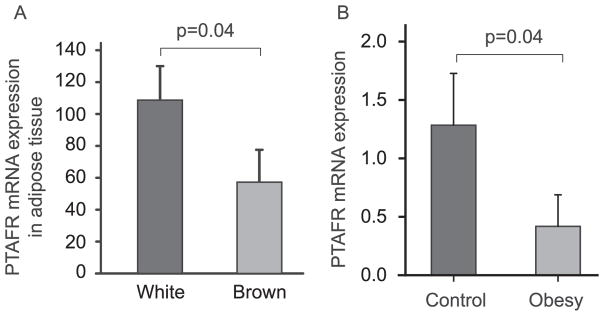
Our findings are supported by several gene expression studies. It has been indicated that obesity-resistant rats have more PTAFR expression in liver (GEO profiles, GDS3677/rn1900/Ptafr). A previous report indicated that the anti-obesity effect of PTAFR results from altered function of brown adipose.9 However, PTAFR expression is significantly lower in the brown adipose tissue than in the white adipose tissue (Fig. 4A) (GDS2813/1427872_at/ Ptafr),16 suggesting that PTAFR may function more on white adipose tissue. This hypothesis is supported by another gene expression experiment, in which expression of PTAFR in omental adipose tissue, a white adipose, was dramatically reduced in obese children (GDS3688/211661_x_at/ PTAFR) (Fig. 4B).
Fig. 4.
Gene Expression Omnibus (GEO) analysis of PTAFR expression. A. PTAFR expression in the white and brown adipose tissue was compared based on the data extracted from GDS2813/1427872_at/Ptafr). B. PTAFR expression in omental adipose tissue in normal and obese children was compared based on data extracted from the GDS3688/211661_x_at/PTAFR. One-tail t test were used for the analysis.
PTAFR is expressed by, and functions in, the central nervous system. PTAFR signaling increases kinase cascades in the hippocampus,17 reduces activity of inhibitory GABA neurons,18 and signals neuronal migration19 and death20 in neurons and microglia. PAF orchestrates complex responses in brain, contributes to long-term potentiation in the somatosensory cortex,21 learning22 and memory.23 PTAFR thus possesses the capacity to regulate behavior through actions in the central nervous system. However, more detailed studies are necessary to clarify how PTAFR affects lifestyles as well as its relationship to the obesity. Our novel findings provide conceptual insights to control obesity, especially in child and young adults, through regulating PTAFR mediated life behavior modifications.
Acknowledgments
We thank Drs. Takao Shimizu (University of Tokyo) and Jeffrey Travers (University of Indiana) for providing the Ptafr−/− mice. We appreciate the advice of Mark Brown, from the Department of Cellular & Molecular Medicine, and the aid provided by Jazmine Danner and Ofer Reizes of the Rodent Behavioral core facility of the Lerner Research Institute. This work was supported by National Institute on Alcohol Abuse and Alcoholism (NIAAA) 017748.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Conflicts of interest
All authors have none to declare.
References
- 1.Kalin S, Heppner FL, Bechmann I, Prinz M, Tschop MH, Yi CX. Hypothalamic innate immune reaction in obesity. Nat Rev Endocrinol. 2015;11:339–351. doi: 10.1038/nrendo.2015.48. [DOI] [PubMed] [Google Scholar]
- 2.Prescott SM, Zimmerman GA, McIntyre TM. Platelet-activating factor. J Biol Chem. 1990;265:17381–17384. [PubMed] [Google Scholar]
- 3.Honda Z, Ishii S, Shimizu T. Platelet-activating factor receptor. J Biochem. 2002;131:773–779. doi: 10.1093/oxfordjournals.jbchem.a003164. [DOI] [PubMed] [Google Scholar]
- 4.Ishii S, Nagase T, Shimizu T. Platelet-activating factor receptor. Prostagl Other Lipid Mediat. 2002;68–69:599–609. doi: 10.1016/s0090-6980(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 5.Marathe GK, Davies SS, Harrison KA, et al. Inflammatory platelet-activating factor-like phospholipids in oxidized low density lipoproteins are fragmented alkyl phosphatidylcholines. J Biol Chem. 1999;274:28395–28404. doi: 10.1074/jbc.274.40.28395. [DOI] [PubMed] [Google Scholar]
- 6.Shimizu T, Mutoh H, Kato S. Platelet-activating factor receptor. Gene structure and tissue-specific regulation. Adv Exp Med Biol. 1996;416:79–84. [PubMed] [Google Scholar]
- 7.McIntyre TM. Bioactive oxidatively truncated phospholipids in inflammation and apoptosis: Formation, targets, and inactivation. Biochim Biophys Acta. 2012;1818:2456–2464. doi: 10.1016/j.bbamem.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Li W, Chen R, McIntyre TM. Circulating biologically active oxidized phospholipids show on-going and increased oxidative stress in older male mice. Redox Biol. 2012;1:110–114. doi: 10.1016/j.redox.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugatani J, Sadamitsu S, Yamaguchi M, et al. Antiobese function of platelet-activating factor: increased adiposity in platelet-activating factor receptor-deficient mice with age. FASEB J. 2014;28:440–452. doi: 10.1096/fj.13-233262. [DOI] [PubMed] [Google Scholar]
- 10.Menezes-Garcia Z, Oliveira MC, Lima RL, et al. Lack of platelet-activating factor receptor protects mice against diet-induced adipose inflammation and insulin-resistance despite fat pad expansion. Obesity. 2014;22:663–672. doi: 10.1002/oby.20142. [DOI] [PubMed] [Google Scholar]
- 11.Malekin SI, Kotelevtsev SV, Gavrilova SA, et al. Long-term normalization of blood pressure in SHR and 1-kidney 1-clip rats by synthetic precursor of stable PAF analogue without systemic effects in normotensive rats. Pathophysiology. 2011;18:151–157. doi: 10.1016/j.pathophys.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Ruiz-Ortega M, Bustos C, Plaza JJ, Egido J. Overexpression of extracellular matrix proteins in renal tubulointerstitial cells by platelet-activating-factor stimulation. Nephrol Dial Transplant. 1998;13:886–892. doi: 10.1093/ndt/13.4.886. [DOI] [PubMed] [Google Scholar]
- 13.Pirotzky E, Page C, Morley J, Bidault J, Benveniste J. Vascular permeability induced by Paf-acether (platelet-activating factor) in the isolated perfused rat kidney. Agents Actions. 1985;16:17–18. doi: 10.1007/BF01999630. [DOI] [PubMed] [Google Scholar]
- 14.Thaler JP, Schwartz MW. Minireview: inflammation and obesity pathogenesis: the hypothalamus heats up. Endocrinology. 2010;151:4109–4115. doi: 10.1210/en.2010-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dixit VD. Adipose-immune interactions during obesity and caloric restriction: reciprocal mechanisms regulating immunity and health span. J Leukoc Biol. 2008;84:882–892. doi: 10.1189/jlb.0108028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seale P, Kajimura S, Yang W, et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moriguchi S, Shioda N, Yamamoto Y, Fukunaga K. Platelet-activating factor-induced synaptic facilitation is associated with increased calcium/calmodulin-dependent protein kinase II, protein kinase C and extracellular signal-regulated kinase activities in the rat hippocampal CA1 region. Neuroscience. 2010;166:1158–1166. doi: 10.1016/j.neuroscience.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Chen C, Bazan NG. Platelet-activating factor inhibits ionotropic GABA receptor activity in cultured hippocampal neurons. Neuroreport. 1999;10:3831–3835. doi: 10.1097/00001756-199912160-00020. [DOI] [PubMed] [Google Scholar]
- 19.Tokuoka SM, Ishii S, Kawamura N, et al. Involvement of platelet-activating factor and LIS1 in neuronal migration. Eur J Neurosci. 2003;18:563–570. doi: 10.1046/j.1460-9568.2003.02778.x. [DOI] [PubMed] [Google Scholar]
- 20.Bate C, Kempster S, Williams A. Platelet-activating factor antagonists protect amyloid-beta damaged neurons from microglia-mediated death. Neuropharmacology. 2006;51:173–181. doi: 10.1016/j.neuropharm.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Heusler P, Boehmer G. Platelet-activating factor contributes to the induction of long-term potentiation in the rat somatosensory cortex in vitro. Brain Res. 2007;1135:85–91. doi: 10.1016/j.brainres.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 22.Row BW, Kheirandish L, Li RC, et al. Platelet-activating factor receptor-deficient mice are protected from experimental sleep apnea-induced learning deficits. J Neurochem. 2004;89:189–196. doi: 10.1111/j.1471-4159.2004.02352.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen C, Bazan NG. Lipid signaling: sleep, synaptic plasticity, and neuroprotection. Prostagl Other Lipid Mediat. 2005;77:65–76. doi: 10.1016/j.prostaglandins.2005.07.001. [DOI] [PubMed] [Google Scholar]